Abstract
The extent of T cell activation, proliferation, and survival that follows T cell receptor ligation is controlled by several factors including the strength of TCR stimulation, the availability of pro-survival cytokines, and the presence or absence of co-stimulatory signals. In addition to engagement of the CD28 co-stimulatory receptor by its natural ligands, B7.1 (CD80) and B7.2 (CD86), recent work has begun to elucidate the mechanisms by which signaling through the OX40 (CD134) co-stimulatory receptor, a member of the TNFR super-family, affects T cell responses. Importantly, OX40 ligation has been shown to augment CD4 and CD8 T cell clonal expansion, effector differentiation, survival, and in some cases, abrogate the suppressive activity of regulatory FoxP3+CD25+CD4+ T cells. In this review, we will focus on the mechanisms regulating OX40 expression on activated T cells as well as the role of OX40-mediated co-stimulation in boosting T cell clonal expansion, effector differentiation, and survival.
Keywords: T lymphocytes, OX40, CD134, co-stimulation, memory
I. Introduction
The initial proliferative response of naïve T cells is driven by T cell receptor (TCR)-mediated recognition of cognate peptide-MHC complexes on antigen presenting cells (APCs). In the absence of co-stimulatory signals, TCR stimulation promotes an abortive proliferative response in which the T cells fail to differentiate and instead, undergo tolerance. The induction of both central (thymic) and peripheral tolerance is essential for removing potentially self-reactive cells from the T cell repertoire and occurs through a variety of mechanisms including anergy, immuno-suppression, and deletion through apoptosis.1,2 In contrast, a productive T cell response requires TCR stimulation in the presence of co-stimulation, which promotes T cell clonal expansion, effector differentiation, and survival. Perhaps the best-characterized T cell co-stimulatory molecule is CD28, a member of the immunoglobulin (Ig) super-family that is constitutively expressed on the surface of naïve T cells and certain subsets of memory T cells. CD28 signaling via its ligands B7.1 (CD80) and B7.2 (CD86) serves to lower the threshold for TCR activation and drives T cell expansion, IL-2 production, differentiation, and the increased expression of anti-apoptotic molecules, such as Bcl-xL.3,4 Importantly, the presence or absence of CD28-B7 co-stimulation is an essential checkpoint that helps to ensure that only activated APCs are able to prime naïve T cells.
Although B7-CD28-specific signaling is a critical component of T cell priming, signaling through CD28 alone is not sufficient to sustain an optimal T cell response. Rather, co-stimulation through various members of the tumor necrosis factor receptor (TNFR) super-family, including OX40 (CD134), 4-1BB (CD137), or GITR is often required to further augment CD4 and CD8 T cell priming and enhance the generation of memory cells.5,6 Our laboratory and others have focused on the mechanisms by which OX40-mediated co-stimulation augments CD4 and CD8 T cell responses. Importantly, OX40 ligation with agonist OX40-specific reagents has been shown to enhance T cell-mediated anti-tumor immunity greatly and to modulate the generation of effector and memory T cells.7,8 In addition to its expression on recently activated CD4 and CD8 T cells, OX40 is also constitutively expressed on the majority of FoxP3+CD25+CD4+ regulatory T cells (Treg) and recent work suggests that OX40-specific signaling can abrogate the suppressive activity of Treg.9-11
Based upon the ability of OX40 ligation to augment effector CD4 and CD8 T cell responses as well as Treg, OX40 could represent an important therapeutic target for human disease. Indeed, inhibition of OX40-mediated signaling can reduce the extent of inflammation and ameliorate the severity of autoimmunity in pre-clinical models of multiple sclerosis (experimental autoimmune encephalomyelitis (EAE)), asthma, arthritis, type 1 (autoimmune) diabetes, and colitis.12-20 Furthermore, recent work has suggested that the extent of OX40 expression might serve as a biomarker that correlates with the initiation and prognosis of human diseases including multiple sclerosis, rheumatoid arthritis, and type 1 diabetes.21-23 OX40-expressing T cells have also been detected in tumor-infiltrating lymphocytes isolated from murine or human tumor biopsies as well as in the tumor-draining lymph nodes of cancer patients.24-26 Importantly, treatment with an agonist anti-OX40 mAb or OX40L-expressing APCs has been shown to boost tumor-specific immunity in a variety of pre-clinical models, including breast, lung, and colon cancer.25,27-30 Based upon these and other pre-clinical data, we recently initiated a Phase I clinical trial with an agonist anti-human OX40 mAb for the treatment of patients with cancer that is measuring immunological endpoints as well as toxicity.7,31
Taken together, these data highlight the critical role of OX40-mediated signaling in boosting T cell responses and suggest that elucidating the cellular and molecular mechanisms regulating OX40-specific co-stimulation may lead to the development of novel therapies for the treatment of human disease. In this review, we will focus on three aspects of OX40-mediated co-stimulation: 1) The mechanisms regulating OX40 and OX40L expression on T cells; 2) The effects of OX40 ligation on T cell clonal expansion and differentiation; and 3) The mechanisms by which OX40-specific signaling augments T cell survival.
II. Regulation of OX40 and OX40L expression on T cells
A. Induction of OX40 expression on activated T cells
OX40 was initially identified on in vitro activated CD4 T cells,32 but subsequent studies revealed that OX40 is also expressed on activated CD8 T cells and neutrophils.33,34 OX40 expression peaks 48-72 hours after TCR stimulation and is down-regulated by approximately 120 hours after the initial TCR stimulation.35 One of the critical factors dictating the extent of OX40 expression is the strength of TCR stimulation. For example, activation of naïve polyclonal CD8 T cells with anti-CD3 alone was shown to induce OX40 in a dose-dependent manner. Similarly, activation of Ag-specific CD8 T cells with high-doses of cognate Ag or a strong TCR agonist in vitro was required to promote optimal OX40 expression, while stimulation with low-dose Ag or a weak TCR agonist resulted in limited or no up-regulation of OX40.36 Although TCR stimulation is clearly required to induce OX40, CD28, IL-2, and IL-4 receptor signals can affect the extent of OX40 expression on activated T cells.37,38 It should be noted that CD28 engagement also leads to enhanced IL-2 production and increased expression of the high-affinity IL-2R on activated T cells. Thus, whether CD28-specific signals regulate OX40 directly or through an IL-2R-dependent mechanism remains to be determined.
One of the most potent effects of OX40-specific T cell co-stimulation is increased IL-2 production and a concomitant increase in expression of the IL-2Rα (CD25) on CD4 and CD8 T cells.39,40 In T cells, signaling through the IL-2R leads to phosphorylation of the JAK1 and JAK3 kinases, which in turn promotes the additional phosphorylation and activation of other signaling molecules including the kinases PKB/AKT and ERK1/2 as well as the transcription factor, STAT5.41,42 Ultimately, IL-2 mediated activation of PKB/AKT promotes survival, while STAT5 activation initiates a positive feedback loop that augments and sustains CD25 expression and IL-2 production. Although OX40 ligation strongly enhances IL-2 production by CD4 T cells, whether IL-2-mediated signaling directly affects OX40 expression on CD4 or CD8 T cells remains unclear. Recent work from Williams et al. demonstrated that CD25-deficient CD4 T cells retained their ability to express OX40 upon TCR stimulation, although IL-2-mediated signaling was required for their differentiation into effector cells following OX40 ligation.43 In contrast, studies from Verdeil et al. have shown that in the presence of sub-optimal TCR stimulation, the addition of exogenous IL-2 enhanced OX40 expression CD8 T cells.36 Similarly, the addition of anti-IL-2 and anti-IL-2Rα monoclonal Abs can reduce the extent of OX40 expression on Treg and inhibit the induction of OX40 on activated CD8 T cells (our unpublished data). We are currently investigating the downstream mechanisms by which IL-2R signaling affects OX40 expression on activated CD8 T cells, including the role of JAK3, STAT5, AKT, and/or ERK1/2 phosphorylation. Interestingly, our preliminary results indicate that OX40 can be induced in an ERK1/2-independent, but JAK3-dependent manner (our unpublished data). Additional studies will be needed to define the downstream signaling molecules that regulate OX40 and to determine whether OX40 is differentially regulated on CD4 and CD8 T cells.
