Abstract
α-Synuclein is a major component of Lewy bodies in Parkinson disease (PD) and dementia with Lewy bodies (DLB). We recently showed that abnormal α-synuclein with resistance to proteinase K (PK) is deposited at presynapses of distinct brain anatomic regions from the early stage of PD and DLB. NUB1, a synphilin-1 binding protein, also accumulates in Lewy bodies but it is not known whether abnormal α-synuclein is associated with NUB1. Here, we demonstrate that in the brains of patients with PD and DLB, NUB1 accumulates in the presynapses in the hippocampus, cerebral neocortex and substantia nigra in which PK-resistant α-synuclein is deposited. Endogenous NUB1 also accumulated with PK-resistant α-synuclein in the presynapses of transgenic mice that express human α-synuclein with an A53T mutation. Immunoelectron microscopy showed that NUB1 was localized to presynaptic nerve terminals where no abnormal filaments were seen. Biochemical analyses showed that NUB1 coexists with abnormal α-synuclein in the brains of DLB patients. These findings suggest that NUB1 along with abnormal α-synuclein is involved in the pathogenesis of Lewy body diseases.
Keywords: α-Synuclein, Dementia with Lewy bodies, NUB1, Parkinson disease, Presynapse, Proteinase K, Synphilin-1
INTRODUCTION
α-Synuclein is a soluble protein primarily expressed at the presynaptic nerve terminals (1, 2). Although the physiological functions of α-synuclein remain uncertain, accumulating data suggest that α-synuclein plays a role in modulation of synaptic plasticity and vesicle pools for multiple neurotransmitters, such as dopamine and glutamate (3–7). Identification of point mutations and multiplications of the α-synuclein gene in familial Parkinson disease (PD) established its association with the pathogenesis of PD (8–13). α-Synuclein accumulates as an insoluble, proteinase K (PK)-resistant protein in neuronal cytoplasm and processes as Lewy bodies (LBs) and Lewy neurites, respectively, in the brains of patients with PD and dementia with LBs (DLB) (14, 15). Recent studies show that PK-resistant α-synuclein is also present at presynaptic nerve terminals in the brains of patients with PD and DLB but not in control subjects, and that the accumulation of PK-resistant α-synuclein in presynapses precedes LB formation in the substantia nigra and hippocampus (16, 17).
NUB1 was originally isolated as an interacting protein of NEDD8, one of the ubiquitin-like proteins (18). NUB1 also interacts with an α-synuclein-binding protein, synphilin-1 (19, 20). Importantly, both NUB1 and synphilin-1 are localized to α-synuclein-positive LBs in PD and DLB brains, as well as to glial cytoplasmic inclusions in brains of patients with multiple system atrophy (21–23). In contrast, NUB1 and synphilin-1 are not accumulated in α-synuclein-negative inclusions in other neurodegenerative disorders (22, 23). These observations imply an association between NUB1 and α-synuclein.
To investigate the possible association between NUB1 and α-synuclein, we performed immunohistochemical, ultrastructural and biochemical analyses using brain samples from LB disease patients and transgenic (Tg) mice that overexpress human α-synuclein with an A53T mutation (SNCA Tg mice). We found that NUB1 is colocalized with non-fibrillar, PK-resistant α-synuclein in presynapses in LB disease patients and SNCA Tg mice.
MATERIALS AND METHODS
Human Subjects
For immunohistochemical analyses, 17 postmortem cases were utilized. These included 6 PD cases (Braak PD stage 4), 6 DLB cases (Braak PD stage 5–6), and 5 normal control subjects (24) (Table). Brain tissues were fixed with 10% buffered formalin for 3 weeks and embedded in paraffin. Serial 4-µm-thick sections were cut from block samples of the following areas in each case: frontal and temporal neocortices, hippocampus, basal ganglia, thalamus, midbrain, pons, medulla oblongata and cerebellum.
Table.
Summary of Human Subjects
| Case No. | Analysis | Pathological diagnosis | Age (years) | Gender | PMI (hour) |
|---|---|---|---|---|---|
| Immunohistochemistry | |||||
| 1 | Normal | 53 | M | 9 | |
| 2 | Normal | 75 | M | 2.5 | |
| 3 | Normal | 80 | F | 1 | |
| 4 | Normal | 81 | M | 3 | |
| 5 | Normal | 84 | M | 10 | |
| 6 | PD | 63 | M | 3 | |
| 7 | PD | 65 | M | 4 | |
| 8 | PD | 69 | M | 1 | |
| 9 | PD | 75 | M | 4 | |
| 10 | PD | 76 | M | 5.5 | |
| 11 | PD | 85 | M | 2 | |
| 12 | DLB | 74 | M | 2 | |
| 13 | DLB | 77 | F | 1 | |
| 14 | DLB | 78 | M | 3 | |
| 15 | DLB | 78 | M | 3 | |
| 16 | DLB | 82 | F | 2 | |
| 17 | DLB | 84 | F | 10 | |
| Biochemistry | |||||
| 18 | Normal | 76 | M | 3.2 | |
| 19 | Normal | 79 | F | 2.5 | |
| 20 | Normal | 82 | F | 4.5 | |
| 21 | DLB | 70 | F | 3 | |
| 22 | DLB | 74 | M | 2.5 | |
| 23 | DLB | 75 | F | 1.5 |
PD, Parkinson disease; DLB, Dementia with Lewy bodies; PMI, post-mortem-interval; F, female; M, male.
For biochemical analyses, frozen brain tissues (middle temporal cortex) from 3 patients with DLB (Braak PD stage 6) and 3 age-matched normal control subjects were obtained from the Brain Research Institute, University of Niigata, Japan (Table). Human brain tissues were dissected at autopsy and immediately frozen at −70°C.
Animals
Tg mice expressing human α-synuclein with an A53T mutation under control of the prion promoter were obtained from Jackson Laboratories, Bar Harbor, ME (25); their wild-type littermates were between 1 and 66 weeks old (homozygous mice, n = 30; heterozygous mice, n = 30; control mice, n = 40). Animals were handled and killed in compliance with institutional and national regulations and policies. The protocol was approved by the Institutional Animal Care and Use Committee at Hirosaki University Graduate School of Medicine, Japan. Tg and control mice were transcardially perfused with phosphate-buffered saline (PBS). The brain was removed and the right hemisphere was fixed with 4% paraformaldehyde for 48 hours. After dehydrating through a graded ethanol series it was embedded in paraffin and cut into 4-µm-thick sections. Left hemispheres were frozen at −80°C until they were used for biochemical analysis. Most homozygous SNCA Tg mice develop complex motor impairment leading to paralysis and death around 40 weeks of age or later, whereas most hemizygous SNCA Tg mice remain healthy at least until they are 70 weeks old (25).
Antibodies
Rabbit anti-NUB1 antibody was generated as previously described (18). Goat antibodies against NUB1 and synphilin-1 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Mouse monoclonal antibodies against phosphorylated α-synuclein (pSyn#64; WAKO, Osaka, Japan) (26), human α-synuclein (LB509; Zymed, South San Francisco, CA) (27), human and mouse α-synuclein (Syn-1; Transduction Laboratory, Lexington, KY), and synaptophysin (SY-38; Boehringer Mannheim, Mannheim, Germany), and rabbit polyclonal antibodies against β-actin (Sigma, Saint Louis, MO) and NEDD8 (28) were used as primary antibodies.
