Abstract
It has been widely presumed that antibody-mediated selective costimulatory molecule blockade of CD28 is superior to CTLA4-Ig. This is based on the premise that specifically blocking CD28 allows inhibitory signals through CTLA-4 to proceed, adding further to the down-regulation of T-cell function. These characteristics of CD28 are likely to be important in efforts to improve upon hematopoietic cell transplantation. Here, we developed for use in the dog model one agonistic and seven antagonistic monoclonal antibodies (mAb) to canine (ca)CD28. Binding studies indicated that an agonistic (5B8) and an antagonistic (1C6) mAb bound equally well to a caCD28/caIgG1 fusion protein and to CD28 expressed on CD4+ and CD8+ peripheral blood T-cells. The antagonistic antibody blocked mixed lymphocyte reactions (MLR) in a dose-dependent manner similar to CTLA4-Ig, while the agonistic antibody to caCD28 enhanced MLR. 5B8 was superior to 1C6 when either was combined with anti-canine CD3 to stimulate lymphocyte proliferation. Furthermore, the agonistic mAb, 5B8, together with anti-CD3 mAb induced 100-fold proliferation of canine regulatory T-cells. Relative to untreated control cells, anti-caCD28 (1C6) and CTLA4-Ig inhibited cytotoxic T lymphocyte (CTL)-mediated killing of alloreactive target cells after a secondary MLR equivalently. These studies demonstrated that mouse anti-caCD28 mAb’s with either agonistic or antagonistic function can be generated.
Keywords: Costimulatory molecule, blockade, CD28, CTLA4-Ig, CTL, regulatory T-cells
INTRODUCTION
Following T-cell receptor interaction with the MHC/peptide complex, T-cells require costimulation for an optimal immune response. Of the multiple costimulatory pathways identified, the interactions of CD28 with its two ligands, B7.1/B7.2 (CD80/CD86), are generally critical for T-cell responses to antigens (1). Targeting T-cell costimulation is used to control T-cell activation in autoimmunity (2,3), allogeneic solid organ, and allogeneic hematopoietic graft rejection (4–7) and graft-versus-host disease (GVHD) (8). CD28 costimulatory blockade is usually accomplished with the cytotoxic T lymphocyte antigen 4 (CTLA-4)-Ig fusion protein (6,9,10). The problem with employing CTLA4-Ig is that, in addition to reducing CD28:B7 interactions, B7 blockade can also prevent CTLA-4 from transmitting inhibitory signals to the T-cells. Structurally related to CD28, CTLA-4 is expressed on the surface of T-cells following activation (11).
An alternative approach to blocking CD28-mediated cell activation is to use anti-CD28 monoclonal antibodies (mAb). This approach is attractive because a CD28 antagonist can block positive signaling through CD28 while leaving the CTLA-4 negative signaling and Treg function intact. However, because of the homodimeric structure of CD28, monoclonal antibodies (mAb), with few exceptions, have been agonists, and their binding results in a positive signal transduction. Because of limited reports of antagonistic anti-CD28 mAb in various transplantation model systems, it is important to expand our understanding of direct blockade versus activation of CD28 by monoclonal antibodies (mAb) relative to blockade of B7 using CTLA4-Ig.
Testing therapeutics for allogeneic hematopoietic cell transplantation (HCT) in the canine model has been remarkably predictive of successful outcomes in the clinic. For example, most of the GVHD prophylactic regimens used in patients have been developed in dogs (12–15). An important goal in the further development of safe HCT for treatment of malignant and nonmalignant diseases has been to reduce or eliminate the amount of total body irradiation (TBI) required by patients before transplantation. In the dog HCT model, stable sustained hematopoietic cell engraftment was established after a sublethal dose of 2 Gy TBI before and a short course of immunosuppression after dog leukocyte antigen-identical marrow transplantation (16). When TBI was reduced to 1 Gy, all grafts were eventually rejected. However, when dogs were treated with human CTLA4-Ig coincident with donor specific infusions of peripheral blood buffy coat cells for 7 days before HCT, 4 of 6 dogs showed persistent mixed donor/host chimerism (7). In an effort to improve on these results, we have produced and characterized several clones of anti-canine (ca)CD28 mAb. Here, we describe the in vitro function of two of these clones, one an agonist and the second an antagonist to CD28 signaling. In addition, we show that anti-caCD28 in combination with anti-CD3 expands canine regulatory T-cells (Tregs) in vitro.