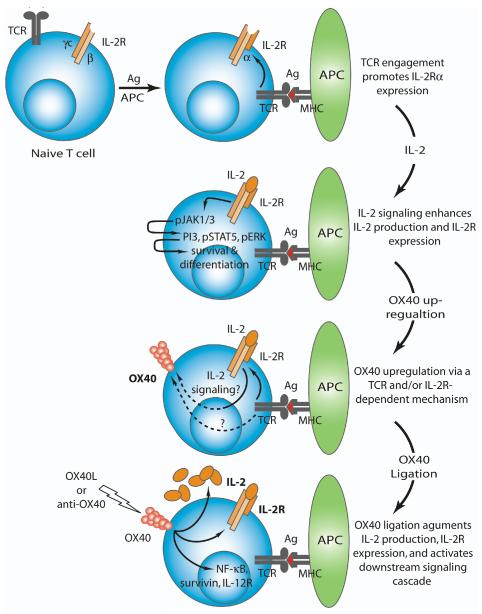
Based upon these and other data, we propose a model for the induction of OX40 on activated T cells. Within several hours of TCR-mediated stimulation of naïve T cells CD25 is expressed, which along with the IL-2Rβ and common γ chain, forms the high-affinity trimeric IL-2R complex and allows for enhanced IL-2 responsiveness (Fig. 1). In addition to IL-2-specific signaling, robust T cell responses require CD28 co-stimulation, which creates a positive feedback loop that serves to further enhance IL-2R expression and signaling. IL-2R-signaling promotes the activation and phosphorylation of the kinases JAK1/3, which initiate a signaling cascade through the activation of PI3K, STAT5, and ERK, which ultimately augments CD8 T cell differentiation and survival. The optimal expression of OX40 occurs 24-48 hours after the activation of naïve T cells and requires the presence of three signals: 1) strong TCR ligation; 2) CD28 engagement; and 3) IL-2/IL-2R signaling. Interestingly, the initial up-regulation of OX40 does not require CD28 ligation as CD28-deficient cells can still express OX40, albeit to a lesser extent than wild-type T cells, suggesting that CD28 ligation and TCR stimulation induce OX40 through partially distinct mechanisms. Ultimately, OX40 stimulation greatly augments IL-2R expression and IL-2 production, which enhances T cell survival and effector differentiation through downstream molecules including NF-κB and survivin (Fig. 1).
Figure 1. Regulation of OX40 expression on activated T cells.
TCR engagement promotes the expression of the IL-2Rα, which along with the IL-2Rβ and IL-2Rγc chains forms the high-affinity IL-2R. IL-2-mediated signaling through the IL-2R promotes the activation (phosphorylation) of several molecules including JAK1/3, PI3K, STAT5, and ERK. Within 24-48 hours post-TCR stimulation the OX40 receptor is expressed on activated T cells. The extent of OX40 expression appears to be regulated through a combination of the strength of TCR stimulation and IL-2R-dependent signaling, although the molecular mechanisms by which IL-2R signaling modulates OX40 expression remain unclear. OX40 ligation via the OX40L or agonist reagents further enhances IL-2 production as well as expression of the IL-2R and ultimately augments T cell differentiation and survival.
Although OX40 is transiently expressed on activated T cells for about 24-72 hours prior to down-regulation, it is constitutively expressed on Treg. This dichotomy could provide insights into the mechanisms that regulate OX40 on Treg versus activated T cells. One unique feature of Treg is that they constitutively express CD25, suggesting that OX40 expression on Treg may be regulated through an IL-2R-dependent mechanism, though this remains to be determined. Alternatively, persistent TCR-mediated recognition of self-peptide-MHC class II complexes could maintain OX40 expression on Treg even in the absence of IL-2 signaling. Interestingly, several groups have shown that Treg are present in MHC class II-deficient hosts,44,45 suggesting that the recognition of MHC class II complexes is not required for the generation of Treg, but whether these cells retain OX40 expression (in the absence of TCR stimulation) is unknown.
B. The regulation of OX40L expression on APCs and T cells
The endogenous ligand for OX40, OX40L (CD134L/CD252), is expressed on activated APCs including B cells, macrophages, dendritic cells, NK cells, human airway smooth muscle (ASM) cells, CD4+CD3− accessory cells, and vascular endothelial cells.46-52 Similar to the expression of OX40, OX40L is not present on quiescent APCs, but is rapidly up-regulated upon activation. OX40L-expressing APCs are often localized at sites of inflammation and it is believed that this likely prevents the widespread activation of OX40 on T cells in vivo since only those T cells capable of migrating into sites of inflammation will be able to receive OX40L-specific co-stimulation. Although the mechanisms regulating OX40L expression on APCs remain poorly defined, several studies have shown that culturing DCs in the presence of TNF-α or anti-CD40 mAb can induce OX40L expression.49,53 Future studies will be needed to explore the molecular mechanisms by which these signals promote the induction of OX40L on APCs and whether the induction of OX40 and its ligand utilize similar or unique signaling pathways. Interestingly, OX40L has also been detected on the surface of activated CD4 and CD8 T cells.54,55 OX40L-deficient CD4 T cells exhibited a decreased proliferative response and impaired survival in vivo suggesting that direct stimulation of OX40L on CD4 T cells may augment the priming of Ag-specific CD4 T cells, although the extent to which T cell-specific expression of OX40L contributes to the CD8 T cell response remains unclear.
III. The effects of OX40 ligation on T cell accumulation and differentiation
A. OX40 signaling enhances T cell clonal expansion
Some of the hallmarks of OX40-specific co-stimulation are enhanced T cell clonal expansion and differentiation. Interestingly, the OX40-mediated increase in expansion of CD4 and CD8 T cells does not appear to be due to an increased rate of T cell proliferation per se, but rather increased survival of the activated T cells.37,56 One mechanism by which OX40 signaling promotes the initial increase in CD4 T cell clonal expansion is through the PKB/AKT-dependent activation of the anti-apoptotic protein, survivin.37,57,58 However, over-expression of wild-type survivin by retro-viral transduction was not sufficient to restore the long-term survival of OX40-deficient CD4 T cells in vivo, suggesting that the ability of OX40 ligation to facilitate the generation of long-lived memory T cells occurs through a survivin-independent mechanism. Since IL-2R signaling activates PKB/AKT and IL-2 blockade can limit the proliferation of OX40-stimulated CD4 T cells in vitro, it may be that IL-2R signaling induces the phosphorylation and subsequent activation of PKB/AKT, which in turn promotes enhanced T cell clonal expansion through a survivin-dependent mechanism. In support of this hypothesis, naïve IL-2-deficient T cells exhibit a similar phenotype to OX40-deficient T cells as they exhibit normal cell cycle entry and proliferation, but reduced accumulation following TCR stimulation.59
B. Effects of OX40 ligation on T cell differentiation
In addition to enhanced T cell clonal expansion, OX40 signaling also augments the accumulation of IL-2, IL-4, IL-5, and IFN-γ-producing effector and memory CD4 T cells.35,60-63 Importantly, despite initial reports suggesting that OX40 signaling preferentially supported the development of Th2 CD4 T cells,48,60 OX40 is expressed at similar levels on Th1 and Th2 CD4 T cells and OX40 signals can enhance both Th1 and Th2 differentiation.64,65 The effects of OX40 stimulation on the generation of Th1 versus Th2 CD4 T cells appears to depend upon the integration of multiple signals, especially the strength of TCR stimulation, Ag dose, and availability of cytokines.66,67 These data suggest that OX40 stimulation serves to augment the overall magnitude of the response, rather than pushing T cell differentiation towards a particular lineage.
In the case of Th2 differentiation, the ability of IL-4R signaling to enhance OX40 expression suggests that there may be a positive feedback loop in which IL-4 signaling in the absence of IL-12 may help drive OX40 expression, which subsequently enhances the production of IL-4. Interestingly, recent work has demonstrated that dendritic cell-specific expression of OX40L is also an important mediator of thymic stromal lymphopoietin-induced inflammatory Th2 responses.68 Further studies revealed that in vivo blockade of OX40L in mouse and non-human primate models of asthma could significantly reduce the extent of Th2-mediated inflammation.69 Together, these studies underscore the important role of OX40 signaling in the induction of Th2-mediated inflammatory responses.
OX40 engagement has also been shown to enhance the effector differentiation of naïve CD8 T cells primed against soluble or tumor-associated Ag, which led to increased CD25 and granzyme B expression as well as enhanced cytolytic activity.40,67 In addition to enhancing the differentiation of tumor-specific T cells in vivo, OX40 ligation promotes the accumulation of CD8 T cells at the tumor site.70 Interestingly, the anti-tumor effects of anti-OX40 treatment were associated with decreased accumulation of potentially immuno-suppressive macrophages in the tumor as well as decreased expression of the immune-suppressive cytokine, TGF-β. In contrast, in this model system OX40 ligation did not abrogate the suppressive function of Treg, suggesting that the anti-tumor effects of anti-OX40 were most likely not due to the inhibition of Treg function.
Several studies have demonstrated that OX40 ligation can reverse peptide or self-Ag-induced CD4 T cell anergy.39,71 In addition, OX40-mediated signals have been shown to augment the priming of tumor-specific low-avidity CD8 T cells.72,73 However, whether OX40 engagement can reverse CD8 T cell anergy to a self-Ag, or more importantly tumor-induced anergy, is unknown. To explore whether OX40 ligation could reverse CD8 T cell anergy to a self-Ag, naïve Ag-specific CD8 T cells were adoptively transferred into POET-1 Tg hosts, which express membrane-bound ovalbumin in the prostate under the control of the rat probasin promoter. Four to 10 weeks later, the donor cells were re-stimulated with exogenous Ag and the in vivo proliferative response was examined. In the absence of OX40 stimulation, the donor cells were rendered anergic and were unable to proliferate. In contrast, OX40 ligation restored the proliferative capacity of the anergic CD8 T cells and enhanced their differentiation into cytolytic effector cells in vivo. OX40-mediated signals were also able to rescue anergic tumor-specific CD8 T cells (up to 7 weeks post-tumor implantation) and increase the survival of tumor-bearing hosts. Importantly, these effects required the combination of TCR stimulation and direct ligation of OX40 on the anergic CD8 T cells, rather than indirect effects through endogenous OX40-expressing CD4 T cells or blockade of Treg function (W.L.R and A.D.W., manuscript submitted).
