Abstract
Objective
To examine the association between fracture and pelvic organ prolapse (POP) in postmenopausal women enrolled in the Women’s Health Initiative Estrogen plus Progestin (WHI-EP) trial.
Methods
POP was assessed as cystocele, rectocele or uterine prolapse, and was graded as “absent-to- mild” or “moderate-to-severe”. Cox proportional hazard analyses (adjusting for age, BMI, race, asthma, emphysema, thyroid disease, family history of fracture, regular menses, age at menopause, nulliparity, history of hormone therapy [HT], history of falls, SES, calcium and vitamin D supplementation and physical activity) explored relationships between moderate-to-severe POP and incident bone fractures.
Results
Moderate-to-severe grade POP was identified in almost 8% of women (n=1,192). Over a follow up duration of 7.41 ± 2.18 years (mean ± SD), 2,156 incident fractures were observed; the most common fracture site was lower arm (n=615, 28.51%) followed by hip (n=205, 9.51%). Adjusted analyses confirmed moderate-to-severe POP (of any type) as an independent risk factor for incident hip fractures (HR 1.83, 95% CI 1.16–2.89, p=0.010). On analyses stratified by assigned treatment (HT versus placebo) moderate-to-severe rectocele emerged as an independent predictor of incident spine (HR 2.61, 95% CI 1.04–6.56, p=0.042) and lower arm fractures (HR 1.87, 95% CI 1.06–3.29, p=0.030) in the placebo group.
Conclusion
We identify moderate-to-severe POP (any type) in postmenopausal women as a risk factor for hip fracture; moderate-to-severe rectocele holds additional risk for spine and lower arm fractures in women not on HT.
Keywords: Prolapse, Fracture, WHI, Estrogen, Progesterone, Rectocele, Hip
Introduction
Pelvic organ prolapse (POP) is a recognized contributor to morbidity in an aging female population (1–2) and to the overall health care cost in the community. More than 300,000 surgical procedures are reportedly performed annually for POP in the USA, with incurred cost in excess of $1 billion (3–4). The prevalence of POP attests to the magnitude of its potential health care burden. As many as 41% of postmenopausal (PM) women enrolled in the Women’s Health Initiative Estrogen Plus Progestin (WHI-EP) Trial demonstrated some degree of POP (2).
Both qualitative and quantitative deficiencies in pelvic collagen are believed to exist in women with POP (5–14). Conversely, a predisposition to POP is identified in women afflicted by generalized connective tissue disorders (15). The role of skeletal collagen in conferring architectural tissue strength is underscored by an increased incidence of fractures in collagen disorders like Marfan and Ehrler Danlos syndromes (16–18). Limited data are suggestive of global collagen deficiency in women with evidence of POP (14, 19–21). Pursuing a hypothesis that PM women with evidence of POP may be at an enhanced lifetime risk for skeletal fragility, we previously identified an association between moderate-to-severe POP and compromised skeletal integrity in the PM population (22). Analyses of baseline data from the WHI-EP trial revealed a higher prevalence of low bone mineral density (BMD) and an enhanced likelihood for fractures (after age 55 years) in PM women identified with moderate-to-severe POP compared to those with absent-to-mild POP (22), thus expanding the spectrum of health concerns associated with POP.
Extending our earlier observations, we herein demonstrate that PM women with moderate-to-severe POP are significantly more likely to experience incident fractures compared to those with absent-to-mild POP. The current study was undertaken utilizing the longitudinal data on PM women recruited in the WHI-EP Trial, details of which have previously been published (23).
METHODS
Study Population
In the WHI-EP randomized controlled trial, 16, 620 postmenopausal women aged 50 to 79 years with an intact uterus at baseline were recruited at 40 US clinical centers between September 1993 and July 2002; 12 were subsequently excluded due to a change in the hysterectomy status. The participants (16, 608) were randomly assigned to receive conjugated equine estrogen, 0.625 mg/d, plus medroxyprogesterone acetate, 2.5 mg/d, (n=8,506) versus placebo (n=8,102).
POP was evaluated at baseline by a standardized pelvic examination(2) and was documented as either absent or, if present, was categorized into distinct anatomical types, i.e. cystocele, rectocele and uterine prolapse. The severity of POP was graded with reference to the introitus. Three grades of increasing descent of pelvic organs were described: mild (descent of the most dependant part of pelvic organs into the vagina but not reaching the introitus), moderate (descent down to the level of the introitus) and severe (descent beyond the introitus). All assessments were performed with the participant in a supine position and performing the Valsalva maneuver (2). Moderate-to-severe POP was stated as the exposure variable (independent) for the assessment of a relationship with incident fracture by bone site (dependent).
