Abstract
Introduction and Aim:
Focussed cardiac intensive care is known to produce better outcomes. We have evaluated the benefits of a dedicated Pediatric Cardiac Intensive Care Unit (PCICU) in the early postoperative outcomes of patients undergoing surgery for congenital heart disease.
Methods:
Prospectively collected data of 634 consecutive patients who underwent congenital heart surgery from September 2008 to September 2009 were analyzed. Midway through this period a dedicated PCICU was started. The patients who were treated in this new PCICU formed the study group (Group B, n = 318). The patients who were treated in a common postoperative cardiac surgery ICU formed the control group (Group A, n = 316). Early postoperative outcomes between the two groups were compared.
Results:
The two groups were comparable with respect to demographic data and intraoperative variables. The duration of mechanical ventilation in the dedicated pediatric cardiac ICU group (32.22 ± 52.02 hours) was lower when compared with the combined adult and pediatric surgery ICU group (42.92 ± 74.24 hours, P= 0.04). There was a shorter duration of ICU stay in the dedicated pediatric cardiac ICU group (2.69 ± 2.9 days vs. 3.43 ± 3.80 days, P = 0.001). The study group also showed a shorter duration of inotropic support and duration of invasive lines. The incidence of blood stream infections was also lower in the dedicated pediatric ICU group (5.03 vs. 9.18%, P = 0.04). A subgroup analysis of neonates and infants <1 year showed that the advantages of a dedicated pediatric intensive care unit were more pronounced in this group of patients.
Conclusions:
Establishment of a dedicated pediatric cardiac intensive care unit has shown better outcomes in terms of earlier extubation, de-intensification, and discharge from the ICU. Blood stream infections were also reduced.
Keywords: Pediatric cardiac intensive care, dedicated intensive care unit, intensivist
INTRODUCTION
Pediatric cardiac intensive care is a rapidly developing speciality that caters to the needs of children with congenital heart disease. With increasing complexity of congenital heart repairs in the current era, dedicated pediatric cardiac intensive care units (PCICU) have become a necessity for the perioperative management of these critically ill children. Dedicated intensive care units (ICU), run by an intensivist, who led a multiprofessional team is known to produce better outcomes.[1–4] However, data specific to dedicated cardiac surgical ICUs are relatively few. Focussed pediatric cardiac programs attached to children's hospitals have become a reality in several developed nations. Pediatric cardiac intensive care is still in the evolving phase in many emerging nations, including India. The majority of pediatric cardiac programs in such nations are attached to well-running, adult cardiac surgical intensive care units. Hence, pediatric patients, after congenital heart surgery, are usually treated in a combined adult and pediatric cardiac surgical ICU. In this study we have tried to evaluate the benefits of a newly formed dedicated pediatric cardiac ICU on the early postoperative outcomes of children after congenital heart surgery, in a high volume center in South India.
METHODS
This prospective observational study included 634 consecutive patients who underwent congenital heart surgery from September 1, 2008 to August 31, 2009. There were no exclusions. A dedicated pediatric cardiac ICU was started on March 1, 2009, six months after initiation of the study. The patients who were treated in the newly formed pediatric cardiac ICU formed the study group (Group B, n = 318). The patients who were treated in the common postoperative cardiac surgical ICU before the establishment of the new unit formed the control group (Group A, n = 316). The early postoperative outcomes, namely, the duration of mechanical ventilation, duration of ICU stay, duration of inotrope therapy and invasive lines, incidence of blood stream infections, and 30-day mortality, were compared between the two groups of patients.