Expression of Recombinant NUB1 in HeLa Cells
To express human or mouse NUB1 tagged with a Flag epitope, human NUB1 (GenBank: AF300717) or mouse NUB1 (AF534114.1) was subcloned into pcDNA3 (Invitrogen, Carlsbad, CA), tagged with MDYKDDDDK at the N-terminus. The plasmid was transfected into HeLa cells using Fugene 6 (Roche Molecular Biochemicals, Indianapolis, IN). After 24 hours, cells were harvested and lysed with lysis buffer containing 4% SDS.
Immunohistochemistry
Because formic acid pretreatment enhanced anti-NUB1 immunostaining of pathological inclusions found in LB disease (23), mouse and human tissue sections were pretreated with 99% formic acid (Wako) for 5 minutes or heated by a microwave oven for 15 minutes in 10 mM citrate buffer (pH 6.0) for antigen retrieval. To examine PK-resistant α-synuclein, PK (Gibco BRL, Gaithersburg, MD; 50 µg/ml) was then applied to the sections. In brief, mouse and human sections were incubated in PK buffer (10 mM Tris-HCl, pH 7.8, 100 mM NaCl, 0.1% Nonidet-P40) for 5 minutes and 10 minutes, respectively, at 37°C. The sections were then subjected to immunohistochemical staining using the avidin-biotin-peroxidase complex method with diaminobenzidine as the chromogen. Antibodies against NUB1 (diluted at 1:100), pSyn#64 (1:5,000), LB509 (1:1,000), Syn-1 (1:1,000), NEDD8 (1:50), synphilin-1 (1:100) and SY-38 (1:1,000) were used as primary antibodies. The sections were counterstained with hematoxylin.
Double immunofluorescent staining was performed to detect co-expression of NUB1 with synaptophysin. For this purpose, sections were blocked with horse serum and then incubated overnight at 4°C with mouse anti-synaptophysin. Following a wash for 3 minutes 5 times with PBS, the sections were incubated for 1 hour with Alexa Fluor 488 conjugated secondary antibody (Invitrogen). After a PBS rinse the sections were examined by a confocal microscope (EZ-Ci, Nikon, Tokyo, Japan). After taking pictures, sections were washed 5 times, treated with 99% formic acid, and incubated overnight at 4°C with rabbit anti-NUB1 antibody. After washing, the sections were incubated with Alexa Fluor 548 conjugated secondary antibody, mounted with Fluoromount G (Southern Biotechnology Inc., Birmingham, AL), and re-examined. The identical regions were captured and merged using Adobe Photoshop CS5 software (Adobe Systems, San Jose, CA).
Immunoelectron Microscopy
Seven-week-old Tg mice (n = 3) were anesthetized with pentobarbital sodium (50 mg/kg) and transcardially perfused with 4% paraformaldehyde and 0.1% glutaraldehyde in 0.1 M phosphate buffer, pH 7.4. The brains were removed and sections of the hippocampus were cut at 50-µm thickness with a vibratome. The sections were incubated with rabbit anti-NUB1 (1:50) for 2 days at 4°C. Some sections were incubated with a biotinylated secondary antibody (1:200) and avidin-biotin complex (1:200). The reaction was developed with DAB (0.1 mg/ml) containing 0.0015% H2O2. The immunolabeled sections were post-fixed in 1% glutaraldehyde and 1% osmium. The other sections were incubated with a 1.4-nm gold-coupled Fab’ fragment of goat anti-rabbit IgG (Nanoprobes, Yaphank, NY). Sections were visualized using a silver enhancing kit (BBInternational, Cardiff, UK), post-fixed in 1% OsO4, and stained with uranyl acetate. All sections were dehydrated in ethanol and embedded in epoxy resin. Finally, ultrathin sections were cut from the resin and viewed with a Hitachi H-300 electron microscope.
Fractionation of Brain Extracts
Frozen brain samples from the patients with DLB and controls were weighed and sequentially extracted with buffers of increasing strength as previously described (29). Briefly, samples were homogenized with 10 volumes of buffer A (10 mM Tris-HCl, pH 7.5, 1 mM EGTA, 10% sucrose, 0.8 M NaCl) with the protease inhibitor cocktail (Roche Applied Science) and centrifuged (Fraction I). Afterwards, another equal volume of buffer A containing 2% Triton X-100 was added. It was then incubated for 30 minutes at 37°C and spun at 100,000 × g for 30 minutes at 4°C (Fraction II). The resultant pellet was homogenized in 5 volumes of buffer A with 1% sarkosyl and incubated for 30 minutes at 37°C. The homogenate was then spun at 100,000 × g for 30 minutes at room temperature (RT) (Fraction III). The sarkosyl-insoluble pellet was homogenized in 4 volumes of buffer A containing 1% 3-[(3-Cholamidopropyl) dimethylammonio] propanesulfonate (CHAPS) and spun at 100,000 × g for 20 minutes at RT (Fraction IV). The pellet was sonicated in 1.5 volumes of 8 M urea buffer (fraction V).
Sucrose Gradient Analysis
We performed sucrose gradient method as previously described (17). Briefly, the temporal neocortex (0.5 g) from the DLB and control subjects was homogenized in Tris-based buffer (TBS; Tris-HCl, pH 7.5, 150 mM NaCl) containing 3 mM CaCl2, 1 mM EDTA, 1 mM EGTA by a Dounce homogenizer for 20 strokes. Tissue homogenates were layered on a linear sucrose gradient (1.2–2.2 M) and centrifuged at 160,000 × g for 2 hours at 4°C using a swing-type rotor S40T (Himac CP-56; Hitachi, Tokyo, Japan). Each fraction was collected from the bottom.
Western Blot Analysis
After SDS-polyacrylamide gel electrophoresis, Western blot analysis was performed as previously described (30). Transfer and detection were carried out according to the protocol provided with the ECL detection system (Amersham Pharmacia Biotech, Piscataway, NJ). Goat anti-NUB1 (1:100), rabbit anti-NUB1 (1:1,000), LB509 (1:1,000), Syn-1 (1:1,000), and rabbit anti-actin (1:3,000) were used as primary antibodies. Horseradish peroxidase-conjugated anti-mouse, -rabbit, or -goat IgG (Santa Cruz Biotechnology) was used as a secondary antibody.
Filter-Trap Analysis
For detection of aggregated α-synuclein we modified the previously described filter-trap analysis (20). Briefly, each fraction of sucrose gradient analysis was subjected to digestion with DNase I (10 µg/ml; AppliChem, Darmstadt, Germany) in TBS for 15 minutes at 37°C and lysed in PK buffer without PK at RT for 10 minutes. The samples were immediately applied to a 0.22-µm cellulose acetate membrane (Millipore, Bedford, MA) on a slot blot apparatus (Bio-Rad, Hercules, CA) using a vacuum manifold. After washing, the membrane was incubated with LB509 and detected by the ECL detection system described above. Semiquantitation of positive signals was done by image analysis using the Image J software (NIH). All values were represented as mean ± SD. Statistical significance was evaluated using the Student t-test when comparing 2 conditions. Probability values less than 0.05 (p < 0.05) were considered significant.