Independent variables of interest included age, race/ethnicity (White, Black, Asian, Hispanic, Other), gynecologic history including age at menarche (categorized as <9, 9–13, ≥14 years), history of regular periods in premenopausal years (yes/no), age at menopause (years), parity, history of prior use of hormone therapy (HT) (never, <5 years, 5–9 years, ≥10 years), history of use of oral contraceptive (OCP) (ever/never), medical history including any history of thyroid disorders (any type, yes/no), asthma (yes/no), emphysema (yes/no), social history including a history of smoking ≥100 cigarettes ever (yes/no), physical activity (energy expenditure in metabolic equivalents [METS], per week), history of falls in past 12 months (yes/no), family history of bone fractures (broken bone in mother or father, yes/no), and current use of calcium and/or vitamin D supplements. Anthropometric measures included body mass index (BMI) and waist to hip ratio (WHR) both as continuous variables. Parity was available as an ordinal variable (−1 to 9; “−1” referred to “never having been pregnant” and “0” referred to “never had a term pregnancy”). Parity was re-coded as “nulliparous” if there was no history of a prior live birth. Age at menarche ≥14 years was coded as “late menarche” and the age of menopause < 45 years was defined as “early menopause”. HT was dichotomized as never/ever. Annual household income, reflecting the socio-economic status (SES), was dichotomized at 50K as previously described (24).
Outcome measures and follow-up
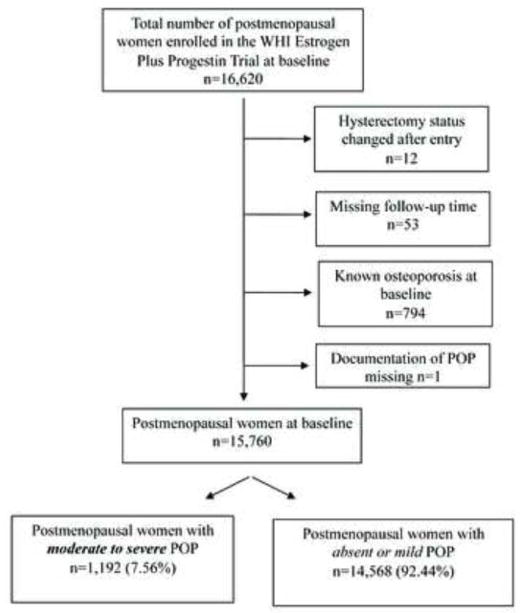
Fracture outcomes were ascertained by a semi-annual questionnaire and included hip, wrist/lower arm, clinical vertebral and total fractures (24). Reported events were verified by local and central adjudication involving review of radiological reports. For the presented analyses, the primary outcome measures were “any fracture” and the site of fracture (hip, spine, and lower arm). The WHI-EP trial was terminated following an average of 5.6 years; in the presented study, participants were followed over 7.41 ± 2.18 years. Follow-up time was calculated from the date of study entry to the date of fracture or date of last exam, whichever came first. Those with missing follow-up data (n=53) and POP status (n=1), and known osteoporosis at baseline (n=794) were excluded for the present analyses; 15, 760 PM women constituted the study sample to explore the hypothesis that moderate-to-severe POP is a risk factor for fracture in PM women (Figure 1).
FIG. 1.
Flowchart of study participants in the WHI Estrogen Plus Progestin clinical trial. WHI, Women’s Health Initiative; POP, pelvic organ prolapse.
Statistical analysis
Demographic and clinical characteristics were compared between those with moderate-to-severe POP and those with absent-to-mild POP using two sample t-tests and chi-square tests for continuous and categorical variables, respectively.
Cox proportional hazard models were constructed to evaluate the effect of POP on fracture. The proportional hazards assumption was tested using Schoenfeld residuals and by comparing the plot of the log (–log [survival]) vs. log of survival time; if the graph resulted in parallel lines, the predictor variable was considered “proportional” (25). Adjustment variables included those independent variables that were previously recognized to be associated with the likelihood for fracture at age >55 in our prior analyses utilizing the baseline data for the cohort (22) and included age, race/ethnicity, early menopause, parity (yes, no), hormone use (ever, never), regular periods in premenopausal years (yes/no), BMI (kg/m2), history of thyroid disorders, history of asthma, history of ever smoking ≥100 cigarettes, history of falls, family history of bone fractures (broken bones in mother or father), SES, METS, current use of calcium and vitamin D supplementation and intervention arm (HT versus placebo). Interaction product terms were created between POP sub-types and intervention arm of the clinical trial (HT or placebo) along with the main effects terms in the fully adjusted models.
Given the known propensity for repeat fracture in those with a history of bone fracture (26), sensitivity analyses were performed after excluding PM women who had previously fractured in their lifetime (ever fracture) to determine the association between moderate-to-severe POP with risk of a first fracture occurring during period of observation. Continuous data are presented as mean ± standard deviation whereas categorical data are shown as percentage (numbers). Magnitude of risk is presented as hazard ratio (HR) ± 95% confidence interval (95% CI). All statistical tests used two t-tailed α of 0.05 and Stata SE 10.1 for Windows (StataCorp, College Station, TX). Approval from the WHI Publication Committee was obtained prior to accessing the study database.
Results
Moderate-to-severe POP (all types) was identified in 1,192 participants (7.56%); cystocele was the most common anatomic variant (5.32%), followed by rectocele (2.72%) whereas uterine prolapse was noted in 1.20%; additional smaller numbers presented with combinations of the various anatomical (cystocele, rectocele or uterine) variants of moderate-to-severe POP. Of the documented incident fractures (n=2,156), the most common site was the lower arm (28.51%; n=615) followed by hip (9.50%; n=205). Of the hip fractures, the majority were the first fracture sustained during the period of observation (81.95%; n=168).
