Abstract
Objective
To assess the efficacy of TU-025, keishibukuryogan, a Japanese prescription herbal medicine used for hot flash management, in American women.
Methods
This randomized, double-blind, placebo-controlled, phase II trial enrolled 178 postmenopausal women, aged 45–58 years, with a Mayo hot flash score greater than 28 per week and who met other inclusion criteria. After a one-week placebo run-in period, participants were randomly assigned to either 12 weeks of placebo, 7.5 g/day or 12.5 g/day. Primary and secondary outcomes were measured by the Mayo Clinic Hot Flash Diary, the Greene Climacteric Index and the Pittsburgh Sleep Quality Index.
Results
At three months, hot flash scores, climacteric symptoms and sleep quality improved by 34% in the placebo group, 40% in the 7.5 g/day group and 38% in the 12.5 g/day group. (p < 0.001). However, the differences in changes between groups were not statistically significant (p = 0.990). Diarrhea unexpectedly developed in 20% of participants receiving active medication.
Conclusions
For American women, unlike the clinical experience for Japanese women, TU-025 did not significantly reduce the frequency and severity of hot flash symptoms, improve climacteric symptoms or benefit sleep quality. This study identified several potentially significant methodological factors to be considered in future scientific assessments of traditional Asian medicines.
Keywords: herbal medicine, hot flash, menopause, alternative medicine
Introduction
Post-menopausal hormone therapy has well-documented positive effects on vasomotor symptoms, sleep quality and even sexual functioning. Yet many post-menopausal women are concerned about the FDA boxed warning regarding slight, but statistically significant, increased risk of substantial harms including dementia1,2 breast cancer,3,4,5 endometrial cancer6, venous thromboembolism7 and gallbladder disease.8
The 2010 North American Menopause Society position statement on postmenopausal hormone therapy addresses these concerns and, based on clinical data, supports use of hormone therapy around the time of menopause to treat significant symptoms or reduce significant risk of fractures.9 The Society notes potential therapeutic benefits and risks need to be considered. For symptomatic women concerned about potentially increased risk of atypical ductal hyperplasia,10 estrogen-receptor positive breast cancer,11 and node-positive breast cancer,12 there is great interest in non-hormonal options.
In Japan, more than 90% of Japanese gynecologists prescribe traditional Japanese multi-herb medicinal formulas for women’s health concerns including menopause.13,14 Termed Kampo, these formulas are regulated as prescription pharmaceutical drugs by the Japanese Ministry of Health and have been covered by the national health care plan for over 25 years.
The 1,800 year old formula termed Keishibukuryogan, whether known as TJ-25 (the Japanese identifier) or TU-025 (the United States identifier), is one of the foremost Kampo agents used for peri-menopausal hot flash management in Japan. Keishibukuryogan consists of Cinnamon bark (Cinnamomum cassia Blume), Peony root (Peonia lactiflora Palls), Peach kernel (Prunus persica Batsch), Poria sclerotium (Poria cocos Wolf) and Moutan bark (Peonia suffuticosa Andrews). The active ingredient (s) are not known. Synergistic interactions are presumed based on historical record and experience.
Kampo agents meet stringent Japanese standards for product manufacturing, consistency and stability. Post-marketing surveillance data obtained from the Japanese Ministry of Health and Welfare and Tsumura & Company demonstrated that TJ-25 used in Japan has an estimated adverse event rate of 0.00356% for the 128,435,263 doses provided to 701,832 unique patients treated for an average of 6 months over 9 years.
Studies conducted using state-of-the-art approaches have demonstrated no estrogenic activity by TJ-25. Noguchi and colleagues in Japan have developed an animal model of menopausal hot flashes focusing on calcitonin gene-related peptide (CGRP).15,16 In vivo interventions of TJ-25 result in equivalence of TJ-25 compared with estrogen in suppressing CGRP-induced hot flashes with no evidence of estrogenic activity.17 Additionally, in the same animal model, TJ-25 inhibits synthesis and release of CGRP.18 Given these mechanistic considerations, we conducted a randomized, double-blind, placebo-controlled, modified phase II trial which enrolled healthy post-menopausal American women to determine the impact of TU-25 on postmenopausal symptoms.
Methods
Design
The study was of twelve weeks duration plus a one week placebo lead-in period. Post menopausal was defined as 1) no menses for consecutive 12 months or 2) previous hysterectomy without oophorectomy and FSH levels ≥ 40 mIU/mL and estradiol (E2) levels ≤ 20 pg/mL or 3) two months past surgery for hysterectomy with oophorectomy. Inclusion Criteria included vasomotor symptoms documented by the Mayo Hot Flash Symptom Diary scoring system: mild = 1, moderate = 2, severe = 3, very severe = 4 for a total score of 28 or greater in a week (i.e. two moderately severe hot flashes/day = 14/week × 2 = 28 score or 4 mild/day = 4×7 = 28). Other criteria included willingness to take an herbal formula, no hormone therapy for eight weeks, smoker of < 10 cigarettes/day, BMI less than 36, Beck Depression Inventory of <11 and not currently taking antidepressant medication. Women with a history of breast or uterine cancer were excluded; however, for other cancers, individuals were eligible if they were cancer-free for five years.