RESULTS
Effect of anti-caCD28 mAb clones 1C6 and 5B8 on MLR
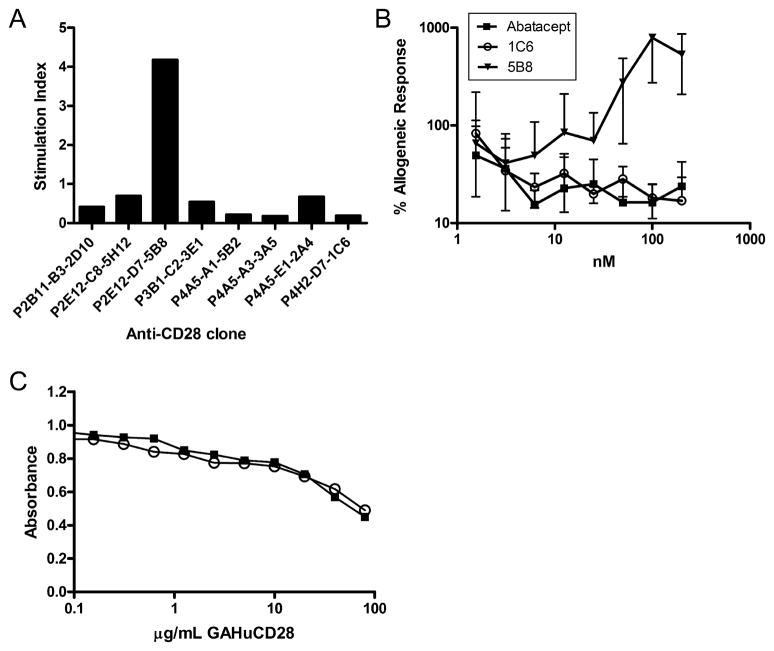
Clones of anti-caCD28 mAb were selected for expression level and ability to suppress or stimulate a 7-day MLR with lymphocytes collected from DLA-nonidentical dog pairs as part of the screening process. Of the eight clones that maintained expression and immunoreactivity long-term, one demonstrated agonistic activity and seven showed antagonistic activity in MLR at a concentration of 20 μg/ml (Figure 1A). Further characterization of the anti-caCD28 mAb was made using the sole agonistic clone (5B8) and one of the antagonistic clones (1C6). The results of a representative MLR showed 5B8 had potent agonistic activity, while clone 1C6 and the recombinant human CTLA4-Ig fusion protein, abatacept, showed antagonistic activities when added in equimolar concentrations to cultured peripheral blood mononuclear cells (PBMC) (Figure 1B). Thus, blockade of CD28 directly by anti-caCD28 mAb was not superior to blockade through B7 by CTLA4-Ig in a 7-day MLR (Figure 1B). Abatacept was used as a comparator as cell binding studies and MLR using canine lymphocytes showed no significant difference in activity between recombinant canine CTLA4-Ig and human CTLA4-Ig (17).
Figure 1.
A) Effect of agonistic and antagonistic anti-CD28 clones on MLR. Supernatants of eight anti-CD28 mAb were purified and tested in a 7-day MLR between DLA-non-identical dogs. Data are presented as stimulation index of CPM of responder cells cultured with anti-CD28 mAb (20 μg/ml) divided by the CPM of responder cells cultured in medium alone. B) Effect of anti-CD28 or CTLA4-Ig on MLR. DLA-nonidentical PBMC were cultured in 7-day MLR in the presence or absence of anti-caCD28 clones 1C6 or 5B8 or human CTLA4-Ig (Abatacept) at concentrations indicated. C) Competitive immunoreactivity of anti-caCD28 mAb and goat anti-human CD28 for binding to caCD28-Ig fusion protein. Murine IgG1 anti-CD28 antibodies (50 ng/ml), 1C6 (closed square) and 5B8 (open circle), were mixed with goat anti-human CD28 and allowed to bind to plates coated with caCD28murineIgG2a fusion protein and evaluated by standard ELISA methods.
Immunoreactivity
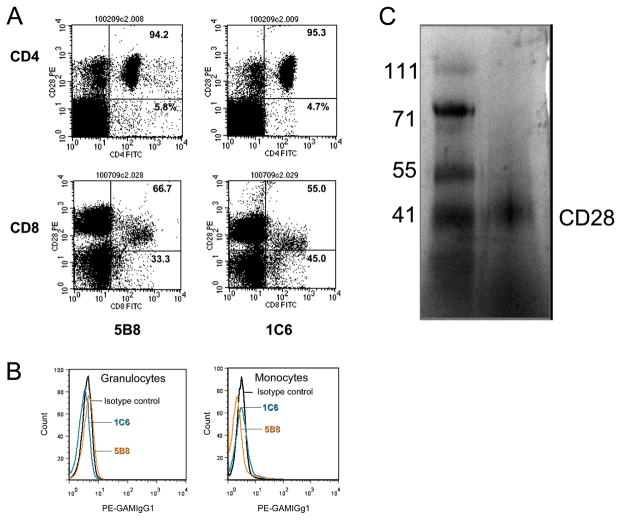
The specificities of two clones of anti-caCD28 mAb for binding to caCD28 were evaluated by ELISA. As shown in Figure 1C, goat anti-human CD28 polyclonal antibody, known to cross-react with canine lymphocytes, competed equally well for binding to the caCD28 fusion protein with either of the anti-caCD28 clones, 1C6 (antagonist) or 5B8 (agonist), in a dose response manner. In order to determine the relative binding of anti-caCD28 mAb to dog CD4+ and CD8+ cells, both 5B8 and 1C6 were analyzed by flow cytometry for binding to freshly isolated dog PBMC. A representative example of five independent CD4+ and CD8+ cell binding studies is shown in Figure 2A. No differences between the two clones were noted for binding CD4+ or CD8+ T-cells. The percent CD4+ cells bound by 5B8 and 1C6 mAb was 94%±1.8% SD and 98%±1.8% SD, respectively, while the percent CD8+ cells bound by the two mAb was 67% ±0.7% SD and 68% ±11.4% SD, respectively. Alternatively, neither clone 1C6 nor 5B8 bound to granulocytes or monocytes (Figure 2B). Anti-CD28 specificity was verified by Western Blot of total PBMC lysates run on a native polyacrylamide gel. A single band of approximately 44 kDa was detected by anti-caCD28 (1C6) (Figure 2C).
FIGURE 2.
Binding of mouse anti-canine CD28 antibodies to canine PBMC. Freshly isolated dog blood was separated on Ficoll into PBMC and granulocyte fractions. Both fractions were stained with 5B8 or 1C6 anti-CD28 antibodies and detected with goat anti-mouse IgG1-PE. A) Cells were also stained with FITC-conjugated anti-CD4 (top row) or anti-CD8 mAb (bottom row). B) Gated granulocytes were stained with FITC conjugated DM5 mAb and were 99.5% pure while gated monocytes were stained with FITC conjugated anti-CD14 mAb and were 99.1% pure. Shown are fluorescently stained cells from these gated populations. Isotype controls were used to establish settings. C) Western blot of 1C6 binding to a 44 kDa protein electrophoresed using native polyacrylamide conditions.
