Abstract
Age-related changes in innate immune function and glial-neuronal communication are early and critical events in brain aging and neurodegenerative disease, and lead to a chronic increase in oxidative stress and inflammation, which initiates neuronal dysfunction and reduced synaptic plasticity, and ultimately disruption in learning and memory in the aged brain. Several lines of evidence suggest a correlation between adult neurogenesis and learning. It has been proposed that a decline in hippocampal neurogenesis contributes to a physiologic decline in brain function. Recently, new and important insights relating to the production of new neurons affecting hippocampal-dependent memory ability have been provided. A multitude of factors have been shown to regulate the production of new neurons in the adult hippocampus, many of which change as a result of aging. Yet, the potential importance of neurogenesis in some affective and cognitive behaviors, as well as endogenous tissue repair mechanisms, makes further investigation of neurogenic regulators warranted. We have recent evidence that key regulators of communication between neurons and microglia are disrupted in the aged brain and may be one of the factors that precedes and initiates the observed increase in chronic inflammatory state. In this review the role of dysfunction in these neuronal-glial communication regulators underlying age-related impairments in cognition and hippocampal neurogenesis will be discussed. An understanding of these mechanisms will lead to the development of preventive or protective therapies.
Keywords: Neurogenesis, aged brain, hippocampus, neuron, microglia
Neurogenesis and Cognition: Is there a link?
Neurogenesis occurs throughout life, predominantly in the subgranular zone (SGZ) of the dentate gyrus in the hippocampal formation and in the subventricular zone (SVZ). Newly generated hippocampal granule cells acquire the morphologic and biochemical properties of neurons. For example, the adult born neurons develop afferent synapses on their cell bodies and dendrites. The new neurons also make efferent synapses, and extend axonal projections along the mossy fibers into the hippocampal CA3 region. Not only do these cells form synapses, they are also electrically active, capable of firing action potentials and receiving synaptic input [1–6]. Thus, the adult born granule neurons become integrated into the hippocampal network.
The functional significance of adult neurogenesis is not fully understood. However, evidence has been provided that newly produced neurons play an important role in functional and dysfunctional events associated with the neurogenic area, such as memory function and neuropathologies [7]. There is strong correlation between the number of new neurons and performance on hippocampal-dependent memory tasks such as the Morris water-maze and trace eye-blink conditioning [3,8–11]. Shors et al. [3] observed a learning deficit in a hippocampal-dependent memory task after reducing the generation of new neurons with an anti-mitotic agent. Other reports, however, either failed to find a correlation between the number of newly generated neurons and memory performance, or observed that aged animals which perform better on a hippocampal-dependent memory task have fewer new neurons compared with animals that perform worse [12,13]. Despite this controversial data, within the last year numerous additional papers have linked hippocampus-dependent learning and neurogenesis. Clear evidence for the functional role of neurogenesis has been provided by Imayoshi et al., who demonstrated that the genetic ablation of newly formed neurons in adult mice led to impairment in hippocampus-dependent cognitive function [14]. Likewise, Jessberger et al. using a different genetic approach demonstrated that adult neurogenesis is correlated with spatial learning [15]. In recent years new and more complex methods have been designed to test the link between neurogenesis and cognition, revealing that neurogenesis is important for specific types of cognitive tasks.
It has been suggested that the age-related decline in hippocampal neurogenesis, together with the decline in other type of synaptic plasticity, contributes to a physiologic decline in memory function [16,17]. In a recent review Drapeau et al. [10] proposed that the differences in the risk of developing age-related memory disorders can be predicted earlier in life. Low hippocampal plasticity may render animals more vulnerable to aging processes. On the other hand, subjects starting off with a high level of neurogenesis may be resistant to the development of age-related memory disorders. In this regard, understanding the mechanisms that regulate the decline in neurogenesis in the aged hippocampus may guide the development of therapeutic strategies aimed at ameliorating age-related cognitive impairment. It was recently demonstrated that decreased neurogenesis in the dentate gyrus during aging is not attributable to the altered number or phenotype of progenitor cells [18]. The precise reason for age-related decrease in neurogenesis is unknown. However, the production of new neurons in the adult hippocampus depends on multiple factors. It has been established that the “environment” of an aged animal has significant impact on stem/progenitor cell function in many tissues of the body. It has been further hypothesized that the age-related decrease in neurogenesis is due to a global, age-related alteration in the microenvironment of the brain. In this review we will discuss the role of age-related chronic inflammatory state in the hippocampal neurogenic environment. We will also highlight an intriguing mechanism concerning interactions between neuron-microglia that regulate inflammation in the hippocampus.
Neurogenesis and inflammation
Two seminal studies, published simultaneously several years ago showed that inflammation tightly regulates neurogenesis. Ekdahl et al. [19] used lipopolysacharide (LPS) continuously delivered into the cortex via an osmotic mini pump and observed that after 28 days of treatment there was a dramatic activation of ED-1+cells. ED-1 (CD68) is a member of the scavenger receptors, which is highly expressed in monocytes and some tissue macrophages. The expression of ED-1 in microglia is normally absent, but can be induced following an inflammatory insult, which is what was found after 28 days of intracortical infusion of LPS. In the young-adult rat, LPS-induced inflammation resulted in an 85% reduction in the number of new neurons born during the inflammatory insult. Monje et al. [20] also found that LPS given systemically caused an increase in microglia activation and a decrease in neurogenesis, which could be prevented by the nonsteroidal anti-inflammatory drug (NSAID) indomethacin.
How does inflammation regulate neurogenesis? One way is via inflammatory mediators produced by microglia. Microglia have the potential to be neurotoxic through the production of reactive oxygen species when engaged in the removal of mature or immature cells [21,22]. The second way that microglia can be involved in regulating neurogenesis is through the production of cytokines. This is particularly true for the key innate cytokines IL-1β and TNF-α, with activated microglia being the major source of TNF-α and IL-1β in the CNS (Gebicke-Haerter, 2001) [23]. The third way in which microglia can regulate neurogenesis is by a pro-repair/pro-neurogenic mechanism, as microglia are also capable of producing a number of growth factors including IGF-1 and BDNF, which have been shown to promote neurogenesis (For review see: [24]). Finally, inflammation also alters the way the new neurons integrate into the existing neuronal circuit [25].
There are many mediators of immunity that could affect neurogensis; however most have not been looked at yet, with the exception of cytokines. The negative regulation of IL-1β in hippocampal neurogenesis has been described by several investigators. We have previously shown that inhibition of caspase-1, which is an enzyme critically important in the production of active IL-1β, could reverse the decrease in hippocampal neurogenesis associated with aging and at the same time improve hippocampal-dependent memory by decreasing pro-inflammatory cytokines and microglia activation [26,27]. The receptor for IL-1β, IL-1RI, is expressed on neural progenitor cells in the SGZ of the hippocampus. The presence of IL-RI on neural progenitor cells suggested that the anti-neurogenic action of IL-1β occurs via a direct effect rather than via polysynaptic or indirect actions on cells surrounding the progenitor cells [28]. Suppression of hippocampal progenitor cell proliferation by IL-1β could result from either a loss of progenitor cells because of cell death, arrest of the cell cycle, or both. However, in vitro studies show that IL-1β suppresses cell proliferation via a reduction in cell cycling rather than increasing cell death [28]. Other negative regulators of stem cell niches have been suggested such as TNFα via TNFR1. Neural progenitor cells have been shown to constitutively express TNFRI and TNFRII [29]. At low concentrations, TNF-α induced proliferation of neural progenitor cells, but at higher concentrations TNF-α induced apoptosis [30,31].
