Abstract
Introduction:
Congenital heart disease (CHD) is associated with multiple risk factors, consanguinity may be one such significant factor. The role of consanguinity in the etiology of CHD is supported by inbreeding studies, which demonstrate an autosomal recessive pattern of inheritance of some congenital heart defects. This study was done to find out the risk factors for CHD.
Methods:
A case-control study was done on pediatric patients at a tertiary care hospital, Aga Khan University Hospital, located in Karachi, Pakistan. A total of 500 patients, 250 cases and 250 controls were included in the study.
Results:
Amongst the 250 cases (i.e. those diagnosed with CHD), 122 patients (48.8%) were born of consanguineous marriages while in the controls (i.e. non-CHD) only 72 patients (28.9%) showed a consanguinity amongst parents. On multivariate analysis, consanguinity emerged as an independent risk factor for CHD; adjusted odds ratio 2.59 (95% C. I. 1.73 - 3.87). Other risk factors included low birth weight, maternal co-morbidities, family history of CHD and first born child. On the other hand, medications used by the mother during the index pregnancy, maternal age and gender of the child did not significantly increase the risk of developing CHD.
Conclusions:
Analyses of our results show that parental consanguinity, family history of CHD, maternal co-morbidities, first born child and low birth weight are independent risk factors for CHD.
Keywords: Cardiovascular malformation, congenital heart defects, consanguinity, risk factors
INTRODUCTION
Approximately about six to eight infants per 1000 live births have cardiovascular malformations.[1] Various studies have shown the etiology of congenital heart disease (CHD) and its pattern of inheritance to be of multifactorial origin.[2] A review of the literature showed that consanguinity,[2–4] and a variety of maternal ailments e.g., infections, maternal smoking, and gestational diabetes mellitus play a major role in the development of CHD. In addition, there are several fetal factors such as prematurity, low birth weight and stillbirth which are found to be associated with CHD.[5]
A number of societies in West and South Asia have a strong preference for consanguineous marriages based on religious or social rationale.[6] A survey conducted in Pakistan in 1990–91, ‘The Pakistan Development and Health Survey’, signified that 61% of women aged 15-49 years are married to their cousins, mostly (50%) to first cousins.[7]
In inbred populations, parental consanguinity has been observed to aggravate the underlying genetic risk factors for CHD,[2,3,8,9] suggesting an autosomal recessive component.[3,9] Studies done in northern India, Beirut and Saudi Arabia have proven this relationship to be statistically significant.[8,9]
However, a descriptive study previously carried out on the Pakistani population concluded that consanguinity and other risk factors like maternal abortions, stillbirths and maternal diabetes mellitus are not significant risk factors for CHD.[8] Due to the contrast between these local findings and recently published international data, we undertook this study to evaluate the association of consanguinity and other factors with CHD in a representative sample of the pediatric population.
MATERIALS AND METHODS
This was designed as a case-control study. The target population was pediatric patients under the age of five years admitted to the Aga Khan University Hospital (AKUH) over a period of one year with a diagnosis of CHD. This hospital is a tertiary care center in the province of Sindh with patients being mostly referred from within the province. The diagnosis of CHD was made by echocardiography done by a pediatric cardiologist.
Based on a review of the literature on similar studies conducted in India,[4] consanguinity in CHD patients was estimated at 46%. Using a ratio of 1:1 for number of cases to number of controls, a sample size of 400 was calculated for a power of 80% at 5% alpha. However, taking into account subjects with incomplete data, the sample size was increased to 500.
Sample selection
Cases were all diagnosed cases of CHD under the age of five years who were admitted to the AKUH. Controls were children under the age of five years admitted to the same hospital on account of another illness and who did not have CHD or any other birth defect. Simple random sampling was used to select 250 controls from the list of all admissions for the year. They were matched for age but not for gender, ethnicity or social class.
Inclusion criteria for both cases and controls were patients aged between 0-5 years, admitted to the pediatric ward at the AKUH. Exclusion criteria for both cases and controls were patients older than five years of age, those seen in the outpatient clinics at the AKUH, and patients whose data regarding risk factors was not available.
Targeting a sample size of 500, data on 265 cases and 265 controls was obtained through file review and phone calls. Three patients refused to give consent.. After accounting for incomplete questionnaires, our final response rate was 500. Here we present the data of these 500 patients, 250 cases (CHD present) and 250 controls (CHD absent).
All data was entered in EpiData and checked twice by two different investigators. The questionnaire data was pre-coded where possible for ease and accuracy of collection. Statistical analysis was performed using statistical package for social science (SPSS version 13.0). Mean ± one standard deviation was computed for continuous data. Frequencies were calculated for categorical variables. Odds ratios (with 95% confidence intervals, CI) were calculated from 2×2 Tables. Univariate logistic regression analysis was used to model CHD as a function of demographic characteristics, characteristics of CHD, and risk factor profiles of cases and controls. Multivariate analysis was than performed to find out the independent predictors of CHD. A P value of < 0.05 was taken to be statistically significant for all analyses.
