Abstract
Anaplastic thyroid carcinoma (ATC) is the most aggressive thyroid cancer variant, accounting for 1–2% of all cases, but 33% of deaths, and exhibiting an average life expectancy of 5 months. ATC is largely unresponsive to radioactive iodine, chemotherapy, external beam radiation or surgery, underscoring the need for new and effective therapies. We evaluated the therapeutic potential of an oncolytic adenovirus, ONYX-411, that replicates selectively in and kills cells with dysfunction of the retinoblastoma (RB) pathway. In the present study, we report that ONYX-411 is able to induce cell death in eight human anaplastic carcinoma cell lines in vitro. The cytopathic effect of the virus is specific to cells with RB dysfunction, which appears to be frequent in ATC. We confirmed the expression of the coxsackie adenovirus receptor, CAR, in all ATC cell lines, demonstrating the potentially universal application of this oncolytic viral therapy to ATC. In addition, the growth of xenograft tumors induced in athymic mice with the ARO and DRO cell lines was significantly reduced by ONYX-411 treatment. These results indicate that ONYX-411 can be a potential therapeutic agent for the treatment of ATC, rendering this class of conditionally replicating adenoviruses an attractive candidate for clinical trials.
Keywords: anaplastic thyroid carcinoma, ONYX-411, retinoblastoma dysfunction, novel therapeutic agent
Introduction
The American Cancer Society estimates that about 37 000 thyroid cancer cases will be diagnosed in the United States in 2008,1 of which 1–2% will be anaplastic thyroid carcinoma (ATC). Though rare, ATC is the most aggressive form of thyroid cancer, with a mean life expectancy of 2–7 months from the time of diagnosis. It accounts for 20–40% of the annual ~1500 thyroid cancer deaths in the United States.2 ATCs are poorly differentiated tumors that exhibit resistance to most treatment modalities, probably because they have sustained alterations in multiple oncogenic pathways, accompanied by a high degree of intratumor clonal diversity. Consequently, the 1-year survival rate after diagnosis is only from 5 to 15%, even with aggressive, multimodal therapy involving surgery, radiotherapy and chemotherapy.2,3 As single-modality therapy has been shown to have a limited effect on ATC, multimodality therapy has become the treatment of choice in recent years with the hope of leading to at least reduced tumor mass and palliative control of the cancer. Keeping in mind the aggressive nature of this cancer and the minimum treatment window, there is a need for the development and evaluation of novel treatment strategies.
Most studies on prevention and therapy of ATC have concentrated on the use of small molecules that can inhibit the growth of ATC cell lines in culture and in mouse tumor explant models. Peroxisome proliferator-activated receptor-γ agonists4 and 17-allylaminogeldanamycin (17-AAG) treatment, respectively, 5 are currently being tested in human clinical trials of differentiated thyroid carcinoma at Mayo Clinic (RC Smallridge, personal communication). There has been interest in exploiting specific genetic abnormalities found in tumors to affect selective and efficient tumor cell killing, and ATCs represent potentially suitable targets for such specific tumor targeting due to the fact that they develop through progressive accumulation of changes in several oncogenic pathways, including p53 and retinoblastoma (RB).6,7 The RB pathway is disrupted in nearly all human cancers.8 RB loss or inactivation is a major mechanism by which tumor cells attain a growth advantage during tumorigenesis.9 RB plays a critical role in cell cycle regulation, stem cell maintenance, tissue regeneration, differentiation and developmental programs. Most of the components of the RB pathway qualify as protooncogenes or tumor suppressors, and each of them can become deregulated through several distinct molecular mechanisms such as gene amplification, chromosomal translocation or inversion, activating or inactivating mutations, promoter silencing by methylation, inactivation by protein–protein sequestration, aberrantly enhanced or inhibited protein turnover and activation by proviral integration.9 The RB pathway is impaired in its function in the majority of advanced human cancers, including ATC, regardless of whether RB itself is mutated or not. RB mutations in thyroid neoplasms have been documented in a small number of cases10 and RB pathway dysfunction appears to be a common feature of thyroid cancer.11 A number of strategies have been employed to target defects in this pathway with the hope of developing new therapeutics.12
With the aim of identifying an effective and fast-acting therapeutic option for ATC, we focused on oncolytic viruses, which target and specifically destroy cancer cells, while leaving normal tissues relatively unharmed. Oncolytic viruses have been used as therapy for several cancers;13 however, limited efforts have thus far gone into understanding their potential for the therapy of ATC. Yu et al.14 evaluated the efficacy of a replication competent, attenuated oncolytic herpes simplex virus, NV1023, which appeared to be effective both in vitro and in vivo. Two different studies have tested conditionally replicative oncolytic adenoviruses as therapeutic agents for ATC. One study involved ONYX-015, which is genetically modified to contain a deletion of the E1B early gene, designed to replicate and cause death preferentially in p53-mutated cells.15 The other study evaluated HILMI, wherein the T-cell factor response elements drive E1A and E1B expression of the adenovirus, thereby allowing the virus to replicate specifically in cells with an active Wnt/β-catenin pathway.16 All these agents showed promise in preclinical studies, but only ONYX-015 has been tested in clinical trials for cancer with excellent safety, but limited efficacy in US trials.17 The limitation of ONYX-015 was its lack of specificity in that it also targeted normal cells although a similar virus has been approved in China for the clinical treatment of head and neck cancer.18 Further, in a comprehensive review of 31 studies, Lam et al.19 demonstrated that only 52% of ATC revealed either p53 protein or TP53 gene alterations when evaluated with a variety of molecular techniques, limiting the efficacy of ONYX-015 as a therapeutic agent for ATC.
