Abstract
The programmed, stepwise acquisition of immunocompetence that marks the development of the fetal immune response proceeds during a period when both T cell receptor and immunoglobulin (Ig) repertoires exhibit reduced junctional diversity due to physiologic terminal deoxynucleotidyl transferase (TdT) insufficiency. To test the effect of N addition on humoral responses, we transplanted bone marrow from TdT-deficient (TdT−/−) and wild-type (TdT+/+) BALB/c mice into recombination activation gene 1-deficient BALB/c hosts. Mice transplanted with TdT−/− cells exhibited diminished humoral responses to the T-independent antigens α-1-dextran and (2,4,6-trinitrophenyl) hapten conjugated to AminoEthylCarboxymethyl-FICOLL, to the T-dependent antigens NP19CGG and hen egg lysozyme, and to Enterobacter cloacae, a commensal bacteria that can become an opportunistic pathogen in immature and immunocompromised hosts. An exception to this pattern of reduction was the T-independent anti-phosphorylcholine response to Streptococcus pneumoniae, which is normally dominated by the N-deficient T15 idiotype. Most of the humoral immune responses in the recipients of TdT−/− bone marrow were impaired, yet population of the blood with B and T cells occurred more rapidly. To further test the effect of N-deficiency on B cell and T cell population growth, transplanted TdT-sufficient and -deficient BALB/c IgMa and congenic TdT-sufficient CB17 IgMb bone marrow were placed in competition. TdT−/− cells demonstrated an advantage in populating the bone marrow, the spleen, and the peritoneal cavity. TdT deficiency, which characterizes fetal lymphocytes, thus appears to facilitate filling both central and peripheral lymphoid compartments, but at the cost of altered responses to a broad set of antigens.
Electronic supplementary material
The online version of this article (doi:10.1007/s00251-011-0543-7) contains supplementary material, which is available to authorized users.
Keywords: Terminal deoxynucleotidyl transferase, B cell development, Immune responses, Mice
Introduction
The ability to respond to specific antigens develops in a sequential, stepwise fashion during ontogeny (Sherwin and Rowlands 1974). The resultant delay in achieving adult immunocompetence is a major contributor to the high risk of morbidity and mortality from infection observed in pre-term and term infants (Cooper 1987; Silverstein 1977; Zinkernagel 2001). This developmental program underlies many vaccination schedules and limits the use of vaccines to protect the very young from a variety of infectious organisms (Stein 1992).
A number of different mechanisms have been invoked to explain the inefficiency of fetal responses to antigen. These include, but are not limited to, immunologic naiveté (Bucy et al. 1990), suboptimal cytokine cell signaling (Chalmers et al. 1998; Cohen et al. 1999), the programmed appearance of lymphocyte subpopulations (e.g., B-1 cells (Herzenberg 2000), splenic marginal zone B cells (Martin and Kearney 2000), and epithelial T cells (Aono et al. 2000; George and Cooper 1990), and restrictions in the diversity of the T cell and B cell antigen receptor repertoires [TCR and immunoglobulin (Ig)] (Schelonka et al. 1998; Schroeder et al. 1998; Schroeder et al. 2001; Yague et al. 1988; Zemlin et al. 2002). This last mechanism is of particular interest because the ability of an individual lymphocyte to recognize and respond to its cognate antigen is generally accepted to reflect the recognition properties of its antigen receptor (Nossal 2003; Rajewsky 1996; Tonegawa 1983).
TdT expression is carefully regulated during ontogeny (Thai et al. 2002). In the mouse fetus, where TdT is not expressed, Ig and TCR V domains contain few, if any, N nucleotides (Feeney 1990; Feeney 1992). Because these restrictions in junctional diversity proceed in parallel with fetal restrictions in immune competence, it has been proposed that the paucity of N addition could be causally related to the suboptimal immune responses that mark the neonate. To test this hypothesis, gene targeting has been used to produce mice that lack TdT (TdT−/−) (Gilfillan et al. 1993; Komori et al. 1993). While TdT−/− mice produce V(D)J junctions with few or no N nucleotides, they have been shown to mount antigen-specific humoral responses (Gilfillan et al. 1995). However, it should be noted that up to the time of birth lymphocyte development in TdT−/− mice should be entirely normal, since the absence of TdT at this stage is physiologic (Nadel et al. 1995). Thus, TdT-deficient mice would be predicted to contain a full complement of TCRs and Igs normally generated by the progeny of fetally derived B cells and T cells. Hence, TdT−/− mice should contain the same progeny of early B cell and T cell progenitors that develop in TdT+/+ mice, including the B-1 population that produces the majority of circulating natural IgM (Benedict et al. 2000).
We have recently shown that the patterns of VDJ usage in early pre-B cell progenitors from the bone marrow of genetically TdT-deficient mice match that of wild-type TdT-sufficient bone marrow and thus differ from those observed in the B cell progenitors from physiologically TdT insufficient perinatal liver. In contrast, the CDR-H3 repertoire expressed by mature bone marrow TdT-deficient B cells is a close, although not identical, homologue of the CDR-H3 repertoire expressed by IgM+IgD+ B cells in the TdT insufficient perinatal liver (Schelonka et al. 2010).
These observations suggested to us that transplantation of bone marrow from wild-type TdT-sufficient (TdT+/+) or -deficient (TdT−/−) mice into congenic recombinase activating gene 1 deficient (RAG1−/−) recipients would enable us to test the role of N addition on humoral immune responses in the absence of the physiologically N nucleotide insufficient B cells that represent the progeny of fetal stem cells. Furthermore, transplantation into an adult RAG1-deficient host would ensure that the accessory cells and immune system components would also be ontogenetically matched.
We show here that although the TdT−/− CD19+ B cells appeared to populate the bone marrow and peripheral lymphoid tissues more rapidly than their TdT+/+ counterparts, the magnitude or kinetics of the responses to both T-dependent and T-independent antigens was consistently diminished when compared to TdT+/+ controls.
Methods
Mice
TdT+/+ BALB/c mice, C.B17 mice congenic for the C57BL/6 H chain locus (IgMb), and RAG1−/− BALB/cJ mice obtained from Jackson Laboratories (Bar Harbor, ME) were used to establish breeding colonies in our animal care facility. TdT−/− mice (Gilfillan et al. 1993) on a C57BL/6 background (the kind gift of Dr. Diane Mathis) were backcrossed for eight to ten generations onto the BALB/c background. All of these mice were maintained in a specific pathogen-free barrier facility in Microisolator® (Lab Products, Inc., Maywood, NJ) caging. All experiments and animal procedures conformed to protocols approved by the University of Alabama Institutional Animal Care and Use Committee.
Host conditioning and transplantation of hematopoetic precursor cells
Except where indicated, 4- to 6-week-old RAG1−/− mice were individually irradiated with 10 cGy/g with a 137Cs source (GammaCell, Toronto, Canada) 2 h prior to transplantation. Single-cell suspensions of bone marrow mononuclear cells were prepared from the marrow of the femurs and tibias of TdT+/+ and TdT−/− mice by flushing with HBSS and passage through a 70-μm nylon mesh. Cells were pooled and 107 mononuclear cells injected into irradiated RAG1−/− mice via the tail vein. These mice exhibited a 99% survival rate to completion of experiments (>6 weeks). Prophylactic antibiotics were not used during this engraftment phase.