As shown in Table 1, compared to women with absent-to-mild POP, those with moderate-to- severe POP were older (p<0.001), were significantly less likely to be of Black race, be nulliparous (p<0.001), were less likely to smoke (p<0.001), to report early menopause (p=0.007), or to acknowledge a prior use of OCP (p=0.040) or HT (p<0.001); those with moderate-to-severe POP were significantly more likely to be of Asian (p<0.001) race, to report a prior history of fracture (p=0.003), to have a higher BMI (p<0.001), and to belong to lower SES as reflected by an annual household income <50K (p=0.016).
Table 1.
Characteristics of the cohort by severity of pelvic organ prolapse (POP).
| Variable | Moderate-to-Severe POP 7.56% (1,192) | Absent-to-Minimal POP 92.44% (14,568) | Total n=15,760 | P value |
|---|---|---|---|---|
| Age at study entry | 64.76 ± 6.74 | 62.92 ± 7.09 | 63.06 ± 7.08 | <0.001 |
| Race | ||||
| White | 82.21 (980) | 84.31 (12,250) | 84.15 (13,230) | 0.056 |
| Black | 4.45 (53) | 7.12 (1,034) | 6.91 (1,087) | <0.001 |
| Asian | 5.20 (62) | 1.94 (282) | 2.19 (344) | <0.001 |
| Hispanic | 6.29 (825) | 5.16 (750) | 5.25 (825) | 0.093 |
| History of late menarche a | 9.66 (115) | 10.86 (1,576) | 10.77 (1,691) | 0.201 |
| History of regular menses | 84.36 (1,003) | 83.93 (12,144) | 83.96 (13,147) | 0.697 |
| Nulliparous b | 1.94 (23) | 10.77 (1,562) | 10.10 (1,585) | <0.001 |
| Age at menopause (years) | 50.10 ± 4.36 | 49.48 ± 4.38 | 49.52 ± 4.38 | <0.001 |
| Early menopause c | 14.99 (155) | 18.34 (2,283) | 18.09 (2,438) | 0.007 |
| History of smoking d | 42.22 (499) | 51.13 (7,397) | 50.46 (7,896) | <0.001 |
| History of previous fracture | 15.66 (174) | 12.60 (1,674) | 12.83 (1,848) | 0.003 |
| History of thyroid disease | 20.86 (247) | 19.64 (2,841) | 19.73 (3,088) | 0.309 |
| History of emphysema | 2.99 (33) | 3.02 (397) | 3.02 (430) | 0.957 |
| History of asthma | 7.33 (86) | 6.25 (896) | 6.33 (982) | 0.146 |
| History of OCP e use (ever) | 40.86 (487) | 43.93 (6,400) | 43.70 (6,887) | 0.040 |
| History of HT f use (ever) | 13.59 (162) | 17.90 (2,608) | 17.58 (2,770) | <0.001 |
| History of falls g | 34.34 (388) | 32.72 (4,437) | 32.84 (4,825) | 0.266 |
| History of family fracture h | 42.37 (509) | 43.62 (6,354) | 43.52 (6,859) | 0.403 |
| Annual income <$50,000 | 71.86 (812) | 68.40 (9,429) | 68.66 (10,241) | 0.016 |
| BMI (kg/m2) | 29.73 ± 5.65 | 28.44 ± 5.88 | 28.53 ± 5.87 | <0.001 |
| Waist-to-Hip Ratio | .84 ± .08 | .82 ± .08 | .82 ± .08 | <0.001 |
| Calcium supplementation i | 49.50 (590) | 48.26 (7,030) | 48.35 (7,620) | 0.411 |
| Vitamin D supplementation j | 43.20 (515) | 42.52 (6,194) | 42.57 (6,709) | 0.646 |
| METS/week k | 10.66 ± 12.58 | 11.60 ± 13.67 | 11.53 ±13.59 | 0.025 |
Data are presented as mean ± standard deviation (continuous) or % (n) (categorical). P-values are calculated by Student’s t-tests for continuous variables and chi-square test for categorical variables.
Late menarche is defined as ≥ 14 years of age.
Nulliparous is defined as no prior history of live birth.
Early menopause is defined as menopause at age ≤45 years
History of smoking defined as ≥100 cigarettes ever.
History of use of oral contraceptives (ever)
History of use of hormone therapy (ever)
History of falls defined as falls within the past 12 months.
Family history of fracture defined as fracture in either or both parents after the age of 40 years.
Calcium and vitamin D supplementation defined as any vs. none.
Metabolic equivalents
Association between the various anatomic variants of POP and site specific incident fractures is presented in Table 2. Adjusted analyses confirmed moderate-to severe POP (any anatomic type, and moderate-to-severe rectocele and cystocele) as independent risks for incident hip fractures.
Table 2.