The present study evaluates ONYX-411, a conditionally replicative oncolytic adenovirus, which has the ability to selectively replicate in a broad array of human tumor cell types with defective RB pathway status. In normal cells, the universal initiator of cell cycle progression, E2F, is bound and inactivated by RB, whereas in RB pathway-deficient cells, E2F levels are high, promoting cell replication. ONYX-411 takes advantage of this by placing the expression of the E1A and E4 genes, which are required for viral replication under the control of the human E2F promoter.20 As E2F levels are high in tumor cells, this promotes viral replication. E1A also binds to RB, releasing E2F and initiating cell cycle progression, which is also crucial for replicating the virus itself. To prevent adenoviral-induced cell cycle progression in normal cells, the E1A gene was modified. For human adenovirus, two non-contiguous domains of E1A, conserved region 1 (CR1, low affinity) and conserved region 2 (CR2, high affinity), are required to mediate RB binding and E2F release, thereby promoting S phase entry.21 ONYX-411 contains a 24-bp deletion within the E1A CR2 domain, preventing RB binding and adenoviral replication in normal cells. When originally developed in 2002, it was demonstrated that the combination of these features in ONYX-411 was crucial for selective viral gene expression, replication and progeny production, specifically in tumor but not in normal human cells.20 These modifications also resulted in reduced in vivo systemic toxicity and a survival benefit in animal models of human cancer following systemic administration.20
In the present report, we demonstrate for the first time that ONYX-411 can be used to selectively target and destroy ATC cell lines with RB dysfunction in vitro and suppress the growth of xenograft tumors in vivo. We also demonstrate that RB dysfunction occurs at a high frequency in ATC. These data together with our observation that the coxsackie adenovirus receptor (CAR) is expressed in all ATC cell lines tested underscore the potential of ONYX-411 as a therapeutic agent for ATC.
Materials and methods
Cell lines
The human embryonic kidney cell line, HEK293 (CRL-1573), was purchased from American Type Culture Collection (ATCC, Manassas, VA) and cultured according to specifications. The ATC-derived cell lines FRO, ARO and DRO were obtained from Dr G Juillard, University of California Los Angeles. SW1736 (gift from Drs Leibowitz and McCombs, Scott and White Memorial Hospital, Temple, TX), BHT101 (gift from Drs Istvan and Palyi, National Institute of Oncology, Budapest, Hungary), KTC-2 and KTC-3 (gift from Dr Junichi Kurebayashi, Kawasaki Medical School, Okayama-Ken, Japan), KAT-4 (gift from Dr Ken Ain, University of Kentucky) and OCUT-1 (gift from Dr Naoyoshi Onoda, Osaka City University, Graduate School of Medicine, Osaka, Japan) were cultured in RPMI-1640 containing 10% charcoal-stripped fetal bovine serum (Biomeda, Foster City, CA), non-essential amino acids, sodium pyruvate, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid buffer and penicillin–streptomycin antimycotic. Normal human thyrocytes were cultured in RPMI-1640 containing 10% fetal bovine serum, insulin, transferrin, selenium (ITS), epidermal growth factor, triiodothyronine and penicillin–streptomycin. All cell lines were grown at 37 °C in a humidified atmosphere of 5% CO2.
Reagents
p53 (DO-1) antibody was purchased from Santa Cruz Biotechnology Inc. (Santa Cruz, CA). RB (4H1) and CAR (RmcB) were purchased from Cell Signaling Technology Inc. (Danvers, MA) and Upstate Biohemicals (Lake Placid, NY), respectively. Anti-human β-actin antibody and MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) reagent were obtained from Sigma-Aldrich (St Louis, MO).
Adenoviral amplification and titer
Original stocks of ONYX-411 were obtained from Onyx Pharmaceuticals (Emoryville, CA). Viruses were amplified in HEK293 cells and purified by CsCl2 gradient ultracentrifugation according to specifications.20 The virus titer was determined by plaque assay on HEK293 cells.
Cell viability assay
ATC cell lines were seeded in 12-well plates (at 5×104 cells per well) and treated with ONYX-411 at multiplicity of infection (MOI) 50 after 24 h,20 except for the dose curve in which cells were infected with MOI 25, 50,100 or 200. Cell survival was evaluated 3 or 6 days after infection using the MTT cell viability assay.22 The percentage of surviving cells was calculated as the ratio of absorbance at 490 nm in the infected cells compared with that of the mock-infected cells. The experiments were repeated three times and each condition was replicated six times in each experiment.
