Abstract
The marine natural product (+)-spongistatin 1 is an extremely potent growth inhibitory agent having activity against a wide variety of cancer cell lines, while exhibiting low cytotoxicity against quiescent human fibroblasts. Consistent with a microtubule-targeting mechanism of action, (+)-spongistatin 1 causes mitotic arrest in DU145 human prostate cancer cells. More importantly, (+)-spongistatin 1 exhibits significant in vivo antitumor activity in the LOX-IMVI human melanoma xenograft model. (+)-Spongistatin 1 is thus an important class of microtubule targeting anticancer agent that warrants further investigation.
Keywords: (+)-Spongistatin 1, anticancer, natural product, mitosis, xenograft model Xu et al. Anticancer Activity of (+)-Spongistatin 1
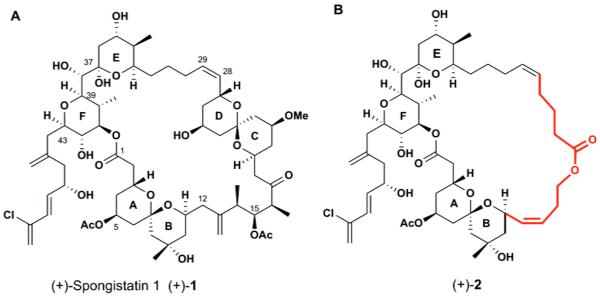
The spongipyrans, a family of architecturally complex marine natural products displaying unique bis-spiroketal subunits, are extraordinarily potent cytotoxic agents. After the initial isolation of spongistatin 4 and 5 in 1982 by Pettit (1), the research groups of Pettit (2,3) Kitagawa (4) and Fusetani (5) independently described structures for spongistatins 1-9, altohyrtin A-C and cinachydrolide A, respectively. Spongistatin 1 [(+)-1, Figure 1A], recognized as the most cytotoxic member of the spongistatin family, has an average IC50 value of 0.12 nM against the National Cancer Institute’s panel of 60 human cancer cell lines (3,6). The initially observed inhibitory activity against in vitro microtubule assembly has been further investigated via tubulin polymerization, competition and turbidity/aggregation assay (7, 8). Results revealed that (+)-spongistatin 1 competitively inhibits tubulin binding of maytansine and rhizoxin as well as GTP exchange; non-competitively inhibits tubulin binding of dolastatin 10, halichondrin B and vinblastine; inhibits formation of the Cys-12-Cys-201/211 cross-link on tubulin and does not cause any tubulin aggregation at substochiometric concentrations. Following these findings, Hamel and coworkers proposed a “polyether” binding site for spongistatins at the tubulin vinca domain, distinct from and in the vicinity of the “peptide” and the “vinca” sites where dolastatin 10/phomopsin A and vinblastine/vincristine bind, respectively (7).
Figure 1.
Spongistatin 1 ((+)-1) and ABEF ring analog ((+)-2).
More recently, Vollmar and coworkers reported that (+)-spongistatin 1 induced apoptotic cell death through both caspase-dependent and -independent mechanisms (9-11). (+)-Spongistatin 1 was shown to induce apoptosis in human primary acute leukemia cells, to prevent long-term survival of leukemia cell lines through caspase-dependent mitochondrial apoptosis pathways, and to enhance synergistically staurosporine-induced cell death through degradation of XIAP, the X-linked inhibitor of apoptosis protein (10). On the other hand, in MCF-7 breast cancer cells, (+)-spongistatin 1 induced apoptosis via a caspase-independent mechanism involving Bim, a pro-apoptotic member of the Bcl-2 family (11). Subsequently, the Vollmar group demonstrated that (+)-spongistatin 1 is a potent antitumor and antimetastatic agent in vitro and in vivo against invasive pancreatic cancer cells (12). To explore further the potential of (+)-spongistatin 1 as a cancer drug lead, we recently disclosed the grams-cale synthesis of (+)-spongistatin 1 for preclinical studies and then initiated an analog program to identify the minimum critical structure required for activity (13). Following the determination of the solution conformation of (+)-spongistatin 1 (14), we have designed and synthesized a simplified ABEF ring analog [(+)-2, Figure 1B] (15, 16), which encouragingly, was shown to have significant in vitro anticancer activity against multiple cancer cell lines. Equally important, the ABEF analog retained the same microtubule targeting mechanism of action as (+)-spongistatin 1 (15, 16).
Here further characterization of the in vitro and in vivo anticancer activity of (+)-spongistatin 1 is reported.