Microglia: the “Immune Cells” of the Brain
The key cellular event signaling ongoing inflammation in the brain is the accumulation of reactive microglia in degenerating areas [32,33]. Aging is associated with inflammation and activation of microglia, as well as with oxidative stress and increased IL-1β levels [34–36]. As compared to the young one, the aged immune system is less able to mount an effective immune response after challenges with infectious pathogens because of complex changes collectively termed “immunosenescence” [37]. Phagocytes are essential during the first phase of the immune responses against pathogens and contribute to inflammatory responses. Thus, macrophages play an essential role as sensors of exogenous and endogenous danger signals through pattern recognition receptors [38]. Microglia are the resident immune cells in the central nervous system where they act as macrophages. Microglia cells in the normal tissue constantly screen their environment. Equipped with receptors for a plethora of molecules, they can immediately sense signs of disturbed structural and functional integrity. For instance, they constitutively express surface receptors that trigger or amplify the innate immune response, including complement receptors, cytokine receptors, chemokine receptors, major histocompatibility complex (MHC) II, and others [39,40]. Upon detection of homeostatic disturbance, microglia can rapidly respond by inducing a protective immune response, which consists of a transient upregulation of inflammatory molecules and neurotrophic factors [41–44]. Neurotrophic factors such as BDNF, glial cell line-derived neurotrophic factor (GDNF), and IGF-1 are secreted by microglia and astrocytes [41–44]. Thus, microglia protect CNS function and remove cells damaged from acute injury. However, microglial neurotoxicity can occur after excessive and uncontrolled stimulation of microglia or when microglia function is impaired [45–47]. Indeed, when chronic inflammation occurs, prolonged activation of microglia triggers the release of a variety of neurotoxic products and proinflammatory cytokines such as IL-1β, IL-6, and TNFα [48]. Protein levels of IL-1β, IL-6, and TNFα are increased in the brain of aged animals [49–51]. Animal studies indicate that increased levels of IL-6 in the hippocampus and cerebral cortex arise primarily from microglia [52]. Activation of microglia leads to the activation of the mitogen-activated protein (MAP) kinase pathways. These kinases are involved in the regulation of a variety of cellular processes, including cell proliferation, differentiation, and death. There are three major MAP kinases: extracellular responsive kinases (ERK), c-Jun N-terminal kinases (JNK), and p38 MAP kinase. Notably, age-related increases in JNK and p38 MAP kinase activation in rat hippocampus have been reported [53,54]. Inhibition of p38 kinase decreases microglial inflammation [55] as well as the production of IL-1β [56]. Indeed, the results of a number of studies indicate that p38 MAP kinase activation is important for IL-1β production [57–60]. In the hippocampus, activation of p38 MAP kinase is coupled with elevated IL-1β levels in aged rats [53]. Moreover, p38 MAP kinase plays a pivotal role in the inhibitory effect of LPS and IL-1β on LTP [61].
Neuron-Microglia Cross-Talk
Recent evidence indicates that neurons are not only passive targets of microglia but rather can control microglia activity. Indeed, neurons may also deliver signals, which keep microglia in their surveillance mode indicating normal function. Under resting conditions there are several neuron-mediated signals that have an anti-inflammatory action at the level of the microglia. In an excellent review Biber and colleagues (2007) described a system of “ON” and “OFF” signals used to regulate microglia activation. “OFF” signals constitutively keep microglia in their resting state and antagonize proinflammatory activity. CD200, CX3CL1, CD47, CD55, HMGB1 are all “OFF” neuroimmunoregulatory proteins constitutively expressed on healthy neurons. “ON” signals are inducible and include chemokines, purine and glutamate and they are found on damaged neurons. Thus, disruption of ongoing communication through calming signals would allow endangered neurons to call for microglia assistance. Strong insults to the CNS or in the aged brain may trigger drastic changes in the functional phenotype of microglia, leading to substantial impairment in neurons and glia [62]. Thus, an important area of investigation is to examine molecules that mediate neuronal-glial interactions, as this is one way neurons can signal inflammatory cells that a response is needed. Two of these molecules that have received attention in the recent literature are CX3CL1 (fractalkine) and CD200.
Function of CD200 receptor and CD200 signaling
CD200 is a highly conserved member of the immunoglobulin family and is expressed on T-cells and B-cells [63–65]. In the CNS, CD200 is primarily localized on neurons and oligodendrocytes, although astrocytes and brain endothelial cells have also been shown to express CD200 [66,67]. CD200 down regulates the activity of microglial cells by ligation of the CD200 receptor (CD200R), which is located selectively on myeloid cells (in the central nervous system the predominant myeloid cells are the microglia).
CD200R, also a member of the immunoglobulin family, has cell-type and species-specific molecular weights (60–90 kDA) [67,68]. CD200R is expressed by a variety of inflammatory cells, including macrophages, neutrophils, microglia, granulocytes, T lymphocytes [69]. Interestingly, in human brain microglia express significantly lower levels of CD200R than blood-derived macrophages [67]. Non-immune associated cells such as, astrocytes, oligodendrocytes, epidermal keratinocytes and Langerhans cells have also been reported to express CD200R. The only known function of CD200 is to bind CD200R.
Binding of CD200 to CD200R activates anti-inflammatory signaling pathways in CD200R expressing cells that downregulate proinflammatory responses [70]. The activation of the MAP kinase family of proteins (ERK, JNK, and p38) is inhibited by CD200R ligation with CD200 [70,71]. Mice lacking the CD200 receptor demonstrate an accelerated microglial response to injury [72]. CD200 protein expression decreases with increasing age and with inflammatory insults such as acute treatment with amyloid β protein [73]. Similarly, in AD pathological brain regions, significantly lower CD200 expression is observed when compared to the same brain regions in age-matched non-demented individuals [67]. Interestingly, the anti-inflammatory cytokine IL-4 can increase CD200 expression on cultured neurons. IL-4 can also abrogate the decrease in CD200 with age [73]. CD200 also appears to be a valid therapeutic target as shown in a recent paper using a model of multiple sclerosis. Using an agonist at CD200R1, in the model of multiple sclerosis there was a decrease in microglia activation and macrophage reactivity and antigen presenting function in the area of disease [74]. These findings lend further support to the potential therapeutic validity of intervening on these pathways in aging and disease.
Function of CX3CL1-CX3CR1 signaling
Fractalkine (CX3CL1), is a chemokine expressed constitutively by neurons and has been identified as a novel neuroimmune regulatory protein, whose function is to send alert signals to microglia and inhibit microglia activity under inflammatory conditions [40,75]. Disruption of this dialog could trigger more drastic changes in the functional phenotype of microglia. Fractalkine is a transmembrane chemokine that exists in both membrane-bound and soluble forms. Its membrane-bound form displays adhesion properties and consists of an intracellular domain and a transmembrane domain [76,77]. Soluble fractalkine is important for chemotaxis.