Western blot analysis
ATC cell lines were plated on 100-cm plates. Forty-eight (48) hours later, cells were harvested and immunoblotting was carried out using standard methods to determine the expression levels of p53, RB and CAR. Human β-actin was evaluated as a control to ensure uniform loading. Antibody binding was visualized by enhanced chemiluminescence (Pierce, Rockford, IL) and images were obtained using a KODAK IS4000MM digital imager.
Xenograft experiments
We evaluated the antitumor effects of ONYX-411 in a xenograft model using the ARO and DRO human anaplastic thyroid cancer cell lines. A total of 1×106 cells were injected subcutaneously into 3- to 4-week-old athymic nude mice (Harlan Sprague Dawley Company, Indianapolis, IN). Tumor nodules were allowed to grow subcutaneously to approximately 50–200 mm3 in size. The mice were randomly divided into two groups that received intratumoral injections of 5×107 plaque-forming units of virus in a total volume of 50 μl per tumor of either the ONYX-411 or the ultraviolet (UV)-inactivated control virus for five consecutive days. Tumor size was measured every 3 days and tumor volume was calculated according to the formula: Vtumor = 0.5236 (l × w × h), where, l, w and h represent length, width and height. The control virus was obtained by UV inactivation of ONYX-411 with three cycles of crosslinking (120 000 μJ) in a UV Stratalinker (Stratagene, La Jolla, CA). All experiments were conducted in accordance with accepted standards of animal care, and this study was approved by the Mayo Institutional Committee on animal care.
Statistical analysis
Quantitative variables such as cell viability measured by MTT assay after virus infection and mouse tumor volumes were analyzed with a two-sample paired t-test. Xenograft tumors were compared by analysis of variance for repeated measures to assess the effect of ONYX-411 treatment. All values with P<0.05 are considered significant.
Results
RB, p53 dysfunction and CAR expression in ATC cell lines
Tumor suppressor gene dysfunction of RB or p53 is usually detected by an increase in the expression of these proteins due to malfunction of the pathway, in that there could be either promoter silencing by methylation, inactivation by protein–protein sequestration or aberrantly enhanced or inhibited protein turnover.9 To determine the susceptibility of ATC cell lines to ONYX-411, we evaluated a panel of nine ATC cell lines to assess whether RB and p53 dysfunction are an inherent property of this cancer. Western blotting of the FRO cell line did not demonstrate either RB or p53 dysfunction, implying that there could be another mechanism involved in the tumorigenic progression of this particular patient-derived cell line (Figure 1). Whereas 8/9 (88.9%) of the ATC cell lines showed variable levels of RB dysfunction (Figure 1), only 5/9 (55.5%) cell lines (ARO, KTC-3, KAT-4, BHT101 and OCUT-1) displayed p53 dysfunction (Figure 1), suggesting that RB rather than p53 dysfunction occurs at a higher frequency in anaplastic thyroid cancer.
Figure 1.
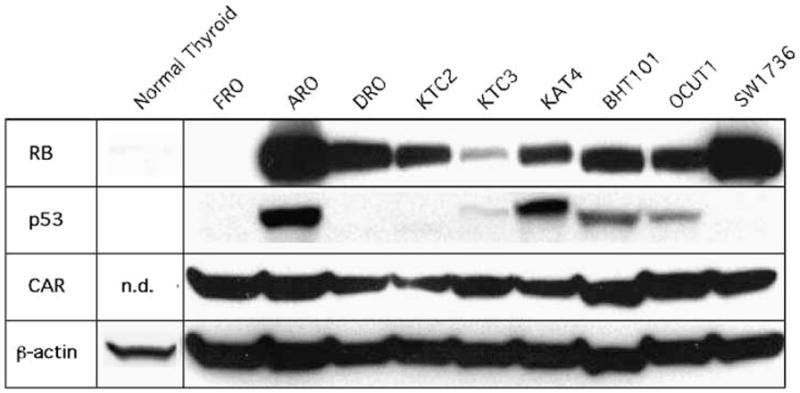
Determination of RB and p53 dysfunction and coxsackie adenovirus receptor (CAR) expression in ATC cell lines. Western blots of 50 μg of whole-cell lysate from each ATC cell line and normal primary thyrocytes as a control were evaluated for RB (top panel), p53 (second panel), CAR (third panel) and β-actin expression as a loading control (bottom panel). The blot is representative of results obtained from three independent experiments. RB, retinoblastoma.
Efficacy of an oncolytic adenovirus is dependent on the expression of the appropriate cellular receptors. Variations or lack of receptor expression could therefore impose limitations on the application of adenovirus-mediated gene therapy. Adenovirus enters the host cell by a receptor-mediated endocytosis mechanism.23 Primary binding to the CAR is followed by internalization mediated by αv-integrins. Although CAR expression has been shown to be variable among cell lines and tissues, it has been shown to be essential for adenoviral entry.23 Evaluation of ATC cell lines by western blot analysis for expression of CAR demonstrated that all cell lines tested expressed CAR to comparable levels, inclusive of FRO (Figure 1).