Our laboratory and others have begun to elucidate the mechanisms by which OX40 engagement augments T cell function. Stimulation of naïve Ag-specific CD4 and CD8 T cells with cognate Ag along with an agonist anti-OX40 mAb in vivo leads to increased expression of genes associated with cell cycle progression as well as several cytokine receptors, particularly the IL-2Rα, IL-7Rα, and IL-12Rβ2, which has been confirmed at the protein level.40,61,62,74 As described above, IL-2/IL-2R-signaling is one of the critical pathways by which OX40-mediated therapy augments T cell differentiation and survival. Recent work from Lee et al. has also shown that OX40 ligation increased IL-7Rα expression on Ag-specific CD8 T cells and that the addition of rIL-7 led to the enhanced survival of anti-OX40 stimulated effector CTL in vitro.75 However, whether OX40 engagement promotes T cell survival through an IL-7-dependent mechanism has not been determined.
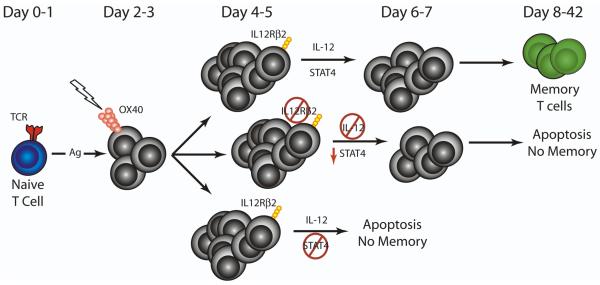
In addition to IL-2 and IL-7, we and others have demonstrated that IL-12 plays a critical role in mediating the effects of OX40 engagement on T cell differentiation and survival. The addition of rIL-12 and rIL-18 was shown to promote robust IFN-γ production by CD4 T cells receiving OX40-mediated co-stimulation, suggesting a link between OX40 stimulation and subsequent IL-12 responsiveness.39 We demonstrated that the IL-12Rβ2 was up-regulated following OX40 engagement of Ag-specific CD4 T cells 3-4 days after Ag challenge.61 Subsequently, we showed that IL-12-specific signaling on the responding CD4 T cells was required for promoting survival following OX40 ligation in vivo (Fig. 2). Further analysis of the downstream molecular mechanisms by which IL-12 signaling enhances OX40-specific CD4 T cell survival demonstrated that IL-12 promotes survival through a STAT4-dependent mechanism (Fig. 2).61 Importantly, the combination of exogenous rIL-12 and anti-OX40 greatly augmented T cell-mediated anti-tumor immunity in several pre-clinical tumor models,61,76,77 suggesting a potent synergistic effect between these two molecules.
Figure 2. OX40-mediated survival occurs through an IL-12-dependent mechanism.
Following Ag-specific activation of naïve CD4 T cells, treatment with an OX40 agonist augments expression of the IL-12Rβ2 and subsequent signaling through the IL-12R is required for the optimal generation of long-lived memory CD4 T cells. In the absence of T cell-specific IL-12R signaling, the extent of STAT4 phosphorylation is reduced, which correlates with increased CD4 T cell apoptosis and the absence of memory T cells. Similarly, STAT4-deficient CD4 T cells also exhibit increased apoptosis and are unable to form memory cells. Together, these data demonstrate that OX40 engagement augments CD4 T cell survival and the formation of long-lived memory CD4 T cells through an IL-12 and STAT4-dependent mechanism.
C. OX40-mediated signaling in the generation of pathogen-specific T cell responses
A variety of laboratories have begun to investigate the role of OX40-OX40L interactions in the generation of pathogen-specific responses. Interestingly, the extent to which OX40 contributes to either CD4 or CD8 T cell responses appears to depend upon the particular pathogen being examined. For example, OX40 signals are dispensable for the generation of primary B and CD8 T cell responses to lymphochoriomeningitis virus (LCMV) and influenza virus, although in some cases the extent of CD4 T cell proliferation and IFN-γ production was somewhat impaired in the absence of OX40 signaling.78-80 Similarly, no differences in the primary CD8 T cell response were observed when OX40-deficient and wild-type mice were challenged with murine cytomegalovirus (mCMV), Listeria monocytogenes, or several other pathogens.78,81,82 However, infection with adenovirus or vaccinia virus (VACV) led to a very different outcome; OX40-deficient mice exhibited greatly reduced accumulation of effector CD8 T cells following viral priming.83,84 In the case of VACV, the decreased CD8 T cell response occurred against a broad range of dominant and subdominant CD8 T cell epitopes and was associated with a reduction in the generation of memory CD8 T cells. The explanation for the variable dependence upon OX40-mediated signals for the generation of effector and memory T cells is unknown, but probably reflects the particular inflammatory signals induced by each specific pathogen.
Although OX40-specific signals are often dispensable for the generation of robust pathogen-specific primary T cell responses, OX40 ligation does provide co-stimulatory signals that are necessary for the optimal generation of memory T cell responses. For example, following infection with mCMV or influenza virus, OX40 ligation was shown to be important for the long-term survival of pathogen-specific memory CD8 T cells.78-81 Additional studies have shown that OX40 engagement is important in the formation and maintenance of memory CD8 T cells following infection with Listeria monocytogenes.82 OX40-mediated co-stimulation was critical for the generation of killer cell lectin-like receptor G1 (KLRG1)low memory precursor effector CD8 T cells. This study also demonstrated that OX40-deficient memory CD8 T cells exhibited reduced homeostatic potential in vivo, suggesting that OX40-specific signals are also important for the maintenance of memory CD8 T cells. It remains to be determined whether the OX40-mediated induction of KLRG1low memory precursor CD8 T cells is unique to Listeria monocytogenes infection or whether this is a general mechanism by which OX40 stimulation augments the generation of memory T cells.
IV. Effects of OX40 ligation on regulatory T cells
In addition to the well-characterized co-stimulatory effects of OX40 ligation on recently activated effector T cells, OX40 has been shown to influence the generation, proliferation, and suppressive function of regulatory CD4 T cells (Treg).9-11 Treg constitute a subset of CD4 T cells that express both CD25 and the transcription factor forkhead box protein 3 (FoxP3) and typically occur in two subsets, natural and induced, which develop in the thymus or periphery, respectively.85,86 A role for co-stimulatory signals in the regulation of Treg was initially identified in CD28-deficient mice, which lack FoxP3+ Treg.87 However, the role of OX40-specific co-stimulation in the induction and suppressive activity of Treg appears to be more complex. Since FoxP3+CD25+CD4+ regulatory T cells constitutively express OX40 on their surface, recent work has examined whether OX40 is necessary for the generation of Treg. An initial study of naïve OX40-deficient mice suggested that there was a delay in the accumulation of CD25+CD4+ Treg in young (<8 week-old) mice compared to wild-type mice, although no differences were observed in older mice.10 Although these data suggested a role for OX40 stimulation in the development of natural Treg, more recent examination of OX40-deficient mice crossed with FoxP-GFP knock-in mice that express a GFP reporter in the endogenous FoxP3 locus demonstrated no significant difference in the number of natural Treg in young OX40-deficient versus wild-type mice.11 Thus, OX40 signaling appears to be dispensable for the development of natural Treg. However, OX40 ligation in the presence of TGF-β and TCR/CD28 stimulation prevented the conversion of CD25- CD4+ T cells into inducible Treg both in vitro and in vivo, in part by down-regulating the expression of FoxP3.11,88,89 There are several potential mechanisms by which OX40 engagement may prevent conversion into Treg, including direct OX40 signaling via PKB/AKT or NF-κB and/or indirect OX40 signaling on other cells present in the local microenvironment. For example, OX40-stimulated memory and effector T cells produced increased levels of cytokines (IFN-γ, IL-4, and IL-6), which can block TGF-β-mediated up-regulation of FoxP3 and their subsequent conversion into Treg.
Although OX40 signals can prevent activated CD4 T cells from converting to Treg, accumulating evidence suggests that OX40 stimulation may also drive the proliferation of existing Treg. Recently, we observed an OX40-mediated increase in Treg in mice where conditions in the local microenvironment favored Treg conversion and in naïve mice lacking polarizing Th1/Th2 signals (C.R. and A.W., manuscript submitted). This novel effect of OX40 on Treg proliferation may also explain the large numbers of Treg that were generated from human umbilical cord blood by in vitro stimulation with anti-CD3/28-coated artificial APCs that were transduced to express OX40L.90 Together, these data support the ability of OX40 engagement to drive the expansion of existing Treg as well as prevent the conversion of Teff into Treg, with both processed dependent upon the local cytokine milieu (Fig. 3).
