Abstract
Coronary artery disease (CAD) is increasingly recognized as an important contributor to morbidity and mortality among persons living with HIV infection. Traditional cardiovascular disease risk factors as well as aspects of HIV infection and its therapy contribute to the increased CAD observed in HIV. Advances in non-invasive imaging methodologies in both computed tomography and magnetic resonance imaging provide opportunities to evaluate coronary artery atherosclerosis in ways not possible by conventional invasive x-ray angiography. Application of these techniques may prove very useful in the study of atherosclerosis in many diseases such as HIV.
Cardiovascular Disease in HIV
Despite clear indications of dramatic declines in all cause mortality following the introduction of potent combination antiretroviral therapy for HIV-infected adults (1), myocardial infarction (MI) is increased in HIV-infected patients compared to the general population (2) and seen in association with increased exposure to antiretroviral therapy (3). The etiology of the increased risk of cardiovascular disease is most likely multifactorial and numerous investigations have been aimed to identify and modify potential risk factors.
Protease inhibitor medications, which were first introduced in the mid-1990’s, are frequently associated with dyslipidemia including elevations in triglycerides as well as total cholesterol (4). The prospective multinational Data Collection on Adverse Events of Anti-HIV Drugs (D.A.D.) Study Group identified a relative rate of MI of 1.16 (95% CI 1.10–1.23) for each year of protease inhibitor therapy when adjusted for exposure to other antiretroviral drug classes and non-lipid traditional cardiovascular disease risk factors. When analyses were adjusted for lipid levels, the attributable risk to protease inhibitor use was reduced but remained a significant risk factor for MI (RR 1.10, 95% CI 1.04–1.18). Once sufficient data were available, subsequent analyses in this same cohort looking at individual medications in various drug classes demonstrated that not all protease inhibitors and that the non-nucleoside reverse transcriptase inhibitors nevirapine and efavirenz were not associated with increased risk of MI (5). Investigation also identified an increased risk of MI with recent exposure to the nucleoside reverse transcriptase inhibitors didanosine and abacavir (6), with evidence of a potential cumulative effect of abacavir exposure (5).
The mechanism by which abacavir conveys this increased cardiovascular risk remains under investigation. Hsue and colleagues identified abacavir use as a risk factor for decreased brachial artery flow mediated dilation, a marker of impaired endothelial function (7). The SMART trial, which evaluated continuous antiviral therapy versus CD4 guided intermittent therapy, identified an increased risk of cardiovascular disease (CVD) events among the HIV-infected participants assigned to continuous viral suppression who were receiving abacavir compared to those not on abacavir(8). In this analysis, baseline levels of the serological biomarkers of inflammation, C-reactive protein and interleukin-6, were also increased in participants on abacavir compared to those on other nucleoside reverse transcriptase inhibitors. However, in a randomized open-label trial in which HIV-infected subjects were assigned either abacavir/lamivudine or tenofovir/emtricitabine as their nucleoside reverse transcriptase inhibitors, there was no difference in the change in multiple serologic markers of inflammation, thrombosis or endothelial function between the two groups after 12 weeks of therapy. Further investigation is needed to fully understand the influence antiretroviral agents exert on the risk and development of CVD in HIV.
The SMART trial also provided compelling evidence that unsuppressed HIV viral replication and accompanying inflammation may increase the risk of cardiovascular disease(9). In this prospective trial of 5472 HIV-infected patients with a CD4 count above 350 cells/mm3 randomized to either continuous antiretroviral therapy or intermittent CD4 count guided therapy, there were 79 composite CVD events with a trend towards a significant increased risk among those in the intermittent therapy arm [HR 1.57 (95% CI 1.00–2.46, p=0.05)] . In a subsequent nested-case control study investigating biomarkers of inflammation and thrombosis, baseline elevations in C-reactive protein, interleukin-6 and D-dimer were predictive of all cause mortality (10). While increased levels of IL-6 and D-dimer correlated with increases in HIV viral load, the pathophysiologic link to atherosclerosis and cardiovascular events for these biomarkers in HIV is under investigation.