RESULTS
Antibody Specificity
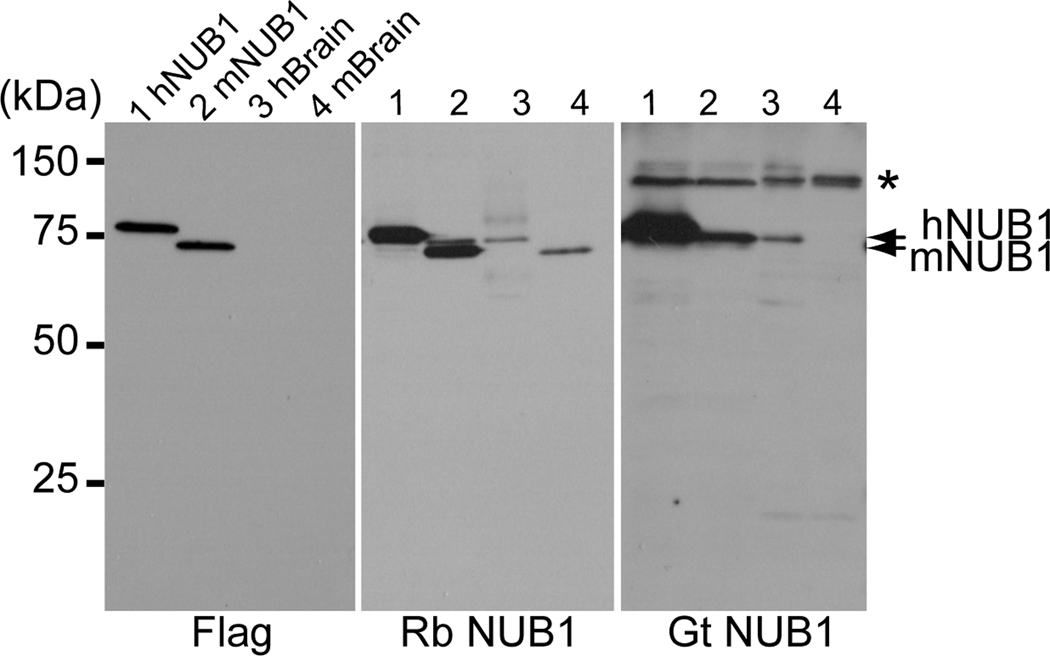
Rabbit anti-NUB1 antibody specifically recognized both human and mouse NUB1. Goat anti-NUB1 antibody reacted with human NUB1, but not with mouse NUB1 (Fig. 1); therefore, we used rabbit anti-NUB1 antibody for immunohistochemical studies.
Figure 1.
Antibody specificity to human and mouse NUB1. Rabbit (Rb) and goat (Gt) anti-NUB1 antibodies detect endogenous NUB1 and flag-tagged human (h) or mouse (m) NUB1 expressed in HeLa cells. An anti-Flag antibody confirms expression of NUB1 tagged with Flag. Lanes 1–2, human or mouse NUB1 tagged with Flag; lanes 3–4, brain lysates from human temporal cortex or mouse brain (arrows). Asterisk indicates non-specific signals.
Immunoreactivity of NUB1 and α-Synuclein in Human Brains
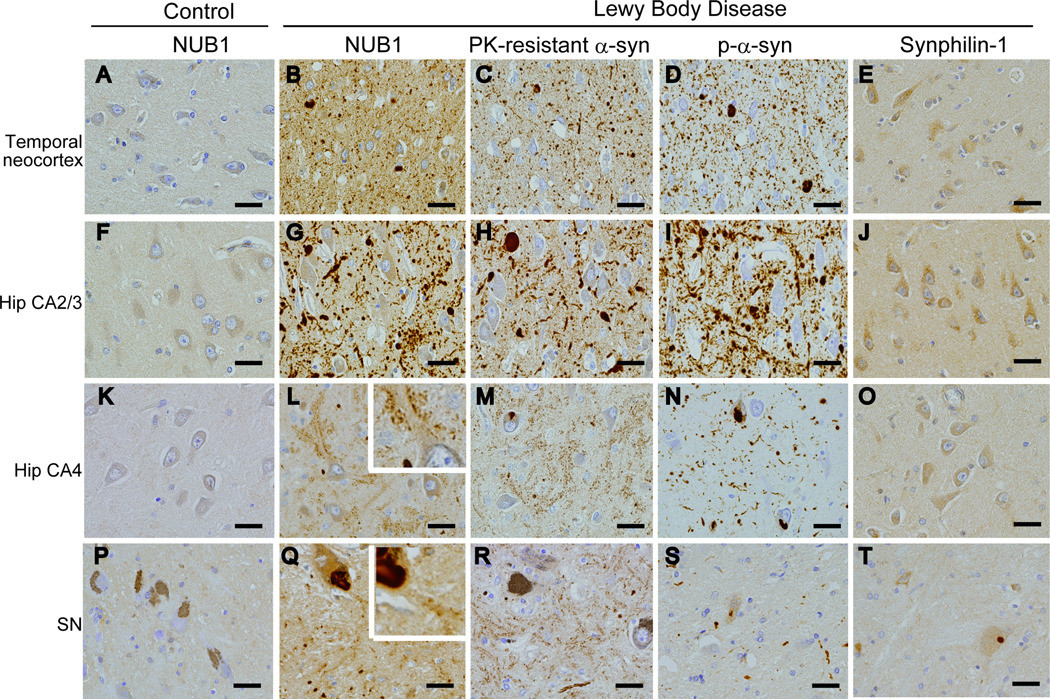
Our previous immunohistochemical studies showed that the anti-NUB1 antibody strongly immunolabels LBs and Lewy neurites in which α-synuclein is highly accumulated (23). Anti-NUB1 antibody barely or weakly immunostained the neuronal perikarya in controls (Fig. 2A, F, K, P). In PD and DLB, however, the anti-NUB1 antibody intensely immunolabeled LBs and Lewy neurites in the cerebral neocortex, hippocampus and brainstem (Fig. 2B, G, Q). The anti-NUB1 antibody also immunolabeled presynapses in the temporal neocortex, hippocampus and substantia nigra in PD and DLB brains (Fig. 2B, G, L, Q). This presynaptic staining was clearly observed in the hippocampal CA4 region and substantia nigra (Fig. 2L, Q). The staining intensity of presynapses was much stronger in DLB brains than in PD brains (data not shown).
Figure 2.
Immunoreactivity of NUB1, proteinase K (PK)-resistant α-synuclein, phosphorylated α-synuclein (p-α-syn) and synphilin-1 in Lewy body disease (LBD) and control brains. (A–T) Temporal neocortex (A–E), hippocampal (Hip) CA2/3 (F–J) and CA4 regions (K–O), and substantia nigra (SN) (P–T) are immunostained. There is weak immunoreactivity for NUB1 in the neuronal cytoplasm in controls (A, F, K, P). Rabbit anti-NUB1 antibody was used on formic acid-treated tissues. There is intense immunoreactivity for NUB1 in abnormal inclusions and in the presynaptic terminals (B, G, L, Q) in Lewy body disease. Insets in (L) and (Q) show presynaptic staining. PK-resistant α-synuclein is demonstrated in abnormal inclusions (C, H) and presynaptic terminals (M, R) in LBD. Phosphorylated α-synuclein immunoreactivity is shown in abnormal inclusions, but not in presynaptic terminals in LBD (D, I, N, S). Synphilin-1 immunoreactivity is not observed in presynaptic terminals (E, J, O, T). Case 3: A, F, K, P; case 16: B–D, G–I, L–N; case 6: Q–S; case 14: E, J, O; case 10: T. Scale bars = 20 µm.