Proportional hazard models demonstrating hazard ratios (HR) and 95% confidence interval (CI) for skeletal site specific fractures sustained during the period of observation in postmenopausal women with moderate- to- severe pelvic organ prolapse (POP) compared to those with absent to minimal POP.
| Outcome | POPŧ | Unadjusted HR (95% CI) | P | Adjusted* HR (95% CI) | P |
|---|---|---|---|---|---|
| All Fractures (2,156) | |||||
| Any POP | 1.00 (0.85, 1.18) | 0.997 | 1.02 (.85, 1.23) | 0.811 | |
| Rectocele | 1.18 (0.92, 1.51) | 0.176 | 1.19 (.91, 1.56) | 0.199 | |
| Cystocele | 0.86 (0.55, 1.33) | 0.494 | 1.02 (0.82, 1.26) | 0.891 | |
| Uterine | 1.00 (0.82, 1.21) | 0.965 | 0.97 (0.60, 1.56) | 0.886 | |
| Hip (n=205) | |||||
| Any POP | 1.85 (1.22, 2.80) | 0.003 | 1.83 (1.16, 2.89) | 0.010 | |
| Rectocele | 2.12 (1.15, 3.88) | 0.016 | 2.18 (1.14, 4.17) | 0.018 | |
| Cystocele | 1.81 (0.67, 4.88) | 0.239 | 1.79 (1.05, 3.07) | 0.034 | |
| Uterine | 1.79 (1.10, 2.91) | 0.018 | 0.95 (0.23, 3.85) | 0.941 | |
| Spine (n=193) | |||||
| Any POP | 0.84 (0.47, 1.50) | 0.550 | 0.76 (0.39, 1.50) | 0.435 | |
| Rectocele | 1.39 (0.65, 2.96) | 0.392 | 1.42 (0.62, 3.23) | 0.407 | |
| Cystocele | 1.44 (0.46, 4.50) | 0.534 | 0.48 (0.18, 1.31) | 0.151 | |
| Uterine | 0.59 (0.26, 1.33) | 0.205 | 1.25 (0.31, 5.06) | 0.758 | |
| Lower Arm (n=615) | |||||
| Any POP† | 0.87 (0.63, 1.20) | 0.397 | 0.93 (0.65, 1.34) | 0.701 | |
| Rectocele† | 1.18 (0.75, 1.86) | 0.481 | 1.17 (0.70, 1.97) | 0.551 | |
| Cystocele | 0.60 (0.22, 1.61) | 0.313 | 0.98 (0.64, 1.49); | 0.923 | |
| Uterine | 0.91 (0.62, 1.31) | 0.607 | 0.59 (0.19, 1.83); | 0.361 | |
Multivariable analyses adjusted for age, BMI, race, asthma, emphysema, thyroid, family history of fracture, regular menses, age at menopause, nulliparity, hormone use, history of falls, income, calcium supplementation, METs per week, and clinical trial intervention arm (HT versus placebo).
Reference group is absent to minimal POP
Denotes a statistically significant interaction between anatomical type of moderate- to- severe POP and HT (p=0.046 and p=0.022 for interaction between moderate to severe POP, any type, and moderate- to- severe Rectocele with HT respectively).
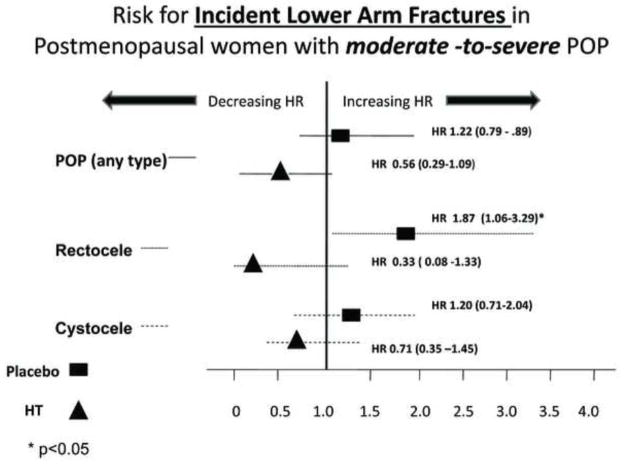
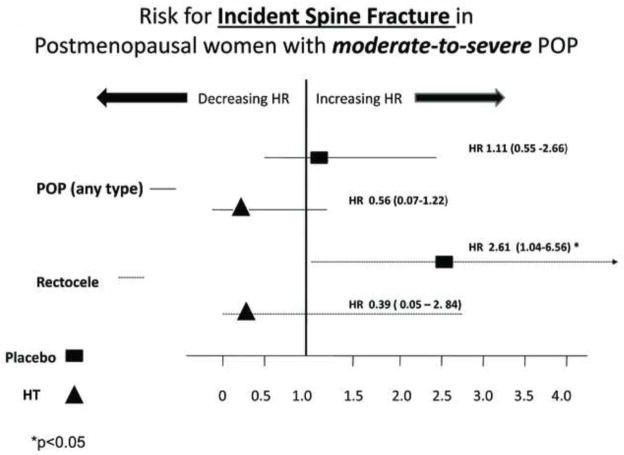
A statistically significant interaction was observed between incident lower arm fracture with moderate-to severe POP and with moderate-to-severe rectocele (p values for interaction = 0.046 and 0.022, respectively) with respect to treatment arm. Stratified analyses by trial assigned intervention (HT versus placebo) identified moderate-to-severe rectocele as an independent risk factor for incident lower arm (Figure 2) and spine (Figure 3) fracture in PM women randomized to placebo but not in those receiving HT.
FIG. 2.
Risk of incident lower arm fracture in postmenopausal women with moderate to severe compared with those with absent to mild POP. POP, pelvic organ prolapse; HR, hazard ratios; HT, hormone therapy.
