Sir,
Dengue fever (DF) is an acute febrile illness found in the tropics, caused by four antigenically related, but distinct viruses viz., DEN-1, DEN-2, DEN-3 and DEN-4 classified under genus Flavivirus, Family Flaviviridae. Dengue is transmitted to humans through the bites of day biting Aedes aegypti. All four serotypes induce a spectrum of illness, ranging from sudden onset of fever, and head ache often accompanied by myalgia, anorexia, arthralgia to increased vascular permeability in dengue haemorrhagic fever (DHF). DF/DHF is confined to the tropics and sub-tropics. Dengue virus infection occurs in >100 countries; with >2.5 billion persons living in these areas. During the past decades, dengue virus has emerged in southern Asia; DF/DHF epidemics have occurred in South East Asia Region (SEAR)1,2. However, dengue has not been reported in Andaman and Nicobar archipelago6.
Andaman and Nicobar islands (92° to 94° East and 6° to 14° North), is an archipelago with > 500 islands/islets, stretching over 700 km from north to south, in the Bay of Bengal. There are 38 inhabited islands with a population of approximately 356,0003. People constantly move between these islands and mainland India, connected by ship and air to Chennai and Kolkata. Periodic outbreaks of dengue fever have been reported in these port cities4,5. Tourism industry is witnessing a significant surge in the number of tourists from mainland India as well as from overseas.
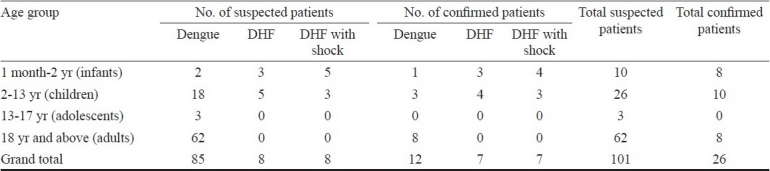
From June through July 2009, we observed an increase in the number of dengue like febrile patients from G.B. Pant Hospital, referral hospital in these islands and from a private child care clinic located in Port Blair. Clinical features in most of the patients were consistent with signs of DF7. Blood specimens were collected from suspected patients at the time of admission and sent to the Regional Medical Research Centre (ICMR), for laboratory diagnosis. All were tested for the presence of anti-dengue virus (DENV) immunoglobulin M (IgM) antibody and anti-chikungunya virus IgM antibody by IgM capture ELISA method by the kits developed by National Institute of Virology (NIV), Pune8. Blood samples were collected between 3-5 days post onset of signs. A total of 101 patients were admitted to hospital/child care/health centres. Dengue virus specific IgM was detected in 26 patients. Suspected and confirmed patients of DF/DHF/DSS (dengue shock syndrome) in different age groups are shown in the Table. Among the patients suspected to be of DHF and DSS, tourniquet test was positive and platelet count was < 100000/mm3. Clinical features and laboratory test findings were consistent with the WHO guidelines7. None of the patients had history of travel to the mainland, or any other dengue endemic countries. All patients were negative for chikungunya virus infection. Concurrently, entomological survey was carried out in one of the locality viz., Garacharma reporting suspected cases. The basic sampling unit was a household, which was systematically searched for water holding containers. Premises were searched, in both indoors and outdoors. Single larval survey (SLS) technique was adopted to assess the larval infestation indices and prevalence of Aedes spp.9. The immatures from different habitats were collected with the help of ladles fabricated with long handles and with glass pipettes where ladles did not enter. Three indices commonly used to assess relative prevalence and monitor Ae. aegypti infestation levels viz., the Breteau index (BI), container index (CI) and house index (HI) were assessed. The BI establishes a relationship between positive containers and houses; CI provides information on the proportion of water holding containers that are positive, while the HI provides information on the proportion of houses positive for the immature larvae9. The immature larvae sampled from different habitats were transferred to plastic containers and were brought to the Centre's laboratory and reared individually in plastic vials up to the emergence of adult. The adults were identified to species10.
Table.
Suspected and confirmed patients of dengue fever/DHF/DHF with shock among different age groups
A total of 147 households were searched randomly. As many as 1001 (mean = 6.81 containers per premise) water holding containers were searched for Aedes larvae, both indoors (143/1001, 14.29%) and outdoors (858/1001, 85.71%); 127 containers (95% CI = 10.73%-14.86%) were found to support Aedes spp. larval breeding (12.69%). Breteau index was 86.39 per cent. From the 127 mosquito immatures emerged, Ae. aegypti (112/127, 88.2%) predominantly followed by Ae. albopictus (15/127, 11.8%). HI for Ae. aegypti and Ae. albopictus were 35.37 and 7.48 per cent respectively. Abundant water holding containers supporting Aedes breeding were cement tanks, metal and plastic drums.
