Abstract
Voltage sensitive phosphatases (VSPs) are unique proteins in which membrane potential controls enzyme activity. They are comprised of the voltage sensor domain of an ion channel coupled to a lipid phosphatase specific for phosphoinositides, and for ascidian and zebrafish VSPs, the phosphatase activity has been found to be activated by membrane depolarization. The physiological functions of these proteins are unknown, but their expression in testis and embryos suggests a role in fertilization or development. Here we investigate the expression pattern and voltage dependence of VSPs in two frog species, Xenopus laevis and Xenopus tropicalis, that are well suited for experimental studies of these possible functions. X. laevis has two VSP genes (Xl-VSP1 and Xl-VSP2), whereas X. tropicalis has only one gene (Xt-VSP). The highest expression of these genes was observed in testis, ovary, liver, and kidney. Our results show that while Xl-VSP2 activates only at positive membrane potentials outside of the physiological range, Xl-VSP1 and Xt-VSP phosphatase activity is regulated in the voltage range that regulates sperm-egg fusion at fertilization.
Keywords: voltage sensitive phosphatase, Xenopus, fertilization
Introduction
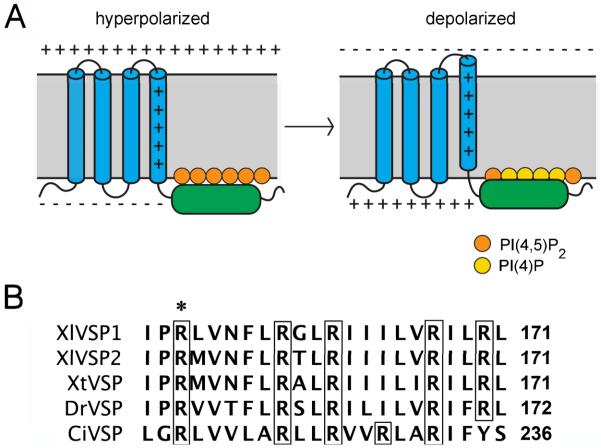
Voltage-sensitive phosphatases (VSPs) are four-transmembrane proteins that contain the characteristic voltage sensor domain of an ion channel, but instead of controlling an ion pore, the voltage sensor is linked to a cytoplasmic phosphoinositide phosphatase (Murata et al., 2005). Studies of the VSPs of the ascidian Ciona intestinalis (Ci-VSP) and the zebrafish Danio rerio VSP (Dr-VSP) have shown that depolarization activates the phosphatase activity (Murata and Okamura, 2007; Hossain et al., 2008). This has been proposed to occur because movement of the voltage sensor results in movement of the phosphatase domain closer to the plasma membrane (Villalba-Galea et al., 2009), where it cleaves the 5′-phosphates of phosphoinositides including PI(4,5)P2 (Iwasaki et al., 2008; Hossain et al., 2008; Halaszovich et al., 2009; Fig. 1A).
Fig. 1.
VSP transmembrane topology and voltage sensor sequence. A: The VSP protein is comprised of four transmembrane domains (blue) and a cytoplasmic phosphatase region (green). Depolarization-induced movement of the positively charged fourth transmembrane domain results in increased dephosphorylation of PI(4,5)P2, possibly due to movement of the phosphatase region closer to its substrate (see Murata and Okamura, 2007; Bezanilla, 2008; Villalba-Galea et al., 2009; Okamura and Dixon, 2011). B: Amino acids of the fourth transmembrane domain of VSPs of Xenopus laevis, Xenopus tropicalis, Danio rerio, and Ciona intestinalis. The transmembrane region is identified by the presence of multiple hydrophobic amino acids. The precise boundaries of this domain are predicted differently by different algorithms, and may vary depending on membrane potential. Arginines (R) are boxed, and R152 of Xl-VSP1 is indicated by an asterisk.
Genes with similar sequences and predicted transmembrane topology to ascidian and zebrafish VSPs have been identified in other vertebrates, and have been named transmembrane phosphatase with tensin homology (TPTE) family genes (Tapparel et al., 2003; Hossain et al., 2008; Neuhaus and Hollemann, 2009). TPTEs are closely related to the phosphatase and tensin homolog (PTEN) gene family (Wu et al., 2001; Okamura and Dixon, 2011). The human genome contains two TPTE gene family members, TPTE and TPTE2 (also known as TPIP) (Tapparel et al., 2003).
Phosphoinositide phosphatase activity has been demonstrated for the ascidian and zebrafish VSP/TPTEs, and for the mouse and human proteins as well (Walker et al., 2001; Wu et al., 2001; Hossain et al., 2008; Iwasaki et al., 2008). So far, however, regulation of the phosphatase activity by voltage has been shown only for the ascidian and zebrafish proteins; for ascidian, the regulation has been established to occur in a physiological voltage range (Murata et al. 2005; Murata and Okamura, 2007; Hossain et al., 2008; Halaszovich et al., 2009).
The broad species distribution of the VSP/TPTE family proteins suggests important biological roles, but their functions are currently unknown. Previous studies have indicated that VSP/TPTE genes have highest expression in the testis and in embryos and larvae, suggesting possible roles in sperm physiology, fertilization, or development (Chen et al., 1999; Walker et al., 2001; Wu et al., 2001; Reymond et al., 2002; Tapparel et al., 2003; Murata et al., 2005; Hossain et al., 2008; Neuhaus and Hollemann, 2009; Ogasawara et al., 2011). Of particular interest is the possibility that VSPs could be voltage sensors accounting for the voltage dependence of fertilization (Jaffe, 1976; Okamura, 2007). In addition, VSP/TPTE expression has been reported for the neural complex and blood cells of adult ascidians (Murata et al., 2005; Ogasawara et al., 2011), and for human brain and stomach (Walker et al., 2001). In contrast, in adult mice, TPTE expression is restricted to testis, among 12 tissues examined (Wu et al., 2001). The tissue distribution of expression of VSP/TPTE genes is unknown for other vertebrates.
