Abstract
Background
Although stem cell therapy has provided a promising treatment for myocardial infarction, the low survival of the transplanted cells in the infarcted myocardium is possibly a primary reason for failure of long-term improvement. Therefore, the development of novel pro-survival strategies to boost stem cell survival will be of significant benefit to this field.
Method and Results
Cardiac progenitor cells (CPCs) were isolated from transgenic mice which constitutively express firefly luciferase and green fluorescent protein. The CPCs were transduced with individual lentivirus carrying the precursor of miR-21, miR-24, and miR-221, a cocktail of these 3 miRNA precursors, or GFP as control. After challenge in serum free medium, CPCs treated with the 3 miRNA cocktail showed significantly higher viability compared to untreated CPCs. Following intramuscular and intramyocardial injections, in vivo bioluminescence imaging (BLI) showed that miRNA cocktail-treated CPCs survived significantly longer after transplantation. Following left anterior descending artery ligation, miRNA cocktail-treated CPCs boosts the therapeutic efficacy in terms of functional recovery. Histological analysis confirmed increased myocardial wall thickness and CPC engraftment in the myocardium with miRNA cocktail. Finally, we used bioinformatics analysis and experimental validation assays to show that Bim, a critical apoptotic activator, is an important target gene of the miRNA cocktail, which collectively can bind to the 3’UTR region of Bim and suppress its expression.
Conclusion
We have demonstrated that a miRNA pro-survival cocktail (miR-21, miR-24, and miR-221) can improve the engraftment of transplanted cardiac progenitor cells and therapeutic efficacy for treatment of ischemic heart disease.
Keywords: microRNA, cardiac progenitor cell, cell therapy, ischemic heart disease
INTRODUCTION
Coronary artery disease (CAD) is a progressive disease with high morbidity and mortality rates in the US. Following myocardial infarction (MI), the limited ability of the surviving cardiac cells to proliferate renders the damaged heart susceptible to unfavorable remodeling processes and morbid sequelae such as heart failure. Current therapies for patients who have CAD or MI include lifestyle modification, drug treatments, percutaneous coronary intervention (PCI), bypass surgery, and orthotopic heart transplantation. In recent years, stem cell therapy has provided a promising means of replacing cardiomyocytes lost during MI via induction of endogenous regenerative processes and transferring of progenitor cells to new myocardium.1 Several types of stem cells have been shown to have a beneficial effect in small animal models, including bone marrow mononuclear cells (BMMNCs),2 mesenchymal stem cells (MSCs),3 endothelial progenitor cells (EPCs),4 and human embryonic stem cell-derived cardiomyocytes (hESC-CMs),5 to name a few. However, large placebo-controlled clinical trials have not shown a consistent benefit in using stem cells to treat patients with ischemic heart disease.6
In recent years, a number of studies have confirmed the presence of resident “progenitor cells” in the myocardium.1 These cells can be isolated and expanded ex vivo, and can also differentiate into cardiomyocytes, smooth muscle cells, and endothelial cells under the appropriate culturing conditions. However, the low survival of the transplanted stem cells, including CPCs, in the infarcted myocardium is a major obstacle to achieving long-term improvements after stem cell therapy.7–13 Therefore, developing novel pro-survival strategies to boost stem cell survival will be highly beneficial to this field.
MicroRNAs (miRNAs) are short 20–22 nucleotide RNA molecules that are expressed in a tissue-specific and developmentally regulated manner. MiRNAs mainly function as negative regulators of gene expression in a variety of organisms, through binding with imperfect complementarity to the 3’ UTR of their target mRNAs14. In the heart, a large number of miRNAs are expressed and are considered to be important regulators in cardiac development and pathophysiology.14 In addition, recent studies indicate that miRNAs can be used as therapeutic targets for various cardiovascular diseases.15–17
In this study, we hypothesize that miRNAs may play a significant role in regulating survival and apoptosis of CPCs and in improving engraftment post-transplantation. We demonstrate for the first time that CPCs treated with a miRNA pro-survival cocktail can significantly improve cell engraftment and functional recovery in a murine model of myocardial infarction.
MATERIALS AND METHODS
Isolation and maintenance of Sca-1+ cardiac progenitor cells (CPCs)
Heart tissue explants are isolated from transgenic mice with ubiquitin promoter constitutively driving firefly luciferase and green fluorescent protein (Fluc-GFP). The minced heart pieces are subjected to enzyme dissociation with 0.2% trypsin and 0.1% collagenase IV (Worthington Biochemical, Lakewood NJ) in PBS three times for 5 minutes at 37°C. Afterwards, the remaining tissue fragments were cultured in the CPC medium (Iscove’s Modified Dulbecco’s IMDM with 15% fetal calf serum, 100 U/mL penicillin G, 100 ug/ml streptomycin, 2 mmol/L L-glutamine, and 0.1 mmoL/L 2-mercaptoethanol) at 37°C. After about 2 weeks, we harvested small, phase-bright cells formed from adherent explants by washing with D-Hanks solution. We then enrich the Sca-1+ cells with anti-Sca-1 microbeads (Miltenyi Biotec) as previously described.18 The enriched Sca-1+ cells were seeded on gelatin coated dishes in CPC medium, including Iscove’s modified Dulbecco’s medium supplemented with 15% fetal bovine serum, 2 mmol/l glutamine, 0.1 mmol/l beta-mercaptoethanol, 100 U/ml penicillin, and 100 ug/ml streptomycin.
