Abstract
A key target for novel stroke therapy is the regulation of post-ischemic inflammatory mechanisms. Recent evidence emphasizes the role of T lymphocytes of differing subtypes in the evolution is ischemic brain damage. We have recently demonstrated the benefit of myelin antigen-specific immunodulatory agents known as recombinant T cell receptor ligands (RTLs) in a standard murine model of focal stroke. The aim of the current study was to extend this initial observation to RTL treatment in a therapeutically relevant timing after middle cerebral artery occlusion (MCAO) and verify functional benefit to complement histological outcome measures. We observed that the administration of mouse-specific RTL551 reduced infarct size and improved sensorimotor outcome when administered within a 3 h post-ischemic therapeutic window. RTL551 treatment reduced cortical, caudate putamen, and total infarct volume as compared to vehicle-treated mice. Using a standard behavioral testing repertoire, we observed that RTL551 reduced sensorimotor impairment 3 days after MCAO. Humanized RTL1000 (HLA-DR2 moiety linked to hMOG-35-55 peptide) also reduced infarct size in HLA-DR2 transgenic mice. These data indicate that this neuroantigen-specific immunomodulatory agent reduces damage when administered in a therapeutically relevant reperfusion timeframe.
Keywords: Cerebral ischemia, Cerebral infarction, Inflammation, T lymphocyte, Immunotherapy, Mouse, Middle cerebral artery occlusion, Stroke
Introduction
Stroke is the third most common cause of death in the USA, affecting approximately 795,000 people each year. Although the annual death rate from stroke decreased approximately 30% from 1995 to 2005, 15% to 30% of stroke survivors remain permanently disabled, 20% require institutional care while 50% to 70% gradually regain functional independence [12]. To date, thrombolytic recombinant tissue plasminogen activator (rtPA) is the only FDA-approved drug for ischemic stroke. If given within 4.5 h from the onset of symptoms, rtPA can significantly reduce the effects of stroke and improve functional recovery. However, use of this drug is very limited, due to the increased incidence of intracranial hemorrhage if given outside the therapeutic window of 4.5 h. Thus, the successful treatment for ischemic stroke remains challenging and the development of new effective therapeutic strategies are greatly needed.
Recent studies have shown that clinical and experimental stroke triggers inflammation in brain and activates the peripheral immune system [15]. Within the first few hours, a rapid activation of resident microglia occurs in the brain, followed by infiltration of peripheral inflammatory cells such as macrophages, neutrophils, natural killer cells, and T and B lymphocytes [5]. The realization that the magnitude of the inflammatory response, including white blood cell count is strongly associated with stroke outcome in patients [3, 20] has generated interest in immunotherapy as a treatment for ischemic stroke [1]. The current study tests a new intervention targeting T cells, chosen with the rationale that these cells may be important in exacerbating ischemic damage following ischemia [9]. We have previously demonstrated that the recombinant T cell receptor ligand (RTL) 551 reduces tissue damage after focal cerebral ischemia when administered at the onset of reperfusion. The current study extends this finding by testing the efficacy of RTL551 when administered at a clinically relevant timepoint, 4 h after the onset of ischemia.
Materials and Methods
All experiments were carried out in accordance with National Institutes of Health guidelines for research animal care and approved by the Institutional Animal Care and Use Committee at Oregon Health & Science University.
Animals
All experiments used age-matched, sexually mature (20–25 g) male mice (C57BL/6, Harlan Laboratories, Indianapolis, IN, USA) or HLA-DRB1*1502 (DR2-Tg) transgenic mice produced by Dr. Chella David [4, 6]. The mice were maintained on a 12/12-h light/dark cycle and permitted ad libitum access to water and standard lab chow. The mice were randomized for surgery and behavioral testing. Exclusions for premature mortality were observed in eight mice in the vehicle group and in four in the RTL551-treated group. Three mice in the vehicle group and two in the RTL551 group were excluded due to intraischemic LDF >25% and one mouse in the vehicle group was excluded due to hypothermia. One mouse in the RTL551-treated group was excluded due to intracranial hemorrhage at the time of euthanasia.