FIG. 3.
Risk of incident spine fracture in postmenopausal women with moderate to severe compared with those with absent to mild POP. POP, pelvic organ prolapse; HR, hazard ratios; HT, hormone therapy.
Hazard ratios for site specific incident fractures were further assessed comparing PM women with moderate-to-severe POP with those with absent POP (i.e. those with mild POP were excluded from the reference group), and confirmed stability of the observed associations between moderate-to-severe POP and incident hip fracture (data not shown Additional sensitivity analyses excluded those with a history of fracture prior to enrollment in the trial (Table 3). Moderate-to-severe POP was again identified as a risk for site specific incident fracture in PM women not on HT; the highest hazard magnitudes were observed in those with moderate-to-severe rectocele.
Table 3.
Proportional hazard models demonstrating adjusted hazard ratios (AHR) for the “first fracture event” sustained during the period of observation in postmenopausal women with moderate to severe pelvic organ prolapse (POP) compared to those with absent to minimal (referent group) POP (note, those with a “history of fracture” prior to enrollment were excluded from these sensitivity analyses).
| Outcome | Overall a AHR (95% CI) | Placebo b AHR (95% CI) | HT c AHR (95% CI) |
|---|---|---|---|
| Any POP † | |||
| Hip fracture | 2.04 (0.99–4.20) | 3.09 (1.24– 7.67) ‡ | 1.12 (0.33–3.82) |
| Spine fracture | 0.83 (0.30–2.30) | 1.11 (0.33–3.69) | 0.43 (0.06–3.20) |
| Lower Arm fracture | 1.12 (0.68–1.85) | 1.64 (0.91–2.94) | 0.54 (0.20–1.47) |
| Rectocele† | |||
| Hip fracture | 1.73 (0.54–5.60) | 5.28 (1.55–17.98) ‡ | § |
| Spine fracture | 2.69 (0.97–7.52) | 3.80 (1.13– 12.73) ‡ | 1.20 (0.16–8.98) |
| Lower Arm fracture | 1.53 (0.75–3.12) | 3.34 (1.61 –6.97) ‡ | § |
| Cystocele† | |||
| Hip fracture | 2.65 (1.24–5.66) ‡ | 3.94 (1.48–10.53) ‡ | 1.67 (0.48–5.74) |
| Spine fracture | 0.57 (0.14–2.35) | 1.07 (0.25–4.55) | § |
| Lower Arm fracture | 1.14 (0.64–2.05) | 1.47 (0.71–3.03) | 0.76 (0.28–2.09) |
AHR ( 95% confidence intervals) are reported for:
overall population,
enrollees in placebo arm,
enrollees in HT arm of the clinical trial.
Models are adjusted for age, BMI, race, asthma, emphysema, thyroid, family history of fracture, regular menses, age at menopause, nulliparity, hormone use, history of falls, income, calcium supplementation and METs per week and for HT intervention arm (a)
Moderate-to-severe grade of POP compared to reference (absent to minimal POP)
Statistically significant p<0.05
No fractures in this group
Given the retrospective cohort study design, a likelihood that the observed differences in incident fractures between the two cohorts (i.e. those with moderate-to-severe compared to absent-to-mild POP) could have resulted from a differential in the duration of follow up was considered. Interestingly, the mean duration of follow up was statistically significantly shorter in women with moderate- to- severe compared to those with absent-to-mild POP (7.78 ± 1.47 versus 7.97 ± 1.57 years, p<0.001). These findings suggest that the actual magnitude of fracture risk relating to moderate -to-severe POP may even be of a greater magnitude than what is observed.
Discussion
In line with our earlier observation (22), our current analyses corroborate our hypothesis relating moderate-to-severe POP in postmenopausal women with skeletal fragility. Our analyses of the longitudinal data available on women enrolled in the WHI-EP trial identify moderate-to-severe rectocele as an independent risk for postmenopausal hip fracture. The skeletal site specific predilection to fracture however is likely a reflection of power related constraints, as an enhanced risk for lower arm and spine fractures was also observed in postmenopausal women with moderate- to- severe POP who were assigned to the placebo arm of the clinical trial. These latter observations imply protective effects of menopausal HT on reducing incident fractures in PM women with moderate-to-severe POP.
We previously described an association between moderate-to-severe POP and likelihood of fracture in aging postmenopausal women (>60 years) (22). Our current and prior observations have been corroborated by Melton et al (27) who, in a retrospective cohort study of 9, 258 women followed for a median of 13.6 years following hysterectomy, described an association between prior surgery for uterine prolapse and fracture risk.
The consistency of the observed relationships between individual anatomical variants of moderate-to-severe POP with incident fractures by site on adjusted analyses as well as on sensitivity analyses add credence to our hypothesis that POP be regarded as a focal manifestation of generalized tissue compromise. A site specific differential in pelvic connective tissue composition and relationship thereof with the skeletal matrix is suggested by our analyses. Qualitative and possibly quantitative distinctions in the pelvic fascia from anatomically distinct compartments are thus implied, albeit remain far from understood. Intra-individual and inter-individual variations in pelvic fascia are well appreciated and this tissue heterogeneity underlies the lack of consensus regarding embryological origins of the pelvic fascia (28); histological distinctions in the fascia of the pelvic compartments are similarly poorly understood. While the underlying mechanisms unifying the consistently observed relationships between rectocele and bone fracture remain unclear, these observations underscore a need for appropriately designed studies focusing on site specific differences in tissue composition within the pelvic compartments.