In vitro differentiation of CPCs
For cardiac and smooth muscle differentiation, CPCs were cultured in poly-D-lysine coated plates in differentiation medium containing 35% Iscove’s modified Dulbecco’s medium with 10% FBS/65% Dulbecco’s modified Eagle medium-Ham F-12 mix containing 2% B27, 0.1 mmol/l 2-mercaptoethanol, 10 ng/ml epidermal growth factor (R&D Systems, Minneapolis, MN), 20 ng/ml basic fibroblast growth factor, 40 nmol/l cardiotrophin-1, 40 nmol/l thrombin (Sigma-Aldrich, St. Louis, MO), 100 U/ml penicillin G, 100 g/ml streptomycin, and 2 mmol/l glutamine. For endothelial differentiation, CPCs were cultured on fibronectin-coated plates with EGM-2 medium (Lonza, Switzerland) with an extra 20 ng/ml of vascular endothelial growth factor (R&D Systems) as previously described.7
Dual-luciferase activity assay
To confirm Bim (a potent apoptotic activator) as the target gene of our miRNA pro-survival cocktail, the 3’ UTR segments of mouse Bim were inserted into the downstream of firefly luciferase in the pGL3 control vector. The Luciferase reporters, pRL-TK control vector plus miRNA oligo precursors (Applied Biosystems) were transfected into NIH 3T3 cells by Lipofectamine 2000 (Invitrogen) in Opti-MEM. Cell lysates were harvested in 24 hours after transfection. Firefly and renilla luciferase activities were measured using Dual-Luciferase Reporter Assay System (Promega) as previously described.19
Intramuscular injection of CPCs for assessment of miRNA cocktail
To compare the survival between CPCs treated with miRNA pro-survival cocktail and CPCs alone, we injected the 2 cell types into the legs of the same animal. This method allowed us to avoid inter-individual variation and perform an unbiased head-to-head comparison. We injected 5×105 cells into hindlimb of adult female SCID/Beige mice (Charles River Laboratories), with a total volume of 10 μl per injection leg (n=8 animals).
Surgical model of murine myocardial infarction and cell delivery
All animal research protocols were approved by the Stanford Animal Research Committee. Ligation of the mid left anterior descending (LAD) artery was performed in adult female SCID Beige mice (Charles River Laboratories, Wilmington, MA) by a single experienced microsurgeon (YG). Myocardial infarction was confirmed by myocardial blanching and EKG changes. Animals (14 weeks old) were randomized into 3 groups (n=60 total): (1) phosphate buffered saline (PBS), (2) 2×106 CPCs alone, and (3) 2×106 CPCs transduced with the 3 miRNA cocktail (n=20/group). After LAD ligation, the animals were injected with CPCs or PBS in the peri-infarct zone at 2 different sites with total volume of 30 μl using a 31-gauge Hamilton syringe.
In vivo optical bioluminescence imaging (BLI)
Please see Supplemental Methods.
Echocardiographic analysis of left ventricular function
Please see Supplemental Methods.
Histological examination
Please see Supplemental Methods.
Statistical analysis
Statistics were calculated using SPSS 12.0 (SPSS Inc, Chicago, Ill). Descriptive statistics included mean and SD. One-way analysis of variance and 2-way repeated-measures analysis of variance with post hoc testing were used. The initial F test was followed up with Bonferroni multiple comparisons procedure. Differences were considered significant at P-values of <0.05.
RESULTS
Characterization and differentiation of CPCs
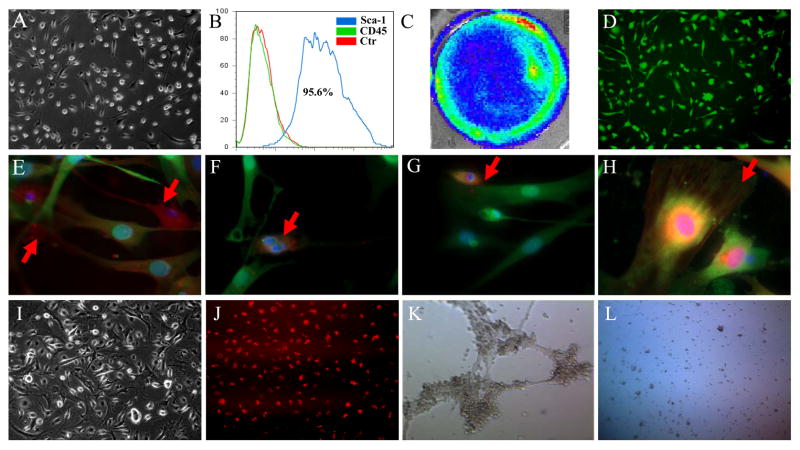
After purification by anti-Sca-1 microbeads, the typical CPCs showed high nucleus-to-cytoplasm ratios. The adherent cells sprouted new round bright cells at their cell body (Figure 1A). Fluorescence-activated cell sorting (FACS) indicated that isolated CPCs were more than 95% Sca-1+ and CD45−; the latter is a marker gene for hematopoietic stem cells (Figure 1B). The isolated CPCs expressed robust levels of Fluc and GFP (Figure 1C and 1D). Next, we differentiated CPCs using induction medium. After 7 days of differentiation into cardiomyocytes and smooth muscle cells, the differentiated cells stained positive for connexin 43 (Figure 1E), cardiac troponin T (cTnT) (Figure 1F), myocyte-specific enhancer factor 2C (MEF2c) (Figure 1G), and α–smooth muscle actin (α-SMA) (Figure 1H). Furthermore, CPCs could be differentiated into endothelial cells (Figure 1I). The differentiated endothelial cells demonstrated DiI-ac-LDL uptake (Figure 1J) and could form tube-like structures on Matrigel angiogenesis assay (Figure 1K). In contrast, the undifferentiated CPCs were not able to form similar tube-like structures (Figure 1L).