Middle Cerebral Artery Occlusion Model
Transient focal cerebral ischemia was induced using reversible middle cerebral artery occlusion (MCAO) via the intraluminal filament technique as described previously [21, 22]. In brief, mice were anesthetized with isoflurane (induction 5.0% and maintenance 1.5–2.0%) delivered via a face mask in O2-enriched air. Head temperature was monitored with a probe placed under the left temporal muscle and was maintained at 36.5±1.0°C throughout MCAO surgery with warm water pads and heating lamp. A small laser Doppler probe (Model MBF3D, Moor Instruments Ltd., Oxford, England) was affixed to the right side of the skull at the mid-ear to eye distance to monitor cortical perfusion and verify vascular occlusion and reperfusion. A 2-cm incision was made in the middle of the anterior neck. After the right common carotid artery was tightened with 6-0 silk (ETHICON, Inc., Somerville, NJ, USA), a 6-0 ETHILON nylon monofilament (ETHICON, Inc., Somerville, NJ, USA) with a heat-blunted silicone-coated tip (about 230 µm diameter) was inserted into the right internal carotid artery via the external carotid artery to approximately 6 mm beyond the internal carotid/pterygopalatine artery bifurcation until the laser Doppler flowmetry (LDF) value dropped to <25% of baseline. After securing the filament in place, the surgical site was closed with 6-0 Vicryl sutures (ETHICON, Inc., Somerville, NJ, USA). Each mouse was then placed in a separate cage. Rectal temperature was monitored and maintained at 36.5±1.0°C throughout the period of occlusion with a warm water pad and heating lamp. At the end of the ischemia period (60 min), the mice were briefly re-anesthetized, the laser Doppler probe was repositioned over the same site on the skull, and the occluding filament was withdrawn for reperfusion. Mice were then allowed to recover and were observed according to the experimental protocol. Sham-operated mice were treated identically with the exception of insertion of the filament to produce an occlusion.
RTL Construction and Production
RTL molecules consist of the α1 and β1 domains of major histocompatibility complex (MHC) II molecules and are expressed as a single polypeptide with or without antigenic amino terminal extensions [2]: RTL551-Ab linked to mouse MOG-35-55 peptide (MEVGW YRSPFSRVVHLYRNGK); and RTL1000 HLA-DR2 linked to human MOG-35-55 (hMOG35-55) peptide (MEVGWYRP- PFSRVVHLYRNGK). RTLs were constructed de novo or by sequential site-directed mutagenesis of previous constructs [18]. Protein purification was as previously described [2].
Treatment with RTL
Mice were randomized to receive 0.1 mL (100 µg) RTL (RTL551 or RTL1000) or 0.1 mL vehicle (Tris-HCl, pH 8.5) by subcutaneous injection 3 h after the onset of reperfusion and at every 24 h of reperfusion. To determine whether RTL551 altered physiological values, we measured glucose, electrolytes, and blood gases in a separate non-surviving cohort of mice before treatment with RTL551 (n=3) or vehicle (n=3) and 3 h post-treatment, and we monitored arterial blood pressure continuously for 3 h.
Determination of Infarct Size
Each brain was sliced into five 2-mm-thick coronal sections. The sections were placed in a 1.2% solution of 2,3,5-triphenyltetrazolium chloride (TTC; Sigma-Aldrich, St. Louis, MO, USA) for 30 min at 37°C and then fixed in 10% formalin for 24 h as described previously [21, 22]. Both sides of each stained coronal slice were photographed using a digital camera, and infarction areas were measured with digital image analysis software (SigmaScan Pro, Jandel, San Rafael, CA, USA) and numerically integrated across the thickness of the slice to obtain an estimate of infarct volume.
Assessment of Functional Outcome
We selected behavioral tests that have consistently provided reliable evaluations of outcome across experimental ischemia studies in our laboratories [7, 22, 23]. All RTL551- and vehicle-treated mice, randomized for behavioral testing, were housed singly in a 12/12-h light/dark cycle, and all assessments were carried out during the second half of the light cycle (1200–1800 hours). The observer who carried out and scored the mouse behavior tests was blind to the treatment group. All equipment was cleaned with 10% ethanol between trials.