There is increasing evidence that HIV infection is associated with increased atherosclerotic burden. For example, HIV infection was associated with increased carotid intima media thickness, a surrogate for coronary artery disease and a marker of increased risk of MI and stroke in the general population(11), in a meta-analysis of over 5456 HIVinfected and 3600 control subjects(12). Utilizing autopsy specimens, Micheletti et al., evaluated coronary artery disease in 66 HIV-infected patients who died of advanced of AIDS under the age of 55 years between 2001 and 2006 and compared them to 19 HIVuninfected controls who died at a similar age of non-coronary arteries disease(13). Thirty percent of individuals with HIV had 50% or greater stenosis in 3 vessels compared with 10.5% of controls and there was an increased lipid content in the plaque of those with HIV infection. These data compliment the observations of Lo and colleagues who directly measured coronary artery plaque burden using CT angiography in 78 HIVinfected asymptomatic men without a history of CVD and 32 uninfected healthy controls (14). HIV-infected men had significantly higher plaque volume and a greater number of coronary artery segments with plaque compared to controls. In addition to traditional risk factors such as age, Framingham risk score and total cholesterol, both duration of HIV infection and lower CD4/CD8 ratio were associated with increased plaque volume in HIV, suggesting a role for HIV and immune activation in atherosclerosis in this population.
It is important to recognize that traditional CVD risk factors play a significant role in the coronary artery disease of patients with HIV. Diabetes, smoking, hyperlipidemia and hypertension were shown to increase the risk of MI in large cohort and retrospective case control studies in HIV (2, 15). In addition to smoking, a substantial history of cocaine use has been associated with acute myocardial infarction. The mechanism for acute MI with cocaine use, however, is not thought be related to increased coronary atherosclerosis(16). In a study of 165 HIV-infected African American adults without known cardiovascular disease or hypertension, Lai and colleagues (17) used CT angiography to assess coronary artery calcification and stenosis and its relationship to cocaine abuse. This study revealed that long-term cocaine use was independently associated with significant coronary artery stenosis (adjusted odds ratio, 7.75; 95% CI 2.26–31.2) in addition to an observed association with exposure to antiretroviral therapy for 6 or more months.
Non-invasive surrogate markers and direct imaging studies that allow quantification of coronary artery disease burden will greatly enhance our ability to understand the pathophysiologic mechanisms of coronary artery disease in HIV and to identify, prevent, and treat cardiovascular disease in these patients.
Advances in Coronary Artery Imaging
Non-invasive coronary imaging has allowed for the early detection of atherosclerosis in patients with HIV by utilizing the advances in both CT(14) and Magnetic Resonance Imaging (MRI) (18) technologies. For many years such a coronary assessment was possible only through invasive and selective X-ray coronary angiography. Such methods require the use of catheters that are introduced in the peripheral arterial system and advanced in the aorta to reach the origins of the coronary arteries for selective contrast injections. However, the success of this method has been attributed mainly to the high spatial resolution of 0.2–0.25 mm and temporal resolution of 33msec provided by conventional X-ray fluoroscopy. This allows for imaging of these relatively small vessels, in the order of 2–4mm in diameter, that are constantly moving due to combined, complex respiration and cardiac motion. Conventional X-ray angiography utilizes a radiation dose of approximately 2.3–22.7 millisievert (mSv)(19). Therefore, for any non-invasive technique to succeed in capturing a diagnostic image of these small vessels, the challenges of both high speed and spatial resolution must be tackled, while attempting to reduce or eliminate radiation use.