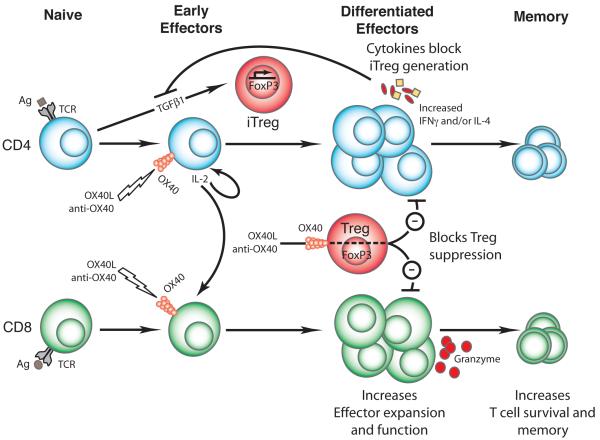
Figure 3. Effects of OX40 ligation on effector versus regulatory T cells.
OX40 engagement by the OX40L or agonist OX40 reagents augments CD4 and CD8 T cell clonal expansion, differentiation, and survival. One of the major effects of OX40 ligation is increased IL-2 production and IL-2R expression, which together serve to enhance CD4 and CD8 T cell effector differentiation and the generation of long-lived memory cells. Alternatively, OX40 is also expressed on regulatory T cells (Treg) and OX40 ligation on Treg can potentially abrogate their suppressive activity. OX40 engagement can also affect the generation of induced Treg (iTreg) as OX40-stimulated effector CD4 T cells can prevent the generation of iTreg through the secretion of pro-inflammatory cytokines, such as IL-4, IL-6, and IFN-γ.
OX40 signaling has also been shown to reverse the suppressive function of Treg. For example, OX40 engagement could block the ability of wild-type Treg to suppress OX40-deficient effector CD4 T cells in vivo.10 Similarly, pre-treatment of Treg with anti-OX40 in vitro was shown to abrogate the ability of Treg to suppress effector CD4 T cells in a model of graft-versus-host disease (GVHD).91 OX40 engagement has been shown to abrogate the suppressive activity of Treg in tumor-bearing mice. In this study, the efficacy of OX40 ligation required expression of OX40 on both the effector and regulatory CD4 T cells, suggesting that a combination of Treg inhibition and enhanced effector CD4 T cell function was necessary for the optimal anti-tumor response.92 In contrast, we were unable to detect any change in Treg function following OX40-mediated therapy of tumor-bearing mice.70 Instead, the beneficial effects of OX40 therapy were mediated by enhanced accumulation of effector CD8 T cells at the tumor site, which led to a favorable ratio of effector to regulatory T cells in vivo. The reasons for these differences remain unclear, but may be due to differences in the dose and/or timing of administration of the agonist anti-OX40 mAb. Given the potent effects of OX40 ligation on the function of Treg in vitro as well as in vivo, it will be of great interest to further examine the mechanisms by which OX40 stimulation affects the function of regulatory T cells.
V. Mechanisms by which OX40-specific signaling augments T cell survival
In addition to enhanced T cell expansion and differentiation, OX40 engagement also profoundly affects T cell survival. Indeed, numerous studies have demonstrated that OX40 stimulation provides critical signals that enhance the long-term survival of CD4 and CD8 T cells in vivo.37,62,93,94 The extent to which OX40 ligation via the endogenous OX40 ligand drives CD4 or CD8 T cell survival is largely dependent upon the stimulatory environment. The optimal generation of memory CD8 T cells to certain pathogens, such as adenovirus, vaccinia virus, influenza virus, and Listeria monocytogenes require OX40-specific signals, whereas other pathogens, including LCMV, can generate normal levels of memory CD8 T cells in an OX40-independent manner. It should be noted that in addition to OX40 engagement, other members of the TNFR super-family, including CD27 and CD137 (4-1BB), also provide critical pro-survival signals that are necessary for the generation of memory T cells in vivo.6 The functional overlap among these molecules likely serves to ensure that potent T cell responses can be generated against a wide range of pathogens and may serve to reduce the incidence of immune evasion.
Expression of endogenous OX40L is strongly dependent on the pro-inflammatory milieu. Therefore, we and others have explored the ability of OX40 agonists to augment T cell priming and survival following activation under non-inflammatory conditions, as is the case with soluble Ag or tumor. Activation of naïve CD4 T cells with soluble Ag and anti-OX40 in vivo led to a 10-15-fold increase in the formation of memory CD4 T cells as compared to soluble Ag alone.62 Similar data were obtained following Ag-specific stimulation of naïve CD8 T cells.95 The molecular mechanisms by which OX40 stimulation enhances T cell memory are still being elucidated, but under certain circumstances, OX40 ligation promotes CD4 T cell survival through the induction of the anti-apoptotic molecules, Bcl-2 and Bcl-xL.37 However, other studies failed to detect significant changes in these molecules compared to control-treated CD4 T cells stimulated in vivo.74,96 These discrepant results likely reflect differences in the stimulatory conditions and whether the cells were activated in vitro versus in vivo.
OX40 signaling also promotes CD4 T cell survival through the action of several other molecules, including TRAF2 and IL-12.61,74 Ligation of various TNFR super-family members leads to the activation of TNFR associated factors (TRAFs), which are adaptor proteins that initiate the activation of downstream signaling cascades.97,98 OX40 signals via TRAF2, TRAF3, and TRAF5, although only TRAF2 and TRAF5 have been associated with OX40 signaling in T cells.74,99-101 The enhanced CD4 T cell survival observed following OX40 engagement appeared to occur through a TRAF2-dependent mechanism; the expression of a dominant-negative form of TRAF2 greatly reduced CD4 T cell survival following OX40 ligation in vivo.74 More recently, we showed that improved CD4 T cell survival following OX40 engagement occurred through an IL-12-dependent mechanism.61 OX40-stimulated CD4 T cells expressed increased levels of the IL-12Rβ2 4-6 days after stimulation in vivo and OX40 ligation of IL-12Rβ2-deficient CD4 T cells was unable to promote their long-term survival. These data suggested that direct stimulation of IL-12 on the responding CD4 T cells was required for the optimal pro-survival effects of anti-OX40 treatment. Although work from Song et al. has shown that OX40 ligation enhanced CD4 T cell survival through a PKB/AKT-dependent mechanism,37 we were unable to detect differences in the expression of active (phosphorylated) AKT between anti-OX40 and control-treated CD4 T cells.61 Instead, IL-12 signaling was shown to augment CD4 T cell survival through a STAT4-dependent pathway (Fig. 2). These apparently conflicting results may reflect the effects of endogenous OX40 signaling versus the use of an agonist anti-OX40 mAb.
In addition to its robust effects on CD4 T cell survival, OX40 engagement can also greatly enhance CD8 T cell survival. One study demonstrated that OX40 engagement enhanced CD8 T cell survival by up-regulating the anti-apoptotic protein, Bcl-xL.102 The induction of Bcl-xL following OX40 ligation may be due, in part, to increased IL-2R signaling as IL-2 can promote the activation of PKB/AKT, which in turns enhances T cell survival through the induction of Bcl-2 and/or Bcl-xL.41,42 Indeed, our work has shown that anti-OX40 treatment greatly increases IL-2Rα expression on activated CD8 T cells, which was associated with increased expression of phospho-AKT and enhanced long-term survival.40,95 OX40 stimulation has also been associated with increased expression of the IL-7Rα (CD127), which is also important for CD8 T cell survival and the generation of memory cells.95 It should be noted that although IL-12 signaling was found to be critical for promoting the OX40-mediated survival of CD4 T cells, OX40 ligation enhanced CD8 T cell memory in an IL-12-independent manner,61 suggesting that OX40 engagement likely enhances CD4 and CD8 T cell survival through distinct mechanisms. Taken together, these data suggest that additional studies will be needed to clarify the precise molecular mechanisms by which OX40 stimulation promotes T cell survival in vivo.
V. Conclusions
OX40-mediated co-stimulation is essential to promote optimal CD4 and CD8 T cell clonal expansion, differentiation, and ultimately the generation of long-lived memory cells (Fig. 3). OX40 signaling can also modulate the function of regulatory T cells, although whether OX40 ligation promotes Treg expansion or, alternatively, abrogates their suppressive activity likely depends upon the specific cytokine milieu in which the responding T cells receive OX40 stimulation. Future studies will be needed to determine the underlying molecular mechanisms that mediate the divergent effects of OX40 ligation on Treg. Additional questions remain regarding the role of IL-2-mediated signaling in modulating OX40 expression and the downstream effects of OX40 ligation on T cell differentiation and survival. For example, is IL-2R signaling required for the induction of OX40 on activated T cells or is TCR stimulation sufficient? Furthermore, what are the signaling pathways regulating OX40 expression and what is the role of IL-2R signaling in this process? Do other TNFR family members utilize the same pathways as OX40 or are distinct mechanisms involved?
