Abstract
The endoplasmic reticulum (ER) is the primary site for synthesis and folding of secreted and membrane-bound proteins. Proteins are translocated into ER lumen in an unfolded state and require protein chaperones and catalysts of protein folding to assist in proper folding. Properly folded proteins traffic from the ER to the Golgi apparatus; misfolded proteins are targeted to degradation. Unfolded protein response (UPR) is a highly regulated intracellular signaling pathway that prevents accumulation of misfolded proteins in the ER lumen. UPR provides an adaptive mechanism by which cells can augment protein folding and processing capacities of the ER. If protein misfolding is not resolved, the UPR triggers apoptotic cascades. Although the molecular mechanisms underlying ER stress-induced apoptosis are not completely understood, increasing evidence suggests that ER and mitochondria cooperate to signal cell death. Mitochondria and ER form structural and functional networks (mitochondria-associated ER membranes [MAMs]) essential to maintain cellular homeostasis and determine cell fate under various pathophysiological conditions. Regulated Ca2+ transfer from the ER to the mitochondria is important in maintaining control of prosurvival/prodeath pathways. We discuss the signaling/communication between the ER and mitochondria and focus on the role of the mitochondrial permeability transition pore in these complex processes.
If protein misfolding in the ER is not resolved by the unfolded protein response (UPR), apoptosis is triggered. The is regulated by Ca2+ transfer from the ER to the mitochondria.
The ER is an elaborate membranous network present in all eukaryotic cells and responsible for many homeostatic responses that include folding and maturation of newly synthesized secretory and transmembrane proteins (Kleizen and Braakman 2004). In addition, this organelle is also the site of cholesterol and steroid biosynthesis, lipid biosynthesis, assembly of core-asparagine linked oligosaccharides, and membrane and secreted protein biosynthesis. Newly synthesized proteins require proper folding within the ER lumen prior to trafficking to specific destinations in the cell. These cellular processes are initiated when nascent polypeptide chains emerge in ER lumen, where posttranslational modifications such as N-linked glycosylation, and intra- and intermolecular disulfide bond formation facilitate the folding of polypeptides to form specific tertiary and quaternary structures for proper protein function (Molinari 2007). Although the amino acid sequence of the protein determines many of these precise processes, numerous proteins, including chaperones and enzymes, aid in proper protein biosynthesis and folding. The key chaperones and folding sensors in the ER include the peptide-binding proteins BiP and GRP94, the lectins calnexin and calreticulin, and the thiol-disulfide oxidoreductases such as protein disulfide isomerase (PDI) and Erp57. The chaperone machinery collectively cooperates to prevent protein misfolding, aberrant interactions, and aggregation. The quality of protein folding is precisely monitored by an ER quality control system that only allows properly folded proteins to be transported to the Golgi compartment and directs misfolded proteins for ER-associated degradation (ERAD) by the 26S proteasome or for degradation through autophagy (Ma and Hendershot 2004; Kincaid and Cooper 2007).
Protein folding in the ER is very sensitive to extracellular stimuli and insults, and intracellular processes that alter Ca2+ homeostasis, redox status, and energy (sugar/glucose) stores. The ER is the central site for Ca2+ storage and homeostasis within the cell. The ER couples its quality control machinery to the storage and utilization of Ca2+. Alterations in intralumenal Ca2+ can cause protein misfolding because both protein folding reactions and protein chaperone functions require high levels of calcium. Under conditions that compromise ER function, particularly the accumulation of newly synthesized unfolded proteins, the organelle signals activation of an elaborate adaptive process known as the unfolded protein response (UPR) (Ron and Walter 2007). Appropriate adaptation to misfolded protein accumulation in the ER lumen requires regulation at all levels of gene expression including transcription, translation, translocation into the ER lumen, and ERAD. Coordinate regulation of all these processes is required to restore proper protein folding and ER homeostasis (Mori et al. 1993; Patil and Walter 2001; Kaufman 2002; Schroder and Kaufman 2005; Wu and Kaufman 2006). Finally, chronic activation of UPR signaling eventually induces an apoptotic (programmed cell death) response. We will briefly discuss below the various signaling arms of the UPR as relevant to cell survival and adaptation and ER stress as it relates to apoptosis and cellular demise.
UPR SIGNALING: CELL SURVIVAL
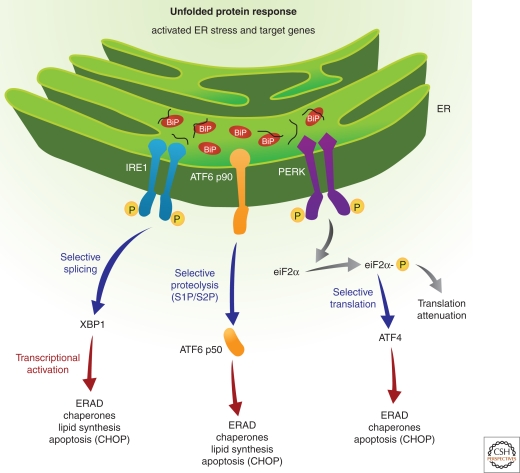
In higher eukaryotic cells, three ER membrane-associated signal transducers sense the presence of misfolded proteins in the ER lumen and initiate adaptive responses (Fig. 1).
Figure 1.
Signaling the unfolded protein response: Three proximal sensors IRE1, PERK, and ATF6 regulate the UPR through their respective signaling cascades. Under nonstressed conditions, BiP binds to the lumenal domains of IRE1 and PERK to prevent their dimerization. On accumulation of unfolded proteins in the ER lumen, IRE1 released from BiP, dimerizes to activate its kinase and RNase activities to initiate XBP1 mRNA splicing, thereby creating a potent transcriptional activator. Primary targets that require IRE1/XBP1 pathway for induction include genes encoding functions in ERAD. Similarly, ATF6 released from BiP transits to the Golgi compartment for cleavage by S1P and S2P proteases to yield a cytosolic fragment that migrates to the nucleus to further activate transcription of UPR-responsive genes. Finally, PERK released from BiP dimerizes, autophosphorylates, and phosphorylates eIF2α on Ser 51 leading to general attenuation of translational initiation. Paradoxically, eIF2α phosphorylation induces translation of ATF4 mRNA. The PERK/eIF2α/ATF4 regulatory axis also induces expression of anti-oxidative stress response genes and expression of genes encoding proteins with proapoptotic functions, such as CHOP.