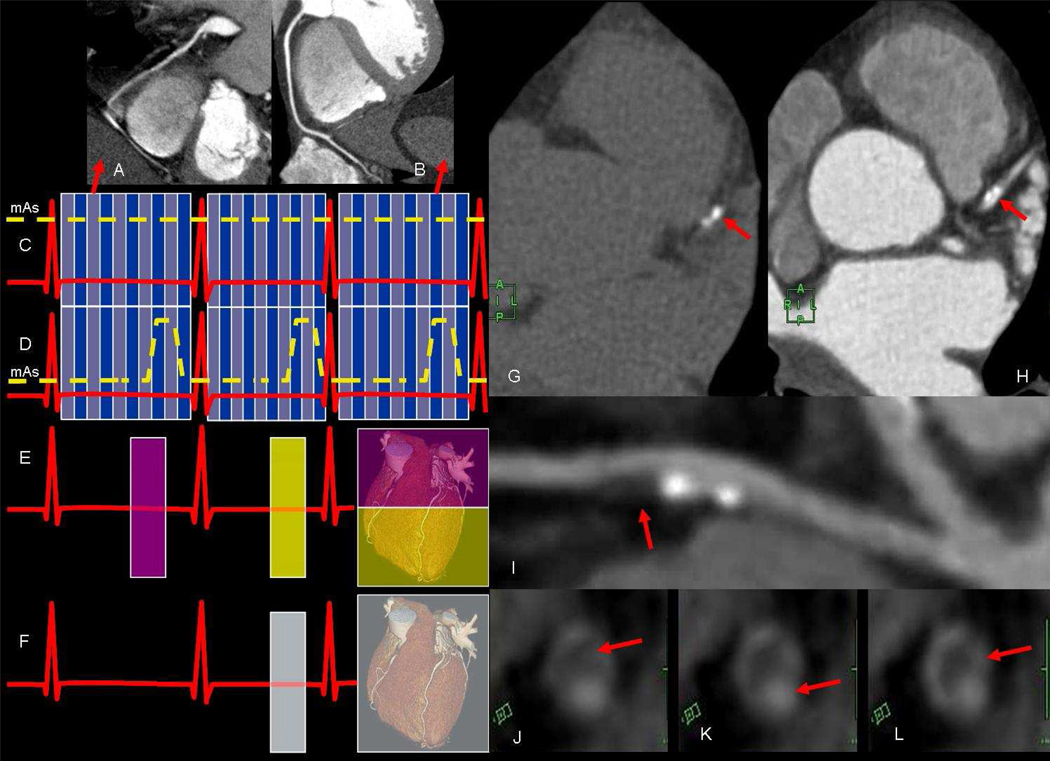
While CT techniques can overcome the respiratory motion challenge by using the breath hold method, the issues of both cardiac motion and reduction of radiation dose need to be addressed. The former may be addressed by taking advantage of the quiescent periods of the cardiac cycle where motion is the least (20). These quiescent periods are usually witnessed at mid-diastole and early systole, which are at approximately 75% and 34% of the cardiac cycle (Figure 1a and b) depending on the heart rate (20). More important than their location in the cardiac cycle is their duration in relation to the temporal resolution of the CT scanner. These durations are inversely related to the heart rate with the end-systolic period lasting for 118msec (range 0–223msec) and the second longer period in mid-diastole lasting and average of 187msec (range 66–330msec). Hence, lengthening the R-R interval, by reducing the heart rate will inevitable lengthen these quiescent periods to act as windows of opportunity to acquire motionless images of the coronary arteries. Such a task is achieved by either orally or intravenously administered β-blockers prior to the scan in order to reduce the heart rate to 60–70 beats per minute. Additionally, this also stabilizes the R-R variability so that these quiescent periods fall in the same position of the cardiac cycle. This is particularly important to avoid deterioration of the imaging quality as a result of increased heart rate that occurs near the end of breath holding (21). In order to capture these relatively dormant periods, the temporal resolution of the CT scanner needs to be shorter than the targeted period in the cardiac cycle. Advancements in gantry technology and the use of dual source/detector combinations have managed to shorten this temporal resolution down to as low as 75msec (22). Such advances may allow for less aggressive use of β-blockers when applying retrospective gating techniques and tube current modulation. The latter optimizes the tube current (Figure 1c and d) to acquire the best image quality during the mid diastolic rest period while the images outside this window are of sufficient quality to cinematically assess other larger non-coronary cardiac anatomy such as the valves and/or myocardium. Although this reduces the effective dose by 10%-40% (23) ranging from 4–14 mSv (19), lowering the heart rate remains an important factor in effective radiation dose reduction (24). Increasing the number of detectors rows to 128, 256, and 320 rows has allowed for complete coverage of the heart in 1–2 cardiac cycles by applying prospective gating (Figure 1e and f) techniques which reduce the effective dose to a reported record of 1mSv (25). Cardiac CT has been useful for the assessment of numerous condition including congenital anomalies and acquired cardiac conditions (26). Multiple studies have shown coronary CT to be a sensitive (97.2%) and specific (87.4%) technique for the detection and evaluation of the degree of coronary artery stenosis (27). However, its greatest potential may lie not only in the assessment of coronary artery disease resulting in stenosis but also in the evaluation, characterization and understanding of the pathophysiology of atherosclerotic plaque. Although calcified plaque resulting in a Agatston score of more than 400 is considered as a risk equivalent for a cardiac event in the intermediate risk group (28), it may represent only the tip of the iceberg (Figure 1g, h and i). Calcified atherosclerotic plaque is one of many stages of plaque (Figure 1j, k and l) development (29). Therefore, this non-invasive technique may open the door for better understanding the relationship of coronary atherosclerosis with various risk factors and associated diseases.