In LB disease, the presynaptic NUB1 was detected predominantly in the temporal neocortex, hippocampus and substantia nigra, and minimally in other regions tested (data not shown). PK-resistant α-synuclein was detected in pathological inclusions (Lewy bodies, Lewy neurites and Lewy dots) as well as diffuse neuropil staining, corresponding to presynaptic nerve terminals (Fig. 2C, H, M, R), particularly in the hippocampal CA4 region and substantia nigra (Fig. 2M, R). Importantly, anti-phosphorylated α-synuclein antibody strongly immunolabeled PK-resistant α-synuclein in pathological inclusions (Fig. 2D, I), but barely immunolabeled PK-resistant α-synuclein in presynaptic nerve terminals (Fig. 2N, S). NUB1 interacts with synphilin-1 (20), but synphilin-1 was not found to be accumulated in the presynapses (Fig 2E, J, O, T).
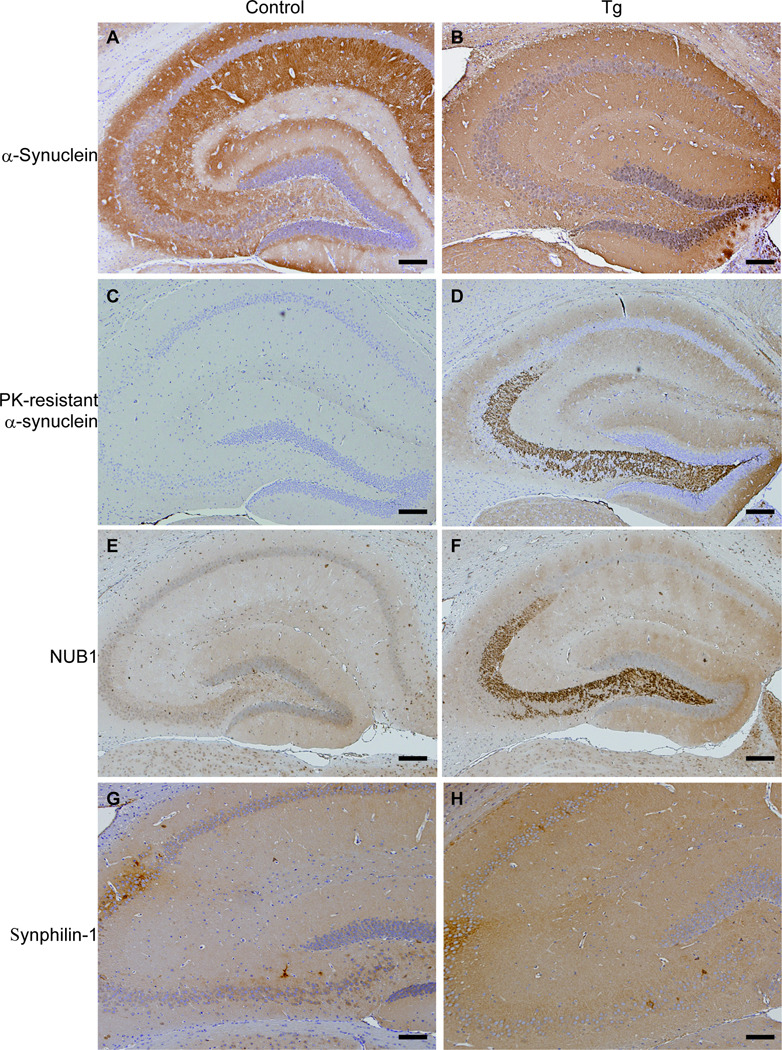
Immunoreactivity of NUB1 and Abnormal α-Synuclein in SNCA Tg Mice
Hippocampal sections were prepared from wild-type and SNCA Tg mice to determine whether NUB1 and PK-resistant α-synuclein are accumulated. PK treatment completely abolished the immunoreactivity of wild-type α-synuclein (Fig. 3A vs. C). In Tg mice, however, although PK-resistant α-synuclein was not found in pathological inclusions, it was detected in presynaptic nerve terminals, particularly in the hippocampus (Fig. 3B, D) (17). Presynaptic accumulation of PK-resistant α-synuclein was also observed in the cerebral cortex (data not shown). Surprisingly, NUB1 was found to be deposited in the hippocampus in Tg mice, but not in controls (Fig. 3E, F). NUB1 was similarly distributed both in heterozygous and homozygous Tg mice. There was no obvious alteration of synphilin-1 immunoreactivity in the Tg mice (Fig. 3G, H).
Figure 3.
Comparison of immunoreactivity for α-synuclein, protein kinase K (PK)-resistant α-synuclein, NUB1 and synphilin-1 in 11-week-old control (A, C, E, G) and heterozygous transgenic (Tg) mice (B, D, F, H). (A, B) Human and mouse α-synuclein are expressed throughout the brain of Tg and control mice. (C–F) PK-resistant α-synuclein and NUB1 immunoreactivity (rabbit anti-NUB1 antibody) is present in presynapses of the hippocampus in Tg mice (D, F), but not in controls (C, E). (G, H) There is no difference in synphilin-1 immunoreactivity between control (G) and Tg (H) mice. Scale bars = 100 µm.