Materials and Methods
Cell culture and cell growth inhibition assay
The chosen cell lines were all human cancer cell lines except for the 4T1 murine breast cancer line, representing a wide variety of cancer types including breast (MDA-MB-453), kidney (A498), lung (H1975), pancreatic (PANC-1) and endometrial cancer (AN3CA, HEC-1A, and RL95-2), glioblastoma (U251 and U-87MG), melanoma (A2058 and LOX-IMVI) and uterine sarcoma (MES-SA). All the cancer cell lines were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) with the exception of U251 which was provided by the National Cancer Institute Tumor Repository (Frederick, MD) and cultured in the standard tissue culture media appropriate for each cell line. All the culture media were supplemented with 10% fetal bovine serum (FBS), 100 I.U./mL penicillin and 100 μg/mL streptomycin. (+)-Spongistatin 1 was synthesized as described previously (13). For the cell growth inhibition assay, the cells were seeded in 96-well tissue culture plates at 500 - 3000 cells/well (seeding density empirically adjusted for each cell line based on growth rate optimization). The cells were allowed to attach for a minimum of 5 h prior to compound administration. (+)-Spongistatin 1 (or DMSO vehicle control) was added to each well at 1:3 serial dilutions starting at 100 nM. The cells were incubated for a period of 4 days after compound addition. Following the incubation period, CellTiter-Glo reagent (Promega, Madison, WI, USA) was added to all the wells to assess cell proliferation/viability. Luminescence was measured using an Envision microplate reader (Perkin Elmer, Waltham, MA, USA). The IC50 values were calculated as the concentration which inhibited cell growth to 50% of DMSO control treated cell populations.
IMR-90 cytotoxicity assay
To evaluate the effect of (+)-spongistatin 1 on non-proliferating normal cells, an in vitro cytotoxicity assay developed to distinguish between true antiproliferative activity and general cellular cytotoxicity unrelated to proliferation was used as described (17). In brief, IMR-90 human fibroblast cells, obtained from ATCC, were grown for 4 days to confluency in MEM containing 10% FBS and supplemented with L-glutamine, penicillin/streptomycin. After washing, the medium was replaced with complete MEM containing 0.1% FBS and the cells were cultured for 3 additional days to achieve complete quiescence. (+)-Spongistatin 1 or vehicle control was then added in the continued presence of 0.1% FBS, followed by incubation for 3 days at 37°C. Cell viability was assessed by the measurement of cellular ATP levels using CellTiter-Glo reagent (Promega).
Immunofluorescence microscopy and mitotic imaging analysis
For the immunofluorescence study, DU145 cells were treated with 1 nM (+)-spongistatin 1 for 24 h, fixed, and then stained with anti-alpha tubulin antibody (Sigma, St. Louis, MO, USA) to visualize microtubules, anti-phospho-histone H3 (pSer28) antibody (Sigma) to visualize mitotic nuclei and DAPI to visualize nuclei in general. Images were captured using a Nikon fluorescent microscope (Nikon Instruments Inc., Melville, NY, USA). To quantitate the mitotic arrest caused by (+)-spongistatin 1, DU145 cells were treated with (+)-spongistatin 1 for 18 h and stained with the same anti-alpha tubulin, anti-phospho-histone H3 antibodies and DAPI. Mitotic imaging analysis and quantification of phospho-histone H3-positive cells were carried out using a Cellomics ArrayScan (Cellomics, Pittsburg, PA, USA).
P-glycoprotein (PgP) sensitivity assay
Test compounds ((+)-spongistatin 1 and paclitaxel) were compared side-by-side in MES-SA human uterine sarcoma cells (ATCC) and a PgP-overexpressing drug resistant subline MES-SA/Dx5-Rx1, derived from MES-SA/Dx5 (ATCC) after long exposure to doxorubicin as described previously (18). Growth inhibitory IC50 values were then calculated using GraphPad Prism (GraphPad Software, Inc, La Jolla, CA, USA).