In addition to the ability of endogenous OX40L-specific signaling to enhance T cell responses against a variety of pathogenic infections, the provision of agonist OX40 signals has also proved valuable for boosting tumor-specific T cell responses in vivo. Indeed, this strategy is currently being examined in a Phase I clinical trial using an anti-human OX40 mAb for the treatment of patients with cancer. Based upon recent pre-clinical data demonstrating the ability of OX40 ligation to abrogate the suppressive function of Treg (Fig. 3), it will be of interest to determine whether OX40-mediated therapy can similarly affect human Treg in vivo. In contrast to the potent effects of OX40 stimulation on T cell priming, blockade of OX40-specific signals can ameliorate the severity of autoimmune diseases in pre-clinical models of type 1 diabetes and multiple sclerosis. Interestingly, OX40 stimulation was shown to promote the expansion of purified human Treg in vitro, suggesting that OX40-specific signaling may provide a novel means of obtaining large numbers of Treg for the adoptive therapy of autoimmune disease. Alternatively, blockade of OX40L has been shown to inhibit TSLP-induced asthma in pre-clinical models suggesting that OX40L may be a useful target for reducing the severity of Th2-mediated allergic asthma. In summary, OX40 signaling can affect several aspects of T cell responses and hence further elucidating the mechanisms by which OX40 ligation affects T cell function may aid in the development of novel therapies for the treatment of several human diseases.
Acknowledgements
We would like to thank Dr. Walter Urba for critical reading of the manuscript and members of the Weinberg lab for helpful discussions.
This work was supported by an American Cancer Society – Sam E. and Kathleen Henry Post-doctoral Fellowship, a Prostate Cancer Foundation Young Investigator Award (W.L.R.), and grants from the M.J. Murdock Charitable Trust and the NIH (A.D.W.).
References
- 1.Heath WR, Carbone FR. Cross-presentation, dendritic cells, tolerance and immunity. Annu Rev Immunol. 2001:1947–64. doi: 10.1146/annurev.immunol.19.1.47. [DOI] [PubMed] [Google Scholar]
- 2.Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22(3):275–84. doi: 10.1016/j.immuni.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 3.Boise LH, Minn AJ, Noel PJ, June CH, Accavitti MA, Lindsten T, Thompson CB. CD28 costimulation can promote T cell survival by enhancing the expression of Bcl-XL. Immunity. 1995;3(1):87–98. doi: 10.1016/1074-7613(95)90161-2. [DOI] [PubMed] [Google Scholar]
- 4.Salomon B, Bluestone JA. Complexities of CD28/B7: CTLA-4 costimulatory pathways in autoimmunity and transplantation. Annu Rev Immunol. 2001:19225–52. doi: 10.1146/annurev.immunol.19.1.225. [DOI] [PubMed] [Google Scholar]
- 5.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3(8):609–20. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 6.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005:2323–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 7.Redmond WL, Weinberg AD. Targeting OX40 and OX40L for the treatment of autoimmunity and cancer. Crit Rev Immunol. 2007;27(5):415–36. doi: 10.1615/critrevimmunol.v27.i5.20. [DOI] [PubMed] [Google Scholar]
- 8.Sugamura K, Ishii N, Weinberg AD. Therapeutic targeting of the effector T-cell co-stimulatory molecule OX40. Nat Rev Immunol. 2004;4(6):420–31. doi: 10.1038/nri1371. [DOI] [PubMed] [Google Scholar]
- 9.Ndhlovu LC, Takeda I, Sugamura K, Ishii N. Expanding role of T-cell costimulators in regulatory T-cell function: recent advances in accessory molecules expressed on both regulatory and nonregulatory T cells. Crit Rev Immunol. 2004;24(4):251–66. doi: 10.1615/critrevimmunol.v24.i4.30. [DOI] [PubMed] [Google Scholar]
- 10.Takeda I, Ine S, Killeen N, Ndhlovu LC, Murata K, Satomi S, Sugamura K, Ishii N. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172(6):3580–9. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 11.Vu MD, Xiao X, Gao W, Degauque N, Chen M, Kroemer A, Killeen N, Ishii N, Chang Li X. OX40 costimulation turns off Foxp3+ Tregs. Blood. 2007;110(7):2501–10. doi: 10.1182/blood-2007-01-070748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nohara C, Akiba H, Nakajima A, Inoue A, Koh CS, Ohshima H, Yagita H, Mizuno Y, Okumura K. Amelioration of experimental autoimmune encephalomyelitis with anti-OX40 ligand monoclonal antibody: a critical role for OX40 ligand in migration, but not development, of pathogenic T cells. J Immunol. 2001;166(3):2108–15. doi: 10.4049/jimmunol.166.3.2108. [DOI] [PubMed] [Google Scholar]
- 13.Ndhlovu LC, Ishii N, Murata K, Sato T, Sugamura K. Critical involvement of OX40 ligand signals in the T cell priming events during experimental autoimmune encephalomyelitis. J Immunol. 2001;167(5):2991–9. doi: 10.4049/jimmunol.167.5.2991. [DOI] [PubMed] [Google Scholar]
- 14.Weinberg AD, Bourdette DN, Sullivan TJ, Lemon M, Wallin JJ, Maziarz R, Davey M, Palida F, Godfrey W, Engleman E, Fulton RJ, Offner H, Vandenbark AA. Selective depletion of myelin-reactive T cells with the anti-OX-40 antibody ameliorates autoimmune encephalomyelitis. Nat Med. 1996;2(2):183–9. doi: 10.1038/nm0296-183. [DOI] [PubMed] [Google Scholar]
- 15.Jember AG, Zuberi R, Liu FT, Croft M. Development of allergic inflammation in a murine model of asthma is dependent on the costimulatory receptor OX40. J Exp Med. 2001;193(3):387–92. doi: 10.1084/jem.193.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Horai R, Nakajima A, Habiro K, Kotani M, Nakae S, Matsuki T, Nambu A, Saijo S, Kotaki H, Sudo K, Okahara A, Tanioka H, Ikuse T, Ishii N, Schwartzberg PL, Abe R, Iwakura Y. TNF-alpha is crucial for the development of autoimmune arthritis in IL-1 receptor antagonist-deficient mice. J Clin Invest. 2004;114(11):1603–11. doi: 10.1172/JCI20742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshioka T, Nakajima A, Akiba H, Ishiwata T, Asano G, Yoshino S, Yagita H, Okumura K. Contribution of OX40/OX40 ligand interaction to the pathogenesis of rheumatoid arthritis. Eur J Immunol. 2000;30(10):2815–23. doi: 10.1002/1521-4141(200010)30:10<2815::AID-IMMU2815>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 18.Pakala SV, Bansal-Pakala P, Halteman BS, Croft M. Prevention of diabetes in NOD mice at a late stage by targeting OX40/OX40 ligand interactions. Eur J Immunol. 2004;34(11):3039–46. doi: 10.1002/eji.200425141. [DOI] [PubMed] [Google Scholar]
- 19.Obermeier F, Schwarz H, Dunger N, Strauch UG, Grunwald N, Scholmerich J, Falk W. OX40/OX40L interaction induces the expression of CXCR5 and contributes to chronic colitis induced by dextran sulfate sodium in mice. Eur J Immunol. 2003;33(12):3265–74. doi: 10.1002/eji.200324124. [DOI] [PubMed] [Google Scholar]
- 20.Higgins LM, McDonald SA, Whittle N, Crockett N, Shields JG, MacDonald TT. Regulation of T cell activation in vitro and in vivo by targeting the OX40-OX40 ligand interaction: amelioration of ongoing inflammatory bowel disease with an OX40-IgG fusion protein, but not with an OX40 ligand-IgG fusion protein. J Immunol. 1999;162(1):486–93. [PubMed] [Google Scholar]
- 21.Aten J, Roos A, Claessen N, Schilder-Tol EJ, Ten Berge IJ, Weening JJ. Strong and selective glomerular localization of CD134 ligand and TNF receptor-1 in proliferative lupus nephritis. J Am Soc Nephrol. 2000;11(8):1426–38. doi: 10.1681/ASN.V1181426. [DOI] [PubMed] [Google Scholar]