In contrast to many other chemokines, fractalkine binds to only one receptor, CX3CR1 [40]. This receptor responds to both membrane-bound and soluble fractalkine. Although there is some debate concerning the cell types expressing these molecules in the CNS, in vivo fractalkine is principally expressed in neurons, whereas its receptor in microglial cells [40,78–82]. Fractalkine acts in vitro as an anti-inflammatory molecule by down regulating production of IL1β, TNFα and IL-6 [45,75]. Several studies have suggested a continuous dialog between neurons and microglia under basal conditions via fractalkine and its receptor CX3CR1 [75,83].
The anatomical expression of fractalkine on neurons and CX3CR1 on microglia has led to the hypothesis of a unique signaling whereby healthy neurons release fractalkine to modulate microglial activation. Thus, interaction between fractalkine and CX3CR1 contributes to maintaining microglia in a resting phase. Disruption of this dialog could trigger changes in the functional phenotype of microglia. When neurons are injured fractalkine levels are down-regulated resulting in microglia recruitment and activation. It has been shown that mice which are deficient in CX3CR1 have marked increase in microglial cell expression of IL-1β in response to LPS stimulation with an associated increase in hippocampal neuronal cell death [75]. CX3CR1-deficent mice also show increased susceptibility to neurotoxins such as MPTP [45], presumably due to increased microglia activation. In addition, it has been shown that microglia from CX3CR1-deficent mice challenged with LPS express distinctly different genes than those expressed by wild-type [45,75]. Decreased protein levels of fractalkine have been detected in plasma from Alzheimer’s disease patients [84] and in the aged rodent hippocampus [85,86]. Additionally, APP transgenic mice showed a decrease in neuronal levels of fractalkine at 9 months of age [87].
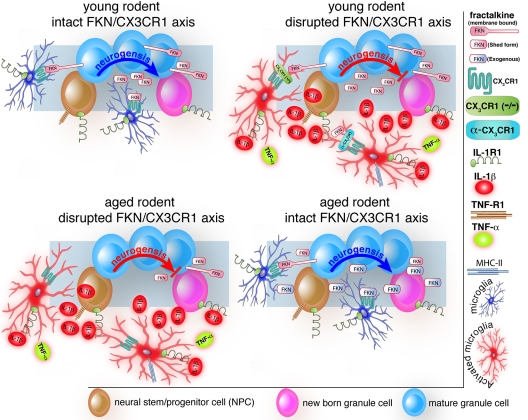
We have recently discovered that fractalkine is reduced in the aged rat hippocampus beginning as early as 12 months of age and that this phenomenon may be one of the players in the imbalance of pro-inflammatory cytokines observed in the aged CNS. However, the decrease in fractalkine observed in the age rats may be due to multiple mechanisms. For instance, an increase in fractalkine cleavage could indirectly contribute to the measured decrease in fractalkine protein. Based on our findings, we hypothesized that, as a consequence of aging, fractalkine signaling becomes disregulated, which leads to an increased microglial activation and decreased cognitive function and neurogenesis. We tested this hypothesis in aged rats and CX3CR1 null mice. The results of our findings are summarized in Figure 1. We found that indeed an impaired fractalkine/CX3CR1 function in the central nervous system could modulate the decrease in hippocampal neurogenesis associated with aging. By administering fractalkine to aged rats we found an increase in hippocampal progenitor cell proliferation in aged animals suggesting that fractalkine modulates at least in part hippocampal neurogenesis in aging. Furthermore, when we administered soluble fractalkine to 22-month-old rats we observed a decrease in microglial MHCII expression coupled to increased neurogenesis. In a final approach, we used a blocking antibody to CX3CR1 in young rats and found that this blocking antibody caused a decrease in neurogensis. Interesting, the anti-proliferative effects of the CX3CR1 blocking antibody was shown to be directly mediated by IL-1β, as IL-1ra was able to significantly reverse the decrease in the number of BrdU+ cells in the SGZ induced by anti-CX3CR1. This suggests that fractalkine and its receptor act through IL-1β to regulate neuronal cell proliferation. The group of rats that received the α-CX3CR1 blocking antibody along with the active IL-1Ra was not significantly different from any of the control groups. This finding is in agreement with our hypothesis that disruption of CX3CR1 function leads to an increase in microglia activation, which could be ultimately responsible in negatively regulating neurogenesis. have also found that CX3CR1−/−mice have reduced neural plasticity as reflected by decreased neurogenesis.
Figure 1.
Dysfunction in Fractalkine/CX3CR1 signaling modulates the decrease in hippocampla neurogenesis associated with aging. In the young rodent brain, interactions between neuronal-derived fractalkine (FKN) and microglial-expressed CX3CR1 contribute to maintain microglia in a resting phase (upper left panel). This contributes to keep the microenvironment of the brain favorable for the ongoing process of neurogenesis. Disruption of this dialog triggers changes in the functional phenotype of microglia with consequent increase in expression of IL-1β and TNFα (upper right panel). A state of chronic inflammation is then established, which negatively regulates hippocampal neurogenesis. The lower left panel illustrates that aging is associated to low expression of FKN and consequent disruption in FKN/CX3CR1 signaling, which is accompanied by increase in microglia activation, and over-expression of proinflammatory cytokines. (Note that the scenario of the aged brain is similar to that of the young brain in which the FKN/CX3CR1 axis is disrupted). The lower right panel illustrates the possibility to rescue neurogenesis in the aged brain by increasing hippocampal FKN protein levels and thus restoring the dialogue between FKN and CX3CR1.
Age-related changes in the fractalkine/CX3CR1 axis have been characterized in other scenarios. For example, in humans there are 2 polymorphisms for the fractalkine receptor, one of which caused reduced adhesion, signaling and chemotaxis. The variant with reduced adhesion is protective for atherosclerosis [88] yet increases the risk of macular degeneration [89]. A dual role for fractalkine and its receptor has been observed in other situations raising the question as to the risk/benefit of fractalkine as a therapeutic tool. For instance, it has been shown that fractalkine deficiency provides protection in an animal model of cerebral ischemia [90], while increased fractalkine expression has been reported during pathologic pain [91]. Enhanced expression of fractalkine has also been observed in HIV-1 associated dementia [92]. One aspect of fractalkine that may also play a role in the differential effects of fractalkine is that fractalkine is a type 1 membrane anchored protein with a mucin-like stalk and can be cleaved by ADAM-10 and cathepsin S to produce soluble fractalkine [93]. The soluble and membrane bound forms may play different functional roles. It has been shown that the membrane bound form plays a role in endothelial cell death [94], whereas soluble fractalkine has been shown to be both neuroprotective [85] and neurodegenerative [95]. A recent paper suggests that CX3CR1 is necessary for cell death in a mouse model of AD [96]; however, in this report the AD mice (3xTg AD crossed with CX3CR1-deficient mice) were examined at an age prior to the development of either extracellular Aβ deposition or intracellular microtubule-associated protein tau (MAPT) aggregation that define AD and thus the nature of the signals that leads to the neurotoxicity in this model is unclear. On the contrary other papers suggest that loss of CX3CR1 exacerbates neurodegeneration [97]. The effect of CX3CR1 deficiency appears to depend on the animal model. For instance, recently has been reported that CX3CR1 deficiency leads to reduced Aβ deposition in two different transgenic mouse models AD [98]; however the same group of investigators showed in a following study that hTau/CX3CR1−/− mice have increased tauophatology [99]. Furthermore, it has been proposed that a tonic neural release of fractalkine provides specific restrain in microglia in the healthy brain, but when the blood brain barrier is disrupted, the role of CX3CR1/Fractalkine may be different [45]. Thus, the relationship remains complex and may relate to the differential processing of fractalkine. Understanding the mechanisms by which fractalkine/CX3CR1 regulate microglia activation in the aged brain can lead to new therapeutic strategies in a variety of neurological disorders characterized by neuroinflammation.