Figure 1. Characterization and differentiation of CPCs.
(A) The morphology of isolated CPCs growing on gelatin coated dish. (B) Flow cytometric analysis of purified Sca-1+ CPCs population. CPCs isolated from transgenic mice express robust (C) firefly luciferase and (D) GFP expression. After culturing in induction medium, differentiated CPCs stained positively with (E) connexin 43, (F) cardiac Troponin T, (G) myocyte-specific enhancer factor 2C, and (H) α-smooth muscle actin. (I) Cell morphology of CPC-derived endothelial cells. (J) These CPC-derived endothelial cells can uptake DiI-Ac-LDL. (K) These CPC-derived endothelial cells can form tube-like structure on Matrigel angiogenesis assay. (L) In contrast, the undifferentiated CPCs cannot form tube-like structure.
Screening of miRNA candidates for improving CPC survival
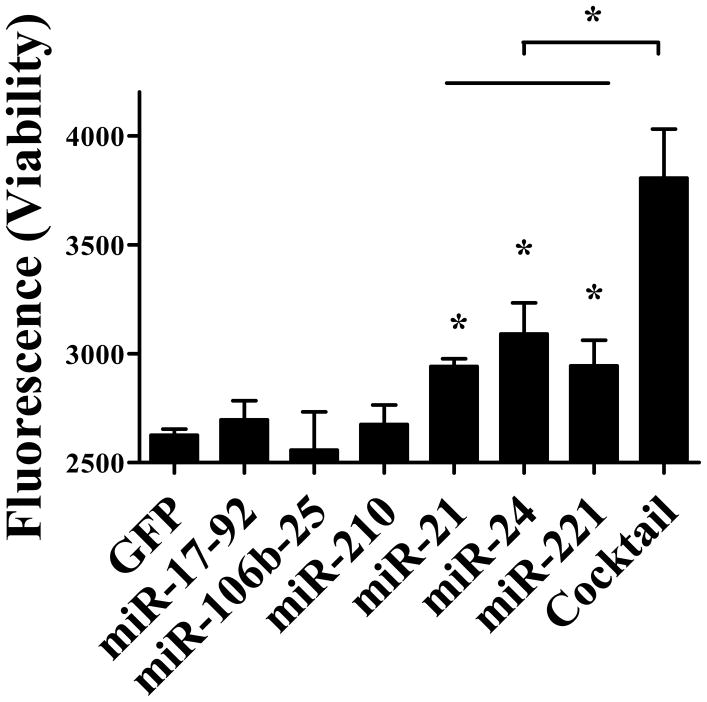
To identify potential pro-survival miRNAs in CPCs, we tested the cell viability after serum free challenge. We tested several miRNAs or miRNA clusters, including miR-21, miR24, miR-210, miR-221, miR-17-92, and miR106b-25, which have been shown to improve cell survival or inhibit cell apoptosis in previous studies.17, 20–23 After transduction with individual miRNA, miRNA cluster, or control lentivirus, CPCs were challenged in serum-free condition for 24 hours. Cell viability was used as a criterion to evaluate miRNA capacity to improve cell survival. Overall, we found that CPCs treated individually with miR-21, miR-24, and miR-221 had significantly higher cell viability, compared to control CPCs that were transduced with GFP (Figure 2). CPCs treated with the 3 miRNA cocktail (miR-21, miR-24, and miR-221) showed the highest cell viability, suggesting synergistic benefits in action.
Figure 2. Screening of miRNAs for improving cell viability.
After CPCs were transduced with several individual miRNAs or miRNAs cluster candidate, cells were exposed to serum free medium. Cell viability was measured after 24 hours of serum free challenge. Note the CPC viability is significantly higher in the presence of the miRNA cocktail (miR-21, miR24, and miR221). One-way ANOVA analysis was used.
Comparison of CPC survival after intramuscular transplantation
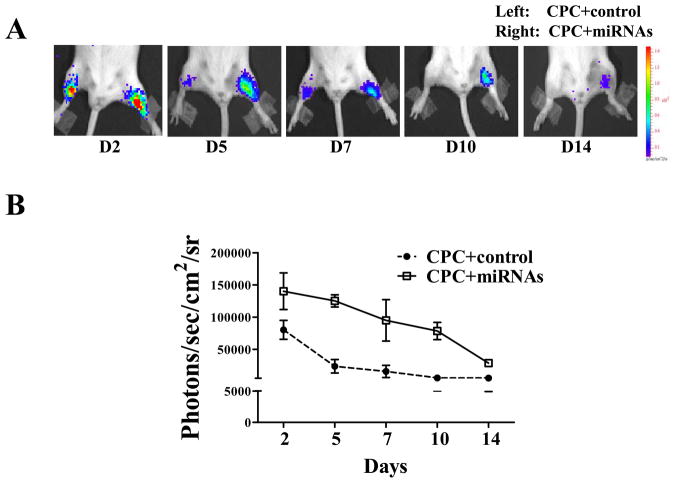
We first evaluated the correlation between Fluc expression and cell numbers (Figure 3A). Our data showed a robust linear correlation (R2=0.99) between bioluminescence signals and cell numbers, which is crucial for accurate tracking of cell fate following in vivo imaging studies (Figure 3B). Next, we assessed cell viability in vivo of CPCs alone versus CPCs treated with the 3 miRNA cocktail following intramuscular injection by bioluminescence imaging (BLI). By injecting the 2 cell types into the same animal, we were able to avoid inter-individual variation and perform a head-to-head comparison of cell survival attributed to the 3 miRNA cocktail. BLI indicated Fluc activity from CPCs treated with miRNA cocktail was stable for at least 14 days after intramuscular injection. In contrast, CPCs alone lost their BLI signal around day 7 post intramuscular injection (Figure 4A). Quantitative analysis of Fluc activity showed significantly stronger signal activities in CPCs treated with miRNA cocktail compared to CPCs alone at all time points: day 2 (14.0×104±1.5×104 vs. 8.0×104±2.8×104), day 5 (12.5×104±9×103 vs. 2.3×104±1.0×104), day 7 (9.5×104±3.2×104 vs. 1.6×104±9.5×103), day 10 (7.8×104±1.3×104 vs. 5.5×103 ± 4.8×102), and day 14 (2.8×104±4.4×103 vs. 5.3×103±3.3×102) (Figure 4B). Overall, these data demonstrated that CPCs treated with miRNA cocktail could survive longer post transplantation than CPCs alone in a hindlimb model without ischemic injury.
