Abstract
In rats expression of the Nav1.7 voltage-gated sodium channel isoform is restricted to the peripheral nervous system and is abundant in the sensory neurons of the dorsal root ganglion. We expressed the rat Nav1.7 sodium channel α subunit together with the rat auxiliary β1 and β2 subunits in Xenopus laevis oocytes and assessed the effects of the pyrethroid insecticide tefluthrin on the expressed currents using the two-electrode voltage clamp method. Tefluthrin at 100 µM modified of Nav1.7 channels to prolong inactivation of the peak current during a depolarizing pulse, resulting in a marked "late current" at the end of a 40-ms depolarization, and induced a sodium tail current following repolarization. Tefluthrin modification was enhanced up to two-fold by the application of a train of up to 100 5-ms depolarizing prepulses. These effects of tefluthrin on Nav1.7 channels were qualitatively similar to its effects on rat Nav1.2, Nav1.3 and Nav1.6 channels assayed previously under identical conditions. However, Nav1.7 sodium channels were distinguished by their low sensitivity to modification by tefluthrin, especially compared to Nav1.3 and Nav1.6 channels. It is likely that Nav1.7 channels contribute significantly to the tetrodotoxin-sensitive, pyrethroid-resistant current found in cultured dorsal root ganglion neurons. We aligned the complete amino acid sequences of four pyrethroid-sensitive isoforms (house fly Vssc1; rat Nav1.3, Nav1.6 and Nav1.8) and two pyrethroid-resistant isoforms (rat Nav1.2 and Nav1.7) and found only a single site, located in transmembrane segment 6 of homology domain I, at which the amino acid sequence was conserved among all four sensitive isoform sequences but differed in the two resistant isoform sequences. This position, corresponding to Val410 of the house fly Vssc1 sequence, also aligns with sites of multiple amino acid substitutions identified in the sodium channel sequences of pyrethroid-resistant insect populations. These results implicate this single amino acid polymorphism in transmembrane segment 6 of sodium channel homology domain I as a determinant of the differential pyrethroid sensitivity of rat sodium channel isoforms.
Keywords: voltage-gated sodium channel, Nav1.7 isoform, pyrethroid, tefluthrin, peripheral nervous system, dorsal root ganglion
1. Introduction
Actions on voltage-gated sodium channels are intrinsic to both the insecticidal effects of pyrethroids and their neurotoxic effects in mammals [1]. Pyrethroids disrupt nerve function by altering the kinetics of the transitions between conducting (open) and nonconducting (closed; inactivated) states of voltage-gated sodium channels that underlie the generation of nerve action potentials [2]. Pyrethroid modification causes persistent channel opening, which in turn causes repetitive nerve firing following single stimuli or use-dependent conduction block depending on the duration of the open time of the pyrethroid-modified channel.
Native sodium channels in mammalian tissues are heteromultimeric complexes comprised of one large (~260 kDa) α subunit and either one or two smaller (33–36 kDa) auxiliary β subunits [3,4]. Sodium channel α subunits form the ion pore and confer the fundamental functional and pharmacological properties of the channel [3]. Sodium channel β subunits modulate channel function, regulate channel expression at the level of the plasma membrane, and contribute to cell adhesion and cell-cell communication [4].
Mammalian genomes contain nine genes encoding sodium channel α subunit isoforms, designated Nav1.1 – Nav1.9 [5,6], and four genes encoding sodium channel β subunits, designated β1 – β4 [4]. Overlapping patterns of sodium channel α subunit expression in the central and peripheral neurons [7,8] limit the utility of native neuronal preparations to identify isoform-dependent differences in pharmacology. This limitation can be overcome by the heterologous expression of cloned individual sodium channel isoforms in the unfertilized oocytes of the frog Xenopus laevis. This system has been employed to assess the pyrethroid sensitivity of four rat sodium channel isoforms. Nav1.2 sodium channels exhibit very low sensitivity to modification by deltamethrin and other pyrethroids [9,10]. By contrast rat Nav1.3, Nav1.6 and Nav1.8 channels are more sensitive to pyrethroid modification [11–16].
The dorsal root ganglion (DRG), a cluster of cell bodies of afferent sensory nerves, has been widely used to study the electrical properties of mammalian peripheral neurons [17]. Electrophysiological studies with cultured DRG neurons have identified two populations of sodium channels that are distinguished by their gating properties and their sensitivity to block by tetrodotoxin (TTX) [18]. The TTX-resistant channel population is sensitive to pyrethroid modification, whereas the TTX-sensitive population is relatively insensitive to pyrethroids [19,20]. The TTX-resistant sodium current has been correlated with expression of the Nav1.8 isoform, but the coordinate expression of multiple TTX-sensitive isoforms within individual DRG cells [17] complicates the identification of the molecular basis of the TTX-sensitive current that is resistant to pyrethroids.
The Nav1.7 sodium channel isoform (formerly designated PN1) was first identified by cDNA cloning from peripheral nervous tissue [21,22] and is abundantly expressed in the dorsal root ganglion [17]. Here we describe the action of the potent pyrethroid insecticide tefluthrin on the rat the Nav1.7 sodium channel α subunit isoform coexpressed with the rat β1 and β2 subunits in Xenopus oocytes. Our results identify Nav1.7 as a pyrethroid-resistant isoform that is likely to contribute to the TTX-sensitive, pyrethroid-resistant sodium current of DRG neurons.
2. Materials and Methods
2.1 Expression in oocytes
The rat Nav1.7 voltage-gated sodium channel α subunit cDNA was provided by G. Mandel (State University of New York, Stony Brook, NY) and the β1 and β2 subunit cDNAs were provided by W.A. Catterall (University of Washington, Seattle, WA). Plasmid cDNAs were digested with restriction enzymes to provide linear templates for cRNA synthesis in vitro using a commercial kit (mMessage mMachine, Ambion, Austin, TX). The integrity of synthesized cRNA was determined by electrophoresis in 1% (w/v) agarose – formaldehyde gels.