No pharmacokinetic data exists on the multiple potentially active ingredients in keishibukuryogan. For this reason, regular dosing was defined as the dosage amount provided on a prescription basis to women in Japan (7.5 grams per day). To assess for a possible dose-response in larger sized American women, a higher-dose arm of 12.5 grams per day was included. Participants were block-randomized to receive one of three treatment protocols: 1) TU-025 at 7.5 g/day, 2) TU-025 at 12.5 g/day or, 3) Placebo. The product was given as five tablets taken twice a day for 12 weeks. Participants were seen monthly for compliance, side effects, laboratory monitoring and outcome measures. Tsumura & Co (Tokyo, Japan) provided product and placebo in capsule form. A separate research pharmacy service distributed placebo and active medication that were identical in appearance and odor. All participants took one-week of placebo after enrollment before active medication was provided.
The study was approved by the University of Minnesota Research Subjects’ Protection Program. All methodologies were reviewed and approved by the United States Food and Drug Administration under their Investigational New Drug (IND) policies. The study was registered at clinicaltrials.gov (NCT00119418).
Sample
From more than 2200 inquiries, 178 healthy postmenopausal women were recruited and enrolled from the Minneapolis, St. Paul metropolitan areal through newspaper, radio and television coverage.
Procedure
Subjects were randomized in blocks using a pseudo-random number that was used to assign participants as they were enrolled in the study to the placebo, low-dose or high-dose groups. The randomization schedule was sent to the research pharmacy to prepare containers for administration to the study participants. All investigators and research staff were blinded to group assignment. The randomization and all analyses were conducted using SAS System™ software.
Analysis followed intention to treat guidelines. Data were analyzed using general linear mixed model methods and generalized linear mixed model methods approach. 19, 20,21 The outcomes were analyzed as either a continuous variable or as a categorical variable when there were clinically accepted cut-points for the measurement. For the continuous variable outcomes (the hot flash scale score, the Greene Climacteric Index, and the Pittsburgh Sleep Quality Scale), we used the PROC MIXED procedure in the SAS System™ software. For categorical outcomes (hot flash scale score ≥ 28 points), the PROC GENMOD and GLIMMIX procedures were used.
For efficacy analysis, the average-daily hot-flash score was weighted based on the number of mild symptoms plus twice the number of moderate hot flashes plus three times the number of severe hot flashes plus four times the number of very severe hot flashes recorded in a given week divided by the number of days. For the primary efficacy analysis, changes in the hot flash score and the number of hot flashes were summarized as the change in percent from the baseline.
The type 3 test of fixed effect provided the F statistic, degrees of freedom, and p values for the effects of the treatment group, month, and treatment group by month. The difference of differences in the means was tested using the latter interaction term.
Statistical power analysis was based upon published data of Loprinzi et al on self-reported frequency and severity of hot flash symptoms with interventions.25 For the within group standard deviation for Composite Hot-flash Score, we used the standard error of the mean and sample size for the placebo group from the Paroxetine randomized controlled trial by Stearns and colleagues.22 Based on their data, we estimated the standard deviation for the Composite Hot-flash Score as 8.76. For statistical power, we assumed a 5% level of significance and a 90 % power for detecting a true group by level difference. We assumed 10% attrition in the study. Based on these assumptions, the study was adequately powered with 60 enrolled participants for each of the three study arms.
Measures
The study included three primary efficacy variables: (1) the frequency and severity of hot flash symptoms, (2) climacteric symptoms, and (3) sleep quality. The daily Mayo Hot Flash Symptom Diary was used for determining the frequency and severity of hot flashes. 23, 24 The daily diary was brought to each monthly visit. The score was aggregated and summarized as a daily average number of hot flashes and hot flash scored based on frequency and severity. Climacteric, or menopausal symptoms were summarized using the Greene Climacteric Index (GCI). 25,26 Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI). 27,28 For the eight PSQI scales, the overall sleep quality and overall indices were treated as primary outcomes. The GCI and PSQI questionnaires were completed in clinic at baseline, one, two and three months after enrollment. Adherence was evaluated by pill counts at each visit. Liver function tests and complete blood counts were obtained at each visit. Hormone measurements were obtained prior to enrollment and at the end of participation. After the last woman completed the study, all participants were contacted by phone and asked to guess their treatment group assignment.