A role for the loss of ovarian hormones in the pathogenesis of POP remains far from clear. An increased risk for POP is described in association with polymorphism in ERα as well as with ERβ (29–30) implying a role for estrogen signaling (or lack of effects thereof) in the pathogenesis of POP. The picture is, however, clouded by inconsistencies in the data relating the presence and progression of POP to the use of menopausal HT or selective estrogen receptor modulators (31–34). Worsening POP was reported in the PM women randomized to the EP arm of the WHI-EP trial (31). A similar lack of efficacy against POP was observed with the use of selective estrogen receptor modulators raloxifene and tamoxifen and conjugated equine estrogen by healthy postmenopausal women (32); following 20 weeks of intervention, progression in POP was observed in 75% of the patients randomized to raloxifene, 60% who received tamoxifen and 22% of the patients receiving conjugated equine estrogen (32). Primary damage to the connective tissue of the pelvic floor occurs during pregnancy and childbirth, and a role for the loss of ovarian hormones concomitant with menopause in the pathogenesis of, or progression in, POP thus remains far from clear.
Based on the current evidence, the role for HT in women with POP is limited to treatment of symptoms relating to vaginal atrophy. Our data suggest beneficial influences of menopausal HT in reducing fracture risk in PM women with moderate-to-severe POP. While these findings reiterate the well recognized protective effects of HT on fracture risk (24), future studies however are needed to help establish a preventive role for postmenopausal HT in mitigating fracture risk in women with moderate-to-severe POP.
Although we herein hypothesize that compromised tissue collagen may be a mechanism that unifies POP (reflective of a weakened pelvic floor) and skeletal fragility, our data cannot elucidate the underlying pathophysiological mechanisms. The relatively subjective nature of the evaluations as well as the supine positioning of the participants at the time of POP grading (33–34) are limitations intrinsic to the study design that may have compromised sensitivity in detecting lesser grades of POP. We attempted to redress this latter concern by targeting obvious descent of pelvic organs as our variable of interest (22), thus confining our definitions to more severe degrees of POP. In the subset of women who underwent bone mineral density (BMD) assessment at baseline, our prior analyses (22) identified significantly lower total body BMD in those with moderate-to-severe POP. While the paucity of incident fracture events in the limited cohort who did undergo BMD testing (BMD was evaluated in only a subset, n=1,024; 46 of 958 with moderate-to-severe POP and 634 of 10,101 with absent-to-mild POP underwent BMD evaluation) limits us from commenting on the contributions of deteriorating BMD to fracture risk, we attempted to address this deficiency by excluding those with a known history of osteoporosis as well as those with prior history of fracture. Although the direction and the magnitude of HR’s for the observed associations between individual anatomical variants of moderate-to-severe POP and fracture site were maintained on sensitivity analyses, the resulting model instability (widening of CI’s) and loss of statistical significance to some of the associations likely reflect power constraints.
Conclusion
Our analyses of longitudinal data for the WHI-EP cohort identify moderate-to-severe POP, a prevalent gynecological entity, as an independent risk factor for incident fracture in postmenopausal women. We reaffirm the previously observed relationships (22) between moderate-to-severe POP in general, and moderate-to-severe rectocele, in particular, with skeletal fragility. Considering the prevalence of POP in PM women (almost 8% of PM women enrolled in WHI-EP Trial demonstrated moderate-to-severe POP) and the observed association with incident hip fractures (which have the highest morbidity and mortality), our findings hold public health implications (35). While our analyses suggest that HT was protective against incident fracture in PM women with moderate-to-severe POP, these findings must be interpreted with caution given the retrospective nature of our analyses, our inability to comment on the occurrence of “non informative” events given the limitations intrinsic to working with an existing dataset, and the wide confidence intervals that are likely reflective of power constraints. It should be noted that comparable efficacy data for commonly used antiresorptive treatments other than HT (e.g. bisphosphonates, SERM’s, calcitonin or parathyroid hormone) have not been specifically substantiated in this population of postmenopausal women identified at risk for fracture. Our findings suggest that considerations on the choice of fracture prevention therapy in postmenopausal women should incorporate the status of pelvic organ descent.
Acknowledgments
Funding/Support: The Women’s Health Initiative program is funded by the National Heart, Lung, and Blood Institute, US Department of Health and Human Services.
Footnotes
This work was presented as an abstract at the annual meeting of the North American Menopause Society, September 2008.