Stage V–VI oocytes were removed from female X. laevis frogs (Nasco, Ft. Atkinson, WI) as described elsewhere [12]. This procedure was performed in accordance with National Institutes of Health guidelines and followed a protocol that was approved by the Cornell University Institutional Animal Care and Use Committee. Each data set was derived from oocytes isolated from at least two different frogs. Oocytes were injected with a 1:1:1 (mass ratio) mixtures of α subunit, β1 subunit and β2 subunit cRNAs (0.5 – 5 ng/oocyte); this mixture provided a ~9-fold molar excess of β1 and β2 cRNAs to ensure the preferential expression of the desired ternary α+β complex [23]. Injected oocytes were incubated in ND-96 medium (in mM: 96 NaCl, 2 KCl, 1.8 CaCl2, 1 MgCl2, and 5 HEPES; adjusted to pH 7.6 at room temperature with NaOH) supplemented with 5% horse serum (Sigma-Aldrich), 1% streptomycin/penicillin, and 1% sodium pyruvate [24] at 19°C for 3–5 days until electrophysiological analysis of sodium currents.
2.2 Electrophysiology
Sodium currents were recorded from oocytes perfused with ND-96 at 21–23°C in the two-electrode voltage clamp configuration using an Axon Geneclamp 500B amplifier (Molecular Devices, Foster City, CA). Microelectrodes were pulled from borosilicate glass capillary tubes (1.0 mm O.D.; 0.5 mm I.D.; World Precision Instruments Inc., Sarasota, FL) and filled with 3 M KCl. Filled electrodes had resistances of 0.7–1.5 MΩ when immersed in ND-96 medium. Currents were filtered at 2 kHz with a low-pass 4-pole Bessel filter and digitized at 50 kHz (Digidata 1320A; Molecular Devices). To determine the voltage dependence of activation, oocytes were clamped at a membrane potential of −100 mV and currents were measured during a 40-ms depolarizing test pulse to potentials from −60 mV to 20 mV in 5-mV increments. Maximal peak transient currents were obtained upon depolarization to −10 mV. To determine the voltage dependence of steady-state inactivation, oocytes were clamped at a membrane potential of −140 mV followed by a 100-ms conditioning prepulse to potentials from −100 mV to 5 mV in 5-mV increments and then a 40-ms test pulse to −10 mV. For determinations of use dependence, oocytes were given trains of 1 to 100 5-ms conditioning prepulses to 10 mV, separated by a 10-ms interpulse interval at the holding potential, followed by a 40-ms test pulse to −10 mV. All experiments employed 10-s intervals between pulses or pulse trains to permit complete recovery from tefluthrin modification. Capacitive transients and leak currents were subtracted using the P/4 method [25].
2.3 Assays with tefluthrin
Tefluthrin (98.8% purity; Syngenta, Bracknell, Berks., UK) was prepared as a stock solution in dimethyl sulfoxide (DMSO) and diluted with ND-96 immediately before use to a final concentration of 100 µM. The final DMSO concentration in the bath did not exceed 0.1%, a concentration that had no effect on sodium currents. Oocytes were perfused at 0.45 ml/min with tefluthrin in ND-96 for 6 min and then with ND-96 for 3 min prior to recording. Washout of tefluthrin during perfusion with ND-96 for up to 30 min was negligible under these conditions. All experiments with pyrethroids employed a disposable capillary perfusion system [20] and custom-fabricated single-use recording chambers [12] to prevent cross-contamination between oocytes.
2.4 Data analysis
Data were acquired and analyzed using pClamp 10.2 (Molecular Devices, Burlingame, CA) and Origin 8.0 (OriginLab Corp., Northampton, MA). The Boltzmann equation [y = (A1 − A2) / (1+e(x−x0)/dx) + A2] was used to fit conductance-voltage and sodium current inactivation data. Time constants for fast sodium channel inactivation were obtained by using the Chebyshev method in Origin 7.0 to fit the falling phase of the peak transient current to a double exponential decay model. Similarly, time constants for pyrethroid-induced sodium tail currents were obtained from fits of currents measured following 40-ms step depolarizations from −100 mV to −10 mV to a double exponential decay model. The conductance of the pyrethroid-induced sodium tail current, extrapolated to the moment of repolarization and normalized to the conductance of the peak current measured in the same oocyte in the absence of pyrethroid, was employed to calculate the fraction of sodium channels modified by each compound in each experiment as described previously [20]. Statistical analyses were performed using the Prism software package (GraphPad Software, La Jolla, CA).
3. Results
3.1 Expression, Gating and Kinetics of Nav1.7 Sodium Channels
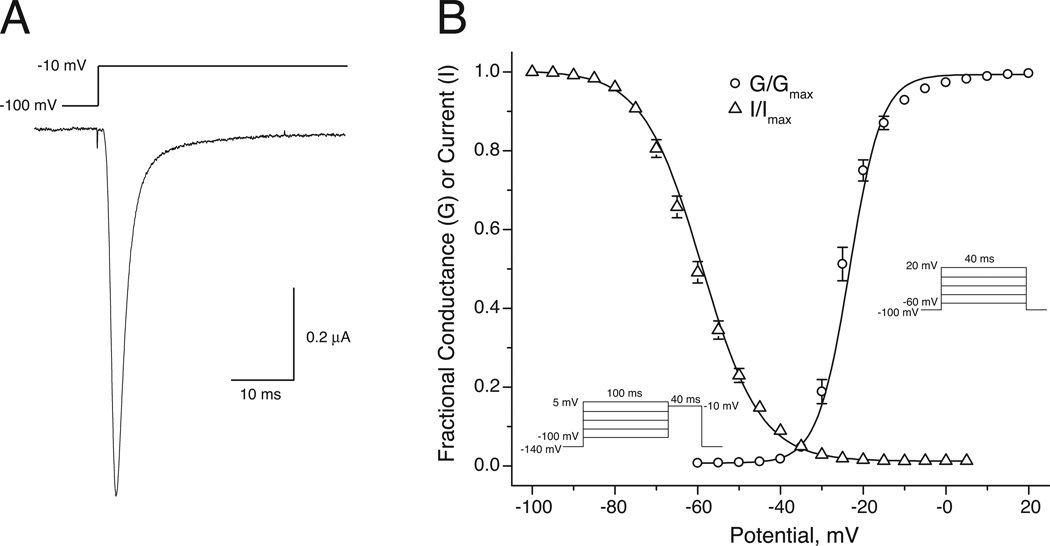
We expressed the Nav1.7 α subunit in combination with the β1 and β2 auxiliary subunits to give channel complexes of comparable subunit composition to those employed in our previous studies of other rat sodium channel isoforms [15,16]. Figure 1A shows a typical sodium current recorded from an oocyte expressing Nav1.7 channels. These currents activated and inactivated within 10 ms of depolarization under conditions that produced maximal peak transient currents. The voltage dependent gating of Nav1.7 channels is illustrated in Fig. 1B and summarized in Table 1.