Anti-CD3/CD25 induced proliferation
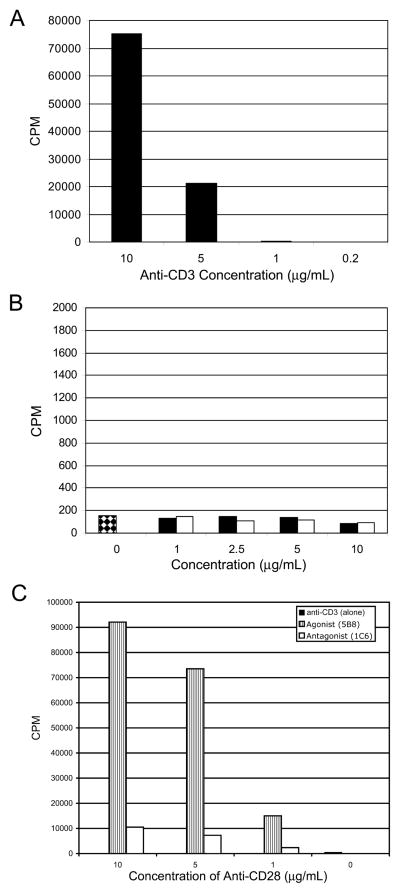
Anti-CD3 mAb alone or combined with anti-caCD28 induces proliferation of T-cells in the absence of mitogens or other stimulation (18). To determine whether the functional differences between antagonistic and agonistic anti-caCD28 were related to their ability to stimulate dog T-cells in the presence of anti-CD3 mAb, escalating doses of both 1C6 and 5B8 were used to coat plates to which dog PBMC were added. As shown in Figure 3A, anti-CD3 alone at 10 and 5 but not at 1.0 μg/ml induced proliferation of canine PBMC Plates coated with 1C6 or 5B8 anti-caCD28 mAbs alone failed to mediate proliferation of canine cells (Figure 3B). Alternatively, a dose dependent proliferation of lymphocytes was seen with anti-caCD28 in the presence of 1 μg/ml anti-CD3 mAb. The agonistic clone (5B8) was superior to the antagonistic clone (1C6) in inducing proliferation of cells when combined with anti-CD3.
FIGURE 3.
Proliferation of PBMC in response to anti-CD3 and anti-caCD28 (5B8 and 1C6). Dog PBMC were isolated from peripheral blood and stimulated for 3 days in microtiter wells coated with anti-CD3 alone (A), or with wells coated with declining concentrations anti-caCD28 clones,5B8 (open bars) or 1C6 (solid bars) (B), or with wells coated with declining concentrations anti-caCD28 clones, 5B8 (striped bars) or 1C6 (open bars) and 1 ug/ml anti-CD3 (C). 3H-thymidine was added to wells 6 hours before harvesting cells.
Anti-CD3/anti-caCD28 expand regulatory T-cells
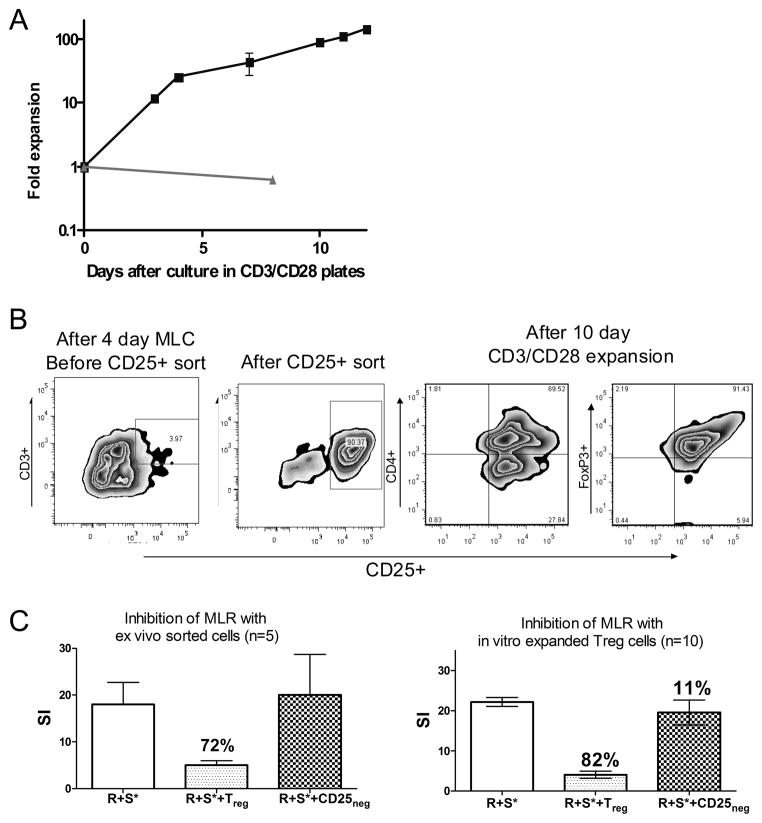
Studies have demonstrated in other model systems that anti-CD3 and anti-CD28 bound to beads can induce regulatory T-cell expansion (19). Here, we sought to determine whether anti-caCD28 (clone 5B8) and anti-CD3 could expand canine CD3+CD25+ T-cells without decreasing regulatory T-cell function. After 4 days of culture, responding T-cells were split and re-plated in freshly prepared CD3/CD28 mAb coated plates. By day 7 of stimulation, cells had expanded 50-fold, compared to the starting population. On day 10, the expansion of plated cells increased to 100-fold (Fig. 4A). The anti-CD3/anti-caCD28-expanded T-cells had phenotypes similar to the starting population of sorted T-cells; Figure 4B shows the population of cells that were CD3+CD4+CD25+ and FoxP3+. The anti-CD3/anti-caCD28-expanded CD3+CD25+ T-cells were tested for their ability to inhibit proliferation of responder (R) cells against irradiated DLA-nonidentical stimulator (S*) cells in a standard MLR. The expanded CD3+CD25+ T-cells were obtained from the same dog as the responder cells. The expanded CD3+CD25+ T-cell: responder: stimulator cell ratio was 1:5:5 (Fig. 4C). The addition of sorted CD3+CD25+ cells obtained from a 4-day MLR functioned as regulatory T-cells and reduced the CPM of the MLR by 72% (mean of 5 experiments), while the anti-caCD3/anti-caCD28-expanded CD3+CD25+ T-cells reduced the CPM of the MLR by 80% (mean of 10 experiments). Adding the sorted CD25 negative cells back to the MLR failed to significantly affect MLR (Figure 4C).