Cylinder Test
We performed the cylinder test to analyze forelimb use bias. Each mouse was placed in a transparent cylinder measuring 9 cm in diameter and 15 cm in height. The cylinder was wide enough for the mouse to move freely and small enough to encourage rearing behavior. Four separate video cameras were placed around the cylinder at 90° intervals to record the rearing behavior from all angles. We recorded a maximum of two paw touches for one rearing event. If the mouse touched the cylinder wall with the same paw twice, we designated these motions as ‘independent.’ If the mouse touched the cylinder wall the first time with one paw and a second time with the other paw, we designated these motions as ‘independent’ and ‘both,’ respectively. A total of 20 forelimb touches were recorded during the 10-min test and were found to be sufficient to determine bias without habituating the mouse to the apparatus. Mice with fewer than 20 forelimb touches in this time period were excluded from behavioral analysis. The final score was calculated as the percentage of total touches that used the impaired forelimb. Baseline paw preference was assessed 1 day before surgery (Pre). Subsequent assessments were made at 3 and 7 days after surgery (day 3 and day 7).
Neurologic Deficit Score
This score was recorded in each mouse on days 1, 2, and 3 after surgery (day 1, day 2, and day 3) as described earlier [17]. The graded scoring systems ranged from 0 to 2, 0 to 3, or 0 to 5 depending on the behavior assessed, with 0 indicating no deficit and the upper limit indicating the most impaired. The behaviors assessed included consciousness (0–3), interaction (0–2), eye appearance (0–2), breathing (0–2), food/water intake (0–2), ability to grab wire top (0–2), motor function (0–5), and activity (0–2).
Latency to Move
Each mouse was placed in the center of a 12-cm diameter circle on a flat surface, and the time required for the mouse to move outside the circle was recorded at day 1, day 2, and day 3, as described earlier [10].
Statistical Analysis
Values are expressed as mean±SEM. Infarction volume was analyzed by two-tailed Student’s t test between two groups and analysis of variance (ANOVA) for multiple comparisons with post hoc Turkey’s test. Laser Doppler flow and physiologic parameters were analyzed by two-way ANOVA with Neuman–Keuls. Behavior testing was analyzed by two-way ANOVA with a post hoc Newman–Keuls test to correct for multiple comparisons