In this study we investigate the expression pattern and voltage dependence of VSPs in frogs of the genus Xenopus. X. laevis has two VSP genes (Xl-VSP1 and Xl-VSP2), whereas X. tropicalis has only one gene (Xt-VSP). We determine the relative abundance of these transcripts in various Xenopus tissues, and find elevated expression not only in testis, but also in ovary, liver, and kidney. Our results show that Xl-VSP1 and Xt-VSP phosphatase activity is regulated in the voltage range that regulates sperm-egg fusion at fertilization (Jaffe et al., 1983; Iwao et al., 1994; Glahn and Nuccitelli, 2003).
Results and Discussion
Identification of VSP/TPTE genes in the Xenopus laevis and Xenopus tropicalis genomes
To identify VSP/TPTE genes in Xenopus, we searched an expressed sequence tag database (NCBI dbEST) using the sequence of the mouse homolog of VSP/TPTE. This search identified two candidate genes in the allotetraploid species X. laevis (Xl-VSP1 and Xl-VSP2) and one in the diploid species X. tropicalis (Xt-VSP). These were confirmed to be VSP/TPTE genes by multiple sequence alignment using Clustal W (Fig. S1, Table S1). We refer to them as VSPs because, as will be described below, they have voltage sensitive phosphatase activity. As for other species, the Xenopus proteins are predicted to have four transmembrane domains. They have multiple conserved arginines in the fourth transmembrane segment, characteristic of a voltage sensor (Fig. 1B), and a cytoplasmic phosphatidylinositide phosphatase region (Fig. S1).
Testis, ovary, kidney, and liver are sites of high Xenopus VSP expression
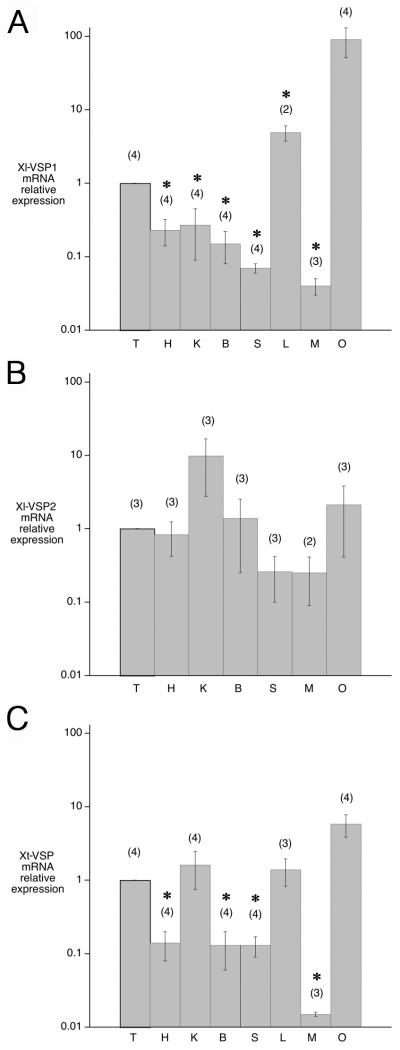
To determine the expression pattern of the Xenopus VSPs, we analyzed the relative levels of Xl-VSP1, Xl-VSP2 and Xt-VSP mRNAs in various X. laevis and tropicalis tissues, using quantitative RT-PCR. Each reaction was normalized for total RNA input. Since testis has been reported to have the highest level of VSP/TPTE RNA among adult tissues of other species (ascidian: Murata et al., 2005; mouse: Wu et al., 2001; human: Chen et al., 1999; Walker et al., 2001; Tapparel et al., 2003), we plotted our results relative to the amount of VSP mRNA in testis (Fig. 2). Xl-VSP1, Xl-VSP2, and Xt-VSP RNAs were all present in testis, but high levels were found in the ovary as well. In fully-grown oocytes that were isolated from the ovary, the Xl-VSP1 RNA concentration was similar to that in the whole ovary from the same frog (data not shown). Liver (Xl-VSP1, Xt-VSP) and kidney (Xl-VSP2, Xt-VSP) also showed a high level of these RNAs.
Fig. 2.
Expression of Xenopus VSP RNAs in various tissues. A: Xl-VSP1, B: Xl-VSP2, C: Xt-VSP. T, testis; H, heart; K, kidney; B, brain; S, stomach; L, liver; M, muscle; O, ovary. Levels of VSP RNAs were determined using quantitative RT-PCR, and values are normalized to that of testis. Error bars indicate mean +/− SEM, (n) = number of animals. Asterisks indicate values that are significantly different from that for testis (P < 0.05). P-values were determined using an unpaired Student’s t-test (Instat, GraphPad Software, San Diego, CA).
In future studies, it will be of particular interest to analyze the cell types within the testis in which the Xenopus VSPs are expressed. In situ hybridization has indicated that RNA encoding mouse VSP/TPTE is localized in secondary spermatocytes and early spermatids (Wu et al., 2001). Immunofluorescence has indicated that Ciona VSP protein is present in sperm (Murata et al., 2005).