ONYX-411-mediated cell killing is specific to cells with RB dysfunction
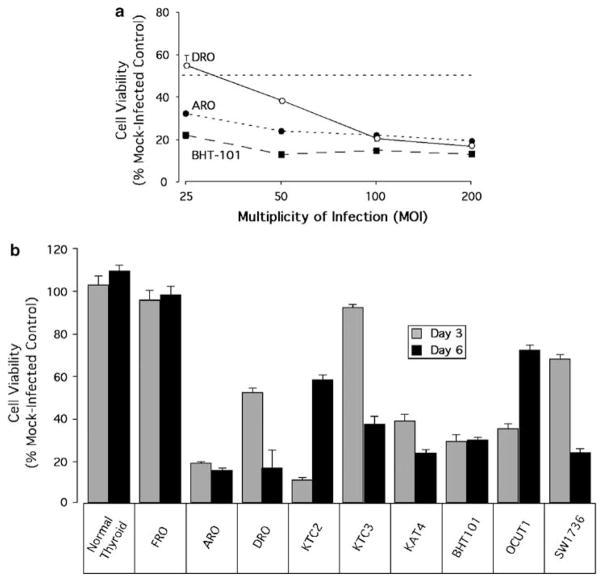
Having determined the RB, p53 status and CAR expression levels in the ATC cells lines, the next step was to evaluate the efficacy of ONYX-411 in these cells. First, we analyzed the sensitivity of three anaplastic thyroid cancer cell lines to ONYX-411 at different MOIs to determine the dose of virus required to obtain 50% or less killing of the cells. ARO, DRO and BHT-101 were infected at MOIs of 25,50,100 and 200 and evaluated for cell viability 72 h later. These three cell lines were susceptible to ONYX-411 even at a low MOI of 25 (Figure 2a). MOI of 50 resulted in a less than 50% survival of the cells, and this MOI was used for subsequent in vitro experiments. No cytopathic effect was observed when the same cells were mock-infected with PBS as a control.
Figure 2.
Efficacy of ONYX-411 is specific to RB dysfunction. (a) Dose effect of ONYX-411 adenovirus treatment of ARO, DRO and BHT101 cells. Cells were infected with ONYX-411 virus at 25, 50, 100 and 200 MOIs. Cell viability was assessed 3 days post-infection by MTT assay. Values are plotted as a percentage of mock-infected controls. The data shown here are the means ± s.d. from two independent experiments with six replicates for each variable. (b) Cell viability in various ATC cell lines after ONYX-411 infection at an MOI of 50 was assessed 3 and 6 days postinfection by MTT assay. Values are plotted as a percentage of mock-infected controls. The data shown here are the mean values ± s.d. from two independent experiments with six replicates of each variable. MOI, multiplicity of infection; MTT, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; RB, retinoblastoma.
The FRO cell line remained unaffected by ONYX-411 treatment (Figure 2b) as expected due to the presence of functional RB (Figure 1). Six of the nine cell lines, ARO, DRO, KTC-2, KAT-4, BHT-101 and OCUT-1, showed greater than 50% cell death by day 3. However, two of these susceptible cell lines, KTC-2 and OCUT-1, which showed dramatic cell killing by day 3 (90 and 65%, respectively), recovered significantly (40 and 30%, respectively) by day 6. Two cell lines, KTC-3 and SW-1736, took longer (6 days) to demonstrate significant cell killing. Thus all cell lines with RB dysfunction exhibited sensitivity to killing by ONYX-411 (Figure 2b).
Normal thyrocytes were incorporated into the study as a control to ensure that ONYX-411 did not affect normal human cells and confirm that its replication was restricted to cells with RB dysfunction. As observed with FRO cells, primary thyrocytes were not affected by ONYX-411 even by day 6 (Figure 2b).
ONYX-411 suppresses growth of ATC xenografts
To determine the efficacy of ONYX-411 in vivo, ARO and DRO xenografts were generated in athymic nude mice. The animals were divided into two groups for injection with either ONYX-411 or UV-inactivated virus. In all cases, each tumor was injected with a total 0.2×109 plaque-forming units of either virus over a period of five consecutive days. Mice were euthanized when tumors reached a volume ≥1000 mm3. None of the mice in this study showed evidence of morbidity.