In vivo tumor xenograft study
Prior to evaluating (+)-spongistatin 1 in tumor models in vivo, the stability of (+)-spongistatin 1 in mouse serum was evaluated by determining the IC50 of (+)-spongistatin 1 in a four-day cell growth inhibition assay, performed with or without a 6 h pre-incubation of compound in 100% mouse serum. For the tumor xenograft study, six-week old NCr female nu/nu mice (Charles River Laboratories, Wilmington, MA, USA) were injected subcutaneously (s.c.) with 1×106 LOX-IMVI human melanoma cells. Tumor-bearing mice were randomized into 5 groups of 6 mice each. The mean group tumor size was approximately 150 mm3 when treatments began on day 7 after tumor implantation. Drug treatments were by i.v. tail vein injection on a Q4Dx3 or Q4Dx2 schedule and consisted of (+)-spongistatin 1 at 0.14, 0.24 or 0.4 mg/kg, paclitaxel at 20 mg/kg (positive control); or vehicle control (saline solution). The tumor volumes and the condition of the mice were recorded every 3-4 days. The tumor volumes were calculated using the formula (length × width2)/2. Unpaired t-test analysis was conducted with GraphPad Prism 5 software.
Results and Discussion
Growth inhibitory activity of (+)-spongistatin 1
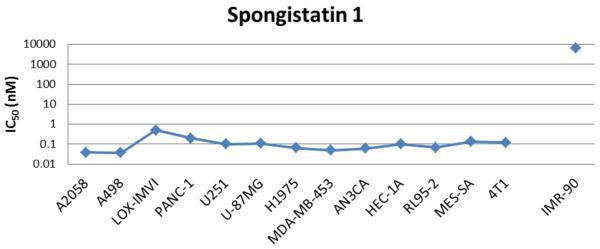
(+)-Spongistatin 1 was previously shown by us and others to display extremely potent sub-nanomolar activity against a variety of cancer cell lines (2, 3, 8-12, 15, 16). We sought to determine the range of anticancer activity of (+)-spongistatin 1 in a larger panel of cancer cell lines. As shown in Figure 2, (+)-spongistatin 1 exhibited consistent sub-nanomolar growth inhibitory activity against all the cancer cell lines examined with IC50 ranging between 0.037-0.5 nM.
Figure 2.
Growth inhibition activity of (+)-Spongistatin 1. Cancer cells assessed by cell growth inhibition and IMR-90 quiescent fibroblasts assessed by cytotoxicity assay after treatment with (+)-spongistatin 1 at a range of concentrations for 4 days and 3 days, respectively.
Differential activity between proliferating cancer cells and quiescent human fibroblasts
To determine if (+)-spongistatin 1 differentially affects cancer cells while sparing normal cells, (+)-spongistatin 1 was evaluated for cell-killing ability against fully-quiescent IMR-90 human fibroblasts. (+)-Spongistatin 1 displayed no cytotoxicity at the low nM levels associated with the antiproliferative activity; the IC50 of (+)-spongistatin 1 was 6.7 micromolar against the quiescent IMR-90 cells (Figure 2). Thus, (+)-spongistatin 1 has a wide in vitro therapeutic window between proliferating and quiescent cells (over 10,000-fold), indicating that the cell growth inhibitory effects observed against human cancer cells result from inhibited proliferative processes, rather than indiscriminant proliferation-independent cytotoxicity.
Effect of (+)-spongistatin 1 on mitosis arrest
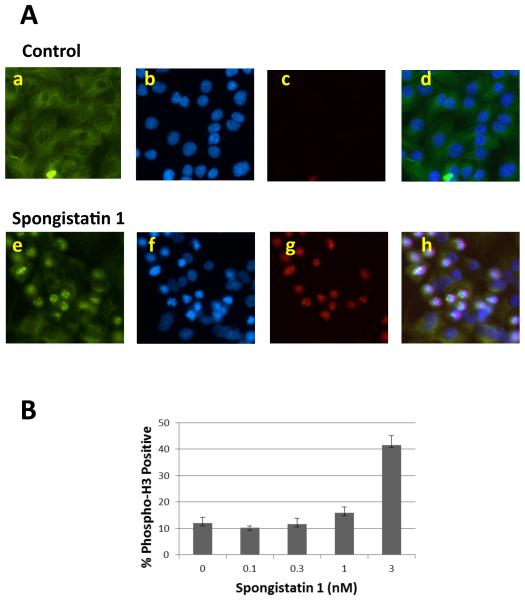
In addition to disruption of tubulin polymerization in vitro (6, 7, 8), (+)-spongistatin 1 also led to enrichment of cells in the G2/M phase of the cell cycle as shown by FACS analysis in U937 lymphoma cells (16). The anti-mitotic effect of (+)-spongistatin 1 was further examined first by immunofluorescence study and then by mitotic imaging analysis of DU145 cells. As shown in Figure 3A, treatment with (+)-spongistatin 1 caused disruption of the mitotic spindles and an increase in phospho-histone H3 positive cells, these observations provided strong evidence for a (+)-spongistatin 1-induced mitotic defect. The percentage of phospho-histone H3-positive cells quantified using Cellomics ArrayScan is shown in Figure 3B. (+)-Spongistatin 1 caused a dose dependent increase in the percentage of cells in mitosis. Taken together, these mitotic imaging studies allowed the conclusion that (+)-spongisitatin 1 caused mitotic arrest.