These transducers are two protein kinases IRE1 (inositol requiring enzyme 1) (Cox et al. 1993; Yoshida et al. 2001), PERK (PKR-like eukaryotic initiation factor 2α kinase) (Harding et al. 2000a,b), and the transcription factor ATF6 (activating transcription factor 6) (Yoshida 2001; Lee et al. 2002; Yoshida et al. 2003; Yamamoto et al. 2007). Under normal cellular conditions in which the ER is presumably “stress-free,” the intralumenal amino-terminal domains of IRE1 and PERK and the carboxy-terminal domain of ATF6 are maintained in an inactive state by interaction with the chaperone BiP/GRP78 (Bertolotti et al. 2000; Liu et al. 2003). This model for negative regulation of the UPR by BiP is also supported by the observation that BiP overexpression prevents activation of the UPR on ER stress (Dorner et al. 1990). Whether BiP is the primary regulator of each UPR sensor is not clearly known, as simple disruption of the interactions between BiP and the UPR sensors may not result in constitutive activation (Oikawa et al. 2007). Recently, the crystal structure of the yeast Ire1p luminal domain (Credle et al. 2005) identified the existence of a deep, long MHC1-type groove in the Ire1p dimer and proposed that unfolded polypeptides directly bind Ire1p to mediate its dimerization. However, although analysis of the human IRE1 indicated a similar structure, the MHC1-type groove was not solvent-accessible (Zhou et al. 2006). In addition, the luminal domain was shown to form dimers in the absence of added polypeptide, bringing into question the requirement for peptide binding to promote dimerization. Because these structures represent static conformations, it is possible the altered conformational states may regulate both BiP and peptide binding. Therefore, it is reasonable to speculate that BiP binding as well as peptide binding act together to regulate IRE1 dimerization. Future studies in this area should resolve this issue. It is believed that the primary trigger for release of BiP from the sensors is the accumulation of misfolded proteins. This, coupled with other unidentified luminal events, results in oligomerization and activation of the IRE1 and PERK kinases and results in the execution of a complex and fascinating downstream intracellular signaling pathway (Bertolotti et al. 2000; Ron and Walter 2007; Aragon et al. 2009; Korennykh et al. 2009; Li et al. 2010). Concomitantly, the third branch of the UPR is activated when ATF6 translocates to the Golgi apparatus where it is cleaved by the serine protease site-1 (S1P) and the metalloprotease site-2 protease (S2P) to generate an active transcription factor (Chen et al. 2002). Interestingly, ATF6 activation is also sensitive to the redox status of the cell and recent evidence suggests that only the reduced monomeric form of ATF6 translocates to the Golgi apparatus (Nadanaka et al. 2007). The overall effect of this tripartite UPR signaling is to attenuate the global mRNA translation and simultaneously up-regulate the expression of chaperones to improve ER folding capacity and ERAD function. The various branches of the UPR are briefly described below.
In the early 1990s, investigations in the budding yeast S. cerevisiae identified the ER stress-regulated kinase and endoribounuclease IRE1 that is conserved from yeast to humans. Two independent groups identified Ire1p/Ern1p as an ER transmembrane protein kinase that acts as a proximal sensor in the yeast UPR that initiates unconventional removal of a 252 base intron within the basic leucine zipper (bZIP) transcription factor Hac1p to induce expression of UPR genes (Cox et al. 1993; Mori et al. 1993). Subsequently, several groups showed that X-box binding protein-1 (XBP1) mRNA is the mammalian homolog of yeast Ire1p and the substrate for the endoribonuclease activity of mammalian IRE1 (Shen et al. 2001; Yoshida et al. 2001; Calfon et al. 2002; Lee et al. 2002). On activation of the UPR, the endoribonuclease activity of IRE1 cleaves XBP1 mRNA to remove a 26 base intron. This splicing reaction creates a translational frame shift to produce the active (or spliced) form of the transcription factor (XBP1s). Spliced Xbp1 is a transcriptional activator for many of the UPR target genes and, in conjunction with ATF6α, launches a transcriptional program to produce ER protein chaperones and proteins involved in ER biogenesis and phospholipid synthesis with the net effect of expanding the folding capacity of the ER to resolve the protein-folding defect (Lee et al. 2003). Some of the genes identified that require the IRE1/XBP1 pathway are those that encode functions involved in ERAD, such as EDEM, ERdj4, and PDI. Indeed, cells that are deficient in either IRE1 or XBP1 are defective in ERAD. Recently, the endoribonuclease activity of IRE1 was suggested to target and degrade ER-associated mRNAs as an additional mechanism to relieve the ER protein-folding load (Merksamer et al. 2008; Hollien et al. 2009).
The bZiP-containing activating transcription factor 6 (ATF6), the second arm of the UPR pathway, was identified as another regulatory protein that, like XBP1, binds the ER stress elements (ERSE-I and II), UPR elements (UPRE), and cAMP response elements (CRE) in the promoters of UPR–responsive genes (Yoshida et al. 1998). In this manner, increased expression of ERAD machinery, such as the ER degradation-enhancing α-mannosidase-like protein (EDEM), increases the clearance and degradation of misfolded proteins in the ER lumen (Kokame et al. 2001). There are two known alleles of ATF6, ATF6α and ATF6β, both synthesized in all tissues as ER transmembrane proteins. ATF6α deletion sensitizes cells and animals to persistent ER stress. In vivo, this failure to recover from ER stress results in fatty liver, uncovering a potential connection between ER stress and lipid metabolism (Wu et al. 2007). It was also reported that ATF6α interacts with CRTC2 to antagonize the ability of CREB to activate gluconeogenesis in the liver (Wang et al. 2009). The transcriptional coactivator PGC-1α, that regulates several exercise-associated aspects of skeletal muscle function, mediates the UPR in myotubes and skeletal muscle through coactivation of ATF6α. Efficient recovery from acute exercise is compromised in Atf6α-/- mice (Wu et al. 2011). Thus, both ATF6 and XBP1, a transcriptional target of ATF6 that requires splicing by the endoribonuclease activity of IRE1, are considered as the predominant regulators of the adaptive UPR transcriptional response to resolve protein misfolding.
