Abstract
Background
Using peer volunteers as delivery agents may improve translation of evidence-based physical activity promotion programs for older adults. This study examined whether tailored support from older peer volunteers could improve initiation and long-term maintenance of physical activity behavior.
Methods
Participants were randomized to two 16-week, group-based programs: (1) peer-delivered, theory-based support for physical activity behavior change; or (2) an intervention typically available in community settings (basic education, gym membership, and pedometer for self-monitoring), attention-matched with health education. Moderate-to-vigorous physical activity (MVPA) was assessed via daily self-report logs at baseline, at the end of the intervention (16 weeks), and at follow-up (18 months), with accelerometry validation (RT3) in a random subsample.
Results
Seven peer volunteers and 81 sedentary adults were recruited. Retention at the end of the trial was 85% and follow-up at 18 months was 61 %. Using intent-to-treat analyses, at 16 weeks, both groups had similar significant improvements in MVPA. At 18 months, the group supplemented with peer support had significantly more MVPA.
Conclusions
Trained peer volunteers may enhance long-term maintenance of physical activity gains from a community-based intervention. This approach has great potential to be adapted and delivered inexpensively in community settings.
Keywords: peer mentors, exercise, aging, translation
Older adults are among the least active segments of the U.S. population1, despite the increasing number of efficacious behavioral interventions targeting physical inactivity in this population. In 2002 the Task Force on Community Preventive Services2 strongly recommended that evidence-based behavioral interventions be an integral part of physical activity (PA) promotion in community settings. Despite this call, and the growing number of evidence-based PA interventions, few programs are currently being translated into community-based settings3 and even fewer are designed at the outset for translation4,5. These factors greatly limit the potential for large-scale public health impacts of physical activity on health in older populations.
One of the primary reasons that PA behavioral interventions have yet to reach widespread dissemination is the reliance on trained professional staff to deliver evidence-based programs. The use of peer volunteers (or peer mentors as we refer to them in the text below) represents a potentially lower cost alternative to trained professional staff that may increase the likelihood of dissemination into community settings. Peer mentors have been adopted for midlife and older adults in other health domains such as nutrition education6, mammography promotion7, and cancer support8. A few recent experimental studies have begun to investigate whether peer volunteers can successfully deliver a PA intervention in older populations. Dorgo et al.9 found in a 14-week supervised exercise program that older adults led by trained peer mentors had similar PA gains relative to older adults led by undergraduate Kinesiology students. In the most direct and rigorous comparison of peer mentors to professional staff, Castro et al.10 found that when delivering the same evidence-based telephone support PA intervention, peer mentors and trained professional staff had significant and equivalent improvements in PA behavior relative to an attention-control at the end of the 12-month intervention. Moreover, peer mentors were found to be able to maintain similar, and in some cases greater, levels of treatment fidelity (i.e., intervention implementation) compared to the trained professional staff.
Despite the appeal of peer-based intervention models to increase translational efforts, there are number of additional questions that need to be addressed. First, can peer mentors enhance PA promotion efforts already being implemented in community settings? These efforts typically consist of providing low-cost access or vouchers for physical activity, basic education, and in some cases, tools for behavioral feedback (e.g., pedometers)11. These strategies have been found to result in small to moderate improvements and are typically short-lived in older populations11 and therefore are unlikely to result in substantial public health impact. Including peer mentors as an adjunct to these approaches has the potential to increase public health impact by providing a cost-conscious way to incorporate more intensive evidence-based behavioral strategies. Moreover, there are likely channels for delivering peer-based interventions already in place in communities throughout the U.S. through agencies and community organizations such as local health departments, senior centers, nonprofit health organizations, and organizations that focus on seniors (e.g., AARP, Administration on Aging). To address this question, of whether peer mentors could enhance existing community-based approaches, we embedded our intervention into community settings where promotion strategies might typically already occur.
A second important question about the use of peer mentors in public health settings is whether appropriately trained peers deliver theoretically-derived intervention components needed to enhance initiation and long-term maintenance of PA behavior. These components include individually adapted self-regulatory and behavior change strategies such as goal setting, problem solving, feedback and reinforcement, and relapse prevention, all of which have been recommended to enhance short-term and long-term PA gains in community settings2, As was preliminarily observed by Castro et al.10, peer mentors may be more effective delivery agents of these theory-based components than professional staff. We believe this may be particularly true when adopting social cognitive12 and self-determination theories13 of behavior change. Both theoretical perspectives place emphasis on interpersonal relationships in promoting motivation, self-efficacy, and behavioral regulation. From a social cognitive perspective, peer mentors may enhance self-efficacy beliefs through vicarious experiences from someone of comparable age, background, and life experience or improve self-regulatory processes linked to overcoming challenges through modeling and verbal persuasion. We also predicted that autonomy would be needed in order to enhance the likelihood of PA adherence once the intervention was completed14.
We conducted a community-based, randomized controlled trial comparing peer-delivered, theory-based advice and support to an intervention designed to mimic what may currently be offered in community settings. Our primary outcome was self-reported physical activity behavior following the intervention period (16 weeks) and at long-term follow-up (18 months). We also explored cardiorespiratory fitness and psychosocial outcomes. We hypothesized that older adults, when exposed to a theory-based PA intervention delivered by peer mentors, would have greater gains in PA following the intervention at long-term follow-up relative to what is currently being offered in community settings.