For transplantation studies of comparative bone marrow development, 3 to 4 h after the irradiation, RAG1−/− mice were reconstituted with a mixture containing 2 × 106 TdT+/+ IgMb (C.B.17) bone marrow cells plus 2 × 106 TdT−/− or TdT+/+ IgMa (BALB/c N10) bone marrow cells. For the reconstitutions, BM was isolated from femora and tibiae of age matched TdT+/+ C.B.17 and BALB/c TdT−/− or TdT+/+ mice. RBC were lysed in ACK lysis buffer, the cells were mixed in 1:1 ratio, and 300 μl of the mix containing 4 × 106 cells total was injected i.v. into the tail vein of the irradiated recipients. The proportion of B cells of each isotype was assessed by flow cytometry 4 weeks post-reconstitution.
Flow cytometric analysis and cell counting
One hundred microliters of whole blood was diluted in 100 μl of 50 mM EDTA. Half of the anticoagulated blood was used to obtain an automated complete blood and differential cell count (Hemavet 850, CDC Technologies, Oxford, CT). The remaining blood was treated with RBC lysing solution (1 mM KHCO3, 0.15 M NH4Cl, and 0.1 mM Na2EDTA), washed with staining buffer (PBS with 2% FBS), and resuspended in a master-mix of staining buffer containing optimal concentrations of antibody reagents. Samples were analyzed on a FACSCalibur (Becton-Dickinson, San Jose, CA). Absolute cell counts for T and B cell populations were calculated by the total lymphocyte count, as determined by the automated cell counter, multiplied by the percentages of CD4+, CD8+, and CD19+ cells from total gated lymphocytes as revealed by FACS analysis.
For FACS analysis of B cell development in the bone marrow, single-cell suspensions of bone marrow from both femurs of individual mice were extracted and prepared as previously described (Hardy and Hayakawa 2001; Ivanov et al. 2005). For each analysis, 1 × 106 cells were stained and analyzed on a LSRII or FACSCalibur (Becton-Dickinson, San Jose, CA).
The following sets of monoclonal antibodies were used: For bone marrow fractions B-C, anti-CD19 APCCy7, anti-CD43 FITC, anti-IgM APC, and anti-BP-1 PE (BD PharMingen, San Diego CA). For bone marrow fractions D-F, anti-CD19 SPRD (Southern Biotech, Birmingham, AL) or anti-CD19 APCCy7, anti-CD43 FITC, anti-IgM APC, anti-IgD PE, anti-IgMa RS3.1, Alexa-488, and anti-IgMb MB86, Alexa-647 (BD PharMingen, San Diego CA). For the spleen, anti-CD21 PE (the kind gift of Dr. J.F. Kearney), anti-CD23 FITC, anti-IgMa RS3.1, Alexa-488, and anti-IgMb MB86, Alexa-647 (all from BD PharMingen, San Diego CA). For the peritoneal cavity, anti-CD5 PE, Mac1 FITC, anti-IgMa RS3.1, Alexa-488, anti-IgMb MB86, Alexa-647 (all from BD PharMingen, San Diego CA), and CD19 SPRD (Southern Biotech, Birmingham, AL)
Serology
To determine immunoglobulin levels in reconstituted mice, class-specific unlabeled and alkaline phosphatase (AP)-labeled antibodies were obtained from Southern Biotechnology Associates (SBA, Birmingham, AL). Mice were bled from the tail vein at weekly intervals prior to and after reconstitution.
To determine antigen-specific antibody levels for primary immunization, beginning 4 weeks following reconstitution, cohorts of mice were immunized and sequentially bled from the tail vein 7, 10, and 14 days later. All antigen challenges were performed on N8 BALB/c TdT−/− mice. Heat-inactivated Enterobacter cloacae (strain MK7) and Streptococcus pneumoniae (strain R36A; the kind gift of Dr. J.F. Kearney) were injected i.v. (108 CFUs/dose) into the tail vein. For the DEX response, mice were immunized i.v. with 100 μg of Dextran B-1355S in HBSS (the kind gift of Dr. J.F. Kearney). For the NP19-CGG response, mice were immunized i.p. with 10 μg of NP19-CGG (Biosearch Technologies, San Francisco, CA) precipitated in potassium aluminum sulfate (alum) in saline. (2,4,6-Trinitrophenyl) hapten conjugated to AminoEthylCarboxymethyl-FICOLL (TNP-FICOLL) was purchased from Biosearch Technologies (San Francisco, CA), and the anti-TNP monoclonal antibody 107.3 was obtained commercially (Becton-Dickinson Biosciences, Pharmingen, San Diego, CA). Mice were immunized i.p. with 250 μg hen egg lysozyme (HEL) emulsified in complete Freund’s adjuvant both purchased from Sigma (St. Louis, MO).
For secondary immunization, mice were given 5 μg of NP19-CGG or 250 μg of HEL emulsified in incomplete Freund’s adjuvant (Sigma St. Louis, MO) i.p. 6 weeks following primary immunization. Antigen-specific antibody levels were sequentially determined from serum obtained from the tail vein 7, 10, and 14 days after immunization.
Quantitative ELISA assays were performed using DEX-BSA (the kind gift of Dr. J.F. Kearney) and NP-BSA (Biosearch Technologies) as antigens coated at 25 μg/ml in PBS onto CoStar EIA/RIA plates. Assays of anti-PC sera of immunized mice were performed as previously described (Kearney et al. 1981) with anti-IgM, PC-BSA, and the T15 anti-idiotypic monoclonal antibody AB1-2. Assays of IgM anti-DEX sera were performed as previously described (Kearney et al. 1985) using MOPC 104E as a standard. Assays of anti-NP sera were performed using the control monoclonal antibody, B1-8. Following a 2-h incubation at 37°C with serum samples and isotype-matched antibody standards, plates were blocked and then incubated with AP-labeled goat anti-mouse IgM, IgG, or Igλ as required (SBA); plates were developed using p-nitrophenyl phosphate substrate (Sigma, St. Louis, MO). For all ELISAs, eight serial dilutions of individual serum samples were made in 1% BSA and 0.05% TWEEN 20 in PBS. All ELISA plates were read at 405 nm using a Bio-Rad Benchmark microplate reader and software (Hercules, CA).
Statistical analysis
Population means were analyzed using an unpaired, two-tailed Student’s t test for populations that were normally distributed, and with the non-parametric Mann–Whitney for those that were not.
Results
Rapid reconstitution of TdT−/− B and T cells
To test the effect of N addition on humoral responses, we transplanted bone marrow from TdT-deficient (TdT−/−) and wild-type (TdT+/+) BALB/c mice into RAG1−/− BALB/c hosts. We tested whether the composition of the starting B cell population might be affected by the absence of TdT, by using the scheme of Hardy and Hayakawa (2001) to compare the prevalence in the bone marrow of B lineage cells belonging to the progenitor (Fr.B-D), immature (Fr.E), and mature (Fr.F) B cell fractions between a cohort of five TdT+/+ and a cohort of five TdT−/− mice. No significant differences in the prevalence of these five bone marrow B cell fractions among the bone marrow mononuclear cells were found (Fig. 1a, b).