Results
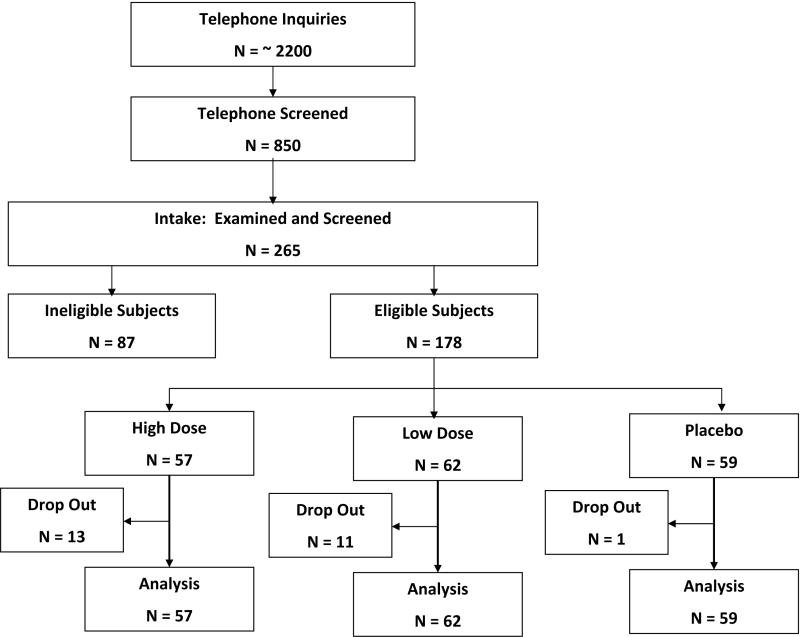
From the 265 women seen at intake and evaluated for inclusion in the study, the final study included 178 eligible women. (Figure 1) Eighty-seven individuals were excluded for on the following reasons in order of frequency: (1) Beck Depression Inventory > 11 (24/87); (2) an insufficient number of baseline hot flashes (20/87); (3) laboratory values out of range (15/87); (4) not in menopause (7/87); and (5) a BMI > 36 kg/meter2 (5/87).
Figure 1.
Disposition of Study Subjects.
Table 1 shows the demographic characteristics of the women enrolled. Participants were nearly evenly divided between the three groups. The groups were comparable with one another in terms of age, racial status, marital status, and educational level. None of the observed differences in characteristics were statistically significant across the study groups. On average, the enrolled women were in their early 50’s (53.6 years of age), white, married and well educated.
Table 1.
Demographic Characteristics of Subjects Enrolled in the TU-025 Hot Flash Management Study.
| Placebo (n = 59) | Low Dose (n = 62) | High Dose (n = 57) | Total (n = 178) | |
|---|---|---|---|---|
| Age* | 53.3 ± 0.38 | 53.7 ± 0.38 | 53.6 ± 0.49 | 53.3 ± 0.24 |
| White Race† | 57 (95.0) | 58 (93.6) | 54 (93.1) | 169 (93.9) |
| Marital Status† | ||||
| Married | 47 (78.3) | 47 (75.8) | 41 (70.7) | 135 (75.0) |
| Divorced | 6 (10.0) | 10 (16.1) | 10 (17.2) | 26 (14.4) |
| Widowed | 0 (0.0) | 0 (0.0) | 2 (3.5) | 2 (1.1) |
| Separated | 1 (1.7) | 0 (0.0) | 0 (0.0) | 1 (0.6) |
| Never married | 4 (6.7) | 2 (3.2) | 2 (3.5) | 8 (4.4) |
| Member unmarried couple | 2 (3.3) | 3 (4.8) | 3 (5.2) | 8 (4.4) |
| Level of Formal Education† | ||||
| High school graduate | 4 (6.7) | 10 (16.1) | 4 (6.9) | 18 (10.0) |
| Some college or technical | 19 (31.7) | 20 (32.3) | 21 (36.2) | 60 (33.3) |
| College graduate | 37 (61.7) | 32 (51.6) | 33 (56.9) | 102 (56.7) |
Values are mean and standard error of mean
Values are count and percent
Approximately 80 percent of the women experienced natural menopause (see Table 2). Based on medical histories obtained, one in nine of the women (12.4%) had had a bilateral oophorectomy. There were no statistically significant differences between the groups in terms of their medical history.
Table 2.