Is it All Innate? What about Adaptive Immunity?
As mentioned earlier, Monje et al [20] found that LPS given systemically caused an increase in microglia activation and a decrease in neurogenesis, which could be prevented by the NSAID indomethacin. This raises the possibility that not only local CNS inflammation, but also systemic inflammation could communicate to the CNS and influence the neurogenic niche. It has been established that the “environment” of an aged animal has significant impact on stem/progenitor cell function in many different tissues. This has been recently examined using the technique of heterochronic parabiosis, which involves connecting the circulation of a young and an aged rat together. Under these circumstances it has been observed that aged progenitor cells in liver, muscle and many other tissues when exposed to the circulation of a young rat increased proliferation and regeneration index [98]. This same effect has been observed ex vivo when young hematopoetic stem cells (HSC’s) are cultured in the presence of serum from young or aged mice. In this paradigm, stem cells take on the phenotype characteristic of the age of the serum, in other words, young serum can rejuvenate aged stem cells, and old serum can “age” young stem cells [99]. This report implicated IGF-1 as one signal present in the aged circulation that may be significant for this “extrinsic aging” effect. We have previously shown that the raw mononuclear fraction of human umbilical cord blood (HUCB) administered intravenously improved the neurogenic niche of the aged brain and stimulated the endogenous stem / progenitor cells to increase proliferation and produce more doublecortin-positive cells, possibly through a decrease in microglia activation [100]. These results are remarkable given that the cells were delivered into the systemic vasculature and not directly into the brain. However, few surviving HUCB-derived cells were found in the brain of animals that received transplants [101–103] and fewer of these surviving cells expressed neural markers, indicating that replacement of lost neurons was not the primary mechanism inducing brain repair. In addition to producing growth/trophic factors, the HUCB mononuclear cells also produce a number of cytokines and chemokines. Indeed, we have consistently found that HUCB administration interrupts the inflammatory cascade observed in the aged brain reduces apoptotic cell death [104] and enhances neurogenesis and angiogenesis [105]. We have administered HUCB in a variety of brain injury and disease models in animals of different ages. When HUCB cells were transplanted after both ischemic and hemorrhagic stroke [101–104,106] and traumatic brain injury [107], there were significant improvements in behavioral indicators. Other negative regulators of stem cell niches have been suggested such as TNFα via TNFR1 [108].
Recently experimental evidence has shown that the integrity of the adaptive immune system plays a pivotal role in cognitive function and hippocampal neurogenesis, both under normal and abnormal conditions [109–111]. Mice with systemic immune deficiency (SCID mice) demonstrated cognitive impairment, which was reversed by replenishment with T cells [110]. In support of the importance of an intact immune system in brain function at physiological levels, Wolf and collaborators identified a specific T cell subtype, CD4+ T cells, as the link between the adaptive immunity and hippocampal neurogenesis. These authors demonstrated that mice null for CD4 + T cells have reduced hippocampal neurogenesis. Conversely, CD8+ T cells do not seem to affect adult hippocampal neurogenesis. Similarly, repopulation of mice lacking CD4+ T cells with CD4 but not CD8 cells brought the number of neuronal proliferative cells back to a level comparable to that of C57BL/6 wild-type mice. These data demonstrated the CD4+ T cells have a key role in the maintenance and control of adult hippocampal neurogenesis under physiological conditions. During aging there is impairment in hippocampal neurogenesis as well as in cognitive function, which is accompanied by a defect in T cell function, including decreased number of CD4+ T cell. Given this observation one has to wonder whether boosting the relevant T cell clones would ameliorate age-related cognitive decline and strengthens hippocampal neurogenesis.
Conclusion
Neuron-microglia signaling is thought to maintain microglia in a resting state, thereby suppressing the neurotoxic activity of these cells. There is an increasing amount of evidence suggesting that interruption of neuron-microglia dialogue is associated with decline in the physiological processes of aging, including hippocampal neurogenesis, as well as with exacerbation of neurodegenerative disease, including Alzheimer’s disease and Parkinson’s disease. In the future, it would be informative to determine how dysfunction in neuron-microglia signaling leads to cognitive impairment and how the disruption in this signaling can be manipulated for preventive and therapeutic purposes.
Acknowledgments
Supported by USPHS grant PO1AG-04418 and the VA Medical Research Service
References
- [1].van PH, Schinder AF, Christie BR, Toni N, Palmer TD, Gage FH. Functional neurogenesis in the adult hippocampus. Nature. 2002;415:1030–1034. doi: 10.1038/4151030a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J. Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- [3].Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410:372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- [4].Lie DC, Song H, Colamarino SA, Ming GL, Gage FH. Neurogenesis in the adult brain: new strategies for central nervous system diseases. Annu. Rev. Pharmacol. Toxicol. 2004;44:399–421. doi: 10.1146/annurev.pharmtox.44.101802.121631. [DOI] [PubMed] [Google Scholar]
- [5].Kempermann G, Wiskott L, Gage FH. Functional significance of adult neurogenesis. Curr. Opin. Neurobiol. 2004;14:186–191. doi: 10.1016/j.conb.2004.03.001. [DOI] [PubMed] [Google Scholar]
- [6].Kuhn HG, ckinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Abrous DN, Koehl M, Le MM. Adult neurogenesis: from precursors to network and physiology. Physiol Rev. 2005;85:523–569. doi: 10.1152/physrev.00055.2003. [DOI] [PubMed] [Google Scholar]
- [8].Dobrossy MD, Drapeau E, Aurousseau C, Le MM, Piazza PV, Abrous DN. Differential effects of learning on neurogenesis: learning increases or decreases the number of newly born cells depending on their birth date. Mol. Psychiatry. 2003;8:974–982. doi: 10.1038/sj.mp.4001419. [DOI] [PubMed] [Google Scholar]