Fig. 1.
Properties of Nav1.7 sodium channels expressed in Xenopus oocytes. (A) Representative current trace recorded during a 40-ms step depolarization from −100 mV to −10 mV. (B) Voltage dependence of activation and steady-state inactivation. For activation, normalized conductance (G/Gmax) was derived from the current-voltage relationship obtained using the indicated pulse protocol by dividing peak transient current (INa) by the driving force (V − Vrev) and normalizing to the maximum conductance observed in each cell. For inactivation, peak transient currents were measured using the indicated pulse protocol and normalized to the maximal peak transient current in each experiment (I/Imax). Values for G/Gmax and I/Imax were plotted as a function of test (activation) or prepulse (inactivation) potential and curves were drawn by fitting mean values to the Boltzmann equation. Each data point is the mean of 17 (activation) or 15 (steady-state inactivation) determinations with different cells; bars show SE values larger than the data point symbols.
Table 1.
Effects of tefluthrin on the voltage dependence of activation and steady-state inactivation of rat Nav1.7 sodium channels expressed in Xenopus oocytes.a
| Activation | Inactivation | |||||
|---|---|---|---|---|---|---|
| Condition | V0.5 | K | n | V0.5 | K | n |
| Control | −24.5 ± 0.7 | 3.96 ± 0.24 | 17 | −59.9 ± 0.9 | 7.03 ± 0.15 | 15 |
| +Tefluthrinb | −24.2 ± 1.4 | 3.24 ± 0.19 | 7 | −58.4 ± 1.1 | 6.82 ± 0.21 | 8 |
Values calculated from fits of the data from the indicated number of individual experiments to the Boltzmann equation; V0.5, midpoint potential (mV) for voltage-dependent activation or inactivation; K, slope factor.
Values obtained in the presence of tefluthrin were not significantly different from control values (unpaired t-tests, P > 0.05).
3.2 Resting modification by tefluthrin
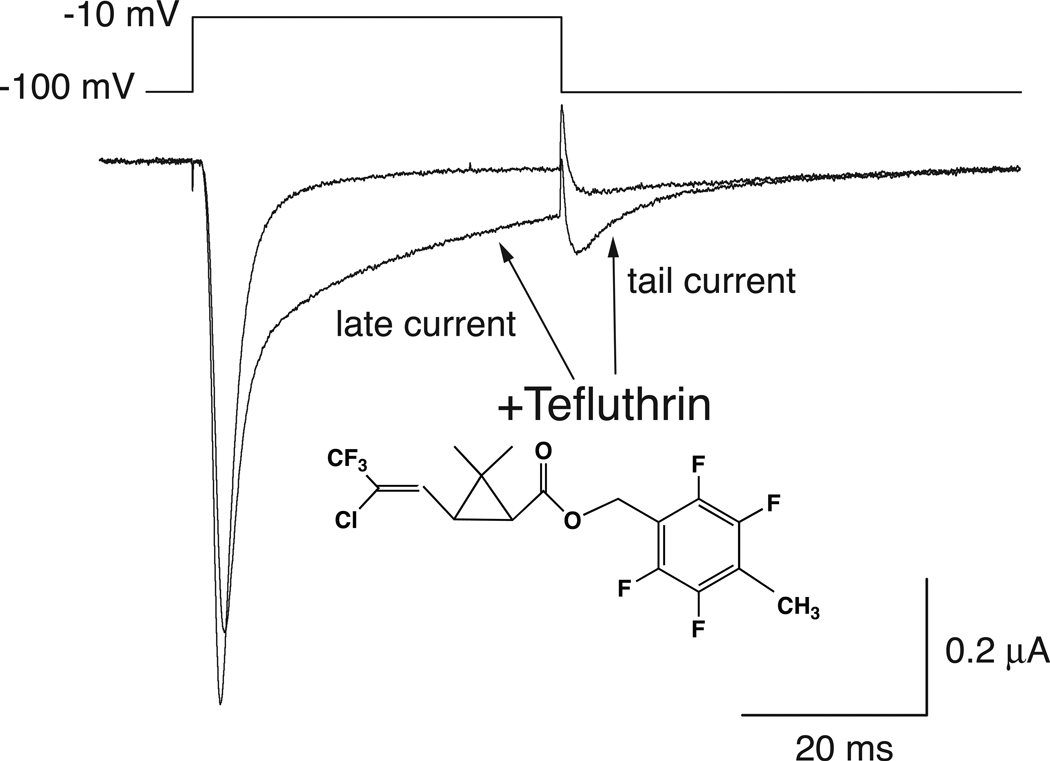
We employed 100 µM tefluthrin, the highest concentration achievable in ND-96 perfusion medium, to maximize the extent of sodium channel modification by this pyrethroid. We assessed the modification of channels by tefluthrin in the resting state by holding the oocyte membrane at a hyperpolarized potential during perfusion and measuring the pyrethroid-modified current during the first depolarizing pulse. Figure 2 show representative currents recorded from a single oocyte expressing Nav1.7 channels before and after exposure to 100 µM tefluthrin. Tefluthrin modification was evident in the prolonged and incomplete inactivation of the peak current during a depolarizing pulse, resulting in a marked "late current" at the end of a 40-ms depolarization, and in the induction of a sodium tail current following repolarization.
Fig. 2.
Representative control and tefluthrin (100 µM)-modified sodium currents recorded from an oocyte expressing rat Nav1.7 sodium channels using the indicated depolarization protocol.