RESULTS
Overall, there were 298 males and 202 females in our study. Of those who had CHD, 151 (60.4%) were males and 99 (39.6%) were females. A similar gender distribution was seen in the controls with 147 (58.8%) males and 103 (41.2%) females.
Out of the 500 study participants, 194 (78%) had parents who had a consanguineous marriage, of which 142 (74%) were first cousins. Of these, 122 (49%) were cases (with 82% being first cousins) and 72 (29%) were controls. Of the cases, 34 (14%) had a family history of CHD, while only four (2%) of the controls had a family history of CHD [Table 1].
Table 1.
Basic characteristics of cases and controls
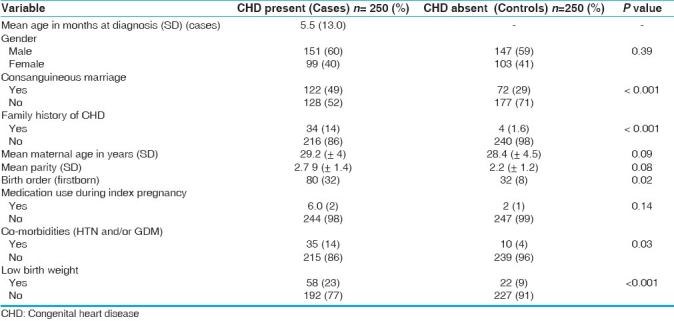
In six (2%) cases, mothers reported use of medication during the index pregnancy; one had used antiepileptic drugs; while the other five could not recall the exact prescription. Mothers of two (0.8%) controls also reported medicine usage, the name of which however, were not known. Mothers of three (1%) cases had rubella infection during the index pregnancy. There were no cases or controls whose mothers had smoked during the index pregnancy. Mothers of 35 (14.0%) of the cases had a co-morbid condition(s) (hypertension and/or gestational diabetes mellitus) at the time of the index pregnancy, compared to 10 (4.0%) mothers of the controls.
Mean age at diagnosis of CHD in our study was 5.5 months ±13.0 months; median being 13 days, with a range of one day to five years. With regard to the birth order, 80 (32.0%) of the cases were firstborn. Significant associations were seen between birth order and presence of CHD, suggesting that most children with CHD were the first child born in their families (P = 0.02).
Forty-nine (20%) of the CHD subjects were preterm and 58 (23%) had a low birth weight, compared to 16 (6%) preterm and 22 (9%) low birth weight in the controls.
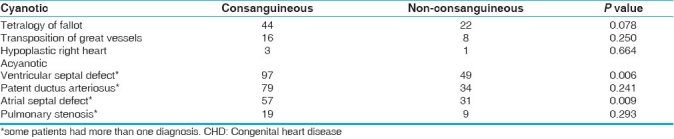
The most common heart defect in our study population was ventricular septal defect (VSD), followed by patent ductus arteriosus (PDA), atrial septal defect (ASD) and tetralogy of Fallot (TOF) [Table 2]. VSD was found to have a significant statistical association with consanguinity (P value = 0.006).
Table 2.
Types of CHD (cases) and their relationship with consanguinity
The most common chromosomal abnormality in children with CHD was Down's syndrome (13 cases) followed by three cases of Di George Syndrome and one case of Turner's syndrome.
Upon univariate analysis, it was seen that children who had CHD were 2.3 times more likely to be born of a consanguineous marriage compared to those without CHD [95% confidence interval (CI) of (1.61–3.38)]. Those who had CHD were also nine times more likely than non-CHD patients to have a positive family history of CHD (95% CI 3.30–27.02). Gender of the child, medication use by the mother during the index pregnancy and maternal age at the index pregnancy were not found to significantly increase the risk of having CHD. On the other hand, the mothers of CHD patients were 3.9 times more likely to have a co-morbid condition like hypertension, or diabetes mellitus as compared to the mothers of non-CHD babies. When the association of hypertension (HTN) and gestational diabetes mellitus (GDM) was individually analyzed, significant relations were found for HTN (P = 0.001); and for GDM (P = 0.008) [Table 3].
Table 3.
Crude and adjusted odds ratios of significant factors associated with congenital heart disease

Upon multivariate analyses, consanguineous marriage, positive family history for CHD and low birth weight were found to be independent predictors of CHD in our study population.
DISCUSSION
The results of our study indicate that the proportion of consanguineous marriages, positive family history, maternal co-morbidities and low birth weight are significantly higher in cases of CHD compared to controls.