Measurement of Xenopus VSP activation using a fluorescent sensor for PI(4,5)P2 coexpressed with VSPs in Xenopus oocytes
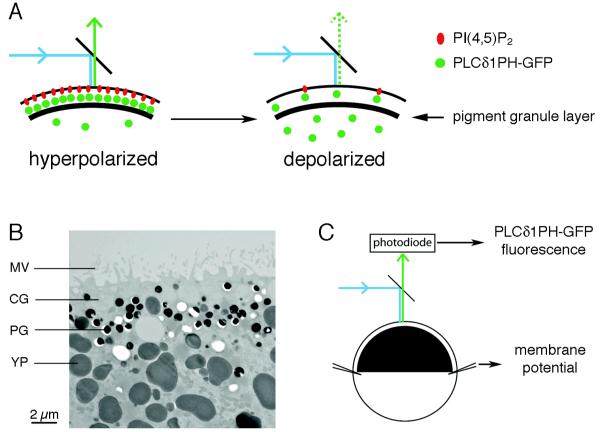
To investigate the voltage-dependence of Xenopus VSP activation, we used a fluorescent sensor for PI(4,5)P2, PLCδ1PH-GFP, which is comprised of the pleckstrin homology domain of PLCδ1 fused to GFP. This sensor localizes to the plasma membrane when bound to PI(4,5)P2, but diffuses into the cytoplasm upon PI(4,5)P2 depletion (Stauffer et al., 1998; Várnai and Balla, 1998). For the experiments to be described, we used fully-grown, prophase-arrested Xenopus oocytes as an expression system. Xenopus oocytes have a dense layer of pigment granules ~3 μm below the plasma membrane in their black “animal” half (Fig. 3A,B). These opaque structures, as well as the yolk platelets throughout the oocyte interior (Fig. 3B), provide an optical mask, shielding most of the cytoplasmic fluorescence. Thus measurement of total PLCδ1PH-GFP fluorescence from the oocyte surface, using a photodiode, provides a measure of PI(4,5)P2 in the oocyte plasma membrane (Fig. 3A,C).
Fig. 3.
Method for measurement of VSP activation in Xenopus oocytes. A: Schematic diagram of the surface of a Xenopus oocyte showing the plasma membrane containing PI(4,5)P2, with the fluorescent sensor PLCδ1PH-GFP bound to it. The thick black band represents the layer of pigment granules underlying the plasma membrane. Application of blue light excites GFP, which emits green light. Activation of the VSP by membrane depolarization depletes PI(4,5)P2, allowing PLCδ1PH-GFP to diffuse away from the membrane, such that its fluorescence is shielded by the pigment granules and yolk platelets in the oocyte cytoplasm. B: Electron micrograph showing the cortical structures in the black half of a Xenopus oocyte. MV, microvilli; CG, cortical granule; PG, pigment granule; YP, yolk platelet. The white areas are PGs and YPs that have been lost from the section. C: Photodiode measurement of PLCδ1PH-GFP emission from a voltage-clamped Xenopus oocyte.
Oocytes coinjected with synthetic mRNAs encoding VSPs and PLCδ1PH-GFP were voltage clamped, and GFP fluorescence was collected from the black half of the oocyte during test pulses to more positive membrane potentials (Figs 3C, 4A). In response to increasing depolarization, the fluorescence detected from the oocyte surface progressively decreased, indicating that depolarization activates the PI(4,5)P2 phosphatase activity of VSP (shown for Xl-VSP1 in Fig. 4A). This result confirms previous studies of ascidian and zebrafish VSPs, using the same PI(4,5)P2 sensor expressed in Xenopus oocytes and detected by confocal microscopy (Murata and Okamura, 2007), or expressed in cultured mammalian cells and detected by total internal reflection (TIRF) (Halaszovich et al. 2009) or Förster resonance energy transfer (FRET) microscopy (Falkenburger et al., 2010).
Fig. 4.
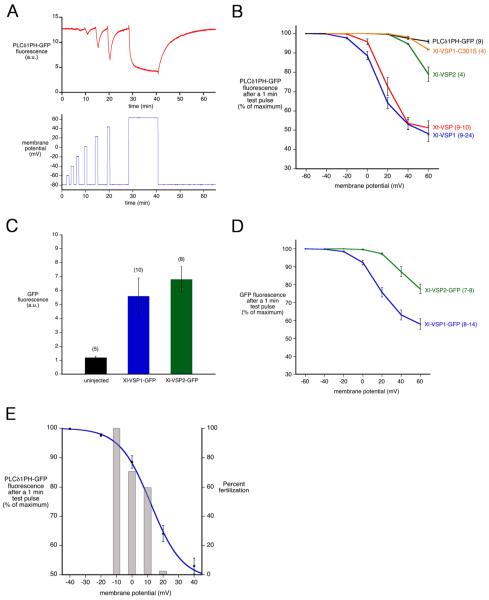
Voltage-dependence of Xenopus VSP activity. A: PLCδ1PH-GFP fluorescence, from an oocyte coexpressing PLCδ1PH-GFP and Xl-VSP1, in response to one minute test pulses to increasingly depolarized membrane potentials. B: Fluorescence intensity as a function of voltage, from oocytes coexpressing PLCδ1PH-GFP and Xl-VSP1, Xl-VSP2, Xt-VSP, Xl-VSP1-C301S, or PLCδPH-GFP alone. C: Measurement of Xl-VSP1-GFP and Xl-VSP2-GFP expression, as determined by GFP fluorescence after injection of Xenopus oocytes with equal amounts of each VSP RNA; PLCδ1PH-GFP was not co-injected. D: Fluorescence intensity as a function of voltage, from oocytes coexpressing PLCδ1PH-GFP and Xl-VSP1-GFP or Xl-VSP2-GFP. The fluorescence intensity shown in (D) is a sum of that from PLCδ1PH-GFP and a small component from the GFP tag on the VSP (< 20%). E: Correlation between the voltage dependence of Xl-VSP1 activity (blue curve, fit with a Boltzmann function to the averaged data from Fig. 4B) and the voltage dependence of Xenopus laevis fertilization (gray bars; data from Iwao et al., 1994). Error bars indicate mean +/− SEM, (n) = number of oocytes, listed as a range in cases where the number of data points varied for different voltages.