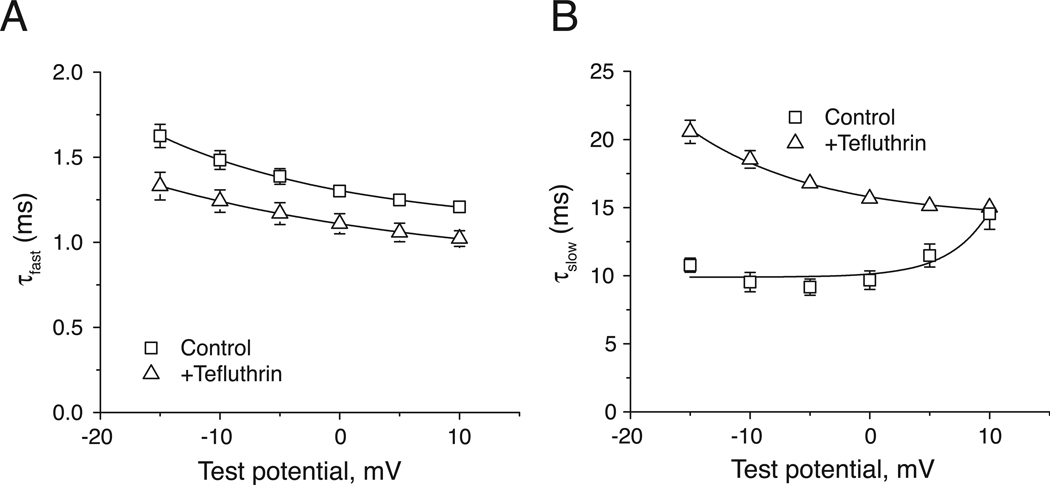
The decay of peak transient currents was fitted best by a two-component, single exponential decay model that yielded first-order decay constants for the fast initial phase (τfast) and slower secondary phase (τslow) of inactivation. Figure 3 shows the effect of tefluthrin on the kinetic components of fast inactivation at test potentials near those producing maximal peak transient currents. Tefluthrin slightly accelerated τfast at all potentials (Fig. 3A) but retarded τslow (Fig. 3B).
Fig. 3.
Effects of tefluthrin on the fast inactivation kinetics of Nav1.7 sodium channels. (A) Effects of tefluthrin (100 µM) on the first-order time constants for the fast component of inactivation (τfast) of Nav1.7 sodium channels obtained from currents recorded during 40-ms depolarizations from −100 mV to the indicated test potential (Vt); values are means ± SE of the indicated number of separate experiments with different oocytes. Each data point is the mean of 17 (control) or 7 (+tefluthrin) determinations with different cells; bars show SE values larger than the data point symbols. (B) Effects of tefluthrin (100 µM) on the first-order time constants for the slow component of inactivation (τslow) of Nav1.7 sodium channels obtained from currents recorded during 40-ms depolarizations from −100 mV to the indicated test potential (Vt); values are means ± SE of the indicated number of separate experiments with different oocytes. Each data point is the mean of 17 (control) or 7 (+tefluthrin) determinations with different cells; bars show SE values larger than the data point symbols.
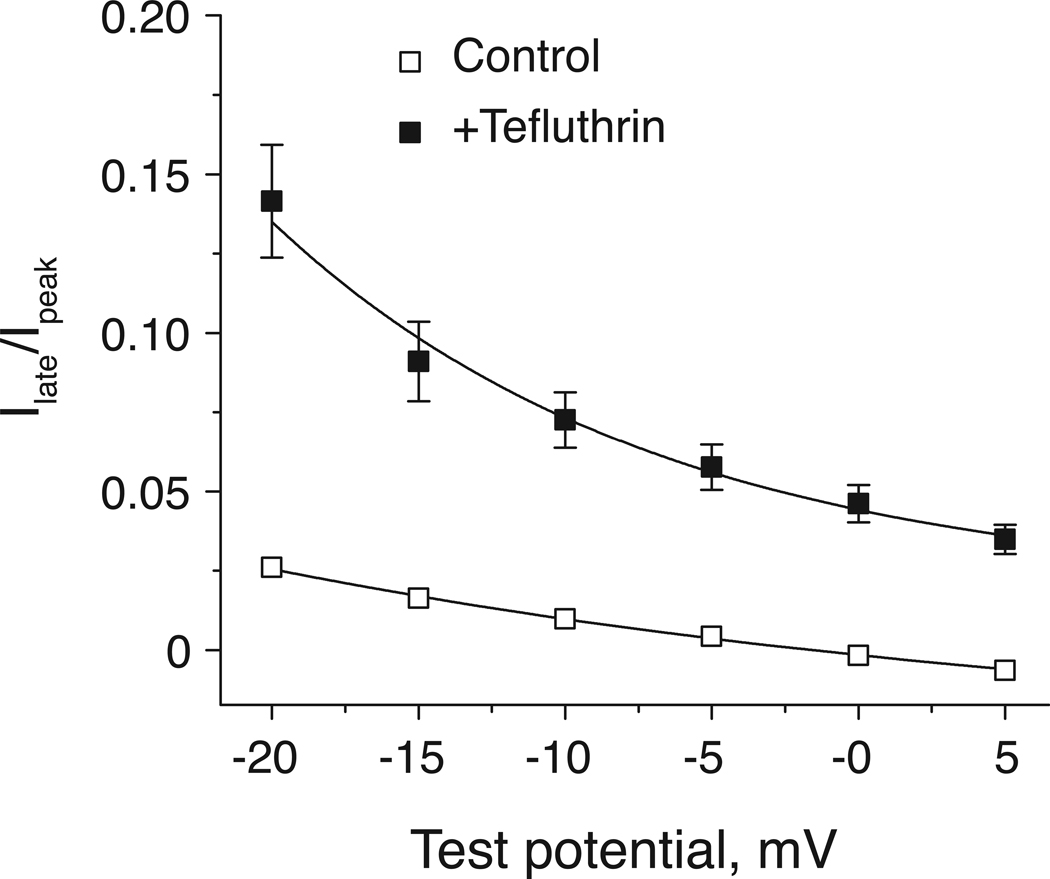
Tefluthrin at 100 µM had no significant effect on the voltage dependence of peak sodium current activation or steady-state fast inactivation (Table 1). However, the altered voltage dependence of activation of tefluthrin-modified channels was indirectly evident in the effects of tefluthrin on the insecticide-induced late current. We determined the late current/peak current (Ilate/Ipeak) ratios in the absence or presence of tefluthrin at test potentials surrounding that producing the maximal peak current for unmodified channels (Fig. 4). In the absence of tefluthrin the Ilate/Ipeak ratio was small and did not vary greatly with test potentials in the range of −20 mV to 5 mV. However, the Ilate/Ipeak ratio measured in the presence of tefluthrin not only was much larger but also exhibited a marked voltage dependence with a strong enhancement of Ilate relative to Ipeak at more negative test potentials.
Fig. 4.
Voltage dependence of late currents measured in the absence or presence of tefluthrin (100 µM). Peak transient currents (Ipeak) and late currents (Ilate ; measured at the end of a 40-ms step depolarization) were recorded during step depolarizations from −100 mV to the indicated test potential. Each data point is the mean of 17 (control) or 7 (+tefluthrin) determinations with different cells; bars show SE values larger than the data point symbols.