Financial Disclosure: None
References
- 1.van der Vaart CH, de Leeuw JR, Roovers JP, Heintz AP. Measuring health-related quality of life in women with urogenital dysfunction: the urogenital distress inventory and incontinence impact questionnaire revisited. Neurourol Urodyn. 2003;22:97–104. doi: 10.1002/nau.10038. [DOI] [PubMed] [Google Scholar]
- 2.Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernam A. Pelvic organ prolapse in the Women’s Health Initiative: Gravity and gravidity. Am J Obstet Gynecol. 2002;186:1160–6. doi: 10.1067/mob.2002.123819. [DOI] [PubMed] [Google Scholar]
- 3.Popovic JR, Kozak LJ. National hospital discharge survey: annual summary, 1998. Vital Health Stat. 2000;148(13):1–194. [PubMed] [Google Scholar]
- 4.Subak LL, Waetjen LE, van den Eeden S, Thom DH, Vittinghoff E, Brown JS. Cost of pelvic organ prolapse surgery in the United States. Obstet Gynecol. 2001;98:646–51. doi: 10.1016/s0029-7844(01)01472-7. [DOI] [PubMed] [Google Scholar]
- 5.Jackson SR, Avery NC, Tarlton JF, Eckford SD, Abrams P, Bailey AJ. Changes in metabolism of collagen in genitourinary prolapse. Lancet. 1996;347 (9016):1658–61. doi: 10.1016/s0140-6736(96)91489-0. [DOI] [PubMed] [Google Scholar]
- 6.Liapis A, Bakas P, Pafiti A, Frangos-Plemenos M, Arnoyannaki N, Creatsas G. Changes of collagen type III in female patients with genuine stress incontinence and pelvic floor prolapse. Eur J Obstet Gynecol Reprod Biol. 2001;97:76–9. doi: 10.1016/s0301-2115(00)00478-4. [DOI] [PubMed] [Google Scholar]
- 7.Soderberg MW, Falconer C, Bystrom B, Malmstrom A, Ekman G. Young women with genital prolapse have a low collagen concentration. Acta Obstet Gynecol Scand. 2004;83:1193–8. doi: 10.1111/j.0001-6349.2004.00438.x. [DOI] [PubMed] [Google Scholar]
- 8.Wong MY, Harmanli OH, Agar M, Dandolu V, Grody MHT. Collagen content of nonsupport tissue in pelvic organ prolapse and stress urinary incontinence. Am J Obstet Gynecol. 2003;189:1597–600. doi: 10.1016/j.ajog.2003.09.043. [DOI] [PubMed] [Google Scholar]
- 9.Takano CC, Girao MJ, Sartori MG, Castro RA, Arruda RM, Simoes MJ, Baracat EC, Rodrigues de Lima G. Analysis of collagen in parametrium and vaginal apex of women with and without uterine prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13:342–5. doi: 10.1007/s001920200076. [DOI] [PubMed] [Google Scholar]
- 10.Keane D, Sims T, Abrams P, et al. Analysis of collagen status in premenopausal nulliparous women with genuine stress incontinence. Br J Obstet Gynaecol. 1997;104:994–998. doi: 10.1111/j.1471-0528.1997.tb12055.x. [DOI] [PubMed] [Google Scholar]
- 11.Stanosz S, Wilanowski K, Zebrowski W, Hajdasz P, Kosciuszkiewicz B. Certain biochemical markers in women with abnormal states of the reproductive system. Ginekol Pol. 1995;66:518–22. [PubMed] [Google Scholar]
- 12.Chen BH, Wen Y, Li H, Polan ML. Collagen metabolism and turnover in women with stress urinary incontinence and pelvic prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2002;13:80–7. doi: 10.1007/s001920200020. [DOI] [PubMed] [Google Scholar]
- 13.Kobak W, Lu J, Hardart A, Zhang C, Syanczyk FZ, Felix JC. Expression of lysyl oxidase and transforming growth factor β2 in women with severe pelvic organ prolapse. J Reprod Med. 2005;50:827–831. [PubMed] [Google Scholar]
- 14.Bai SW, Choe BH, Kim JY, Park KH. Pelvic organ prolapse and connective tissue abnormalities in Korean women. J Reprod Med. 2002;47:231–4. [PubMed] [Google Scholar]
- 15.Carley ME, Schaffer J. Urinary incontinence and pelvic organ prolapse in women with Marfan or Ehlers Danlos syndrome. Am J Obstet Gynecol. 2000;182:1021–3. doi: 10.1067/mob.2000.105410. [DOI] [PubMed] [Google Scholar]
- 16.Boskey AL, Wright TM, Blank RD. Collagen and bone strength. JBMR. 1999;14:330–337. doi: 10.1359/jbmr.1999.14.3.330. [DOI] [PubMed] [Google Scholar]
- 17.Kohlmeier L, Gasner C, Marcus R. Bone mineral status of women with Marfan syndrome. Am J Med. 1993;95:568–72. doi: 10.1016/0002-9343(93)90351-o. [DOI] [PubMed] [Google Scholar]
- 18.Carbone L, Tylavsky FA, Bush AJ, Koo W, Orwoll E, Cheng S. Bone density in Ehlers-Danlos syndrome. Osteoporos Int. 2000;11:388–92. doi: 10.1007/s001980070104. [DOI] [PubMed] [Google Scholar]
- 19.AL-Rawi ZS, Al-Rawi ZT. Joint hypermobility in women with genital prolapse. Lancet. 1982;1(8287):1439–41. doi: 10.1016/s0140-6736(82)92453-9. [DOI] [PubMed] [Google Scholar]