FIGURE 4.
Expansion of canine regulatory T-cells using anti-caCD28 (5B8) and anti-CD3. After 4 days, cultures of DLA-nonidentical lymphocytes were transferred to antiCD3/anti-CD28 coated plates and expanded for an additional 4 days. Cells were split and cultured in antibody coated plates with (■) or without antibodies (▲) and evaluated for proliferation (A). The phenotype of the cells after the initial 4-day culture period, after CD25+ cell sorting, and after a 10-day expansion was determined by flow cytometry using anti-CD3-PE and anti-CD25-FITC or anti-CD4-PE and anti-FoxP3-FITC labeled antibodies (B). Proliferation of responder cells (R) with stimulator cells (S) in MLR was determined in the presence or absence of sorted regulatory T-cells or expanded regulatory T-cells (C).
CD28 costimulatory molecule blockade suppresses CTL function
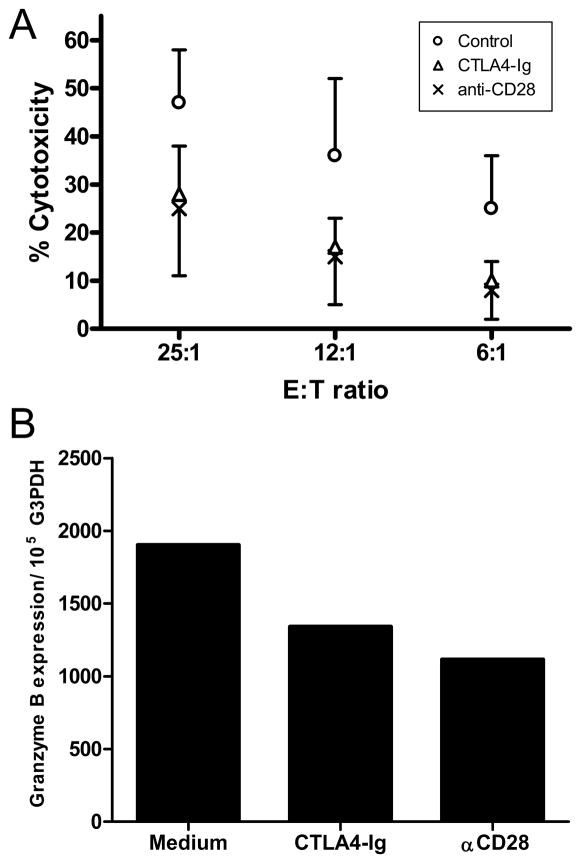
Previous studies indicated no superiority in costimulatory molecule blockade by anti-caCD28 compared to blockade of B7 by CTLA4-Ig in 7-day MLR (Fig. 1B). This posed the question as to whether CTL development, manifested by longer culture periods, would show different sensitivity to an anti-CD28 (clone 1C6) relative to CTLA4-Ig. Previous studies demonstrated that canine CTL could be generated from MLR of peripheral blood lymphocytes collected from two DLA-nonidentical dogs after two in vitro stimulations with irradiated stimulator cells (20). Responder cells were collected from MLR treated with medium alone, 100 nM CTLA4-Ig, or 100 nM anti-CD28 (IC6), and co-cultured with irradiated stimulator cells with or without costimulatory molecule blockade for an additional 4 days and tested in a 4-hour cytotoxicity assay. Figure 5A shows the combined results of three CTL assays. Anti-CD28 was equally effective as CTLA4-Ig in inhibiting CTL activity relative to untreated controls (effector to target cell ratios of 25:1, 12:1 and 6:1, p<0.02, Mann Whitney U-test). Messenger RNA collected from 11 day MLR of control (medium only), CTLA4-Ig and anti-caCD28 (clone 1C6) treated cells was used to assess expression of granzyme B in each treated population of cells. Expression of granzyme B was reduced relative to G3PDH in both CD28 blockade groups compared to the control population, suggesting blockade reduced the levels of granzyme B in the cultured cell population (Figure 5B). In separate studies, addition of anti-caCD28 (1C6) to the 4-hour 51Cr release assay failed to suppress or enhance lysis of PHA blasted target cells (data not shown).
FIGURE 5.
Costimulatory molecule blockade by CTLA4-Ig (abatacept) or anti-caCD28 in a CTL assay. A) Lymphocytes from DLA-nonidentical dogs were cultured in a MLR for 7 days in the presence or absence of 100 nM CTLA4-Ig or anti-caCD28 (1C6). Cells were harvested and restimulated for an additional 4 days with or without anti-caCD28 or CTLA4-Ig. Responding cells were tested for cytotoxicity against PHA-blasted, 51Cr-labeled target cells at 3 different effector to target cell ratios. Untreated controls differed significantly from CTLA4-Ig and anti-CD28 (1C6) treated cells at each effector to target cell ratio (p<0.02). Data are expressed as the mean and standard deviation of 3 experiments. B) RT-PCR analysis of 11-day cultures of responder cells treated with medium alone, 100 nM CTLA4-Ig or 100 nM 1C6, anti-caCD2. Results are presented as copy number proportional to G3PDH expression.