Only a small voltage-dependent decrease in PLCδ1PH-GFP fluorescence was seen in oocytes expressing PLCδ1PH-GFP alone (Fig. 4B), indicating that although VSP RNA is endogenously expressed in the oocyte, little VSP protein is present in the oocyte plasma membrane. We also made similar measurements in oocytes that had been treated with progesterone to cause them to resume meiosis and progress from prophase (the stage in the ovary) to second metaphase (the stage at which ovulation and fertilization occur). Like the prophase stage oocytes, those at second metaphase had little or no functionally detectable VSP protein in their plasma membrane (data not shown). Thus, like many RNAs in oocytes, VSP mRNA may be present in preparation for embryogenesis, but not translated in the oocyte, due to repressive complexes that interact with regions of the endogenous RNA outside of the coding sequence (Richter, 2007; Evsikov and Marín de Evsikova, 2009). The VSP RNAs that we injected were not subject to this inhibition because they included only the coding region and not the flanking regulatory sequences.
Upon return to resting membrane potential (or a holding potential of −80 mV), the PLCδ1PH-GFP fluorescence signal recovered to baseline (Fig. 4A), indicating that an active PIP5-kinase rapidly resynthesizes PI(4,5)P2.
Xl-VSP1 and Xt-VSP activate in a physiological range of membrane potentials, but Xl-VSP2 activates at more positive potentials
To evaluate the voltage-dependence of the activation of Xl-VSP1, Xl-VSP2, and Xt-VSP, we measured PLCδ1PH-GFP fluorescence after one minute membrane depolarizations. The oocyte membrane potential was clamped in +20 mV increments from a holding potential of −80 mV, allowing the fluorescence signal to recover between test pulses (Fig. 4A). By this method, activation of Xl-VSP1 and Xt-VSP was detectable at −20 mV and 0 mV respectively, whereas Xl-VSP2 did not show activation until +40 mV (Fig. 4B). Oocytes expressing a catalytically-inactive mutant of Xl-VSP1 (C301S), showed little decrease in fluorescence even at +60 mV (Fig. 4B).
To examine whether the shift in voltage dependence of the phosphatase activity seen with Xl-VSP2 might be due to lower membrane expression, we tagged Xl-VSP1 and Xl-VSP2 with GFP to compare their expression levels. Both tagged proteins localized to the plasma membrane in Xenopus oocytes, and for oocytes injected with the same amount of RNA for Xl-VSP1-GFP or Xl-VSP2-GFP, the same amount of fluorescence was measured from each, indicating that the same amount of each protein was expressed (Fig. 4C). With the amounts of RNA that we injected, Xl-VSP1-GFP and Xl-VSP2-GFP emitted ~5x less fluorescence than PLCδ1PH-GFP, which allowed us to use this sensor together with the GFP-tagged VSPs. These measurements showed an ~40 mV positive shift in the voltage required to activate Xl-VSP2-GFP relative to that required for Xl-VSP1-GFP (Fig. 4D). Thus under conditions where we demonstrated equivalent protein expression, we confirmed that Xl-VSP1 and Xl-VSP2 differ in their voltage dependence.
Mutation of an arginine in the fourth transmembrane domain shifts the activation of Xl-VSP1 to more negative membrane potentials
To further characterize the properties of Xl-VSP1, and to generate a tool for future studies of Xl-VSP1 function, we investigated the effect of mutating an arginine near the extracellular side of the fourth transmembrane domain (R152Q; Fig. 1B) on the voltage-dependence of the activation of phosphatase activity. Mutation of the analogous arginine in VSPs of other species results in a negative shift in the voltage dependence of the motion of the voltage sensor domain as detected by a FRET sensor (Ci-VSP; Dimitrov et al., 2007), by the “gating” or “sensing” current (Dr-VSP; Hossain et al., 2008), and by fluorometry (Ci-VSP; Kohout et al., 2008). Correspondingly, the phosphatase activity of Xl-VSP1-R152Q activated at an ~20 mV more negative voltage than that of Xl-VSP1, with a half-maximal response at −11 mV vs +12 mV (Fig. 5A). This mutation also shifted the voltage dependence of Xl-VSP1-GFP activation, with a half-maximal response occuring at −31 mV vs +17 mV for the wildtype protein. It is unknown why the negative shift caused by the R152Q mutation was more pronounced for the GFP-tagged protein.
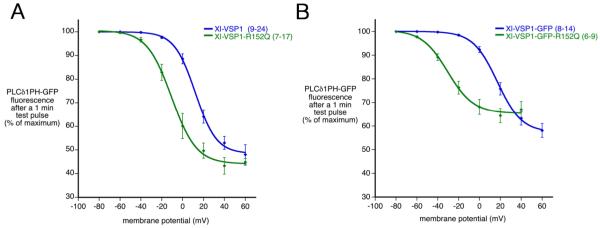
Fig. 5.
