Abstract
Background
There is controversy regarding the definition and characteristics of mild cognitive impairment in Parkinson’s disease.
Objective
The Movement Disorders Society commissioned a Task Force to critically evaluate the literature and determine the frequency and characteristics of Parkinson’s disease-mild cognitive impairment and its association with dementia.
Methods
Comprehensive PubMed literature review using systematic inclusion and exclusion criteria.
Results
A mean of 26.7% (range, 18.9–38.2%) of non-demented Parkinson’s disease patients have mild cognitive impairment. The frequency of Parkinson’s disease mild cognitive impairment increases with age, disease duration, and disease severity. Impairments occur in a range of cognitive domains, but single domain impairment is more common than multiple domain impairment, and within single domain impairment, non-amnestic is more common than amnestic impairment. A high proportion of patients with Parkinson’s disease-mild cognitive impairment progress to dementia in a relatively short period of time.
Conclusions
The primary conclusions of the Task Force are that: (1) Parkinson’s disease-mild cognitive impairment is common; (2) there is significant heterogeneity within Parkinson’s disease-mild cognitive impairment in the number and types of cognitive domain impairments; (3) Parkinson’s disease-mild cognitive impairment appears to place patients at risk of progressing to dementia; and (4) formal diagnostic criteria for Parkinson’s disease-mild cognitive impairment are needed.
Keywords: mild cognitive impairment, Parkinson’s disease, systematic review
INTRODUCTION
Cognitive impairment is common in Parkinson’s disease (PD), with long-term longitudinal studies reporting that most PD patients develop dementia (PDD).1–3 The impact of PDD is substantial, with major consequences for functioning,4, 5, 6 nursing home admission,7 psychiatric morbidity,8 caregiver burden 9, 10 and mortality.11, 12
Mild cognitive impairment in PD (PD-MCI), defined as a cognitive decline that is not normal for age but with essentially normal functional activities, also appears to be common, even at the time of PD diagnosis and prior to initiation of dopaminergic therapy.13 Although the term MCI applied to PD is not without controversy,14, 15 it is more frequently used and more widely accepted than alternative terms. Identifying PD-MCI is important clinically, as these patients appear to be at increased risk for developing PDD.16 The biological validity of PD-MCI is supported by preliminary structural17 and functional18, 19 neuroimaging, electroencephalography,20, 21 genetic,22, 23 cerebrospinal fluid,24–26 and autopsy studies27 showing an association between a range neuropathophysiological variables and either cognitive impairment or cognitive decline in non-demented PD patients. From a scientific standpoint, studying PD-MCI offers insight into the neural substrate of the earliest stage of cognitive decline in PD that may lead to early intervention and may guide drug development focused on preventing or delaying the onset of PDD.
In spite of what is known about PD-MCI, the heterogeneity of cognitive deficits from the initial stages of the disease and the relative scarcity of longitudinal studies have made it difficult to definitively determine the following: (1) whether there are different and reproducible subtypes of PD-MCI; (b) what proportion of PD-MCI patients progress to PDD; (3) whether the term MCI can be defined and operationalized in PD to determine those patients at imminent risk of PDD; and (4) whether this risk differs on the basis of MCI subtype.
Given the critical importance of having uniform criteria for PD-MCI both for the identification and management of PD patients and for future therapeutic trials, the Movement Disorders Society (MDS) commissioned a Task Force to: (1) critically evaluate the literature; (2) more accurately determine the frequency and characteristics of PD-MCI and its conversion rate to PDD; and (3) propose formal diagnostic criteria for PD-MCI. The present paper deals with the first two of these issues since they are necessary to address prior to proposing new criteria. Criteria and guidelines for the definition and ascertainment of MCI in PD based on the present “state of the art” will be addressed in a future manuscript.
MATERIALS AND METHODS
Literature Search and Selection of Articles
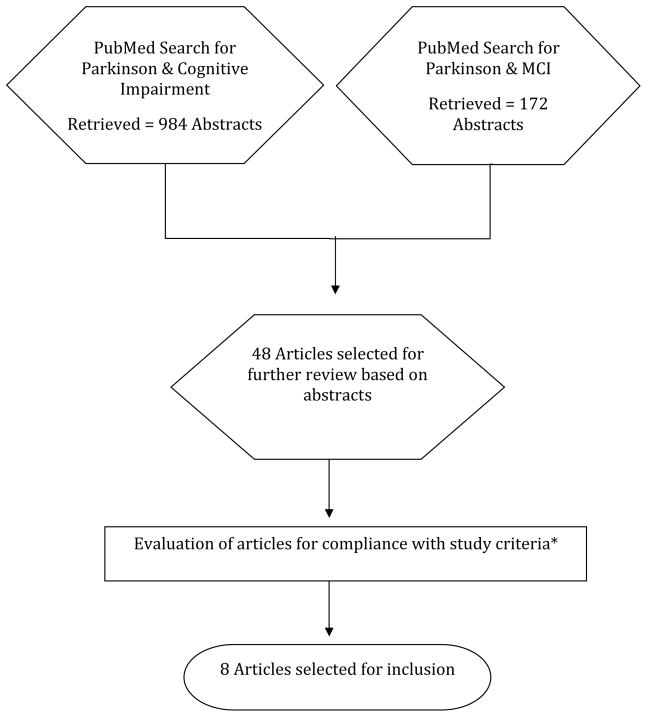
A comprehensive review of the literature through September 1, 2010 was conducted through Medline (PubMed) using combined free search terms that included “Parkinson,” “cognitive impairment,” and “mild cognitive impairment.” The search was limited to empirical English-language articles. The search retrieved 984 articles using the key terms “Parkinson and cognitive impairment” and 172 articles using “Parkinson and mild cognitive impairment,” with most of the latter articles already retrieved in the first search.
Abstracts (or papers when abstracts lacked information on inclusion and exclusion criteria) were further scrutinized to include only those reports that fit study inclusion criteria: (1) a minimum of 100 non-demented PD patients in cross-sectional studies, or 50 patients in prospective studies; (2) presence of quantitative neuropsychological information covering at least three of five cognitive domains: memory, executive, attention/working memory, visuospatial, and language; and (3) comparison of PD to a local control group or use of normative values. Exclusion criteria were: (1) lack of definition of impaired cognition or dementia, review articles, guidelines, meta-analyses, clinical therapeutic trials (unless the trial was negative), and (2) cognitive studies in demented or surgically-treated PD patients and in other neurological diseases, and articles focused on depression, REM sleep behavior disorder, olfactory dysfunction, impulse control disorders or psychosis without relevant cognitive data. Articles with abstracts that did not disclose all inclusion/exclusion criteria explicitly were included for further review.
Forty-eight articles met study inclusion criteria based on the review. The 48 articles were reviewed by five pairs of Task Force members (approximately 10 articles per pair) who independently extracted key data from the identified articles.1, 3, 12, 13, 16, 28–70 The most common reasons for exclusion were lack of definition of impaired cognition or failure to explicitly exclude patients with dementia, 1, 3, 29, 30, 33, 36, 42, 44–46, 48, 49, 51, 55–57, 59, 62, 64, 66, 67 sample size not meeting inclusion criteria 31, 32, 35, 37, 52, 58, 60, 71, 72, or evaluation of less than 3 domains of cognition. 3, 12, 61, 63, 65, 69
Data Extraction and Quality Assessment
The Task Force members rated the manuscripts using a structured form that included: (1) type of study (random, door-to-door; multiple sources; hospital based); (2) number of PD patients and percentage of patients with MCI, (3) demographics; (4) diagnostic criteria for PD, MCI, and PDD; (5) neuropsychological tests utilized; (6) number of cognitive domains tested; and (7) the presence of a normal control group or use of tests with normative values. For our final analysis we selected articles that had clearly defined cognitive and PD diagnostic criteria, studied at least three cognitive domains with standard versions of published neuropsychological tests, either included a control group matched by age and education evaluated with the same protocol or utilized test normative values to define MCI, and did not include the same study population as another study (except for prospective studies).
RESULTS
A total of 8 papers met all the inclusion/exclusion criteria. The studies included a total of 776 PD patients from 6 cross-sectional studies and 198 from 2 prospective studies (Table 1) and varied widely regarding design, population, and criteria and methods for defining MCI and dementia.
Table 1.