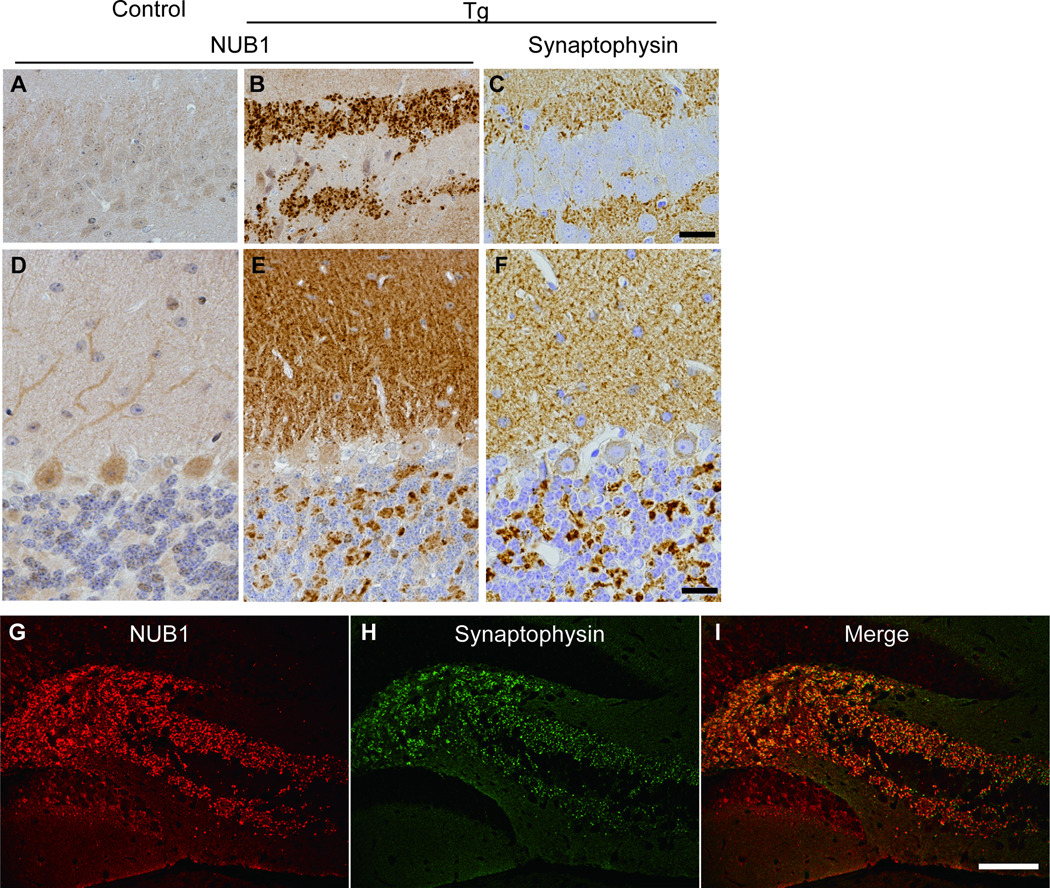
Because the distribution of NUB1-positive punctate structures resembled presynaptic staining pattern, we carried out an immunohistochemical analysis using an antibody against synaptophysin, a presynaptic marker protein. As expected, the staining pattern of synaptophysin was identical to that of NUB1 (Fig. 4), indicating that endogenous NUB1 was deposited together with PK-resistant α-synuclein in presynaptic nerve terminals in Tg mice.
Figure 4.
Distribution of NUB1 (A, B, D, E, G) and synaptophysin (C, F, H) in the hippocampus (A–C, G–I) and cerebellum (D–F) in 12-week-old control (A, D) and heterozygous transgenic (Tg) (B, C, E–I) mice. (A, D) NUB1 is mainly expressed in the neuronal cytoplasm in controls. (B) Rabbit anti-NUB1 antibody reveals positive deposits along with the region surrounding the CA3 pyramidal cell layers in Tg mice. (C) Synaptophysin immunoreactivity is seen in the same regions. (E, F) NUB1 (E)-and synaptophysin (F)-positive signals are located in presynapses in the molecular and granular layers in the cerebellum of Tg mice. (G–I) Double immunofluorescence labeling shows immunoreactivity of NUB1 (red) and synaptophysin (green) in the hippocampal regions of a Tg mouse. Yellow indicates the colocalization of NUB1 and synaptophysin. Scale bars: A–F = 20 µm; G–I = 50 µm.
Developmental Accumulation of NUB1 in SNCA Tg Mice
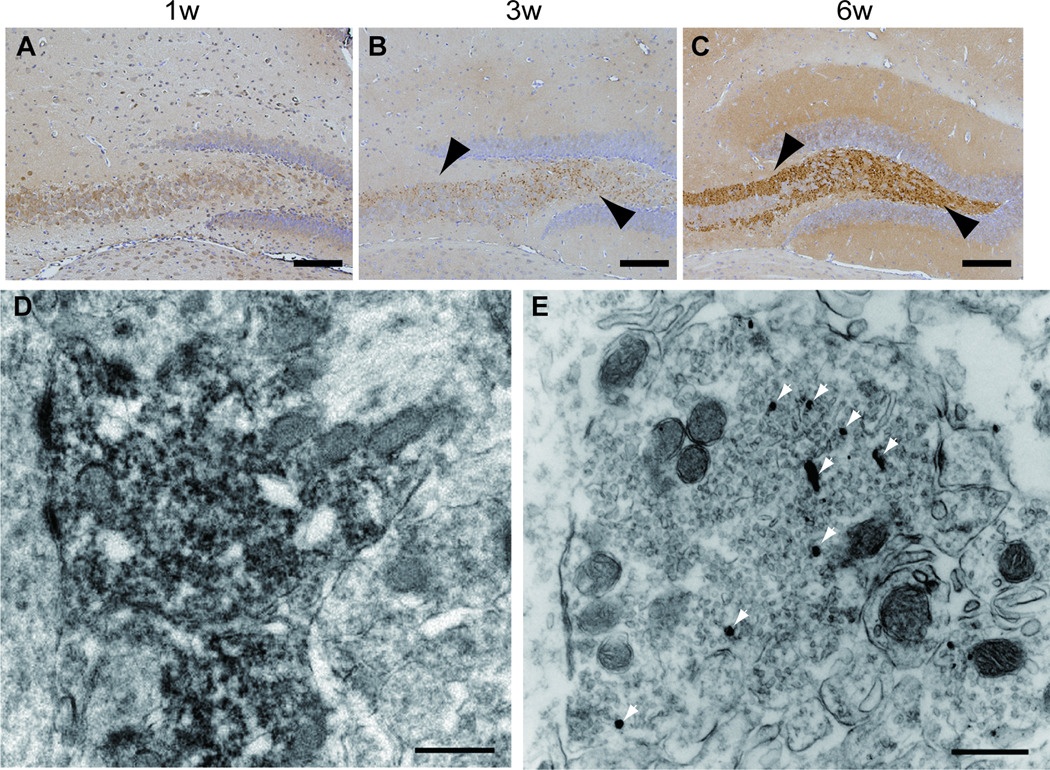
PK-resistant α-synuclein deposited in presynapses in Tg mice even at the age of 1 week (data not shown). To determine when NUB1 starts depositing at presynapses, hippocampal sections from a series of young Tg mice (1-, 3-, and 6-weeks old) were immunostained with anti-NUB1 antibody. Presynaptic NUB1 deposits were observed at ages of 3 and 6 weeks, but not at the age of 1 week. By immunoelectron microscopy, presynaptic deposits of NUB1 in the hippocampus of SNCA Tg mice were localized to synaptic vesicles and the cytoplasm adjacent to vesicles (Fig. 5D, E). Importantly, despite an extensive search, no filamentous structures were found in NUB1-positive presynapses (Fig. 5E).
Figure 5.
Appearance of presynaptic NUB1 in the hippocampus of heterozygous Tg mice. (A) No deposits are seen in a 1-week-old mouse. (B, C) Presynaptic NUB1 (arrowheads) is demonstrated in 3-week-old (B) and 6-week-old (C) Tg mice. (D, E) Immunoelectron micrographs for NUB1 of the mossy fiber terminals in the hippocampal CA3 region of 7-week-old SNCA Tg mice using 3,3’ diaminobenzidine reaction (D) and silver enhancement method (E). The immunolabeling is distributed throughout the presynaptic terminal (D). Silver-enhanced deposits (arrowheads) are associated with synaptic vesicles; no aberrant aggregates are seen in the terminals. Rabbit anti-NUB1 antibody was used for A–E. Scale bars: A–C = 100 µm; D, E = 500 nm.