Mutation of arginine 152 to glutamine shifts Xl-VSP1 phosphatase activation to more negative membrane potentials. A: Xl-VSP1-R152Q activates at voltages ~20 mV more negative than Xl-VSP1, as measured by PLCδPH-GFP fluorescence intensity at one minute after a depolarizing test pulse. B: Xl-VSP1-GFP-R152Q activates at voltages ~50 mV more negative than Xl-VSP1-GFP, as measured by GFP fluorescence intensity at one minute after a depolarizing test pulse. The fluorescence intensity in (B) is a sum of that from PLCδ1PH-GFP and a small component from the GFP tag on the VSP (< 20%). The curves are Boltzmann fits to the averaged data. Error bars indicate mean +/− SEM, (n) = number of oocytes, listed as a range in cases where the number of data points varied for different voltages, except for Xl-VSP1-R152Q at +60 mV, where n = 3 (Fig. 5A).
Possible physiological functions of voltage-sensitive phosphatases in fertilization and development
Our findings show that Xl-VSP1 and Xt-VSP phosphatase activities can be activated at voltages that occur naturallya in cells. In particular, the membrane potential of Xenopus laevis eggs changes during fertilization from ~−20 mV to ~+20 mV (as measured in a solution containing 30 mM Cl−; Jaffe et al., 1983). In several species, including Xenopus laevis, this rapid depolarization of the egg plasma membrane establishes a block to polyspermy, since the positive shift in membrane potential inhibits sperm-egg fusion (Jaffe, 1976; Grey et al., 1982; Jaffe et al., 1983; Jaffe and Gould, 1985). The voltage-dependence of Xl-VSP1 activation closely parallels the voltage-dependence of the inhibition of sperm-egg fusion in Xenopus laevis (Iwao et al., 1994; Fig. 4E). Although the molecular basis of this electrically mediated process is not understood, cross-species fertilization experiments have indicated that the depolarization of the fertilized egg membrane is sensed by a component of the sperm cell membrane (Jaffe et al., 1982, 1983; Iwao and Jaffe, 1989). These findings, as well as evidence indicating a role for PI(4,5)P2 in regulating cell membrane fusion (Barrero-Villar et al., 2008), suggest VSPs as candidate molecules capable of translating depolarization of the egg membrane by the fertilizing sperm into prevention of fusion of additional sperm.
The results presented here support this hypothesis by showing that Xenopus VSPs are expressed in testis (Fig. 2), and that the voltage ranges for activation of Xl-VSP1 and inhibition of sperm-egg fusion are similar (Fig. 4E). The method we used to measure VSP activation does not provide exact information about how rapidly PI(4,5)P2 is dephosphorylated after depolarization, since time is required for the released sensor to diffuse away from the plasma membrane into the cytosolic space behind the pigment granule screen. However, a previous study in which the activity of Ci-VSP (from the ascidian Ciona intestinalis) was monitored by use of a PI(4,5)P2-sensitive ion channel (GIRK2) showed that the PI(4,5)P2 decrease in response to depolarization to +20 mV occured with a time constant of ~1 second (Murata and Okamura, 2007). As in Xenopus, polyspermy prevention in ascidians is electrically mediated, and the voltages that regulate sperm-egg fusion (Goudeau et al., 1994) are close to those that regulate VSP activity (Murata and Okamura, 2007; Halaszovich et al., 2009). Thus, for both Xenopus and Ciona, activation of a voltage-sensitive phosphatase in the sperm, in response to depolarization of the egg membrane at fertilization, could be relevant for prevention of polyspermy.
We also established that an R152Q mutation in the Xl-VSP1 sequence shifts the voltage dependence of the phosphatase activation. This finding should allow us to test whether sperm from transgenic frogs expressing this mutated form of Xl-VSP1 show a shift in the voltage dependence of sperm-egg fusion. Xenopus is well suited for such studies because both the production of transgenic frogs, and electrophysiological studies of fertilization, are practical. Such a transgenic approach could also be used to investigate possible functions of Xl-VSP1 in spermatogenesis, sperm motility, and the acrosome reaction, as well as in physiological processes in other tissues.
Although VSP/TPTEs were initially identified as predominantly localized in testis (Chen et al., 1999; Murata et al., 2005), subsequent studies including ours have indicated expression in other tissues as well. In particular, Xl-VSP1 RNA is found in the oocyte, although little functional protein expression is detectable in the oocyte or mature egg. Thus masked mRNAs encoding VSPs may be present in preparation for an as yet unidentified function in early development.
Materials and Methods
In silico identification of Xenopus VSPs
The National Center for Biotechnology Information (NCBI) BLAST engine was used for GenBank searches. ESTs and cDNAs were downloaded from NCBI. EST sequences were assembled in consensus sequences using CAP3 software (Huang and Madan, 1999), and the encoded protein sequences identified using ORF finder (NCBI). Searches against the genome assembly for Xenopus tropicalis were performed using online search tools of ENSEMBL project (http://www.ensembl.org/). Multiple sequence alignments and their analyses were performed using MEGA 4.0 (Tamura et al., 2007). The cDNA and protein sequences of VSP/TPTE family members mentioned or identified in this study are provided in Table S1.
Quantitative RT-PCR
Total RNA was prepared from freshly collected X. laevis and tropicalis tissues, obtained as approved by the University of Connecticut Health Center Animal Care Committee. RNA was isolated with Trizol reagent (Invitrogen, Carlsbad, CA), and its concentration and integrity were determined by OD260 measurement and agarose gel electrophoresis. Random-primed cDNA for each tissue was reverse transcribed from DNAase-treated total RNA with a Superscript III First Strand Synthesis kit (Invitrogen) and amplified using iQ SYBR Green Supermix reagent on an iCycler iQ Real Time PCR Detection System (BioRad, Hercules, CA). Each PCR reaction contained cDNA from 80 ng total RNA, and was run in triplicate. Validated qPCR primers specific for each target gene were as follows: Xl-VSP1: CAGCACACAGTAGTAGAAGCC, GCTGTGTATGTGGTCAGAACAC; Xl-VSP2: CCAGATACACAGCCAAAGTGC, GGGTAATAGTACGTTAAGAAGTG; Xt-VSP: GTGTAAGCACCAAGTTCCAAGG, CTGATACTCCGGATTTTGAGTTG.