and also by one-way repeated measure ANOVA with the post hoc Newman–Keuls test to correct for multiple comparisons for paw preference test in each group. Survival rates were analyzed by log-rank test in behavior testing. A P<0.05 was considered statistically significant. All statistical analyses were carried out using SigmaStat Statistical Software (Version 3.0; SPSS, Chicago, IL, USA).
Results
RTL Treatment Significantly Reduces Infarction Size After 96 h Reperfusion
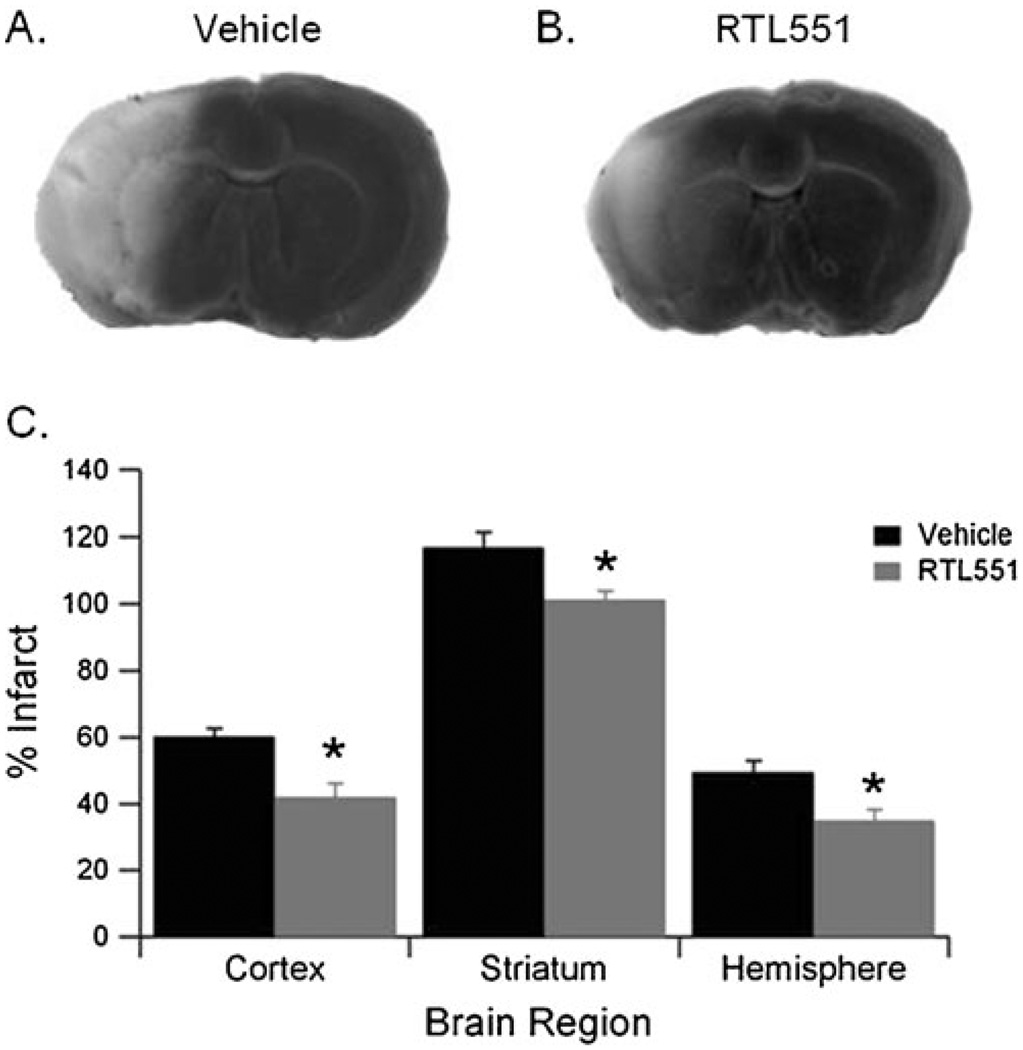
We have previously demonstrated that the administration of RTL551 to adult male C57Bl/6 mice at the onset of occlusion reduced infarct volume 96 h after 60 min MCAO. In order to assess the therapeutic potential of RTL treatment, male mice were given RTL551 3 h after reperfusion. All mice were subjected to 60 min MCAO and were randomly assigned to vehicle or RTL551 group 3 h after reperfusion. Drug injections were repeated every 24 h until mice were euthanized at 96 h reperfusion. Compared to the vehicle-treated mice, RTL551-treated mice had significantly smaller infarct volumes in cortex (60.2±2.5% in vehicle; n=9 and 41.9±4.1% in RTL551; n=11, P<0.05), striatum (117.0±4.4% in vehicle and 101.3±2.7% in RTL551; P<0.05), and in hemisphere (49.5±3.5% in vehicle and 35.0±3.3% in RTL551; P<0.05) (Fig. 1a–c).
Fig. 1.
RTL551 treatment 4 h after the onset of ischemia reduces infarct volume in C57Bl/6 mice. Representative TTC-stained brains from vehicle- (a) and RTL551-treated (b) mice. Sixty-minute transient MCAO was followed by four daily treatments with vehicle (Tris-HCl) or 100 µg RTL, and the infarction volume was quantified as a percentage of the non-ischemic contralateral hemisphere. (c) Quantification of infarct volume of vehicle- and RTL551-treated mice. Group sizes were n=9 for vehicle and n=11 for RTL551. Data are presented as mean±SEM of individual mice. *Different than vehicle, P<0.05
Intra-Ischemic Physiological Parameters
In order to evaluate the physiological effects of RTL treatment, we measured blood pressure and arterial blood gases during and 3 h after MCAO. No differences in intra-ischemic physiologic parameters and LDF reduction were observed among experimental groups. In a separate, non-surviving cohort, no significant differences in arterial blood gases, glucose, electrolytes, and mean arterial blood pressure were observed between the RTL551 and vehicle groups. All values remained within physiologic limits.