- [9].Drapeau E, Montaron MF, Aguerre S, Abrous DN. Learning-induced survival of new neurons depends on the cognitive status of aged rats. J. Neurosci. 2007;27:6037–6044. doi: 10.1523/JNEUROSCI.1031-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Drapeau E, Nora AD. Role of neurogenesis in age-related memory disorders. Aging Cell. 2008 doi: 10.1111/j.1474-9726.2008.00369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Leuner B, Mendolia-Loffredo S, Kozorovitskiy Y, Samburg D, Gould E, Shors TJ. Learning enhances the survival of new neurons beyond the time when the hippocampus is required for memory. J. Neurosci. 2004;24:7477–7481. doi: 10.1523/JNEUROSCI.0204-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Bizon JL, Lee HJ, Gallagher M. Neurogenesis in a rat model of age-related cognitive decline. Aging Cell. 2004;3:227–234. doi: 10.1111/j.1474-9728.2004.00099.x. [DOI] [PubMed] [Google Scholar]
- [13].Merrill DA, Karim R, Darraq M, Chiba AA, Tuszynski MH. Hippocampal cell genesis does not correlate with spatial learning ability in aged rats. J. Comp Neurol. 2003;459:201–207. doi: 10.1002/cne.10616. [DOI] [PubMed] [Google Scholar]
- [14].Imayoshi I, Sakamoto M, Ohtsuka T, Takao K, Miyakawa T, Yamaguchi M, Mori K, Ikeda T, Itohara S, Kageyama R. Roles of continuous neurogenesis in the structural and functional integrity of the adult forebrain. Nat. Neurosci. 2008;11:1153–1161. doi: 10.1038/nn.2185. [DOI] [PubMed] [Google Scholar]
- [15].Jessberger S, Clark RE, Broadbent NJ, Clemenson GD, Jr, Consiglio A, Lie DC, Squire LR, Gage FH. Dentate gyrus-specific knockdown of adult neurogenesis impairs spatial and object recognition memory in adult rats. Learn. Mem. 2009;16:147–154. doi: 10.1101/lm.1172609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Bizon JL, Gallagher M. Production of new cells in the rat dentate gyrus over the lifespan: relation to cognitive decline. Eur. J. Neurosci. 2003;18:215–219. doi: 10.1046/j.1460-9568.2003.02733.x. [DOI] [PubMed] [Google Scholar]
- [17].Drapeau E, Mayo W, Aurousseau C, Le MM, Piazza PV, Abrous DN. Spatial memory performances of aged rats in the water maze predict levels of hippocampal neurogenesis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:14385–14390. doi: 10.1073/pnas.2334169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hattiangady B, Shetty AK. Aging does not alter the number or phenotype of putative stem/progenitor cells in the neurogenic region of the hippocampus. Neurobiol. Aging. 2006 doi: 10.1016/j.neurobiolaging.2006.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ekdahl CT, Claasen JH, Bonde S, Kokaia Z, Lindvall O. Inflammation is detrimental for neurogenesis in adult brain. Proc. Natl. Acad. Sci. U. S. A. 2003;100:13632–13637. doi: 10.1073/pnas.2234031100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Monje ML, Toda H, Palmer TD. Inflammatory blockade restores adult hippocampal neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- [21].Wakselman S, Bechade C, Roumier A, Bernard D, Triller A, Bessis A. Developmental neuronal death in hippocampus requires the microglial CD11b integrin and DAP12 immunoreceptor. J. Neurosci. 2008;28:8138–8143. doi: 10.1523/JNEUROSCI.1006-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Marin-Teva JL, Dusart I, Colin C, Gervais A, van RN, Mallat M. Microglia promote the death of developing Purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- [23].Gebicke-Haerter PJ. Microglia in neurodegeneration: molecular aspects. Microsc. Res. Tech. 2001;54:47–58. doi: 10.1002/jemt.1120. [DOI] [PubMed] [Google Scholar]
- [24].Ziv Y, Schwartz M. Immune-based regulation of adult neurogenesis: implications for learning and memory. Brain Behav. Immun. 2008;22:167–176. doi: 10.1016/j.bbi.2007.08.006. [DOI] [PubMed] [Google Scholar]
- [25].Jakubs K, Bonde S, Iosif RE, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O. Inflammation regulates functional integration of neurons born in adult brain. J. Neurosci. 2008;28:12477–12488. doi: 10.1523/JNEUROSCI.3240-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Gemma C, Fister M, Hudson C, Bickford PC. Improvement of memory for context by inhibition of caspase-1 in aged rats. Eur. J. Neurosci. 2005;22:1751–1756. doi: 10.1111/j.1460-9568.2005.04334.x. [DOI] [PubMed] [Google Scholar]
- [27].Gemma C, Bachstetter AD, Cole MJ, Fister M, Hudson C, Bickford PC. Blockade of caspase-1 increases neurogenesis in the aged hippocampus. Eur. J. Neurosci. 2007;26:2795–2803. doi: 10.1111/j.1460-9568.2007.05875.x. [DOI] [PubMed] [Google Scholar]
- [28].Koo JW, Duman RS. IL-1beta is an essential mediator of the antineurogenic and anhedonic effects of stress. Proc. Natl. Acad. Sci. U. S. A. 2008;105:751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J. Neurosci. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Bernardino L, Agasse F, Silva B, Ferreira R, Grade S, Malva JO. Tumor necrosis factor-alpha modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cells. 2008;26:2361–2371. doi: 10.1634/stemcells.2007-0914. [DOI] [PubMed] [Google Scholar]
- [31].Sheng WS, Hu S, Ni HT, Rowen TN, Lokensgard JR, Peterson PK. TNF-alpha-induced chemokine production and apoptosis in human neural precursor cells. J. Leukoc. Biol. 2005;78:1233–1241. doi: 10.1189/jlb.0405221. [DOI] [PubMed] [Google Scholar]
- [32].David JP, Ghozali F, Fallet-Bianco C, Wattez A, Delaine S, Boniface B, Di MC, Delacourte A. Glial reaction in the hippocampal formation is highly correlated with aging in human brain. Neurosci. Lett. 1997;235:53–56. doi: 10.1016/s0304-3940(97)00708-8. [DOI] [PubMed] [Google Scholar]
- [33].Streit WJ, Walter SA, Pennell NA. Reactive microgliosis. Prog. Neurobiol. 1999;57:563–581. doi: 10.1016/s0301-0082(98)00069-0. [DOI] [PubMed] [Google Scholar]
- [34].Conde JR, Streit WJ. Microglia in the aging brain. J. Neuropathol. Exp. Neurol. 2006;65:199–203. doi: 10.1097/01.jnen.0000202887.22082.63. [DOI] [PubMed] [Google Scholar]
- [35].Sugaya K. [Glial activation and brain aging] Nippon Yakurigaku Zasshi. 2001;118:251–257. doi: 10.1254/fpj.118.251. [DOI] [PubMed] [Google Scholar]
- [36].Griffin WS. Inflammation and neurodegenerative diseases. Am. J. Clin. Nutr. 2006;83:470S–474S. doi: 10.1093/ajcn/83.2.470S. [DOI] [PubMed] [Google Scholar]
- [37].Solana R, Pawelec G, Tarazona R. Aging and innate immunity. Immunity. 2006;24:491–494. doi: 10.1016/j.immuni.2006.05.003. [DOI] [PubMed] [Google Scholar]