Baseline Menopausal History for Subjects Enrolled in the TU-025 Hot Flash Management Study.
| History of Menopause | Placebo (n = 59) | Low Dose (n = 62) | High Dose (n = 57) | Total (n = 178) |
|---|---|---|---|---|
| Natural Menopause | 46 (78.0) | 50 (80.7) | 44 (77.2) | 140 (78.7) |
| Hysterectomy Without Oophorectomy | 4 (6.8) | 5 (8.1) | 8 (8.8) | 14 (7.9) |
| Partial Oophorectomy | 0 (0.0) | 1 (1.6) | 1 (1.8) | 2 (1.1) |
| Oophorectomy | 9 (15.3) | 6 (9.7) | 7 (12.3) | 22 (12.4) |
Values are mean and standard error of mean
Values are count and percent
Table 3 shows core physical findings for these women at baseline. Overall, the majority of women subjects enrolled were either slightly overweight or obese. The mean body mass index (BMI) for these women was 26.1 kg/meter2. Between the intervention conditions, there were no statistically significant differences in these core physical findings at the time of intake.
Table 3.
Physical Findings at Baseline for Subjects Enrolled in the TU-025 Hot Flash Management Study.
| Placebo (n = 59) | Low Dose (n = 62) | High Dose (n = 57) | Total (n = 178) | |
|---|---|---|---|---|
| Anthropometric | ||||
| Measures* | ||||
| Height at Intake (cm) | 165.6 ± 0.8 | 163.9 ± 0.9 | 53.6 ± 0.5 | 165.3 ± 0.8 |
| Weight at Intake (KG) | 71.3 ± 1.5 | 70.9 ± 1.8 | 53.6 ± 0.5 | 71.5 ± 1.7 |
| Body Mass Index (KG/meter2) | 26.0 ± 0.5 | 26.3 ± | 53.6 ± 0.5 | 26.2 ± 0.6 |
| Obesity Classification† | ||||
| Underweight (< 18.5) | 0 (0.0) | 1 (1.6) | 0 (0.0) | 1 (0.6) |
| Normal Weight (18.5 – 24.9) | 25 (42.4) | 25 (40.3) | 27 (47.4) | 77 (43.3) |
| Overweight (25.0 – 29.9) | 26 (44.1) | 22 (35.5) | 18 (31.6) | 66 (37.1) |
| Obesity Class I (30.0 – 34.9) | 5 (8.5) | 11 (17.7) | 8 (14.0) | 24 (13.5) |
| Obesity Class II (35.0 <36 in this study) | 3 (4.8) | 3 (4.8) | 4 (7.0) | 10 (5.6) |
| Blood Pressure (mm Hg) * | ||||
| Systolic Blood Pressure | 123.6 ± 1.9 | 126.1 ± 2.0 | 53.6 ± 0.5 | 124.2 ± 1.8 |
| Diastolic Blood Pressure | 72.0 ± 1.1 | 71.3 ± 1.2 | 53.6 ± 0.5 | 70.7 ± 1.2 |
| Pulse Rate (beats per minute) * | 71.9 ± 1.4 | 69.3 ± 1.5 | 53.6 ± 0.5 | 71.9 ± 1.7 |
Values are mean and standard error of mean
Values are count and percent
Baseline estradiol and follicle stimulating hormone (FSH) levels are shown in Table 4. No statistically significant differences exist between the intervention conditions. Approximately, 3 in 5 women were found to have estradiol levels less than 21 pg/ml and 3 in 4 women had FHS levels greater than or equal to 60 IU/L. These levels are consistent with those found among women at menopause.
Table 4.
Baseline Hormone Levels for Subjects Enrolled in the TU-025 Hot Flash Management Study.