Behavioral Testing
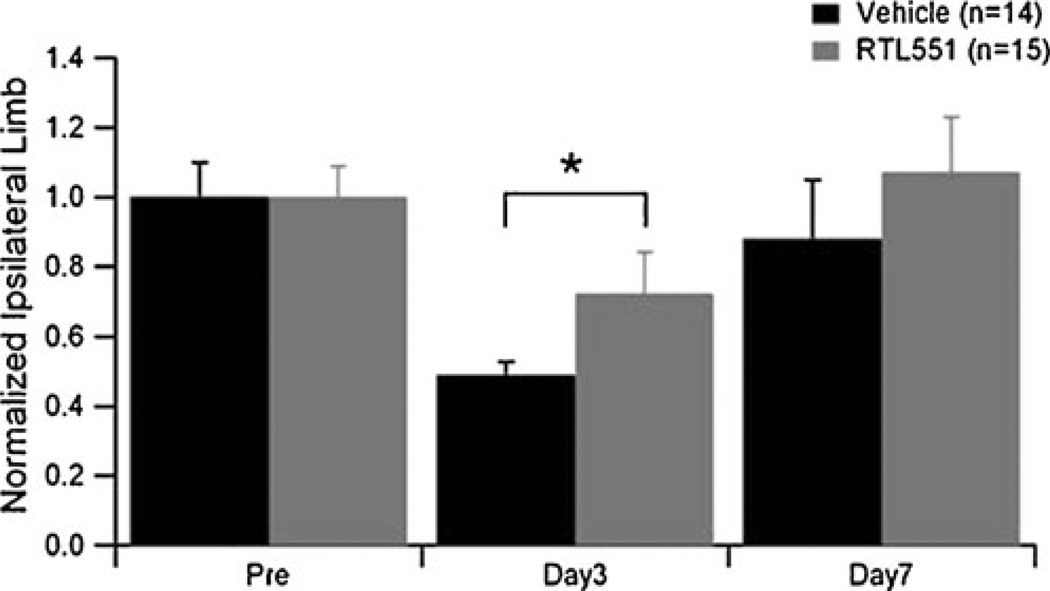
Neurobehavioral testing was performed over a 7-day period in sham and MCAO mice treated with vehicle or RTL551. The survival rate in each group was as follows: vehicle MCAO, 67% (18/27 in total); RTL551 MCAO, 50% (17/34 in total); vehicle sham MCAO, 100% (12/12 in total); and RTL551 sham MCAO, 93% (13/14 in total). There was no significant difference between the vehicle and the RTL551 groups with regard to survival rate after MCAO (P=0.43). No mice were excluded due to inability of performing the tasks. Neurological deficit score and latency to move were assessed at days 1 and 3 following MCAO as indicators of general health. There were no statistical differences in neurological deficit scores or latency to move between experimental groups on either day tested (data not shown). Motor dysfunction on the affected paw after MCAO was assessed using the cylinder test. We observed no asymmetries in pre-surgery tests (Fig. 2) or in sham-operated animals (data not shown). However, right forelimb use was significantly reduced in vehicle MCAO-treated mice at day 3 (P<0.05), but not in RTL551 MCAO mice. By day 7, the mice in both vehicle and RTL551 treatment groups recovered to pre-surgical values with no difference between groups. At the conclusion of behavioral testing, infarct volume was assessed and no differences between vehicle and RTL551 groups were observed. As expected, no infarction was detected in the surgical sham groups.
Fig. 2.