Profound tumor growth was observed in all groups treated with inactivated control virus (Figures 3b and d), and tumor growth reached 1000 mm3 by weeks 5–6. On the other hand, tumor growth was significantly suppressed in tumors that were treated with ONYX-411, the effect being most dramatic in the DRO xenografts compared with the ARO xenografts (Figures 3a and c). All tumors that were injected with ONYX-411 were suppressed by 2.3- to 3-fold. Figures 3b and d are representative images of the suppressive effect of ONYX-411 on ATC tumors in comparison to their inactivated virus-treated controls. In addition, as shown in Figures 4e and f, DRO tumors appeared to exhibit neovasculo-genesis, as suggested by the deep-blue coloration of the tumors in nude mice treated with the UV-inactivated virus (Figure 3d). ONYX-411 treatment of the tumors resulted in significant reduction of the coloration in tumors (Figure 3d) as well as reduced levels of red blood cells in the hematoxylin and eosin-stained tumor sections (Figures 4g and h). Both ARO and DRO tumors are highly de-differentiated, although there is evidence of poorly organized thyroid follicles in ARO (Figure 4b), but not in DRO cells (Figure 4f). Collectively, these data confirm the efficacy of this virus against ATC cell line xenografts in vivo.
Figure 3.
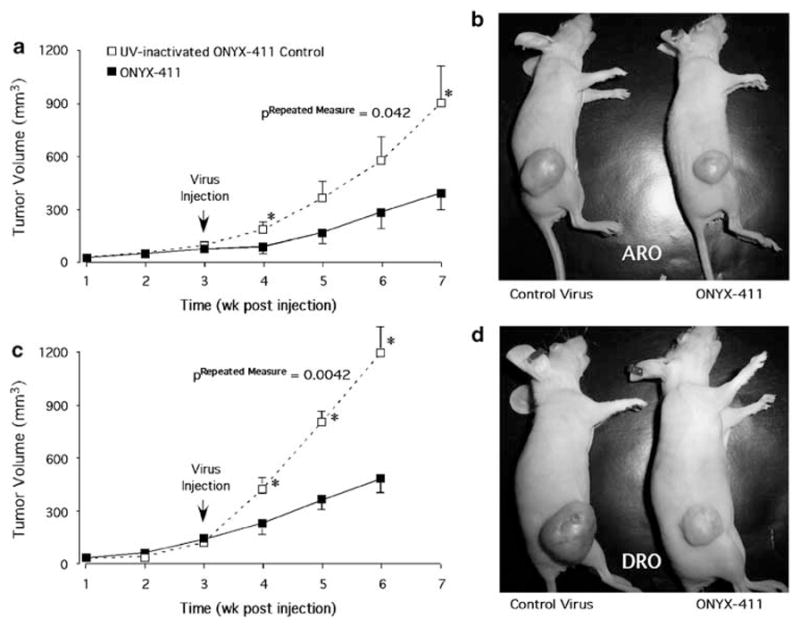
Tumoricidal effect of ONYX-411 on ATC cell line-induced xenografts. Cells (106) from each of the cell lines, ARO (a and b) and DRO (c and d) were injected s.c. into nude mice to generate xenograft tumors. Three weeks post-injection, mice were divided into two groups per cell line, and 5×107 pfu per 50 μl of either UV-inactivated control or intact ONYX-411 virus was injected intratumorally per day per tumor for five consecutive days. Tumor volumes were monitored every week for 6–7 weeks and plotted as a function of time (a and c). Animals were killed in cases where tumor volumes exceeded 1000 mm3. The values represent means ± s.e. from seven (ARO) and 10 (DRO) independent tumors. (b and d) Representative images of animals with tumors demonstrating the suppressive effect of ONYX-411-treated tumors (animals on the right) in comparison to their UV-inactivated virus-treated controls (animals on the left). Analysis of variance for repeated measures indicated that the treatment differences between UV-inactivated and ONYX-411 virus with both ARO and DRO cells were statistically significant at the P=0.0442 and 0.0042 levels, respectively. S.c., subcutaneously.
Figure 4.
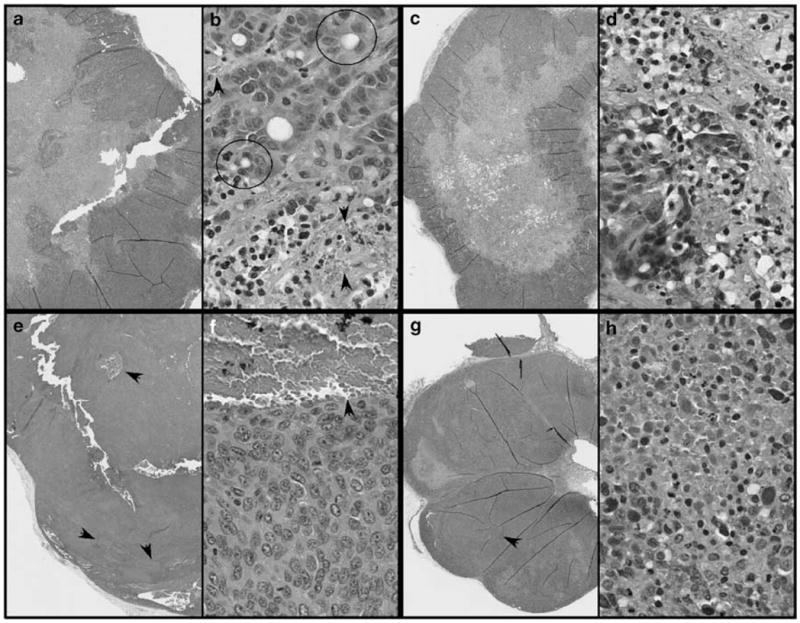
Hematoxylin and eosin-stained sections of ARO (a–d) and DRO (e–h) xenograft tumors from nude mice treated with UV-inactivated (a, b, e and f) or intact ONYX-411 virus (c, d, g and h). In each case, the panels (b, d, f and h) are higher magnification images of the cells. Arrows indicate regions of blood vessel formation. The circled structures represent empty follicles with poorly organized follicular thyroid cells, which were frequently observed in ARO cell tumors (b). Similar structures were not observed in the case of DRO cell tumors (f). UV, ultraviolet.