- [38].Galea I, Bechmann I, Perry VH. What is immune privilege (not)? Trends Immunol. 2007;28:12–18. doi: 10.1016/j.it.2006.11.004. [DOI] [PubMed] [Google Scholar]
- [39].Aloisi F. Immune function of microglia. Glia. 2001;36:165–179. doi: 10.1002/glia.1106. [DOI] [PubMed] [Google Scholar]
- [40].Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, Botti P, Bacon KB, Feng L. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc. Natl. Acad. Sci. U. S. A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Batchelor PE, Liberatore GT, Wong JY, Porritt MJ, Frerichs F, Donnan GA, Howells DW. Activated macrophages and microglia induce dopaminergic sprouting in the injured striatum and express brain-derived neurotrophic factor and glial cell line-derived neurotrophic factor. J. Neurosci. 1999;19:1708–1716. doi: 10.1523/JNEUROSCI.19-05-01708.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Miwa T, Furukawa S, Nakajima K, Furukawa Y, Kohsaka S. Lipopolysaccharide enhances synthesis of brain-derived neurotrophic factor in cultured rat microglia. J. Neurosci. Res. 1997;50:1023–1029. doi: 10.1002/(SICI)1097-4547(19971215)50:6<1023::AID-JNR13>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- [43].O'Donnell SL, Frederick TJ, Krady JK, Vannucci SJ, Wood TL. IGF-I and microglia/macrophage proliferation in the ischemic mouse brain. Glia. 2002;39:85–97. doi: 10.1002/glia.10081. [DOI] [PubMed] [Google Scholar]
- [44].Nakajima K, Kohsaka S. Microglia: neuroprotective and neurotrophic cells in the central nervous system. Curr. Drug Targets. Cardiovasc. Haematol. Disord. 2004;4:65–84. doi: 10.2174/1568006043481284. [DOI] [PubMed] [Google Scholar]
- [45].Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- [46].van RD, Hanisch UK. Microglia. Metab Brain Dis. 2004;19:393–411. doi: 10.1023/b:mebr.0000043984.73063.d8. [DOI] [PubMed] [Google Scholar]
- [47].Streit WJ. Microglial senescence: does the brain's immune system have an expiration date? Trends Neurosci. 2006;29:506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- [48].Colton CA, Gilbert DL. Production of superoxide anions by a CNS macrophage, the microglia. FEBS Lett. 1987;223:284–288. doi: 10.1016/0014-5793(87)80305-8. [DOI] [PubMed] [Google Scholar]
- [49].Gemma C, Mesches MH, Sepesi B, Choo K, Holmes DB, Bickford PC. Diets enriched in foods with high antioxidant activity reverse age-induced decreases in cerebellar beta-adrenergic function and increases in proinflammatory cytokines. J. Neurosci. 2002;22:6114–6120. doi: 10.1523/JNEUROSCI.22-14-06114.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Terao A, pte-Deshpande A, Dousman L, Morairty S, Eynon BP, Kilduff TS, Freund YR. Immune response gene expression increases in the aging murine hippocampus. J. Neuroimmunol. 2002;132:99–112. doi: 10.1016/s0165-5728(02)00317-x. [DOI] [PubMed] [Google Scholar]
- [51].Weindruch R, Prolla TA. Gene expression profile of the aging brain. Arch. Neurol. 2002;59:1712–1714. doi: 10.1001/archneur.59.11.1712. [DOI] [PubMed] [Google Scholar]
- [52].Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. J. Neuroimmunol. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- [53].O'Donnell E, Vereker E, Lynch MA. Age-related impairment in LTP is accompanied by enhanced activity of stress-activated protein kinases: analysis of underlying mechanisms. Eur. J. Neurosci. 2000;12:345–352. doi: 10.1046/j.1460-9568.2000.00900.x. [DOI] [PubMed] [Google Scholar]
- [54].Vereker E, O'Donnell E, Lynch MA. The inhibitory effect of interleukin-1beta on long-term potentiation is coupled with increased activity of stress-activated protein kinases. J. Neurosci. 2000;20:6811–6819. doi: 10.1523/JNEUROSCI.20-18-06811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Lund S, Porzgen P, Mortensen AL, Hasseldam H, Bozyczko-Coyne D, Morath S, Hartung T, Bianchi M, Ghezzi P, Bsibsi M, Dijkstra S, Leist M. Inhibition of microglial inflammation by the MLK inhibitor CEP-1347. J. Neurochem. 2005;92:1439–1451. doi: 10.1111/j.1471-4159.2005.03014.x. [DOI] [PubMed] [Google Scholar]
- [56].Kim SH, Smith CJ, Van Eldik LJ. Importance of MAPK pathways for microglial pro-inflammatory cytokine IL-1 beta production. Neurobiol. Aging. 2004;25:431–439. doi: 10.1016/S0197-4580(03)00126-X. [DOI] [PubMed] [Google Scholar]
- [57].Baldassare JJ, Bi Y, Bellone CJ. The role of p38 mitogen-activated protein kinase in IL-1 beta transcription. J. Immunol. 1999;162:5367–5373. [PubMed] [Google Scholar]
- [58].Carter AB, Monick MM, Hunninghake GW. Both Erk and p38 kinases are necessary for cytokine gene transcription. Am. J. Respir. Cell Mol. Biol. 1999;20:751–758. doi: 10.1165/ajrcmb.20.4.3420. [DOI] [PubMed] [Google Scholar]
- [59].Scherle PA, Jones EA, Favata MF, Daulerio AJ, Covington MB, Nurnberg SA, Magolda RL, Trzaskos JM. Inhibition of MAP kinase kinase prevents cytokine and prostaglandin E2 production in lipopolysaccharide-stimulated monocytes. J. Immunol. 1998;161:5681–5686. [PubMed] [Google Scholar]
- [60].Yoshinari D, Takeyoshi I, Koibuchi Y, Matsumoto K, Kawashima Y, Koyama T, Ohwada S, Morishita Y. Effects of a dual inhibitor of tumor necrosis factor-alpha and interleukin-1 on lipopolysaccharide-induced lung injury in rats: involvement of the p38 mitogen-activated protein kinase pathway. Crit Care Med. 2001;29:628–634. doi: 10.1097/00003246-200103000-00029. [DOI] [PubMed] [Google Scholar]
- [61].Kelly A, Vereker E, Nolan Y, Brady M, Barry C, Loscher CE, Mills KH, Lynch MA. Activation of p38 plays a pivotal role in the inhibitory effect of lipopolysaccharide and interleukin-1 beta on long term potentiation in rat dentate gyrus. J. Biol. Chem. 2003;278:19453–19462. doi: 10.1074/jbc.M301938200. [DOI] [PubMed] [Google Scholar]
- [62].Biber K, Neumann H, Inoue K, Boddeke HW. Neuronal ‘On’ and ‘Off’ signals control microglia. Trends Neurosci. 2007;30:596–602. doi: 10.1016/j.tins.2007.08.007. [DOI] [PubMed] [Google Scholar]
- [63].Morris RJ, Beech JN. Sequential expression of OX2 and Thy-1 glycoproteins on the neuronal surface during development. An immunohistochemical study of rat cerebellum. Dev. Neurosci. 1987;9:33–44. doi: 10.1159/000111606. [DOI] [PubMed] [Google Scholar]
- [64].Webb M, Barclay AN. Localisation of the MRC OX-2 glycoprotein on the surfaces of neurones. J. Neurochem. 1984;43:1061–1067. doi: 10.1111/j.1471-4159.1984.tb12844.x. [DOI] [PubMed] [Google Scholar]