Figure 1.
a) coronary CT. scan angiography during systole and b) diastole showing superior image quality of the latter due to longer quiescent period in diastole. Schematic scan modes during c) retrospective electrocardiographic (ECG) gating without dose modulation (blue lines show division of the R-R interval into 10 phases of the cardiac cycle) d) retrospective ECG gating with dose modulation to reduce effective ration dose. Schematic diagram of prospective scan modes using e) step-and shot technique in 2 heart beats (e.g. using 256 detector rows) f) or single heart beat for wider coverage (320 detector rows). Coronary CT g) without and h) with contrast showing calcification (red arrows) in the left anterior descending coronary artery (LAD). Multi-planer reformatted image of the same LAD showing the extent of the non-calcified plaque which appears to as large as the calcified portion. Cross sectional CT images of another coronary artery showing the j) low density non-calcified plaque k) causing severe narrowing of the lumen and l) enhancement of the vessel wall.
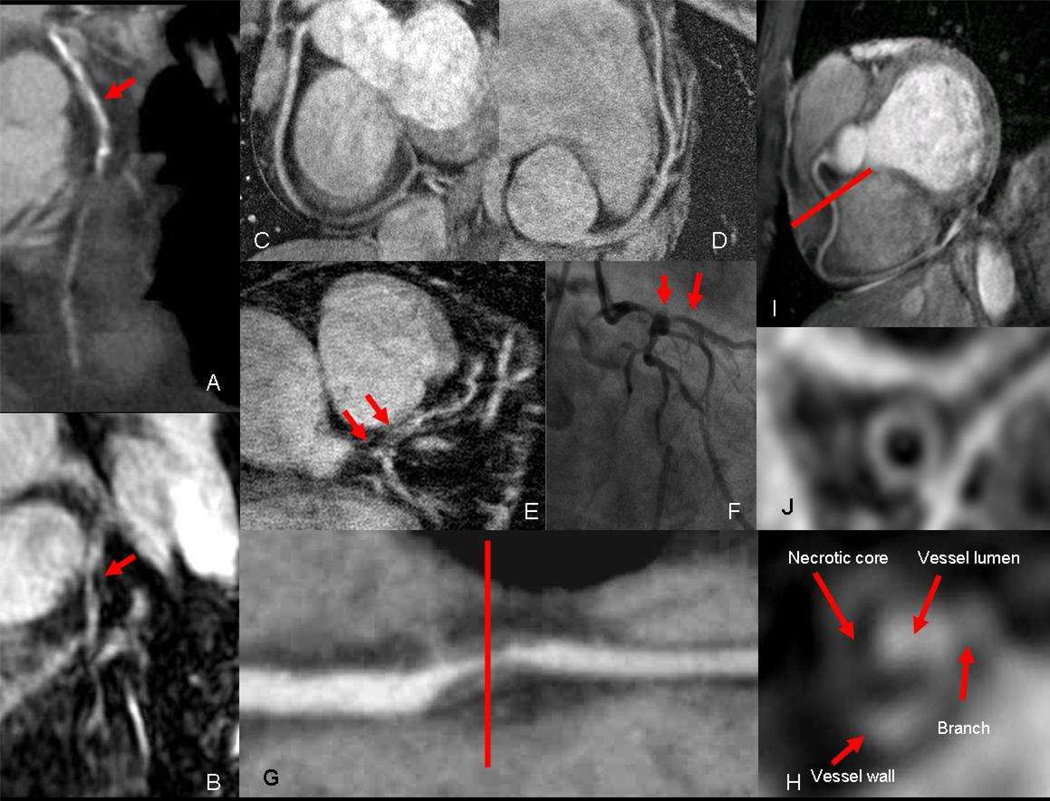
Despite its value, coronary CT angiography is limited by the use of potentially nephrotoxic contrast agents and ionizing radiation. Additionally, CT image quality is degraded by blooming artifacts (Figure 2a and b) from coronary calcifications (30) and inadequate heart rate control, such as for patients with contraindications to β blockers. MR angiography, therefore, may play an alterative or supplemental role in such situations. A meta analysis study of coronary MR angiography has shown a sensitivity of 87.1% and specificity of 70.3% for this modality in the detections of significant coronary artery stenosis (27). However, this performance appears to have improved with the use contrast agents and high magnetic field (3T) to reach a sensitivity and specificity of 94% and 82% receptivity (31). Such an improvement is attributed mainly to the higher signal-to-noise ratio (SNR) available at 3T compared to 1.5T. This improvement in SNR has also allowed for an improvement in temporal resolution which would allow a shorter acquisition window of approximately 35msec. Higher temporal resolution permits coronary imaging in the systolic rest period (32) thereby avoiding the need of heart rate