Fig. 1.
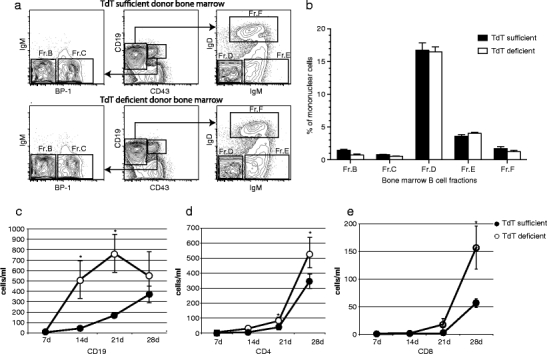
Lymphocyte populations in RAG1−/− mice after reconstitution with either TdT+/+or TdT−/− bone marrow cells. a TdT−/− and TdT+/+ donor cells within the lymphocyte gate were first distinguished on the basis of the expression of CD19 and CD43. Early B cell progenitors (CD19+CD43+) were divided into fractions B and C on the basis of the expression of BP-1. Late pre-B, immature B, and mature B cells (CD19+CD43−) were divided into fractions D, E, and F on the basis of the surface expression of IgM and IgD. b Comparison between the relative numbers of cells within the Hardy fractions B-F from TdT−/− and TdT+/+ donor bone marrow mononuclear cells. c Absolute numbers of CD19+ B cells in peripheral blood at 7-day intervals following reconstitution. d Absolute numbers of CD4+ T cells. e Absolute numbers of CD8+ T cells in peripheral blood. Closed circles are RAG1−/− mice (n = 10) that received TdT+/+ bone marrow and open circles are RAG1−/− (n = 10) mice that received TdT−/− bone marrow cells
Four weeks after transplantation with 107 BALB/c TdT+/+ bone marrow mononuclear cells, RAG1−/− mice that had been preconditioned with a single dose of 10 cGy/g irradiation prior to transplantation exhibited high numbers of circulating CD19+ B cells and CD4+ and CD8+ T cells (Supplemental Figure 1). On the 7th day after transplant, the numbers of B cells and T cells in the blood in TdT−/− and TdT+/+ RAG1−/− recipient mice were similar and low (Fig. 1c), supporting that there were no excess numbers of mature B cells coming from either the TdT−/− or TdT+/+ donor bone marrow and thus cells that populated peripheral lymphocyte niches over time would represent the progeny of de novo development.
Two weeks after transplant, TdT−/− cells achieved greater lymphocyte numbers than BALB/c TdT+/+ cells. TdT−/− CD19+ B cell numbers exceeded TdT+/+ at 14 and 21 days, and then normalized by 28 days (Fig. 1c). TdT−/− CD4+ T cells consistently exceeded TdT+/+ at 14, 21, and 28 days where they averaged 483 ± 102 versus 346 ± 48 leukocytes per microliter (p = 0.004) (Fig. 1d). Similarly, TdT−/− CD8+ T cells exceeded TdT+/+ numbers at 21 and at 28 days where they reached 157 ± 39 versus 57 ± 8 cells per microliter (p < 0.001) (Fig. 1e).
A developmental advantage for TdT−/− B cells in bone marrow and peripheral lymphoid tissues
To clarify whether N-deficient B and T cells had a growth advantage over N-sufficient cells, RAG1−/− mice were reconstituted with equivalent numbers of congenic total bone marrow cells from C.B17 IgMb and TdT−/− or TdT+/+ IgMa littermates. Four weeks after transplantation at a time when our initial studies suggested that the numbers of CD19+ B cells in the blood were most likely to have converged (Fig. 1), the proportions of CD19+ IgMa and IgMb B cells in bone marrow, spleen, and peritoneal cavity were determined by flow cytometry in the TdT/C.B17 chimeras. The blood was also re-examined. In the bone marrow, there was a 3-fold increase in the proportion of CD19+ cells from TdT−/− versus TdT+/+ precursors (p < 0.001). Bone marrow immature Fraction E and mature, recirculating Fraction F cells demonstrated a 2-fold increase in the proportion of cells from TdT−/− versus TdT+/+ precursors (p = 0.02 and p < 0.001, respectively). In the marginal zone of the spleen, the proportion of cells from TdT−/− precursors was 2-fold greater than TdT+/+ (p < 0.001) and the follicular zone proportion was less at 1.4 (p = 0.004). In the peritoneal cavity, there was a 2-fold increase in the proportion of B-1a and B-2 cells from TdT−/− versus TdT+/+ precursors (p < 0.001). The proportion of TdT−/− versus TdT+/+ precursors in the B-1b population was similar. In the blood, there was a 1.4-fold increase in the proportion of CD19+ cells from TdT−/− versus TdT+/+ precursors (p = 0.005) (Fig. 2).
Fig. 2.

TdT−/− cells exhibit a selective advantage for populating peripheral lymphoid tissues. Cell numbers in selected tissues obtained from RAG1−/− mice receiving 2 × 106 bone marrow cells from wild-type CB17 (IgMb) mice and from either congenic TdT−/− or TdT+/+ (IgMa) mice were evaluated by flow cytometry 4 weeks after transplantation. Values given are the ratio of either TdT−/− IgMa (open circle) or TdT+/+ IgMa (closed circle) to IgMb-bearing cells
Increased serum immunoglobulin levels in TdT−/− reconstituted mice
We began our analysis by measuring the baseline, pre-immunization IgM, IgA, and IgG levels 28 days post transplant. IgM and IgA levels were higher in the TdT−/− mice (133 ± 15 versus 75 ± 8 μg IgM/ml, p = 0.01, and 45 ± 4 versus 26 ± 2 μg IgA/ml, p < 0.01, respectively). IgG levels in the TdT−/− reconstituted mice exceeded TdT+/+ for all subclasses, although statistical significance was achieved only for IgG1 and IgG2a. The levels for IgG1 were 160 ± 7 versus 102 ± 11 μg/ml; p < 0.01; for IgG2a, the levels were 45 ± 4 versus 26 ± 2 μg/ml, p < 0.01); for IgG2b, the levels were 198 ± 34 versus 143 ± 18 μg/ml, p = 0.19; and for IgG3, the levels were 117 ± 14 versus 97 ± 26 μg/ml, (p = 0.51) (Fig. 3).
Fig. 3.