- [65].Barclay AN. Different reticular elements in rat lymphoid tissue identified by localization of Ia, Thy-1 and MRC OX 2 antigens. Immunology. 1981;44:727–736. [PMC free article] [PubMed] [Google Scholar]
- [66].Koning N, Bo L, Hoek RM, Huitinga I. Downregulation of macrophage inhibitory molecules in multiple sclerosis lesions. Ann. Neurol. 2007;62:504–514. doi: 10.1002/ana.21220. [DOI] [PubMed] [Google Scholar]
- [67].Walker DG, Dalsing-Hernandez JE, Campbell NA, Lue LF. Decreased expression of CD200 and CD200 receptor in Alzheimer's disease: a potential mechanism leading to chronic inflammation. Exp. Neurol. 2009;215:5–19. doi: 10.1016/j.expneurol.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Gorczynski R, Chen Z, Kai Y, Lee L, Wong S, Marsden PA. CD200 is a ligand for all members of the CD200R family of immunoregulatory molecules. J. Immunol. 2004;172:7744–7749. doi: 10.4049/jimmunol.172.12.7744. [DOI] [PubMed] [Google Scholar]
- [69].Rijkers ES, de RT, Baridi A, Veninga H, Hoek RM, Meyaard L. The inhibitory CD200R is differentially expressed on human and mouse T and B lymphocytes. Mol. Immunol. 2008;45:1126–1135. doi: 10.1016/j.molimm.2007.07.013. [DOI] [PubMed] [Google Scholar]
- [70].Hatherley D, Barclay AN. The CD200 and CD200 receptor cell surface proteins interact through their N-terminal immunoglobulin-like domains. Eur. J. Immunol. 2004;34:1688–1694. doi: 10.1002/eji.200425080. [DOI] [PubMed] [Google Scholar]
- [71].Zhang S, Cherwinski H, Sedgwick JD, Phillips JH. Molecular mechanisms of CD200 inhibition of mast cell activation. J. Immunol. 2004;173:6786–6793. doi: 10.4049/jimmunol.173.11.6786. [DOI] [PubMed] [Google Scholar]
- [72].Hoek RM, Ruuls SR, Murphy CA, Wright GJ, Goddard R, Zurawski SM, Blom B, Homola ME, Streit WJ, Brown MH, Barclay AN, Sedgwick JD. Down-regulation of the macrophage lineage through interaction with OX2 (CD200) Science. 2000;290:1768–1771. doi: 10.1126/science.290.5497.1768. [DOI] [PubMed] [Google Scholar]
- [73].Lyons A, Downer EJ, Crotty S, Nolan YM, Mills KH, Lynch MA. CD200 ligand receptor interaction modulates microglial activation in vivo and in vitro: a role for IL-4. J. Neurosci. 2007;27:8309–8313. doi: 10.1523/JNEUROSCI.1781-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Liu Y, Bando Y, Vargas-Lowy D, Elyaman W, Khoury SJ, Huang T, Reif K, Chitnis T. CD200R1 Agonist Attenuates Mechanisms of Chronic Disease in a Murine Model of Multiple Sclerosis. J. Neurosci. 2010;30:2025–2038. doi: 10.1523/JNEUROSCI.4272-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Ransohoff RM, Liu L, Cardona AE. Chemokines and chemokine receptors: multipurpose players in neuroinflammation. Int. Rev. Neurobiol. 2007;82:187–204. doi: 10.1016/S0074-7742(07)82010-1. [DOI] [PubMed] [Google Scholar]
- [76].Hundhausen C, Misztela D, Berkhout TA, Broadway N, Saftig P, Reiss K, Hartmann D, Fahrenholz F, Postina R, Matthews V, Kallen KJ, Rose-John S, Ludwig A. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood. 2003;102:1186–1195. doi: 10.1182/blood-2002-12-3775. [DOI] [PubMed] [Google Scholar]
- [77].Tsou CL, Haskell CA, Charo IF. Tumor necrosis factor-alpha-converting enzyme mediates the inducible cleavage of fractalkine. J. Biol. Chem. 2001;276:44622–44626. doi: 10.1074/jbc.M107327200. [DOI] [PubMed] [Google Scholar]
- [78].Yoshida H, Imaizumi T, Fujimoto K, Matsuo N, Kimura K, Cui X, Matsumiya T, Tanji K, Shibata T, Tamo W, Kumagai M, Satoh K. Synergistic stimulation, by tumor necrosis factor-alpha and interferon-gamma, of fractalkine expression in human astrocytes. Neurosci. Lett. 2001;303:132–136. doi: 10.1016/s0304-3940(01)01699-8. [DOI] [PubMed] [Google Scholar]
- [79].Meucci O, Fatatis A, Simen AA, Miller RJ. Expression of CX3CR1 chemokine receptors on neurons and their role in neuronal survival. Proc. Natl. Acad. Sci. U. S. A. 2000;97:8075–8080. doi: 10.1073/pnas.090017497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Kawaguchi N, Kume T, Akaike A, Satoh M. Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia? FEBS Lett. 1998;429:167–172. doi: 10.1016/s0014-5793(98)00583-3. [DOI] [PubMed] [Google Scholar]
- [81].Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, Vath J, Gosselin M, Ma J, Dussault B, Woolf E, Alperin G, Culpepper J, Gutierrez-Ramos JC, Gearing D. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387:611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- [82].Hatori K, Nagai A, Heisel R, Ryu JK, Kim SU. Fractalkine and fractalkine receptors in human neurons and glial cells. J. Neurosci. Res. 2002;69:418–426. doi: 10.1002/jnr.10304. [DOI] [PubMed] [Google Scholar]
- [83].Tran PB, Miller RJ. Chemokine receptors: signposts to brain development and disease. Nat. Rev. Neurosci. 2003;4:444–455. doi: 10.1038/nrn1116. [DOI] [PubMed] [Google Scholar]
- [84].Kim TS, Lim HK, Lee JY, Kim DJ, Park S, Lee C, Lee CU. Changes in the levels of plasma soluble fractalkine in patients with mild cognitive impairment and Alzheimer's disease. Neurosci. Lett. 2008;436:196–200. doi: 10.1016/j.neulet.2008.03.019. [DOI] [PubMed] [Google Scholar]
- [85].Bachstetter AD, Morganti JM, Jernberg J, Schlunk A, Mitchell SH, Brewster KW, Hudson CE, Cole MJ, Harrison JK, Bickford PC, Gemma C. Fractalkine and CX(3)CR1 regulate hippocampal neurogenesis in adult and aged rats. Neurobiol. Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.11.022. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Lyons A, Lynch AM, Downer EJ, Hanley R, O'Sullivan JB, Smith A, Lynch MA. Fractalkine-induced activation of the phosphatidylinositol-3 kinase pathway attentuates microglial activation in vivo and in vitro. J. Neurochem. 2009;110:1547–1556. doi: 10.1111/j.1471-4159.2009.06253.x. [DOI] [PubMed] [Google Scholar]
- [87].Duan RS, Yang X, Chen ZG, Lu MO, Morris C, Winblad B, Zhu J. Decreased fractalkine and increased IP-10 expression in aged brain of APP(swe) transgenic mice. Neurochem. Res. 2008;33:1085–1089. doi: 10.1007/s11064-007-9554-z. [DOI] [PubMed] [Google Scholar]