DISCUSSION
Costimulatory molecule blockade using CTLA4-Ig has been used clinically for the prevention of kidney graft rejection (21) and for the treatment of both rheumatoid arthritis (22) and psoriasis (23). For applications such as transplantation tolerance, CD80/86 blocking strategies are expected to be inferior to CD28 specific blockade. The main reason for this assumption is that antagonistic antibodies specific for CD28 maintain CTLA-4 signaling which is important for the activation of regulatory T-cells and the development of transplant tolerance (24,25). Only a few antagonistic anti-CD28 mAb have been described. Blockade of CD28 can be accomplished with single chain Fv antibodies (26). Single chain antibodies have rapid clearance due to their size but remain antagonists with an acceptable circulating half life when conjugated to large molecular weight proteins such as α1-antitrypsin (26). A humanized murine anti-CD28 mAb, FK734, enhances proliferation of CD4+ and CD8+ cells as well as secretion of interleukin-2 and interferon-γ in vitro yet conversely inhibits the rejection of human skin transplanted with human peripheral blood lymphocytes in SCID/beige mice (27) Using anti-CD28 mAb (JJ319) or anti-CD154 alone in a rat cardiac allograft model results in delay in graft rejection, while co-administration of the two costimulatory blocking molecules leads to long-term graft acceptance in 60% of the recipients in (28).
In previous studies, we examined human CTLA4-Ig in the dog HCT model for prevention of DLA-identical marrow graft rejection following sub-optimal conditioning of 100 cGy TBI (7). While 4 of 6 animals engrafted compared to 0 of 12 controls, we posed the question whether species-specific reagents would give even better results. To this end, we generated both a recombinant caCTLA4-Ig and anti-caCD28 mAb. Surprisingly, in vitro studies showed that the caCTLA4-Ig was not significantly different from the recombinant human CTLA4-Ig (abatacept) in suppressing MLR or binding to canine monocytes and dendritic cells (29). Given that CTLA4-Ig also blocks potentially down-regulating signals through CTLA-4, we have turned to the development and characterization of anti-caCD28 mAb.
Cell binding studies against a caCD28-Ig fusion protein indicated that binding of either an agonist or antagonist mAb was equally inhibited by cross reacting goat anti-human CD28 antibodies. One clone, 5B8, had agonist activity in MLR, suggesting an ability to cross-link and activate CD28 above activation by cell-cell contact of responder and stimulator cells. More commonly, our clones of anti-caCD28 were antagonistic and showed inhibition on a molar basis equivalent to recombinant canine or human CTLA4-Ig. However, when the anti-caCD28 clones, 5B8, and, to a significantly lesser extent,1C6, were bound to tissue culture plates, both mAb demonstrated agonistic activity synergistic with anti-CD3 mAb. The switch from antagonistic to modest agonistic function by 1C6 when substrate-bound was presumably due to an increased ability of 1C6 to cross-link and activate CD28. Podesta et al. (30) showed that antagonistic antibodies against the lutropin receptor on Leydig cells could become agonistic once cross-linked by a second antibody.
Flow cytometry binding studies indicated there was a difference in binding of the two anti-CD28 mAb to CD4+ versus CD8+ cells isolated from the peripheral blood. Anti-CD28 mAbs on average bound 96% of CD4+ cells and only 67% of CD8+ cells. In the mouse, expression of CD28 was found to be dependent upon cell maturity with greater antigen expression found on mature cells (31). Virtually all murine CD4+ and CD8+ cells in the spleen, lymph node and blood were shown to express CD28 antigen (31). These results suggest canine CD8+ T-cells express CD28 differently than the mouse.
Canine regulatory T-cells express CD25, FoxP3, IL10 and TGFB as detected by mAb and RT-PCR (17). This cell population, when added to MLR, suppressed 3H Thymidine incorporation (17). However, in order to utilize the suppressive properties of regulatory T-cells in vivo, methods are needed for ex vivo expansion of these cells. It has been described previously that anti-CD28 acted synergistically with anti-CD3 in other model systems and induced proliferation of Tregs (19). In our studies, we demonstrated that canine regulatory T-cells could be expanded ex vivo with an anti-CD3 mAb in conjunction with an agonistic anti-caCD28 mAb. Furthermore, 100-fold expanded regulatory T-cells maintained their suppressor activity when added to MLR. These results are important when considering future studies in the canine transplantation model in which expanded regulatory T-cells are required for induction or maintenance of immune tolerance.
In an attempt to delineate the superiority of antagonistic anti-caCD28 over that of recombinant CTLA4-Ig in vitro, we evaluated the blocking properties of the two reagents for longer incubation periods and using CTL assays. To this end, we tested the effect of equal molar concentrations of CTLA4-Ig and anti-caCD28 on the generation of CTL following double-stimulated MLR. Both CTLA4-Ig and anti-caCD28 (1C6) produced equivalent suppression of CTL activity after a secondary MLR. Similarly, Haspot et al. (32), found that CTLA4-Ig and an anti-CD28 fab blocked cell proliferation in both a primary and secondary MLR in an inbred Lewis rat model. It appears that identification of other in vitro assays or use of in vivo studies will be required to demonstrate functional differences between CTLA4-Ig and specific antibody-mediated blockade of CD28.
Here, we showed that anti-caCD28 mAb clones could be generated with either antagonistic or agonistic function and that an antagonist mAb could function as an agonist when plastic-bound. The agonistic antibody along with anti-CD3 was very effective in expanding cells with regulatory T-cell phenotype. The antagonistic anti-caCD28 effectively blocked MLR and CTL development. Both of these antibodies have applications as valuable reagents for the induction of tolerance in the canine HCT model.