| Placebo (n = 59) | Low Dose (n = 62) | High Dose (n = 57) | Total (n = 178) | |
|---|---|---|---|---|
| Estradiol Levels | 22.9 ± 1.8 | 20.6 ± 2.2 | 21.4 ± 1.9 | 21.6 ± 1.2 |
| ≤ 20 | 34 (57.6) | 39 (62.9) | 34 (59.7) | 107 (60.1) |
| 21 – 50 | 21 (35.6) | 22 (35.5) | 21 (35.6) | 64 (36.0) |
| ≥ 50 | 4 (6.8) | 1 (1.2) | 2 (3.5) | 7 (3.9) |
| Follicle Stimulating Hormone | 81.5 ± 3.5 | 79.1 ± 3.2 | 79.5 ± 3.2 | 80.0 ± 1.9 |
| ≤ 39 | 1 (1.7) | 2 (3.2) | 1 (1.8) | 4 (2.3) |
| 40 – 60 | 12 (20.3) | 14 (22.6) | 11 (19.6) | 37 (20.9) |
| ≥ 60 | 46 (78.0) | 46 (74.2) | 44 (78.6) | 136 (76.8) |
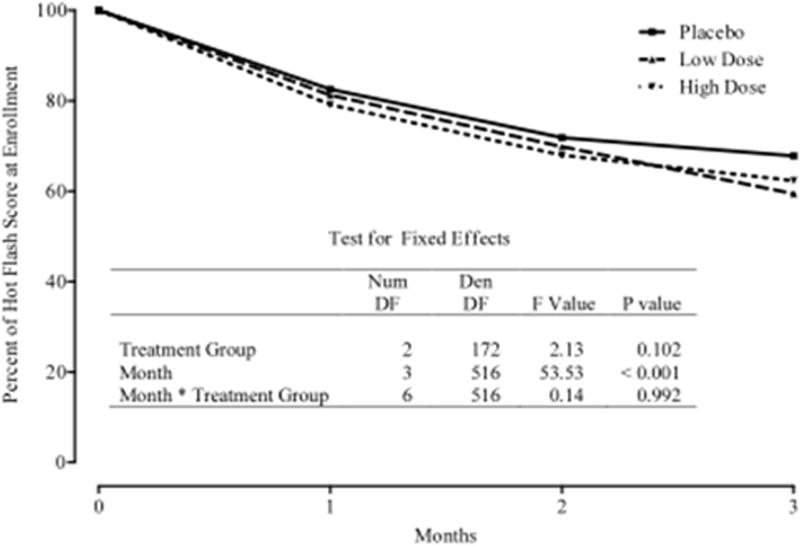
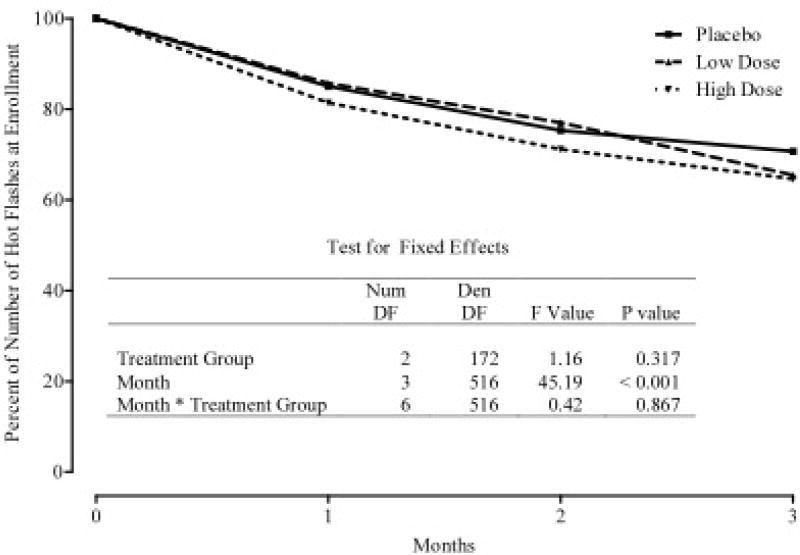
Figures 2 and 3 summarize the mean Hot Flash diary scores at baseline, 1-month, 2-months, and 3-months. Figure 2 depicts the change and percentage change in average monthly hot flash scores. Figure 3 depicts the change and percentage change in numbers of hot flashes at monthly visits. At three months, the results from the hot flash scores showed a decrease in all three groups; 34% in the placebo group, 40% in the 7.5 g/day group and 38% in the 12.5 g/day group. While these declines in mean scores were significant (p < 0.001) within each group, the differences between groups were not statistically significant (p=0.990)
Figure 2.
Percent of Hot Flash Score at Enrollment According to Treatment Group and Month Following Enrollment.
Figure 3.
Percent of Number of Hot Flashes at Enrollment According to Treatment and Month Following Enrollment.
The mean scores for the Greene Climacteric Index, which includes Depression, Anxiety, Psychosocial, Somatic, Vasomotor, and Sexual Dysfunction Indexes declined significantly across the study (all p values < 0.001). However, no statistically significant treatment group by month interaction was observed.
The Pittsburgh Sleep Quality Index and its associated sub-scales demonstrated a statistically significant attenuation in all scores for all participants across the study (all p values < 0.001) except for sleep medication use, which remained constant across the study. The Beck Depression Inventory summarized at intake and conclusion demonstrated some improvement in mood (p = 0.044) with no significant differences between treatment groups. (data not shown)
Twenty-five women (14.0%) withdrew before completing the 12 week study. There were no statistically significant differences in the likelihoods of withdrawing across the treatment conditions. (Chi-square=4.12; DF=2; p=0.127). The primary reason for withdrawal was diarrhea (15/25) followed by persistent hot flashes (6/25). Diarrhea was present in approximately 20% of participants receiving active medication. (Table 5) This diarrhea was statistically significantly more prevalent in the Low-Dose and High-Dose groups compared with the Placebo group. (Chi-square=12.05; DF=2; p=0.002)
Table 5.