Biochemical Analyses of NUB1 in DLB
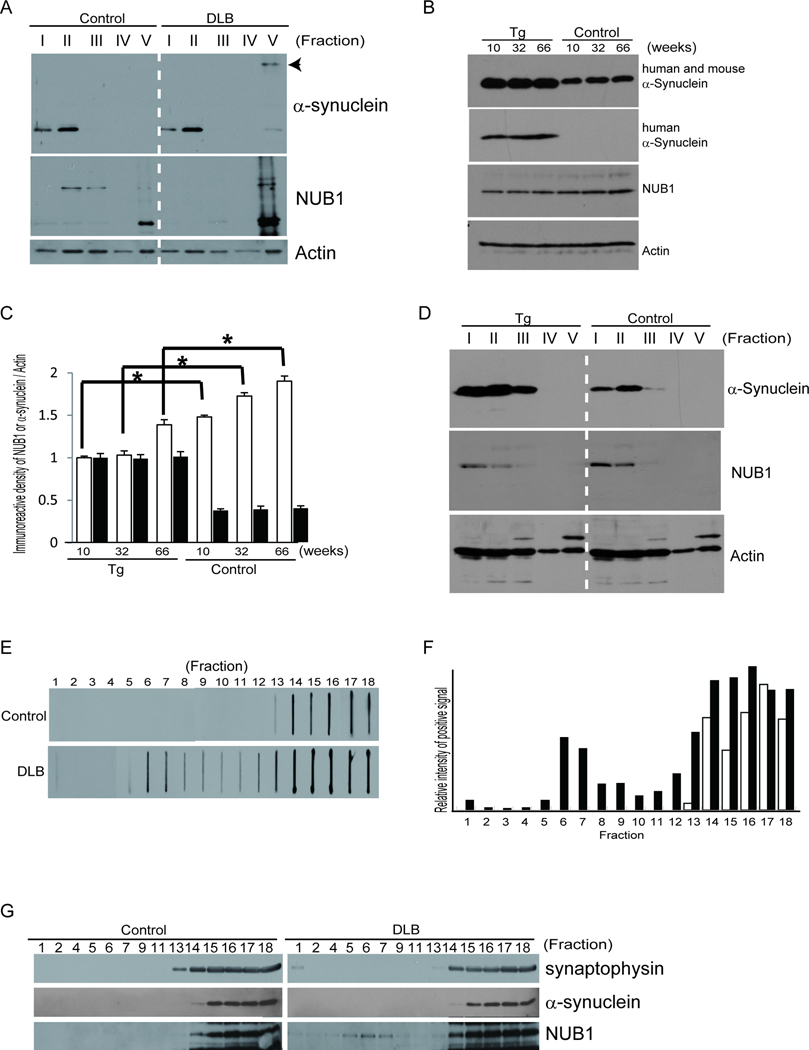
Previous reports showed that α-synuclein is modified by ubiquitination, phosphorylation and nitration (26, 31, 32), generating insoluble α-synuclein in DLB brain (33). We fractionated the brain samples using increasing detergent strengths. Immunoblotting showed that the solubility of α-synuclein was altered in fraction V of DLB brains, consistent with a previous report (33) (Fig. 6A). Importantly, NUB1 was strongly detected in insoluble protein fraction V both in DLB and controls and its amount was increased in DLB vs. controls (Fig. 6A). Decreased levels of NUB1 vs. age-matched controls were found in SNCA Tg mice (Fig. 6B) and quantitative data supported the conclusion that the amount of NUB1 was significantly decreased in SNCA Tg mice (Fig. 6C). Therefore, we performed fractionated examination but were not able to find differences of solubility between SNCA Tg mice and controls (Fig. 6D). It is noteworthy that the detergent solubility of mouse NUB1 different from that of human NUB1, i.e. mouse NUB1 was detected only in soluble fractions (Fig. 6D, fractions I–III). Considering that α-synuclein was not detected in the insoluble fraction in SNCA Tg mice, the biochemical property of molecular components such as NUB1 and α-synuclein seem to differ between human subjects and model mice.
Figure 6.
Biochemical profiles of NUB1 protein in the brains of patients with dementia with Lewy bodies (DLB), SNCA transgenic (Tg) mice and controls. (A) Sequential fractionation reveals that NUB1 is composed of soluble (fractions I–IV) and insoluble (fraction V) forms based on detergent solubility. The amount of insoluble NUB1 is increased in a patient with DLB (case 22) vs. a control (case 18). Actin is used as loading control. Unless otherwise indicated, goat anti-NUB1 antibody was used for biochemical studies. Arrowhead indicates abnormal α-synuclein. (B) The hippocampal region was removed from the brains of hemizygous SNCA Tg mice and controls (male, 10-, 32-, and 66-weeks old) and lysed in 4% SDS sample buffer. Samples were analyzed by immunoblotting using rabbit anti-NUB1, anti-α-synuclein (Syn-1 and LB509), and β-actin. The amounts of endogenous NUB1 increase with age in both Tg and control mice. NUB1 expression is decreased in SNCA Tg mice compared to controls. (C) Quantitative analysis showing that the amount of NUB1 is significantly reduced in SNCA Tg mice and the amount of α-synuclein is ~2.5 times higher in Tg mice than that in controls. Values were normalized to the amount of actin. The value in SNCA Tg mice (lane 1) is defined as 100% in immunoreactive densities of NUB1 and α-synuclein, respectively. Open and solid bars indicate expression levels of NUB1 and α-synuclein, respectively. *p < 0.01 for age-matched comparisons. (D) Sequential fractionation of the hippocampal region from brains of hemizygous SNCA Tg mice and controls (male, 66-week-old) reveals that mouse NUB1 is mainly composed of a soluble form (fractions I–III). α-synuclein is also seen in soluble fractions in both SNCA Tg and control mice. β-actin is used as control for loading amount. Rabbit anti-NUB1 antibody was used in these studies. (E) After a sucrose density gradient centrifugation, samples were applied to a cellulose acetate membrane on a slot blot apparatus. Filter trap analysis shows abnormal α-synuclein in almost all fractions in a DLB patient brain (case 23); lower-density fractions contain α-synuclein in a control (case 19). (F) Quantitative analysis showing that α-synuclein is distributed in almost all fractions in DLB (solid bars); α-synuclein is detected in fractions 13–18 in controls (open bars). (G) Western blot analysis showing that NUB1 is distributed in almost all fractions in DLB, whereas NUB1 expression is restricted to fractions 14–18 in controls. NUB1 distribution is similar to that of abnormal α-synuclein.
Finally, we performed sucrose density gradient analysis using human frozen tissues. It is known that there is a higher density of α-synuclein in DLB vs. controls (16, 17). Indeed, filter-trap assay demonstrated that abnormally aggregated α-synuclein existed in all fractions in DLB (Fig. 6E, F). NUB1 was mainly localized to fractions 14–18 in controls and DLB (Fig. 6G). Additionally, however, NUB1 was distributed to fractions 1–13 only in DLB. Quantitative analysis implied that the distribution pattern of NUB1 was similar to that of abnormally aggregated α-synuclein (Fig. 6F).