Patterns of care and staffing arrangements in the intensive care units
Common postoperative cardiac surgical ICU (September 1, 2008 to February 29, 2009)
The pediatric patients, after congenital heart surgery, were cared for in a common postoperative cardiac surgical ICU. The unit operated without a dedicated pediatric cardiac intensivist. The postoperative care for both adult and pediatric cardiac surgical patients was provided by a 24-hour on-call cardiac anesthesiologist and a cardiac surgeon from a monthly rotating duty roster. A pediatric cardiology resident who also had responsibilities in the emergency department, wards, and cardiac catheterization laboratory provided assistance in the clinical management, when required. Consultants from pediatric cardiac surgery, cardiac anesthesiology, and pediatric cardiology team took independent rounds. Clinical decision-making was on an individual basis and on consultation with senior staff members of the anesthesia and surgical teams, as and when needed. The management of mechanical ventilation and weaning from the ventilator was done by the on-call anesthesiologist, on the advice of senior anesthesiology and surgical consultants. The responsibility for order writing was with the surgical team. Decisions regarding tapering of inotropic supports, removal of invasive lines, and any other invasive procedure in the ICU, were taken on the basis of advice from senior members of the anesthesia and surgical team. Perioperative management of the individual cardiac lesions was according to the standard institutional practices. Decision for discharge from the ICU was made by the admitting surgeon. The nursing team worked in eight-hour shifts and was led by a single charge nurse of the ICU, looking after both adult and pediatric cardiac surgical patients. Nurse patient ratio was 1:1.
Dedicated pediatric cardiac intensive care unit (March 1, 2009 to August 31, 2009)
The pediatric cardiac surgical patients were cared for in the dedicated pediatric cardiac ICU. A newly trained pediatric cardiac intensivist was appointed as the coordinator of the ICU. The intensivist was committed to the ICU 12 hours/day, six days a week, and was available on phone during the night hours and holidays. The intensivist was free from any responsibility outside the ICU while on service (operating room, cardiac catheterization laboratory, emergency department, etc.). A 24-hour, on-call cardiac anesthesiologist, under the supervision of the intensivist, and a cardiac surgeon also provided primary care to the postoperative patients from a monthly roster. The nurse-patient ratio was 1:1. Fixed multidisciplinary rounds, including by the pediatric cardiac intensivist, cardiac anesthesiologist, pediatric cardiac surgeon, pediatric cardiologist, respiratory therapist, bedside nurse, and the charge nurse of the ICU were conducted every day. A detailed clinical case presentation and discussion was carried out for each patient, at the end of which the plan for the day was formulated for each patient. The physician orders were written by the intensive care team. The enactment of the daily plan was done under the supervision of the intensivist. Protocol-guided weaning from mechanical ventilation, with focus on early extubation, directly supervised by the intensivist was followed. The weaning of inotropes, removal of invasive lines, any invasive procedure in the ICU, and discharge from the ICU was also as per the guidance of the dedicated intensivist. ICU policies and protocols were established based on discussion among senior members of the anesthesia, cardiology, and surgery teams. A monthly morbidity and mortality meeting was also organized on a regular basis.
Data collection and statistical analysis
Data was collected prospectively, using a proforma, at the point of transfer out of each patient from the intensive care unit. Institutional approval for data collection was obtained before the initiation of the study. The preoperative and intraoperative variables collected for each patient included demographic data, admission diagnosis, surgical procedure, use of cardiopulmonary bypass (CPB), CPB time, and the aortic cross clamp (ACC) time. The outcome variables that were analyzed, included the duration of mechanical ventilation, duration of inotrope therapy, duration of invasive lines, incidence of blood stream infections, duration of ICU stay, and 30-day mortality.
Analysis of data was done using the SPSS software version 17 (SPSS Inc). The analysis included frequency distributions, percentages, and calculation of mean, median, and standard deviations. Comparison of groups for categorical variables was done using the chi-square test. Non-parametric data were compared with independent samples, with “t” test and Mann Whitney-U test where appropriate. P values <0.05 were considered to be significant.