The decay of tefluthrin-induced tail currents (as in Fig. 2) was fitted best by a two-component, single exponential decay model that yielded first-order time constants for the fast initial phase and slower secondary phase of decay with values of 6.6 ± 0.5 and 37.8 ± 2.2 ms, respectively (means ± SE, n = 15). We employed the normalized conductance of the tefluthrin-induced sodium tail current to calculate the fraction of sodium channels that were modified by tefluthrin [20]. By this criterion tefluthrin at 100 µM modified only 8.9 ± 0.7% (mean ± SE, n = 10) of Nav1.7 sodium channels in the resting state.
3.3 Use-dependent modification by tefluthrin
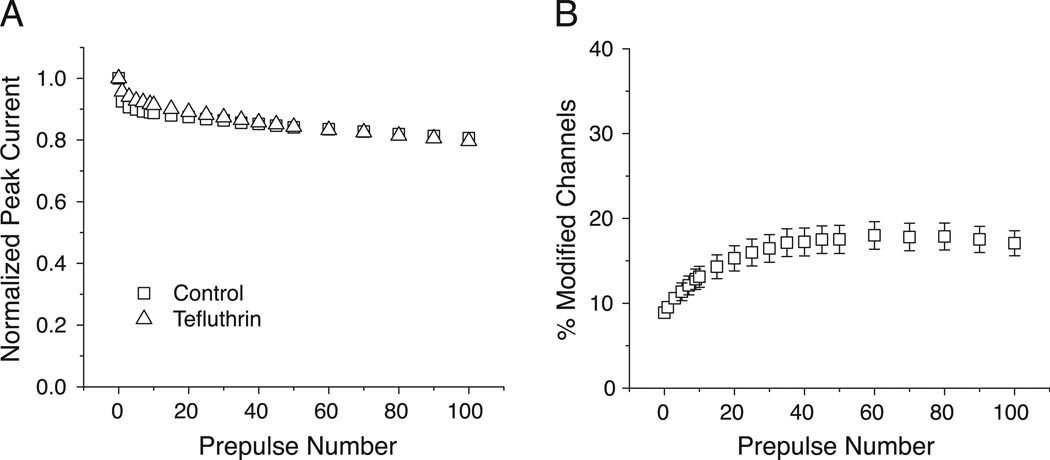
Modification of both insect and mammalian sodium channels by some pyrethroids is enhanced by repeated depolarization [26]. We therefore determined the effect of repeated high-frequency depolarization on the extent of modification of Nav1.7 sodium channels by tefluthrin. In the absence of tefluthrin, repeated stimulation caused an initial decline in the amplitudes of peak transient sodium currents obtained during the first five depolarizing pulses, after which current amplitudes declined more slowly and then stabilized at ~80% of the initial amplitude (Fig. 5A). Tefluthrin had no effect on the stability of peak currents following repeated depolarizing prepulses (Fig. 5A). Fig. 5B shows the effect of 0–100 depolarizing prepulses on the extent of modification of Nav1.7 sodium channels by 100 µM tefluthrin, corrected for the use-dependent changes in control peak current stability shown in Fig. 5A. The extent of modification increased as a function of prepulse number over the first 40 prepulses and then stabilized. Maximal use-dependent modification (at 60 prepulses) was 18.0 ± 1.6% (n = 10), approximately two-fold greater than resting modification.
Fig. 5.
Effect of repeated depolarization on sodium current stability and modification by tefluthrin of Nav1.7 sodium channels. (A) Effect of repeated depolarizing pulses on the normalized amplitude of peak transient sodium currents measured in the absence or presence of tefluthrin (100 µM); values were normalized to the amplitude of the peak current from the same oocyte prior to repetitive depolarization and are expressed as means of 14 paired experiments with different oocytes; in all cases SE values were smaller than the data point symbols. (B) Effect of repeated depolarizing pulses on the extent of tefluthrin modification of Nav1.7 channels; values for fractional modification were calculated from the normalized conductances of sodium tail currents and are expressed as means of 6 separate experiments with different oocytes; bars show SE values larger than the data point symbols.
4. Discussion
The Nav1.7 sodium channel α subunit is expressed together with the β1 and β2 subunits in DRG neurons [21,27], but the native subunit structure of Nav1.7-containing sodium channel complexes is not known. Expression of Nav1.7 channels in Xenopus oocytes in the absence of β subunits gives channels with abnormally slow inactivation kinetics [21,28]. The first study of Nav1.7 expression in oocytes [21] reported no effect of either the β1 or β2 subunit on the properties of expressed channels, but a subsequent study [28] found that coexpression with the β1 subunit accelerated channel inactivation and altered the gating properties of the channel. We chose to express Nav1.7 channels in combination with the β1 and β2 subunits to facilitate direct comparison of our results with the results of previous studies of rat Nav1.2, Nav1.3 and Nav1.6 channels in this system [15,16]. The kinetics of inactivation and the voltage dependence of activation of Nav1.7+β1+β2 channels in the present study agree well with the results obtained for Nav1.7+β1 channels [28] but the voltage dependence of steady-state fast inactivation of Nav1.7+β1+β2 channels was shifted by approximately 10 mV in the direction of depolarization compared to Nav1.7+β1 channels. This difference may be due to the inclusion of the β2 subunit in our experiments.
We employed 100 µM tefluthrin, the highest nominal concentration achievable in ND-96 perfusion medium, to maximize the extent of sodium channel modification and to facilitate comparison of the effects of tefluthrin on Nav1.7 channels with effects on other rat sodium channel isoforms under the same assay conditions [11,15,16]. Studies of pyrethroid action modification of sodium currents under voltage clamp conditions, such as those in the present study, typically employ high concentrations of insecticide to produce detectable populations of pyrethroid-modified channels. However, the concentrations of pyrethroid required to disrupt normal action potential generation in neurons are orders of magnitude lower because only a small fraction of the available population of channels must be modified in order to disrupt electrical signaling [29].
Tefluthrin modified Nav1.7 sodium channels by causing a slowly-inactivating late current during a depolarizing pulse and a prominent tail current following repolarization. These effects, which are the hallmarks of pyrethroid action on sodium channels in native neurons and heterologous expression systems, were also qualitatively similar to the effects of tefluthrin on other rat sodium channel isoforms in the oocyte system [11,15,16]. Modification of Nav1.7 channels by tefluthrin was enhanced by approximately two-fold by the application of trains of brief depolarizing prepulses. This result is in good agreement with previous studies of Nav1.2, Nav1.3 and Nav1.6 channels whose modification by tefluthrin was enhanced two- to four-fold by repetitive depolarization [15,16]. By contrast, repetitive depolarization did not significantly enhance the modification of Nav1.8 channels by tefluthrin [11]. This difference may reflect an intrinsic difference in the properties of Nav1.8 channels compared to the other four isoforms, but it also may reflect the omission of β subunits in experiments with the Nav1.8 isoform.