- 22.Carboni S, Aboul-Enein F, Waltzinger C, Killeen N, Lassmann H, Pena-Rossi C. CD134 plays a crucial role in the pathogenesis of EAE and is upregulated in the CNS of patients with multiple sclerosis. J Neuroimmunol. 2003;145(1-2):1–11. doi: 10.1016/j.jneuroim.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 23.Endl J, Rosinger S, Schwarz B, Friedrich SO, Rothe G, Karges W, Schlosser M, Eiermann T, Schendel DJ, Boehm BO. Coexpression of CD25 and OX40 (CD134) receptors delineates autoreactive T-cells in type 1 diabetes. Diabetes. 2006;55(1):50–60. [PubMed] [Google Scholar]
- 24.Morris A, Vetto JT, Ramstad T, Funatake CJ, Choolun E, Entwisle C, Weinberg AD. Induction of anti-mammary cancer immunity by engaging the OX-40 receptor in vivo. Breast Cancer Res Treat. 2001;67(1):71–80. doi: 10.1023/a:1010649303056. [DOI] [PubMed] [Google Scholar]
- 25.Kjaergaard J, Tanaka J, Kim JA, Rothchild K, Weinberg A, Shu S. Therapeutic efficacy of OX-40 receptor antibody depends on tumor immunogenicity and anatomic site of tumor growth. Cancer Res. 2000;60(19):5514–21. [PubMed] [Google Scholar]
- 26.Vetto JT, Lum S, Morris A, Sicotte M, Davis J, Lemon M, Weinberg A. Presence of the T-cell activation marker OX-40 on tumor infiltrating lymphocytes and draining lymph node cells from patients with melanoma and head and neck cancers. Am J Surg. 1997;174(3):258–65. doi: 10.1016/s0002-9610(97)00139-6. [DOI] [PubMed] [Google Scholar]
- 27.Biagi E, Dotti G, Yvon E, Lee E, Pule M, Vigouroux S, Gottschalk S, Popat U, Rousseau R, Brenner M. Molecular transfer of CD40 and OX40 ligands to leukemic human B cells induces expansion of autologous tumor-reactive cytotoxic T lymphocytes. Blood. 2005;105(6):2436–42. doi: 10.1182/blood-2004-07-2556. [DOI] [PubMed] [Google Scholar]
- 28.Dannull J, Nair S, Su Z, Boczkowski D, DeBeck C, Yang B, Gilboa E, Vieweg J. Enhancing the immunostimulatory function of dendritic cells by transfection with mRNA encoding OX40 ligand. Blood. 2005;105(8):3206–13. doi: 10.1182/blood-2004-10-3944. [DOI] [PubMed] [Google Scholar]
- 29.Andarini S, Kikuchi T, Nukiwa M, Pradono P, Suzuki T, Ohkouchi S, Inoue A, Maemondo M, Ishii N, Saijo Y, Sugamura K, Nukiwa T. Adenovirus vector-mediated in vivo gene transfer of OX40 ligand to tumor cells enhances antitumor immunity of tumor-bearing hosts. Cancer Res. 2004;64(9):3281–7. doi: 10.1158/0008-5472.can-03-3911. [DOI] [PubMed] [Google Scholar]
- 30.Weinberg AD, Rivera MM, Prell R, Morris A, Ramstad T, Vetto JT, Urba WJ, Alvord G, Bunce C, Shields J. Engagement of the OX-40 receptor in vivo enhances antitumor immunity. J Immunol. 2000;164(4):2160–9. doi: 10.4049/jimmunol.164.4.2160. [DOI] [PubMed] [Google Scholar]
- 31.Weinberg AD, Thalhofer C, Morris N, Walker JM, Seiss D, Wong S, Axthelm MK, Picker LJ, Urba WJ. Anti-OX40 (CD134) administration to nonhuman primates: immunostimulatory effects and toxicokinetic study. J Immunother (1997) 2006;29(6):575–85. doi: 10.1097/01.cji.0000211319.00031.fc. [DOI] [PubMed] [Google Scholar]
- 32.Mallett S, Fossum S, Barclay AN. Characterization of the MRC OX40 antigen of activated CD4 positive T lymphocytes--a molecule related to nerve growth factor receptor. Embo J. 1990;9(4):1063–8. doi: 10.1002/j.1460-2075.1990.tb08211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baumann R, Yousefi S, Simon D, Russmann S, Mueller C, Simon HU. Functional expression of CD134 by neutrophils. Eur J Immunol. 2004;34(8):2268–75. doi: 10.1002/eji.200424863. [DOI] [PubMed] [Google Scholar]
- 34.al-Shamkhani A, Birkeland ML, Puklavec M, Brown MH, James W, Barclay AN. OX40 is differentially expressed on activated rat and mouse T cells and is the sole receptor for the OX40 ligand. Eur J Immunol. 1996;26(8):1695–9. doi: 10.1002/eji.1830260805. [DOI] [PubMed] [Google Scholar]
- 35.Gramaglia I, Weinberg AD, Lemon M, Croft M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J Immunol. 1998;161(12):6510–7. [PubMed] [Google Scholar]
- 36.Verdeil G, Puthier D, Nguyen C, Schmitt-Verhulst AM, Auphan-Anezin N. STAT5-mediated signals sustain a TCR-initiated gene expression program toward differentiation of CD8 T cell effectors. J Immunol. 2006;176(8):4834–42. doi: 10.4049/jimmunol.176.8.4834. [DOI] [PubMed] [Google Scholar]
- 37.Rogers PR, Song J, Gramaglia I, Killeen N, Croft M. OX40 promotes Bcl-xL and Bcl-2 expression and is essential for long-term survival of CD4 T cells. Immunity. 2001;15(3):445–55. doi: 10.1016/s1074-7613(01)00191-1. [DOI] [PubMed] [Google Scholar]
- 38.Toennies HM, Green JM, Arch RH. Expression of CD30 and Ox40 on T lymphocyte subsets is controlled by distinct regulatory mechanisms. J Leukoc Biol. 2004;75(2):350–7. doi: 10.1189/jlb.0803401. [DOI] [PubMed] [Google Scholar]
- 39.Lathrop SK, Huddleston CA, Dullforce PA, Montfort MJ, Weinberg AD, Parker DC. A signal through OX40 (CD134) allows anergic, autoreactive T cells to acquire effector cell functions. J Immunol. 2004;172(11):6735–43. doi: 10.4049/jimmunol.172.11.6735. [DOI] [PubMed] [Google Scholar]
- 40.Redmond WL, Gough MJ, Charbonneau B, Ratliff TL, Weinberg AD. Defects in the Acquisition of CD8 T Cell Effector Function after Priming with Tumor or Soluble Antigen Can Be Overcome by the Addition of an OX40 Agonist. J Immunol. 2007;179(11):7244–53. doi: 10.4049/jimmunol.179.11.7244. [DOI] [PubMed] [Google Scholar]
- 41.Gaffen SL. Signaling domains of the interleukin 2 receptor. Cytokine. 2001;14(2):63–77. doi: 10.1006/cyto.2001.0862. [DOI] [PubMed] [Google Scholar]
- 42.Nelson BH, Willerford DM. Biology of the interleukin-2 receptor. Adv Immunol. 1998:701–81. doi: 10.1016/s0065-2776(08)60386-7. [DOI] [PubMed] [Google Scholar]
- 43.Williams CA, Murray SE, Weinberg AD, Parker DC. OX40-mediated differentiation to effector function requires IL-2 receptor signaling but not CD28, CD40, IL-12Rbeta2, or T-bet. J Immunol. 2007;178(12):7694–702. doi: 10.4049/jimmunol.178.12.7694. [DOI] [PubMed] [Google Scholar]
- 44.Kish DD, Gorbachev AV, Fairchild RL. Regulatory function of CD4+CD25+ T cells from Class II MHC-deficient mice in contact hypersensitivity responses. J Leukoc Biol. 2007;82(1):85–92. doi: 10.1189/jlb.0207089. [DOI] [PubMed] [Google Scholar]
- 45.Shimoda M, Mmanywa F, Joshi SK, Li T, Miyake K, Pihkala J, Abbas JA, Koni PA. Conditional ablation of MHC-II suggests an indirect role for MHC-II in regulatory CD4 T cell maintenance. J Immunol. 2006;176(11):6503–11. doi: 10.4049/jimmunol.176.11.6503. [DOI] [PubMed] [Google Scholar]
- 46.Kim MY, Anderson G, White A, Jenkinson E, Arlt W, Martensson IL, Erlandsson L, Lane PJ. OX40 ligand and CD30 ligand are expressed on adult but not neonatal CD4+CD3− inducer cells: evidence that IL-7 signals regulate CD30 ligand but not OX40 ligand expression. J Immunol. 2005;174(11):6686–91. doi: 10.4049/jimmunol.174.11.6686. [DOI] [PubMed] [Google Scholar]
- 47.Burgess JK, Carlin S, Pack RA, Arndt GM, Au WW, Johnson PR, Black JL, Hunt NH. Detection and characterization of OX40 ligand expression in human airway smooth muscle cells: a possible role in asthma? J Allergy Clin Immunol. 2004;113(4):683–9. doi: 10.1016/j.jaci.2003.12.311. [DOI] [PubMed] [Google Scholar]