control which is particularly useful for imaging children and patients with contraindications to the use of β-blockers. The use of higher magnetic field has also resulted in improvement of spatial resolution (Figure 2c, d, e and f) to reach 350μm (33), matching that of CT. These advances help improve coronary MR performance, however, the greater potential of this technology is improvements in tissue characterization of atherosclerotic plaque (Figure 2g and h). For example, over-expression of αvβ3 indicating angiogenesis (34), will probably present as delayed enhancement of vulnerable plaque (29, 35) and vessel wall (Figure 2h). Other MR capabilities that are not possible with CT include the assessment of coronary artery endothelial dysfunction (36), coronary vessel wall imaging (37) (Figure 2i and j) and measuring coronary blood flow velocities (38). Overall, advances in both CT and MR coronary imaging will provide opportunities to evaluate coronary artery atherosclerosis not only non-invasively, but also in ways not possible by conventional invasive x-ray angiography that is limited to luminography. Such opportunities may prove very useful in the understanding of atherosclerosis in many diseases such as HIV.
Figure 2.
a) Coronary CT of the left anterior descending coronary artery (LAD) showing severe calcification (red arrow) of the proximal and mid segment that obscure visualization of the lumen. However, corresponding b) coronary MR with contrast of the same LAD is not affected by calcium and reveals the area of critical narrowing (red arrow). High resolution coronary MR with 350 μm in plane resolution of the c) right coronary artery (RCA) and d) LAD. e) 350 μm coronary MR angiography showing severe narrowing of left main and LAD which is confirmed on f) conventional X-ray angiography. f) Multi-planer reformatted image of diseased RCA showing severe narrowing from non-calcified plaque, which demonstrates a g) necrotic core and enhancing wall on coronary NR with contrast. h) Coronary MR without contrast of normal RCA and i) corresponding black blood image showing the normal vessel wall of the mid segment.
Acknowledgements
This work was funded by the National Institutes of Health Intramural Research Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no conflicts of interest to report.