Figure 3.
Effect of (+)-spongistatin 1 on mitosis. A. Fluorescence microscopic images of DU145 cells treated with either 1 nM (+)-spongistatin 1 or DMSO control for 24 h, then fixed and stained with anti-alpha tubulin antibody (a, e, green), anti-phospho-histone H3 antibody (b, f, red) and DAPI (c, g, blue). Merged images are shown in d and h. B. Quantitation of mitotic nuclei (phospho-histone H3 positive) using Cellomics ArrayScan.
(+)-Spongistatin 1 as a moderate PgP substrate
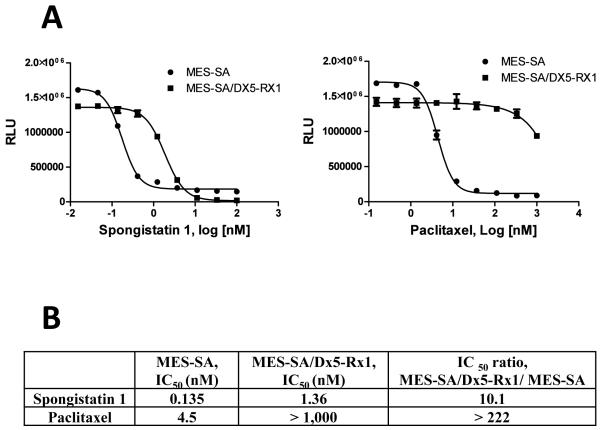
Multidrug resistance (MDR) of anticancer drugs represents a significant clinical problem (19). A well-established driver of MDR is the drug efflux transporter P-glycoprotein (PgP; 19). The susceptibility of (+)-spongistatin 1 to PgP-mediated efflux was therefore evaluated by determining if growth inhibitory activity of (+)-spongistatin 1 was reduced in PgP-overexpressing cancer cells. As shown in Figure 4, (+)-spongistatin 1 was only moderately sensitive to PgP-mediated drug efflux compared to the high sensitivity of paclitaxel, a known PgP substrate. The IC50 values for growth inhibition in the PgP-over expressing MES-SA/Dx5-Rx1 cells were 10-fold higher than the IC50 values in the parental MES-SA cells for (+)-spongistatin 1, versus more than 224-fold for paclitaxel. These data thus indicated that (+)-spongistatin 1 had moderate susceptibility to PgP-mediated drug efflux. Chemical modification of (+)-spongistatin 1 to reduce PgP susceptibility would therefore be desirable.
Figure 4.
Susceptibility to PgP-mediated efflux. (+)-Spongistatin 1 and paclitaxel were tested side by side for growth inhibitory activity in the MES-SA parental cell line and its PgP overexpressing subline MES-SA/Dx5-Rx1. Growth inhibition curves are shown in A. IC50s determined using GraphPad Prism (average of results of two experiments for spongistatin1) are shown in B.
Stability of (+)-spongistatin 1 in mouse serum
(+)-Spongistatin 1 was found to be very stable in mouse serum with little change in measured IC50 values after 6 h pre-incubation with mouse serum (data not shown). Under similar conditions, certain halichondrin B lactone analogs have been found to lose activity in mouse serum (20).
In vivo antitumor activity of (+)-spongistatin 1
Having demonstrated potent antiproliferative activity in conjunction with a wide in vitro window between proliferating and quiescent cells, the question of whether (+)-spongistatin 1 exerts in vivo anticancer activity was addressed. As shown in Figure 5, (+)-spongistatin 1 displayed a dose-dependent inhibition of LOX-IMVI tumor growth, with a maximum observed body weight loss of 18%. Tumor growth inhibition at 0.24 mg/kg on a Q4D x 2 schedule was found to be statistically significant (p = 0.001 on day 18); No statistically significant efficacy was identified at the 0.14 mg/kg dose on a q4d x 3 schedule (Table I), while 0.4 mg/kg caused overt toxicity. Taken together, these results demonstrated that (+)-spongistatin 1 displayed significant antitumor activity on established human tumor xenografts.
Figure 5.