Methods
Design and Procedures
The AAMP (Active Adult Mentoring Program) study was a 16-week randomized controlled trial with an 18-month follow-up. Data were collected between 2006 and 2008 and analyzed in 2010 and 2011. Participants were drawn from a university community in the southeastern United States. Recruitment efforts included announcements in a local newspaper, a university older adult participant registry, and flyers in community gathering places (e.g., grocery stores, recreation/community centers, retirement communities). Interested community members were invited to call the study office to undergo telephone screening. Participants had to meet the following eligibility criteria: (a) 50 years of age or older; (b) currently inactive or insufficiently active, as defined by not meeting national PA recommendations15 during the past 6 months; (c) free of any medical factors that would prohibit unsupervised exercise (e.g., major cardiovascular disease, pulmonary disease, recent cancer treatment) or impact study compliance or assessment (e.g., cognitive impairment [as defined by a cutoff score of ≤27 on the Telephone Interview for Cognitive Status16], prescription of heart-rate attenuating medication, hearing or speech impairment); and (d) be willing to accept random assignment, comply with study procedures, and commit to the entire study period. If determined to be eligible, participants were invited to the baseline assessment visit where they underwent informed consent procedures. Following this visit, participants were randomized to one of the two study arms and contacted with group meeting details. Institutional approval was obtained for all aspects of the study protocol.
Intervention
Participants were randomized to one of two 16-week, group-based study arms: (1) peer-led advice and support for physical activity initiation and maintenance (active intervention); or (2) a “standard” community-based PA promotion intervention, attention-matched with peer health education (standard community intervention). Both arms were given access to the community exercise facility in which the study was housed during the program and were given a pedometer for PA self-monitoring. Intervention format, staff time, and attention were identical across study arms. In total, 8 group “replicates” were conducted. A replicate was comprised of an active intervention and a standard community intervention group. Recruitment was ongoing such that when sufficient numbers of eligible participants were available in a given replicate, the replicate was randomized into active intervention and standard community intervention groups. A blocked randomization scheme was used with a 1:1 allocation ratio to ensure equivalent group sizes within each replicate. Each group consisted of 3 to 7 participants and a peer mentor. Intervention sessions from both arms were conducted in one of two community-based exercise facilities: (1) a University-owned exercise facility typically available for staff and faculty; or (2) an exercise facility at a suburban church in the local community.
Active Intervention Arm
Initial sessions (weeks 1–3) focused on basic trust and rapport building between the mentor and group members. During these sessions the peer mentors were trained to ask open-ended questions and participants were invited to share information about their PA history. Subsequent sessions (weeks 4–10) were semi-structured in nature and grounded in social cognitive12 and self-determination13 theories. The goal of these sessions was for the mentor to provide support to learn self-management skills for PA initiation and maintenance. Participants were encouraged to engage in a variety of lifestyle physical activities including walking and resistance exercises. This was accomplished through self-efficacy and self-determined behavior enhancing activities such as encouragement and regular feedback, goal setting, building a PA supportive social support system, and problem solving exercises. Mental imagery was another component of the intervention given its links to social cognitive theory and associations between self-efficacy beliefs and physical activity17, 18. The [mal sessions (weeks 10–16) focused on relapse prevention skills and developing a specific plan to transition to a home- or community-based exercise routine.
Standard Community Intervention Arm
This arm was designed to mimic a “standard” approach to PA promotion that may typically exist in community settings. This included two educational sessions which covered the benefits of physical activity and basic feedback for exercise prescription. Access to an exercise facility during the intervention period and pedometers for self-monitoring were also provided, as these strategies may also be present in many community settings. In order to attention-match for the additional sessions provided in the active intervention arm, basic health education sessions were facilitated by trained peer mentors on a broad range of aging-tailored topics based on public ally available materials from the National Institutes of Health senior health topical guide (http://nihseniorhealth.gov/). Homework assignments, discussion questions, and appropriate praise and reinforcement were elicited by the peer mentors in a similar framework as the active intervention arm. Peer mentors were also selected from a similar pool of community volunteers and appropriate training and quality control took place prior to and during the study for this arm.
Peer Mentors and Quality Control
Peer mentors were recruited from a registry of research participants from previous health promotion studies and through a local health fair. Mentors were eligible if they reported having a regular physical activity routine (for active intervention arm) or had a basic background in health education (for standard community intervention arm), were willing to commit to regular participation in group meetings, and were open to completing all training and receiving regular feedback about their performance. Peer mentors generally volunteered their time without remuneration; however, in a few cases mentors were modestly reimbursed for their travel (approximately $15/session). All peer mentors received a manual of the weekly activities and underwent a 4 hour training session. Intervention sessions in both arms were audio-recorded and monitored by the research team for content and proper deli very of materials. Quality control checklists and scoring procedures were used to give the peer mentors feedback about ways to improve their efforts to facilitate group meetings. Program staff met weekly with the mentor after each of the first five sessions to give feedback and coaching. Additional support and feedback was provided as needed throughout the intervention.
Measurements
Measurements were administered at baseline prior to randomization, at the end of the 16-week intervention period, and at 18 months following the intervention.
Physical Activity
The Leisure-Time Exercise Questionnaire (LTEQ) was used to assess self-report PA behavior at baseline, following the intervention at 16 weeks, and during the follow-up week at 18 months. The LTEQ is a three-item scale that asks participants to rate how often they engaged in mild, moderate, and strenuous leisure-time exercise19. Although typically used as a 7-day recall of PA behavior, in the present study the LTEQ was used as a daily measure and summed across the week to reduce recall bias. Recent literature has chosen to not include mild minutes in calculations of PA20 given that currently moderate or vigorous activity is emphasized for health benefit15. Minutes of moderate- to-vigorous physical activity (MVPA) were computed from the LTEQ by adding the number of bouts reported and multiplying by 20. This value was then summed across the week to represent minutes of MVPA per week. Previous research has supported the validity and reliability of LTEQ score interpretations with adults21 and older adult20,21 populations.