Figure 3. Correlation between CPC numbers and bioluminescence signals.

(A) CPCs were seeded into 6-well plate with increasing cell numbers. (B) Assessment of bioluminescence signals showed a robust linear correlation (R2=0.99) between the cell number and Fluc expression, which is crucial for accurate tracking of cell survival by in vivo imaging. Pearson’s correlation was used. Each data point is from individual observation.
Figure 4. Direct comparison of cell survival in vivo by bioluminescence imaging.
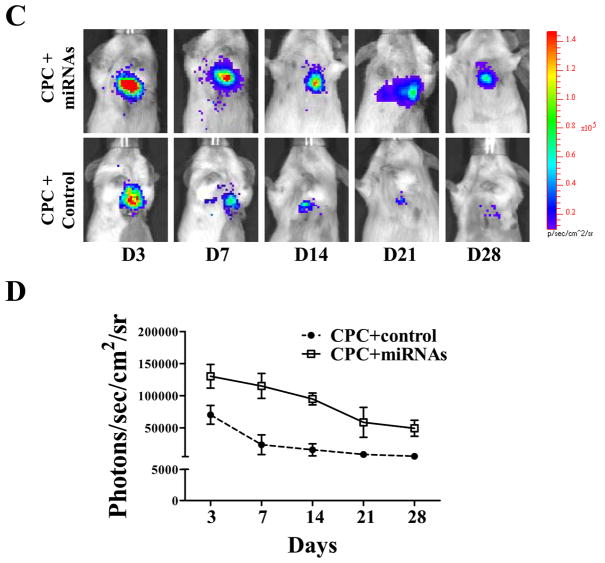
(A) Representative bioluminescence images of animals injected with CPCs alone (left leg) vs. CPCs treated with miRNA cocktail (right leg). Note this is in the setting of an uninjured hindlimb injection model. (B) Quantitative analysis of longitudinal BLI signal following intramuscular hindlimb injection. Two-way RMANOVA was used. (C) Following myocardial infarction, adult mice were injected intramyocardially with CPCs alone vs. CPCs treated with miRNA cocktail. (D) Quantitative analysis of BLI signals demonstrated that CPCs treated with miRNA cocktail showed significantly better survival at all time points. 2-way ANOVA was used for statistical analysis; two-way RMANOVA was used.
Assessment of CPC fate and function following myocardial infarction
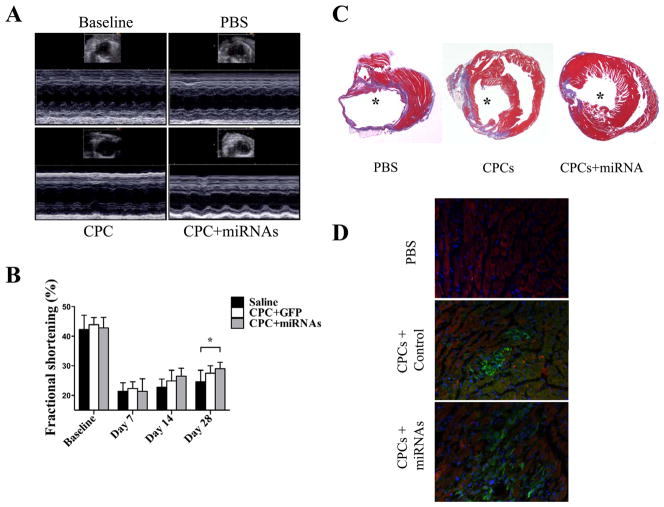
To examine whether miRNA cocktail can also enhance cell survival in an ischemic injury milieu, mice underwent LAD ligation and intramyocardial injection with (1) PBS, (2) CPCs alone, and (3) CPCs treated with miRNA cocktail. To investigate the systemic cell distribution of CPCs after intramyocardial cell delivery, we harvested the whole heart, liver, spleen, and lung tissues for individual tissue bioluminescence analysis ex vivo. At 72 hours after cell delivery, we found majority of bioluminescence signal in explanted heart but not in lung, liver, and spleen (Supplemental Figure 3). In vivo BLI demonstrated significantly stronger Fluc activity in the infarcted hearts of animals injected with CPCs treated with miRNA cocktail compared to CPCs alone at all time points: day 3, day 7, day 14, day 21, and day 28 (Figure 4C, 4D). For functional assessment, the baseline left ventricular fractional shortening (FS) was comparable in all 3 groups (Figure 5A, 5B). After LAD ligation, CPCs treated with miRNA cocktail had significantly higher FS compared with the PBS control group at day 28 (28.8±2.4% vs. 24.6±3.9%; P<0.05), whereas the CPC alone group showed a favorable trend toward FS improvement compared with the PBS control group (27.5±2.5% vs. 24.6±3.9%; P=0.18).