MATERIALS AND METHODS
Experimental animals and blood cell preparation
RBF/DnJ mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Beagles, mini-mongrel, basenji and golden retriever crossbreeds were raised at the Fred Hutchinson Cancer Research Center or purchased from commercial kennels. Animals were housed in AAALAC-accredited facilities, and study designs were approved by the Institutional Animal Care and Use Committee. Selection of dogs for MLR and CTL assays required typing of litters and parents using highly polymorphic microsatellite markers within dog leukocyte antigen (DLA) class I and class II regions (33) and DLA-DRB1 gene sequencing (34).
Assembly of caCD28/murineIg2a and caCD28/caIgG1 fusion vectors
Details of the methods used to produce caCD28/murineIg2a and caCD28/caIgG1 fusion vectors is available online.
Cell culture and protein production
Murine myeloma cells, NS0, were electroporated with linearized fusion plasmids. Expression levels were monitored by ELISA specific for either mouse IgG2a or canine IgG1. Transfected cells were grown to extinction in serum-free medium and supernatant was collected. CD28murineIgG2a fusion was purified over a HiTrap Protein A (GE Healthcare, Piscataway, NJ) column. The caCD28caIgG1 fusion was purified over a HiTrapNHS-activated HP column (GE Healthcare) covalently coupled to goat anti-dog IgG1 antibody (Bethyl Laboratories, Montgomery, TX).
Monoclonal antibody production
NS0 were electroporated with caCD28/pcDNA3.1 plasmid in Opti-MEM (Invitrogen). caCD28-expressing cells were sorted by flow cytometry with goat anti-human CD28 (R&D Systems, Minneapolis, MN). RBF/DnJ mice were immunized with irradiated caCD28-expressing cells (2000 cGy) using Ribi adjuvant and boosted with irradiated cells or purified caCD28 murine Ig2a fusion protein (35). Spleens were harvested and hybridomas were generated using accepted methods (36). Hybridomas were screened for caCD28 reactivity by ELISA. Positive clones were further tested by flow cytometry for binding to canine T-cells. Unlabeled and peroxidase-conjugated secondary antibodies were purchased from Southern Biotech (Birmingham, AL). PE or FITC-conjugated secondary antibodies were purchased from Jackson ImmunoResearch (West Grove, PA). Hybridomas were purified from serum-free culture medium over HiTrap Protein A column.
Functional assays
The agonistic/antagonistic activity of anti-caCD28 antibodies was tested in MLR (37). Details of this procedure are available online.
Expansion of regulatory T-cells was done in 24-well TC plates coated with anti-CD3 mAb and anti-caCD28 (5B8) under conditions described above. Sorted CD3+CD25+ T-cells that had been alloantigen-activated in 4 day MLR were placed in the anti-CD3 and anti-caCD28 mAb coated plates at 2×105 per well in CDM. Stimulation index (SI) was determined by counts per minute (CPM) of 3H thymidine incorporation of allogeneic responder and irradiated stimulator cells (R+S*)/autologous responder cells ±irradiation (R+R*). Incorporation of 3H Thymidine was determined as described above.
Cells were stained for CD3 and CD4 expression using canine-specific mAb, CA17.6F9 and 13.1E4, respectively, provided by Dr. Peter Moore, University of California, Davis. CD25 expression was assessed with FITC-conjugated clone ACT-1 (Dako, Carpentaria, CA). Antibody specificity was determined by electrophoresis of a lysate of canine lymphocytes on a NativePAGE Novex Bis-Tris Gel system (Invitrogen) and transferring the proteins to a PVDF membrane. CD28 antigen was detected with anti-caCD28 (1C6) followed by HRP-labeled goat anti-mouse IgG1 and stained with TMB (Vector Labs).
CTL assay was a modification of the method described by Deeg et al. (20). Details of this assay are available online. RT-PCR was performed by extracting mRNA from cultured cells and transcribed into cDNA using uMACs One-Step cDNA kits (Miltenyi Biotec, Auburn,CA). Absolute quantitative PCR was used to measure granzyme B expression using primers and Taqman probes designed by Primer Express (Applied Biosystems, Foster City, CA) based on a previously reported sequence (1). Absolute copy numbers were calculated based on granzyme B standard curves and normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (G3PDH) by methods previously reported (38).
Acknowledgments
Grant Support: The authors are grateful for research funding from the National Institutes of Health, Bethesda, MD grants P01CA078902, P30CA015704 and U19AI067770. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health nor its subsidiary Institutes and Centers.
Support was also provided by awards from the Joseph Steiner Krebsstifung, Bern, Switzerland and Lupin Foundation, Metairie, Louisiana (both to R.S.).
The authors thank Liz Wayner, Ph.D. in the Fred Hutchinson Cancer Research Center, Antibody Development Program for assistance in generating anti-CD28 mAB and the Biologics Facility for producing and purifying anti-CD28 mAbs. They also thank Helen Crawford and Bonnie Larson for help with manuscript preparation.
ABBREVIATIONS
- caCD28
canine CD28
- caCD28caIgG1
canine CD28 and canine IgG1 fusion protein
- caCD28murineIgG2a
canine CD28 and murine IgG2a fusion protein
- CTL
cytotoxic T lymphocyte
- CTLA-4
cytotoxic T lymphocyte antigen 4
- DLA
dog leukocyte antigen
- G3PDH
glyceraldehyde-3-phosphate dehydrogenase
Footnotes
AUTHORSHIP:
- Scott S. Graves drafted and revised the manuscript, co-supervised the study, analyzed and interpreted data. No conflicts of interest.