Objective Physical Activity Validation
A random subs ample of 22 participants (balanced by study arm) wore an RT3 triaxial accelerometer (Stayhealthy, Monrovia, CA) at baseline and following the intervention at16 weeks to verify the self-reported PA data. Participants were instructed to (a) secure the unit over the dominant hip; (b) wear the device while they were awake; and (c) take off the unit for swimming or bathing. Data were collected in 1-min epochs. Data compliance and cleaning procedures were consistent with large objective PA monitoring studies1,22. The RT3 accelerometer has previously been validated against self-reported PA (7-Day PA Recall) in a sample of cancer survivors and was found to have moderate agreemenr23. To determine time spent in MVPA, an existing proprietary calibration equation supplied by the manufacturer (Stayhealthy, Monrovia, CA), which has been validated against direct calorimetry24, was used to convert vector magnitude units into metabolic equivalent units (METS). All 1-min epochs of recording ≥3.0 METS were classified as MVPA.
Cardiorespiratory Fitness
A submaximal graded exercise test was used to obtain estimations of cardiorespiratory fitness (V02peak) at baseline and following the 16 week intervention period. A modified Balke treadmill protocol25 with continuous heart rate monitoring was used. Permission from the participant's physician was granted prior to administration. It should be noted that such an approach is known to provide less accurate estimations of cardiorespiratory fitness than a maximal graded exercise test; however, for a population of sedentary older adults it is considered a safe and adequate alternative26.
Self-Efficacy Beliefs
Barriers self-efficacy27 and exercise self-efficacy28, both common measures in the field, were used to assess the self-efficacy construct at baseline and following the intervention period at 16 weeks. Both BSE (α = .95) and EXSE (α = .99) showed excellent internal consistency.
Self-Determined Behavior Beliefs
The Exercise Motivation Scale29 was assessed at baseline, following the 16-week intervention period, and at 18 months. This 31-item measure of exercise motivation was designed to assess motivational tendencies in the exercise context according to a self-determination framework. The EMS has shown adequate factorial evidence to support its 8-factor structure, but can also be weighted across the 8-subscales to form a single indicator of self-determined behavior along the self-determination continuunr30, which is how we have used the EMS in our current investigation. The overall measure internal consistency was very good (α = .85).
Sample Size and Statistical Analyses
Sample size estimates for study outcomes were derived from improvements in a pilot study conducted in preparation for this larger trial. This study yielded effect size estimates ranging from 0.38 to 1.28 (depending on study outcome. It should be noted, however, that this was an intervention-only pilot study that was shorter in duration (8 weeks) than the current trial. Therefore, the most conservative effect size was used. The sample size need to detect an effect size of 0.38 or greater with an alpha at .05 and power of .80 was 35 participants per arm. Recruitment efforts were adjusted by 20% to protect against dropout. Thus, total sample size required was 84. No effect size estimates were available for 18-month outcomes.
To address the primary research hypothesis of whether the active intervention arm had improved outcomes relative to the standard community intervention arm, repeated measures analyses of variance (R-ANOVA) were conducted for all study variables, with 16-week and 18-month outcomes initially examined in separate models. Among outcomes that were followed to 18 months follow-up (MVPA, EMS), additional R-ANOVAs were used to assess differential trajectories of change across the three assessment points by treatment arm (i.e., time x treatment interaction effect). Pearson product moment conelations were computed to validate the self-reported PA against the accelerometer data in the subsample where both sources of data were available. Intent-to-treat principles were used for all study variables such that when data were missing at 16 weeks or 18 months, baseline values were used. Effect size estimates were calculated for all outcomes. Alpha was set at p < .05 and an analyses were two-tailed. The analyses were carried out using SPSS 17.0 (SPSS, Chicago, IL).
Results
Sample Characteristics and Study Flow
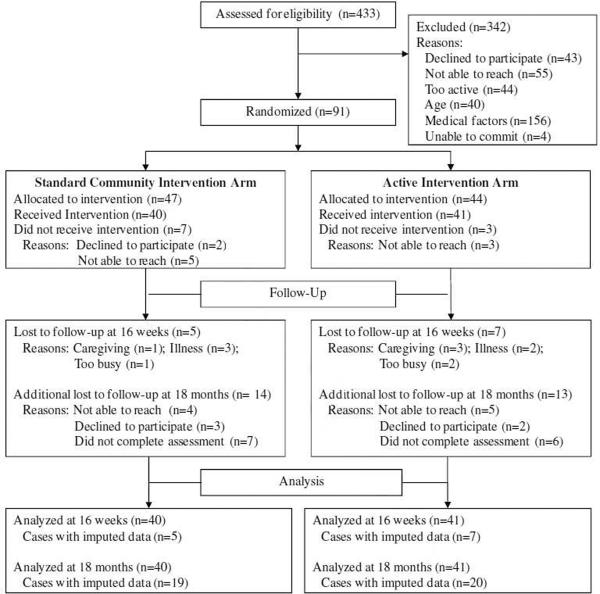
Figure 1 describes the study flow of the intervention. A total of 433 participants were assessed for eligibility. Among those excluded at the screening stage (n=342), 28.7% declined to participate (n=43) or were not able to be reached by phone (n=55). An additional 24.6% were found to be too active (n=44) or too young (n=40), thus did not undergo the medical history portion of the screening. An additional 45.6% were excluded due to medical factors which included the following: (a) prescription of heart rate attenuating medication (n=36); (b) history of epilepsy, head injuring requiring hospitalization, or diagnosed stroke in the last year (n=16); (c) cognitive impairment (n=15); (d) ambulation requiring assistive device (n=14); (e) hearing or speech impairments making verbal communication difficult in a group setting (n=10); (f) diagnosis Of schizophrenia, clinical depression, bipolar disorder or other major psychiatric illness (n= 10); (g) history of major cardiovascular disease (n=4); (h) current pulmonary disease (n=4); (i) recent history of drug or alcohol abuse (n=4); or G) treatment for cancer in the past year (n=3). Finally, an additional 1.2% indicated they were unable to commit to the entire study period (n=4) but were otherwise eligible.