RTL551 treatment improved functional recovery after MCAO-affected paw use in vehicle- (Tris-HCl) or RTL551-treated mice were tested 1 day before MCAO (pre), 3 days after MCAO (day 3) and 7 days after MCAO (day 7). Group sizes were n=18 and n=12 for vehicle-treated MCAO and sham mice, respectively, and n=17 and n=13 for RTL551-treated MCAO and sham mice. Data are presented as mean±SEM of individual mice. *Different than vehicle, P<0.05
RTL1000
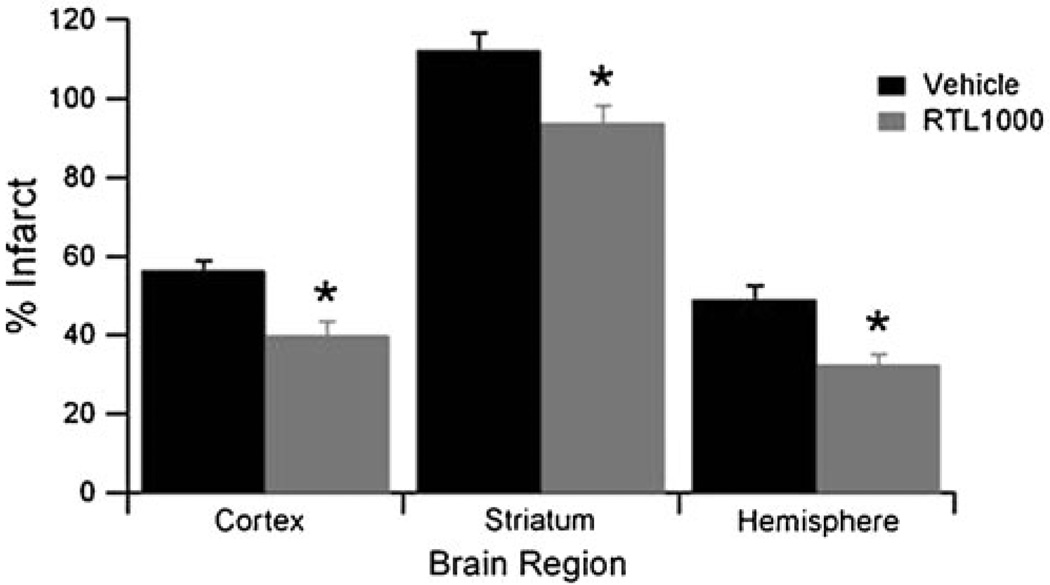
To further assess the therapeutic possibility of humanized RTL1000, containing the HLA-DR2 moiety covalently linked to hMOG-35-55 peptide, we injected DR2-Tg mice with RTL1000 3 h after MCAO. Injection was repeated every 24 h until the mice were euthanized at 96 h reperfusion. As shown in Fig. 3, RTL1000-treated mice had significantly smaller infarct volumes than did vehicle-treated mice in the cortex (56.3±2.4% in vehicle; n=10 and 39.9±3.6% in RTL551; n=10, P<0.05), striatum (112.2±4.4% in vehicle and 93.8±4.5% in RTL551; P<0.05), and in hemisphere (48.9±3.5% in vehicle and 32.4±2.7% in RTL551; P<0.05). These data demonstrate that RTL1000, containing a neuroantigen peptide and a matched MHC II moiety, can significantly reduce stroke lesion size in the mice that contain the same neuroantigen peptide.
Fig. 3.
RTL1000 treatment 4 h after the onset of ischemia reduces infarct volume in DR2 transgenic mice. Sixty-minute transient MCAO was followed by four daily treatments with vehicle (Tris-HCl) or 100 µg RTL, and infarction volume was quantified as a percentage of the non-ischemic contralateral hemisphere. Quantification of infarct volume of vehicle- and RTL551-treated mice. Group sizes were n=10 for vehicle and n=10 for RTL1000. Data are presented as mean±SEM of individual mice. *Different than vehicle, P<0.05
Discussion
Our results demonstrate that RTL treatment remains effective when administered at a clinically relevant timepoint of 3 h after reperfusion, 4 h after onset. We observed that both RTL551 and RTL1000 significantly reduced infarct size 4 days after MCAO. Similarly, we demonstrate that RTL551 improved functional outcome measured by paw preference test 3 days after MCAO. These data suggest that RTLs remain a promising class of drug for the treatment of ischemic stroke.
Recombinant T cell receptor (TCR) ligands are partial MHC class II molecules comprised of covalently linked β1 and α1 chains tethered to antigenic peptides. These unique molecules were designed as minimal ligands for the TCR of peptide-specific T cells. Unlike four-domain MHC II molecules that can induce T cell activation and apoptosis, RTLs are partial agonists that cause autoreactive T cells to become nonpathogenic. RTLs are effective at minimizing symptoms of mice in experimental autoimmune encephalomyelitis, although the mechanism of the drug has not been completely elucidated. It has been demonstrated that RTL suppresses proliferative responses and inflammatory cytokine production [8, 19], inflammatory response in the spleen [19], and RTL upregulates the expression of the FoxP3 gene in splenocytes [8], which is reported to work as a neuroprotective [11]. Similarly, we previously reported that RTL reduces infarct size in a mouse MCAO model when given at the onset of reperfusion followed by injections every 24 h [21]. Further, RTL treatment reduced peripheral immunocyte infiltration into the brain following MCAO, while also partially preventing the splenic atrophy that accompanies the downstream immunosuppressive phase of stroke [21]. While the exact mechanism of RTL action remains elusive, the ability to reduce neuroinflammation following MCAO without concurrent peripheral immunosuppression makes this an appealing therapeutic for immunotherapy. The current study furthered our understanding of RTL as a potential therapeutic by extending the timing of administration to 4 h after the onset of ischemia.