Activation of the third arm of the UPR is mediated through PERK, an ER-associated transmembrane serine/threonine protein kinase. On accumulation of unfolded proteins in the ER lumen, PERK dimerization and trans-autophosphorylation leads to activation of the kinase function that phosphorylates the α subunit of eukaryotic translation initiation factor (eIF2α) at Ser51. This phosphorylation attenuates mRNA translation initiation to reduce protein synthesis and the protein folding demand on the ER (Harding et al. 1999, 2000a,b, 2001; Morimoto et al. 2004). There are three additional eIF2α kinases, PKR (double-stranded RNA-activated protein kinase), GCN2 (general control nonderepressible kinase 2), and HRI (heme-regulated inhibitor kinase) that also phosphorylate Ser51 on eIF2α. The precise role of the individual eIF2α kinases is somewhat unclear because a single stress can activate more than a single eIF2α kinase. For example, PKR is also activated by ER stress and PKR can protect cells from ER stress and initiate inflammatory response signaling (Nakamura et al. 2010). In addition to translational attenuation, activation of PERK branch of the UPR also decreases transcription of several dependent genes such as that of ribosomal RNA (DuRose et al. 2009). Although phosphorylation of eIF2α inhibits general translation initiation, it is required for the selective translation of several mRNAs. Two transcription factors that require eIF2α phosphorylation are the activating transcription factors 4 and 5 (ATF4, ATF5). Expression profiling studies identified several genes, including those encoding amino acid biosynthesis and transport functions, antioxidative stress responses, and apoptosis genes, such as growth arrest and DNA damage 34 (GADD34) and CAAT/Enhancer binding protein (C/EBP) homologous protein (CHOP) (Harding et al. 2000; Ma et al. 2002) that require PERK, eIF2α phosphorylation, and ATF4 (Scheuner et al. 2001; Ron 2002; Harding et al. 2003). Lack of eIF2α phosphorylation in β cells caused a severe diabetic phenotype because of heightened and unregulated proinsulin translation, defective folding, and trafficking of ER cargo proteins, reduced expression of ER stress response and β cell-specific genes, increased oxidative damage, and apoptosis. However, glucose intolerance and β cell death in these mice were attenuated by a diet containing antioxidants (Back et al. 2009). It seems that phosphorylation of eIF2α coordinately attenuates mRNA translation, prevents oxidative stress, and optimizes ER protein folding to support insulin production. The finding that increased proinsulin synthesis is sufficient to cause oxidative damage in β cells may reflect events in the β cell failure associated with insulin resistance in type 2 diabetes that include decreased insulin production, loss of β cell-specific gene expression, increased expression of UPR genes, oxidative stress, and apoptosis (Huang and Tindall 2007; Laybutt et al. 2007).
ER STRESS-DEATH RESPONSE
Apparently, execution of the UPR program does not always result in successful and efficient alleviation of the ER stress and therefore under conditions of severe or prolonged stress signals, the UPR can also culminate in induction of apoptosis (Rao et al. 2004). Both mitochondrial-dependent and -independent cell death pathways likely mediate apoptosis in response to ER stress. The ER might actually serve as a site where apoptotic signals are generated and integrated to elicit the death response. Several mechanisms by which apoptotic signals are generated at the ER include: (1) pro-apoptotic Bcl-2 proteins Bak and Bax are switched on by the IRE1α pathway leading to regulated Ca2+ release from the ER (Hetz et al. 2006); (2) cleavage and activation of procaspase-12; (3) IRE1-mediated activation of ASK1 (apoptosis signal-regulating kinase 1)/JNK (c-Jun amino terminal kinase); and finally, (4) PERK/eIF2α-dependent induction of the proapoptotic transcription factor CEBP homologous protein (CHOP). CHOP is a downstream transcriptional target of ATF6 and PERK/eIF2/ATF4. CHOP is a bZIP-containing transcription factor that inhibits the expression of Bcl-2, and activates transcription of several genes that encode apoptotic functions including GADD34, DR5, and TRB3 (McCullough et al. 2001). In addition, ER stress-induced IRE1α phosphorylation leads to recruitment of TRAF2 (tumor necrosis factor receptor-associated factor 2) and ASK1 to the cytosolic leaflet of ER membrane (Kawamori et al. 2003). Simultaneous activation of the PERK and IRE1 pathways also impacts NF-ĸB-IKk signaling pathway during ER stress by either activation of IKk or degradation of the p65 subunit (Hu et al. 2006).
The mechanism of ER stress-induced apoptosis through Bak and Bax, that localize to both ER and mitochondria, has been shown to be associated with release of ER calcium with concomitant increase of mitochondrial calcium (Nutt et al. 2002). The Ca2+ released from the ER enters the mitochondria leading to depolarization of the inner membrane, cytochrome c release, and activation of the Apaf-1 (apoptosis protease-activating factor 1)/procaspase-9-regulated apoptosis pathway. The mechanism by which ER stress is directly coupled to activation of caspases, particularly caspase 12, which was initially characterized by Nakagawa and Yuan (2000), remains somewhat elusive. The significance of ER stress-associated caspase 12 activation remains enigmatic because a functional caspase 12 is not conserved in humans.
Finally, analysis of gene-deleted mice has provided additional insight into ER stress-induced apoptosis. Cells from Apaf-1-deficient mice are susceptible to ER stress-induced apoptosis, indicating the existence of non-mitochondrial cell death pathways. Similarly, Bak/Bax double knockout, Caspase-12−/− and Chop−/− MEFs all show partial resistance to ER stress-induced apoptosis, further supporting the idea that they facilitate the apoptotic response on ER stress. Although, caspase-12-deficient and CHOP-deficient mice show no developmental defects, they display protection to genetically imposed or environmentally imposed ER stress. There are studies that indicate that ER stress-induced apoptosis may have a mitochondrial component (Deniaud et al. 2008). Overall, it seems PERK serves as a critical control point that determines commitment to cell death or promotes survival (Rutkowski et al. 2006; Ron and Walter 2007). However, there remain critical gaps in our understanding of the ability of individual UPR initiators to recognize and respond to various forms of ER stress and then engage distinct survival or death responses under different cellular environments.