Figure 1.
AAMP Study Flow.
The remaining 91 eligible participants were randomized to the two study arms. Descriptive information for the sample is reported in Table 1. Age was similar across groups, with a relatively equal proportion between 50–64 and 65+ years of age categories. More than half of the sample was married, with progressively fewer numbers of participants divorced, widowed, or never married respectively. The sample was primarily female, white, of non- Hispanic descent, and college-educated. Participants, on average, were moderately overweight. In total, 7 mentors completed the 8 replicates (16 total groups). No demographics differences between study mentors were observed by study arm. Mentors, on average, were 67.29 ± 4.19 years of age (range = 61 to 72), had 18.29 ± 1.80 years of education (Master's degree equivalent), and completed 2.29 ± 1.38 groups.
Table 1.
Baseline demographic characteristics by study arm (N=81).
| Characteristic | Active Intervention (n=41) | Standard Community Intervention (n = 40) | Total Sample |
|---|---|---|---|
| Age ± SD, years | 63.49 ± 8.26 | 63.35 ± 9.07 | 63.42 ± 8.62 |
| Age group, n (%) | |||
| 50 to 64 years | 19 (46.3) | 20 (50.0) | 39 (48.1) |
| 65 years and over | 22 (53.7) | 20 (50.0) | 42 (51.9) |
| Gender, n (%) | |||
| Female | 35 (85.4) | 32 (80.0) | 67 (82.7) |
| Male | 6 (14.6) | 8 (20.0) | 14 (17.3) |
| Marital status, n (%) | |||
| Married | 22 (53.7) | 22 (55.0) | 44 (54.3) |
| Divorced | 11 (26.8) | 12 (30.0) | 23 (28.4) |
| Widowed | 5 (12.2) | 2 (5.0) | 7 (8.6) |
| Single, or never married | 3 (7.3) | 4 (10.0) | 7 (8.6) |
| Education ± SD, years | 15.93 ± 2.20 | 16.38 ± 2.31 | 16.15 ± 2.25 |
| Educational status, n (%) | |||
| High school/GED | 3 (7.3) | 2 (5.0) | 5 (6.2) |
| Some college or vocational training | 10 (24.4) | 11 (27.5) | 21 (25.9) |
| College graduate | 11 (26.8) | 8 (20.0) | 19 (23.5) |
| Some postbaccalaureate or Master's degree | 15 (36.6) | 14 (35.0) | 29 (35.8) |
| Doctoral degree | 2 (4.9) | 5 (12.5) | 7 (8.6) |
| Race, n (%) | |||
| White | 37 (90.2) | 37 (92.5) | 74 (91.4) |
| African-American | 2 (4.9) | 1 (2.5) | 3 (3.7) |
| Asian | 0 (0.0) | 1 (2.5) | 1 (1.2) |
| Biracial | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Other | 2 (4.9) | 0 (0.0) | 2 (2.5) |
| Ethnicity, n (%) | |||
| Hispanic/Latino | 2 (4.9) | 1 (2.5) | 3 (3.7) |
| BMI, kg/m2 | 28.39 ± 6.53 | 26.77 ± 5.68 | 27.56 ± 6.12 |
Note. All group differences were not significant (p > .10).
Overall, 85% of participants (83% in active intervention and 87% in the standard community intervention; n=69 of 81 who received any component of the intervention) completed the intervention and the assessment following the intervention period at 16 weeks. Study completers were not statistically different from those lost to follow-up on all demographic variables (ps>.10), with the exception of BMI (t (72) = −2.67, p = .009), which was lower among study completers. Post hoc exploration indicated that BMI difference was due to a single extreme value (BMI = 47.80), who reported attrition due to caregiving responsibilities. The elimination of this value from the analysis yielded a non significant BMI difference. The relatively small sample size precluded meaningful analyses of differential lost to follow-up by study arm; however, the distribution of lost to follow-up and the reasons provided appeared equal across arms. All mentors completed the group(s) to which they were initially assigned.
Among study completers, 61% (38% in the active intervention and 40% in the standard community intervention, n=42 of 69 study completers) completed the assessment at 18 months following the intervention period. This represented an overall 52% retention rate among those who were randomized and received any exposure to the intervention at 18 months (n=81). Those who were lost to follow-up at 18 months were not statistically different from those who completed this assessment on any of the demographic variables (ps>.10).