Figure 5. Evaluation of cardiac functional recovery after myocardial infarction.
(A) Representative echocardiographic images of healthy heart vs. infarcted hearts receiving PBS, CPCs alone, and CPCs treated with miRNA cocktail at day 28. (B) Comparison of fractional shortening (FS) among the 3 groups. Compared with PBS control, CPCs treated with miRNA cocktail group had significantly higher FS (P<0.05). The CPC alone group only showed a positive trend toward improvement (P=0.18). Two-way RMANOVA was used for statistical analysis. (C) Representative Masson trichrome staining of explanted heart showed increased wall thickness for CPCs treated with miRNA cocktail group, confirming the positive echocardiographic data. * represents left ventricle. (D) Engraftment of transplanted CPCs was confirmed by GFP staining. As expected, CPCs treated with miRNA cocktail group showed significantly higher engraftment compared with CPC alone group. Green: GPF; Red: cTnT; Blue: DAPI.
Ex vivo histological confirmation of bioluminescence and echocardiographic data
After imaging, animals were euthanized and hearts explanted for histological analysis. Masson trichrome staining indicated a reduced infarction size for the CPC group treated with miRNA cocktail (21.5±3.4%) compared with the CPCs alone (26.5±3.9%) and PBS control groups (32.5±5.4%; P<0.05) at day 28 (Figure 5C). Immunostaining of explanted hearts also confirmed that the population of GFP+ cells in CPCs treated with miRNA cocktail is significantly higher than CPC alone and PBS control groups (Figure 5D).
Prediction and confirmation of miRNA targets
To elucidate the potential targets of these miRNAs, we predicted the target genes of miR-21, miR-24, and miR-221 using both TargetScan and MicroCosm, which are based on different prediction algorithms.24 Irrespective of site conservation, 1791 genes were predicted as potential target genes of mouse miR-21, 3048 genes as the potential target genes of mouse miR-24, and 2295 genes as the potential target genes of mouse miR-221. Bioinformatics analysis indicated many apoptotic genes are the targets of these 3 miRNAs, which are putatively suppressed by these miRNAs. These apoptotic genes include Casp3, Casp8ap2, Bax, Pdcd4, and Fasl. Interestingly, 106 genes are predicted as the common targets of all 3 miRNAs. Some of these 106 genes include apoptotic activators or effectors (including Bcl2l11, Foxo3, and Ak2), which are potential suppression targets by all of the three miRNAs (Supplementary Figure 1). Bcl2l11, also named as Bim, belongs to Bcl-2 protein family. Interestingly, Bim has shown to be a critical apoptotic activator, which prompted us to perform further experimental analysis.25
There are a total of 6 binding sites on the 3’UTR of Bim, including 1 binding site of miR-21, 2 binding sites of miR-24, and 3 binding sites of miR-221 (Figure 6A, Supplementary Figure 2). To experimentally validate this bioinformatic prediction, we employed luciferase reporter assay. The 6 miRNAs binding segments of 3’UTR of Bim were amplified by PCR and inserted into downstream of the luciferase reporter gene separately in the pGL3 control vector for the Dual-luciferase assay, including 21, 24_1, 24_2, 221_1, 221_2, and 221_3 recombinant reporter (Figure 6B). For reporter 21, 24_1, 24_2, 221_2, 221_3, compared to the precursor miRNA control, their luciferase activity was significantly suppressed by their relative miRNAs. Furthermore, the luciferase activity could not be suppressed by using mismatched miRNAs. For example, luciferase activity of reporter 21 could be suppressed by miR-21, but not by miR-24. However, for the reporter 221_1, the luciferase activity was not suppressed by miR-221, indicating that this binding site is dispensable for Bim suppression by miR-221.
Figure 6. Schematic diagram for constitutive reporter vector and confirmation of the target genes of miRNAs.
(A) For luciferase assay, the segment of Bim 3’ UTR containing miRNA binding site was amplified by PCR and inserted downstream of Fluc driven by SV40 promoter. (B) Dual luciferase assay indicated the luciferase activity of the reporters could be suppressed by relative miRNAs, except for reporter 221_1. One-way ANOVA was used. (C) Western blot showed the isoforms of Bim in CPCs could be inhibited by miRNA cocktails endogenously.
Next, we assessed whether miRNAs could regulate Bim expression endogenously. CPCs were transduced with these three miRNAs to evaluate whether miRNAs could regulate Bim expression by Western blotting (Figure 6C). Compared with control, the levels of Bim isoforms proteins were significantly down-regulated by miRNAs cocktails. Collectively, these data suggest that Bim is a real target of these miRNA cocktails, as confirmed by bioinformatic prediction and experimental validation. To further elucidate the downstream target of miRNA cocktail treatment, the transduced CPCs were exposed to serum free condition and the activated caspase 3 protein levels were evaluated by Western blotting method. Compared with control, the protein levels of active caspase 3 were down-regulated by miRNA cocktails (Figure 6D).
