Abstract
Background:
Standard assay has limited utility in diagnosing HIV reactivity among infants till the age of 18 months by which time, many HIV-infected infants expire. The test for diagnosing infant and children below 18 months is DNA polymerase chain reaction (DNAPCR) either by dried blood spot (DBS) or whole blood sample (WBS). Early infant diagnosis (EID) project is implemented in 18 districts of Gujarat through 33 PPTCT centers from 1st April 2010. Present analysis is done to evaluate factors curbing mother to child HIV transmission.
Materials and Methods:
Study included all children (< 18 months) who are born to HIV-positive mothers or referred children with signs/ symptoms of HIV with unknown parent status or children already on anti-retroviral therapy whose status could not be confirmed by antibody tests. Data was compiled and analyzed according to the infant's age at testing, type of feeding, history of Anti retero viral (ARV) prophylaxis, and type of delivery. Data compiled between April and August 2010 was used for the analysis.
Results:
Cohort of 326 infants was followed up, fewer infants (14/270) who received ARV prophylaxis tested positive than those who did not (23/56). Transmission was more in normal delivery (29/252) than cesarean (8/74). Low transmission rate was seen in replacement feeding (13/208) than breast/mixed feeding (24/94). Out of 37 samples found positive by the DBS, 17 were sent for WBS and all were found to be positive.
Conclusion:
DBS test results were found as accurate as WBS. So DBS (less cumbersome and cost effective) can be used in future exclusively. Nevirapine administration at birth as mother baby pair showed 36% decrease in MTCT.
Keywords: Dried Blood spot, early infant diagnosis, hiv testing in children, whole blood sample
Introduction
HIV-infected children are most vulnerable of all patients. In infants who acquire HIV at the time of delivery, disease progresses rapidly in the first few months of life, often leading to death.(1–4) While 35% children do not see their first birthday, 53% do not celebrate their second birthday. The exposed child when receives prophylactic antibiotics (Cotrimoxazole) and anti-retroviral therapy (ART) as soon as is medically indicated, there is a significant chance of long and healthy survival.
The antibody-specific methods of diagnosing HIV infection among adults are enzyme-linked immunoassay (ELISA), simple and rapid tests based on different methodology. Mothers transfer antibodies passively to babies in utero; these standard assays have limited utility until the age of 18 months at which the mother's antibodies totally clear from the infant's blood and infected child develop its own antibody to HIV. There is an ongoing risk for HIV acquisition during breast feeding; therefore, HIV infection can be excluded after breast feeding is stopped for 6 weeks. HIV antibody testing can be done at 9 to 12 month of age; by then 74% and 96% of HIV-uninfected children respectively will have a negative HIV antibody test. However, it is recommended that if the infant is diagnosed at 9-12 months, a confirmatory test should be done after 18 months of age and infant should have stopped breast feeding for more than 6 weeks.
More precise option is the antigen-specific test for diagnosing infants/children below 18 months is DNA polymerase chain reaction (DNA PCR),(5–7) which detects HIV-1 pro-viral DNA integrated to human genome. There are two methods namely whole blood sample (WBS) and dried blood spot (DBS) method. Out of these, WBS requires sophisticated, expensive equipment, and the samples have to be sent to the laboratory within 24 hours under proper cold chain maintenance, while DBS can be stored and shipped to testing facilities without refrigeration within 15 days, so DBS is user friendly and cost-effective mode in resource-limited settings.(5) National AIDS Control Organization (NACO) suggests to use the DBS as a prime test for all the children and if it is found positive then to use a WBS as a confirmatory test. DBS is advised for infants below 6 months of age, while for those above 6 months of age first a rapid test is to be done and the DBS is to be done only if the rapid test is found positive.(8)
The present analysis was done to (1) find out HIV reactivity at the earlier stage of life of an exposed child so as to link the reactive infant to appropriate care, support, and treatment facilities, (2) correlate HIV positivity with factors like type of feeding, history of ARV prophylaxis and type of delivery, and (3) quantify the reduction achieved in MTCT by SD-NVP.
Materials and Methods
Under the guidance of NACO, Gujarat State AIDS Control Society (GSACS) is implementing an EID project in 18 districts through 33 PPTCT centers (where ANC positivity is relatively high) from the 1 April 2010. GSACS trained lab technicians, counselors and medical officers of these 33 PPTCT project-supported testing centers.
Inclusion criteria
All children with age < 18 months born to HIV-positive mothers, referred children with signs and symptoms of HIV with unknown parent status and children already on ART based on presumptive diagnosis but their confirmatory status is not known.