Self-Reported Physical Activity Outcomes
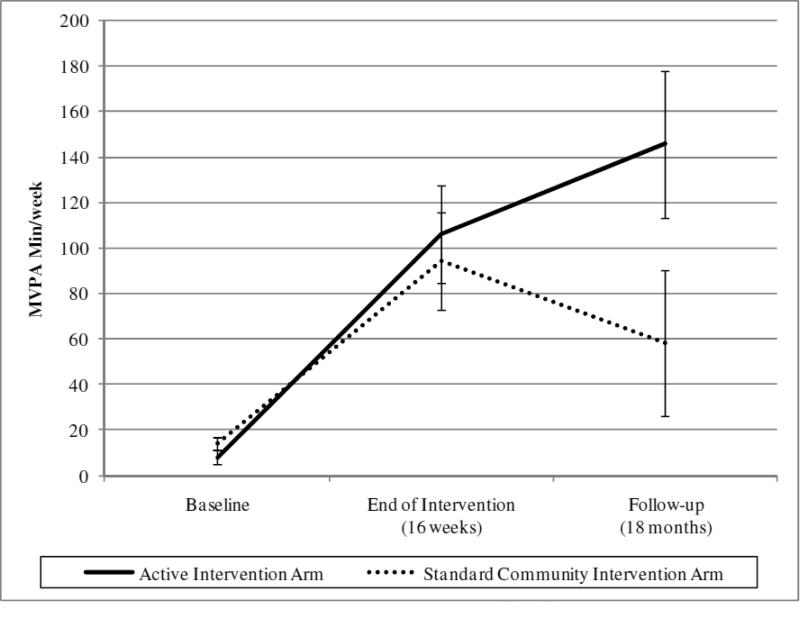
Means and standard deviations for all study outcomes are reported in Table 2. Self-reported PA outcomes (MVPA min/week) were assessed at baseline, at the end of the intervention (16 weeks), and at follow-up (18 months). Figure 2 displays these outcomes graphically. At 16 weeks, both study arms reported significantly more MVPA min/week relative to baseline, F(2,79)=37.61, p<.001, effect size=1.38. However, the active intervention arm did not report greater gains relative to the standard community intervention arm. At 18 months, the active intervention arm reported significantly more MVPA minutes/week relative to the standard community intervention arm (F[2,79]=4.35, p=.04, effect size=.32). The repeated measures ANOVA model (accounting for all three timepoints) revealed a marginally significant time × group interaction, F(1.60,123.22)=2.92, p=.07, η2=.05.
Table 2.
Means and standard deviations fur study outcomes (N =81).
| Baseline |
End of intervention (16 weeks) |
Follow-up (18 months) |
|
|---|---|---|---|
| Variable | M (SD) | M (SD) | M(SD) |
| Self-reported Physical Activity (min·wk−1) | |||
| Active Intervention | 8.08 (9.28) | 106.06 (140.14)† | 145.73 (264.42)* |
| Standard Community Intervention | 13.86 (23.62) | 94.41 (128.45) | 58.32 (103.58) |
| Cardio Respiratory Fitness (V02peak) | |||
| Active Intervention | 27.37 (7.47) | 29.07 (8.02)† | - |
| Standard Community Intervention | 28.40 (7.61) | 30.94 (9.03) | - |
| Barriers Self-Efficacy | |||
| Active Intervention | 70.38 (21.98) | 69.36 (23.83) | |
| Standard Community Intervention | 63.65 (24.13) | 60.92 (27.13) | - |
| Exercise Self-Efficacy | |||
| Active Intervention | 80.79 (25.17) | 72.10 (28.87) | - |
| Standard Community Intervention | 67.25 (27.86) | 63.72 (31.43) | - |
| Self-Determined Behavior | |||
| Active Intervention | 16.93 (6.64) | 18.05 (5.43)* | 36.10 (27.56)* |
| Standard Community Intervention | 15.87 (5.79) | 15.13 (6.67) | 22.81 (17.35) |
Note.
Between-arm difference, p<.05;
Main effect for time, p<.05; Intent-to-treat values are imputed.
Figure 2.
Between-group differences in MVPA minutes/week† at baseline, following the intervention (16 weeks), and at follow-up (18 months)
†Based on intent-to-treat data; MVPA = Reported moderate-to-vigorous physical activity as measured by the Leisure Time Exercise Questionnaire (LTEQ).
Objective Physical Activity Validation
During the baseline and the 16-week assessment periods, 27% of participants were randomly selected, balanced by study arm (n=22, 11 in each arm), to wear the RT3 accelerometer. Participants wore the accelerometer for an average of 8.83 (range=6–14) days at baseline and 6.71 (range=4–10) days at 16 weeks, with a total of 302 daily observations. Wear time was similar by study arm (p >.10). Minutes of accelerometer-derived MVPA were significantly correlated with minutes of LTEQ-reported MVPA (r=.48, p <.001). Despite the small sample size, at 16 weeks the active intervention aim showed marginal significance with more minutes of accelerometer-derived MYPA relative to the standard community intervention arm (F[1,19]=3.61, p=.059).
Cardiorespiratory Fitness
Results suggested that both study arms significantly improved their fitness levels at 16 weeks relative to baseline, F(1,79)=7.93, p=.006, effect size=.63. The active intervention arm did not show significantly different changes in fitness relative to the standard community intervention arm.
Self-Efficacy and Self-Determination
For both self-efficacy variables (BSE and EXSE), there were no significant differences observed at 16 weeks. For the self-determined behavior outcome (EMS), the active intervention arm showed significantly higher scores relative to the standard community intervention arm at 16 weeks (F[2,79]=4.19, p=.045, effect size=.46) and 18 months (F[2,79]=5.33, p=.02, effect size=.52). The repeated measures ANOVA model (accounting for all three timepoints) confirmed a significant time × group interaction, F(1.08,77.43)=4.93, p=.03, η2=.10.