Demographics of studies that met systematic review study inclusion criteria.
| Cross Sectional Studies | Sample size (N) | Age (years) | Education (years) | Disease Duration (years) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Authors | Total | PD-NC | PD-MCI | PD-NC | PD-MCI | PD-NC | PD-MCI | PD-NC | PD-MCI |
| Aarsland et al., 200928 | 196 | 159 (81.1%) | 37 (18.9%) | 67.0±9.4* | 70.2±7.6* | 10.9±3.2* | 11.2±3.7* | 2.3±1.7* | 2.4±2.1* |
| Foltynie et al., 200434 | 142 (T=159) | 92 (64.8%) | 50 (35.2%) | 66.5** | 73.7** | NA | NA | 3.1 | 2.2 |
| Hoops et al., 200940 | 115 (T=132) | 92 (80.0%) | 23 (20.0%) | 63.9±9.7 | 68.1±9.2π | 16.5±3.1 π | 16.2±3.1 | 5.5±4.7 | 8.2±5.9 π |
| Mamikonyan et al., 200947 | 106 | 75 (70.7%) | 31 (29.2%) | 64.6±10.3#* | 15.6±3.0#* | 6.5±5.8#* | |||
| Muslimovic et al., 200513 | 115 | 88 (76.5%) | 27 (23.5%) | 64.9 ±10.4* | 70.3±8.1* | 11.6±2.3 | 11.7±2.7 | 1.5±0.9 | 1.66±0.9 |
| Pai et al., 200153 | 102 | 63 (61.8%) | 39 (38.2%) | 68.0# | 6.2# | NA | NA | ||
| Total | 776 | 569 (73.3%) | 207 (26.7%) | ||||||
|
| |||||||||
| Longitudinal Studies | |||||||||
| Janvin et al., 2006 16 | 72b,♮ | 34 (47.2%) b | 38 (52.8%) b | 68.1±9.2* | 73.2* | 10.2±3.9* | 8.8* | 12.2±4.6* | 11.2* |
| Williams-Gray et al., 200768 Δ | 126b | 41 (32.5%)f, ♮♮ | 72 (57.1%) f | NA | NA | NA | NA | NA | NA |
| Total | 198 | 75 (38%) | 110 (55.6%) | ||||||
N, Participants in study with Parkinson’s disease (PD) who met inclusion criteria; PD-NC, PD without MCI; PD-MCI, PD with MCI; NA: T=total sample size includes demented patients; data not available;
data includes PD-MCI and PDD;
data of the whole sample, not specified by groups;
59 (82%) completed followed up of whom 24 developed dementia;
at baseline;
longitudinal study of Foltynie et al.34 sample;
at follow-up;
13 (10.3%) developed PDD.
Significant between-group (PD-NC vs PD-MCI) differences:
p<0.05,
p<0.001
Cross-sectional Studies
Table 2 shows the neuropsychological tests and domains explored, MCI criteria used, and MCI subtypes found in each study. Overall, the studies demonstrate that PD-MCI is common in PD patients without dementia (mean cross-sectional prevalence =26.7%, range =18.9–38.2%), its frequency increases with age and duration of PD, single domain impairment is more common than multiple domain impairment, and in the case of single domain impairment, non-amnestic MCI is more common than amnestic MCI.
Table 2.
Assessments, PD-MCI criteria and cognitive profiles.
| Author, Year | Domains (Tests) Assessed | Depression Scale | MCI Definition | Subjective Impairment Required | Cognitive Profile Found |
|---|---|---|---|---|---|
| Aarsland et al., 200928 |
Verbal Memory (California Verbal Learning Test II, total immediate recall, short-delay and long-delay free recall) Visuospatial Ability (VOSP Silhouette & Cube Subtests Attention, Executive Function (Animal Names, MMSE Serial 7s, Stroop Color-Word Test) |
MADRS | ≥ 1.5 SD below the z- score in at least one of three domains | No | Total MCI: 18.9% 86.5% SD 62.2% non-amnestic MCI-SD 24.3% amnestic MCI-SD 13.5% MD 2.7% non-amnestic MCI-MD 10.8% amnestic MCI-MD |
| Foltynie et al., 200434 |
Executive function (Animal fluency and FAS fluency; modified Tower of London task) Spatial Memory (CANTAB Spatial Recognition Subtest) Pattern Recognition Memory (CANTAB Pattern Recognition Memory Subtest) |
No Depression Scale | ≥ 1 SD below the normative data in at least one test | No | Total MCI: 35.2% 58.0% SD 34.0% fronto-striatal deficits-SD 24% temporal lobe deficits-SD 42.0% fronto-striatal & temporal deficits-MD |
| Hoops et al., 200940 |
Memory (Hopkins Verbal Learning Test, at least one of two measures impaired) Executive Function (Tower of London) Attention (Backward digit span) Visuospatial (Cube Copying) |
GDS-15 | ≥ 1.5 SD below the normative data on tests on at least two cognitive domains112 | Yes and preserved Independent Activities of Daily Living | Total MCI: 20.0% MCI Subtypes not described |
| Mamikonyan et al., 200947 |
Memory (Hopkins Verbal Learning Test-Revised, abnormal if impaired in two of three components: immediate free recall total score, retention percentage and recognition) Executive Function (Stroop Color-Word Test; the Tower of London; semantic verbal fluency) (abnormal if impairment in 2 of the 3 tests) Attention (Digit Span) |
Inventory of Depression Symptomatology | ≥ 1.5 SD below the normative data in at least one domain | No | Total MCI: 29.2% 61.3% SD 29% attention-SD 19.3% amnestic-SD 13% executive--SD 38.7% MD |
| Muslimovic et al., 200513 |
Executive function (Trails A and B, Stroop Color-Word Test, Tower of London- Drexel test, modified Wisconsin Card Sorting Test) WAIS-R digit symbol test, Control Word Association Test, category fluency, WAIS-III similarities) Memory (Auditory Verbal Learning Test, Rivermead Behavioral Memory Test, Logical Memory Test, Wechsler Memory Scale III, Visual Association Test) Attention (WAIS-R digits forward and backward) Language (Boston Naming Test) Visuospatial and constructive function (Judgment of Line Orientation, Groningen Intelligence Test-spatial subtest, Clock Drawing Test) |
Hospital Anxiety and Depression Scale | ≥2 SD below the normative data on ≥3 neuropsychological tests | No | Total Impaired: 23.5% MCI subtypes not described (reported overall % of impaired tests) |
| Pai et al., 2001 53 |
Memory (Cognitive Ability Screening Instrument, CASI subtest) Executive Function (CASI subtest) Attention (CASI subtest) Language (CASI subtest) Visuospatial (CASI subtest) |
No Depression Scale | ≥ 1.5 SD below the mean in at least one subtest | No | Total MCI: 38.2% MCI subtypes not described (reported overall % of impaired tests) |
|
| |||||
| Prospective Author, Year | Domains (Tests) Assessed | Depression Scale | MCI Criteria | Subjective Impairment Required | Cognitive Profile Found |
|
| |||||
| Janvin et al., 2006 16 |
Short Term Visual Memory (Benton Visual Retention Test) Visuospatial Abilities (Judgment of Line Orientation test) Executive Function (Stroop Color-Word Test) |
BDI | ≥ 1.5 SD below control group in at least one test | Total MCI: 52.8% b 60.5% SD b 44.7% non-amnestic-SD b (26.3% executive-SD; 18.4% visuospatial-SD) 15.8% amnestic-SD b 39.5%-MD b |
|
|
| |||||
| Author, Year | Domains (Tests) Assessed | Depression Scale | MCI Definition | Subjective Impairment Required | Cognitive Profile Found |
|
| |||||
| Williams-Gray et al, 200768 |
Executive (Animal Fluency & FAS Fluency, Tower of London) Pattern Recognition Memory (CANTAB Pattern Recognition Memory Subtest) Visuospatial (copy of the MMSE pentagon) Spatial Memory (CANTAB Spatial Recognition Memory Subtests) |
BDI | ≥ 1 SD below the control group on at least one test | No | Total MCI: 57.1% MCIf fronto-striatal-SD (“predominant”) |
BDI: Beck Depression Inventory; MADRS: Montgomery and Asberg Depression Rating Scale; GDS: Geriatric Depression Scale; VOSP: Visual Object and Spatial Perception Battery; MMSE: Mini Mental Status Exam; WAIS-R: Weschler Adult Intelligence Scale-Revised; WMS: Wechsler Memory Scale; WCST: Wisconsin Card Sorting Test; COWAT: Controlled Oral Word Association Test; CANTAB: Cambridge Neuropsychological Test Automated Battery; FAS Fluency: A test of fluency for words starting with F, A, & S;
Subset of the PANDA: Parkinson Neuropsychometric Dementia Assessment. Subjects with MCI were categorized as “Single Domain Only” (cognitive impairment in a single domain, SD), “Multiple Domain” (cognitive impairment in two or more cognitive domains, MD), and “Overall Impairment” (sum of patients who had either single-or multiple- domain cognitive impairment);
at baseline;
at follow-up.
Aarsland et al.28 evaluated a community-based incident cohort of 196 non-demented, drug-naïve PD patients73 and 201 healthy controls (HC). The neuropsychological battery measured global cognition with the Mini-Mental State Examination (MMSE) and evaluated three cognitive domains with additional neuropsychological testing. The authors calculated z-scores for the PD patients based on control data. They categorized MCI cases into one of four subtypes (Table 2). Compared with HC, PD patients were impaired on all neuropsychological tests, and 18.9% met criteria for MCI (Table 1), with a risk ratio (RR) of 2.1 compared with controls. Older PD patients (≥65 years) had a higher RR (2.6) of having MCI than younger cases (<65 years; RR=1.5).