Molecular cloning and in vitro transcription
Plasmids encoding Xl-VSP1 (BC099069, clone # 6868954) and Xl-VSP2 (CF520610, clone # 7008398) were purchased from Open Biosystems (Huntsville, AL), and a plasmid encoding Xt-VSP (NM_001015951.2, clone # 77E02) was purchased from GeneService (Cambridge, UK). The protein coding sequences of the VSPs were inserted into pcDNA3 (Invitrogen). C-terminal GFP-tagged Xenopus VSPs were constructed by cloning the VSP coding sequences into peGFP-N2 (Clontech, Mountain View, CA), and then inserting the VSP-GFP sequences into pcDNA3. Mutant VSP constructs were made using a Quikchange IIXL Site directed mutagenesis kit (Stratagene, Santa Clara, CA). In vitro transcribed VSP mRNAs were generated with a mMessage mMachine T7 kit (Ambion, Austin, TX) from plasmid DNA that was linearized with DraIII. pOMU-PLCδ1PH-GFP was generously provided by Drs M. Takano (Jichi Medical University) and N. Uozumi (Tohoku University) (Zhang et al., 2004), and was linearized with XbaI for making RNA. RNAs were assayed for integrity and concentration by agarose gel electrophoresis and fluorometry (Q-bit, Invitrogen) and stored at −80°C.
Expression of proteins in Xenopus oocytes
X. laevis ovary was obtained surgically from healthy female frogs as approved by the University of Connecticut Health Center Animal Care Committee. Ovary pieces were digested at 22-25°C for 90-100 min in 1.25 - 2.0 % collagenase (C0130, Sigma, St. Louis, MO), 0.1% soybean trypsin inhibitor (T-9003, Sigma), and 0.1% BSA (Bovuminar, Serologicals Corp., Norcross, GA) in Modified Ringer’s solution (100 mM NaCl, 2 mM CaCl2, 1 mM MgCl2, 1.8 mM KCl, 10 mM HEPES, pH 7.8); the oocytes were then washed in a high potassium buffer, pH 6.5, and Modified Ringer’s solution (Duesbury and Masui, 1996). Fully grown defolliculated oocytes were injected using a Picospritzer II (General Valve, Fairfield, NJ) with ~0.8 pg VSP RNA and ~1 pg PLCδ1PH-GFP RNA. Xl-VSP1-GFP-R152Q RNA was injected at ~1/3 the concentration of other VSP RNAs, to limit its toxic effects on the oocyte, which may have resulted from activation of a small amount of phosphatase activity at the resting membrane potential (see Results and Discussion). Oocytes were cultured at 18°C for 2-4 days before recording, in 50% Leibovitz’s L-15 media (Invitrogen), supplemented with 15 mM HEPES, pH 7.8, and 20 U penicillin, 20 U streptomycin, 40 U nystatin, and 100 μg gentamycin per ml. To cause the meiotic cell cycle to progress from prophase to second metaphase, oocytes were incubated with 1 μg/ml progesterone (Q2600, Steraloids, Inc., Newport RI).
Electrophysiology and fluorescence measurement
Glass microelectrodes (TW150F, World Precision Instruments, Sarasota, FL) were filled with 3M KCl (~0.6 MΩ tip resistance for passing current, ~5-10 MΩ for recording voltage). Oocytes were positioned on a nylon mesh (500 × 500 μm openings) with the pigmented animal hemisphere facing up, and were imaged using a 40X, 0.8 NA water immersion Achroplan objective (#44 00 95, Zeiss, Thornwood NY) on the stage of a Axioskop 2 FS plus microscope (Zeiss). GFP fluorescence was excited using a 100 watt halogen lamp and a 450-490 nm bandpass filter, and collected through a 515 nm longpass emission filter. Fluorescence signal intensity was measured using a photodiode (model 71882, Oriel Instruments, Stratford, CT) from an ~60 μm diameter spot near the oocyte animal pole. Similar results were obtained using Modified Ringer’s bath solution, or Modified Ringer’s with choline chloride replacing all but 1.8 mM of the sodium chloride, to prevent a large depolarization-induced sodium current (Kado and Baud, 1981). Two electrode voltage clamp was established using a modified AM-2 amplifier from Biodyne (Santa Monica, CA; Kline et al., 1986). Membrane voltage and fluorescence signals were digitized (Digidata 1322A, Axon Instruments, Foster City, CA) and analyzed using pClamp 9.2 software (Axon Instruments). For Figs 4E, 5A, and 5B, Prism software (GraphPad, La Jolla, CA) was used to fit fluorescence-voltage relations with the Boltzmann function:
where V50 is the voltage of half maximal activation. Measured values for y, ymax, and x were used to generate the curve; ymin was not measured. The plateau at ~50% of maximum fluorescence is most likely due primarily to cytoplasmic fluorescence that is not shielded by the cortical pigment layer.
Electron microscopy
Xenopus oocytes were fixed and embedded as previously described (Terasaki et al., 2001). Thin sections were stained with uranyl acetate and lead citrate, and imaged with a Hitachi 87650 transmission electron microscope (Pleasanton, CA).