ER-MITOCHONDRIA INTERACTIONS
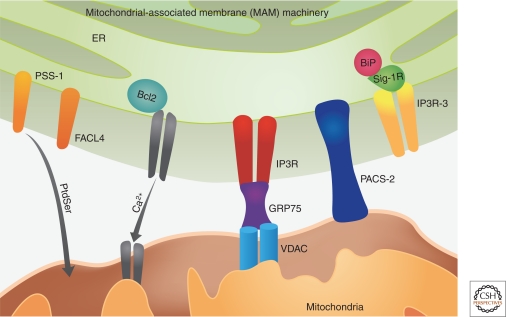
The classical concept of mitochondria as the cell’s powerhouse and as an isolated organelle has been profoundly challenged over the last two decades with the realization that mitochondria function within a highly dynamic integrated reticular network that is continually remodeled by both fusion and fission events. Both the ER and mitochondria are presently accepted as dynamic organelles capable of modifying their structure and function in response to changing environmental conditions. ER and mitochondria interact both physiologically and functionally, and one of the most critical aspects of this interaction is calcium signaling between the two organelles (Fig. 2). ER and mitochondria form close contacts with 20% of the mitochondrial surface in direct contact with the ER (Kornmann et al. 2009). These contacts through which the ER communicates with mitochondria are referred to as mitochondrial-associated membranes (MAM) (Vance 1990). These physical associations have pivotal roles in numerous cellular functions including Ca2+ signaling, lipid transport, energy metabolism, and cell survival. The ER-contiguous membranes also contain multiple phospholipids and glycosphingolipid synthesizing enzymes including long chain fatty acid–CoA ligase type 4 (FACl4) and phosphatidylserine synthase-1(PSS-1), and support direct transfer of lipids between the ER and mitochondria (Piccini et al. 1998; Stone and Vance 2000). The interaction between the two organelles is mediated by mitochondrial shaping proteins and key chaperones including calnexin, calreticulin, ERp44, ERp57, grp75, and the sigma-1 receptor. Over the years, a number of MAM-specific proteins have been identified including many ion channel and transporter proteins (IP3 receptors [IP3R], VDAC, Ca2+ ATPase, etc.), ubiquitin ligases, vesicular-sorting proteins, electron transport chain proteins, and mitochondrial fusion proteins. Most of these proteins are ER proteins with only a few belonging to the mitochondria such as the VDAC and the uniporters. The mitochondrial-shaping proteins that are involved in modulating these two organelles are Dynamin-related protein-1 (DRP1) (Smirnova et al. 2001) and mitofusin 1 and 2 (Mfn-1 and dMfn2) that regulate mitochondrial fission and fusion, respectively (Chen et al. 2003). The molecular machinery mediating fusion and fission events are very intricate requiring the independent but coordinated processing of both the outer and inner mitochondrial membranes. These proteins, including DRP1, Mfn1, and Mfn2, were originally identified in yeast but many of these genes have orthologs in mammals and belong to a large GTPase protein family. The vesicular-sorting protein, PACS-2 (phosphor-acidic cluster sorting protein 2) is a multifunctional sorting protein that controls the ER-mitochondria axis and the role of this axis in cellular homeostasis and apoptosis (Simmen et al. 2005). PACS-2 is required for the intimate association of mitochondria with ER. PACS-2 depletion results in mitochondrial fragmentation and uncouples this organelle from ER indicating that PACS-2 might be involved in ER protein folding and Ca2+ homeostasis (Szabadkai et al. 2004).
Figure 2.
MAMs support the lipid transfer from ER to mitochodria through enzymes FACL4 and PSS-1. MAMs are enriched in chaperones like sigma-1Rs and they colacalize with BiP and IP3R. A multifunctional sorting protein that controls the ER-mitochondria axis Mitochondrial chaperone grp75 is a link between ER Ca2+-release channel IP3R and isoform of VDAC.
Ca2+ SIGNALING AT THE MAM
An increasing body of evidence unequivocally suggests that the function of the ER is intimately connected with that of the mitochondria with Ca2+ signaling being at the hub of this interorganelle interaction. Mitochondria play significant roles in shaping the Ca2+ signal released from the ER. Under normal physiological conditions, the bulk of the Ca2+ resides within the ER lumen and, during cellular events requiring a Ca2+ signal, a small bolus is released into the cytoplasm only to be resequestered later and with a small proportion crossing the outer mitochondrial membrane. The ER Ca2+ functions both as a reservoir and simultaneously controls the activity of chaperones responsible for protein folding and processing (Rizzuto and Pozzan 2006). A great deal of recent evidence also shows that Ca2+ uptake into the mitochondria is controlled by specific proteins residing at the outer and inner mitochondrial membranes interface, namely the voltage-dependent anion channel (VDAC) and the Ca2+ uniporter (Duchen and Szabadkai 2010) and with mitochondrial Ca2+ being expelled by antiporters in an exchange process for either Na+ or H+. Thus, the antiporter and the exchanger maintain mitochondrial membrane potential and optimal Ca2+ concentrations in the mitochondria. At the same time, important cellular processes that connect apoptosis to ER-mitochondria interactions is manifested when alterations in Ca2+ homeostatic mechanisms result in massive and/or a prolonged mitochondrial Ca2+ overload.
The most important molecular component of Ca2+ handling machinery of the ER is represented by the IP3Rs that are primarily clustered in the MAM regions where ER is closely juxtaposed to the mitochondria and thereby delineating these zones as primary subcellular microdomains of Ca2+ transfer from the ER to the mitochondria (Rizzuto et al. 1998). The release of Ca2+ from ER stores by IP3Rs has implications in numerous models of apoptosis as deletion of IP3R gene by genetic ablation or antisense strategy increases resistance to apoptosis (Blackshaw et al. 2000). There are three isoforms of the IP3R gene and recent data shows that the type 3 gene (IP3R-3) localized to the MAM plays a selective role in apoptosis induction by selectively transmitting apoptotic Ca2+ signals into mitochondria, whereas the type 1 gene (IP3R-1) preferentially mediates cytosolic Ca2+ mobilization (Mendes et al. 2005). Finally, a fascinating aspect of this interaction is the finding that in response to survival signals, Akt interacts and phosphorylates IP3Rs, significantly reducing their Ca2+ release activity (Szado et al. 2008).
MOLECULAR CHAPERONES AT THE MAM
Both Ca2+-binding and glucose-regulated chaperones are abundantly found on the membranes as well as lumens of both ER and mitochondria. These chaperones serve as constitutive ER Ca2+ pools and also facilitate proper protein folding in a Ca2+-dependent manner. Some of these chaperones also couple to and regulate the activities of specific Ca2+ channels. A novel chaperone that specifically targets the MAM is the sigma-1R receptor, a ligand-operated Ca2+-sensitive ER chaperone that colocalizes with IP3R at the MAM. Sigma 1Rs form a Ca2+-sensitive machinery or complex at the MAM along with GRP78/BiP, and are now believed to prolong calcium signaling from the ER to mitochondria by stabilizing IP3R at the MAMs (Hayashi and Su 2007). The IP3 receptors are vulnerable to ubiquitylation and proteasomal degradation on stimulation by IP3, and thus stabilization of the IP3 receptors during intracellular signaling by the Sigma 1R is critical to maintain proper Ca2+ signaling both in the cytosol and in the mitochondria. Another important chaperone found at the MAM is the Grp75, and a recent study showed that cytosolic Grp75 tethers the ligand-binding domain of the IP3 receptors to VDAC1. The mitochondrial chaperone Grp75 regulates IP3R-mediated mitochondrial Ca2+ signaling (Szabadkai et al. 2006). Isoform 1 of VDAC is physically linked to the ER Ca2+ release channel IP3R through Grp75, highlighting chaperone-mediated conformational coupling between the IP3R and the mitochondrial Ca2+ uptake machinery. ER chaperones calnexin and calreticulin are also compartmentalized at MAM (Hayashi and Su 2007; Myhill et al. 2008). In addition to providing buffering capacity in ER, calreticulin inhibits the IP3 receptor-mediated Ca2+ signaling by using its high-affinity, low-capacity, Ca2+-binding domain (Camacho and Lechleiter 1995). Calnexin can regulate the Ca2+ ATPase activity via protein–protein interaction (Roderick et al. 2000). Another ER chaperone ERp57 can work in conjunction with calreticulin and facilitate in regulating the activity of Ca2+-ATPase (Li and Camacho 2004). ERp44 chaperone can inhibit type I IP3 receptors in a planar lipid bilayer system thereby modulating the IP3 receptor signaling (Anelli et al. 2003).