Foltynie et al.34 assessed cognitive function in an incident cohort of 159 PD patients.74 Thirteen (8%) of the patients74 scored <24 on MMSE and were considered to have dementia, even though dementia at diagnosis was an exclusion criterion. Of the remaining 146 cases, 142 were included in the study, and more than one-third were considered cognitively impaired, defined as scoring ≥1 SD below the normative mean in a pattern recognition task (temporal lobe impairment), the Tower of London task (fronto-striatal impairment), or on both tests (global impairment). The term “MCI” was not used in this study.
Hoops et al.40 compared the discriminant validity of the Montreal Cognitive Assessment (MoCA) and MMSE at diagnosing MCI in PD, using a neuropsychological test battery as a gold standard. Among a convenience sample of 132 PD cases at a movement disorders clinic,73 12.9% had PDD and 20% had MCI based on a test battery that evaluated four cognitive domains.
Mamikonyan et al.,47 explored the cognitive performance of 106 PD patients (a convenience sample at a movement disorders clinic), who had intact global cognition based on ther age- and education-adjusted MMSE score. Thirty-one patients (29.2%) were classified as having MCI, defined as scoring ≥1.5 SD below the normative data in at least one of the three domains assessed. The authors concluded that cognitive impairment is frequent in PD patients with normal cognition based on MMSE score, and that memory deficits are common at the stage of PD-MCI.
Muslimovic et al.,13 studied 115 PD patients without “global cognitive deterioration” defined as MMSE score <24 and 70 elderly HC in one of the most detailed investigations of cognition in non-demented PD patients. A comprehensive battery of 28 neuropsychological tests was administered, and PD patients were significantly impaired on 20 of these. Twenty-seven patients (23.5%) were cognitively impaired, defined as scoring ≥2 SD below the normative mean on ≥3 cognitive tests, compared with 4% of HC. The domains most commonly impaired were attention/executive function, psychomotor speed and memory.
Pai et al.,53 studied cognitive abilities in 102 non-demented PD patients recruited from a behavioral clinic. They used the Chinese version of the Cognitive Ability Screening Instrument (CASI), a comprehensive screening instrument with normative data that includes subscores for five cognitive domains. They found that 38.2% fulfilled MCI criteria, defined as ≥1.5 SD below the mean in at least one subtest.
Longitudinal Studies
One of the studies reviewed68 included the longitudinal assessment of patients on whom baseline data was reported in the cross-sectional studies selected above. 34 Janvin et al.16 conducted a longitudinal study of cognitive function in a community-based sample of 145 PD cases. Cases and controls were assessed at baseline and at 4 years. Patients with MMSE score <25 at baseline were considered demented and excluded. Of the 145 original cases, 72 PD non-demented cases were studied and compared to 38 HC. After four years, for those who completed follow-up, dementia developed in 18/29 (62.0%) of patients with PD-MCI at baseline, compared with 6/30 (20.0%) of PD patients with normal cognition at baseline. This sample consisted of established PD patients, explaining the higher overall conversion rate to PDD over the same time period used in the CamPaign study (see below). The proportion converting to PDD was numerically higher among those with single domain non-amnestic MCI (69%) compared to amnestic MCI (40%), and in a logistic regression analysis this MCI subtype at baseline predicted PDD development. The authors concluded that PD-MCI is a risk factor for developing PDD.
Williams-Gray et al.,68 reassessed 126 non-demented PD patients between 3–5 years after their baseline evaluation, and found that 10% developed PDD over this time period. In this first wave of follow-up, older age, non-tremor dominant phenotype, higher Unified Parkinson’s Disease Rating Scale motor scores, and below average performance on tests of semantic fluency, pentagon copying, spatial recognition memory, and Tower of London were associated with a more rapid rate of decline on the MMSE and progression to PDD. The authors concluded that posterior cortical cognitive deficits increased the risk for the development of PDD, whereas fronto-striatal cognitive deficits did not.
DISCUSSION
The results of our systematic literature search and review are that: (1) an average of 26.7% (range 18.9–38.2%) of non-demented PD patients have PD-MCI; (2) cognitive deficits can be detected in some patients even at the time of PD diagnosis; (3) the frequency of MCI increases with age, and duration and severity of PD; (4) impairments can occur in a range of cognitive domains; (5) non-amnestic single domain MCI is more common than amnestic single domain MCI; and (6) PD-MCI appears to be a risk factor for the development of PDD.
Prevalence and Correlates
The majority of PD patients will develop dementia.3, 22 The point prevalence of PDD is approximately 30%, 75 and the cumulative prevalence is at least 75% for PD patients surviving more than 10 years.2, 3 As MCI precedes PDD, the cumulative prevalence of PD-MCI must be at least as high as that of PDD. Consequently, our finding that approximately 27% of PD patients meet criteria for PD-MCI at any given time is not surprising. Our results are similar to those recently reported by Aarsland et al.,76 who used a common methodology for the definition of MCI on pooled data of over 1,000 non-demented PD patients from multiple centers, and found that 25.8% (23.5–28.2%) had MCI.
The lowest proportion of patients with MCI was found in a study of patients at early PD stages,13 and the highest proportion in studies including cases with more advanced disease severity and duration.16, 68, 76 The variation in MCI frequency present in the articles reviewed also reflects differences in methodology (e.g., study settings and populations, recruitment methods, MCI diagnostic criteria, number of cognitive domains assessed, number of tests used for each domain, and how impairment on a test was defined). The fact that cognitive deficits in PD are detectible in some patients even at the time of clinical diagnosis highlights complexities in differentiating PD from dementia with Lewy bodies (DLB).
The correlates of PD-MCI have not been studied extensively. Of the studies included in this review, there was evidence that increasing age, 34, 47 more severe PD, 34, 47 late onset of disease, 13 and lower educational levels 53 were associated with PD-MCI.
Profile of Cognitive Impairment
Although the cognitive deficits in PD have traditionally been classified as being “subcortical” in nature77 (i.e., relatively greater impairments in executive abilities, information processing speed, and working memory compared with episodic memory storage and language), our review showed that a range of cognitive domains are impaired in PD patients without dementia. Other research in PD has demonstrated deficits in executive (i.e., impaired planning and working memory),78 visuospatial,79 attentional,80 memory,81 and even language abilities.82–85
In all of the studies reviewed herein, single domain MCI was more common than multiple domain MCI, and non-amnestic MCI was more common than amnestic MCI in patients with impairment in a single domain. Debate exists about the extent to which the mild memory and language deficits in PD are secondary to executive and working memory problems. Additionally, as prospective studies using formal definitions for MCI subtypes are almost non-existent, the usefulness and predictive value of this PD-MCI classification structure is currently hypothetical.
Epidemiology of Progression from PD-MCI to Dementia
The few longitudinal studies of non-demented PD patients find that 20–60% develop PDD over a period of 2–5 years,22, 46, 76, 86–89 and even newly diagnosed PD patients on average experience significant decline in a range of cognitive domains over a several-year period.49,90 The finding that PD-MCI patients are at higher risk for developing dementia is consistent with a clinico-pathologic study reporting cognitive impairment in the earliest stages of clinically manifested PD, possibly related to changes in brainstem monoaminergic nuclei or early involvement of forebrain cholinergic nuclei.91
Preliminary research suggests that the majority of PD-MCI cases convert to PDD over a several-year period.16, 22, 68 The two longitudinal studies included in this review differed in terms of design and methodology. Williams-Gray et al.68 followed an incident cohort of non-demented PD patients in two waves, but did not specifically examine progression from a state of “mild impairment” to PDD. The focus of this research to date has been to determine which demographic (increasing age), neuropsychological (semantic verbal fluency and visuospatial deficits), and genetic factors (MAPT H1/H1 tau genotype) predicted conversion to PDD at the second wave of follow-up22. The other study used a cross-sectional sample of survivors from a prevalence sample.16 In this study, Janvin et al. found that 62% of PD-MCI patients converted to PDD over a 4-year period, compared with 20% of PD patients with normal cognition. The frequency of conversion to PDD over a 4-year period was: multiple domain MCI (63%), single, non-memory domain MCI (69%), single domain, amnestic MCI (40%), and normal cognition (20%).
Regarding other risk factors, in one of the longitudinal studies reviewed increasing severity of depression was associated with an increased risk of conversion from PD-MCI to PDD.16 In other research not covered in this manuscript, demographic and clinical correlates or risk factors for PDD development have included older age, male sex, lower educational level, longer duration of PD, and greater motor impairment.22, 64, 83, 86, 90, 92, 93
Clinical Impact
PD-MCI appears to be a clinically significant syndrome, as even mild cognitive deficits or self-rated cognitive deficits in early PD are associated with functional impairment5, 94 and worse quality of life (QoL).95, 96 Thus, identification and intervention at the earliest stage of PD-MCI is a crucial unmet need for the overall care of PD patients. The high frequency of MCI in PD highlights the need for clinicians to routinely screen for cognitive impairment in PD, as the results, including their prognostic implications, may influence clinical decision-making. However, research in this area is preliminary, and additional studies are needed to validate measures that are sensitive to initial changes in independent activities of daily living (IADLs), QoL and interpersonal relationships that can occur at the stage of PD-MCI.