References
- 1.Bozzette SA, Ake CF, Tam HK, et al. Long-term survival and serious cardiovascular events in HIV-infected patients treated with highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:338–341. doi: 10.1097/QAI.0b013e31815e7251. [DOI] [PubMed] [Google Scholar]
- 2.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friis-Moller N, Reiss P, Sabin CA, et al. Class of antiretroviral drugs and the risk of myocardial infarction. N Engl J Med. 2007;356:1723–1735. doi: 10.1056/NEJMoa062744. [DOI] [PubMed] [Google Scholar]
- 4.Kotler DP. HIV and antiretroviral therapy: lipid abnormalities and associated cardiovascular risk in HIV-infected patients. J Acquir Immune Defic Syndr. 2008;49 Suppl 2:S79–S85. doi: 10.1097/QAI.0b013e318186519c. [DOI] [PubMed] [Google Scholar]
- 5.Worm SW, Sabin C, Weber R, et al. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D:A:D) study. J Infect Dis. 2010;201:318–330. doi: 10.1086/649897. [DOI] [PubMed] [Google Scholar]
- 6.Sabin CA, Worm SW, Weber R, et al. Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients enrolled in the D:A:D study: a multi-cohort collaboration. Lancet. 2008;371:1417–1426. doi: 10.1016/S0140-6736(08)60423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsue PY, Hunt PW, Wu Y, et al. Association of abacavir and impaired endothelial function in treated and suppressed HIV-infected patients. AIDS. 2009 doi: 10.1097/QAD.0b013e32832e7140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Use of nucleoside reverse transcriptase inhibitors and risk of myocardial infarction in HIV-infected patients. AIDS. 2008;22:F17–F24. doi: 10.1097/QAD.0b013e32830fe35e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips AN, Carr A, Neuhaus J, et al. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antivir Ther. 2008;13:177–187. doi: 10.1177/135965350801300215. [DOI] [PubMed] [Google Scholar]
- 10.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS Med. 2008;5:e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340:14–22. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 12.Hulten E, Mitchell J, Scally J, Gibbs B, Villines TC. HIV positivity, protease inhibitor exposure and subclinical atherosclerosis: a systematic review and meta-analysis of observational studies. Heart. 2009;95:1826–1835. doi: 10.1136/hrt.2009.177774. [DOI] [PubMed] [Google Scholar]
- 13.Micheletti RG, Fishbein GA, Fishbein MC, et al. Coronary atherosclerotic lesions in human immunodeficiency virus-infected patients: a histopathologic study. Cardiovasc Pathol. 2009;18:28–36. doi: 10.1016/j.carpath.2007.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lo J, Abbara S, Shturman L, et al. Increased prevalence of subclinical coronary atherosclerosis detected by coronary computed tomography angiography in HIV-infected men. AIDS. 2010;24:243–253. doi: 10.1097/QAD.0b013e328333ea9e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 16.Chang AM, Walsh KM, Shofer FS, McCusker CM, Litt HI, Hollander JE. Relationship between cocaine use and coronary artery disease in patients with symptoms consistent with an acute coronary syndrome. Acad Emerg Med. 18:1–9. doi: 10.1111/j.1553-2712.2010.00955.x. [DOI] [PubMed] [Google Scholar]
- 17.Lai S, Fishman EK, Lai H, et al. Long-Term Cocaine Use and Antiretroviral Therapy Are Associated with Silent Coronary Artery Disease in African Americans with HIV Infection Who Have No Cardiovascular Symptoms. Clin Infect Dis. 2008;46:600–610. doi: 10.1086/526782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mikhail IJ, Purdy JB, Dimock DS, et al. High Rate of Coronary Artery Abnormalities in Adolescents and Young Adults Infected with Human Immunodeficiency Virus Early in Life. Pediatr Infect Dis J. 2011 doi: 10.1097/INF.0b013e31820f6575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Einstein AJ, Moser KW, Thompson RC, Cerqueira MD, Henzlova MJ. Radiation dose to patients from cardiac diagnostic imaging. Circulation. 2007;116:1290–1305. doi: 10.1161/CIRCULATIONAHA.107.688101. [DOI] [PubMed] [Google Scholar]
- 20.Johnson KR, Patel SJ, Whigham A, Hakim A, Pettigrew RI, Oshinski JN. Three-dimensional, time-resolved motion of the coronary arteries. J Cardiovasc Magn Reson. 2004;6:663–673. doi: 10.1081/jcmr-120038086. [DOI] [PubMed] [Google Scholar]