- 48.Flynn S, Toellner KM, Raykundalia C, Goodall M, Lane P. CD4 T cell cytokine differentiation: the B cell activation molecule, OX40 ligand, instructs CD4 T cells to express interleukin 4 and upregulates expression of the chemokine receptor, Blr-1. J Exp Med. 1998;188(2):297–304. doi: 10.1084/jem.188.2.297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ohshima Y, Tanaka Y, Tozawa H, Takahashi Y, Maliszewski C, Delespesse G. Expression and function of OX40 ligand on human dendritic cells. J Immunol. 1997;159(8):3838–48. [PubMed] [Google Scholar]
- 50.Baum PR, Gayle RB, 3rd, Ramsdell F, Srinivasan S, Sorensen RA, Watson ML, Seldin MF, Baker E, Sutherland GR, Clifford KN, et al. Molecular characterization of murine and human OX40/OX40 ligand systems: identification of a human OX40 ligand as the HTLV-1-regulated protein gp34. Embo J. 1994;13(17):3992–4001. doi: 10.1002/j.1460-2075.1994.tb06715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zingoni A, Sornasse T, Cocks BG, Tanaka Y, Santoni A, Lanier LL. Cross-talk between activated human NK cells and CD4+ T cells via OX40-OX40 ligand interactions. J Immunol. 2004;173(6):3716–24. doi: 10.4049/jimmunol.173.6.3716. [DOI] [PubMed] [Google Scholar]
- 52.Imura A, Hori T, Imada K, Ishikawa T, Tanaka Y, Maeda M, Imamura S, Uchiyama T. The human OX40/gp34 system directly mediates adhesion of activated T cells to vascular endothelial cells. J Exp Med. 1996;183(5):2185–95. doi: 10.1084/jem.183.5.2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fillatreau S, Gray D. T cell accumulation in B cell follicles is regulated by dendritic cells and is independent of B cell activation. J Exp Med. 2003;197(2):195–206. doi: 10.1084/jem.20021750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Soroosh P, Ine S, Sugamura K, Ishii N. OX40-OX40 ligand interaction through T cell-T cell contact contributes to CD4 T cell longevity. J Immunol. 2006;176(10):5975–87. doi: 10.4049/jimmunol.176.10.5975. [DOI] [PubMed] [Google Scholar]
- 55.Mendel I, Shevach EM. Activated T cells express the OX40 ligand: requirements for induction and costimulatory function. Immunology. 2006;117(2):196–204. doi: 10.1111/j.1365-2567.2005.02279.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Weatherill AR, Maxwell JR, Takahashi C, Weinberg AD, Vella AT. OX40 ligation enhances cell cycle turnover of Ag-activated CD4 T cells in vivo. Cell Immunol. 2001;209(1):63–75. doi: 10.1006/cimm.2001.1783. [DOI] [PubMed] [Google Scholar]
- 57.Song J, So T, Cheng M, Tang X, Croft M. Sustained survivin expression from OX40 costimulatory signals drives T cell clonal expansion. Immunity. 2005;22(5):621–31. doi: 10.1016/j.immuni.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 58.Song J, Salek-Ardakani S, Rogers PR, Cheng M, Van Parijs L, Croft M. The costimulation-regulated duration of PKB activation controls T cell longevity. Nat Immunol. 2004;5(2):150–8. doi: 10.1038/ni1030. [DOI] [PubMed] [Google Scholar]
- 59.D’Souza WN, Lefrancois L. IL-2 is not required for the initiation of CD8 T cell cycling but sustains expansion. J Immunol. 2003;171(11):5727–35. doi: 10.4049/jimmunol.171.11.5727. [DOI] [PubMed] [Google Scholar]
- 60.Ohshima Y, Yang LP, Uchiyama T, Tanaka Y, Baum P, Sergerie M, Hermann P, Delespesse G. OX40 costimulation enhances interleukin-4 (IL-4) expression at priming and promotes the differentiation of naive human CD4(+) T cells into high IL-4-producing effectors. Blood. 1998;92(9):3338–45. [PubMed] [Google Scholar]
- 61.Ruby CE, Montler R, Zheng R, Shu S, Weinberg AD. IL-12 Is Required for Anti-OX40-Mediated CD4 T Cell Survival. J Immunol. 2008;180(4):2140–8. doi: 10.4049/jimmunol.180.4.2140. [DOI] [PubMed] [Google Scholar]
- 62.Evans DE, Prell RA, Thalhofer CJ, Hurwitz AA, Weinberg AD. Engagement of OX40 enhances antigen-specific CD4(+) T cell mobilization/memory development and humoral immunity: comparison of alphaOX-40 with alphaCTLA-4. J Immunol. 2001;167(12):6804–11. doi: 10.4049/jimmunol.167.12.6804. [DOI] [PubMed] [Google Scholar]
- 63.Weinberg AD, Vella AT, Croft M. OX-40: life beyond the effector T cell stage. Semin Immunol. 1998;10(6):471–80. doi: 10.1006/smim.1998.0146. [DOI] [PubMed] [Google Scholar]
- 64.De Smedt T, Smith J, Baum P, Fanslow W, Butz E, Maliszewski C. Ox40 costimulation enhances the development of T cell responses induced by dendritic cells in vivo. J Immunol. 2002;168(2):661–70. doi: 10.4049/jimmunol.168.2.661. [DOI] [PubMed] [Google Scholar]
- 65.Gramaglia I, Jember A, Pippig SD, Weinberg AD, Killeen N, Croft M. The OX40 costimulatory receptor determines the development of CD4 memory by regulating primary clonal expansion. J Immunol. 2000;165(6):3043–50. doi: 10.4049/jimmunol.165.6.3043. [DOI] [PubMed] [Google Scholar]
- 66.Rogers PR, Croft M. CD28, Ox-40, LFA-1, and CD4 modulation of Th1/Th2 differentiation is directly dependent on the dose of antigen. J Immunol. 2000;164(6):2955–63. doi: 10.4049/jimmunol.164.6.2955. [DOI] [PubMed] [Google Scholar]
- 67.Jenkins SJ, Perona-Wright G, Worsley AG, Ishii N, MacDonald AS. Dendritic cell expression of OX40 ligand acts as a costimulatory, not polarizing, signal for optimal Th2 priming and memory induction in vivo. J Immunol. 2007;179(6):3515–23. doi: 10.4049/jimmunol.179.6.3515. [DOI] [PubMed] [Google Scholar]
- 68.Ito T, Wang YH, Duramad O, Hori T, Delespesse GJ, Watanabe N, Qin FX, Yao Z, Cao W, Liu YJ. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202(9):1213–23. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seshasayee D, Lee WP, Zhou M, Shu J, Suto E, Zhang J, Diehl L, Austin CD, Meng YG, Tan M, Bullens SL, Seeber S, Fuentes ME, Labrijn AF, Graus YM, Miller LA, Schelegle ES, Hyde DM, Wu LC, Hymowitz SG, Martin F. In vivo blockade of OX40 ligand inhibits thymic stromal lymphopoietin driven atopic inflammation. J Clin Invest. 2007;117(12):3868–78. doi: 10.1172/JCI33559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gough MJ, Ruby CE, Redmond WL, Dhungel B, Brown A, Weinberg AD. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 2008;68(13):5206–15. doi: 10.1158/0008-5472.CAN-07-6484. [DOI] [PubMed] [Google Scholar]
- 71.Bansal-Pakala P, Jember AG, Croft M. Signaling through OX40 (CD134) breaks peripheral T-cell tolerance. Nat Med. 2001;7(8):907–12. doi: 10.1038/90942. [DOI] [PubMed] [Google Scholar]
- 72.Murata S, Ladle BH, Kim PS, Lutz ER, Wolpoe ME, Ivie SE, Smith HM, Armstrong TD, Emens LA, Jaffee EM, Reilly RT. OX40 costimulation synergizes with GM-CSF whole-cell vaccination to overcome established CD8+ T cell tolerance to an endogenous tumor antigen. J Immunol. 2006;176(2):974–83. doi: 10.4049/jimmunol.176.2.974. [DOI] [PubMed] [Google Scholar]
- 73.Lustgarten J, Dominguez AL, Cuadros C. The CD8+ T cell repertoire against Her-2/neu antigens in neu transgenic mice is of low avidity with antitumor activity. Eur J Immunol. 2004;34(3):752–61. doi: 10.1002/eji.200324427. [DOI] [PubMed] [Google Scholar]
- 74.Prell RA, Evans DE, Thalhofer C, Shi T, Funatake C, Weinberg AD. OX40-mediated memory T cell generation is TNF receptor-associated factor 2 dependent. J Immunol. 2003;171(11):5997–6005. doi: 10.4049/jimmunol.171.11.5997. [DOI] [PubMed] [Google Scholar]
- 75.Lee SJ, Rossi RJ, Lee SK, Croft M, Kwon BS, Mittler RS, Vella AT. CD134 Costimulation Couples the CD137 Pathway to Induce Production of Supereffector CD8 T Cells That Become IL-7 Dependent. J Immunol. 2007;179(4):2203–14. doi: 10.4049/jimmunol.179.4.2203. [DOI] [PubMed] [Google Scholar]