Exclusion criteria
Children whose age >18 months and their testing is done under antibody specified testing as per National Protocol.
Data of 5 months (April to August 2010) was considered for the analysis. During this period, 326 samples were collected in PPTCT clinics and out of them 103 were found negative in the rapid test; rest 223 samples (either found positive on rapid or were less than 6 months of age) were linked PCR laboratory for DBS testing. Out of these 223, 37 tested positive for HIV. All infants found DBS positive have to be sent to ART centers to collect the WBS samples. Out of which up to August 10, 17 WBS were sent to PCR lab Mumbai for testing and all of them were found positive on WBS as well. All 17 positive children referred to the ART centers for the pediatric ART, where they underwent further evaluation for medical eligibility for antiretroviral therapy (ART). Those who met the criteria were put on treatment and rest are followed up closely and provided Cotrimoxazole prophylactic therapy (CPT) and nutritional counseling.
Results
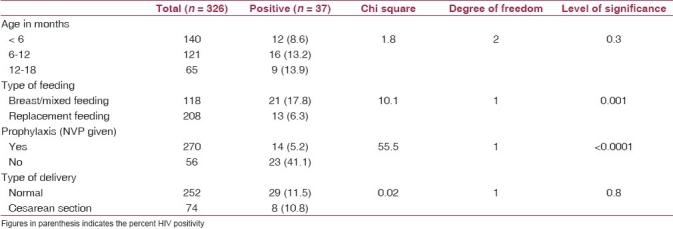
Out of total 326 infants born out of HIV-positive pregnant women, 37 were found positive with 11.4% HIV positivity. There were 170 (52%) males and 156 (48%) females with 24 and 13 respectively being HIV reactive. Sero-positivity was higher in males (14.2%) than females (8.3%). Most children belonged to the age group of less than 6 months (43.0%) followed by 6–12 months (37.1%) and 12–18 months of age (20.0%). HIV positivity was high in 6–18 months of age (13.2–13.9%) compared to less than 6 months of age (8.6%). However, this difference was not significant [Table 1]. Following the advice of health providers, 208 (63.8%) infants were kept on replacement feeding and they had significantly low HIV positivity (6.3%) than those who were either on breast/mixed feeding. Out of 270 infants who received ARV prophylaxis, 14 became HIV positive with a positivity of 5.2% compared to those who did not receive the ARV prophylaxis (41.1%) and this difference was highly significant. HIV positivity rates in normal delivery (11.5%) and in cesarean section (10.8%) were comparable and difference was insignificant [Table 1]. Till Aug 2010, out of 37 samples found positive by the DBS, 17 samples were sent for WBS and all of them were found positive by WBS as well.
Table 1.
HIV test result and basic profile of the children tested (n = 326) (April to August 2010)
Discussion
The role of gender in influencing HIV positivity in new born child is not known. In the present study, HIV positivity was high in male than female children. A study in Kenya(9) also found more positivity in male (23%) than female (11%) children. The present study also found almost three times high positivity in those children who are kept on breast or mixed feeding. Exclusive breast feeding for first 6 months for such children is recommended(8) but in field situations, it is difficult to ensure this so ultimately it leads to the mixed feeding – which is worse than the replacement feeding. Administration of SD-NVP to mother and newborn at the time of delivery is the most effective tool under the NACP III for reducing the MTCT. Here too in the present study, HIV positivity was significantly less in those children who received the ARV prophylaxis than those who could not. This difference of eight times less positivity when SD-NVP coverage is ensured emphasizes the need for strengthening the strategy of giving ARVs for MTCT. Studies from other countries such as Botswana(10) and Tanzania(11) also show low prevalence in children receiving the Nevirapine at the time of birth however, the prevalence of HIV in such groups vary between 7% and 17%. Kenyan study(9) showed proportion of HIV as 15% and 30% in children who were “given ARV” and “not given ARV” respectively.
Cesarean section (CS) is believed to decrease transmission of HIV as documented in a Kenyan study.(9) However, there is statistically insignificant difference between vaginal delivery and Cesarean section. A complete agreement was found between the results of DBS and the WBS as all 17 DBS-positive samples sent for WBs have also given the positive results. This discordant result in this series till date is nil. Since DBS is user friendly and cost effective, these results can provide an evidence of changing the current protocol of doing the WBS in all those found positive with the DBS. In that case, the results of DBS can be taken as final and can form the basis declaring the status of the child. Elsewhere(5) too, DBS has been shown as effective as WBS, with a sensitivity of 100% and specificity of 99.6%. However, this finding is needs validation with more evidences.