Complexities in the Assessment of Cognition in PD
Assessment of cognition in PD can be complicated by disease or medication-related effects, such as bradykinesia, fatigue, sleepiness, and mood disorders, which can adversely impact test results regardless of cognitive abilities. Specifically, motor slowing (i.e., bradykinesia) and resting or intentional tremor may lead to impaired performance on any timed test, while tremor can interfere with performance on any test requiring motor abilities (e.g., use of a pencil to complete a task). However, there has been very little research examining the impact of these factors on cognitive performance specifically.
Another issue is that the definition of MCI forces a dichotomization (present-absent) of a continuous variable (cognitive test performance), and debate continues regarding the appropriate cut-off score and number of tests used to define PD-MCI. Caviness et al.32 reported that 21% of subjects had PD-MCI if an abnormality on multiple tests within a domain was required, but this rose to 42% if an abnormality on only one test was required. There is concern that the commonly-used definitions of MCI may lack sensitivity to detect early cognitive decline (rather than impairment) in high functioning persons.97 Persons functioning at a high level (i.e., above average) premorbidly have to experience sizeable declines before scoring at least 1.5 SD below normative means. Consequently, a considerable proportion of such patients with cognitive decline would be classified as having “normal cognition.” While it can be argued that high premorbid functioning protects against MCI in much the same manner as it does against dementia, this argument ignores the fact that high functioning persons may be in more demanding work or social settings in which even small cognitive declines translate into subtle functional impairments. On the other hand, a key feature in diagnosing MCI pertains to a change in cognition. Thus, MCI is not just a value on a cognitive test relative to the mean; rather, it is critical that the person has experienced a change in cognition compared with baseline.
Additional unresolved issues are whether cognitive decline in PD is linear and whether different profiles of cognitive deficits may have a different evolution and prognosis. Previous studies have noted that time to PDD diagnosis is highly variable98 and that cognitive changes after relatively long-term follow-up are not too consistent.97 Although a distinct evolution of different cognitive domains cannot be inferred from a study using only the MMSE, the re-analysis of data from a long-term prevalence study identified a variation in the slope of decline, specifically a rapid cognitive deterioration after a relatively stable period.99 These data should be examined in light of neuroimaging18, 19 and clinical52, 68 data showing that the transition from MCI to dementia in PD is characterized by the addition of posterior cortical deficits upon frontal-subcortical ones.
Biomarkers
None of the reviewed papers examined biomarkers specifically as they pertain to PD-MCI. One of the incident cohorts included in this review underwent a second wave follow-up, 22 and in that research the tau MAPT H1/H1 genotype (but not COMT genotype) was found to be a risk factor for PDD, a finding recently confirmed.22 Other genetic polymorphisms (e.g., COMT polymorphisms and BDNF Val66Met genotype 100, 101) have been shown to be associated with impairments in specific cognitive domains or abilities in PD. Several CSF biomarkers for cognitive decline in PD have been proposed.102 Recent research suggests decreased CSF β-amyloid (Aβ) 1–42 is associated with the early stages of cognitive decline in PD.24, 26 This decrease might be due to specific Aβ plaque pathology, but it may be nonspecific, as Aβ 1–42 has been shown to be decreased in neurodegenerative disorders lacking distinct plaque pathology103–105 and in vivo plaque imaging (PET imaging with the Aβ-binding Pittsburgh Compound B) shows no correlation between the plaque load and cognition in PD. 106 Instead, the findings suggest a different mechanism of Aβ processing, perhaps due to synaptic α-synuclein pathology. 107, 108 Structural neuroimaging, including diffusion tensor imaging, have reported white matter abnormalities in non-demented PD patients.108 In another study17 that classified patients as PD normal cognition (PD-NC), PD-MCI, or PDD and used two different imaging analyses, PD-MCI patients compared with PD-NC either had reduced gray matter in the prefrontal cortex and temporal lobes,17 or were found to have similar regional brain volumes. Using FDG-PET, a PD-related cognitive pattern in non-demented PD patients has been reported, characterized by metabolic reductions in frontal and parietal association areas, and relative increases in the cerebellar vermis and dentate nuclei.109 The pattern predicted memory and visuospatial performance, and in a subsequent study single domain MCI patients had a PD-related cognitive pattern expression intermediate (but not statistically significantly different) between normal and multiple domain MCI patients.18 Clearly there is a need for the prospective and longitudinal assessment of accessible biomarkers (including CSF, blood, and neuroimaging) and neuropathological examination to further address this issue.
Conclusions and Future Directions
There has been limited research on the epidemiology and prognostic utility of PD-MCI as a clinical syndrome. Nonetheless, the studies selected for review here, and other studies of PD-MCI not meeting inclusion criteria, yield relatively consistent prevalence estimates of MCI and its subtypes. They also show that single domain MCI is more common than multiple domain MCI, and that non-amnestic, single domain MCI is more common than amnestic, single domain MCI.
The Task Force has used this critical review of the literature and consensus of experts to formulate PD-MCI diagnostic criteria that will be published separately. Once diagnostic criteria for PD-MCI are proposed, prospective studies enrolling subjects with a wide range of pre-morbid ability will be needed to examine the predictive value of both MCI overall and MCI subtypes. Intervention trials can target MCI as a clinical syndrome, perhaps stratifying MCI by subtype to determine if particular interventions (pharmacological vs. behavioral) have differential acute or long-term effects. Although it is not known if or how a diagnosis of PD-MCI should impact clinical management, at a minimum these patients should be carefully monitored for ongoing cognitive decline.
Figure. Search Results.
*Study criteria: see text
Acknowledgments
Irene Litvan is funded by 5R01AG024040-04.
Author Roles
1. Research project: A. Conception, B. Organization, C. Execution
2. Statistical Analysis: A. Design, B. Execution, C. Review and Critique
3. Manuscript: A. Writing of the first draft, B. Review and Critique
| Authors | Research Project | Statistical Analysis | Manuscript |
|---|---|---|---|
| Litvan I | A, B, C | B, C | A, B |
| Aarsland D | A, C | C | A, B |
| Adler C | A, C | C | A, B |
| Goldman J | A, C | C | A, B |
| Kulisevsky J | A, C | C | A, B |
| Mollenhauer B | A, C | C | A, B |
| Rodriguez-Oroz MC | A, C | C | A, B |
| Tröster AI | A, C | C | A, B |
| Weintraub D | A, C | C | A, B |
Financial disclosure related to research covered in this article: A statement that documents all funding sources and potential conflicts of interest from each author that relate to the research covered in the article submitted must be included on the title page, regardless of date. This material will be available online and may be printed at the discretion of the editors.
| Irene Litvan | |
|---|---|
| Stock Ownership in medically-related fields: None | Intellectual Property Rights: None |
| Consultancies: None | Expert Testimony: None |
| Advisory Boards: None | Employment: None |
| Partnerships: None | Contracts: None |
| Honoraria: None | Royalties: None |
| Grants: None | Other |
| Dag Aarsland | |
|---|---|
| Stock Ownership in medically-related fields: None | Intellectual Property Rights: None |
| Consultancies: None | Expert Testimony: None |
| Advisory Boards: None | Employment: None |
| Partnerships: None | Contracts: None |
| Honoraria: None | Royalties: None |
| Grants: None | Other |
| Charles Adler | |
|---|---|
| Stock Ownership in medically-related fields: None | Intellectual Property Rights: None |
| Consultancies: Ipsen, Merck Serono | Expert Testimony: None |
| Advisory Boards: Biogen Idec, Eli Lilly, Medtronic, Merz | Employment: None |
| Partnerships: None | Contracts: None |
| Honoraria: None | Royalties: None |
| Grants: Arizona Biomedical Research Commission, Michael J. Fox Foundation | Other |
| Jennifer G. Goldman | |
|---|---|
| Stock Ownership in medically-related fields None | Intellectual Property Rights None |
| Consultancies None | Expert Testimony None |
| Advisory Boards None | Employment None |
| Partnerships None | Contracts None |
| Honoraria None | Royalties None |
| Grants None | Other None |
| Jaime Kulisevsky | |
|---|---|
| Stock Ownership in medically-related fields: None | Intellectual Property Rights: None |
| Consultancies: None | Expert Testimony: None |
| Advisory Boards: None | Employment: None |
| Partnerships: None | Contracts: None |
| Honoraria: None | Royalties: None |
| Grants: None | Other |
| Brit Mollenhauer | |
|---|---|
| Stock Ownership in medically-related fields: None | Intellectual Property Rights: None |
| Consultancies: None | Expert Testimony: None |
| Advisory Boards: None | Employment: None |
| Partnerships: None | Contracts: None |
| Honoraria: None | Royalties: None |
| Grants: None | Other: None |
| Maria C. Rodriguez-Oroz | |
|---|---|
| Stock Ownership in medically-related fields: None | Intellectual Property Rights.: None |
| Consultancies: None | Expert Testimony None |
| Advisory Boards. None | Employment None |
| Partnerships. None | Contracts None |
| Honoraria None | Royalties None |
| Grants. None | Other None |
| Alexander I. Tröster | |
|---|---|
| Stock Ownership in medically-related fields None | Intellectual Property Rights: None |
| Consultancies Medtronic, Inc., St Jude, Boston Scientific | Expert Testimony: None |
| Advisory Boards Medtronic, Inc. St Jude | Employment: None |
| Partnerships None | Contracts: None |
| Honoraria Medtronic, Boehringer Ingelheim | Royalties: None |
| Grants National Parkinson Foundation, Glaxo Smith Kline, Medtronic, Inc. | Other |
| Daniel Weintraub | |
|---|---|
| Stock Ownership in medically-related fields | Intellectual Property Rights: None |
| Consultancies: None | Expert Testimony: None |
| Advisory Boards: Merck Serono, Novartis | Employment: None |
| Partnerships: None | Contracts: None |
| Honoraria: None | Royalties: None |
| Grants: None | Other: None |
Full financial disclosure for the previous 12 months: A statement that documents all funding sources, regardless of relationship to the current research in the article, from each author must be attached to the article at the end of the manuscript on the last page. This material will be printed or posted on the journal website at the Editors’ discretion.