DISCUSSION
Myocardial infarction (MI) is the leading cause of morbidity and mortality in the western world. MI leads to thinning of the infarct wall, cardiac dilatation, and adverse cardiac remodeling. At the cellular level, MI causes cellular apoptosis, necrosis, and fibrosis. Stem cell therapy, which is designed to replenish the infarct area with new cells, is therefore a promising therapeutic strategy. Cardiac progenitor cells, which can be committed to cardiomyocytes, smooth muscle cells, and endothelial cell lineages, are one of many potential candidate cell types for cardiovascular regenerative medicine. Although several studies have demonstrated improvement in cardiac function after CPC transplantation,26, 27 the low survival of the transplanted cells is believed to be one of the main hurdles for the failure to show long-term improvements after cell therapy.7
MicroRNAs are a class of small non-coding RNAs, mainly functioning as negative regulators of protein coding genes. In this study, we report that a novel miRNA pro-survival cocktail can improve the engraftment of the transplanted CPCs. Consequently, the longer surviving CPCs could lead to better functional recovery. We first isolated CPCs from transgenic mice that constitutively express Fluc and GFP. These isolated CPCs can differentiate into cardiomyocytes, smooth muscle cells, and endothelial cells under proper induction protocols.7, 28 Afterward, we selected several miRNA candidates that have been shown to improve cell survival or inhibit cell apoptosis. Cell viability analysis demonstrated that miR-21, miR-24, and miR-211 were particularly potent compared with control. Furthermore, when transduced with these three miRNAs as a cocktail, we found that CPCs had significantly better cell viability both in vitro and in vivo, which led to improved function as demonstrated by echocardiography and histology.
MiR-21 displays strong evolutionary conservation across a wide range of species, and is expressed in mammalian organ systems such as heart, spleen, small intestine, and colon.29 miR-21 is overexpressed in many tumor samples with anti-apoptotic roles by inhibiting several apoptotic genes. Most recently, miR-21 has been found to play important roles in cardiovascular biology in the areas of vascular smooth muscle cell proliferation and apoptosis, cardiac cell growth and death, and cardiac fibroblast functions.30 Overexpression of miR-21 can significantly decrease infarct size in mouse acute MI models by targeting PDCD4 and its downstream molecule AP1.31 In addition, miR-21 has a predominant effect on cardiac fibroblasts in response to ischemia/reperfusion in the mouse heart by suppression of PTEN.32 MiR-24 also has been shown to be an anti-apoptotic miRNA in the developing neural retina22 and tumorigenesis.33 miR-221 is involved as a miRNA in several cancer cells by functioning as an anti-apoptotic regulator by suppressing PTEN. 34 Antisense inhibition of miR-21 or miR-221 can arrest cell cycle and induce apoptosis in pancreatic adenocarcinoma.21
To elucidate the molecular mechanism of CPC survival by miRNA pro-survival cocktail, we performed target prediction of these three miRNAs. We found these miRNAs potentially suppress many classic apoptotic genes, including Casp3, Casp8ap2, Bax, Pdcd4, and Fasl, as well as Bim, Foxo3 and Ak2, which can be inhibited by all the three miRNAs. Bim is of particular interest because it belongs to the Bcl-2 protein family and can interact with other members of the family, including Bcl2, Bcl-xL, and Mcl-125. Bim mainly functions as a critical apoptotic activator. Bim gives rise to a variety of isoforms, including the most extensively studied cytotoxic splice variants, Bims, BimL, and BimEL.25 Overexpression of Bim in cardiac myocytes can induce apoptosis.35 Our dual-luciferase assay indicated that all three miRNAs can bind to the 3’UTR of Bim transcript and suppress its expression. Bim has been shown to activate Caspase cascades by inducing cytochrome c release from mitochondria and induce apoptosis of cells.13, 36, 37 Furthermore, western blotting indicated that the miRNA cocktail inhibits Bim and subsequently suppresses activation of caspase 3, contributing to the survival of CPCs. Our data demonstrated that miR-21, miR-24, and miR-221 can inhibit the apoptotic Bim, and can improve cell survival after challenge with serum-free stress in vitro and ischemic stress in vivo. Taken together, we believe the inhibition of potent pro-apoptotic Bim by the microRNA cocktail presents a novel and an important avenue for promoting cell survival.
Several cell types have been used for clinical trials.37, 38 However, there is still little convincing evidence that stem cells can engraft significantly in the myocardium.6 It is thought that massive cell death following transplantation may be a major limiting factor that impedes long-term cardiac recovery. Therefore, developing pro-survival or anti-apoptosis strategies to boost stem cell survival after transplantation may benefit this field. Cellular preconditioning by physical, chemical, genetic, and pharmacological manipulation has shown that the cells can better withstand the rigors of lethal ischemia in vitro as well as post-transplantation.39 Here we report that a novel miRNA pro-survival cocktail (miR-21, miR-24 and miR221) can also significantly improve CPC engraftment via suppression of pro-apoptotic Bim. Transplantation of CPCs treated with the miRNA cocktail can lead to significant improvement in cardiac function in a murine MI model. Hence miRNAs may provide a valuable method to promote cell survival by regulating multiple target genes, or even an entire gene network.
Supplementary Material
Acknowledgments
Funding Sources. This work was supported in part by grants from NIH HL099117, EB009689, Burroughs Wellcome Foundation (JCW), HL099776 (RCR), and AHA Postdoctoral Fellowship (SH).