Ig isotype values in RAG1−/− mice after reconstitution with either TdT+/+ or TdT−/− bone marrow cells. Closed circles are RAG1−/− mice (n = 10) that received TdT+/+ bone marrow cells and open circles are RAG1−/− (n = 10) mice that received TdT−/− bone marrow cells. Serum immunoglobulin concentrations were determined by ELISA 4 weeks after transplantation
Increased responses to heat-inactivated S. pneumoniae, but decreased responses to E. cloacae
We further analyze the effect of N addition on humoral responses to bacterial antigens. At 7 days after immunization with heat-inactivated S. pneumoniae, TdT−/− reconstituted mice demonstrated higher levels of IgM anti-phosphorylcholine (anti-PC) than TdT+/+ (Fig. 4a). The disparity was greatest at 7 days (258 ± 25 μg/ml versus 58 ± 12 μg/ml, p < 0.001). In wild-type BALB/c mice, the T15 idiotype is the dominant anti-PC idiotype (Claflin and Cubberley 1980; Claflin and Rudikoff 1976). At 7 days post-immunization, robust T15 levels were observed in the TdT−/− reconstituted mice whereas four of nine TdT+/+ reconstituted mice expressed no T15-bearing antibodies and the remaining five expressed low levels (148 ± 15 versus 20 ± 3 μg/ml, p < 0.001). T15 idiotype-specific anti-PC antibodies were ultimately expressed in all the TdT+/+ reconstituted mice by 10 days post-immunization, albeit still at low levels (Fig. 4b).
Fig. 4.

Anti-PC and anti-DEX responses after immunization with heat-killed bacteria. ELISA analysis of RAG1−/− reconstituted mice immunized with Streptococcus pneumoniae or Enterobacter cloacae. a Anti-PC response at time points following immunization. b Anti-idiotypic T-15 response to S. pneumoniae. c Anti-idiotypic, α1→3 dextran response to E. cloacae. The titers in pre-immune sera were <1 μg/ml (data not shown). Closed circles are RAG1−/− mice (n = 10) that received TdT+/+ bone marrow and open circles are RAG1−/− (n = 10) mice that received TdT−/− bone marrow cells
The response to E. cloacae is dominated by anti-α1→3 dextran antibodies of the DEX idiotype (anti-DEX), which use a λ light chain (Kearney et al. 1985). TdT−/− reconstituted mice exhibited lower anti-DEX levels than TdT+/+ 7 days post-immunization (42 ± 24 versus 111 ± 28 μg/ml, p = 0.02; Fig. 4c). By days 10 and 14, however, the differences in the anti-DEX response to E. cloacae no longer achieved statistical significance.
Diminished responses to the T-independent antigens α1→3 dextran and TNP-FICOLL
To evaluate the responses to T-independent antigens, the reconstituted mice were challenged with either α1→3 dextran (DEX) or TNP-FICOLL 4 weeks after transplant. Mice reconstituted with TdT−/− bone marrow cells had lower DEX-specific antibodies at 7, 10, and 14 days than TdT+/+ (p ≤ 0.002 (Fig. 5a)). At 7 days, the response to TNP-FICOLL in TdT−/− reconstituted mice was also less robust in the mice reconstituted with TdT+/+ cells [124 ± 67 versus 218 ± 77 (p<0.001)]. Beyond 7 days, the level of TNP-specific antibodies converged (Fig. 5b).
Fig. 5.

Humoral response to T-independent antigens. ELISA analysis of RAG1−/− reconstituted mice immunized with model antigens α1→3 dextran or TNP-Ficoll. a Anti-DEX response at time points following immunization. b Anti-TNP response. Titers in pre-immune sera were <1 μg/ml (data not shown). Closed circles are RAG1−/− mice (n = 10) that received TdT+/+ bone marrow and open circles are RAG1−/− (n = 10) mice that received TdT−/− bone marrow cells
Diminished secondary IgG responses to the T-dependent antigens NP19-CGG and HEL
To evaluate the responses to T-dependent antigens, the reconstituted mice were challenging with either NP19-CGG or HEL. At 7, 10, and 14 days following primary immunization, anti-NP and anti-HEL IgG titers were similar between TdT−/− and TdT+/+ reconstituted mice (Fig. 6). On re-challenge, the TdT−/− reconstituted mice failed to boost antibody levels beyond those achieved following primary immunization (p < 0.001).
Fig. 6.

Primary and secondary humoral response to T-dependent protein antigens. ELISA analysis of RAG1−/− reconstituted mice immunized with egg lysozyme (HEL) or NP19-CGG. a Anti-HEL response at time points following primary and secondary immunization. b Anti-NP response. Titers in pre-immune sera were <1 μg/ml (data not shown). Closed circles are RAG1−/− mice (n = 10 for primary and n = 6 for secondary immunization) that received TdT+/+ bone marrow and open circles are RAG1−/− (n = 10 for primary and secondary immunization) mice that received TdT−/− bone marrow cells
Discussion
These studies were undertaken to assess the impact of the absence of N nucleotide addition on the growth of the lymphocyte population and on the humoral response to representative antigens. We used a model of transplanting adult stem cells into adult animals to avoid the potentially confounding influences of persistent fetal-derived lymphocyte subpopulations as well as that of the fetal liver and thymic microenvironments. In these transplanted mice, we show that while restricting the initial receptor repertoire to a fetal-like, N-less pattern facilitates rapid population of lymphoid tissues; this potential benefit has incurred the cost of degrading the humoral immune response to five of the six antigens tested, including bacteria.
Our findings of an accelerated population of lymphoid tissues with B cells expressing BCRs without N addition are in concert with previous observations that TdT−/− B cells pass through the splenic transitional stages more efficiently than TdT+/+ B cells (Carey et al. 2008) and that thymocyte development is facilitated in the absence of TdT (Aono et al. 2000; Bogue et al. 1992). This rapid “filling” of the peripheral lymphocyte pool by TdT−/− progenitors could reflect differences in survival, differences in homeostatic proliferation, or differences in responses to either exogenous or endogenous antigens.
Allowing unencumbered passage of N-less lymphocytes with canonical, germline-encoded antigen receptors though developmental checkpoints appears to be important in the generation of the natural antibody repertoire (Martin et al. 2001) and may protect against pathogenic self-reactivity (Feeney et al. 2001; Robey et al. 2004). Our transplantation studies were designed to specifically exclude the contribution of physiologically TdT-deficient fetal cells. The higher levels of IgM, IgG, and IgA in baseline, pre-immunization sera of mice transplanted with N-less precursors may thus reflect facilitated development of the natural antibody repertoire by adult B cell progenitors, including B-1 cell progenitors (Esplin et al. 2009; Montecino-Rodriguez et al. 2006; Tung et al. 2006), which normally would introduce N nucleotides into VDJ joins and thus have difficulty producing fetal-like canonical, germline-encoded natural antibodies.