Prevalence of Unanticipated Diarrhea (Adverse Event) among the 178 Women Subjects Enrolled in the Hot Flash Study.
| Frequency | Placebo (n = 59) | Low Dose (n = 62) | High Dose (n = 57) | Total (n = 178) | ||||
|---|---|---|---|---|---|---|---|---|
| No | 58 | 98.3 % | 48 | 77.4 % | 46 | 80.7 % | 152 | 85.4 % |
| Yes | 1 | 1.7 % | 14 | 22.6 % | 11 | 19.3 % | 26 | 14.6 % |
Discussion
Despite supportive animal model data in peer-reviewed literature as well as the widespread use of prescription TJ-25 in Japan for peri-menopausal women, this rigorous randomized controlled trial failed to demonstrate efficacy of TU-025 beyond placebo for reducing the severity and frequency of hot flash symptoms, climacteric symptoms or disrupted sleep symptoms in post-menopausal American women. Failure to find a treatment benefit was unlikely related to breaches in study protocol, subject adherence, statistical power and length of follow-up.
Despite use of a one-week placebo lead-in period to minimize enrollment of placebo responders, and despite the additional 12 weeks of study duration, clinically significant reductions in hot flash severity and frequency were experienced in a significant portion of the subjects receiving placebo. The 34% rate of placebo response is consistent with that reported in other clinical trials. However, the placebo effect was expected to resolve before 12 weeks of therapy. Future herbal medicine trials should measure participant expectations at the time of enrollment. In this study, several participants noted afterward that they enrolled to “prove” herbal medicines work. This suggests the presence of a significant meaning response.29
Unlike the study of a single Western herb with candidate marker ingredients for monitoring serum levels, the translation of a unique healing system, such as Kampo diagnoses and therapeutics, into a Western model is faced with limitations that may have not been appreciated. Despite the strengths of the study, three methodologic concerns are important to recognize for future trials of a products taken out of their whole-systems context.
The first concern is the selection of the study population. Following FDA guidelines, we restricted entrance into this study to women greater than one year since the cessation of menses. This meant that many women who requested enrollment were excluded. Traditional Kampo practitioners do not include a women’s menstrual status in formula decisions. Additionally, women with depressed mood as determined by a Beck Depression Inventory scores (>11) were turned away from the study and requested to work with a physician regarding the possibility of depression. The reasoning was that subjects at risk for significant depression should not be enrolled when proven effective anti-depressant therapy that also addressed hot flashes was already available. We did not realize that nearly 100% of these women refused to consider prescription anti-depressant therapy and attributed their depressive symptoms to severe life disruption by hot flashes/night sweats. Exclusions due to menopausal status or an elevated depression score may have introduced bias in enrollment.
Second, inclusion criteria did not consider traditional Kampo assessments of each subject’s sho or constitutional state. Although completely contrary to Kampo practice, every woman who met the FDA entrance criteria was enrolled in the study. Per Kampo tradition, keishibukuryogan is best matched to women with hiesho (subjective coldness) and ouketsu (metaphoric blood stagnation). Women would a qi stagnation pattern, for example, would be more likely to respond to another commonly used formula, kamishoyosan. The substantially more drop-outs in the treated rather than the placebo group suggests a problem existed with the treatment itself in American women. The prominent diarrhea noted by 20% of American women receiving TU-025 at approximately 6 weeks of treatment is consistent with a constitutional state and formula mismatch.
A third concern is appropriate dosing of the product. TU-025, keishibukuryogan, does not have active marker ingredients for monitoring. In the absence of pharmacokinetic data and monitoring capacities, no rational guidance exists for determining total dose and dose frequency required for a non-Japanese population. Dosing for this study was based on extrapolation from Japanese tradition adopted for American women with distinct preference for twice a day pill dosing and a higher mean body mass index. Although a higher total dose was used in one arm, the dose may not have been sufficient or sufficiently frequent to effect a change.
Future trials of traditional Asian medicines will need to consider these three concerns. Most importantly, however, for future trials, Watanabe et al have recently proposed two innovative methodologies that would include use of both Western and Kampo diagnoses with randomization and placebo controls.30 These new methodologies may not only meet FDA requirements for generalizable inclusion criteria but would match traditional clinical assessments and treatments. Future studies should be developed on one of these templates. Additionally, since pharmacokinetic data cannot be developed on formulas without a known, single active ingredient, smaller preliminary dose-escalation trials may be necessary to develop rational dosing guidelines.