ER Ca2+ AND MITOCHONDRIAL PERMEABILITY TRANSITION
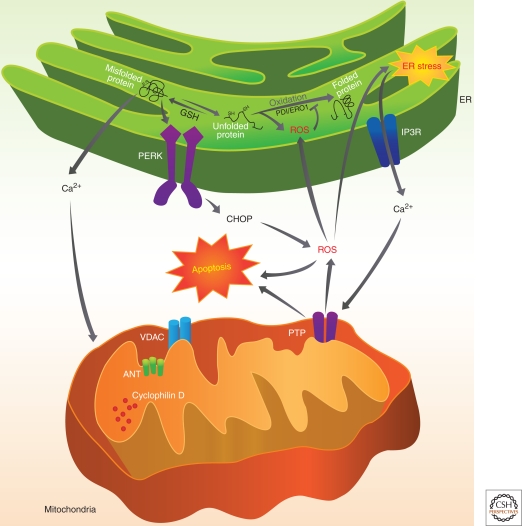
From the previous sections, it is clear that the function of the ER is intimately connected with that of the mitochondria, and a key process that links these ER-mitochondria interactions is the control of Ca2+ signaling. The mitochondria have a unique structure that contains two membranes that separates four distinct compartments, the outer mitochondrial membrane (OMM), the inner mitochondrial membrane (IMM), the space between these two membranes, and finally the matrix. The recent discovery that a massive and/or a prolonged influx of calcium into mitochondria can lead to the formation and opening of a large high-conductivity pore in the IMM, known as the mitochondrial permeability transition pore (PTP), a channel or “uniporter” driven by a large electrochemical gradient is critical for maintaining IMM stability. Mitochondrial Ca2+ overload and cellular redox status are considered the most potent inducers of permeability transition. The molecular identity of the PTP is not very clear but it seems to be comprised of the voltage-dependent anion channel (VDAC) localized in the OMM, the adenine nucleotide translocase (ANT) in the IMM, and cyclophilin D (a peptidyl-prolyl isomerase) localized in the matrix. Under normal physiological conditions, the opening and closing of PTP controls the homeostasis of mitochondria and regulates the matrix volume, Ca2+ flux, and the redox equilibrium and matrix pH. Under various pathophysiological conditions, permeabilization of the OMM can result in the release into the cytosol of a series of pro-apoptotic proteins such as cytochrome c, apoptosis-inducing factor (AIF), and smac/Diablo resulting in the demise of the cell through the execution of the apoptotic program involving proteases and nucleases. Recent studies have shown that the mitochondrial PTP plays a significant role in ischemia reperfusion injury in the heart as well as after myocardial infarction leading to breakdown of mitochondria and necrotic cell death (Halestrap 2010). The permeabilization of the OMM is also determined primarily by an interaction between the pro-Bax and Bak and antiapoptotic Bcl-2 family members (Welch et al. 2009). Intriguingly, the MAM has emerged as a key point in the regulation of mitochondrial Ca2+ and the redox equilibrium, functioning as a central hub of cellular signaling. The two abundant MAM-associated ion channels, the IP3R and the VDAC resident on the OMM, primarily mediate Ca2+ transfer between the two organelles and ultimately determining Ca2+ load (Fig. 3). Both these channels also function as redox sensors, and several proteins including regulators of autophagy (Beclin-1) and apoptosis (Bcl-2 and Bad) cluster around this core platform of Ca2+ channels. Antiapoptotic Bcl-2 members (Bcl-2 and Bcl2-XL) have been suggested to exert their effect by suppressing Ca2+ transfer from the ER to mitochondria. Overexpression of Bcl-2 decreases ER luminal Ca2+, thereby inhibiting Ca2+- and oxidative stress-mediated cell death (Pinton et al. 2000). On the other hand, studies have shown that knockdown of the proapoptotic members Bax and Bak increases the interaction of Bcl-2 with type-1 IP3Rs and promotes both the phosphorylation of the IP3R and constitutive Ca2+ leak through the IP3Rs (Oakes et al. 2005). Thus, Bcl-2 family members regulate IP3R-1 phosphorylation to control the rate of ER Ca2+ release and in a way regulate cell fate by determining the probability of opening the mitochondrial PTP.
Figure 3.
ER-mitochondria cross talk is mediated by protein misfolding within the ER, which results in release of calcium from the intracellular stores into the cytosol through IP3Rs. Ca2+ has a critical role in this ER and mitochondrial cross talk. Ca2+ released from ER is taken up by mitochondria and results in calcium overload and induces depolarization of permeability transition pore (PTP) and induces apoptotic stimuli to release caspases.
FUTURE DIRECTIONS
There has been tremendous progress over the past two decades in comprehending the mechanisms underlying ER stress-dependent UPR activation. The cellular processes linking protein folding, oxidative stress, and ER stress are tightly linked, and how aberrations in this signaling network communicate to the mitochondria to regulate cell death or survival is a fascinating emerging area of investigation. Future studies are required to understand how these stresses affect protein folding, misfolding, and secretion in vivo. These studies should identify under what physiological and pathological states these pathways are activated in vivo and how they finally influence disease outcome. A coherent understanding of the ER-mitochondria cross talk and nexus will certainly aid in the development of specific therapeutic strategies to treat diseases associated with protein misfolding and inflammation such as obesity, diabetes, and neurodegeneration, as well as those associated with aging.
ACKNOWLEDGMENTS
The authors appreciate the work of Janet L. Mitchell in preparing this article and Jennifer Harley in assisting with the illustrations. Portions of this work were supported by NIH grants DK042394, HL052173, and HL057346 (R.J.K.). Additionally, J.D.M. is supported by AHA grant 10SDG2610338.