Discussion
In the present study, we have demonstrated for the first time that RB pathway dysfunction is a common attribute of anaplastic thyroid cancer (Figure 1). We have utilized this inherent dysfunction of RB in ATC to validate the efficacy of ONYX-411, a conditionally replicative oncolytic adenovirus, as a potential therapeutic agent for this aggressive cancer. We evaluated ATC cell lines for expression of CAR, the primary receptor for adenovirus, and demonstrated that receptor expression is not a limitation to the use of this agent as a therapeutic agent for ATC, as CAR was expressed on all the cell lines analyzed (Figure 1). Our data also clearly indicate that ONYX-411 replication is restricted to cells with RB dysfunction (Figures 2a and b) and that it is not capable of replication in normal cells thereby enabling it potentially to be used for systemic therapy. Further, we also show that the virus can significantly suppress the growth of ATC xenografts in vivo (Figure 3). The fact that ATCs commonly demonstrate RB dysfunction and efficient expression of CAR strengthens the candidacy of ONYX-411 as a potential therapeutic agent for anaplastic thyroid cancer.
The role of tumor suppressor gene loss or mutation has not been extensively investigated in thyroid neoplasms. With respect to ATC, both RB and p53 status has been documented using various molecular techniques,10,11,19 indicating that RB rather than p53 dysfunction occurs at higher frequency. The present study further emphasizes these observations, as eight of the nine cell lines tested had RB dysfunction, whereas only five of the nine cell lines exhibited p53 pathway dysfunction. These data argue that the RB pathway could prove to be a very important therapeutic target in ATC, underscoring the potential of ONYX-411 as a novel therapeutic agent for ATC.
Oncolytic viruses that exploit tumor-specific genetic abnormalities to affect selective and efficient tumor cell killing have been tested in other cancers with moderate success. For example, the conditionally replicating adenovirus, AD5-D24RGD, which replicates selectively in cells with malfunctioning RB pathway, is currently being tested clinically in ovarian cancer patients.24 However, limited effort has gone into understanding their potential for the therapy of ATC, an otherwise untreatable thyroid malignancy. ONYX-015 was developed to specifically replicate in cells with p53 dysfunction,15 and HILMI was developed to exploit the Wnt/β-catenin pathway.16 Although we have not tested our panel of cell lines for the Wnt/β-catenin pathway, we have observed that RB rather than p53 dysfunction occurs at a higher frequency in ATC, suggesting that ONYX-411 may be a more useful therapeutic agent for the treatment of ATC.
Our cell line data clearly indicate that ONYX-411-mediated cell killing is very specific to cells with RB dysfunction and there is no apparent toxicity to normal cells (Figure 2b). The observation that KTC-2 and OCUT-1 recovered significantly by day 6 suggests that these cell lines might have been a mixed population that included a small fraction of cells displaying resistance to ONYX-411. This could be due to cells with either CAR deficiency or presence of normal RB. The delay in killing of KTC-3 can be attributed to the lower extent of RB dysfunction (Figure 1), which could be a delaying factor for viral replication. However, the explanation for the delayed cell death observed in SW-1736, despite pronounced RB dysfunction and strong CAR expression, is not known.
The nude mice xenograft experiments validate the in vitro tumoricidal efficacy of ONYX-411 in vivo. Saito et al.25 have recently demonstrated that the E1A region of adenovirus suppressed the production of vascular endothelial growth factor and inhibited tumor angiogenesis by binding with p300, further emphasizing the potential utility of adenovirus-mediated gene therapy. The decreased neovasculogenesis might also be an indirect effect of tumor size. As increasing tumor size stimulates new blood vessel growth through hypoxic signals,26,27 it is possible that the smaller tumors exhibit reduced blood vessel numbers due to a reduction in neovasculogenic signals. In the case of the DRO tumors, we also observed a more robust therapeutic effect when smaller tumors between 50 and 100 mm3 are treated with virus in comparison to larger tumors (>100 mm3) (data not shown). This could be because the DRO tumors are so aggressive, the rate of tumor growth may have outstripped the rate of oncolysis in the larger tumors and might account for the absence of complete eradication of tumor growth. This effect might be overcome by increasing virus dose or identifying combinatorial therapies that would enhance treatment efficacy. In this regard, peroxisome proliferator-activated receptor-γ agonists in conjunction with paclitaxel have shown some promise in the treatment of ATC cell lines in vitro and in vivo.4
While this paper was being prepared, we learned that doubts had arisen with respect to the authenticity of DRO cells as an ATC cell line (B Haugen, personal communication). It was suggested that these cells might be derived from the melanoma A375 cell line. We therefore undertook studies to determine the phenotypic and genotypic characteristics of several of the cell lines used in this study, including DRO and A375 cells that were obtained from ATCC. A very high degree of genotype concordance was observed between DRO and A375 cells using an 11-marker tandem-repeat panel, suggesting that they were likely derived from the same individual (data not shown). As to the determination of the respective cell type of origin of DRO and A375, which cannot be determined by tandem-repeat genotyping, the expression patterns of proteins considered to be lineage-specific were equivocal (data not shown). Therefore, the tissues of origin of DRO and A375 cells remain uncertain at this time. Caution must therefore be exercised when interpreting experiments performed with A375 or DRO cells in a tissue-specific context. However, the concordance between RB dysfunction and ONYX-411 response is an important and valid observation regardless of whether DRO cells are derived from thyrocytes or melanocytes.