Supplementary Material
Acknowledgments
We thank Alan Fein, Lixia Yue, Tamas Balla, Bertil Hille, Björn Falkenburger, Kazufumi Takamune, Yasuhiro Iwao, Souhei Sakata, and Tatsuki Kurokawa for helpful discussions and advice, and Art Hand and Mark Terasaki for their help with electron microscopy. This research was supported by NIH R21HD067017 to Laurinda A. Jaffe, by a Human Frontiers Science Program to Yasushi Okamura, and by travel funds from the University of Connecticut Health Center Auxillary to William J. Ratzan.
Contract grant sponsor: NIH/NICHD
Contract grant number: R21HD067017
Literature Cited
- Barrero-Villar M, Barroso-González J, Cabrero JR, Gordón-Alonso M, Álvarez-Losada S, Muñoz-Fernández MA, Sánchez-Madrid F, Valenzuela-Fernández A. PI4P5-kinase Iα is required for efficient HIV-1 entry and infection of T cells. J Immunol. 2008;181:6882–6888. doi: 10.4049/jimmunol.181.10.6882. [DOI] [PubMed] [Google Scholar]
- Bezanilla F. How membrane proteins sense voltage. Nat Rev Mol Cell Biol. 2008;9:323–332. doi: 10.1038/nrm2376. [DOI] [PubMed] [Google Scholar]
- Chen H, Rossier C, Morris MA, Scott HS, Gos A, Bairoch A, Antonarakis SE. A testis-specific gene, TPTE, encodes a putative transmembrane tyrosine phosphatase and maps to the pericentromeric region of human chromosomes 21 and 13, and to chromosomes 15, 22, and Y. Hum Genet. 1999;105:399–409. doi: 10.1007/s004390051122. [DOI] [PubMed] [Google Scholar]
- Dimitrov D, He Y, Mutoh H, Baker BJ, Cohen L, Akemann W, Knöpfel T. Engineering and characterization of an enhanced fluorescent protein voltage sensor. PLoS ONE. 2007;2:e440. doi: 10.1371/journal.pone.0000440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesbery N, Masui Y. The role of Ca2+ in progesterone-induced germinal vesicle breakdown of Xenopus laevis oocytes: the synergic effects of microtubule depolymerization and Ca2+ Dev Genes Evol. 1996;206:110–124. doi: 10.1007/s004270050037. [DOI] [PubMed] [Google Scholar]
- Evsikov AV, Marín de Evsikova C. Gene expression during the oocyte-to-embryo transition in mammals. Mol Reprod Dev. 2009;76:805–818. doi: 10.1002/mrd.21038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkenburger BH, Jensen JB, Hille B. Kinetics of PIP2 metabolism and KCNQ2/3 channel regulation studied with a voltage-sensitive phosphatase in living cells. J Gen Physiol. 2010;135:99–114. doi: 10.1085/jgp.200910345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glahn D, Nuccitelli R. Voltage-clamp study of the activation currents and fast block to polyspermy in the egg of Xenopus laevis. Develop Growth Differ. 2003;45:187–197. doi: 10.1034/j.1600-0854.2004.00684.x. [DOI] [PubMed] [Google Scholar]
- Goudeau H, Depresle Y, Rosa A, Goudeau M. Evidence by a voltage clamp study of an electrically mediated block to polyspermy in the egg of the ascidian Phallusia mammillata. Dev Biol. 1994;166:489–501. doi: 10.1006/dbio.1994.1332. [DOI] [PubMed] [Google Scholar]
- Grey RD, Bastiani MJ, Webb DJ, Schertel ER. An electrical block is required to prevent polyspermy in eggs fertilized by natural mating of Xenopus laevis. Dev Biol. 1982;89:475–484. doi: 10.1016/0012-1606(82)90335-9. [DOI] [PubMed] [Google Scholar]
- Halaszovich CR, Schrieber DN, Oliver D. Ci-VSP is a depolarization-activated phosphatidylinositol-4,5-bisphosphate and phosphatidylinositol-3,4,5-trisphosphate 5-phosphatase. J Biol Chem. 2009;284:2106–2113. doi: 10.1074/jbc.M803543200. [DOI] [PubMed] [Google Scholar]
- Hossain MI, Iwasaki H, Okochi Y, Chahine M, Higashijima S, Nagayama K, Okamura Y. Enzyme domain affects the movement of the voltage sensor in ascidian and zebrafish voltage-sensing phosphatases. J Biol Chem. 2008;283:18248–18259. doi: 10.1074/jbc.M706184200. [DOI] [PubMed] [Google Scholar]
- Huang X, Madan A. CAP3: A DNA sequence assembly program. Genome Res. 1999;9:868–877. doi: 10.1101/gr.9.9.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwao Y, Jaffe LA. Evidence that the voltage-dependent component in the fertilization process is contributed by the sperm. Dev Biol. 1989;134:446–451. doi: 10.1016/0012-1606(89)90117-6. [DOI] [PubMed] [Google Scholar]
- Iwao Y, Miki A, Kobayashi M, Onitake K. Activation of Xenopus eggs by an extract of Cynops sperm. Develop Growth Differ. 1994;36:469–479. doi: 10.1111/j.1440-169X.1994.00469.x. [DOI] [PubMed] [Google Scholar]
- Iwasaki H, Murata Y, Kim Y, Hossain MI, Worby CA, Dixon JE, McCormack T, Sasaki T, Okamura Y. A voltage-sensing phosphatase, Ci-VSP, which shares sequence identity with PTEN, dephosphorylates phosphatidylinositol 4,5-bisphosphate. Proc Natl Acad Sci USA. 2008;105:7970–7975. doi: 10.1073/pnas.0803936105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe LA. Fast block to polyspermy in sea urchin eggs is electrically mediated. Nature. 1976;261:68–71. doi: 10.1038/261068a0. [DOI] [PubMed] [Google Scholar]