| Irene Litvan | |
|---|---|
| Stock Ownership in medically-related fields:None | Intellectual Property Rights: None |
| Consultancies: None | Expert Testimony: None |
| Advisory Boards: General Electric | Employment: University of Louisville |
| Partnerships: None | Contracts: None |
| Honoraria: None | Royalties: Elsevier |
| Grants: 5R01AG024040-04, Parkinson Study Group, Allon Therapeutics, Noscira, National Parkinson Foundation, CurePSP, Signature Partnership (University of Louisville), Litvan Neurological Research Foundation | Other: Endowment/Donations Parkinson Support Center of Kentuckiana |
| Dag Aarsland | |
|---|---|
| Stock Ownership in medically-related fields: None | Intellectual Property Rights: none |
| Consultancies: None | Expert Testimony None |
| Advisory Boards: Diagenic | Employment: none |
| Partnerships None | Contracts: none |
| Honoraria Lundbeck, Novartis, Diagenis | Royalties: none |
| Grants Merck Seronon, Lundbeck | |
| Charles Adler | |
|---|---|
| Stock Ownership in medically-related fields | Intellectual Property Rights: None |
| Consultancies: Ipsen, Merck Serono | Expert Testimony: None |
| Advisory Boards Biogen Idec, Eli Lilly, Medtronic | Employment: None |
| Partnerships | Contracts: None |
| Honoraria | Royalties: None |
| Grants Arizona Biomedical Research Commission, Michael J. Fox Foundation | Other |
| Jennifer G. Goldman | |
|---|---|
| Stock Ownership in medically-related fields None | Intellectual Property Rights None |
| Consultancies None | Expert Testimony None |
| Advisory Boards None | Employment None |
| Partnerships None | Contracts None |
| Honoraria None | Royalties None |
| Grants: NIH/NINDS, Parkinson’s Disease | Other Merz, Boehringer- |
| Foundation | Ingelheim |
| Jaime Kulisevsky | |
|---|---|
| Stock Ownership in medically-related fields: None | Intellectual Property Rights: None |
| Consultancies: None | Expert Testimony: None |
| Advisory Boards: Merck-Serono | Employment: None |
| Partnerships: None | Contracts: None |
| Honoraria: Lundbeck, Boheringer | Royalties: None |
| Grants: Boheringer, Merck-Serono, CIBERNED | Other |
| Brit Mollenhauer | |
|---|---|
| Stock Ownership in medically-related fields:None | Intellectual Property Rights: None |
| Consultancies: None | Expert Testimony: None |
| Advisory Boards: Novartis | Employment: None |
| Partnerships: None | Contracts: None |
| Honoraria: GlaxoSmithKline, Orion Pharma | Royalties: None |
| Grants: Michael J. Fox Foundation for Parkinson’s Research | Other Research support TEVA Pharma, Desitin, GE Healthcare, Boehringer-Ingelheim |
| Maria C Rodriguez-Oroz | |
|---|---|
| Stock Ownership in medically-related fields None | Intellectual Property Rights: None |
| Consultancies None | Expert Testimony: None |
| Advisory Boards UCB | Employment: None |
| Partnerships None | Contracts: None |
| Honoraria GlasoSmithKline, UCB, Lundbeck, Medtronic | Royalties: None |
| Grants. FIS, Spanish Government; Health Navarrian Government, | Other None |
| Alexander Tröster | |
|---|---|
| Stock Ownership in medically-related fields | Intellectual Property Rights: None |
| Consultancies Medtronic, St Jude | Expert Testimony: None |
| Advisory BoardsMedtronic, St Jude | Employment: None |
| Partnerships | Contracts: None |
| Honoraria Medtronic | Royalties: None |
| Grants | Intellectual Property Rights: None |
| Daniel Weintraub | |
|---|---|
| Stock Ownership in medically-related fields: None | Intellectual Property Rights: Assigned copyright of QUIP to University of Pennsylvania |
| Consultancies: None | Expert Testimony: None |
| Advisory Boards: GE, | Employment: None |
| Partnerships: None | Contracts: None |
| Honoraria: Boehringer Ingelheim, Sanofi Aventis, Johnson and Johnson, Solvay | Royalties: None |
| Grants: NIH, Fox Foundation | Other: None |
References
- 1.Buter TC, van den Hout A, Matthews FE, Larsen JP, Brayne C, Aarsland D. Dementia and survival in Parkinson disease: a 12-year population study. Neurology. 2008;70:1017–1022. doi: 10.1212/01.wnl.0000306632.43729.24. [DOI] [PubMed] [Google Scholar]
- 2.Hely MA, Reid WG, Adena MA, Halliday GM, Morris JG. The Sydney multicenter study of Parkinson’s disease: the inevitability of dementia at 20 years. Mov Disord. 2008;23:837–844. doi: 10.1002/mds.21956. [DOI] [PubMed] [Google Scholar]
- 3.Aarsland D, Andersen K, Larsen JP, Lolk A, Kragh-Sorensen P. Prevalence and characteristics of dementia in Parkinson disease: an 8-year prospective study. Arch Neurol. 2003;60:387–392. doi: 10.1001/archneur.60.3.387. [DOI] [PubMed] [Google Scholar]
- 4.Bronnick K, Ehrt U, Emre M, et al. Attentional deficits affect activities of daily living in dementia-associated with Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2006;77:1136–1142. doi: 10.1136/jnnp.2006.093146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rosenthal E, Brennan L, Xie S, et al. Association between cognition and function in patients with Parkinson disease with and without dementia. Mov Disord. 2010;25:1170–1176. doi: 10.1002/mds.23073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uc EY, Rizzo M, Anderson SW, Sparks JD, Rodnitzky RL, Dawson JD. Driving with distraction in Parkinson disease. Neurology. 2006;67:1774–1780. doi: 10.1212/01.wnl.0000245086.32787.61. [DOI] [PubMed] [Google Scholar]
- 7.Aarsland D, Larsen JP, Tandberg E, Laake K. Predictors of nursing home placement in Parkinson’s disease: a population-based, prospective study. J Am Geriatr Soc. 2000;48:938–942. doi: 10.1111/j.1532-5415.2000.tb06891.x. [DOI] [PubMed] [Google Scholar]
- 8.Aarsland D, Bronnick K, Ehrt U, et al. Neuropsychiatric symptoms in patients with Parkinson’s disease and dementia: frequency, profile and associated care giver stress. J Neurol Neurosurg Psychiatry. 2007;78:36–42. doi: 10.1136/jnnp.2005.083113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aarsland D, Larsen JP, Karlsen K, Lim NG, Tandberg E. Mental symptoms in Parkinson’s disease are important contributors to caregiver distress. Int J Geriatr Psychiatry. 1999;14:866–874. [PubMed] [Google Scholar]
- 10.Schrag A, Hovris A, Morley D, Quinn N, Jahanshahi M. Caregiver-burden in parkinson’s disease is closely associated with psychiatric symptoms, falls, and disability. Parkinsonism Relat Disord. 2006;12:35–41. doi: 10.1016/j.parkreldis.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 11.Levy G, Tang MX, Louis ED, et al. The association of incident dementia with mortality in PD. Neurology. 2002;59:1708–1713. doi: 10.1212/01.wnl.0000036610.36834.e0. [DOI] [PubMed] [Google Scholar]
- 12.Lo RY, Tanner CM, Albers KB, et al. Clinical features in early Parkinson disease and survival. Arch Neurol. 2009;66:1353–1358. doi: 10.1001/archneurol.2009.221. [DOI] [PubMed] [Google Scholar]
- 13.Muslimovic D, Post B, Speelman JD, Schmand B. Cognitive profile of patients with newly diagnosed Parkinson disease. Neurology. 2005;65:1239–1245. doi: 10.1212/01.wnl.0000180516.69442.95. [DOI] [PubMed] [Google Scholar]
- 14.Troster AI. Neuropsychological characteristics of dementia with Lewy bodies and Parkinson’s disease with dementia: differentiation, early detection, and implications for “mild cognitive impairment” and biomarkers. Neuropsychol Rev. 2008;18:103–119. doi: 10.1007/s11065-008-9055-0. [DOI] [PubMed] [Google Scholar]
- 15.Dubois B, Burn D, Goetz C, et al. Diagnostic procedures for Parkinson’s disease dementia: recommendations from the movement disorder society task force. Mov Disord. 2007;22:2314–2324. doi: 10.1002/mds.21844. [DOI] [PubMed] [Google Scholar]