In conclusion, we show that anaplastic thyroid cancer cell lines exhibit a high frequency of RB dysfunction, and in targeting this pathway, tumor-specific killing was achieved both in vitro and in vivo using a novel oncolytic adenovirus ONYX-411 with no discernable effect on normal thyrocytes or ATC cell lines lacking RB pathway dysfunction. Further, we demonstrate that ATC does express the primary CAR emphasizing the potential of this oncolytic adenovirus as a novel therapeutic agent for the treatment of ATC, an otherwise untreatable malignancy.
Acknowledgments
This work was supported by NIH Grant CA80117 (NLE), a generous donation from the Price Foundation, Florida Department of Health Bankhead-Coley grant (JAC) and a grant for rare cancers from Dr Ellis and Dona Brunton (JAC). We wish to extend their appreciation to Dr Lori Erickson for help with the interpretation of the hematoxylin and eosin-stained slides of ATC-xenografted tumors in nude mice. We are also indebted to Dr Henry J Hiddinga for assistance with the animal studies.
Footnotes
Disclosure The authors have nothing to disclose.
References
- 1.http://www.cancer.org/docroot/stt/stt_0.asp
- 2.Are C, Shaha AR. Anaplastic thyroid carcinoma: biology, pathogenesis, prognostic factors, and treatment approaches. Ann Surg Oncol. 2006;13:453–464. doi: 10.1245/ASO.2006.05.042. [DOI] [PubMed] [Google Scholar]
- 3.Haigh PI. Anaplastic thyroid carcinoma. Curr Treat Options Oncol. 2000;1:353–357. doi: 10.1007/s11864-000-0051-8. [DOI] [PubMed] [Google Scholar]
- 4.Copland JA, Marlow LA, Kurakata S, Fujiwara K, Wong AK, Kreinest PA, et al. Novel high-affinity PPARgamma agonist alone and in combination with paclitaxel inhibits human anaplastic thyroid carcinoma tumor growth via p21WAF1/CIP1. Oncogene. 2006;25:2304–2317. doi: 10.1038/sj.onc.1209267. [DOI] [PubMed] [Google Scholar]
- 5.Elisei R, Vivaldi A, Ciampi R, Faviana P, Basolo F, Santini F, et al. Treatment with drugs able to reduce iodine efflux significantly increases the intracellular retention time in thyroid cancer cells stably transfected with sodium iodide symporter complementary deoxyribonucleic acid. J Clin Endocrinol Metab. 2006;91:2389–2395. doi: 10.1210/jc.2005-2480. [DOI] [PubMed] [Google Scholar]
- 6.Santarpia L, El-Naggar AK, Cote GJ, Myers JN, Sherman SI. Phoshatidylinositol 3-kinase/Akt and Ras/Raf-mitogen-activated kinase pathway mutations in anaplastic thyroid cancer. J Clin Endocrinol Metab. 2008;93:278–284. doi: 10.1210/jc.2007-1076. [DOI] [PubMed] [Google Scholar]
- 7.Saltman B, Singh B, Hedvat CV, Wreesmann VB, Ghossein R. Patterns of expression of cell cycle/apoptosis genes along the spectrum of thyroid carcinoma progression. Surgery. 2006;140:899–905. doi: 10.1016/j.surg.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 9.Bartek J, Bartkova J, Lukas J. The retinoblastoma protein pathway in cell cycle control and cancer. Exp Cell Res. 1997;237:1–6. doi: 10.1006/excr.1997.3776. [DOI] [PubMed] [Google Scholar]
- 10.Anwar F, Emond MJ, Schmidt RA, Hwang HC, Bronner MP. Retinoblastoma expression in thyroid neoplasms. Mod Pathol. 2000;13:562–569. doi: 10.1038/modpathol.3880097. [DOI] [PubMed] [Google Scholar]
- 11.Volante M, Croce S, Pecchioni C, Papotti M. E2F-1 transcription factor is overexpressed in oxyphilic thyroid tumors. Mod Pathol. 2002;15:1038–1043. doi: 10.1097/01.MP.0000028645.36632.A8. [DOI] [PubMed] [Google Scholar]
- 12.McNeish IA, Bell SJ, Lemoine NR. Gene therapy progress and prospects: cancer gene therapy using tumour suppressor genes. Gene Therapy. 2004;11:497–503. doi: 10.1038/sj.gt.3302238. [DOI] [PubMed] [Google Scholar]