- 20.Norton PA, Baker JE, Sharp HC, Warenski JC. Genitourinary prolapse and joint hypermobility in women. Obstet Gynecol. 1995;85:225–8. doi: 10.1016/0029-7844(94)00386-R. [DOI] [PubMed] [Google Scholar]
- 21.Strinic T, Bukovic D, Eterovic D, Stipic I, Silovski H, Stanceric T, Videc L. Pulmonary ventilatory function in premenopausal women with and without genital descensus. Coll Antropol. 2002;26(Suppl):139–42. [PubMed] [Google Scholar]
- 22.Pal L, Hailpern SM, Santoro NF, Freeman R, Barad D, Kipersztok S, Barnabei VM, Wassertheil-Smoller S. Association of pelvic organ prolapse and fractures in postmenopausal women: analysis of baseline data from the Women’s Health Initiative Estrogen Plus Progestin trial. Menopause. 2008;15:59–66. doi: 10.1097/gme.0b013e3181151444. [DOI] [PubMed] [Google Scholar]
- 23.Stefanick ML, Cochrane BB, Hsia J, Barad DH, Liu JH, Johnson SR. The Women’s Health Initiative postmenopausal hormone trials: overview and baseline characteristics of participants. Ann Epidemiol. 2003;13:S78–86. doi: 10.1016/s1047-2797(03)00045-0. [DOI] [PubMed] [Google Scholar]
- 24.Cauley JA, Robbins J, Chen Z, Cummings SR, Jackson RD, LaCroix AZ, LeBoff M, Lewis CE, McGowan J, Neuner J, Pettinger M, Stefanick ML, Wactawski-Wende J, Watts NB Women’s Health Initiative Investigators. Effects of estrogen plus progestin on risk of fracture and bone mineral density: the Women’s Health Initiative randomized trial. JAMA. 2003;290:1729–38. doi: 10.1001/jama.290.13.1729. [DOI] [PubMed] [Google Scholar]
- 25.Stare J, Pohar M, Henderson R. Goodness of fit of relative survival models. Stat Med. 2005;24:3911–3925. doi: 10.1002/sim.2414. [DOI] [PubMed] [Google Scholar]
- 26.Klotzbuecher CM, Ross PD, Landsman PB, Abbott TA, 3rd, Berger M. Patients with prior fractures have an increased risk of future fractures: a summary of the literature and statistical synthesis. J Bone Miner Res. 2000;15:721–739. doi: 10.1359/jbmr.2000.15.4.721. [DOI] [PubMed] [Google Scholar]
- 27.Melton LJ, 3rd, Achenbach SJ, Gebhart JB, Babalola EO, Atkinson EJ, Bharucha AE. Influence of hysterectomy on long-term fracture risk. Fertil Steril. 2007;88:156–62. doi: 10.1016/j.fertnstert.2006.11.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.van Ophoven A, Roth S. The Anatomy and Embryological Origins of the Fascia of Denonvilliers: A Medico-Historical Debate. Review Article. The Journal of Urology. 1997;157:3–9. doi: 10.1097/00005392-199701000-00003. [DOI] [PubMed] [Google Scholar]
- 29.Chen HY, Wan L, Chung YW, Chen WC, Tsai FJ, Tsai CH. Estrogen receptor beta gene haplotype is associated with pelvic organ prolapse. Eur J Obstet Gynecol Reprod Biol. 2008;138:105–9. doi: 10.1016/j.ejogrb.2007.12.013. [DOI] [PubMed] [Google Scholar]
- 30.Chen HY, Chung YW, Lin WY, Chen WC, Tsai FJ, Tsai CH. Estrogen receptor alpha polymorphism is associated with pelvic organ prolapse risk. Int Urogynecol J Pelvic Floor Dysfunct 2008. 2008;19:1159–63. doi: 10.1007/s00192-008-0603-1. [DOI] [PubMed] [Google Scholar]
- 31.Vardy MD, Lindsay R, Scotti RJ, Mikhail M, Richart RM, Nieves J, Zion M, Cosman F. Short-term urogenital effects of raloxifene, tamoxifen, and estrogen. Am J Obstet Gynecol. 2003;189:81–8. doi: 10.1067/mob.2003.374. [DOI] [PubMed] [Google Scholar]
- 32.Kudish BI, Iglesia CB, Sokol RJ, Cochrane B, Richter HE, Larson J, Hendrix SL, Howard BV. Effect of weight change on natural history of pelvic organ prolapse. Obstet Gynecol. 2009;113:81–8. doi: 10.1097/AOG.0b013e318190a0dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barber MD, Lambers AR, Visco AG, Bump RC. Effect of patient position on clinical evaluation of pelvic organ prolapse. Obstet Gynecol. 2000;96:18–22. doi: 10.1016/s0029-7844(00)00859-0. [DOI] [PubMed] [Google Scholar]
- 34.Muir TW, Stepp KJ, Barber MD. Adoption of the pelvic organ prolapse quantification system in peer-reviewed literature. Am J Obstet Gynecol. 2003;189:1632–5. doi: 10.1016/j.ajog.2003.09.010. [DOI] [PubMed] [Google Scholar]
- 35.Bruyere O, Brandi ML, Burlet N, Harvey N, Lyritis G, Minne H, Boonen S, Reginster JY, Rizzoli R, Akesson K. Post-fracture management of patients with hip fracture: a perspective. Curr Med Res Opin. 2008;24:2841–51. doi: 10.1185/03007990802381430. [DOI] [PubMed] [Google Scholar]