In vivo anticancer activity of (+)-spongistatin 1 in a LOX human melanoma xenograft model. LOX melanoma xenografts (approximately 150 mm3) were treated by i.v. tail vein injection of (+)-spongistatin 1 or paclitaxel positive control or saline vehicle control. X axes, arrows indicate days on which compounds were administered.
Table I. Unpaired t-test analysis based on day 15 and day 18 tumor volume.
| Day 15 | Day 18 | |
|---|---|---|
| 0.14 mg/kg vs. Vehicle | 0.1442 | 0.1627 |
| 0.24 mg/kg vs. Vehicle | 0.0024 | 0.001 |
The in vivo antitumor and antimetastatic activities of (+)-spongistatin 1, administered before sizable tumors were formed, were recently reported by Vollmar’s group using an orthotopic pancreatic cancer model (12). Under those conditions, (+)-spongistatin 1 inhibited pancreatic tumor growth and metastasis to the lymph nodes and liver at fairly low doses (daily injection of 10 μg/kg). In the present study using the LOX-IMVI melanoma model, drug treatment began after the tumors had reached larger size, ~150 mm3 and therefore the result further established the in vivo antitumor activity of spongistatin 1 on established tumors.
In summary, spongistatin 1 has consistent sub- nanomolar growth inhibitory potency in a broad range of human cancer cell lines in vitro. Consistent with its microtubule targeting mechanism of action, spongistatin 1 causes significant mitotic arrest in cancer cells as evidenced by an increase in phospho-histone H3 positive cells. Importantly, spongistatin 1 causes statistically significant tumor growth inhibition in vivo in a human melanoma xenograft model. These results thus further substantiate the notion that spongistatin 1 represents an important class of tubulin agents whose anticancer therapeutic potential warrants further investigation.
Acknowledgements
The authors would like to thank the National Institutes of Health (National Cancer Institute) for support of this work at the University of Pennsylvania through grant CA-070329-01-11 and the National Cancer Institute Tumor Repository for providing the U251 cell line under a Material Transfer Agreement.
References
- 1.Pettit GR. Antineoplastic agents. 317. Marine animal and terrestrial plant anticancer constituents. Pure Appl Chem. 1994;66:2271–2281. [Google Scholar]
- 2.Pettit GR, Cichacz ZA, Gao F, Herald CL, Boyd MR. Isolation and structure of the remarkable human cancer cell growth inhibitors spongistatins 2 and 3 from an eastern Indian Ocean Spongia sp. J Chem Soc, Chem Commun. 1993;14:1166–1168. [Google Scholar]
- 3.Pettit GR, Chicacz ZA, Gao F, Herald CL, Boyd MR, Schmidt JM, Hooper JNA. Antineoplastic agents. 257 Isolation and structure of spongistatin 1. J Org Chem. 1993;58:1302–1304. [Google Scholar]
- 4.Kobayashi M, Aoki S, Sakai H, Kawazoe K, Kihara N, Sasaki T, Kitagawa I. Altohyrtin A, a potent antitumor macrolide from the Okinawan marine sponge Hyrtios altum. Tetrahedron Lett. 1993;34:2795–2798. doi: 10.1248/cpb.41.989. [DOI] [PubMed] [Google Scholar]
- 5.Fusetani N, Shinoda K, Matsunaga S. Bioactive marine metabolites. 48. Cinachyrolide A: A potent cytotoxic macrolide possessing two spiro ketals from marine sponge Cinachyra sp. J Am Chem Soc. 1993;115:3977–3981. [Google Scholar]
- 6.Bai R, Cichacz ZA, Herald CL, Pettit GR, Hamel E. Spongistatin 1, a highly cytotoxic, sponge-derived, marine natural product that inhibits mitosis, microtubule assembly, and the binding of vinblastine to tubulin. Mol Pharmacol. 1993;44:757–766. [PubMed] [Google Scholar]
- 7.Bai R, Taylor GF, Cichacz ZA, Herald CL, Kepler JA, Pettit GR, Hamel E. The spongistatins, potently cytotoxic inhibitors of tubulin polymerization, bind in a distinct region of the vinca domain. Biochemistry. 1995;34:9714–9721. doi: 10.1021/bi00030a009. [DOI] [PubMed] [Google Scholar]