- 16.Janvin CC, Larsen JP, Aarsland D, Hugdahl K. Subtypes of mild cognitive impairment in Parkinson’s disease: progression to dementia. Mov Disord. 2006;21:1343–1349. doi: 10.1002/mds.20974. [DOI] [PubMed] [Google Scholar]
- 17.Beyer MK, Janvin CC, Larsen JP, Aarsland D. A magnetic resonance imaging study of patients with Parkinson’s disease with mild cognitive impairment and dementia using voxel-based morphometry. J Neurol Neurosurg Psychiatry. 2007;78:254–259. doi: 10.1136/jnnp.2006.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang C, Mattis P, Perrine K, Brown N, Dhawan V, Eidelberg D. Metabolic abnormalities associated with mild cognitive impairment in Parkinson disease. Neurology. 2008;70:1470–1477. doi: 10.1212/01.wnl.0000304050.05332.9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hosokai Y, Nishio Y, Hirayama K, et al. Distinct patterns of regional cerebral glucose metabolism in Parkinson’s disease with and without mild cognitive impairment. Mov Disord. 2009;24:854–862. doi: 10.1002/mds.22444. [DOI] [PubMed] [Google Scholar]
- 20.Onofrij M, Gambi D, Malatesta G, Ferracci F, Fulgente T. Electrophysiological techniques in the assessment of aging brain: lacunar state and differential diagnosis. Eur Neurol. 1989;29 (Suppl 2):44–47. doi: 10.1159/000116468. [DOI] [PubMed] [Google Scholar]
- 21.Caviness JN, Hentz JG, Evidente VG, et al. Both early and late cognitive dysfunction affects the electroencephalogram in Parkinson’s disease. Parkinsonism Relat Disord. 2007;13:348–354. doi: 10.1016/j.parkreldis.2007.01.003. [DOI] [PubMed] [Google Scholar]
- 22.Williams-Gray CH, Evans JR, Goris A, et al. The distinct cognitive syndromes of Parkinson’s disease: 5 year follow-up of the CamPaIGN cohort. Brain. 2009;132:2958–2969. doi: 10.1093/brain/awp245. [DOI] [PubMed] [Google Scholar]
- 23.Seto-Salvia N, Clarimon J, Pagonabarraga J, et al. Dementia Risk in Parkinson Disease: Disentangling the Role of MAPT Haplotypes. Arch Neurol. 2011;68:359–364. doi: 10.1001/archneurol.2011.17. [DOI] [PubMed] [Google Scholar]
- 24.Alves G, Bronnick K, Aarsland D, et al. CSF amyloid-{beta} and tau proteins, and cognitive performance, in early and untreated Parkinson’s Disease: the Norwegian ParkWest study. J Neurol Neurosurg Psychiatry. 2010;81:1080–1086. doi: 10.1136/jnnp.2009.199950. [DOI] [PubMed] [Google Scholar]
- 25.Ballard C, Jones EL, Londos E, Minthon L, Francis P, Aarsland D. alpha-Synuclein antibodies recognize a protein present at lower levels in the CSF of patients with dementia with Lewy bodies. Int Psychogeriatr. 2010;22:321–327. doi: 10.1017/S1041610209991049. [DOI] [PubMed] [Google Scholar]
- 26.Siderowf A, Xie SX, Hurtig H, et al. CSF amyloid {beta} 1–42 predicts cognitive decline in Parkinson disease. Neurology. 2010;75(12):1055–1061. doi: 10.1212/WNL.0b013e3181f39a78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Adler CH, Caviness JN, Sabbagh MN, et al. Heterogeneous neuropathological findingsin Parkinson’s disease with mild cognitive impairment. Acta Neuropathol. 2010;120:827–828. doi: 10.1007/s00401-010-0744-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest study. Neurology. 2009;72:1121–1126. doi: 10.1212/01.wnl.0000338632.00552.cb. [DOI] [PubMed] [Google Scholar]
- 29.Athey RJ, Porter RW, Walker RW. Cognitive assessment of a representative community population with Parkinson’s disease (PD) using the Cambridge Cognitive Assessment-Revised (CAMCOG-R) Age Ageing. 2005;34:268–273. doi: 10.1093/ageing/afi098. [DOI] [PubMed] [Google Scholar]
- 30.Athey RJ, Walker RW. Demonstration of cognitive decline in Parkinson’s disease using the Cambridge Cognitive Assessment (Revised) (CAMCOG-R) Int J Geriatr Psychiatry. 2006;21:977–982. doi: 10.1002/gps.1595. [DOI] [PubMed] [Google Scholar]
- 31.Caparros-Lefebvre D, Pecheux N, Petit V, Duhamel A, Petit H. Which factors predict cognitive decline in Parkinson’s disease? J Neurol Neurosurg Psychiatry. 1995;58:51–55. doi: 10.1136/jnnp.58.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Caviness JN, Driver-Dunckley E, Connor DJ, et al. Defining mild cognitive impairment in Parkinson’s disease. Mov Disord. 2007;22:1272–1277. doi: 10.1002/mds.21453. [DOI] [PubMed] [Google Scholar]
- 33.Cooper CA, Mikos AE, Wood MF, et al. Does laterality of motor impairment tell us something about cognition in Parkinson disease? Parkinsonism Relat Disord. 2009;15:315–317. doi: 10.1016/j.parkreldis.2008.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Foltynie T, Brayne CE, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson’s patients in the UK. The CamPaIGN study. Brain. 2004;127:550–560. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- 35.Gill DJ, Freshman A, Blender JA, Ravina B. The Montreal cognitive assessment as a screening tool for cognitive impairment in Parkinson’s disease. Mov Disord. 2008;23:1043–1046. doi: 10.1002/mds.22017. [DOI] [PubMed] [Google Scholar]
- 36.Girotti F, Soliveri P, Carella F, et al. Dementia and cognitive impairment in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:1498–1502. doi: 10.1136/jnnp.51.12.1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goldman WP, Baty JD, Buckles VD, Sahrmann S, Morris JC. Cognitive and motor functioning in Parkinson disease: subjects with and without questionable dementia. Arch Neurol. 1998;55:674–680. doi: 10.1001/archneur.55.5.674. [DOI] [PubMed] [Google Scholar]
- 38.Hayashi R, Hanyu N, Tamaru F. Cognitive impairment in Parkinson’s disease: a 6year follow-up study. Parkinsonism Relat Disord. 1998;4:81–85. doi: 10.1016/s1353-8020(98)00018-2. [DOI] [PubMed] [Google Scholar]
- 39.Hobson P, Meara J. The detection of dementia and cognitive impairment in a community population of elderly people with Parkinson’s disease by use of the CAMCOG neuropsychological test. Age Ageing. 1999;28:39–43. doi: 10.1093/ageing/28.1.39. [DOI] [PubMed] [Google Scholar]
- 40.Hoops S, Nazem S, Siderowf AD, et al. Validity of the MoCA and MMSE in the detection of MCI and dementia in Parkinson disease. Neurology. 2009;73:1738–1745. doi: 10.1212/WNL.0b013e3181c34b47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Janvin C, Aarsland D, Larsen JP, Hugdahl K. Neuropsychological profile of patients with Parkinson’s disease without dementia. Dement Geriatr Cogn Disord. 2003;15:126–131. doi: 10.1159/000068483. [DOI] [PubMed] [Google Scholar]
- 42.Janvin CC, Aarsland D, Larsen JP. Cognitive predictors of dementia in Parkinson’s disease: a community-based, 4-year longitudinal study. J Geriatr Psychiatry Neurol. 2005;18:149–154. doi: 10.1177/0891988705277540. [DOI] [PubMed] [Google Scholar]
- 43.Kalbe E, Calabrese P, Kohn N, et al. Screening for cognitive deficits in Parkinson’s disease with the Parkinson neuropsychometric dementia assessment (PANDA) instrument. Parkinsonism Relat Disord. 2008;14:93–101. doi: 10.1016/j.parkreldis.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 44.Klepac N, Hajnsek S, Trkulja V. Cognitive performance in nondemented nonpsychotic Parkinson disease patients with or without a history of depression prior to the onset of motor symptoms. J Geriatr Psychiatry Neurol. 2010;23:15–26. doi: 10.1177/0891988709351831. [DOI] [PubMed] [Google Scholar]
- 45.Locascio JJ, Corkin S, Growdon JH. Relation between clinical characteristics of Parkinson’s disease and cognitive decline. J Clin Exp Neuropsychol. 2003;25:94–109. doi: 10.1076/jcen.25.1.94.13624. [DOI] [PubMed] [Google Scholar]