For example, the humoral response to PC in mice is considered to be a component of the natural antibody repertoire and is normally dominated by antibodies expressing canonical heavy and light chains lacking N addition in CDR-H3. B cells that spontaneously produce these antibodies typically develop in the perinatal period and belong to the long-lived CD5+ B-1a subset (Feeney 1991; Hardy et al. 1994; Herzenberg 2000; Martin et al. 2001; Masmoudi et al. 1990; Sigal et al. 1977). However, the primary source of adaptive antibody responses induced by pneumococcal polysaccharide, which are essential for long-term protection, has been attributed to the B-1b subset (Haas et al. 2005). The robust PC and T-15 idiotypic responses to S. pneumoniae that we and others (Mi et al. 2002) have shown in mice expressing Igs devoid of N-nucleotide addition were expected and likely due to the increased availability and abundance of germline-encoded canonical DFL16.1-containing CDR-H3s (Schelonka et al. 2010; and manuscript in preparation). The converse appears to be true as well. Transgenic mice that express TdT and force the introduction of N nucleotides into V(D)J junctions during fetal life exhibit decreased levels of anti-PC antibodies bearing the T15 idiotype and lack protection against infection with live S. pneumoniae (Benedict and Kearney 1999). The strong PC and T-15 idiotypic responses observed in our mice, which were transplanted with adult bone marrow B cell progenitors only, are in concert with recent studies that have revealed the presence of a small subset of phenotypically distinct B-1 progenitors in adult bone marrow that can efficiently generate B-1 cells, most of which belong to the B-1b sister population (Esplin et al. 2009; Montecino-Rodriguez et al. 2006; Tung et al. 2006).
Despite the increase in both the absolute number of lymphocytes and in the serum concentration of immunoglobulin, mice transplanted with N-less precursor cells exhibited diminished humoral responses to the prototypic T cell independent, type II antigens TNP-Ficoll and α1→3 dextran. These antigens contain repetitive determinants allowing for a high degree of sIgM cross-linking and thus independent B cell activation and antibody production (Mond et al. 1995). Intravenous challenge with α1→3 dextran elicits a response that is dominated by the J558 idiotype (Blomberg et al. 1972; Schilling et al. 1980; Stohrer and Kearney 1983). α1→3 Dextran is expressed on the surface of E. cloacae, a commensal microorganism of the digestive tract and skin of mouse and human that can be an opportunistic pathogen in immune compromised hosts. The anti-DEX response typically dominates after intravenous challenge with heat-killed bacteria. Mice transplanted with N-less precursors had a diminished response to the heat-killed bacteria. This may be due to an N-less B cell repertoire that is unable to generate plasmablasts with the very short CDR-H3s that characterize the anti-DEX response (Blomberg et al. 1972; Schilling et al. 1980; Stohrer and Kearney 1983). Our findings are in accordance with Mahmoud and Kearney that have recently proposed that TdT activity is required for the generation of optimal anti-DEX Ab response and the dominance of the J558 idiotype in adult BALB/c mice (Mahmoud and Kearney 2010).
Although the DEX response has been primarily considered to be independent of T cell function, studies have shown that it can be modified by T suppressor cells (Specht et al. 2003; Stab et al. 1990). Suppressor T cells with a germline αβTCR recognize a component of the J558 epitope and provide a gate, which precludes variability in the CDR-H3 occurring in the periphery (Clemens et al. 1998). Similarly, lymphokines secreted by T cells modulate the type II TI response (Pecanha et al. 1992). It remains possible that alterations in the TCR repertoire induced by an absence of N addition could also have diminished the anti-DEX response.
We examined the role of N addition in T-dependent responses more directly by challenging the transplanted mice with HEL and NP19-CGG. HEL elicits a T cell dependent, class II MHC response with a type-2 cytokine signature (Yip et al. 1999). The vast majority of the HEL-specific T cell response is directed towards a small proportion of HEL peptides (Velazquez et al. 2001). Vβ and Vα T cell CDR3s of 7–31 and 103–117 HEL peptide regions have been shown to be heterogeneous in sequence and commonly contain N nucleotides (Gapin et al. 1998). Affinity maturation by means of somatic hypermutation alters the structural conformation of CDR-H3, thereby improving the binding of HEL (Li et al. 2003). This process appears to spread structural diversity generated by V-D-J recombination from central to peripheral regions of the antibody binding site (Tomlinson et al. 1996). It has been postulated that dendritic cells play a major role in the focusing of the immune response against a few antigenic determinants, while B lymphocytes may diversify the T cell response by presenting a more heterogeneous set of peptide–MHC complexes (Gapin et al. 1998). TdT−/− T lymphocytes may be unable to escape the constraints of the CDR-H3 diversity limitations, while B lymphocytes with a restricted immune repertoire may be unable to sufficiently diversify the T cell response by presenting only a set limited of peptide–MHC complexes.
In BALB/c mice, the primary response to the nitrophenylacetyl hapten of nitrophenylacetyl-chicken gamma globulin (NP19-CGG) is dominated by IgG1λ anti-NP antibodies with a restricted idiotype (Cumano and Rajewsky 1985). The CDR-H3 sequences of anti-NP antibodies often contain N nucleotides and these junctional nucleotides often determine the maturation pathway of the antibody (Furukawa et al. 1999). However, after primary intraperitoneal challenge with NP19-CGG, the anti-NP IgG in mice transplanted with TdT+/+ or TdT−/− precursors had a similar response, suggesting that N nucleotides in CDR-H3 were not essential for creating robust antigen binding sites. Still, on secondary immunization, mice transplanted with TdT−/− bone marrow had only a modest increase in antigen-specific antibody titers, whereas mice transplanted with TdT+/+ bone marrow demonstrated nearly a full log increase in anti-NP IgG titers. Similarly, after primary challenge, the IgG anti-lysozyme response in mice transplanted with TdT+/+ or TdT−/− precursors was equivalent. And, on secondary immunization, mice limited to an N-less repertoire also had only a modest increase in antigen-specific antibody titers. Both the NP19-CGG and lysozyme responses normally undergo affinity maturation as well as class switching after secondary immunization (Weiss and Rajewsky 1990; Weiss et al. 1992). The diminished secondary response to NP19-CGG and to lysozyme in the TdT−/− mice could thus have been influenced by suboptimal T cell help, by an altered initial antibody repertoire enriched for antibodies expressing lesser antigen affinity due to absence of N addition, or both.
The transplantation model we have developed here presents us with the opportunity to test these hypotheses further. Mutant mouse strains exist where B cell or T cell development have been selectively abrogated, e.g., JH−/− or TCRβ−/− mice. Transplantation studies mixing and matching JH−/−, TCRβ−/−, and TdT−/− genotypes and bone marrow should enable sequential analysis of the contribution of N addition to Ig versus TCR function in diminishing the immune response to these and other antigens. These studies are currently underway in our laboratory.