- 8.Luduena RF, Roach MC, Prasad V, Pettit GR, Cichacz ZA, Herald CL. Interaction of three sponge-derived macrocyclic lactone polyethers (spongistatin 3, halistatins 1 and 2) with tubulin. Drug Dev Res. 1995;35:40–48. [Google Scholar]
- 9.Schneiders UM, Schyschka L, Barth N, Vollmar AM. Characterisation of apoptosis signal transduction induced by spongistatin 1 in MCF-7 cells. Naunyn-Schmiede-bergs Archives of Pharmacology. 2007;375:399. [Google Scholar]
- 10.Schyschka L, Rudy A, Jeremias I, Barth N, Pettit GR, Vollmar AM. Spongistatin 1: a new chemosensitizing marine compound that degrades XIAP. Leukemia. 2008;22:1737–1745. doi: 10.1038/leu.2008.146. [DOI] [PubMed] [Google Scholar]
- 11.Schneiders UM, Schyschka L, Rudy A, Vollmar AM. BH3-only proteins Mcl-1 and Bim as well as endonuclease G are targeted in spongistatin 1-induced apoptosis in breast cancer cells. Mol Cancer Ther. 2009;8:2914–2925. doi: 10.1158/1535-7163.MCT-08-1179. [DOI] [PubMed] [Google Scholar]
- 12.Rothmeier AS, Schneiders UM, Wiedmann RM, Ischenko I, Bruns CJ, Rudy A, Zahler S, Vollmar AM. The marine compound spongistatin 1 targets pancreatic tumor progression and metastasis. Int J Cancer. 2010;127:1096–1105. doi: 10.1002/ijc.25241. [DOI] [PubMed] [Google Scholar]
- 13.Smith AB, Sfouggatakis C, Risatti CA, Sperry JB, Zhu W, Doughty VA, Tomioka T, Gotchev DB, Bennett CS, Sakamoto S, Atasoylu O, Shirakami S, Bauer D, Takeuchi M, Koyanagi J, Sakamoto Y. Spongipyran synthetic studies. Evolution of a scalable total synthesis of (+)-spongistatin 1. Tetrahedron. 2009;65:6489–6509. doi: 10.1016/j.tet.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Atasoylu O, Furst G, Risatti C, Smith AB., III The solution structure of (+)-spongistatin 1 in DMSO. Org Lett. 2010;12:1788–1791. doi: 10.1021/ol100417d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith AB, III, Risatti CA, Atasoylu O, Benett CS, TenDyke K, Xu Q. Design, synthesis, and biological evaluation of EF- and ABEF- analogues of (+)-spongistatin 1. Org Lett. 2010;12:1792–1795. doi: 10.1021/ol100418n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith AB, III, Risatti CA, Atasoylu O, Bennett CS, Liu L, Cheng H, TenDyke K, Xu Q. Design, synthesis, and biological evaluation of diminutive forms of (+)-spongistatin 1: lessons learned. 2011 doi: 10.1021/ja2046167. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rudolph-Owen LA, Salvato K, Cheng C, Wu J, Towle MJ, Littlefield BA. A 96-well plate cell-based assay to quantify undesired cytotoxic effects against quiescent non-dividing cells. Proc Amer Assoc Cancer Res. 2004;45:264–265. [Google Scholar]
- 18.Liu JW, Cui GH, Zhao M, Cui CY, Ju JF, Peng SQ. Dual-acting agents that possess reversing resistance and anticancer activities: design, synthesis, MES-SA/Dx5 cell assay, and SAR of benzyl 1,2,3,5,11,11a-hexahydro-3,3-dimethyl-1-oxo-6H-imidazo 3′,4′:1,2 pyridin-3,4-b-indol-2-substitutedacetates. Bioorg & Med Chem. 2007;15:7773–7788. doi: 10.1016/j.bmc.2007.08.061. [DOI] [PubMed] [Google Scholar]
- 19.Baguley BC. Multiple drug resistance mechanisms in cancer. Mol Biotechnol. 2010;46:308–316. doi: 10.1007/s12033-010-9321-2. [DOI] [PubMed] [Google Scholar]
- 20.Towle MJ, Wels BF, Cheng H, Budrow J, Zheng W, Seletsky BM, Hawkins LD, Palme MH, Habgood GJ, Singer LA, DiPietro LV, Wang Y, Chen JJ, Lydon PJ, Quincy DA, Tagami K, Kishi Y, Yu MJ, Littlefield BA. Halichondrin B macrocyclic ketone analog E7389: medicinal chemistry repair of lactone ester instability generated during structural simplification to clinical candidate; Annual Meeting Amer Assoc Cancer Res; San Francisco, CA. 2002; Abstract 5721. [Google Scholar]