Footnotes
Conflict of interest. None
References
- 1.Gonzales C, Pedrazzini T. Progenitor cell therapy for heart disease. Exp Cell Res. 2009;315:3077–3085. doi: 10.1016/j.yexcr.2009.09.006. [DOI] [PubMed] [Google Scholar]
- 2.Waksman R, Baffour R. Bone marrow and bone marrow derived mononuclear stem cells therapy for the chronically ischemic myocardium. Cardiovasc Radiat Med. 2003;4:164–168. doi: 10.1016/S1522-1865(03)00163-X. [DOI] [PubMed] [Google Scholar]
- 3.Dai W, Hale SL, Martin BJ, Kuang JQ, Dow JS, Wold LE, Kloner RA. Allogeneic mesenchymal stem cell transplantation in postinfarcted rat myocardium: Short- and long-term effects. Circulation. 2005;112:214–223. doi: 10.1161/CIRCULATIONAHA.104.527937. [DOI] [PubMed] [Google Scholar]
- 4.Narmoneva DA, Vukmirovic R, Davis ME, Kamm RD, Lee RT. Endothelial cells promote cardiac myocyte survival and spatial reorganization: Implications for cardiac regeneration. Circulation. 2004;110:962–968. doi: 10.1161/01.CIR.0000140667.37070.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kolossov E, Bostani T, Roell W, Breitbach M, Pillekamp F, Nygren JM, Sasse P, Rubenchik O, Fries JW, Wenzel D, Geisen C, Xia Y, Lu Z, Duan Y, Kettenhofen R, Jovinge S, Bloch W, Bohlen H, Welz A, Hescheler J, Jacobsen SE, Fleischmann BK. Engraftment of engineered es cell-derived cardiomyocytes but not bm cells restores contractile function to the infarcted myocardium. J Exp Med. 2006;203:2315–2327. doi: 10.1084/jem.20061469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rosenzweig A. Cardiac cell therapy--mixed results from mixed cells. N Engl J Med. 2006;355:1274–1277. doi: 10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
- 7.Li Z, Lee A, Huang M, Chun H, Chung J, Chu P, Hoyt G, Yang P, Rosenberg J, Robbins RC, Wu JC. Imaging survival and function of transplanted cardiac resident stem cells. J Am Coll Cardiol. 2009;53:1229–1240. doi: 10.1016/j.jacc.2008.12.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Bogt KE, Schrepfer S, Yu J, Sheikh AY, Hoyt G, Govaert JA, Velotta JB, Contag CH, Robbins RC, Wu JC. Comparison of transplantation of adipose tissue- and bone marrow-derived mesenchymal stem cells in the infarcted heart. Transplantation. 2009;87:642–652. doi: 10.1097/TP.0b013e31819609d9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Terrovitis JV, Smith RR, Marban E. Assessment and optimization of cell engraftment after transplantation into the heart. Circ Res. 2010;106:479–494. doi: 10.1161/CIRCRESAHA.109.208991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li Z, Wu JC, Sheikh AY, Kraft D, Cao F, Xie X, Patel M, Gambhir SS, Robbins RC, Cooke JP. Differentiation, survival, and function of embryonic stem cell derived endothelial cells for ischemic heart disease. Circulation. 2007;116:I46–54. doi: 10.1161/CIRCULATIONAHA.106.680561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.van der Bogt KE, Sheikh AY, Schrepfer S, Hoyt G, Cao F, Ransohoff KJ, Swijnenburg RJ, Pearl J, Lee A, Fischbein M, Contag CH, Robbins RC, Wu JC. Comparison of different adult stem cell types for treatment of myocardial ischemia. Circulation. 2008;118:S121–129. doi: 10.1161/CIRCULATIONAHA.107.759480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sheikh AY, Lin SA, Cao F, Cao Y, van der Bogt KE, Chu P, Chang CP, Contag CH, Robbins RC, Wu JC. Molecular imaging of bone marrow mononuclear cell homing and engraftment in ischemic myocardium. Stem Cells. 2007;25:2677–2684. doi: 10.1634/stemcells.2007-0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao F, Wagner RA, Wilson KD, Xie X, Fu JD, Drukker M, Lee A, Li RA, Gambhir SS, Weissman IL, Robbins RC, Wu JC. Transcriptional and functional profiling of human embryonic stem cell-derived cardiomyocytes. PLoS One. 2008;3:e3474. doi: 10.1371/journal.pone.0003474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mishra PK, Tyagi N, Kumar M, Tyagi SC. Micrornas as a therapeutic target for cardiovascular diseases. J Cell Mol Med. 2009;13:778–789. doi: 10.1111/j.1582-4934.2009.00744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matkovich SJ, Wang W, Tu Y, Eschenbacher WH, Dorn LE, Condorelli G, Diwan A, Nerbonne JM, Dorn GW., 2nd Microrna-133a protects against myocardial fibrosis and modulates electrical repolarization without affecting hypertrophy in pressure-overloaded adult hearts. Circ Res. 2010;106:166–175. doi: 10.1161/CIRCRESAHA.109.202176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Zhang X, Ren XP, Chen J, Liu H, Yang J, Medvedovic M, Hu Z, Fan GC. Microrna-494 targeting both proapoptotic and antiapoptotic proteins protects against ischemia/reperfusion-induced cardiac injury. Circulation. 2010;122:1308–1318. doi: 10.1161/CIRCULATIONAHA.110.964684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu S, Huang M, Li Z, Jia F, Ghosh Z, Lijkwan MA, Fasanaro P, Sun N, Wang X, Martelli F, Robbins RC, Wu JC. Microrna-210 as a novel therapy for treatment of ischemic heart disease. Circulation. 2010;122:S124–131. doi: 10.1161/CIRCULATIONAHA.109.928424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tang YL, Shen L, Qian K, Phillips MI. A novel two-step procedure to expand cardiac sca-1+ cells clonally. Biochem Biophys Res Commun. 2007;359:877–883. doi: 10.1016/j.bbrc.2007.05.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hu SJ, Ren G, Liu JL, Zhao ZA, Yu YS, Su RW, Ma XH, Ni H, Lei W, Yang ZM. Microrna expression and regulation in mouse uterus during embryo implantation. J Biol Chem. 2008;283:23473–23484. doi: 10.1074/jbc.M800406200. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Tan W, Neo TW, Aung MO, Wasser S, Lim SG, Tan TM. Role of the mir-106b-25 microrna cluster in hepatocellular carcinoma. Cancer Sci. 2009;100:1234–1242. doi: 10.1111/j.1349-7006.2009.01164.x. [DOI] [PubMed] [Google Scholar]