The existential issue of why the mouse limits the introduction of N nucleotides into both its B cell and T cell receptors has been a difficult one to address. Coupled with previous observations from other investigators (Aono et al. 2000; Bogue et al. 1992) that thymocyte development is facilitated in the absence of TdT and our previous studies demonstrating relatively few changes in the composition of the CDR-H3 repertoire in terms of length and amino acid content in the transition from late pre-B cells to mature B cells in the bone marrow (Schelonka et al. 2010), the results obtained in this study provide support for the hypothesis that an absence of N addition facilitates both B cell and T cell development because the germline repertoire is predisposed to fit within the boundaries of receptors that are acceptable for passage through late developmental checkpoints in the primary lymphoid organs (Ippolito et al. 2006; Ivanov et al. 2005). If so, this would suggest that there is a developmental advantage to prioritizing the population and thus the full development of lymphoid tissues over robust immune responses to certain antigens. The exigencies of creating fully populated lymphoid organs may thus outweigh the increased threat from infection that occurs as a result of limiting the diversity of these antigen receptor repertoires; especially since other mechanisms are typically in play to provide maternally derived passive immunity during the period of greatest vulnerability.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Lymphocyte populations at time points following reconstitution. a Absolute numbers of CD19+ B cells in peripheral blood following reconstitution at 7-day intervals. b Absolute numbers of CD4+ T cells. c Absolute numbers of CD8+ T cells in peripheral blood. Black boxes are RAG1−/− mice (n = 10) that received 10 cGy/g preconditioning irradiation and gray boxes are RAG1−/− mice (n = 10) that received no irradiation. (JPEG 293 kb)
Acknowledgments
The authors wish to thank G. Ippolito, J. Kearney, F. Martin, M. Cooper, P. Burrows, and C. Klug for their invaluable advice and support. This work was supported in part by HD043327 (RLS), AI48115 (HWS), and AI090742 (HWS); and by NIH support for flow cytometry through P30 AR48311 and P30 AI027767. The authors declare that they have no competing financial interests.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Abbreviations
- AP
Alkaline phosphatase
- BCR
B cell receptor
- BM
Bone marrow
- BSA
Bovine serum albumin
- DEX
α1→3 Dextran
- FACS
Fluorescence-activated cell sorting
- HEL
Hen egg lysozyme
- Ig
Immunoglobulin
- NP19-CGG
Nitrophenylacetyl-chicken gamma globulin
- PC
Phosphorylcholine
- RAG1
Recombination activation gene 1
- TCR
T cell receptor
- TdT
Terminal deoxynucleotidyl transferase
- TNP-FICOLL
(2,4,6-Trinitrophenyl) hapten conjugated to AminoEthylCarboxymethyl-FICOLL
- WT
Wild type
References
- Aono A, Enomoto H, Yoshida N, Yoshizaki K, Kishimoto T, Komori T. Forced expression of terminal deoxynucleotidyl transferase in fetal thymus resulted in a decrease in gammadelta T cells and random dissemination of Vgamma3Vdelta1 T cells in skin of newborn but not adult mice. Immunol. 2000;99:489–497. doi: 10.1046/j.1365-2567.2000.00987.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedict CL, Kearney JF. Increased junctional diversity in fetal B cells results in a loss of protective anti-phosphorylcholine antibodies in adult mice. Immunity. 1999;10:607–617. doi: 10.1016/S1074-7613(00)80060-6. [DOI] [PubMed] [Google Scholar]
- Benedict CL, Gilfillan S, Thai TH, Kearney JF. Terminal deoxynucleotidyl transferase and repertoire development. Immunol Rev. 2000;175:150–157. doi: 10.1111/j.1600-065X.2000.imr017518.x. [DOI] [PubMed] [Google Scholar]
- Blomberg B, Geckeler WR, Weigert M. Genetics of the antibody response to dextran in mice. Science. 1972;177:178–180. doi: 10.1126/science.177.4044.178. [DOI] [PubMed] [Google Scholar]
- Bogue M, Gilfillan S, Benoist C, Mathis D. Regulation of N-region diversity in antigen receptors through thymocyte differentiation and thymus ontogeny. Proc Natl Acad Sci USA. 1992;89:11011–11015. doi: 10.1073/pnas.89.22.11011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucy RP, Chen CH, Cooper MD. Ontogeny of T cell receptors in the chicken thymus. J Immunol. 1990;144:1161–1168. [PubMed] [Google Scholar]
- Carey JB, Moffatt-Blue CS, Watson LC, Gavin AL, Feeney AJ. Repertoire-based selection into the marginal zone compartment during B cell development. J Exp Med. 2008;205:2043–2052. doi: 10.1084/jem.20080559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalmers IM, Janossy G, Contreras M, Navarrete C. Intracellular cytokine profile of cord and adult blood lymphocytes. Blood. 1998;92:11–18. [PubMed] [Google Scholar]
- Claflin JL, Cubberley M. Clonal nature of the immune response to phosphocholine. VII. Evidence throughout inbred mice for molecular similarities among antibodies bearing the T15 idiotype. J Immunol. 1980;125:551–558. [PubMed] [Google Scholar]
- Claflin JL, Rudikoff S. Uniformity in the clonal repertoire for the immune response to phosphorylecholine in mice: a case for a germline basis of antibody diversity. CSH Symp Quan Biol. 1976;41:725. doi: 10.1101/sqb.1977.041.01.083. [DOI] [PubMed] [Google Scholar]
- Clemens A, Rademaekers A, Specht C, Kolsch E. The J558 VH CDR3 region contributes little to antibody avidity; however, it is the recognition element for cognate T cell control of the alpha(1–>3) dextran-specific antibody response. Int Immunol. 1998;10:1931–1942. doi: 10.1093/intimm/10.12.1931. [DOI] [PubMed] [Google Scholar]
- Cohen SB, Perez-Cruz I, Fallen P, Gluckman E, Madrigal JA. Analysis of the cytokine production by cord and adult blood. Hum Immunol. 1999;60:331–336. doi: 10.1016/S0198-8859(98)00126-8. [DOI] [PubMed] [Google Scholar]
- Cooper MD. B lymphocytes: normal development and function. N Eng J Med. 1987;317:1452–1456. doi: 10.1056/NEJM198712033172306. [DOI] [PubMed] [Google Scholar]
- Cumano A, Rajewsky K. Structure of primary anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibodies in normal and idiotypically suppressed C57BL/6 mice. Eur J Immunol. 1985;15:512–520. doi: 10.1002/eji.1830150517. [DOI] [PubMed] [Google Scholar]