Amongst the 250 cases of CHD in our study, 122 patients (49%) were children of consanguineous marriages while in the controls only 72 patients (29%) showed a consanguineous background. Previous studies reported from the Middle East also show that the proportion of consanguinity and first-cousin marriages among cases of CHD is appreciably higher than controls.[2,3] Another study carried out on a Muslim population in Northern India also showed a higher percentage (3.37% out of 741 children) of CHD in the offspring of consanguineous marriages as compared to offspring of non-consanguineous marriages (1.22% in 980 children).[10]
According to studies carried out previously, the percentage of consanguineous marriages in Pakistan is almost 60%, with 80% of these being amongst first cousins. The surveys carried out by the Pakistan Development and Health Survey (PDHS) reveal that prevalence of consanguinity, in the past three to four decades, has shown much consistency with the previous statistics.[6]
Although according to PDHS two-thirds of marriages in Pakistan are consanguineous, results of a study carried out by Hussain R. show that consanguineous marriages are considerably favored in every part of the country regardless of the ethnicity, caste, creed or religion. In Pakistani society the decision for marriages of both sons and daughters are predominantly taken by the parents. It has been inferred that the rationale behind inclination towards consanguineous unions are social and cultural rather than any monetary settlements.[11] The prevailing clan or tribe-oriented culture gives importance to commonality and cohesion in societal groups, thereby supporting consanguinity in Pakistan.[12]
The association of consanguinity with various diseases in children has formerly been investigated. The results of these studies show that, despite fertility being greater in consanguineous than in non-consanguineous marriages, percentage of living children are approximately equivalent in both groups, due to increased child mortality in the former.[11,13–15] Another study has shown a high incidence of mental retardation and physical handicap as well in children born of consanguineous than non-consanguineous marriages.[16]
The results of these studies emphasize the role of homozygous recessive genes in the causation of different types of congenital heart malformations, which may appear to follow a multi-factorial pattern of inheritance. Therefore, educating the community on the deleterious effects of inbreeding, especially in countries with overall high consanguinity rates and increased disease burden, is imperative.[2]
Maternal co-morbidities such as hypertension and diabetes mellitus were seen in 35 of the CHD cases (14%) and only 10 of the controls (4%) in our study. Similarly, in a study carried out in Egypt, diabetes in the mother was seen to be independently associated with increased risk of CHD among patients with Down's syndrome along with the following factors: parental consanguinity, use of antibiotics in pregnancy and oral contraceptive pills.[17] This indicates the interaction between the genetic and environmental factors in the pathogenesis of CHD.
The mean age at diagnosis of the CHD patients in our study was 5.5 months (SD 13.0). In a study carried out in Southern Beirut, of 249 cases of CHD, 81.5% were diagnosed in the neonatal period.[3]
Amongst the 250 CHD cases, 69 (21%) patients had a diagnosis of cyanotic heart disease and 266 (79%) were diagnosed with acyanotic disease. The most common cardiovascular malformation was VSD, seen in 97 cases (39%). A similar observation has been made in studies carried out in Turkey[18,19] and Beirut.[9]
A study carried out in Pakistan[8] showed that there was no association between CHD and factors such as consanguinity and maternal diabetes. The difference in results observed in our study and the one carried out previously on the same population may be accounted for by the different study design that we employed, namely case-control compared to the descriptive study design used earlier.
This particular study design allowed us to compare the rates of consanguinity and multiple risk factors in CHD patients with those in controls in a short time period. It was less cumbersome financially and could be carried out by the limited number of students present in our team. However, there were a number of limitations in our study design. These included limitation in recall of the parents while giving information about exposure to risk factors when the phone calls were made, or during history-taking at the time of admission. A limitation of case-control study designs which also applies to our study is that the absolute risk of any individual factor cannot be indicated.
We conducted our study at a single tertiary care hospital, due to easy availability of patient records. Ethical issues were adequately addressed by our team through the use of consent forms when making phone calls. Furthermore, written permission was obtained from the primary physician of the study subjects before viewing any patient records. One problem faced by certain members of the team was the unwillingness of the parents of deceased patients to cooperate with the investigator. However, in such cases, parents were not coerced into participating.
In conclusion, our study suggests that children with CHD have several risk factors, and are more likely to be offspring of consanguineous parents, as compared to children without CHD.
The results from our study can benefit the population of the South East Asian region in general and our country in particular. The results can be disseminated to basic healthcare units throughout Pakistan which would result in greater awareness about the deleterious effects of consanguinity and its association with CHD.[19] This may serve to discourage the custom of inbreeding and henceforth cause a decline in the rates of CHD and other congenital malformations in our population in the coming future.
ACKNOWLEDGMENTS
Department of Community Health Sciences, Aga Khan University Hospital, Karachi, Pakistan.