DISCUSSION
We demonstrated that NUB1 is colocalized with PK-resistant α-synuclein at presynapses in LB disease patients and SNCA Tg mice, but not in controls. α-Synuclein is phosphorylated in pathological inclusions such as LBs and Lewy neurites of LB disease (26). Ultrastructurally, phosphorylated α-synuclein-positive inclusions are composed of filamentous structures (10–16 nm in diameter) in SNCA Tg mice (25). Our immunoelectron microscopy revealed that no filamentous structures are seen in NUB1-positive presynapses in SNCA Tg mice, suggesting that fibril formation does not participate in acquiring PK-resistance of α-synuclein and that NUB1 coexists with abnormal α-synuclein without fibril formation in presynapses in LB disease.
What causes the accumulation of NUB1 in presynapses in LB disease? Although α-synuclein is primarily expressed at presynaptic nerve terminals, “normal or physiological” α-synuclein is unlikely to be associated with NUB1. This is supported by our previous findings that α-synuclein does not interact with NUB1 in yeast cells (23). Furthermore, overexpression of wild-type or mutant α-synuclein had no effect on the subcellular localization of endogenous NUB1 in mammalian cells (unpublished data). Alternatively, it is possible that abnormal (i.e. PK-resistant) α-synuclein plays a crucial role in the alteration of NUB1 distribution. In support of this hypothesis we found that NUB1 accumulates in the same regions where PK-resistant α-synuclein does. Biochemical analyses further indicated that NUB1 coexists with abnormal α-synuclein in almost all fractions from DLB brains, but not from control subjects. Furthermore, deposition of presynaptic PK-resistant α-synuclein preceded that of NUB1 in SNCA Tg mice. Taken together, α-synuclein may change its conformation at presynapses, resulting in the alteration of susceptibility to PK, and subsequently NUB1 is recruited to the corresponding presynapses.
It is noteworthy that presynaptic PK-resistant α-synuclein is unlikely to be phosphorylated or ubiquitinated, raising the question of how NUB1 recognizes presynaptic PK-resistant α-synuclein. Given that PK treatment has a large effect on differentiation of normal prion protein from the abnormal form (34), it is plausible that presynaptic α-synuclein changes its conformation in distinct brain regions in LB disease. Actually, α-synuclein aggregates into a β-pleated sheet conformation in vitro and within LBs and glial cytoplasmic inclusions (15, 35–37). Hidalgo-de-Quintana et al reported that NUB1-binding protein, aryl-hydrocarbon receptor interacting protein-like 1 (AIPL1) functions as a co-chaperone protein in cooperate with a molecular chaperone Hsp70 (38). Moreover, NUB1 also coexists with another molecular chaperone Hsp90 in a fraction with a molecular mass of approximately 443–669 kDa in retinoblastoma cells (38). This raises the possibility that a chaperoning machinery containing NUB1 recognizes abnormal α-synuclein through chaperone activity in the presynapses.
In addition to AIPL1, NUB1 and NUB1L (the latter being a longer isoform with additional 14 amino acids) are known to bind several molecules such as synphilin-1, NEDD8, FAT10 and UbC1 (20, 28, 39–41). Although synphilin-1 is a presynaptic protein, we found no alteration in the distribution of synphilin-1 in SNCA Tg mice. Our previous study showed that NUB1 suppresses the formation of LB-like inclusions by accelerating synphilin-1 degradation in cultured cells (20). Thus, we cannot rule out the possibility that NUB1 efficiently degrades synphilin-1, resulting in no synphilin-1 immunoreactivity in presynapses of SNCA Tg mice.
In conclusion, we provide evidence that NUB1 coexists with abnormal, PK-resistant α-synuclein in LB disease patients and SNCA Tg mice. Because occurrence of presynaptic PK-resistant α-synuclein precedes α-synuclein modified with phosphorylation in distinct brain regions in human LB disease, NUB1 may recognize abnormal α-synuclein from the early stage of protein aggregation. Further study is needed to test this hypothesis and to elucidate the involvement of these two closely related proteins in the pathobiology of LB disease.
ACKNOWLEDGMENTS
The authors wish to express their gratitude to M. Nakata for her technical assistance.
This work was supported by Grants-in-Aid 20590335 (K.T.), 20591361 (F.M.) and 20300123 (K.W.) for Scientific Research from the Ministry of Education, Culture, Sports, Science, and Technology, Japan, a Grant for Hirosaki University Institutional Research (K.W.), the Collaborative Research Project (2010–2209) of the Brain Research Institute, Niigata University (F.M.), and an Intramural Research Grant (21B-4) for Neurological and Psychiatric Disorders of NCNP (K.W.), and a Grant-in-Aid from the Research Committee for CNS Degenerative diseases (H.T.), the Ministry of Health, Labour and Welfare, Japan, and National Institutes of Health Grant R01 AG024497 (T.K.). This work was also supported by the Memorial Foundation of Suzuken (K.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Maroteaux L, Scheller RH. The rat brain synucleins: family of proteins transiently associated with neuronal membrane. Brain Res Mol Brain Res. 1991;11:335–343. doi: 10.1016/0169-328x(91)90043-w. [DOI] [PubMed] [Google Scholar]
- 2.Iwai A, Masliah E, Yoshimoto M, et al. The precursor protein of non-Aβ component of Alzheimer’s disease amyloid is a presynaptic protein of the central nervous system. Neuron. 1995;14:467–475. doi: 10.1016/0896-6273(95)90302-x. [DOI] [PubMed] [Google Scholar]
- 3.Abeliovich A, Schmitz Y, Farinas I, et al. Mice lacking α-synuclein display functional deficits in the nigrostriatal dopamine system. Neuron. 2000;25:239–252. doi: 10.1016/s0896-6273(00)80886-7. [DOI] [PubMed] [Google Scholar]
- 4.George JM, Jin H, Woods WS, et al. Characterization of a novel protein regulated during the critical period for song learning in the zebra finch. Neuron. 1995;15:361–372. doi: 10.1016/0896-6273(95)90040-3. [DOI] [PubMed] [Google Scholar]
- 5.Murphy DD, Rueter SM, Trojanowski JQ, et al. Synucleins are developmentally expressed, and α-synuclein regulates the size of the presynaptic vesicular pool in primary hippocampal neurons. J Neurosci. 2000;20:3214–3220. doi: 10.1523/JNEUROSCI.20-09-03214.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu S, Ninan I, Antonova I, et al. α-Synuclein produces a long-lasting increase in neurotransmitter release. EMBO J. 2004;23:4506–4516. doi: 10.1038/sj.emboj.7600451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yavich L, Oksman M, Tanila H, et al. Locomotor activity and evoked dopamine release are reduced in mice overexpressing A30P-mutated human α-synuclein. Neurobiol Dis. 2005;20:303–313. doi: 10.1016/j.nbd.2005.03.010. [DOI] [PubMed] [Google Scholar]