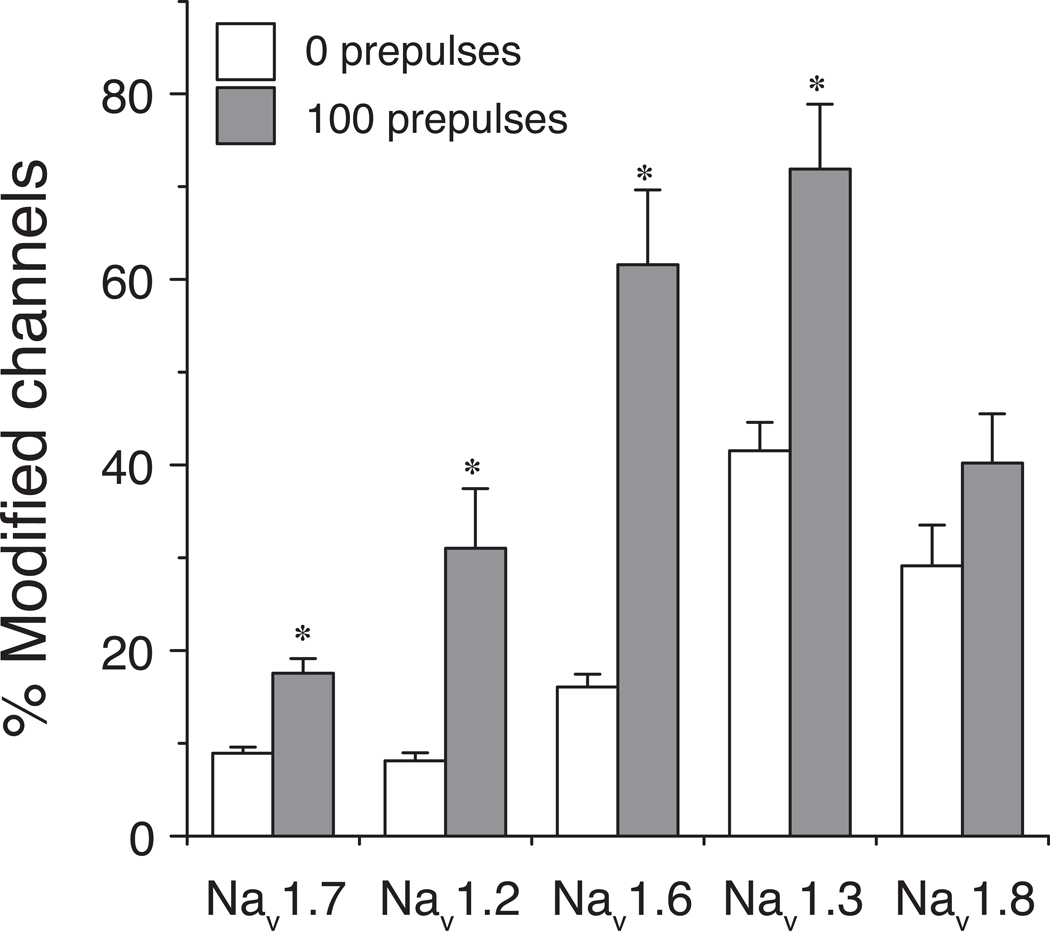
Nav1.7 sodium channels were distinguished principally by their very low sensitivity to modification by tefluthrin. Figure 6 compares the extent of both resting and use-dependent (after 100 prepulses) modification of Nav1.7 channels by 100 µM tefluthrin to corresponding data from previous studies of the rat Nav1.2, Nav1.3, Nav1.6 and Nav1.8 channels [11,15,16]. Our results identify Nav1.7 channels as having the lowest sensitivity to pyrethroid modification of any of the five rat isoforms studied to date. In view of the widespread expression of Nav1.7 channels in DRG neurons, it is likely that they contribute significantly to the TTX-sensitive, pyrethroid-resistant current found in these cells [19,20].
Fig. 6.
Comparison of the resting (0 prepulses) and use-dependent (after 100 prepulses) modification of five rat sodium channel isoforms expressed in Xenopus oocytes. Data for rat Nav1.7 channels are taken from the data set shown in Fig 5. Data for rat Nav1.2, rat Nav1.3, Nav1.6 and Nav1.8 channels are from previous studies in this laboratory [11,15,16]. Asterisks indicate significant use-dependent enhancement of channel modification (paired t-tests, P < 0.05).
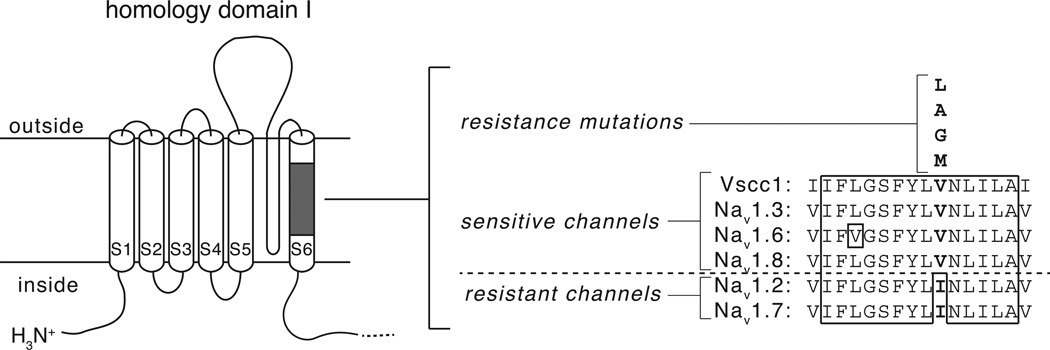
Prior to the present study, Nav1.2 was the only rat isoform identified as being pyrethroid-resistant. The identification of Nav1.7 as a second pyrethroid-resistant isoform provided the opportunity to search for amino acid sequence differences between channels characterized as either pyrethroid-sensitive or pyrethroid-resistant. We aligned the complete amino acid sequences of four pyrethroid-sensitive isoforms (house fly Vssc1 and rat Nav1.3, Nav1.6 and Nav1.8) and the two pyrethroid-resistant isoforms (rat Nav1.2 and Nav1.7) and found only a single site in the sodium channel amino acid sequence at which the amino acid sequence was conserved among all four sensitive isoform sequences but differed in the two resistant isoform sequences. This residue, located in transmembrane segment 6 of homology domain I (Fig. 7) is a conserved valine in the sensitive isoform sequences but is replaced with an isoleucine in the Nav1.2 and Nav1.7 sequences. The site of this polymorphism lies within a block of highly conserved sequence in a region of the channel that is thought to form part of the inner pore. It is also noteworthy that this residue (Val410 in Vssc1) has been identified as the site of multiple point mutations resulting in replacement by leucine, alanine, glycine or methionine in sodium channel sequences from pyrethroid-resistant insects [30–32]. Moreover, Vssc1 sodium channels containing the V410M mutation were resistant to modification by cismethrin when expressed in Xenopus oocytes [33]. It is of interest that such a wide variety of substitutions are tolerated at this valine residue despite its highly conserved sequence context.