RESULTS
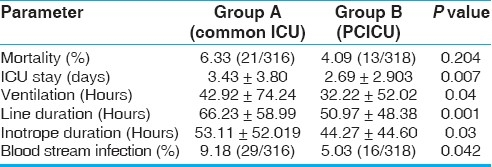
Data are presented as mean ± SD (standard deviation) or median and range, as appropriate. The two groups were comparable with respect to demographic data, use of CPB, CPB time, and aortic cross clamp time [Table 1]. The percentage of neonates and children less than one year were also comparable between the two groups. Both groups of patients were operated upon by the same surgical team. There was a significant reduction in the duration of mechanical ventilation in the dedicated pediatric ICU group (Group B, 32.22 ± 52.02 hours) compared to the common postoperative cardiac surgical ICU group (Group A, 42.92 ± 74.24 hours). The duration of ICU stay was also significantly lower in Group B than in Group A (2.69 ± 2.9 days vs. 3.43 ± 3.80 days). The inotropic supports and invasive lines were removed earlier in the dedicated pediatric ICU group [Table 2]. There was a significantly lower incidence of blood stream infections in Group B. Although the mortality in Group B was lower than in the control group, this did not reach statistical significance.
Table 1.
Demographic and intraoperative variables
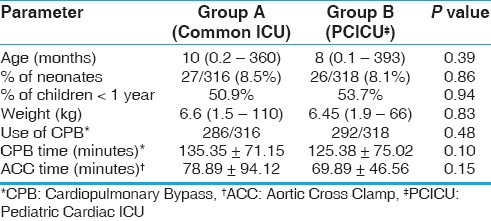
Table 2.
Outcome data for the entire group
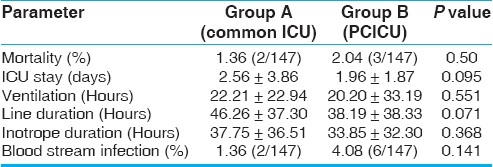
We have also done a subgroup analysis of patients less than one year, as infants and neonates together constitute 50% of our surgical volumes. This subgroup analysis showed a further striking reduction in the duration of ventilation, ICU stay, duration of invasive lines and inotropes, and the incidence of blood stream infections in children less than one year, in the dedicated ICU group [Table 3]. However, these parameters did not reach statistical significance when they were evaluated for the older age group (children more than one year) [Table 4].
Table 3.
Outcome data in patients aged less than one year
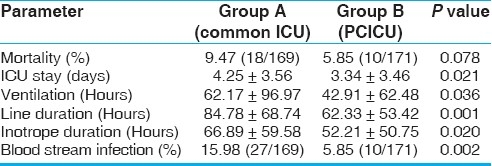
Table 4.
Outcome data in patients aged more than one year
DISCUSSION
The organizational model and staffing patterns of ICU has always been a focus of interest. There is adequate evidence of the beneficial effects of focussed intensive care on the clinical outcomes.[1–3] A systematic review of 26 studies analyzing the staffing patterns of ICU has found that the greater use of intensivists in the ICU led to a significant reduction in ICU and hospital mortality and length of stay.[4] The Leapfrog group, a United States business consortium of healthcare sector purchasers, had recommended that board-certified, critical care specialists be available during the daytime hours and be able to return to the ICU or arrange for an onsite physician to do so within five minutes of being paged.[5] A subsequent literature review by Young et al. concluded that using the Leapfrog standards would save approximately 54,000 lives per year in the United States.[6]
The outcome benefits of dedicated PCICV have not been evaluated so far. The most commonly recommended model of a Pediatric Cardiac Intensive Care Unit has been an intensivist-led multiprofessional team approach in a dedicated intensive care unit.[7–9] In a recent survey of various pediatric cardiac intensive care programs in the United States and Europe, 19 (35%) institutions had a dedicated cardiac ICU, whereas, in 35 hospitals (65%) pediatric cardiac patients were cared for within a general pediatric cardiac intensive care unit.[10]
In developing countries, pediatric cardiac surgical programs have always been under the ‘shadow’ of successful adult cardiac surgical programs. As a result, pediatric cardiac surgical patients share a common postoperative cardiac surgical ICU with the adult cardiac patients. Lack of trained personnel and limited infrastructure have been recognized as major limiting factors in the development of focussed pediatric cardiac care units in these emerging nations.[11]
It is beyond the scope of this manuscript to elaborate the recommendations for an ideal PCICU.[8,9,12] Briefly, based on the available evidence, an ideal pediatric cardiac intensive care unit should be built on a multidisciplinary cohesive team, comprising of pediatric subspecialties of cardiology, cardiac surgery, critical care medicine, cardiac anesthesia, nursing, and respiratory therapy, and can also incorporate pediatric pulmonology, adult cardiology, and occupational and physical therapy. The unit should be specifically organized to provide postoperative care for pediatric heart patients or it should be a pediatric intensive care unit that satisfies the American academy of pediatrics (AAP) recommendations for level I pediatric intensive care units.[9,12] The unit should be a physically independent one and should have a physician leadership, in the form of a medical director, to build a cohesive team. The unit should have its own policies and protocols to minimize practice variations and should also have a provision for staff training, research, and quality improvement.