- 21.Park JK, Lee EJ, Esau C, Schmittgen TD. Antisense inhibition of microrna-21 or -221 arrests cell cycle, induces apoptosis, and sensitizes the effects of gemcitabine in pancreatic adenocarcinoma. Pancreas. 2009;38:e190–199. doi: 10.1097/MPA.0b013e3181ba82e1. [DOI] [PubMed] [Google Scholar]
- 22.Walker JC, Harland RM. Microrna-24a is required to repress apoptosis in the developing neural retina. Genes Dev. 2009;23:1046–1051. doi: 10.1101/gad.1777709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong L, Lai M, Chen M, Xie C, Liao R, Kang YJ, Xiao C, Hu WY, Han J, Sun P. The mir-17-92 cluster of micrornas confers tumorigenicity by inhibiting oncogene-induced senescence. Cancer Res. 2010;70:8547–8557. doi: 10.1158/0008-5472.CAN-10-1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomas M, Lieberman J, Lal A. Desperately seeking microrna targets. Nat Struct Mol Biol. 2010;17:1169–1174. doi: 10.1038/nsmb.1921. [DOI] [PubMed] [Google Scholar]
- 25.Gillings AS, Balmanno K, Wiggins CM, Johnson M, Cook SJ. Apoptosis and autophagy: Bim as a mediator of tumour cell death in response to oncogene-targeted therapeutics. FEBS J. 2009;276:6050–6062. doi: 10.1111/j.1742-4658.2009.07329.x. [DOI] [PubMed] [Google Scholar]
- 26.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation. 2007;115:896–908. doi: 10.1161/CIRCULATIONAHA.106.655209. [DOI] [PubMed] [Google Scholar]
- 27.Tang XL, Rokosh G, Sanganalmath SK, Yuan F, Sato H, Mu J, Dai S, Li C, Chen N, Peng Y, Dawn B, Hunt G, Leri A, Kajstura J, Tiwari S, Shirk G, Anversa P, Bolli R. Intracoronary administration of cardiac progenitor cells alleviates left ventricular dysfunction in rats with a 30-day-old infarction. Circulation. 2010;121:293–305. doi: 10.1161/CIRCULATIONAHA.109.871905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res. 2004;95:911–921. doi: 10.1161/01.RES.0000147315.71699.51. [DOI] [PubMed] [Google Scholar]
- 29.Lagos-Quintana M, Rauhut R, Yalcin A, Meyer J, Lendeckel W, Tuschl T. Identification of tissue-specific micrornas from mouse. Curr Biol. 2002;12:735–739. doi: 10.1016/s0960-9822(02)00809-6. [DOI] [PubMed] [Google Scholar]
- 30.Cheng Y, Zhang C. Microrna-21 in cardiovascular disease. J Cardiovasc Transl Res. 2010;3:251–255. doi: 10.1007/s12265-010-9169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dong S, Cheng Y, Yang J, Li J, Liu X, Wang X, Wang D, Krall TJ, Delphin ES, Zhang C. Microrna expression signature and the role of microrna-21 in the early phase of acute myocardial infarction. J Biol Chem. 2009;284:29514–29525. doi: 10.1074/jbc.M109.027896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy S, Khanna S, Hussain SR, Biswas S, Azad A, Rink C, Gnyawali S, Shilo S, Nuovo GJ, Sen CK. Microrna expression in response to murine myocardial infarction: Mir-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc Res. 2009;82:21–29. doi: 10.1093/cvr/cvp015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin W, Shi Y, Zhao B, Yao C, Jin L, Ma J, Jin Y. Mir-24 regulates apoptosis by targeting the open reading frame (orf) region of faf1 in cancer cells. PLoS One. 2010;5:e9429. doi: 10.1371/journal.pone.0009429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chun-Zhi Z, Lei H, An-Ling Z, Yan-Chao F, Xiao Y, Guang-Xiu W, Zhi-Fan J, Pei-Yu P, Qing-Yu Z, Chun-Sheng K. Microrna-221 and microrna-222 regulate gastric carcinoma cell proliferation and radioresistance by targeting pten. BMC Cancer. 2010;10:367. doi: 10.1186/1471-2407-10-367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Suzuki S, Yokoyama U, Abe T, Kiyonari H, Yamashita N, Kato Y, Kurotani R, Sato M, Okumura S, Ishikawa Y. Differential roles of epac in regulating cell death in neuronal and myocardial cells. J Biol Chem. 2010;285:24248–24259. doi: 10.1074/jbc.M109.094581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tanaka S, Wakeyama H, Akiyama T, Takahashi K, Amano H, Nakayama KI, Nakamura K. Regulation of osteoclast apoptosis by bcl-2 family protein bim and caspase-3. Adv Exp Med Biol. 2010;658:111–116. doi: 10.1007/978-1-4419-1050-9_12. [DOI] [PubMed] [Google Scholar]
- 37.Mummery CL, Davis RP, Krieger JE. Challenges in using stem cells for cardiac repair. Sci Transl Med. 2010;2:27ps17. doi: 10.1126/scitranslmed.3000558. [DOI] [PubMed] [Google Scholar]
- 38.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 39.Haider H, Ashraf M. Preconditioning and stem cell survival. J Cardiovasc Transl Res. 2010;3:89–102. doi: 10.1007/s12265-009-9161-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.