Conclusion
Unlike clinical experience in Japan, for American women, use of a standardized 1800 year-old traditional Japanese multiherb forumula, termed kesihibukuryogan or TU-025, did not significantly reduce the frequency and severity of hot flash symptoms, improve climacteric symptoms or benefit sleep quality. Also unlike clinical experience in Japan, significant diarrhea developed in 20% of participants receiving the active agent. Despite the use of a one-week placebo lead-in to minimize placebo response, the placebo agent significantly reduced hot flash severity and frequency. This study identified several potentially significant methodological factors unique to the scientific study of a traditional herbal formula to be considered in future assessments of traditional Asian medicines.
Acknowledgments
Funding: Tsumura & Co, National Institutes of Health grant M01-RR00400
Drs. Plotnikoff and Watanabe have received funding from Tsumura & Company for research, presentations and consultations. Drs. Plotnikoff and Watanabe have received honorariums from Tsumura & Company for speaking engagements.
The authors want to highlight the significant contributions by Douglas Yee, MD, study directors Lisa Morley and Emily Lindell, study nurse Deb Spindler, RN, the staff at the University of Minnesota General Clinical Research Center, the staff at the Fairview-University Research Pharmacy and the members of the Keio University Center for Kampo Medicine. The study team deeply appreciates the technical and financial support by Tsumura & Company, Tokyo, Japan. This trial was supported in part by NIH grant M01-RR00400.
Footnotes
The other authors report no conflicts of interest or need for any disclosures.
ClinicalTrials.gov identifier NCT00119418
References
- 1.Rapp SR, Espeland MA, Shumaker SA, Henderson VW, Brunner P, Manson JE, et al. Effect of estrogen plus progestin on global cognitive function in postmenopausal women: the Women’s Health Inititive Memory Study: a randomized controlled trial. JAMA. 2003;289:2663–72. doi: 10.1001/jama.289.20.2663. [DOI] [PubMed] [Google Scholar]
- 2.Shumaker SA, Legault C, Rapp SR, Thai L, Wallace RB, Ockene J, et al. Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA. 2003;289:2651–62. doi: 10.1001/jama.289.20.2651. [DOI] [PubMed] [Google Scholar]
- 3.Chelbowski RT, Hendrix SL, Langer RD, et al. Influence of estrogen plus progestin on breast cancer and mammography in healthy postmenopausal women: the Womeh’s Health Initiative Randomized Trial. JAMA. 2003;289:3242–53. doi: 10.1001/jama.289.24.3243. [DOI] [PubMed] [Google Scholar]
- 4.Li CI, Malone KE, Porter PL, et al. Relationship between long durations and different regimes of hormone therapy and risk of breast cancer. JAMA. 2003;289:3254–63. doi: 10.1001/jama.289.24.3254. [DOI] [PubMed] [Google Scholar]
- 5.Beral V Million Women Study Coordinators. Breast cancer and hormone-replacement therapy in the Million Women Study. Lancet. 2003;362:419–27. doi: 10.1016/s0140-6736(03)14065-2. [DOI] [PubMed] [Google Scholar]
- 6.Grady D, Gebrestsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta-analysis. Obstet Gyencol. 1995;85:304–13. doi: 10.1016/0029-7844(94)00383-O. [DOI] [PubMed] [Google Scholar]
- 7.Miller J, Chan BK, Nelson HD. Postmenopausal estrogen replacement and risk for venous thromboembolism: a systematic review and meta-analysis for the U.S. Preventive Services Task Force. Ann Intern Med. 2002;136:680–90. doi: 10.7326/0003-4819-136-9-200205070-00011. [DOI] [PubMed] [Google Scholar]
- 8.Hulley S, Furberg C, Barrett-Connor F, Cauley J, Grady D, Haskell W, et al. Noncardiovascular disease outcomes during 6.8 years of hormone therapy. HERS II. JAMA. 2002;288:58–66. doi: 10.1001/jama.288.1.58. [DOI] [PubMed] [Google Scholar]
- 9.North American Menopause Society. Estrogen and progestogen use in postmenopausal women: 2010 position statement of the The North American Menopause Society. Menopause. 2010;17:242–55. doi: 10.1097/gme.0b013e3181d0f6b9. [DOI] [PubMed] [Google Scholar]