Discussion
These results suggest that both the active intervention arm, which consisted of a peer-delivered, theory-based intervention, and the standard community intervention, were effective at significantly increasing PA behavior and improving cardiorespiratory fitness following the intervention period at 16 weeks. By 18 months follow-up, it appeared that the active intervention arm was able to maintain (and slightly increase) their PA behavior while the standard community intervention arm began to return back to their baseline levels. 'While exercise-related self-efficacy (both ESE and EXSE) did not appear to be impacted by the intervention (in either arm), self-determined behavior (i.e., intrinsic motivation) showed modest but significant increases at 16 weeks in the active intervention arm that continued to increase during the follow-up period at 18 months.
Since both groups improved their PA behavior at equal rates following the intervention period at 16 weeks, we were not able to conclude from this study that peer mentors delivering a theory-based intervention were more effective than standard community approaches at initiating PA. This finding, however, is not surprising when one considers the strength of the comparison arm in this trial. The standard community intervention included basic education about PA, access to an exercise facility, and a pedometer for self-monitoring. Moreover, participants in both arms responded to health promotion materials to be recruited into the study where PA promotion efforts were expected. Anyone of these factors, or more likely a combination, was likely to explain the substantial initial gains that were observed in the comparison group. Previous trials that have explored using peer mentors to promote PA have limited generalizability given the types of comparison/control groups that were selected, including (a) the use of health education delivery by trained health educators10; (b) identical program delivered by undergraduate students9; (c) no-contact delayed intervention31; and (d) a lack of a comparison/control condition altogether32. This is the first study that we are aware of to compare a peer-delivered program to a type of program that is likely to be present in diverse community settings across the country.
Perhaps the most striking result of this study was the substantial maintenance of PA behavior in the active intervention arm after 18 months of follow-up. It should be emphasized that these results were observed despite no formal contact between the program staff or peer mentors with the participants and the exercise membership was also withdrawn from both arms at the end of the 16-week intervention. We attribute the success of this program at improving long-term maintenance to at least two things: (a) the theory-based content of the program appropriately taught the behavioral skills needed to maintain behavior after the intense support of group participation was withdrawn; and (b) the peer mentors provided the context and supportive environment needed in which to learn the behavioral skills and practice them during the 16-week intervention phase that were then applied during the follow-up phase of the trial. More specifically, the PA gains observed during follow-up should also be viewed in light of changes in self-determined PA over time. Despite not seeing differences in PA behavior at 16 weeks, self-determined PA did increase in the active intervention arm only, suggesting that the peer mentors were able to provide a behavioral context that supported autonomy, perceptions of competence, and meaningful relationships with participants. These constructs are basic psychological needs which are linked to intrinsically motivated reasons for performing PA such as enjoyment, personal satisfaction, and health benefits13. Once participants were left to maintain their behavior on their own, self-determined behavior continued to increase suggesting that the individuals were able to internalize autonomous forms of behavioral regulation which facilitated adherence to PA behavior. This finding is in line with previous trials from a recent RCT where clinically significant reductions in weight and higher levels of PA were observed after 12 months of a self-determination theory-based intervention with overweight and obese women14.
With respect to self-efficacy, a similar pattern of results was not observed, as self-efficacy did not change. This may have occurred for a number of reasons: (a) the specific intervention content was not appropriately focused to increase self-efficacy or the peer mentors were not successful at delivering these components of the intervention; (b) high baseline levels of self-efficacy may have resulted in a ceiling effect; or (c) inappropriate/inconsistent behavioral targets in the wording of the questions may have resulted in confusion for the participants. As far as we are aware, the role of self-efficacy in peer-delivered PA interventions has not been studied, so future studies should continue to explore this variable to determine whether this lack of effect is real or has methodological origins.
Unlike many other PA efficacy trials, this intervention was designed with translation and dissemination in mind from the outset. There are three key components which suggest a high likelihood that this intervention may successfully be adopted in other community settings. First, the cost-sensitive nature of using peer volunteers as delivery agents, relative to trained professionals, suggest that community organizations such as YMCA's, senior centers, and other health agencies may be more willing to adopt such a program. Our experience through this trial suggests that recruiting peer volunteers with the necessary background, training them to deliver the intervention appropriately, and maintaining their participation through the program, is a feasible goal under most conditions. Anecdotally, we observed that the peer volunteers perceived substantial personal benefit from their participation and would have likely continued their efforts for longer periods of time. Second, the fact that our comparison arm was a standard approach to delivering PA promotion in the field and our results suggest enhanced maintenance of PA behavior, we believe provides convincing evidence that the program can improve outcomes in community settings. Finally, the nature of the intervention was designed to be adaptive and easily adopted. For example, this intervention has been standardized for both the peer mentors and the participants. the group-based format can be adopted in a number of diverse community settings, and no special equipment is needed to deliver the intervention.
The primary strengths of this study were the rigorous research design and the collection of outcomes at 18 months following the intervention. Randomized designs where interventions are compared to standards in the field are needed to provide an evidence-base and compelling rationale for program adoption in the field. Likewise, long-term maintenance of PA is a critical behavioral target to increase overall public health impact5. A final strength of this study was the inclusion of objective measures of physical activity and cardiorespiratory fitness as validation of the self-reported improvements in PA. The results of these assessments corroborate what was observed in the 16-week self-reported outcomes and lend confidence to what was observed at 18 months. In fact, the marginally significant group effect observed in the accelerometry data at 16 weeks suggests that (a) perceptions of PA improvements may have lagged behind actual improvements in the active intervention arm; or (b) a modest, but systematic difference was present at 16 weeks favoring the active intervention arm but the small sample size and imprecise measure of self-reported PA was not able to detect it. Either way, future studies should combine self-reported and objective methods of monitoring PA when evaluating peer-based interventions.