Fig. 7.
Identification of a sodium channel amino acid sequence polymorphism associated with differential pyrethroid sensitivity. Left: diagram of sodium channel homology domain I showing the location of a conserved block of amino acid sequence in transmembrane segment 6. Right: Aligned amino acid sequences in transmembrane segment 6 from four pyrethroid-sensitive channels (house fly Vssc1, rat Nav1.3, rat Nav1.6 and rat Nav1.8) and two pyrethroid-resistant channels (rat Nav1.2 and rat Nav1.7). Residues enclosed in the solid line are identical in all six sequences. Polymorphic residues aligning with V410 of Vssc1 are shown in boldface type; substitutions at the valine residue aligning with V410 of Vssc1 that are associated with pyrethroid resistance in insects are shown above the aligned sequences.
Whereas our results implicate this amino acid polymorphism in domain IS6 as a determinant of relative pyrethroid sensitivity among rat sodium channel isoforms it is not the only such determinant in mammalian sodium channels. Comparisons of rat and human Nav1.3 channels in the oocyte expression system [15] revealed that the human channel was much less sensitive to modification by tefluthrin (similar in sensitivity to the rat Nav1.7 channel described here) than the rat Nav1.3 ortholog. The rat and human Nav1.3 amino acid sequences differ at only 54 of 1951 amino acid sequence positions and share the valine residue in domain IS6 that is identified in the present study as being associated with pyrethroid sensitivity. We conclude that other determinants of differential sensitivity among mammalian sodium channel isoforms remain to be discovered.
Research Highlights.
We expressed rat Nav1.7 voltage-gated sodium channels in Xenopus laevis oocytes.
The effects of tefluthrin on Nav1.7 channels were enhanced by repeated depolarization.
Nav1.7 channels were relatively insensitive to tefluthrin compared to other isoforms.
Pyrethroid sensitivity was correlated with an amino acid polymorphism in domain IS6.
The domain IS6 polymorphism occurs at a site of knockdown resistance mutations.
Acknowledgments
This work was supported in part by a grant (R01-ES013686) from the National Institute of Environmental Health Sciences, National Institutes of Health. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of the National Institute of Environmental Health Sciences. We thank P. Adams and S. Kopatz for technical assistance, and we thank R. Araujo, S. McCavera and R. von Stein for critical reviews of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Soderlund DM, Clark JM, Sheets LP, Mullin LS, Piccirillo VJ, Sargent D, Stevens JT, Weiner ML. Mechanisms of pyrethroid toxicity: implications for cumulative risk assessment. Toxicology. 2002;171:3–59. doi: 10.1016/s0300-483x(01)00569-8. [DOI] [PubMed] [Google Scholar]
- 2.Soderlund DM. Mode of action of pyrethrins and pyrethroids. In: Casida JE, Quistad GB, editors. Pyrethrum Flowers: Production, Chemistry, Toxicology, and Uses. New York: Oxford University Press; 1995. pp. 217–233. [Google Scholar]
- 3.Catterall WA. From ionic currents to molecular mechanisms: structure and function of voltage-gated sodium channels. Neuron. 2000;26:13–25. doi: 10.1016/s0896-6273(00)81133-2. [DOI] [PubMed] [Google Scholar]
- 4.Patino GA, Isom LL. Electrophysiology and beyond: multiple roles of Na+ channel β subunits in development and disease. Neuroscience Letters. 2010;486:53–59. doi: 10.1016/j.neulet.2010.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldin AL. Resurgence of sodium channel research. Annual Review of Physiology. 2001;63:871–894. doi: 10.1146/annurev.physiol.63.1.871. [DOI] [PubMed] [Google Scholar]
- 6.Yu FH, Catterall WA. Overview of the voltage-gated sodium channel family. Genome Biology. 2003;4:207.1–207.7. doi: 10.1186/gb-2003-4-3-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Felts PA, Yokoyama S, Dib-Hajj S, Black JA, Waxman SG. Sodium channel α-subunit mRNAs I, II, III, NaG, Na6 and hNE (PN1): different expression patters in developing rat nervous system. Molecular Brain Research. 1997;45:71–82. doi: 10.1016/s0169-328x(96)00241-0. [DOI] [PubMed] [Google Scholar]
- 8.Whitaker WRJ, Faull RLM, Waldvogel HJ, Plumpton CJ, Emson PC, Clare JJ. Comparative distribution of voltage-gated sodium channel proteins in human brain. Molecular Brain Research. 2001;88:37–53. doi: 10.1016/s0169-328x(00)00289-8. [DOI] [PubMed] [Google Scholar]
- 9.Smith TJ, Soderlund DM. Action of the pyrethroid insecticide cypermethrin on rat brain IIa sodium channels expressed in Xenopus oocytes. NeuroToxicology. 1998;19:823–832. [PubMed] [Google Scholar]
- 10.Vais H, Atkinson S, Eldursi N, Devonshire AL, Williamson MS, Usherwood PNR. A single amino acid change makes a rat neuronal sodium channel highly sensitive to pyrethroid insecticides. FEBS Letters. 2000;470:135–138. doi: 10.1016/s0014-5793(00)01305-3. [DOI] [PubMed] [Google Scholar]
- 11.Choi J-S, Soderlund DM. Structure-activity relationships for the action of 11 pyrethroid insecticides on rat Nav1.8 sodium channels expressed in Xenopus oocytes. Toxicology and Applied Pharmacology. 2006;211:233–244. doi: 10.1016/j.taap.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 12.Smith TJ, Soderlund DM. Potent actions of the pyrethroid insecticides cismethrin and cypermethrin on rat tetrodotoxin-resistant peripheral nerve (SNS/PN3) sodium channels expressed in Xenopus oocytes. Pesticide Biochemistry and Physiology. 2001;70:52–61. [Google Scholar]
- 13.Soderlund DM, Lee SH. Point mutations in homology domain II modify the sensitivity of rat Nav1.8 sodium channels to the pyrethroid cismethrin. Neurotoxicology. 2001;22:755–765. doi: 10.1016/s0161-813x(01)00065-1. [DOI] [PubMed] [Google Scholar]