To summarize the key findings of the present study, we found similar patient profiles in both groups. The dedicated PCICU showed better outcomes in terms of shorter extubation time, shorter ICU stay, shorter duration of inotropes and invasive lines, and a reduction in blood stream infections. There was a trend toward lower mortality, although this did not reach statistical significance. A protocolized weaning approach with emphasis on early extubation under the direct supervision of the intensivist may have contributed to the significantly shorter times to extubation in the study cohort. The continuous presence of a skilled intensivist can facilitate the recognition of opportunities for early weaning from inotropic support and de-intensification. Early extubation and de-intensification probably resulted in a significant reduction in the ICU length of stay in the dedicated ICU group. Prolonged residence of invasive lines has been associated with increased risk of blood stream infections.[13] The earlier removal of invasive lines as soon as it is deemed appropriate may have helped to reduce the incidence of blood stream infections in the dedicated ICU group. Although the mortality was lower in the dedicated ICU group, this did not reach statistical significance. We believe that if the study had been followed up for one more year the numbers would have reached significance.
The subgroup analysis based on the age of the patients reveals that the beneficial effects of a dedicated team are far more pronounced for neonates and infants than for the older age group. This becomes more relevant in the present era, where a large number of infants and neonates present for early corrective surgery.
Novick et al., in a study of 2599 adult cardiac surgical patients had demonstrated significant reduction in the postoperative ventilation hours, shorter hospital length of stay, and reduced transfusion of blood components, after the opening of a specialized cardiac surgery recovery unit, manned by 24-hour, in-house intensivists.[14] Kumar et al., in a large prospective cohort study had also demonstrated a significantly shorter ICU and hospital stay following postoperative care in a dedicated adult cardiac surgical unit.[15]
The dedicated ICU employed a multidisciplinary approach to patient care. The daily multidisciplinary rounds formed a platform to integrate the inputs from various members of the care team and formulated a coordinated daily plan for each patient. This approach, together with the continuity of care provided by the constant presence of the intensivist to supervise the day-to-day management of the patient might have contributed to the overall improved performance of the dedicated unit.
Limitations
A randomized controlled trial was not possible in this investigation. However, the two groups were matched with regard to the demographic characteristics and intraoperative variables. The risk scoring for congenital heart disease of all patients was not done for the common postoperative ICU group (Group A). Hence, a subgroup analysis based on the complexity of the lesions was not feasible. Finally, the cost effectiveness of this model has also not been analyzed.