Footnotes
Editors: Richard I. Morimoto, Dennis Selkoe, and Jeff Kelly
Additional Perspectives on Protein Homeostasis available at www.cshperspectives.org
REFERENCES
- Anelli T, Alessio M, Bachi A, Bergamelli L, Bertoli G, Camerini S, Mezghrani A, Ruffato E, Simmen T, Sitia R 2003. Thiol-mediated protein retention in the endoplasmic reticulum: The role of ERp44. EMBO J 22: 5015–5022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aragón T, van Anken E, Pincus D, Serafimova IM, Korennykh AV, Rubio CA, Walter P 2009. Messenger RNA targeting to endoplasmic reticulum stress signalling sites. Nature 457: 736–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back SH, Scheuner D, Han J, Song B, Ribick M, Wang J, Gildersleeve RD, Pennathur S, Kaufman RJ 2009. Translation attenuation through eIF2α phosphorylation prevents oxidative stress and maintains the differentiated state in β cells. Cell Metab 10: 13–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D 2000. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol 2: 326–332 [DOI] [PubMed] [Google Scholar]
- Blackshaw S, Sawa A, Sharp AH, Ross CA, Snyder SH, Khan AA 2000. Type 3 inositol 1,4,5-trisphosphate receptor modulates cell death. FASEB J 14: 1375–1379 [DOI] [PubMed] [Google Scholar]
- Calfon M, Zeng H, Urano F, Till JH, Hubbard SR, Harding HP, Clark SG, Ron D 2002. IRE1 couples endoplasmic reticulum load to secretory capacity by processing the XBP-1 mRNA. Nature 415: 92–96 [DOI] [PubMed] [Google Scholar]
- Camacho P, Lechleiter JD 1995. Calreticulin inhibits repetitive intracellular Ca2+ waves. Cell 82: 765–771 [DOI] [PubMed] [Google Scholar]
- Chen X, Shen J, Prywes R 2002. The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J Biol Chem 277: 13045–13052 [DOI] [PubMed] [Google Scholar]
- Chen H, Detmer SA, Ewald AJ, Griffin EE, Fraser SE, Chan DC 2003. Mitofusins Mfn1 and Mfn2 coordinately regulate mitochondrial fusion and are essential for embryonic development. J Cell Biol 160: 189–200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox JS, Shamu CE, Walter P 1993. Transcriptional induction of genes encoding endoplasmic reticulum resident proteins requires a transmembrane protein kinase. Cell 73: 1197–1206 [DOI] [PubMed] [Google Scholar]
- Credle JJ, Finer-Moore JS, Papa FR, Stroud RM, Walter P 2005. On the mechanism of sensing unfolded protein in the endoplasmic reticulum. Proc Natl Acad Sci 102: 18773–18784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deniaud A, Sharaf el dein O, Maillier E, Poncet D, Kroemer G, Lemaire C, Brenner C 2008. Endoplasmic reticulum stress induces calcium-dependent permeability transition, mitochondrial outer membrane permeabilization and apoptosis. Oncogene 27: 285–299 [DOI] [PubMed] [Google Scholar]
- Dorner AJ, Wasley LC, Kaufman RJ 1990. Protein dissociation from GRP78 and secretion are blocked by depletion of cellular ATP levels. Proc Natl Acad Sci 87: 7429–7432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchen MR, Szabadkai G 2010. Roles of mitochondria in human disease. Essays Biochem 47: 115–137 [DOI] [PubMed] [Google Scholar]
- DuRose JB, Scheuner D, Kaufman RJ, Rothblum LI, Niwa M 2009. Phosphorylation of eukaryotic translation initiation factor 2α coordinates rRNA transcription and translation inhibition during endoplasmic reticulum stress. Mol Cell Biol 29: 4295–4307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halestrap AP 2010. A pore way to die: The role of mitochondria in reperfusion injury and cardioprotection. Biochem Soc Trans 38: 841–860 [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Ron D 1999. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 397: 271–274 [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Zhang Y, Zeng H, Wek R, Schapira M, Ron D 2000a. Regulated translation initiation controls stress-induced gene expression in mammalian cells. Mol Cell 6: 1099–1108 [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Bertolotti A, Zeng H, Ron D 2000b. Perk is essential for translational regulation and cell survival during the unfolded protein response. Mol Cell 5: 897–904 [DOI] [PubMed] [Google Scholar]
- Harding HP, Novoa I, Bertolotti A, Zeng H, Zhang Y, Urano F, Jousse C, Ron D 2001. Translational regulation in the cellular response to biosynthetic load on the endoplasmic reticulum. Cold Spring Harb Symp Quant Biol 66: 499–508 [DOI] [PubMed] [Google Scholar]
- Harding HP, Zhang Y, Zeng H, Novoa I, Lu PD, Calfon M, Sadri N, Yun C, Popko B, Paules R, et al. 2003. An integrated stress response regulates amino acid metabolism and resistance to oxidative stress. Mol Cell 11: 619–633 [DOI] [PubMed] [Google Scholar]
- Hayashi T, Su TP 2007. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca2+ signaling and cell survival. Cell 131: 596–610 [DOI] [PubMed] [Google Scholar]
- Hetz C, Bernasconi P, Fisher J, Lee AH, Bassik MC, Antonsson B, Brandt GS, Iwakoshi NN, Schinzel A, Glimcher LH, et al. 2006. Proapoptotic BAX and BAK modulate the unfolded protein response by a direct interaction with IRE1α. Science 312: 572–57616645094 [Google Scholar]
- Hollien J, Lin JH, Li H, Stevens N, Walter P, Weissman JS 2009. Regulated Ire1-dependent decay of messenger RNAs in mammalian cells. J Cell Biol 186: 323–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu P, Han Z, Couvillon AD, Kaufman RJ, Exton JH 2006. Autocrine tumor necrosis factor α links endoplasmic reticulum stress to the membrane death receptor pathway through IRE1α-mediated NF-kappaB activation and down-regulation of TRAF2 expression. Mol Cell Biol 26: 3071–3084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H, Tindall DJ 2007. Dynamic FoxO transcription factors. J Cell Sci 120: 2479–2487 [DOI] [PubMed] [Google Scholar]
- Kaufman RJ 2002. Orchestrating the unfolded protein response in health and disease. J Clin Invest 110: 1389–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamori D, Kajimoto Y, Kaneto H, Umayahara Y, Fujitani Y, Miyatsuka T, Watada H, Leibiger IB, Yamasaki Y, Hori M 2003. Oxidative stress induces nucleo-cytoplasmic translocation of pancreatic transcription factor PDX-1 through activation of c-Jun NH(2)-terminal kinase. Diabetes 52: 2896–2904 [DOI] [PubMed] [Google Scholar]