In terms of limitations, it is difficult to conclude what the “key ingredient” of the intervention was that resulted in the long-term maintenance. While it was clear that the peer mentors, delivering a theory-based intervention, produced favorable long-term outcomes in PA behavior and intrinsic motivation, we were not able to conclude whether it was the peer mentor facilitation alone, the use of goal setting, mental imagery exercises, or problem-solving activities, or a combination of these components which resulted in these improvements. Likewise, despite careful training and quality control, there were notable differences in the style and effectiveness of specific peer mentors. Due to small sample size we were unable to explore these observations empirically to understand what attributes distinguished successful from unsuccessful mentors. Future studies should include larger sample sizes and more extensive process-level data in order to explore these factors. The generalizability of this study is also limited given the underrepresentation of men and minorities in our sample. Finally, as is the case with many long-term follow-up studies, our study suffered from considerable attrition. While attrition in our study did not appear to be systematically related to group assignment or demographic characteristics, and we implemented a very conservative, intent-to-treat analytical strategy, our interpretations regarding long-term maintenance should be viewed with some caution.
There are a number of future directions of study in light of these findings. First, we believe this intervention has significant promise to be translated on a larger scale into more diverse community settings. This should likely be done through developing community partnerships with existing organizations. Also, formal cost-benefit and cost-effective analyses of peer-led interventions are needed. These types of studies should compare a peer-led intervention to existing community interventions as was used in the current trial and professionally-led programs. Finally, peer-based interventions need to be tested head-to-head with different delivery channels such as face-to-face, group-based, telephone-based, and internet-based. This will be useful to understand for whom and under what conditions these channels may be more or less effective and feasible.
In conclusion, evidence-based PA interventions that can be successfully translated into community settings for older populations are needed. Using peer volunteers as delivery agents is an appealing alternative to trained professional staff given the reduced cost and its success in other domains. In the current study we found that trained peer mentors delivering atheory-based intervention can enhance long-term maintenance of physical activity gains relative to a standard approach to PA promotion. This approach has potential to be delivered in community settings.
Acknowledgements
This work was supported in part by a Research Opportunity Fund in the College of Health and Human Performance at the University of Florida, an Age Network Multidisciplinary Research Enhancement grant at the University of Florida, a Mentorship Opportunity Grant from the Graduate Student Council at the University of Florida, institutional training grants awarded to Stanford University (5T32-HL-007034, M. P. B) and the University of Florida (T32-AG-020499, J. M. D.), and individual training grants awarded to the University of Florida (F31-AG-032802, J. M. D.; 1R36AG029664, A. A. M.). We are grateful to Mary Branagan, Bill Burk, Christine Dietrich, Dyke Farrow, Gail Harris, George Lebo, Bob Millot, and Milledge Murphy for their outstanding efforts as peer mentors.
References
- 1.Troiano RP, Berrigan D, Dodd KW, Masse LC, Tilert T, McDowell M. Physical activity in the United States measured by accelerometer. Med Sci Sports Exerc. 2008;40:181–188. doi: 10.1249/mss.0b013e31815a51b3. [DOI] [PubMed] [Google Scholar]
- 2.Kahn EB, Ramsey LT, Brownson RC, et al. The effectiveness of interventions to increase physical activity - A systematic review. Am J Prev Med. 2002;22:73–108. doi: 10.1016/s0749-3797(02)00434-8. [DOI] [PubMed] [Google Scholar]
- 3.Wilcox S, Dowda M, Leviton LC, et al. Active for Life - Final results from the translation of two physical activity programs. Am J Prev Med. 2008;35:340–351. doi: 10.1016/j.amepre.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 4.Estabrooks PA, Glasgow RE. Translating effective clinic-based physical activity interventions into practice. Am J Prev Med. 2006;31:545–556. doi: 10.1016/j.amepre.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 5.Glasgow RE, Lichtenstein E, Marcus AC. Why don't we see more translation of health promotion research to practice? Rethinking the efficacy-to-effectiveness transition. Am J Public Health. 2003;93:1261–1267. doi: 10.2105/ajph.93.8.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perez-Escamilla R, Hrorni-Fiedler A, Vega-Lopez S, Bermudez-Millan A, Segura-Perez S. Impact of peer nutrition education on dietary behaviors and health outcomes among Latinos: a systematic literature review. J Nutr Educ Behav. 2008;40:208–225. doi: 10.1016/j.jneb.2008.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Derose KP, Fox SA, Reigadas E, Hawes-Dawson J. Church-based telephone mammography counseling with peer counselors. J Health Commun. 2000;5:175–188. doi: 10.1080/108107300406884. [DOI] [PubMed] [Google Scholar]