CONCLUSIONS
The establishment of a multidisciplinary cohesive pediatric team led by a dedicated pediatric intensivist in a dedicated PCICU has resulted in better outcomes in terms of shorter extubation time, shorter ICU stay, shorter duration of invasive lines, and lower blood stream infections. The improvement in postoperative outcomes were specifically noted in pediatric patients less than one year of age.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Topeli A, Laghi F, Tobin MJ. Effect of closed unit policy and appointing an intensivist in a developing country. Crit Care Med. 2005;33:299–306. doi: 10.1097/01.ccm.0000153414.41232.90. [DOI] [PubMed] [Google Scholar]
- 2.Fuchs RJ, Berenholtz SM, Dorman T. Do intensivists in ICU improve outcome? Best Pract Res Clin Anaesthesiol. 2005;19:125–35. [PubMed] [Google Scholar]
- 3.Chittawatanarat K, Pamorsinlapathum T. The impact of closed ICU model on mortality in general surgical intensive care unit. J Med Assoc Thai. 2009;92:1627–34. [PubMed] [Google Scholar]
- 4.Pronovost PJ, Angus DC, Dorman T, Robinson KA, Dremsizov TT, Young TL. Physician staffing patterns and clinical outcomes in critically ill patients: A systematic review. JAMA. 2002;288:2151–62. doi: 10.1001/jama.288.17.2151. [DOI] [PubMed] [Google Scholar]
- 5.Milstein A, Galvin RS, Delbanco SF, Salber P, Buck CR., Jr Improving the safety of health care: The leapfrog initiative. Eff Clin Pract. 2000;3:313–6. [PubMed] [Google Scholar]
- 6.Young MP, Birkmeyer JD. Potential reduction in mortality rates using an intensivist model to manage intensive care units. Eff Clin Pract. 2000;3:284–9. [PubMed] [Google Scholar]
- 7.Baden HP, Zimmerman JJ, Brilli RJ, Wong H, Wetzel RC, Burns JP, et al. Intensivist-led team approach to critical care of children with heart disease. Pediatrics. 2006;117:1854–6. doi: 10.1542/peds.2006-0353. author reply 185 6-7. [DOI] [PubMed] [Google Scholar]
- 8.Chang AC. How to start and sustain a successful pediatric cardiac intensive care program: A combined clinical and administrative strategy. Pediatr Crit Care Med. 2002;3:107–111. doi: 10.1097/00130478-200204000-00003. [DOI] [PubMed] [Google Scholar]
- 9.Guidelines for pediatric cardiovascular centers. Pediatrics. 2002;109:544–9. doi: 10.1542/peds.109.3.544. [DOI] [PubMed] [Google Scholar]
- 10.Stromberg D. Pediatric cardiac intensivists: Are enough being trained? Pediatr Crit Care Med. 2004;5:391–2. doi: 10.1097/01.pcc.0000128606.13235.37. [DOI] [PubMed] [Google Scholar]
- 11.Balachandran R, Nair SG, Kumar RK. Establishing a pediatric cardiac intensive care unit - Special considerations in a limited resources environment. Ann Pediatr Cardiol. 2010;3:40–9. doi: 10.4103/0974-2069.64374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rosenberg DI, Moss MM. Guidelines and levels of care for pediatric intensive care units. Crit Care Med. 2004;32:2117–27. doi: 10.1097/01.ccm.0000142704.36378.e9. [DOI] [PubMed] [Google Scholar]
- 13.O’Grady NP, Alexander M, Dellinger EP, Gerberding JL, Heard SO, Maki DG, et al. Guidelines for the prevention of intravascular catheter-related infections. The Hospital Infection Control Practices Advisory Committee, Center for Disease Control and Prevention, U.S. Pediatrics. 2002;110:e51. doi: 10.1542/peds.110.5.e51. [DOI] [PubMed] [Google Scholar]
- 14.Novick RJ, Fox SA, Stitt LW, Butler R, Kroh M, Hurlock-Chorostecki C, et al. Impact of the opening of a specialized cardiac surgery recovery unit on postoperative outcomes in an academic health sciences centre. Can J Anaesth. 2007;54:737–43. doi: 10.1007/BF03026870. [DOI] [PubMed] [Google Scholar]
- 15.Kumar K, Zarychanski R, Bell DD, Manji R, Zivot J, Menkis AH, et al. Impact of 24-hour in-house intensivists on a dedicated cardiac surgery intensive care unit. Ann Thorac Surg. 2009;88:1153–61. doi: 10.1016/j.athoracsur.2009.04.070. [DOI] [PubMed] [Google Scholar]