- 13.Vaha-Koskela MJ, Heikkila JE, Hinkkanen AE. Oncolytic viruses in cancer therapy. Cancer Lett. 2007;254:178–216. doi: 10.1016/j.canlet.2007.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu Z, Eisenberg DP, Singh B, Shah JP, Fong Y, Wong RJ. Treatment of aggressive thyroid cancer with an oncolytic herpes virus. Int J Cancer. 2004;112:525–532. doi: 10.1002/ijc.20421. [DOI] [PubMed] [Google Scholar]
- 15.Portella G, Scala S, Vitagliano D, Vecchio G, Fusco A. ONYX-015, an E1B gene-defective adenovirus, induces cell death in human anaplastic thyroid carcinoma cell lines. J Clin Endocrinol Metab. 2002;87:2525–2531. doi: 10.1210/jcem.87.6.8529. [DOI] [PubMed] [Google Scholar]
- 16.Abbosh PH, Li X, Li L, Gardner TA, Kao C, Nephew KP. A conditionally replicative, Wnt/beta-catenin pathway-based adenovirus therapy for anaplastic thyroid cancer. Cancer Gene Ther. 2007;14:399–408. doi: 10.1038/sj.cgt.7701024. [DOI] [PubMed] [Google Scholar]
- 17.Cohen EE, Rudin CM. ONYX-015. Onyx pharmaceuticals. Curr Opin Investig Drugs. 2001;2:1770–1775. [PubMed] [Google Scholar]
- 18.Liu TC, Galanis E, Kirn D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nat Clin Pract Oncol. 2007;4:101–117. doi: 10.1038/ncponc0736. [DOI] [PubMed] [Google Scholar]
- 19.Lam KY, Lo CY, Chan KW, Wan KY. Insular and anaplastic carcinoma of the thyroid: a 45-year comparative study at a single institution and a review of the significance of p53 and p21. Ann Surg. 2000;231:329–338. doi: 10.1097/00000658-200003000-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson L, Shen A, Boyle L, Kunich J, Pandey K, Lemmon M, et al. Selectively replicating adenoviruses targeting deregulated E2F activity are potent, systemic antitumor agents. Cancer Cell. 2002;1:325–337. doi: 10.1016/s1535-6108(02)00060-0. [DOI] [PubMed] [Google Scholar]
- 21.Dyson N, Guida P, McCall C, Harlow E. Adenovirus E1A makes two distinct contacts with the retinoblastoma protein. J Virol. 1992;66:4606–4611. doi: 10.1128/jvi.66.7.4606-4611.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Denizot F, Lang R. Rapid colorimetric assay for cell growth and survival. Modifications to the tetrazolium dye procedure giving improved sensitivity and reliability. J Immunol Methods. 1986;89:271–277. doi: 10.1016/0022-1759(86)90368-6. [DOI] [PubMed] [Google Scholar]
- 23.Meier O, Greber UF. Adenovirus endocytosis. J Gene Med. 2004;6(Suppl 1):S152–S163. doi: 10.1002/jgm.553. [DOI] [PubMed] [Google Scholar]
- 24.Fueyo J, Alemany R, Gomez-Manzano C, Fuller GN, Khan A, Conrad CA, et al. Preclinical characterization of the antiglioma activity of a tropism-enhanced adenovirus targeted to the retinoblastoma pathway. J Natl Cancer Inst. 2003;95:652–660. doi: 10.1093/jnci/95.9.652. [DOI] [PubMed] [Google Scholar]
- 25.Saito Y, Sunamura M, Motoi F, Abe H, Egawa S, Duda DG, et al. Oncolytic replication-competent adenovirus suppresses tumor angiogenesis through preserved E1A region. Cancer Gene Ther. 2006;13:242–252. doi: 10.1038/sj.cgt.7700902. [DOI] [PubMed] [Google Scholar]
- 26.Mizukami Y, Kohgo Y, Chung DC. Hypoxia inducible factor-1 independent pathways in tumor angiogenesis. Clin Cancer Res. 2007;13:5670–5674. doi: 10.1158/1078-0432.CCR-07-0111. [DOI] [PubMed] [Google Scholar]
- 27.Mukhopadhyay D, Datta K. Multiple regulatory pathways of vascular permeability factor/vascular endothelial growth factor (VPF/VEGF) expression in tumors. Semin Cancer Biol. 2004;14:123–130. doi: 10.1016/j.semcancer.2003.09.019. [DOI] [PubMed] [Google Scholar]