- [88].McDermott DH, Fong AM, Yang Q, Sechler JM, Cupples LA, Merrell MN, Wilson PW, D'Agostino RB, O'Donnell CJ, Patel DD, Murphy PM. Chemokine receptor mutant CX3CR1-M280 has impaired adhesive function and correlates with protection from cardiovascular disease in humans. J. Clin. Invest. 2003;111:1241–1250. doi: 10.1172/JCI16790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Tuo J, Smith BC, Bojanowski CM, Meleth AD, Gery I, Csaky KG, Chew EY, Chan CC. The involvement of sequence variation and expression of CX3CR1 in the pathogenesis of age-related macular degeneration. FASEB J. 2004;18:1297–1299. doi: 10.1096/fj.04-1862fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Soriano SG, Amaravadi LS, Wang YF, Zhou H, Yu GX, Tonra JR, Fairchild-Huntress V, Fang Q, Dunmore JH, Huszar D, Pan Y. Mice deficient in fractalkine are less susceptible to cerebral ischemia-reperfusion injury. J. Neuroimmunol. 2002;125:59–65. doi: 10.1016/s0165-5728(02)00033-4. [DOI] [PubMed] [Google Scholar]
- [91].Milligan ED, Sloane EM, Watkins LR. Glia in pathological pain: a role for fractalkine. J. Neuroimmunol. 2008;198:113–120. doi: 10.1016/j.jneuroim.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Pereira CF, Middel J, Jansen G, Verhoef J, Nottet HS. Enhanced expression of fractalkine in HIV-1 associated dementia. J. Neuroimmunol. 2001;115:168–175. doi: 10.1016/s0165-5728(01)00262-4. [DOI] [PubMed] [Google Scholar]
- [93].Re DB, Przedborski S. Fractalkine: moving from chemotaxis to neuroprotection. Nat. Neurosci. 2006;9:859–861. doi: 10.1038/nn0706-859. [DOI] [PubMed] [Google Scholar]
- [94].Yoneda O, Imai T, Goda S, Inoue H, Yamauchi A, Okazaki T, Imai H, Yoshie O, Bloom ET, Domae N, Umehara H. Fractalkine-mediated endothelial cell injury by NK cells. J. Immunol. 2000;164:4055–4062. doi: 10.4049/jimmunol.164.8.4055. [DOI] [PubMed] [Google Scholar]
- [95].Shan S, Hong-Min T, Yi F, Jun-Peng G, Yue F, Yan-Hong T, Yun-Ke Y, Wen-Wei L, Xiang-Yu W, Jun M, Guo-Hua W, Ya-Ling H, Hua-Wei L, Ding-Fang C. NEW evidences for fractalkine/CX3CL1 involved in substantia nigral microglial activation and behavioral changes in a rat model of Parkinson's disease. Neurobiol. Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.03.004. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- [96].Fuhrmann M, Bittner T, Jung CK, Burgold S, Page RM, Mitteregger G, Haass C, LaFerla FM, Kretzschmar H, Herms J. Microglial Cx3cr1 knockout prevents neuron loss in a mouse model of Alzheimer's disease. Nat. Neurosci. 2010;13:411–413. doi: 10.1038/nn.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, Lee JC, Cook DN, Jung S, Lira SA, Littman DR, Ransohoff RM. Control of microglial neurotoxicity by the fractalkine receptor. Nat. Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- [98].Lee S, Varvel NH, Konerth ME, Xu G, Cardona AE, Ransohoff RM, Lamb BT. CX3CR1 Deficiency Alters Microglial Activation and Reduces Beta-Amyloid Deposition in Two Alzheimer's Disease Mouse Models. Am. J. Pathol. 2010;177:2549–2562. doi: 10.2353/ajpath.2010.100265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Bhaskar K, Konerth M, Kokiko-Cochran ON, Cardona A, Ransohoff RM, Lamb BT. Regulation of tau pathology by the microglial fractalkine receptor. Neuron. 2010;68:19–31. doi: 10.1016/j.neuron.2010.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433:760–764. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- [101].Mayack SR, Shadrach JL, Kim FS, Wagers AJ. Systemic signals regulate ageing and rejuvenation of blood stem cell niches. Nature. 2010;463:495–500. doi: 10.1038/nature08749. [DOI] [PubMed] [Google Scholar]
- [102].Bachstetter AD, Pabon MM, Cole MJ, Hudson CE, Sanberg PR, Willing AE, Bickford PC, Gemma C. Peripheral injection of human umbilical cord blood stimulates neurogenesis in the aged rat brain. BMC. Neurosci. 2008;9:22. doi: 10.1186/1471-2202-9-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Sorg RV, Andres S, Kogler G, Fischer J, Wernet P. Phenotypic and functional comparison of monocytes from cord blood and granulocyte colony-stimulating factor-mobilized apheresis products. Exp. Hematol. 2001;29:1289–1294. doi: 10.1016/s0301-472x(01)00735-4. [DOI] [PubMed] [Google Scholar]
- [104].Araki H, Hino N, Karasawa Y, Kawasaki H, Gomita Y. Effect of dopamine blockers on cerebral ischemia-induced hyperactivity in gerbils. Physiol Behav. 1999;66:263–268. doi: 10.1016/s0031-9384(98)00293-5. [DOI] [PubMed] [Google Scholar]
- [105].Willing AE, Lixian J, Milliken M, Poulos S, Zigova T, Song S, Hart C, Sanchez-Ramos J, Sanberg PR. Intravenous versus intrastriatal cord blood administration in a rodent model of stroke. J. Neurosci. Res. 2003;73:296–307. doi: 10.1002/jnr.10659. [DOI] [PubMed] [Google Scholar]
- [106].Borlongan CV, Cahill DW, Sanberg PR. Locomotor and passive avoidance deficits following occlusion of the middle cerebral artery. Physiol Behav. 1995;58:909–917. doi: 10.1016/0031-9384(95)00103-p. [DOI] [PubMed] [Google Scholar]
- [107].Pennypacker KR, Kassed CA, Eidizadeh S, Saporta S, Sanberg PR, Willing AE. NF-kappaB p50 is increased in neurons surviving hippocampal injury. Exp. Neurol. 2001;172:307–319. doi: 10.1006/exnr.2001.7817. [DOI] [PubMed] [Google Scholar]
- [108].Yamamoto T, Araki H, Futagami K, Kawasaki H, Gomita Y. Dopaminergic neurotransmission triggers ischemia-induced hyperactivity in Mongolian gerbils. Acta Med. Okayama. 2001;55:277–282. doi: 10.18926/AMO/32017. [DOI] [PubMed] [Google Scholar]
- [109].Ford AL, Goodsall AL, Hickey WF, Sedgwick JD. Normal adult ramified microglia separated from other central nervous system macrophages by flow cytometric sorting. Phenotypic differences defined and direct ex vivo antigen presentation to myelin basic protein-reactive CD4+ T cells compared. J. Immunol. 1995;154:4309–4321. [PubMed] [Google Scholar]
- [110].Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJ, Bonde S, Kokaia Z, Jacobsen SE, Lindvall O. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. J. Neurosci. 2006;26:9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Ziv Y, Ron N, Butovsky O, Landa G, Sudai E, Greenberg N, Cohen H, Kipnis J, Schwartz M. Immune cells contribute to the maintenance of neurogenesis and spatial learning abilities in adulthood. Nat. Neurosci. 2006;9:268–275. doi: 10.1038/nn1629. [DOI] [PubMed] [Google Scholar]
- [112].Kipnis J, Cohen H, Cardon M, Ziv Y, Schwartz M. T cell deficiency leads to cognitive dysfunction: implications for therapeutic vaccination for schizophrenia and other psychiatric conditions. Proc. Natl. Acad. Sci. U. S. A. 2004;101:8180–8185. doi: 10.1073/pnas.0402268101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Wolf SA, Steiner B, Akpinarli A, Kammertoens T, Nassenstein C, Braun A, Blankenstein T, Kempermann G. CD4-positive T lymphocytes provide a neuroimmunological link in the control of adult hippocampal neurogenesis. J. Immunol. 2009;182:3979–3984. doi: 10.4049/jimmunol.0801218. [DOI] [PubMed] [Google Scholar]