- Esplin BL, Welner RS, Zhang O, Borghesi LA, Kincade PW. A differentiation pathway for B1 cells in adult bone marrow. Proc Natl Acad Sci USA. 2009;106:5773–5778. doi: 10.1073/pnas.0811632106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney AJ. Lack of N regions in fetal and neonatal mouse immunoglobulin V-D-J junctional sequences. J Exp Med. 1990;172:1377–1390. doi: 10.1084/jem.172.5.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feeney AJ. Predominance of the prototypic T15 anti-phosphorylcholine junctional sequence in neonatal pre-B cells. J Immunol. 1991;147:4343–4350. [PubMed] [Google Scholar]
- Feeney AJ. Comparison of junctional diversity in the neonatal and adult immunoglobulin repertoires. Int Rev Immunol. 1992;8:113–122. doi: 10.3109/08830189209055567. [DOI] [PubMed] [Google Scholar]
- Feeney AJ, Lawson BR, Kono DH, Theofilopoulos AN. Terminal deoxynucleotidyl transferase deficiency decreases autoimmune disease in MRL-Fas(lpr) mice. J Immunol. 2001;167:3486–3493. doi: 10.4049/jimmunol.167.6.3486. [DOI] [PubMed] [Google Scholar]
- Furukawa K, Akasako-Furukawa A, Shirai H, Nakamura H, Azuma T. Junctional amino acids determine the maturation pathway of an antibody. Immunity. 1999;11:329–338. doi: 10.1016/S1074-7613(00)80108-9. [DOI] [PubMed] [Google Scholar]
- Gapin L, Bravo de Alba Y, Casrouge A, Cabaniols JP, Kourilsky P, Kanellopoulos J. Antigen presentation by dendritic cells focuses T cell responses against immunodominant peptides: studies in the hen egg-white lysozyme (HEL) model. J Immunol. 1998;160:1555–1564. [PubMed] [Google Scholar]
- George JF, Cooper MD. Gamma/delta T cells and alpha/beta T cells differ in their developmental patterns of receptor expression and modulation requirements. Eur J Immunol. 1990;20:2177–2181. doi: 10.1002/eji.1830201005. [DOI] [PubMed] [Google Scholar]
- Gilfillan S, Dierich A, Lemeur M, Benoist C, Mathis D. Mice lacking TdT: mature animals with an immature lymphocyte repertoire. Science. 1993;261:1175–1178. doi: 10.1126/science.8356452. [DOI] [PubMed] [Google Scholar]
- Gilfillan S, Bachmann M, Trembleau S, Adorini L, Kalinke U, Zinkernagel R, Benoist C, Mathis D. Efficient immune responses in mice lacking N-region diversity. Eur J Immunol. 1995;25:3115–3122. doi: 10.1002/eji.1830251119. [DOI] [PubMed] [Google Scholar]
- Haas KM, Poe JC, Steeber DA, Tedder TF. B-1a and B-1b cells exhibit distinct developmental requirements and have unique functional roles in innate and adaptive immunity to S. pneumoniae. Immunity. 2005;23:7–18. doi: 10.1016/j.immuni.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Hardy RR, Hayakawa K. B cell development pathways. Annu Rev Immunol. 2001;19:595–621. doi: 10.1146/annurev.immunol.19.1.595. [DOI] [PubMed] [Google Scholar]
- Hardy RR, Carmack CE, Li YS, Hayakawa K. Distinctive developmental origins and specificities of murine CD5+ B cells. Immunol Rev. 1994;137:91–118. doi: 10.1111/j.1600-065X.1994.tb00660.x. [DOI] [PubMed] [Google Scholar]
- Herzenberg LA. B-1 cells: the lineage question revisited. Immunol Rev. 2000;175:9–22. doi: 10.1111/j.1600-065X.2000.imr017520.x. [DOI] [PubMed] [Google Scholar]
- Ippolito GC, Schelonka RL, Zemlin M, Ivanov, Kobayashi R, Zemlin C, Gartland GL, Nitschke L, Pelkonen J, Fujihashi K, Rajewsky K, Schroeder HW., Jr Forced usage of positively charged amino acids in immunoglobulin CDR-H3 impairs B cell development and antibody production. J Exp Med. 2006;203:1567–1578. doi: 10.1084/jem.20052217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov, Schelonka RL, Zhuang Y, Gartland GL, Zemlin M, Schroeder HW., Jr Development of the expressed immunoglobulin CDR-H3 repertoire is marked by focusing of constraints in length, amino acid utilization, and charge that are first established in early B cell progenitors. J Immunol. 2005;174:7773–7780. doi: 10.4049/jimmunol.174.12.7773. [DOI] [PubMed] [Google Scholar]
- Kearney JF, Barletta R, Quan ZS, Quintans J. Monoclonal vs. heterogeneous anti-H-8 antibodies in the analysis of anti-phosphorylcholine response in BABL/c mice. Eur J Immunol. 1981;11:877–883. doi: 10.1002/eji.1830111106. [DOI] [PubMed] [Google Scholar]
- Kearney JF, McCarthy MT, Stohrer R, Benjamin WH., Jr Induction of germ-line anti-alpha 1-3 dextran antibody responses in mice by members of the Enterobacteriaceae family. J Immunol. 1985;135:3468–3472. [PubMed] [Google Scholar]
- Komori T, Okada A, Stewart V, Alt FW. Lack of N regions in antigen receptor variable region genes of TdT-deficient lymphocytes. Science. 1993;261:1171–1175. doi: 10.1126/science.8356451. [DOI] [PubMed] [Google Scholar]
- Li Y, Li H, Yang F, Smith-Gill SJ, Mariuzza RA. X-ray snapshots of the maturation of an antibody response to a protein antigen. Nat Struct Biol. 2003;10:482–488. doi: 10.1038/nsb930. [DOI] [PubMed] [Google Scholar]
- Mahmoud TI, Kearney JF. Terminal deoxynucleotidyl transferase is required for an optimal response to the polysaccharide alpha-1,3 dextran. J Immunol. 2010;184:851–858. doi: 10.4049/jimmunol.0902791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”. Immunol Rev. 2000;175:70–79. doi: 10.1111/j.1600-065X.2000.imr017515.x. [DOI] [PubMed] [Google Scholar]
- Martin F, Oliver AM, Kearney JF. Marginal zone and B1 B cells unite in the early response against T-independent blood-borne particulate antigens. Immunity. 2001;14:617–629. doi: 10.1016/S1074-7613(01)00129-7. [DOI] [PubMed] [Google Scholar]
- Masmoudi H, Mota Santos TA, Huetz F, Coutinho AA, Cazenave PA. All T15 Id-positive antibodies (but not the majority of VHT15+ antibodies) are produced by peritoneal CD5+ B lymphocytes. Int Immunol. 1990;2:515–520. doi: 10.1093/intimm/2.6.515. [DOI] [PubMed] [Google Scholar]
- Mi QS, Rezanka LJ, Lustig A, Zhou L, Longo DL, Kenny JJ. The M603 idiotype is lost in the response to phosphocholine in terminal deoxynucleotidyl transferase-deficient mice. Eur J Immunol. 2002;32:1139–1146. doi: 10.1002/1521-4141(200204)32:4<1139::AID-IMMU1139>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez E, Leathers H, Dorshkind K. Identification of a B-1 B cell-specified progenitor. Nat Immunol. 2006;7:293–301. doi: 10.1038/ni1301. [DOI] [PubMed] [Google Scholar]
- Nadel B, Tehranchi S, Feeney AJ. Coding end processing is similar throughout ontogeny. J Immunol. 1995;154:6430–6436. [PubMed] [Google Scholar]
- Nossal GJV. The double helix and immunology. Nature. 2003;421:440–444. doi: 10.1038/nature01409. [DOI] [PubMed] [Google Scholar]
- Pecanha LM, Snapper CM, Lees A, Mond JJ. Lymphokine control of type 2 antigen response. IL-10 inhibits IL-5- but not IL-2-induced Ig secretion by T cell-independent antigens. J Immunol. 1992;148:3427–3432. [PubMed] [Google Scholar]