- Kincaid MM, Cooper AA 2007. Misfolded proteins traffic from the endoplasmic reticulum (ER) due to ER export signals. Mol Biol Cell 18: 455–463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleizen B, Braakman I 2004. Protein folding and quality control in the endoplasmic reticulum. Curr Opin Cell Biol 16: 343–349 [DOI] [PubMed] [Google Scholar]
- Kokame K, Kato H, Miyata T 2001. Identification of ERSE-II, a new cis-acting element responsible for the ATF6-dependent mammalian unfolded protein response. J Biol Chem 276: 9199–9205 [DOI] [PubMed] [Google Scholar]
- Korennykh AV, Egea PF, Korostelev AA, Finer-Moore J, Zhang C, Shokat KM, Stroud RM, Walter P 2009. The unfolded protein response signals through high-order assembly of Ire1. Nature 457: 687–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornmann B, Currie E, Collins SR, Schuldiner M, Nunnari J, Weissman JS, Walter P 2009. An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 325: 477–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laybutt DR, Hawkins YC, Lock J, Lebet J, Sharma A, Bonner-Weir S, Weir GC 2007. Influence of diabetes on the loss of β cell differentiation after islet transplantation in rats. Diabetologia 50: 2117–2125 [DOI] [PubMed] [Google Scholar]
- Lee K, Tirasophon W, Shen X, Michalak M, Prywes R, Okada T, Yoshida H, Mori K, Kaufman RJ 2002. IRE1-mediated unconventional mRNA splicing and S2P-mediated ATF6 cleavage merge to regulate XBP1 in signaling the unfolded protein response. Genes Dev 16: 452–466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AH, Iwakoshi NN, Glimcher LH 2003. XBP-1 regulates a subset of endoplasmic reticulum resident chaperone genes in the unfolded protein response. Mol Cell Biol 23: 744–759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Camacho P 2004. Ca2+-dependent redox modulation of SERCA 2b by ERp57. J Cell Biol 164: 35–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Korennykh AV, Behrman SL, Walter P 2010. Mammalian endoplasmic reticulum stress sensor IRE1 signals by dynamic clustering. Proc Natl Acad Sci 107: 16113–16118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CY, Xu Z, Kaufman RJ 2003. Structure and intermolecular interactions of the luminal dimerization domain of human IRE1α. J Biol Chem 278: 17680–17687 [DOI] [PubMed] [Google Scholar]
- Ma Y, Hendershot LM 2004. ER chaperone functions during normal and stress conditions. J Chem Neuroanat 28: 51–65 [DOI] [PubMed] [Google Scholar]
- Ma Y, Brewer JW, Diehl JA, Hendershot LM 2002. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J Mol Biol 318: 1351–1365 [DOI] [PubMed] [Google Scholar]
- McCullough KD, Martindale JL, Klotz LO, Aw TY, Holbrook NJ 2001. Gadd153 sensitizes cells to endoplasmic reticulum stress by down-regulating Bcl2 and perturbing the cellular redox state. Mol Cell Biol 21: 1249–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes CC, Gomes DA, Thompson M, Souto NC, Goes TS, Goes AM, Rodrigues MA, Gomez MV, Nathanson MH, Leite MF 2005. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J Biol Chem 280: 40892–40900 [DOI] [PubMed] [Google Scholar]
- Merksamer PI, Trusina A, Papa FR 2008. Real-time redox measurements during endoplasmic reticulum stress reveal interlinked protein folding functions. Cell 135: 933–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molinari M 2007. N-glycan structure dictates extension of protein folding or onset of disposal. Nat Chem Biol 3: 313–320 [DOI] [PubMed] [Google Scholar]
- Mori K, Ma W, Gething MJ, Sambrook J 1993. A transmembrane protein with a cdc2+/CDC28-related kinase activity is required for signaling from the ER to the nucleus. Cell 74: 743–756 [DOI] [PubMed] [Google Scholar]
- Morimoto H, Okamura H, Yoshida K, Kitamura S, Haneji T 2004. Okadaic acid induces apoptosis through double-stranded RNA-dependent protein kinase/eukaryotic initiation factor-2α pathway in human osteoblastic MG63 cells. J Biochem 136: 433–438 [DOI] [PubMed] [Google Scholar]
- Myhill N, Lynes EM, Nanji JA, Blagoveshchenskaya AD, Fei H, Carmine Simmen K, Cooper TJ, Thomas G, Simmen T 2008. The subcellular distribution of calnexin is mediated by PACS-2. Mol Biol Cell 19: 2777–2788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadanaka S, Okada T, Yoshida H, Mori K 2007. Role of disulfide bridges formed in the luminal domain of ATF6 in sensing endoplasmic reticulum stress. Mol Cell Biol 27: 1027–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa T, Zhu H, Morishima N, Li E, Xu J, Yankner BA, Yuan J 2000. Caspase-12 mediates endoplasmic-reticulum-specific apoptosis and cytotoxity by amyloid-beta. Nature 6: 98–103 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Furuhashi M, Li P, Cao H, Tuncman G, Sonenberg N, Gorgun CZ, Hotamisligil GS 2010. Double-stranded RNA-dependent protein kinase links pathogen sensing with stress and metabolic homeostasis. Cell 140: 338–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutt LK, Pataer A, Pahler J, Fang B, Roth J, McConkey DJ, Swisher SG 2002. Bax and Bak promote apoptosis by modulating endoplasmic reticular and mitochondrial Ca2+ stores. J Biol Chem 277: 9219–9225 [DOI] [PubMed] [Google Scholar]
- Oakes SA, Scorrano L, Opferman JT, Bassik MC, Nishino M, Pozzan T, Korsmeyer SJ 2005. Proapoptotic BAX and BAK regulate the type 1 inositol trisphosphate receptor and calcium leak from the endoplasmic reticulum. Proc Natl Acad Sci 102: 105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oikawa D, Kimata Y, Kohno K 2007. Self-association and BiP dissociation are not sufficient for activation of the ER stress sensor Ire1. J Cell Sci 120: 1681–1688 [DOI] [PubMed] [Google Scholar]
- Patil C, Walter P 2001. Intracellular signaling from the endoplasmic reticulum to the nucleus: The unfolded protein response in yeast and mammals. Curr Opin Cell Biol 13: 349–355 [DOI] [PubMed] [Google Scholar]