- Diane M. Stone produced fusion proteins, tested anti-CD28 clones. No conflicts of interest.
- Carol Loretz performed MLR. No conflicts of interest.
- Laura J. Peterson performed cell binding studies. No conflicts of interest.
- Marina Lesnikova performed regulatory T-cell experiments. No conflicts of interest.
- Billanna Hwang conducted PCR experiments. No conflicts of interest.
- George E. Georges analyzed and interpreted data. No conflicts of interest.
- Richard Nash reviewed the manuscript. No conflicts of interest.
- Rainer Storb designed and supervised the study, revised manuscript. No conflicts of interest.
References
- 1.Linsley PS, Ledbetter JA. The role of the CD28 receptor during T cell responses to antigen (Review) Annu Rev Immunol. 1993;11:191–212. doi: 10.1146/annurev.iy.11.040193.001203. [DOI] [PubMed] [Google Scholar]
- 2.Daikh D, Wofsy D, Imboden JB. The CD28-B7 costimulatory pathway and its role in autoimmune disease (Review) J Leukoc Biol. 1997;62:156–162. doi: 10.1002/jlb.62.2.156. [DOI] [PubMed] [Google Scholar]
- 3.Buch MH, Vital EM, Emery P. Abatacept in the treatment of rheumatoid arthritis (Review) Arthritis Research and Therapy. 2008;10 (Suppl 1):S5. doi: 10.1186/ar2416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Turka LA, Linsley PS, Lin H, et al. T-cell activation by the CD28 ligand B7 is required for cardiac allograft rejection in vivo. Proc Natl Acad Sci USA. 1992;89:11102–11105. doi: 10.1073/pnas.89.22.11102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearson TC, Alexander DZ, Winn KJ, Linsley PS, Lowry RP, Larsen CP. Transplantation tolerance induced by CTLA4-Ig. Transplantation. 1994;57:1701–1706. [PubMed] [Google Scholar]
- 6.Kean LS, Adams AB, Strobert E, et al. Induction of chimerism in rhesus macaques through stem cell transplant and costimulation blockade-based immunosuppression. Am J Transplant. 2007;7:320–335. doi: 10.1111/j.1600-6143.2006.01622.x. [DOI] [PubMed] [Google Scholar]
- 7.Storb R, Yu C, Zaucha JM, et al. Stable mixed hematopoietic chimerism in dogs given donor antigen, CTLA4Ig, and 100 cGy total body irradiation before and pharmacologic immunosuppression after marrow transplant. Blood. 1999;94:2523–2529. [PubMed] [Google Scholar]
- 8.Yu XZ, Bidwell SJ, Martin PJ, Anasetti C. CD28-specific antibody prevents graft-versus-host disease in mice. J Immunol. 2000;164:4564–4568. doi: 10.4049/jimmunol.164.9.4564. [DOI] [PubMed] [Google Scholar]
- 9.Snanoudj R, de Preneuf H, Creput C, et al. Costimulation blockade and its possible future use in clinical transplantation (Review) Transpl Int. 2006;19:693–704. doi: 10.1111/j.1432-2277.2006.00332.x. [DOI] [PubMed] [Google Scholar]
- 10.Vincenti F. Costimulation blockade in autoimmunity and transplantation (Review) J Allergy Clin Immunol. 2008;121:299–306. doi: 10.1016/j.jaci.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Rothstein DM, Sayegh MH. T-cell costimulatory pathways in allograft rejection and tolerance (Review) [erratum appears in Immunol Rev. 2004 Feb;197:243] Immunol Rev. 2003;196:85–108. doi: 10.1046/j.1600-065x.2003.00088.x. [DOI] [PubMed] [Google Scholar]
- 12.Storb R, Epstein RB, Graham TC, Thomas ED. Methotrexate regimens for control of graft-versus-host disease in dogs with allogeneic marrow grafts. Transplantation. 1970;9:240–246. doi: 10.1097/00007890-197003000-00007. [DOI] [PubMed] [Google Scholar]
- 13.Deeg HJ, Storb R, Weiden PL, et al. Cyclosporin A and methotrexate in canine marrow transplantation: engraftment, graft-versus-host disease, and induction of tolerance. Transplantation. 1982;34:30–35. doi: 10.1097/00007890-198207000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Storb R, Raff RF, Appelbaum FR, et al. FK506 and methotrexate prevent graft-versus-host disease in dogs given 9. 2 Gy total body irradiation and marrow grafts from unrelated DLA-nonidentical donors. Transplantation. 1993;56:800–807. doi: 10.1097/00007890-199310000-00005. [DOI] [PubMed] [Google Scholar]
- 15.Yu C, Seidel K, Nash RA, et al. Synergism between mycophenolate mofetil and cyclosporine in preventing graft-versus-host disease among lethally irradiated dogs given DLA-nonidentical unrelated marrow grafts. Blood. 1998;91:2581–2587. [PubMed] [Google Scholar]
- 16.Storb R, Yu C, Wagner JL, et al. Stable mixed hematopoietic chimerism in DLA-identical littermate dogs given sublethal total body irradiation before and pharmacological immunosuppression after marrow transplantation. Blood. 1997;89:3048–3054. [PubMed] [Google Scholar]
- 17.Abrams VK, Hwang B, Lesnikova M, et al. A novel monoclonal antibody specific for canine CD25 (P4A10): selection and evaluation of canine Tregs. Vet Immunol Immunopathol. 2010;135:257–265. doi: 10.1016/j.vetimm.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baroja ML, Lorre K, van Vaeck F, Ceuppens JL. The anti-T cell monoclonal antibody 9. 3 (anti-CD28) provides a helper signal and bypasses the need for accessory cells in T cell activation with immobilized anti-CD3 and mitogens. Cell Immunol. 1989;120:205–217. doi: 10.1016/0008-8749(89)90188-3. [DOI] [PubMed] [Google Scholar]