Medical Record Office at Aga Khan University Hospital, Karachi, Pakistan.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–900. doi: 10.1016/s0735-1097(02)01886-7. [DOI] [PubMed] [Google Scholar]
- 2.Nabulsi MM, Tamim H, Sabbagh M, Obeid MY, Yunis KA, Bitar FF. Parental consanguinity and congenital heart malformations in a developing country. Am J Med Genet A. 2003;116:342–7. doi: 10.1002/ajmg.a.10020. [DOI] [PubMed] [Google Scholar]
- 3.Becker SM, Al Halees Z, Molina C, Paterson RM. Consanguinity and congenital heart disease in Saudi Arabia. Am J Med Genet. 2001;99:8–13. doi: 10.1002/1096-8628(20010215)99:1<8::aid-ajmg1116>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 4.Venugopalan P, Agarwal AK. Spectrum of congenital heart defects associated with down syndrome in high consanguineous Omani population. Indian Pediatr. 2003;40:398–403. [PubMed] [Google Scholar]
- 5. [Last accessed on 2007 Apr 26]. Available from: http://www.lpch.org/DiseaseHealthInfo/Health Library/cardiac/fcchd.html .
- 6.Hussain R, Bittles AH. The prevalence and demographic characteristics of consanguineous marriages in Pakistan. J Biosoc Sci. 1998;30:261–75. doi: 10.1017/s0021932098002612. [DOI] [PubMed] [Google Scholar]
- 7.Afzal M, Ali SM, Siyal HB. Consanguineous marriages in Pakistan. Pak Dev Rev. 1994;33:663–74. [PubMed] [Google Scholar]
- 8.Hassan I, Haleem AA, Bhutta ZA. Profile and risk factors for congenital heart disease. J Pak Med Assoc. 1997;47:78–81. [PubMed] [Google Scholar]
- 9.Chehab G, Bittar Z. Cumulative incidence and distribution of congenital heart diseases in newborns in Beirut and its southern suburb (1999-2002) J Med Liban. 2004;52:121–5. [PubMed] [Google Scholar]
- 10.Badaruddoza, Afzal M, Akhtaruzzaman Inbreeding and congenital heart diseases in a north Indian population. Clin Genet. 1994;45:288–91. doi: 10.1111/j.1399-0004.1994.tb04032.x. [DOI] [PubMed] [Google Scholar]
- 11.Hussain R. Community perceptions of reasons for preference for consanguineous marriages in Pakistan. J Biosoc Sci. 1999;31:449–61. doi: 10.1017/s0021932099004496. [DOI] [PubMed] [Google Scholar]
- 12.Hussain R. The effect of religious, cultural and social identity on population genetic structure among Muslims in Pakistan. Ann Hum Biol. 2005;32:145–53. doi: 10.1080/03014460500075167. [DOI] [PubMed] [Google Scholar]
- 13.Asha Bai PV, John TJ, Subramaniam VR. Reproductive wastage and developmental disorders in relation to consanguinity in south India. Trop Geogr Med. 1981;33:275–80. [PubMed] [Google Scholar]
- 14.Bittles AH, Grant JC, Shami SA. Consanguinity as a determinant of reproductive behaviour and mortality in Pakistan. Int J Epidemiol. 1993;22:463–7. doi: 10.1093/ije/22.3.463. [DOI] [PubMed] [Google Scholar]
- 15.Hussain R. The impact of consanguinity and inbreeding on perinatal mortality in Karachi, Pakistan. Paediatr Perinat Epidemiol. 1998;12:370–82. doi: 10.1046/j.1365-3016.1998.00146.x. [DOI] [PubMed] [Google Scholar]
- 16.Abdulrazzaq YM, Bener A, al-Gazali LI, al-Khayat AI, Micallef R, Gaber T. A study of possible deleterious effects of consanguinity. Clin Genet. 1997;51:167–73. doi: 10.1111/j.1399-0004.1997.tb02447.x. [DOI] [PubMed] [Google Scholar]
- 17.Mokhtar MM, Abdel-Fattah M. Major birth defects among infants with down syndrome in Alexandria, Egypt (1995-2000): trends and risk factors. East Mediterr Health J. 2001;7:441–51. [PubMed] [Google Scholar]
- 18.Tanner K, Sabrine N, Wren C. Cardiovascular malformations among preterm infants. Pediatrics. 2005;116:e833–8. doi: 10.1542/peds.2005-0397. [DOI] [PubMed] [Google Scholar]
- 19.Güçer S, Ince T, Kale G, Akçören Z, Ozkutlu S, Talim B, et al. Noncardiac malformations in congenital heart disease: a retrospective analysis of 305 pediatric autopsies. Turk J Pediatr. 2005;47:159–66. [PubMed] [Google Scholar]