- 8.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 9.Krüger R, Kuhn W, Muller T, et al. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson’s disease. Nat Genet. 1998;18:106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 10.Chartier-Harlin MC, Kachergus J, Roumier C, et al. α-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364:1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 11.Bradbury J. α-synuclein gene triplication discovered in Parkinson’s disease. Lancet Neurol. 2003;2:715. doi: 10.1016/s1474-4422(03)00601-x. [DOI] [PubMed] [Google Scholar]
- 12.Goedert M. Familial Parkinson’s disease. The awakening of α-synuclein. Nature. 1997;388:232–233. doi: 10.1038/40767. [DOI] [PubMed] [Google Scholar]
- 13.Zarranz JJ, Alegre J, Gomez-Esteban JC, et al. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann Neurol. 2004;55:164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 14.Spillantini MG, Schmidt ML, Lee VM, et al. α-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 15.Baba M, Nakajo S, Tu PH, et al. Aggregation of α-synuclein in Lewy bodies of sporadic Parkinson’s disease and dementia with Lewy bodies. Am J Pathol. 1998;152:879–884. [PMC free article] [PubMed] [Google Scholar]
- 16.Kramer ML, Schulz-Schaeffer WJ. Presynaptic α-synuclein aggregates, not Lewy bodies, cause neurodegeneration in dementia with Lewy bodies. J Neurosci. 2007;27:1405–1410. doi: 10.1523/JNEUROSCI.4564-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanji K, Mori F, Mimura J, et al. Proteinase K-resistant α-synuclein is deposited in presynapses in human Lewy body disease and A53T α-synuclein transgenic mice. Acta Neuropathol. 2010;120:145–154. doi: 10.1007/s00401-010-0676-z. [DOI] [PubMed] [Google Scholar]
- 18.Kito K, Yeh ET, Kamitani T. NUB1, a NEDD8-interacting protein, is induced by interferon and down-regulates the NEDD8 expression. J Biol Chem. 2001;276:20603–20609. doi: 10.1074/jbc.M100920200. [DOI] [PubMed] [Google Scholar]
- 19.Engelender S, Kaminsky Z, Guo X, et al. Synphilin-1 associates with α-synuclein and promotes the formation of cytosolic inclusions. Nat Genet. 1999;22:110–114. doi: 10.1038/8820. [DOI] [PubMed] [Google Scholar]
- 20.Tanji K, Tanaka T, Mori F, et al. NUB1 suppresses the formation of Lewy body-like inclusions by proteasomal degradation of synphilin-1. Am J Pathol. 2006;169:553–565. doi: 10.2353/ajpath.2006.051067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wakabayashi K, Engelender S, Yoshimoto M, et al. Synphilin-1 is present in Lewy bodies in Parkinson’s disease. Ann Neurol. 2000;47:521–523. [PubMed] [Google Scholar]
- 22.Wakabayashi K, Engelender S, Tanaka Y, et al. Immunocytochemical localization of synphilin-1, an α-synuclein-associated protein, in neurodegenerative disorders. Acta Neuropathol. 2002;103:209–214. doi: 10.1007/s004010100451. [DOI] [PubMed] [Google Scholar]
- 23.Tanji K, Mori F, Kakita A, et al. Immunohistochemical localization of NUB1, a synphilin-1-binding protein, in neurodegenerative disorders. Acta Neuropathol. 2007;114:365–371. doi: 10.1007/s00401-007-0238-1. [DOI] [PubMed] [Google Scholar]
- 24.Braak H, Del Tredici K, Rub U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 25.Giasson BI, Duda JE, Quinn SM, et al. Neuronal α-synucleinopathy with severe movement disorder in mice expressing A53T human α-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- 26.Fujiwara H, Hasegawa M, Dohmae N, et al. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 27.Jakes R, Crowther RA, Lee VM, et al. Epitope mapping of LB509, a monoclonal antibody directed against human α-synuclein. Neurosci Lett. 1999;269:13–16. doi: 10.1016/s0304-3940(99)00411-5. [DOI] [PubMed] [Google Scholar]
- 28.Kamitani T, Kito K, Fukuda-Kamitani T, et al. Targeting of NEDD8 and its conjugates for proteasomal degradation by NUB1. J Biol Chem. 2001;276:46655–46660. doi: 10.1074/jbc.M108636200. [DOI] [PubMed] [Google Scholar]
- 29.Tanji K, Kamitani T, Mori F, et al. TRIM9, a novel brain-specific E3 ubiquitin ligase, is repressed in the brain of Parkinson’s disease and dementia with Lewy bodies. Neurobiol Dis. 2010;38:210–218. doi: 10.1016/j.nbd.2010.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang HX, Tanji K, Mori F, et al. Epitope mapping of 2E2-D3, a monoclonal antibody directed against human TDP-43. Neurosci Lett. 2008;434:170–174. doi: 10.1016/j.neulet.2008.01.060. [DOI] [PubMed] [Google Scholar]
- 31.Giasson BI, Duda JE, Murray IV, et al. Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science. 2000;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 32.Hasegawa M, Fujiwara H, Nonaka T, et al. Phosphorylated α-synuclein is ubiquitinated in α-synucleinopathy lesions. J Biol Chem. 2002;277:49071–49076. doi: 10.1074/jbc.M208046200. [DOI] [PubMed] [Google Scholar]
- 33.Lippa CF, Fujiwara H, Mann DM, et al. Lewy bodies contain altered α-synuclein in brains of many familial Alzheimer’s disease patients with mutations in presenilin and amyloid precursor protein genes. Am J Pathol. 1998;153:1365–1370. doi: 10.1016/s0002-9440(10)65722-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Prusiner SB, Groth DF, Bolton DC, et al. Purification and structural studies of a major scrapie prion protein. Cell. 1984;38:127–134. doi: 10.1016/0092-8674(84)90533-6. [DOI] [PubMed] [Google Scholar]
- 35.Hashimoto M, Hsu LJ, Sisk A, et al. Human recombinant NACP/α-synuclein is aggregated and fibrillated in vitro: relevance for Lewy body disease. Brain Res. 1998;799:301–306. doi: 10.1016/s0006-8993(98)00514-9. [DOI] [PubMed] [Google Scholar]
- 36.Hashimoto M, Hsu LJ, Xia Y, et al. Oxidative stress induces amyloid-like aggregate formation of NACP/α-synuclein in vitro. Neuroreport. 1999;10:717–721. doi: 10.1097/00001756-199903170-00011. [DOI] [PubMed] [Google Scholar]
- 37.Trojanowski JQ, Lee VM. Parkinson’s disease and related neurodegenerative synucleinopathies linked to progressive accumulations of synuclein aggregates in brain. Parkinsonism Relat Disord. 2001;7:247–251. doi: 10.1016/s1353-8020(00)00065-1. [DOI] [PubMed] [Google Scholar]
- 38.Hidalgo-de-Quintana J, Evans RJ, Cheetham ME, et al. The Leber congenital amaurosis protein AIPL1 functions as part of a chaperone heterocomplex. Invest Ophthalmol Vis Sci. 2008;49:2878–2887. doi: 10.1167/iovs.07-1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Akey DT, Zhu X, Dyer M, et al. The inherited blindness associated protein AIPL1 interacts with the cell cycle regulator protein NUB1. Hum Mol Genet. 2002;11:2723–2733. doi: 10.1093/hmg/11.22.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hipp MS, Raasi S, Groettrup M, et al. NEDD8 ultimate buster-1L interacts with the ubiquitin-like protein FAT10 and accelerates its degradation. J Biol Chem. 2004;279:16503–16510. doi: 10.1074/jbc.M310114200. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka T, Yeh ET, Kamitani T. NUB1-mediated targeting of the ubiquitin precursor UbC1 for its C-terminal hydrolysis. Eur J Biochem. 2004;271:972–982. doi: 10.1111/j.1432-1033.2004.03999.x. [DOI] [PubMed] [Google Scholar]