The observations of clinical dengue, DHF and DSS supported by evidence for recent infections are perhaps the first ever from this archipelago. DHF appears when more than one serotype circulates in an area11. Reasons for emergence of DF/DHF are complex. However, plausible factors that appear to have contributed to the present scenario can be identified. First, demographic changes, most importantly rapid urbanization and population growth, eventually leading to inadequate water, necessitating storage of water in containers, this leads to increase in Ae. aegypti population densities facilitating transmission. Second, domestic/peri-domestic breeding of mosquitoes contained significant numbers of Ae. aegypti vis-a-vis Ae. albopictus with high larval indices; abundance of a particular species is considered as one criterion for determining the vector status. Finally, increased air travel serves transportation of dengue viruses between endemic locations on the mainland.
Ever since the documentation of prevalence of antibodies for dengue fever (DEN-2) in these islands12, the health personnel in the islands have been keeping a vigil on the emergence of disease. During the 2006 chikungunya epidemic6 over 150 subjects were tested for dengue fever and none turned positive.
Ae . aegypti has a wide distribution within urban Port Blair13 and is infiltrating to other peri-urban/rural areas14. Consequently, the adjoining peri-urban and rural areas are receptive to DENV. Therefore, continued risk of dengue infection cannot be ignored. Besides, inadequate health infrastructure with competing priorities, geographical remoteness of the areas within island, promotion of Andaman and Nicobar archipelago as an international tourist destination, DF/DHF is an emerging problem. Moreover, it is known that DHF occurs where multiple serotypes of dengue virus are simultaneously or sequentially transmitted11. Current situation warrants extreme vigil from public health perspective for its effective control.
Footnotes
Conflicts of interest: None whatsoever.
References
- 1.Pandey BD, Morita K, Khanal SR, Takasaki T, Miyazaki I, Ogawa T, et al. Dengue virus, Nepal. Emerg Infect Dis. 2008;14:514–5. doi: 10.3201/eid1403.070473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Dengue outbreak in Bhutan. Commun Dis Newslett. 2007;4:1–2. [Google Scholar]
- 3.Know Andaman database. [accessed on June 18, 2010]. Available from: http://www.and.nic.in .
- 4.Victor TJ, Malathi M, Asokan R, Padmanaban P. Laboratory-based dengue fever surveillance in Tamil Nadu, India. Indian J Med Res. 2007;126:112–5. [PubMed] [Google Scholar]
- 5.Basu M, Dasgupta MK, Kundu TK, Sengupta B, De GK, Roy BN. Profile of pediatric dengue cases from a tertiary care hospital in Kolkata. Indian J Public Health. 2007;51:234–6. [PubMed] [Google Scholar]
- 6.Manimunda SP, Singh SS, Sugunan AP, Singh O, Roy S, Shriram AN, et al. Chikungunya fever, Andaman and Nicobar islands, India. Emerg Infect Dis. 2007;13:1259–60. doi: 10.3201/eid1308.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dengue haemorrhagic fever: diagnosis, treatment, prevention and control. 2nd ed. Geneva: World Health Organization; 1997. World Health Organization. [Google Scholar]
- 8.Yergolkar PN, Tandale BV, Arankalle VA, Sathe PS, Sudeep AB, Gandhe SS, et al. Chikungunya outbreaks caused by African genotype, India. Emerg Infect Dis. 2006;12:1580–83. doi: 10.3201/eid1210.060529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dengue and dengue hemorrhagic fever in the Americas: guidelines for prevention and control. Washington: Pan American Health Organization; 1994. PAHO. (Scientific Publication No 548) [Google Scholar]
- 10.Barraud PJ. Family Culicidae. Tribes Megarhinini and Culicini. V. London: Taylor and Francis; 1934. The fauna of British India including Ceylon and Burma. Diptera; pp. 1–452. [Google Scholar]
- 11.Halstead SB. Nelson text book of pediatrics. New Delhi: Elsevier (Reed Elsevier India); 2004. Dengue fever and dengue haemorrhagic fever; p. 1092. [Google Scholar]
- 12.Padbidri VS, Wairagkar NS, Joshi GD, Umarani UB, Risbud AR, Gaikwad DL, et al. A serological survey of arboviral diseases among the human population of the Andaman and Nicobar Islands, India. Southeast Asian J Trop Med Public Health. 2002;33:794–800. [PubMed] [Google Scholar]
- 13.Shriram AN, Sehgal SC. Aedes aegypti (L) in Port Blair, Andaman and Nicobar islands- distribution and larval ecology. J Commun Dis. 1999;31:185–92. [PubMed] [Google Scholar]
- 14.Shriram AN, Sugunan AP, Vijayachari P. Infiltration of Aedes aegypti into peri-urban areas in South Andaman. Indian J Med Res. 2008;127:618–20. [PubMed] [Google Scholar]