- 21.Holland AE, Goldfarb JW, Edelman RR. Diaphragmatic and cardiac motion during suspended breathing: preliminary experience and implications for breath-hold MR imaging. Radiology. 1998;209:483–489. doi: 10.1148/radiology.209.2.9807578. [DOI] [PubMed] [Google Scholar]
- 22.Flohr TG, Leng S, Yu L, et al. Dual-source spiral CT with pitch up to 3.2 and 75 ms temporal resolution: image reconstruction and assessment of image quality. Med Phys. 2009;36:5641–5653. doi: 10.1118/1.3259739. [DOI] [PubMed] [Google Scholar]
- 23.Poll LW, Cohnen M, Brachten S, Ewen K, Modder U. Dose reduction in multi-slice CT of the heart by use of ECG-controlled tube current modulation ("ECG pulsing"): phantom measurements. Rofo. 2002;174:1500–1505. doi: 10.1055/s-2002-35945. [DOI] [PubMed] [Google Scholar]
- 24.Jakobs TF, Becker CR, Ohnesorge B, et al. Multislice helical CT of the heart with retrospective ECG gating: reduction of radiation exposure by ECG-controlled tube current modulation. Eur Radiol. 2002;12:1081–1086. doi: 10.1007/s00330-001-1278-x. [DOI] [PubMed] [Google Scholar]
- 25.Duarte R, Bettencourt N, Costa JC, Fernandez G. Coronary computed tomography angiography in a single cardiac cycle with a mean radiation dose of approximately 1 mSv: initial experience. Rev Port Cardiol. 2010;29:1667–1676. [PubMed] [Google Scholar]
- 26.Hendel RC, Patel MR, Kramer CM, et al. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475–1497. doi: 10.1016/j.jacc.2006.07.003. [DOI] [PubMed] [Google Scholar]
- 27.Dewey M. Coronary CT versus MR angiography: pro CT--the role of CT angiography. Radiology. 2011;258:329–339. doi: 10.1148/radiol.10100161. [DOI] [PubMed] [Google Scholar]
- 28.Greenland P, LaBree L, Azen SP, Doherty TM, Detrano RC. Coronary artery calcium score combined with Framingham score for risk prediction in asymptomatic individuals. Jama. 2004;291:210–215. doi: 10.1001/jama.291.2.210. [DOI] [PubMed] [Google Scholar]
- 29.Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953–957. doi: 10.1038/nature06803. [DOI] [PubMed] [Google Scholar]
- 30.Liu X, Zhao X, Huang J, et al. Comparison of 3D free-breathing coronary MR angiography and 64-MDCT angiography for detection of coronary stenosis in patients with high calcium scores. AJR Am J Roentgenol. 2007;189:1326–1332. doi: 10.2214/AJR.07.2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Q, Li K, Liu X, et al. Contrast-enhanced whole-heart coronary magnetic resonance angiography at 3.0-T: a comparative study with X-ray angiography in a single center. J Am Coll Cardiol. 2009;54:69–76. doi: 10.1016/j.jacc.2009.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gharib AM, Herzka DA, Ustun AO, et al. Coronary MR angiography at 3T during diastole and systole. J Magn Reson Imaging. 2007;26:921–926. doi: 10.1002/jmri.21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ustun A, Desai M, Abd-Elmoniem KZ, Schar M, Stuber M. Automated identification of minimal myocardial motion for improved image quality on MR angiography at 3 T. AJR Am J Roentgenol. 2007;188:W283–W290. doi: 10.2214/AJR.06.0334. [DOI] [PubMed] [Google Scholar]
- 34.Heroux J, Gharib AM, Danthi NS, Cecchini S, Ohayon J, Pettigrew RI. High-affinity alphavbeta3 integrin targeted optical probe as a new imaging biomarker for early atherosclerosis: initial studies in Watanabe rabbits. Mol Imaging Biol. 2010;12:2–8. doi: 10.1007/s11307-009-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ishida M, Kato S, Sakuma H. Cardiac MRI in ischemic heart disease. Circ J. 2009;73:1577–1588. doi: 10.1253/circj.cj-09-0524. [DOI] [PubMed] [Google Scholar]
- 36.Hays AG, Hirsch GA, Kelle S, Gerstenblith G, Weiss RG, Stuber M. Noninvasive visualization of coronary artery endothelial function in healthy subjects and in patients with coronary artery disease. J Am Coll Cardiol. 2010;56:1657–1665. doi: 10.1016/j.jacc.2010.06.036. [DOI] [PubMed] [Google Scholar]
- 37.Abd-Elmoniem KZ, Weiss RG, Stuber M. Phase-sensitive black-blood coronary vessel wall imaging. Magn Reson Med. 2010;63:1021–1030. doi: 10.1002/mrm.22286. [DOI] [PubMed] [Google Scholar]
- 38.Johnson K, Sharma P, Oshinski J. Coronary artery flow measurement using navigator echo gated phase contrast magnetic resonance velocity mapping at 3.0 T. J Biomech. 2008;41:595–602. doi: 10.1016/j.jbiomech.2007.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]