- 76.Kuriyama H, Watanabe S, Kjaergaard J, Tamai H, Zheng R, Weinberg AD, Hu HM, Cohen PA, Plautz GE, Shu S. Mechanism of third signals provided by IL-12 and OX-40R ligation in eliciting therapeutic immunity following dendritic-tumor fusion vaccination. Cell Immunol. 2006;243(1):30–40. doi: 10.1016/j.cellimm.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 77.Pan PY, Zang Y, Weber K, Meseck ML, Chen SH. OX40 ligation enhances primary and memory cytotoxic T lymphocyte responses in an immunotherapy for hepatic colon metastases. Mol Ther. 2002;6(4):528–36. doi: 10.1006/mthe.2002.0699. [DOI] [PubMed] [Google Scholar]
- 78.Kopf M, Ruedl C, Schmitz N, Gallimore A, Lefrang K, Ecabert B, Odermatt B, Bachmann MF. OX40-deficient mice are defective in Th cell proliferation but are competent in generating B cell and CTL Responses after virus infection. Immunity. 1999;11(6):699–708. doi: 10.1016/s1074-7613(00)80144-2. [DOI] [PubMed] [Google Scholar]
- 79.Hendriks J, Xiao Y, Rossen JW, van der Sluijs KF, Sugamura K, Ishii N, Borst J. During viral infection of the respiratory tract, CD27, 4-1BB, and OX40 collectively determine formation of CD8+ memory T cells and their capacity for secondary expansion. J Immunol. 2005;175(3):1665–76. doi: 10.4049/jimmunol.175.3.1665. [DOI] [PubMed] [Google Scholar]
- 80.Dawicki W, Bertram EM, Sharpe AH, Watts TH. 4-1BB and OX40 act independently to facilitate robust CD8 and CD4 recall responses. J Immunol. 2004;173(10):5944–51. doi: 10.4049/jimmunol.173.10.5944. [DOI] [PubMed] [Google Scholar]
- 81.Humphreys IR, Loewendorf A, de Trez C, Schneider K, Benedict CA, Munks MW, Ware CF, Croft M. OX40 costimulation promotes persistence of cytomegalovirus-specific CD8 T Cells: A CD4-dependent mechanism. J Immunol. 2007;179(4):2195–202. doi: 10.4049/jimmunol.179.4.2195. [DOI] [PubMed] [Google Scholar]
- 82.Mousavi SF, Soroosh P, Takahashi T, Yoshikai Y, Shen H, Lefrancois L, Borst J, Sugamura K, Ishii N. OX40 costimulatory signals potentiate the memory commitment of effector CD8+ T cells. J Immunol. 2008;181(9):5990–6001. doi: 10.4049/jimmunol.181.9.5990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Salek-Ardakani S, Moutaftsi M, Crotty S, Sette A, Croft M. OX40 drives protective vaccinia virus-specific CD8 T cells. J Immunol. 2008;181(11):7969–76. doi: 10.4049/jimmunol.181.11.7969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lee SW, Park Y, Song A, Cheroutre H, Kwon BS, Croft M. Functional dichotomy between OX40 and 4-1BB in modulating effector CD8 T cell responses. J Immunol. 2006;177(7):4464–72. doi: 10.4049/jimmunol.177.7.4464. [DOI] [PubMed] [Google Scholar]
- 85.O’Garra A, Vieira P. Regulatory T cells and mechanisms of immune system control. Nat Med. 2004;10(8):801–5. doi: 10.1038/nm0804-801. [DOI] [PubMed] [Google Scholar]
- 86.Bluestone JA, Abbas AK. Natural versus adaptive regulatory T cells. Nat Rev Immunol. 2003;3(3):253–7. doi: 10.1038/nri1032. [DOI] [PubMed] [Google Scholar]
- 87.Salomon B, Lenschow DJ, Rhee L, Ashourian N, Singh B, Sharpe A, Bluestone JA. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12(4):431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 88.So T, Croft M. Cutting edge: OX40 inhibits TGF-beta- and antigen-driven conversion of naive CD4 T cells into CD25+Foxp3+ T cells. J Immunol. 2007;179(3):1427–30. doi: 10.4049/jimmunol.179.3.1427. [DOI] [PubMed] [Google Scholar]
- 89.Xiao X, Kroemer A, Gao W, Ishii N, Demirci G, Li XC. OX40/OX40L costimulation affects induction of Foxp3+ regulatory T cells in part by expanding memory T cells in vivo. J Immunol. 2008;181(5):3193–201. doi: 10.4049/jimmunol.181.5.3193. [DOI] [PubMed] [Google Scholar]
- 90.Hippen KL, Harker-Murray P, Porter SB, Merkel SC, Londer A, Taylor DK, Bina M, Panoskaltsis-Mortari A, Rubinstein P, Van Rooijen N, Golovina TN, Suhoski MM, Miller JS, Wagner JE, June CH, Riley JL, Blazar BR. Umbilical cord blood regulatory T-cell expansion and functional effects of tumor necrosis factor receptor family members OX40 and 4-1BB expressed on artificial antigen-presenting cells. Blood. 2008;112(7):2847–57. doi: 10.1182/blood-2008-01-132951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Valzasina B, Guiducci C, Dislich H, Killeen N, Weinberg AD, Colombo MP. Triggering of OX40 (CD134) on CD4(+)CD25+ T cells blocks their inhibitory activity: a novel regulatory role for OX40 and its comparison with GITR. Blood. 2005;105(7):2845–51. doi: 10.1182/blood-2004-07-2959. [DOI] [PubMed] [Google Scholar]
- 92.Piconese S, Valzasina B, Colombo MP. OX40 triggering blocks suppression by regulatory T cells and facilitates tumor rejection. J Exp Med. 2008;205(4):825–39. doi: 10.1084/jem.20071341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weinberg AD, Evans DE, Thalhofer C, Shi T, Prell RA. The generation of T cell memory: a review describing the molecular and cellular events following OX40 (CD134) engagement. J Leukoc Biol. 2004;75(6):962–72. doi: 10.1189/jlb.1103586. [DOI] [PubMed] [Google Scholar]
- 94.Maxwell JR, Weinberg A, Prell RA, Vella AT. Danger and OX40 receptor signaling synergize to enhance memory T cell survival by inhibiting peripheral deletion. J Immunol. 2000;164(1):107–12. doi: 10.4049/jimmunol.164.1.107. [DOI] [PubMed] [Google Scholar]
- 95.Ruby CE, Redmond WL, Haley D, Weinberg AD. Anti-OX40 stimulation in vivo enhances CD8+ memory T cell survival and significantly increases recall responses. Eur J Immunol. 2007;37(1):157–66. doi: 10.1002/eji.200636428. [DOI] [PubMed] [Google Scholar]
- 96.Huddleston CA, Weinberg AD, Parker DC. OX40 (CD134) engagement drives differentiation of CD4+ T cells to effector cells. Eur J Immunol. 2006;36(5):1093–103. doi: 10.1002/eji.200535637. [DOI] [PubMed] [Google Scholar]
- 97.Dempsey PW, Doyle SE, He JQ, Cheng G. The signaling adaptors and pathways activated by TNF superfamily. Cytokine Growth Factor Rev. 2003;14(3-4):193–209. doi: 10.1016/s1359-6101(03)00021-2. [DOI] [PubMed] [Google Scholar]
- 98.Chung JY, Park YC, Ye H, Wu H. All TRAFs are not created equal: common and distinct molecular mechanisms of TRAF-mediated signal transduction. J Cell Sci. 2002;115(Pt 4):679–88. doi: 10.1242/jcs.115.4.679. [DOI] [PubMed] [Google Scholar]
- 99.Arch RH, Thompson CB. 4-1BB and Ox40 are members of a tumor necrosis factor (TNF)-nerve growth factor receptor subfamily that bind TNF receptor-associated factors and activate nuclear factor kappaB. Mol Cell Biol. 1998;18(1):558–65. doi: 10.1128/mcb.18.1.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kawamata S, Hori T, Imura A, Takaori-Kondo A, Uchiyama T. Activation of OX40 signal transduction pathways leads to tumor necrosis factor receptor-associated factor (TRAF) 2- and TRAF5-mediated NF-kappaB activation. J Biol Chem. 1998;273(10):5808–14. doi: 10.1074/jbc.273.10.5808. [DOI] [PubMed] [Google Scholar]
- 101.Takaori-Kondo A, Hori T, Fukunaga K, Morita R, Kawamata S, Uchiyama T. Both amino- and carboxyl-terminal domains of TRAF3 negatively regulate NF-kappaB activation induced by OX40 signaling. Biochem Biophys Res Commun. 2000;272(3):856–63. doi: 10.1006/bbrc.2000.2860. [DOI] [PubMed] [Google Scholar]
- 102.Bansal-Pakala P, Halteman BS, Cheng MH, Croft M. Costimulation of CD8 T cell responses by OX40. J Immunol. 2004;172(8):4821–5. doi: 10.4049/jimmunol.172.8.4821. [DOI] [PubMed] [Google Scholar]