- Piccini M, Vitelli F, Bruttini M, Pober BR, Jonsson JJ, Villanova M, Zollo M, Borsani G, Ballabio A, Renieri A 1998. FACL4, a new gene encoding long-chain acyl-CoA synthetase 4, is deleted in a family with Alport syndrome, elliptocytosis, and mental retardation. Genomics 47: 350–358 [DOI] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Magalhães P, Schulze-Osthoff K, Di Virgilio F, Pozzan T, Rizzuto R 2000. Reduced loading of intracellular Ca2+ stores and downregulation of capacitative Ca2+ influx in Bcl-2-overexpressing cells. J Cell Biol 148: 857–862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RV, Ellerby HM, Bredesen DE 2004. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Differ 11: 372–380 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pozzan T 2006. Microdomains of intracellular Ca2+: Molecular determinants and functional consequences. Physiol Rev 86: 369–408 [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T 1998. Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses. Science 280: 1763–1766 [DOI] [PubMed] [Google Scholar]
- Roderick HL, Lechleiter JD, Camacho P 2000. Cytosolic phosphorylation of calnexin controls intracellular Ca2+ oscillations via an interaction with SERCA2b. J Cell Biol 149: 1235–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D 2002. Proteotoxicity in the endoplasmic reticulum: Lessons from the Akita diabetic mouse. J Clin Invest 109: 443–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ron D, Walter P 2007. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol 8: 519–529 [DOI] [PubMed] [Google Scholar]
- Rutkowski DT, Arnold SM, Miller CN, Wu J, Li J, Gunnison KM, Mori K, Sadighi Akha AA, Raden D, Kaufman RJ 2006. Adaptation to ER stress is mediated by differential stabilities of prosurvival and proapoptotic mRNAs and proteins. PLoS Biol 4: e374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheuner D, Song B, McEwen E, Liu C, Laybutt R, Gillespie P, Saunders T, Bonner-Weir S, Kaufman RJ 2001. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Mol Cell 7: 1165–1176 [DOI] [PubMed] [Google Scholar]
- Schroder M, Kaufman RJ 2005. The mammalian unfolded protein response. Annu Rev Biochem 74: 739–789 [DOI] [PubMed] [Google Scholar]
- Shen X, Ellis RE, Lee K, Liu CY, Yang K, Solomon A, Yoshida H, Morimoto R, Kurnit DM, Mori K, et al. 2001. Complementary signaling pathways regulate the unfolded protein response and are required for C. elegans development. Cell 107: 893–903 [DOI] [PubMed] [Google Scholar]
- Simmen T, Aslan JE, Blagoveshchenskaya AD, Thomas L, Wan L, Xiang Y, Feliciangeli SF, Hung CH, Crump CM, Thomas G 2005. PACS-2 controls endoplasmic reticulum-mitochondria communication and Bid-mediated apoptosis. EMBO J 24: 717–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnova E, Griparic L, Shurland DL, van der Bliek AM 2001. Dynamin-related protein Drp1 is required for mitochondrial division in mammalian cells. Mol Biol Cell 12: 2245–2256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SJ, Vance JE 2000. Phosphatidylserine synthase-1 and -2 are localized to mitochondria-associated membranes. J Biol Chem 275: 34534–34540 [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Simoni AM, Chami M, Wieckowski MR, Youle RJ, Rizzuto R 2004. Drp-1-dependent division of the mitochondrial network blocks intraorganellar Ca2+ waves and protects against Ca2+-mediated apoptosis. Mol Cell 16: 59–68 [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Simoni AM, Bianchi K, De Stefani D, Leo S, Wieckowski MR, Rizzuto R 2006. Mitochondrial dynamics and Ca2+ signaling. Biochim Biophys Acta 1763: 442–449 [DOI] [PubMed] [Google Scholar]
- Szado T, Vanderheyden V, Parys JB, De Smedt H, Rietdorf K, Kotelevets L, Chastre E, Khan F, Landegren U, Söderberg O, et al. 2008. Phosphorylation of inositol 1,4,5-trisphosphate receptors by protein kinase B/Akt inhibits Ca2+ release and apoptosis. Proc Natl Acad Sci 105: 2427–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance JE 1990. Phospholipid synthesis in a membrane fraction associated with mitochondria. J Biol Chem 265: 7248–7256 [PubMed] [Google Scholar]
- Wang Y, Vera L, Fischer WH, Montminy M 2009. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature 460: 534–537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch C, Santra MK, El-Assaad W, Zhu X, Huber WE, Keys RA, Teodoro JG, Green MR 2009. Identification of a protein, G0S2, that lacks Bcl-2 homology domains and interacts with and antagonizes Bcl-2. Cancer Res 69: 6782–6789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Kaufman RJ 2006. From acute ER stress to physiological roles of the unfolded protein response. Cell Death Differ 13: 374–384 [DOI] [PubMed] [Google Scholar]
- Wu J, Rutkowski DT, Dubois M, Swathirajan J, Saunders T, Wang J, Song B, Yau GD, Kaufman RJ 2007. ATF6α optimizes long-term endoplasmic reticulum function to protect cells from chronic stress. Dev Cell 13: 351–364 [DOI] [PubMed] [Google Scholar]
- Wu J, Ruas JL, Estall JL, Rasbach KA, Choi JH, Ye L, Boström P, Tyra HM, Crawford RW, Campbell KP, et al. 2011. The unfolded protein response mediates adaptation to exercise in skeletal muscle through a PGC-1α/ATF6α complex. Cell Metab 13: 160–169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto K, Sato T, Matsui T, Sato M, Okada T, Yoshida H, Harada A, Mori K 2007. Transcriptional induction of mammalian ER quality control proteins is mediated by single or combined action of ATF6α and XBP1. Dev Cell 13: 365–376 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Haze K, Yanagi H, Yura T, Mori K 1998. Identification of the cis-acting endoplasmic reticulum stress response element responsible for transcriptional induction of mammalian glucose-regulated proteins. Involvement of basic leucine zipper transcription factors. J Biol Chem 273: 33741–33749 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Yamamoto A, Okada T, Mori K 2001. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell 107: 881–891 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Matsui T, Hosokawa N, Kaufman RJ, Nagata K, Mori K 2003. A time-dependent phase shift in the mammalian unfolded protein response. Dev Cell 4: 265–271 [DOI] [PubMed] [Google Scholar]
- Zhou J, Liu CY, Back SH, Clark RL, Peisach D, Xu Z, Kaufman RJ 2006. The crystal structure of human IRE1 luminal domain reveals a conserved dimerization interface required for activation of the unfolded protein response. Proc Natl Acad Sci 103: 14343–14348 [DOI] [PMC free article] [PubMed] [Google Scholar]