- Jaffe LA, Gould M. Polyspermy-preventing mechanisms. In: Metz C, Monroy A, editors. Biology of Fertilization. Vol. 3. Academic Press; Orlando: 1985. pp. 223–250. [Google Scholar]
- Jaffe LA, Gould-Somero M, Holland LZ. Studies of the mechanism of the electrical polyspermy block using voltage clamp during cross-species fertilization. J Cell Biol. 1982;92:616–621. doi: 10.1083/jcb.92.3.616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe LA, Cross NL, Picheral B. Studies of the voltage-dependent polyspermy block using cross-species fertilization of amphibians. Dev Biol. 1983;98:319–326. doi: 10.1016/0012-1606(83)90362-7. [DOI] [PubMed] [Google Scholar]
- Kado RT, Baud C. The rise and fall of electrical excitability in the oocyte of Xenopus laevis. J Physiol (Paris) 1981;77:1113–1117. [PubMed] [Google Scholar]
- Kline D, Jaffe LA, Kado RT. A calcium-activated sodium conductance contributes to the fertilization potential in the egg of the nemertean worm Cerebratulus lacteus. Dev Biol. 1986;117:184–193. doi: 10.1016/0012-1606(86)90360-x. [DOI] [PubMed] [Google Scholar]
- Kohout SC, Ulbrich MH, Bell SC, Isacoff EY. Subunit organization and functional transitions in Ci-VSP. Nat Struct Mol Biol. 2008;15:106–108. doi: 10.1038/nsmb1320. [DOI] [PubMed] [Google Scholar]
- Murata Y, Okamura Y. Depolarization activates the phosphoinositide phosphatase Ci-VSP, as detected in Xenopus oocytes coexpressing sensors of PIP2. J Physiol. 2007;583:875–889. doi: 10.1113/jphysiol.2007.134775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- Neuhaus H, Hollemann T. Kidney specific expression of cTPTE during development of the chick embryo. Gene Expression Patterns. 2009;9:568–571. doi: 10.1016/j.gep.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Ogasawara M, Sasaki M, Nakazawa N, Nishino A, Okamura Y. Gene expression profile of Ci-VSP in juveniles and adult blood cells of ascidian. Gene Expression Patterns. 2011;11:233–238. doi: 10.1016/j.gep.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Okamura Y. Biodiversity of voltage sensor domain proteins. Pflug Arch. 2007;454:361–371. doi: 10.1007/s00424-007-0222-6. [DOI] [PubMed] [Google Scholar]
- Okamura Y, Dixon JE. Voltage-sensing phosphatase: its molecular relationship with PTEN. Physiology. 2011;26:6–13. doi: 10.1152/physiol.00035.2010. [DOI] [PubMed] [Google Scholar]
- Reymond A, et al. Human chromosome 21 gene expression atlas in the mouse. Nature. 2002;420:582–586. doi: 10.1038/nature01178. [DOI] [PubMed] [Google Scholar]
- Richter JD. CPEB: a life in translation. Trends Biochem Sci. 2007;32:279–285. doi: 10.1016/j.tibs.2007.04.004. [DOI] [PubMed] [Google Scholar]
- Stauffer TP, Ahn S, Meyer T. Receptor-induced transient reduction in plasma membrane PtdIns(4,5)P2 concentration monitored in living cells. Curr Biol. 1998;8:343–346. doi: 10.1016/s0960-9822(98)70135-6. [DOI] [PubMed] [Google Scholar]
- Tamura K, Dudley J, Nei M, Kumar S. MEGA4: Molecular Evolutionary Genetics Analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599. doi: 10.1093/molbev/msm092. [DOI] [PubMed] [Google Scholar]
- Tapparel C, Reymond A, Girardet C, Guillou L, Lyle R, Lamon C, Hutter P, Antonarakis SE. The TPTE gene family: cellular expression, subcellular localization and alternative splicing. Gene. 2003;323:189–199. doi: 10.1016/j.gene.2003.09.038. [DOI] [PubMed] [Google Scholar]
- Terasaki M, Runft LL, Hand AR. Changes in organization of the endoplasmic reticulum during Xenopus oocyte maturation and activation. Mol Biol Cell. 2001;12:1103–1116. doi: 10.1091/mbc.12.4.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Várnai P, Balla T. Visualization of phosphoinositides that bind pleckstrin homology domains: calcium- and agonist-induced dynamic changes and relationship to myo-[3H]inositol-labeled phosphoinositide pools. J Cell Biol. 1998;143:501–510. doi: 10.1083/jcb.143.2.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villalba-Galea CA, Miceli F, Taglialatela M, Bezanilla F. Coupling between the voltage-sensing and phosphatase domains of Ci-VSP. J Gen Physiol. 2009;134:5–14. doi: 10.1085/jgp.200910215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker SM, Downes CP, Leslie NR. TPIP: a novel phosphoinositide 3-phosphatase. Biochem J. 2001;360:277–283. doi: 10.1042/0264-6021:3600277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Dowbenko D, Pisabarro MT, Dillard-Telm L, Koeppen H, Lasky LA. PTEN 2, a Golgi-associated testis-specific homologue of the PTEN tumor suppressor lipid phosphatase. J Biol Chem. 2001;276:21745–21753. doi: 10.1074/jbc.M101480200. [DOI] [PubMed] [Google Scholar]
- Zhang L, Lee J-K, John SA, Uozumi N, Kodama I. Mechanosensitivity of GIRK channels is mediated by protein kinase C-dependent channel-phosphatidylinositol 4,5-bisphosphate interaction. J Biol Chem. 2004;279:7037–7047. doi: 10.1074/jbc.M307323200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.