- 46.Mahieux F, Fenelon G, Flahault A, Manifacier MJ, Michelet D, Boller F. Neuropsychological prediction of dementia in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1998;64:178–183. doi: 10.1136/jnnp.64.2.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mamikonyan E, Moberg PJ, Siderowf A, et al. Mild cognitive impairment is common in Parkinson’s disease patients with normal Mini-Mental State Examination (MMSE) scores. Parkinsonism Relat Disord. 2009;15:226–231. doi: 10.1016/j.parkreldis.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mavandadi S, Nazem S, Ten Have TR, et al. Use of latent variable modeling to delineate psychiatric and cognitive profiles in Parkinson disease. Am J Geriatr Psychiatry. 2009;17:986–995. doi: 10.1097/JGP.0b013e3181b215ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muslimovic D, Post B, Speelman JD, De Haan RJ, Schmand B. Cognitive decline in Parkinson’s disease: a prospective longitudinal study. J Int Neuropsychol Soc. 2009;15:426–437. doi: 10.1017/S1355617709090614. [DOI] [PubMed] [Google Scholar]
- 50.Nazem S, Siderowf AD, Duda JE, et al. Montreal cognitive assessment performance in patients with Parkinson’s disease with “normal” global cognition according to mini-mental state examination score. J Am Geriatr Soc. 2009;57:304–308. doi: 10.1111/j.1532-5415.2008.02096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Oh JY, Kim YS, Choi BH, Sohn EH, Lee AY. Relationship between clinical phenotypes and cognitive impairment in Parkinson’s disease (PD) Arch Gerontol Geriatr. 2009;49:351–354. doi: 10.1016/j.archger.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 52.Pagonabarraga J, Kulisevsky J, Llebaria G, Garcia-Sanchez C, Pascual-Sedano B, Gironell A. Parkinson’s disease-cognitive rating scale: a new cognitive scale specific for Parkinson’s disease. Mov Disord. 2008;23:998–1005. doi: 10.1002/mds.22007. [DOI] [PubMed] [Google Scholar]
- 53.Pai MC, Chan SH. Education and cognitive decline in Parkinson’s disease: a study of 102patients. Acta Neurol Scand. 2001;103:243–247. [PubMed] [Google Scholar]
- 54.Palazzini E, Soliveri P, Filippini G, et al. Progression of motor and cognitive impairment in Parkinson’s disease. J Neurol. 1995;242:535–540. doi: 10.1007/BF00867426. [DOI] [PubMed] [Google Scholar]
- 55.Pedersen KF, Larsen JP, Alves G, Aarsland D. Prevalence and clinical correlates of apathy in Parkinson’s disease: a community-based study. Parkinsonism Relat Disord. 2009;15:295–299. doi: 10.1016/j.parkreldis.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 56.Riedel O, Klotsche J, Spottke A, et al. Cognitive impairment in 873 patients with idiopathic Parkinson’s disease. Results from the German Study on Epidemiology of Parkinson’s Disease with Dementia (GEPAD) J Neurol. 2008;255:255–264. doi: 10.1007/s00415-008-0720-2. [DOI] [PubMed] [Google Scholar]
- 57.Riepe MW, Kassubek J, Tracik F, Ebersbach G. Screening for cognitive impairment in Parkinson’s disease--which marker relates to disease severity? J Neural Transm. 2006;113:1463–1468. doi: 10.1007/s00702-006-0433-6. [DOI] [PubMed] [Google Scholar]
- 58.Santangelo G, Trojano L, Vitale C, et al. A neuropsychological longitudinal study in Parkinson’s patients with and without hallucinations. Mov Disord. 2007;22:2418–2425. doi: 10.1002/mds.21746. [DOI] [PubMed] [Google Scholar]
- 59.Santangelo G, Vitale C, Trojano L, Verde F, Grossi D, Barone P. Cognitive dysfunctions and pathological gambling in patients with Parkinson’s disease. Mov Disord. 2009;24:899–905. doi: 10.1002/mds.22472. [DOI] [PubMed] [Google Scholar]
- 60.Starkstein SE, Bolduc PL, Mayberg HS, Preziosi TJ, Robinson RG. Cognitive impairments and depression in Parkinson’s disease: a follow up study. J Neurol Neurosurg Psychiatry. 1990;53:597–602. doi: 10.1136/jnnp.53.7.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Taylor JP, Rowan EN, Lett D, O’Brien JT, McKeith IG, Burn DJ. Poor attentional function predicts cognitive decline in patients with non-demented Parkinson’s disease independent of motor phenotype. J Neurol Neurosurg Psychiatry. 2008;79:1318–1323. doi: 10.1136/jnnp.2008.147629. [DOI] [PubMed] [Google Scholar]
- 62.Uc EY, McDermott MP, Marder KS, et al. Incidence of and risk factors for cognitive impairment in an early Parkinson disease clinical trial cohort. Neurology. 2009;73:1469–1477. doi: 10.1212/WNL.0b013e3181bf992f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.van Rooden SM, Visser M, Verbaan D, Marinus J, van Hilten JJ. Patterns of motor and non-motor features in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2009;80:846–850. doi: 10.1136/jnnp.2008.166629. [DOI] [PubMed] [Google Scholar]
- 64.Verbaan D, Marinus J, Visser M, et al. Cognitive impairment in Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2007;78:1182–1187. doi: 10.1136/jnnp.2006.112367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Verbaan D, van Rooden SM, Visser M, Marinus J, Emre M, van Hilten JJ. Psychotic and compulsive symptoms in Parkinson’s disease. Mov Disord. 2009;24:738–744. doi: 10.1002/mds.22453. [DOI] [PubMed] [Google Scholar]
- 66.Verleden S, Vingerhoets G, Santens P. Heterogeneity of cognitive dysfunction in Parkinson’s disease: a cohort study. Eur Neurol. 2007;58:34–40. doi: 10.1159/000102164. [DOI] [PubMed] [Google Scholar]
- 67.Vingerhoets G, Verleden S, Santens P, Miatton M, De Reuck J. Predictors of cognitive impairment in advanced Parkinson’s disease. J Neurol Neurosurg Psychiatry. 2003;74:793–796. doi: 10.1136/jnnp.74.6.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Williams-Gray CH, Foltynie T, Brayne CE, Robbins TW, Barker RA. Evolution of cognitive dysfunction in an incident Parkinson’s disease cohort. Brain. 2007;130:1787–1798. doi: 10.1093/brain/awm111. [DOI] [PubMed] [Google Scholar]
- 69.Zadikoff C, Fox SH, Tang-Wai DF, et al. A comparison of the mini mental state exam to the Montreal cognitive assessment in identifying cognitive deficits in Parkinson’s disease. Mov Disord. 2008;23:297–299. doi: 10.1002/mds.21837. [DOI] [PubMed] [Google Scholar]
- 70.Zakzanis KK, Freedman M. A neuropsychological comparison of demented and nondemented patients with Parkinson’s disease. Appl Neuropsychol. 1999;6:129–146. doi: 10.1207/s15324826an0603_1. [DOI] [PubMed] [Google Scholar]
- 71.Hayashi A, Nagaoka M, Yamada K, Ichitani Y, Miake Y, Okado N. Maternal stress induces synaptic loss and developmental disabilities of offspring. Int J Dev Neurosci. 1998;16:209–216. doi: 10.1016/s0736-5748(98)00028-8. [DOI] [PubMed] [Google Scholar]
- 72.Elgh E, Domellof M, Linder J, Edstrom M, Stenlund H, Forsgren L. Cognitive function in early Parkinson’s disease: a population-based study. Eur J Neurol. 2009;16:1278–1284. doi: 10.1111/j.1468-1331.2009.02707.x. [DOI] [PubMed] [Google Scholar]
- 73.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56:33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 74.Gibb WR, Lees AJ. The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry. 1988;51:745–752. doi: 10.1136/jnnp.51.6.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Aarsland D, Zaccai J, Brayne C. A systematic review of prevalence studies of dementia in Parkinson’s disease. Mov Disord. 2005;20:1255–1263. doi: 10.1002/mds.20527. [DOI] [PubMed] [Google Scholar]
- 76.Aarsland D, Bronnick K, Williams-Gray C, et al. Mild cognitive impairment in Parkinson disease: a multicenter pooled analysis. Neurology. 2010;75:1062–1069. doi: 10.1212/WNL.0b013e3181f39d0e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Pillon B, Deweer B, Agid Y, Dubois B. Explicit memory in Alzheimer’s, Huntington’s, and Parkinson’s diseases. Arch Neurol. 1993;50:374–379. doi: 10.1001/archneur.1993.00540040036010. [DOI] [PubMed] [Google Scholar]