- 14.Meacham CA, Brodfuehrer PD, Watkins JA, Shafer TJ. Developmentally-regulated sodium channel subunits are differentially sensitive to α-cyano containing pyrethroids. Toxicology and Applied Pharmacology. 2008:273–281. doi: 10.1016/j.taap.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 15.Tan J, Soderlund DM. Human and rat Nav1.3 voltage-gated sodium channels differ in inactivation properties and sensitivity to the pyrethroid insecticide tefluthrin. Neurotoxicology. 2009;30:81–89. doi: 10.1016/j.neuro.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tan J, Soderlund DM. Divergent actions of the pyrethroid insecticides S-bioallethrin, tefluthrin, and deltamethrin on rat Nav1.6 sodium channels. Toxicology and Applied Pharmacology. 2010;247:229–237. doi: 10.1016/j.taap.2010.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rush AM, Cummins TR, Waxman SG. Multiple sodium channels and their role in electrogenesis within dorsal root ganglion neurons. Journal of Physiology. 2007;579:1–14. doi: 10.1113/jphysiol.2006.121483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roy ML, Narahashi T. Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. Journal of Neuroscience. 1992;12:2104–2111. doi: 10.1523/JNEUROSCI.12-06-02104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tabarean IV, Narahashi T. Potent modulation of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels by the Type II pyrethroid deltamethrin. Journal of Pharmacology and Experimental Therapeutics. 1998;284:958–965. [PubMed] [Google Scholar]
- 20.Tatebayashi H, Narahashi T. Differential mechanism of action of the pyrethroid tetramethrin on tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels. Journal of Pharmacology and Experimental Therapeutics. 1994;270:595–603. [PubMed] [Google Scholar]
- 21.Sangameswaran L, Fish LM, Koch BD, Rabert DK, Delgado SG, Ilnicka M, Jakeman LB, Novakovic S, Wong K, Sze P, Tzoumaka E, Stewart GR, Herman RC, Chan H, Eglen RM, Hunter JC. A novel tetrodotoxin-sensitive, voltage-gated sodium channel expressed in rat and human dorsal root ganglia. Journal of Biological Chemistry. 1997;272:14805–14809. doi: 10.1074/jbc.272.23.14805. [DOI] [PubMed] [Google Scholar]
- 22.Toledo-Aral JJ, Moss BL, He Z-J, Koszowski AG, Whisenand T, Levinson SR, Wolf JJ, Silos-Santiago I, Halegoua S, Mandel G. Identification of PN1, a predominant voltage-dependent sodium channel expressed principally in peripheral neurons. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:1527–1532. doi: 10.1073/pnas.94.4.1527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tan J, Soderlund DM. Independent and joint modulation of rat Nav1.6 voltage-gated sodium channels by coexpression with the auxiliary β1 and β2 subunits. Biochemical and Biophysical Research Communications. 2011;407:788–792. doi: 10.1016/j.bbrc.2011.03.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldin AL. Maintenance of Xenopus laevis and oocyte injection. Methods in Enzymology. 1992;207:266–297. doi: 10.1016/0076-6879(92)07017-i. [DOI] [PubMed] [Google Scholar]
- 25.Bezanilla F, Armstrong CM. Inactivation of the sodium channel. Journal of General Physiology. 1977;70:549–566. doi: 10.1085/jgp.70.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Soderlund DM. State-dependent modification of voltage-gated sodium channels by pyrethroids. Pesticide Biochemistry and Physiology. 2010;97:78–86. doi: 10.1016/j.pestbp.2009.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Black JA, Dib-Hajj SD, McNabola K, Jeste S, Rizzo MA, Kocsis JD, Waxman SG. Spinal sensory neurons express multiple sodium channel α subunit mRNAs. Molecular Brain Research. 1996;43:117–131. doi: 10.1016/s0169-328x(96)00163-5. [DOI] [PubMed] [Google Scholar]
- 28.Vijayaragavan K, O'Leary ME, Chahine M. Gating properties of Nav1.7 and Nav1.8 peripheral nerve sodium channels. Journal of Neuroscience. 2001;21:7909–7918. doi: 10.1523/JNEUROSCI.21-20-07909.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Song J-H, Narahashi T. Modulation of sodium channels of rat cerebellar Purkinje neurons by the pyrethroid tetramethrin. Journal of Pharmacology and Experimental Therapeutics. 1996;277:445–453. [PubMed] [Google Scholar]
- 30.Park Y, Taylor MFJ, Feyereisen R. A valine421 to methionine mutation in IS6 of the hscp voltage-gated sodium channel associated with pyrethroid resistance in Heliothis virescens F. Biochemical and Biophysical Research Communications. 1997;239:688–691. doi: 10.1006/bbrc.1997.7511. [DOI] [PubMed] [Google Scholar]
- 31.Yoon KS, Kwon DH, Strychartz JP, Hollingsworth CS, Lee SH, Clark JM. Biochemical and molecular analysis of deltamethrin resistance in the common bed bug (Hemiptera: Cimicidae) Journal of Medical Entomology. 2008;45:1092–1101. doi: 10.1603/0022-2585(2008)45[1092:bamaod]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 32.Hopkins BW, Pietrantonio PV. The Helicoverpa zea (Boddie) (Lepidoptera: Noctuidae) voltage-gated sodium channel and mutations associated with pyrethroid resistance in field-collected adult males. Insect Biochemistry and Molecular Biology. 2010;40:385–393. doi: 10.1016/j.ibmb.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 33.Lee SH, Soderlund DM. The V410M mutation associated with pyrethroid resistance in Heliothis virescens reduces the pyrethroid sensitivity of house fly sodium channels expressed in Xenopus oocytes. Insect Biochemistry and Molecular Biology. 2001;31:19–29. doi: 10.1016/s0965-1748(00)00089-8. [DOI] [PubMed] [Google Scholar]