- 8.Macvean ML, White VM, Sanson-Fisher R. One-to-one volunteer support programs for people with cancer: A review of the literature. Pat Educ Counsel. 2008;70:10–24. doi: 10.1016/j.pec.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 9.Dorgo S, King GA, Brickey GD. The Application of Peer Mentoring to Improve Fitness in Older Adults. J. Aging Phys. Act. 2009;17:344–361. doi: 10.1123/japa.17.3.344. [DOI] [PubMed] [Google Scholar]
- 10.Castro CM, Pruitt L, Buman MP, King AC. Older adults peer mentors to promote physical activity: Results of the TEAM randomized controlled clinical trial. Health Psychol. in press. [Google Scholar]
- 11.van der Bij AK, Laurant MGH, Wensing M. Effectiveness of physical activity interventions for older adults - A review. Am J Prev Med. 2002;22:120–133. doi: 10.1016/s0749-3797(01)00413-5. [DOI] [PubMed] [Google Scholar]
- 12.Bandura A. Social foundations of thought and action: A social cognitive theory. Prentice-Hall; Englewood Cliffs, NJ: 1986. [Google Scholar]
- 13.Deci EL, Ryan AM. Intrinsic motivation and self-determination in human behavior. Plenum; New York: 1985. [Google Scholar]
- 14.Silva MN, Vieira PN, Coutinho SR, Meinderico CS, Matos MG, Sardinha LB. Using self-determination theory to promote physical activity and weight control: A randomized controlled trial in women. J Behav Med. 2010;33:110–122. doi: 10.1007/s10865-009-9239-y. [DOI] [PubMed] [Google Scholar]
- 15.Physical Activity Guidelines Advisory Committee . Physical Activity Guidelines Advisory Committee Report. U.S. Department of Health and Human Services; Washington, DC: 2008. [DOI] [PubMed] [Google Scholar]
- 16.Brandt J, Spencer M, Folstein M. The telephone interview for cognitive status. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- 17.Giacobbi PR, Jr., Tuccitto D, Buman MP, Munro-Chandler K. A measurement and conceptual investigation of exercise imagery establishing construct validity. Res Q Exerc Sport. 2010;18:485–493. doi: 10.1080/02701367.2010.10599710. [DOI] [PubMed] [Google Scholar]
- 18.Kim BH, Giacobbi PR., Jr. The use of exercise related mental imagery by middle-aged adults. J Imagery Res Sport Phys Act. 2008;4:1–38. [Google Scholar]
- 19.Godin G, Shephard RJ. A simple method to assess exercise behavior in the community. Can J Applied Sport Science. 1985;10:141–146. [PubMed] [Google Scholar]
- 20.Karvinen KH, Courneya KS, Campbell KL, et al. Correlates of exercise motivation and behavior in a population-based sample of endometrial cancer survivors: an application of the Theory of Planned Behavior. Int J Behav Nutr Phys Act. 2007;4:1–10. doi: 10.1186/1479-5868-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs DR, Ainsworth BE, Hartman TJ, Leon AS. A simultaneous evaluation of 10 commonly used physical-activity questionanires. Med Sci Sports Exerc. 1993;25:81–91. doi: 10.1249/00005768-199301000-00012. [DOI] [PubMed] [Google Scholar]
- 22.Buman MP, Hekler EB, Haskell WL, et al. Objective light intensity physical activity associations with rated health in older adults. Am J Epidemiol. 2010;172:1155–1165. doi: 10.1093/aje/kwq249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sloane R, Snyder DC, Demark-Wahnefried W, Lobach D, Kraus WE. Comparing the 7-Day Physical Activity Recall with a Triaxial Accelerometer for Measuring Time in Exercise. Med Sci Sports Exerc. 2009;41:1334–1340. doi: 10.1249/MSS.0b013e3181984fa8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothney MP, Schaefer EV, Neumann MM, Choi L, Chen KY. Validity of physical activity intensity predictions by ActiGraph, Actical, and RT3 accelerometers. Obesity. 2008;16:1946–52. doi: 10.1038/oby.2008.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.American College of Sports Medicine . ACSM's Guidelines for Exercise Testing and Prescription. 7th edition Williams & Wilkins; Philadephia: Lippincott: 2006. [Google Scholar]
- 26.Gill TM, DiPietro L, Krumholz HM. Role of exercise stress testing and safety monitoring for older persons starting an exercise program. J Am Med Assoc. 2000;284:342–349. doi: 10.1001/jama.284.3.342. [DOI] [PubMed] [Google Scholar]
- 27.MaAuley E. The role of efficacy cognitions in the prediction of exercise behavior in middle-aged adults. J Behav Med. 1992;15:65–88. doi: 10.1007/BF00848378. [DOI] [PubMed] [Google Scholar]
- 28.McAuley E. Self-efficacy and the maintenance of exercise participattion in older adults. J Behav Med. 1993;16:103–113. doi: 10.1007/BF00844757. [DOI] [PubMed] [Google Scholar]
- 29.Li E. The exercise motivation scale: Its multifaceted structure and construct validity. J Applied Sport Psych. 1999;11:97–115. [Google Scholar]
- 30.Vallerand RJ, Rousseau FL. Intrinsic and extrinsic motivation in sport and exercise: A review using the hierarchical model of intrinsic and extrinsic motivation. In: Singer RN, Hausenblas HA, Janelle CM, editors. Handbook of Sport Psychology. John Wiley & Sons; New York, NY: 2001. [Google Scholar]
- 31.Batik O, Phelan EA, Walwick JA, Wang GQ, LoGerfo JP. Translating a community-based motivational support program to increase physical activity among older adults with diabetes at community clinics: A pilot study of physical activity for a lifetime of success. Prev Chron Dis. 2008;5 http://www.cdc.gov/pcd/issues/2008/jan/07_0142.htm. [PMC free article] [PubMed] [Google Scholar]
- 32.Etkin CD, Prohaska TR, Harris BA, Latham N, Jette A. Feasibility of implementing the strong for life program in community settings. Gerontologist. 2006;46:284–292. doi: 10.1093/geront/46.2.284. [DOI] [PubMed] [Google Scholar]