Conclusions
There is a need to advocate the exclusive breast feeding, and allow replacement feeding only when it is acceptable, feasible, affordable, sustainable, safe (AFASS) while the mixed feeding should be contraindicated.
There is a need to strengthen the coverage of current strategy of giving ARV prophylaxis to both mother and newborn.
Based on the evidence from this study, the revision of protocol may be considered whereby the DBS results can be considered as final.
Limitations
Present communication is based on the observations of only 326 children and is part of an ongoing intervention; therefore the data needs a conscious interpretation. Moreover, many studies have shown a concern on development of NVP resistance to the mother due to the administration of MB pair.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Mphatswe W, Blanckenberg N, Tudor-Williams G, Prendergast A, Thobakgale C, Mkhwanazi N, et al. High frequency of rapid immunological progression in African infants infected in the era of perinatal HIV prophylaxis. AIDS. 2007;21:1253–61. doi: 10.1097/QAD.0b013e3281a3bec2. [DOI] [PubMed] [Google Scholar]
- 2.Newell ML, Coovadia H, Cortina-Borja M, Rollins N, Gaillard P, Dabis F. Mortality of infected and uninfected infants born to HIV-infected mothers in Africa: A pooled analysis. Lancet. 2004;364:1236–43. doi: 10.1016/S0140-6736(04)17140-7. [DOI] [PubMed] [Google Scholar]
- 3.Little K, Thorne C, Luo C, Bunders M, Ngongo N, McDermott P, et al. Disease progression in children with vertically-acquired HIV infection in sub-Saharan Africa: Reviewing the need for HIV treatment. Curr HIV Res. 2007;5:139–53. doi: 10.2174/157016207780077002. [DOI] [PubMed] [Google Scholar]
- 4.Violari A, Cotton MF, Gibb DM, Babiker AG, Steyn J, Madhi SA, et al. Early antiretroviral therapy and mortality among HIV-infected infants. N Engl J Med. 2008;359:2233–44. doi: 10.1056/NEJMoa0800971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sherman GG, Cooper PA, Coovadia AH, Puren AJ, Jones SA, Mokhachane M, et al. Polymerase chain reaction for diagnosis of human immunodeficiency virus infection in infancy in low resource settings. Pediatr Infect Dis J. 2005;24:993–7. doi: 10.1097/01.inf.0000187036.73539.8d. [DOI] [PubMed] [Google Scholar]
- 6.Creek TL, Sherman GG, Nkengasong J, Lu L, Finkbeiner T, Fowler MG, et al. Infant human immunodeficiency virus diagnosis in resource-limited settings: Issues, technologies, and country experiences. Am J Obstet Gynecol. 2007;197(3 Suppl):S64–71. doi: 10.1016/j.ajog.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 7.Stevens W, Sherman G, Downing R, Parsons LM, Ou CY, Crowley S, et al. Role of the laboratory in ensuring global access to ARV treatment for HIV-infected children: Consensus statement on the performance of laboratory assays for early infant diagnosis. Open AIDS J. 2008;2:17–25. doi: 10.2174/1874613600802010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.New Delhi: National AIDS Control Organization; 2010. Jan, NACO, Guidelines for Care of HIV exposed infants and children less than 18 months; pp. 24–36. [Google Scholar]
- 9.Pathfinder International/ Kenya's Prevention of Mother to Child Transmission Project Early Infant Diagnosis of HIV through Dried Blood Spot Testing. 2007. Oct, [Last accessed on 2010 Oct 7]. Available from: http://www.pathfind.org/site/DocServer/Kenya_EID.pdf?docID=10201 .
- 10.Creek, Tracy Early diagnosis of human immunodeficiency virus in infants using polymerase chain reaction on dried blood spots in Botswana's national program for prevention of mother-to-child transmission. Pediatr Infect Dis J. 2008;27:22–6. doi: 10.1097/INF.0b013e3181469050. [DOI] [PubMed] [Google Scholar]
- 11.Nuwagaba-Biribonwoha, Werq-Semo B, Abdallah A, Cunningham A, Gamaliel JG, Mtunga S, et al. Introducing a multi-site program for early diagnosis of HIV infection among HIV-exposed infants in Tanzania. BMC Pediatr. 2010;10:44. doi: 10.1186/1471-2431-10-44. [DOI] [PMC free article] [PubMed] [Google Scholar]