- 10.Menes TS, Kerlikowske K, Jaffer S, Seger D, Milioretti DL. Rates of atypical ductal hyperplasia have declined with less us of postmenopausal hormone treatment: finding from the Breast Cancer Surveillance Consortium. Cancer Epidemiol Biomarkers Prev. 2009;18:2822–8. doi: 10.1158/1055-9965.EPI-09-0745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jemal A, Ward E, Thun MJ. Recent trends in breast cancer incidence rates by age and tumor characterizstics among U.S. women. Breast Cancer Res. 2007;9 (3):R28. doi: 10.1186/bcr1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chlebowski RT, Anderson GL, Gass M, et al. Estrogen plus progestin and breast cancer incidence and mortality in postmenopausal women. JAMA. 2010;304:1684–92. doi: 10.1001/jama.2010.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ishibashi A, Kosoto H, Ohno S, et al. The Japanese Society of Oriental Medicine. Introduction to Kampo, Japanese Traditional Medicine. Elsevier; Tokyo: 2005. General Introduction to Kampo; p. 2. [Google Scholar]
- 14.Nikkei Medical. Utilization survey of Kampo medicines. 2007;10(Suppl):41–7. [Google Scholar]
- 15.Noguchi M, Yuzurihara M, Ikarashi Y. Effects of the vasoactive neuropeptides aclcitonin gene-related peptide, substance P and vasoactive intestinal peptide on skin temperature in ovariectomized rats. Neuropeptides. 2002;36:327–32. doi: 10.1016/s0143-4179(02)00090-2. [DOI] [PubMed] [Google Scholar]
- 16.Noguchi M, Ikarashi Y, Yuzurihara M, et al. Up-regulation of calcitonin gene-related peptide receptors underlying elevation of skin temperature in ovariectomized rats. J Endocrinol. 2002;175:177–83. doi: 10.1677/joe.0.1750177. [DOI] [PubMed] [Google Scholar]
- 17.Noguchi M, Ikarashi Y, Yuzirihara M, et al. Effects of the Japanese herbal medicine Keishi-bukuryo-gan and 17β estradiol on calcitonin gene-related peptide-induced elevation of skin temperature in ovariectomized rats. J Endocrinol. 2003;176:359–66. doi: 10.1677/joe.0.1760359. [DOI] [PubMed] [Google Scholar]
- 18.Noguchi M, Ikarashi Y, Yuzurihara M, et al. Effects of 17-β-estradiol and the Japanese herbal medicine Keishi bukuryo-gan on the release and synthesis of calcitonin gene-related peptide in ovariectomized rats. J Pharmacol Sci. 2003;93:80–6. doi: 10.1254/jphs.93.80. [DOI] [PubMed] [Google Scholar]
- 19.Brown H, Prescott R. Applied Mixed Models in Medicine. Chichester, United Kingdom: John Wiley & Sons; 1999. [Google Scholar]
- 20.Dobson AJ. An Introduction to Generalized Linear Models. 2. Boca Raton, FL: Chapman & Hall/CRC; 2002. [Google Scholar]
- 21.Stokes ME, Davis CS, Koch GG. Categorical Data Analysis Using the SAS System. Cary, NC: SAS Institute Inc; 2000. [Google Scholar]
- 22.Stearns V, Beebe KL, Iyengar M, Dube E. Paroxetine controlled release n the treatment of menopausal hot flashes: a randomized controlled trial. JAMA. 2003 Jun 4;289(21):2827–34. doi: 10.1001/jama.289.21.2827. [DOI] [PubMed] [Google Scholar]
- 23.Sloan JA, Loprinzi CL, Novotny PJ, Barton DL, Lavasseur BI, Windschitl H. Methologic lessons learned from hot flash studies. J Clin Oncol. 2001;19 (23):4280–90. doi: 10.1200/JCO.2001.19.23.4280. [DOI] [PubMed] [Google Scholar]
- 24.Loprinzi CL, Kugler JW, Sloan JA, Mailliard JA, LaVasseur BI, Barton DL, et al. Venlaxafine in management of hot flashes in survivors of breast cancer: a randomised controlled trial. Lancet. 2000;356 (9247):2059–63. doi: 10.1016/S0140-6736(00)03403-6. [DOI] [PubMed] [Google Scholar]
- 25.Greene JG. A factor analytic study of climacteric symptoms. Journal of Psychomatic Medicine. 1976;20:425–430. doi: 10.1016/0022-3999(76)90005-2. [DOI] [PubMed] [Google Scholar]
- 26.Greene JG. Constructing a standard climacteric scale. Mauritas. 1998;29:25–31. doi: 10.1016/s0378-5122(98)00025-5. [DOI] [PubMed] [Google Scholar]
- 27.Buysee DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Research. 1998;28 (2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 28.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. Jounal of Psychosomatic Resarch. 1998;45 (1):5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 29.Moerman D. Meaning, Medicine and the ‘Placebo Effect’. Cambridge: Cambridge UniversityPress; 2005. [Google Scholar]
- 30.Watanabe K, Matsuura K, Gao P, et al. Traditional Japanese Kampo medicine: clinical research between modernity and traditional medicine — the state of research and methodological suggestions for the future. [accessed June 16, 2010];eCAM. 2010 doi: 10.1093/ecam/neq067. [DOI] [PMC free article] [PubMed] [Google Scholar]