- 19.Oberg HH, Wesch D, Lenke J, Kabelitz D. An optimized method for the functional analysis of human regulatory T cells. Scand J Immunol. 2006;64:353–360. doi: 10.1111/j.1365-3083.2006.01825.x. [DOI] [PubMed] [Google Scholar]
- 20.Deeg HJ, Storb R, Raff RF, Weiden PL, DeRose S, Thomas ED. Marrow grafts between phenotypically DLA-identical and haploidentical unrelated dogs: Additional antigens controlling engraftment are not detected by cell-mediated lympholysis. Transplantation. 1982;33:17–21. doi: 10.1097/00007890-198201000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Bluestone JA, Liu W, Yabu JM, et al. The effect of costimulatory and interleukin 2 receptor blockade on regulatory T cells in renal transplantation. Am J Transplant. 2008;8:2086–2096. doi: 10.1111/j.1600-6143.2008.02377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuemmerle-Deschner JB, Benseler S. Abatacept in difficult-to-treat juvenile idiopathic arthritis. Biologics. 2008;2:865–874. doi: 10.2147/btt.s3355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abrams JR, Kelley SL, Hayes E, et al. Blockade of T lymphocyte costimulation with cytotoxic T lymphocyte-associated antigen 4-immunoglobulin (CTLA4Ig) reverses the cellular pathology of psoriatic plaques, including the activation of keratinocytes, dendritic cells, and endothelial cells. J Exp Med. 2000;192:681–694. doi: 10.1084/jem.192.5.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kirk AD, Tadaki DK, Celniker A, et al. Induction therapy with monoclonal antibodies specific for CD80 and CD86 delays the onset of acute renal allograft rejection in non-human primates. Transplantation. 2001;72:377–384. doi: 10.1097/00007890-200108150-00005. [DOI] [PubMed] [Google Scholar]
- 25.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanhove B, Laflamme G, Coulon F, et al. Selective blockade of CD28 and not CTLA-4 with a single-chain Fv-alpha1-antitrypsin fusion antibody. Blood. 2003;102:564–570. doi: 10.1182/blood-2002-08-2480. [DOI] [PubMed] [Google Scholar]
- 27.Shiao SL, McNiff JM, Masunaga T, Tamura K, Kubo K, Pober JS. Immunomodulatory properties of FK734, a humanized anti-CD28 monoclonal antibody with agonistic and antagonistic activities. Transplantation. 2007;83:304–313. doi: 10.1097/01.tp.0000251426.46312.d5. [DOI] [PubMed] [Google Scholar]
- 28.Guillonneau C, Seveno C, Dugast AS, et al. Anti-CD28 antibodies modify regulatory mechanisms and reinforce tolerance in CD40Ig-treated heart allograft recipients. J Immunol. 2007;179:8164–8171. doi: 10.4049/jimmunol.179.12.8164. [DOI] [PubMed] [Google Scholar]
- 29.Graves SS, Stone D, Loretz C, et al. Establishment of long-term tolerance to SRBC in dogs by recombinant canine CTLA4-Ig. Transplantation. 2009;88:317–322. doi: 10.1097/TP.0b013e3181ae3285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Podesta EJ, Solano AR, Attar R, Sanchez ML, Vedia L. Receptor aggregation induced by antilutropin receptor antibody and biological response in rat testis Leydig cells. PNAS. 1983;80:3986–3990. doi: 10.1073/pnas.80.13.3986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gross JA, Callas E, Allison JP. Identification and distribution of the costimulatory receptor CD28 in the mouse. J Immunol. 1992;149:380–388. [PubMed] [Google Scholar]
- 32.Munn DH, Sharma MD, Mellor AL. Ligation of B7-1/B7-2 by human CD4+ T cells triggers indoleamine 2,3-dioxygenase activity in dendritic cells. J Immunol. 2004;172:4100–4110. doi: 10.4049/jimmunol.172.7.4100. [DOI] [PubMed] [Google Scholar]
- 33.Wagner JL, Burnett RC, DeRose SA, Francisco LV, Storb R, Ostrander EA. Histocompatibility testing of dog families with highly polymorphic microsatellite markers. Transplantation. 1996;62:876–877. doi: 10.1097/00007890-199609270-00032. [DOI] [PubMed] [Google Scholar]
- 34.Wagner JL, Works JD, Storb R. DLA-DRB1 and DLA-DQB1 histocompatibility typing by PCR-SSCP and sequencing (Brief Communication) Tissue Antigens. 1998;52:397–401. doi: 10.1111/j.1399-0039.1998.tb03063.x. [DOI] [PubMed] [Google Scholar]
- 35.Taggart RT, Samloff IM. Stable antibody-producing murine hybridomas. Science. 1983;219:1228–1230. doi: 10.1126/science.6402815. [DOI] [PubMed] [Google Scholar]
- 36.Köhler G, Milstein C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature. 1975;256:495–497. doi: 10.1038/256495a0. [DOI] [PubMed] [Google Scholar]
- 37.Lee WS, Suzuki Y, Graves SS, et al. Canine bone marrow derived mesenchymal stromal cells suppress allo-reactive lymphocyte prolifearion in vitro but fail to enhance engraftment in canine bone marrow transplantation. Biol Blood Marrow Transplant. :9999. doi: 10.1016/j.bbmt.2010.04.016. prepublished online May 10, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nash RA, Yunusov M, Abrams K, et al. Immunomodulatory effects of mixed hematopoietic chimerism: immune tolerance in canine model of lung transplantation. Am J Transplant. 2009;9:1037–1047. doi: 10.1111/j.1600-6143.2009.02619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]