- Rajewsky K. Clonal selection and learning in the antibody system. Nature. 1996;381:751–758. doi: 10.1038/381751a0. [DOI] [PubMed] [Google Scholar]
- Robey IF, Peterson M, Horwitz MS, Kono DH, Stratmann T, Theofilopoulos AN, Sarvetnick N, Teyton L, Feeney AJ. Terminal deoxynucleotidyltransferase deficiency decreases autoimmune disease in diabetes-prone nonobese diabetic mice and lupus-prone MRL-Fas(lpr) mice. J Immunol. 2004;172:4624–4629. doi: 10.4049/jimmunol.172.7.4624. [DOI] [PubMed] [Google Scholar]
- Schelonka RL, Raaphorst FM, Infante D, Kraig E, Teale JM, Infante AJ. T cell receptor repertoire diversity and clonal expansion in human neonates. Pediatr Res. 1998;43:396–402. doi: 10.1203/00006450-199803000-00015. [DOI] [PubMed] [Google Scholar]
- Schelonka RL, Ivanov, Vale AM, Szymanska E, Zemlin M, Gartland GL, Schroeder HW., Jr The CDR-H3 repertoire from TdT-deficient adult bone marrow is a close, but not exact, homologue of the CDR-H3 repertoire from perinatal liver. J Immunol. 2010;185:6075–6084. doi: 10.4049/jimmunol.1001419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilling J, Clevinger B, Davie JM, Hood L. Amino acid sequence of homogeneous antibodies to dextran and DNA rearrangements in heavy chain V-region gene segments. Nature. 1980;283:35–40. doi: 10.1038/283035a0. [DOI] [PubMed] [Google Scholar]
- Schroeder HW, Jr, Ippolito GC, Shiokawa S. Regulation of the antibody repertoire through control of HCDR3 diversity. Vaccine. 1998;16:1383–1390. doi: 10.1016/S0264-410X(98)00096-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder HW, Jr, Zhang L, Philips JB., III Slow, programmed maturation of the immunoglobulin HCDR3 repertoire during the third trimester of fetal life. Blood. 2001;98:2745–2751. doi: 10.1182/blood.V98.9.2745. [DOI] [PubMed] [Google Scholar]
- Sherwin WK, Rowlands JDT. Development of humoral immunity in lethally irradiated mice reconstituted with fetal liver. J Immunol. 1974;113:1353–1360. [PubMed] [Google Scholar]
- Sigal NH, Pickard AR, Metcalf ES, Gearhart PJ, Klinman NR. Expression of phosphorylcholine-specific B cells during murine development. J Exp Med. 1977;146:933–948. doi: 10.1084/jem.146.4.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverstein AM. Ontogeny of the immune response: a perspective. In: Cooper MD, editor. Development of Host Defense. New York: Raven; 1977. pp. 1–10. [Google Scholar]
- Specht C, Schluter B, Rolfing M, Bruning K, Pauels HG, Kolsch E. Idiotype-specific CD4+CD25+ T suppressor cells prevent, by limiting antibody diversity, the occurrence of anti-dextran antibodies crossreacting with histone H3. Eur J Immunol. 2003;33:1242–1249. doi: 10.1002/eji.200323273. [DOI] [PubMed] [Google Scholar]
- Stab F, Austrup F, Kolsch E. Regulation of the anti alpha dextran IgG antibody response of BALB/c mice by idiotype-specific T suppressor lymphocytes. J Immunol. 1990;144:53–59. [PubMed] [Google Scholar]
- Stein KE. Thymus-independent and thymus-dependent responses to polysaccharide antigens. J Infect Dis. 1992;165:S49–S52. doi: 10.1093/infdis/165-Supplement_1-S49. [DOI] [PubMed] [Google Scholar]
- Stohrer RC, Kearney JF. Fine idiotype analysis of B cell precursors in the T-dependent and T-independent responses to alpha 1-3 dextran in BALB/c mice. J Exp Med. 1983;158:2081–2094. doi: 10.1084/jem.158.6.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thai TH, Purugganan MM, Roth DB, Kearney JF. Distinct and opposite diversifying activities of terminal transferase splice variants. Nat Immunol. 2002;3:457–462. doi: 10.1038/ni788. [DOI] [PubMed] [Google Scholar]
- Tomlinson IM, Walter G, Jones PT, Dear PH, Sonnhammer EL, Winter G. The imprint of somatic hypermutation on the repertoire of human germline V genes. J Mol Biol. 1996;256:813–817. doi: 10.1006/jmbi.1996.0127. [DOI] [PubMed] [Google Scholar]
- Tonegawa S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]
- Tung JW, Mrazek MD, Yang Y, Herzenberg LA. Phenotypically distinct B cell development pathways map to the three B cell lineages in the mouse. Proc Natl Acad Sci USA. 2006;103:6293–6298. doi: 10.1073/pnas.0511305103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velazquez C, DiPaolo R, Unanue ER. Quantitation of lysozyme peptides bound to class II MHC molecules indicates very large differences in levels of presentation. J Immunol. 2001;166:5488–5494. doi: 10.4049/jimmunol.166.9.5488. [DOI] [PubMed] [Google Scholar]
- Weiss U, Rajewsky K. The repertoire of somatic antibody mutants accumulating in the memory compartment after primary immunization is restricted through affinity maturation and mirrors that expressed in the secondary response. J Exp Med. 1990;172:1681–1689. doi: 10.1084/jem.172.6.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss U, Zoebelein R, Rajewsky K. Accumulation of somatic mutants in the B cell compartment after primary immunization with a T cell-dependent antigen. Eur J Immunol. 1992;22:511–517. doi: 10.1002/eji.1830220233. [DOI] [PubMed] [Google Scholar]
- Yague J, Blackman MA, Born WK, Marrack PC, Kappler JW, Palmer E. The structure of V alpha and J alpha segments in the mouse. Nucl Acids Res. 1988;16:11355–11364. doi: 10.1093/nar/16.23.11355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip HC, Karulin AY, Tary-Lehmann M, Hesse MD, Radeke H, Heeger PS, Trezza RP, Heinzel FP, Forsthuber T, Lehmann PV. Adjuvant-guided type-1 and type-2 immunity: infectious/noninfectious dichotomy defines the class of response. J Immunol. 1999;162:3942–3949. [PubMed] [Google Scholar]
- Zemlin M, Schelonka RL, Bauer K, Schroeder HW., Jr Regulation and chance in the ontogeny of B and T cell antigen receptor repertoires. Immunol Res. 2002;26:265–278. doi: 10.1385/IR:26:1-3:265. [DOI] [PubMed] [Google Scholar]
- Zinkernagel RM. Maternal antibodies, childhood infections, and autoimmune diseases. N Eng J of Med. 2001;345:1331–1335. doi: 10.1056/NEJMra012493. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Lymphocyte populations at time points following reconstitution. a Absolute numbers of CD19+ B cells in peripheral blood following reconstitution at 7-day intervals. b Absolute numbers of CD4+ T cells. c Absolute numbers of CD8+ T cells in peripheral blood. Black boxes are RAG1−/− mice (n = 10) that received 10 cGy/g preconditioning irradiation and gray boxes are RAG1−/− mice (n = 10) that received no irradiation. (JPEG 293 kb)