- 78.Siegert RJ, Weatherall M, Taylor KD, Abernethy DA. A meta-analysis of performance on simple span and more complex working memory tasks in Parkinson’s disease. Neuropsychology. 2008;22:450–461. doi: 10.1037/0894-4105.22.4.450. [DOI] [PubMed] [Google Scholar]
- 79.Cronin-Golomb A, Braun AE. Visuospatial dysfunction and problem solving in Parkinson’s disease. Neuropsychology. 1997;11:44–52. doi: 10.1037//0894-4105.11.1.44. [DOI] [PubMed] [Google Scholar]
- 80.Dujardin K, Degreef JF, Rogelet P, Defebvre L, Destee A. Impairment of the supervisory attentional system in early untreated patients with Parkinson’s disease. J Neurol. 1999;246:783–788. doi: 10.1007/s004150050455. [DOI] [PubMed] [Google Scholar]
- 81.Weintraub D, Moberg PJ, Culbertson WC, Duda JE, Stern MB. Evidence for impaired encoding and retrieval memory profiles in Parkinson disease. Cogn Behav Neurol. 2004;17:195–200. [PubMed] [Google Scholar]
- 82.Dubois B, Pillon B. Cognitive deficits in Parkinson’s disease. J Neurol. 1997;244:2–8. doi: 10.1007/pl00007725. [DOI] [PubMed] [Google Scholar]
- 83.Green J, McDonald WM, Vitek JL, et al. Cognitive impairments in advanced PD without dementia. Neurology. 2002;59:1320–1324. doi: 10.1212/01.wnl.0000031426.21683.e2. [DOI] [PubMed] [Google Scholar]
- 84.Levin BE, Katzen HL. Early cognitive changes and nondementing behavioral abnormalities in Parkinson’s disease. Adv Neurol. 1995;65:85–95. [PubMed] [Google Scholar]
- 85.Lewis SJ, Cools R, Robbins TW, Dove A, Barker RA, Owen AM. Using executive heterogeneity to explore the nature of working memory deficits in Parkinson’s disease. Neuropsychologia. 2003;41:645–654. doi: 10.1016/s0028-3932(02)00257-9. [DOI] [PubMed] [Google Scholar]
- 86.Aarsland D, Andersen K, Larsen JP, Lolk A, Nielsen H, Kragh-Sorensen P. Risk of dementia in Parkinson’s disease: a community-based, prospective study. Neurology. 2001;56:730–736. doi: 10.1212/wnl.56.6.730. [DOI] [PubMed] [Google Scholar]
- 87.Levy G, Schupf N, Tang MX, et al. Combined effect of age and severity on the risk of dementia in Parkinson’s disease. Ann Neurol. 2002;51:722–729. doi: 10.1002/ana.10219. [DOI] [PubMed] [Google Scholar]
- 88.Marder K, Tang MX, Cote L, Stern Y, Mayeux R. The frequency and associated risk factors for dementia in patients with Parkinson’s disease. Arch Neurol. 1995;52:695–701. doi: 10.1001/archneur.1995.00540310069018. [DOI] [PubMed] [Google Scholar]
- 89.Stern Y, Marder K, Tang MX, Mayeux R. Antecedent clinical features associated with dementia in Parkinson’s disease. Neurology. 1993;43:1690–1692. doi: 10.1212/wnl.43.9.1690. [DOI] [PubMed] [Google Scholar]
- 90.Kandiah N, Narasimhalu K, Lau PN, Seah SH, Au WL, Tan LC. Cognitive decline in early Parkinson’s disease. Mov Disord. 2009;24:605–608. doi: 10.1002/mds.22384. [DOI] [PubMed] [Google Scholar]
- 91.Braak H, Rub U, Del Tredici K. Cognitive decline correlates with neuropathological stage in Parkinson’s disease. J Neurol Sci. 2006;248:255–258. doi: 10.1016/j.jns.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 92.Aarsland D, Tandberg E, Larsen JP, Cummings JL. Frequency of dementia in Parkinson disease. Arch Neurol. 1996;53:538–542. doi: 10.1001/archneur.1996.00550060082020. [DOI] [PubMed] [Google Scholar]
- 93.Tomer R, Levin BE, Weiner WJ. Side of onset of motor symptoms influences cognition in Parkinson’s disease. Ann Neurol. 1993;34:579–584. doi: 10.1002/ana.410340412. [DOI] [PubMed] [Google Scholar]
- 94.Martin RC, Okonkwo OC, Hill J, et al. Medical decision-making capacity in cognitively impaired Parkinson’s disease patients without dementia. Mov Disord. 2008;23:1867–1874. doi: 10.1002/mds.22170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Klepac N, Trkulja V, Relja M, Babic T. Is quality of life in non-demented Parkinson’s disease patients related to cognitive performance? A clinic-based cross-sectional study. Eur J Neurol. 2008;15:128–133. doi: 10.1111/j.1468-1331.2007.02011.x. [DOI] [PubMed] [Google Scholar]
- 96.Marras C, McDermott MP, Rochon PA, Tanner CM, Naglie G, Lang AE. Predictors of deterioration in health-related quality of life in Parkinson’s disease: results from the DATATOP trial. Mov Disord. 2008;23:653–659. doi: 10.1002/mds.21853. quiz 776. [DOI] [PubMed] [Google Scholar]
- 97.Troster AI, Woods SP, Morgan EE. Assessing cognitive change in Parkinson’s disease: development of practice effect-corrected reliable change indices. Arch Clin Neuropsychol. 2007;22:711–718. doi: 10.1016/j.acn.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 98.Aarsland D, Kvaloy JT, Andersen K, et al. The effect of age of onset of PD on risk of dementia. J Neurol. 2007;254:38–45. doi: 10.1007/s00415-006-0234-8. [DOI] [PubMed] [Google Scholar]
- 99.Aarsland D, Muniz G, Matthews F. Nonlinear decline of mini-mental state examination in Parkinson’s disease. Mov Disord. 2010 doi: 10.1002/mds.23416. [DOI] [PubMed] [Google Scholar]
- 100.Foltynie T, Lewis SG, Goldberg TE, et al. The BDNF Val66Met polymorphism has a gender specific influence on planning ability in Parkinson’s disease. J Neurol. 2005;252:833–838. doi: 10.1007/s00415-005-0756-5. [DOI] [PubMed] [Google Scholar]
- 101.Guerini FR, Beghi E, Riboldazzi G, et al. BDNF Val66Met polymorphism is associated with cognitive impairment in Italian patients with Parkinson’s disease. Eur J Neurol. 2009;16:1240–1245. doi: 10.1111/j.1468-1331.2009.02706.x. [DOI] [PubMed] [Google Scholar]
- 102.Mollenhauer B, Trenkwalder C. Neurochemical biomarkers in the differential diagnosis of movement disorders. Mov Disord. 2009;24:1411–1426. doi: 10.1002/mds.22510. [DOI] [PubMed] [Google Scholar]
- 103.Otto M, Esselmann H, Schulz-Shaeffer W, et al. Decreased beta-amyloid1-42 in cerebrospinal fluid of patients with Creutzfeldt-Jakob disease. Neurology. 2000;54:1099–1102. doi: 10.1212/wnl.54.5.1099. [DOI] [PubMed] [Google Scholar]
- 104.Holmberg B, Johnels B, Blennow K, Rosengren L. Cerebrospinal fluid Abeta42 is reduced in multiple system atrophy but normal in Parkinson’s disease and progressive supranuclear palsy. Mov Disord. 2003;18:186–190. doi: 10.1002/mds.10321. [DOI] [PubMed] [Google Scholar]
- 105.Noguchi M, Yoshita M, Matsumoto Y, Ono K, Iwasa K, Yamada M. Decreased beta-amyloid peptide42 in cerebrospinal fluid of patients with progressive supranuclear palsy and corticobasal degeneration. J Neurol Sci. 2005;237:61–65. doi: 10.1016/j.jns.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 106.Gomperts SN, Rentz DM, Moran E, et al. Imaging amyloid deposition in Lewy body diseases. Neurology. 2008;71:903–910. doi: 10.1212/01.wnl.0000326146.60732.d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kramer ML, Schulz-Schaeffer WJ. Presynaptic alpha-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci. 2007;27:1405–1410. doi: 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schulz-Schaeffer WJ. The synaptic pathology of alpha-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol. 2010;120:131–143. doi: 10.1007/s00401-010-0711-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang C, Mattis P, Tang C, Perrine K, Carbon M, Eidelberg D. Metabolic brain networks associated with cognitive function in Parkinson’s disease. Neuroimage. 2007;34:714–723. doi: 10.1016/j.neuroimage.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]