The data presented in the current study demonstrates that RTL1000 provides significant protection against MCAO when administered 4 h after the onset of ischemia. RTL1000 is a humanized RTL comprised of MOG-35-55 peptide covalently linked to an HLA-DR2 moiety. The use of DR2-Tg mice provides a unique experimental tool to test the efficacy of RTL1000 which is targeted to use in humans, in fact has finished phase I clinical trial for multiple sclerosis in human patients and its safety has been already proven [16]. Natural variations in vascular anatomy results are differing ischemic sensitivity among mouse strains [13], making it difficult to compare the outcomes of WT and DR2-Tg mice. However, the controlled nature of our experimental design makes it clear that RTL1000 minimizes ischemic damage in these transgenic mice. These reports strongly suggest potential efficacy for the treatment of human stroke.
Our experiments do not provide an evidence for long-term benefit as MCAO-induced impairment in motor function was not apparent 7 days after MCAO. Therefore, further work is warranted to evaluate the long-term functional benefit of post-stroke RTL therapy. However, our neurobehavioral data do provide evidence that post-stroke RTL treatment minimizes sensorimotor impairment 3 days after MCAO. This observation is consistent with the histological data showing decreased infarct volume at 4 days, indicating that post-stroke RTL therapy is a promising approach worth further study. Interestingly, both vehicle MCAO-treated mice and RTL551 MCAO mice showed impairment on ipsilateral side contrary to the previous reports [10, 22]. This surprising observation is not without precedence, as several reports demonstrated ipsilateral motor dysfunction after unilateral stroke in human patients [14, 17]. Regardless the carefully controlled nature of our experiments and consistency with histological data provide confidence that RTL improves sensorimotor function after MCAO.
In conclusion, we demonstrated the therapeutic activity of recombinant TCR ligands, RTL551 and RTL1000, when administered 4 h after the onset of ischemia. RTL improved both histological and functional outcome. RTL1000, a humanized RTL comprised of MOG-35-55 peptide covalently linked to an HLA-DR2 moiety, is currently in clinical trials in multiple sclerosis and potentially might be useful for treatment of human patients with stroke.
Acknowledgments
The authors thank Ms. Kathy Gage, grants and publications writer for the Department of Anesthesiology and Perioperative Medicine, OHSU, for her outstanding editorial work in the preparation of this paper. This material is based upon work supported in part by the Department of Veterans Affairs, Veterans Health Administration, Office of Research and Development, Biomedical Laboratory Research and Development. The contents do not represent the views of the Department of Veterans Affairs or the US government.
Contributor Information
Kozaburo Akiyoshi, Department of Anesthesiology and Perioperative Medicine, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd., Portland, OR 97239, USA.
Suzan Dziennis, Department of Anesthesiology and Perioperative Medicine, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd., Portland, OR 97239, USA; Neuroimmunology Research, Portland VA Medical Center, R&D-31, 3710 SW US Veterans Hospital Rd., Portland, OR 97239, USA.
Julie Palmateer, Department of Anesthesiology and Perioperative Medicine, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd., Portland, OR 97239, USA.
Xuefang Ren, Department of Anesthesiology and Perioperative Medicine, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd., Portland, OR 97239, USA; Neuroimmunology Research, Portland VA Medical Center, R&D-31, 3710 SW US Veterans Hospital Rd., Portland, OR 97239, USA.
Arthur A. Vandenbark, Neuroimmunology Research, Portland VA Medical Center, R&D-31, 3710 SW US Veterans Hospital Rd., Portland, OR 97239, USA Research Service, Department of Veterans Affairs Medical Center, 3710 SW US Veterans Hospital Rd., Portland, OR 97239, USA; Department of Molecular Microbiology & Immunology, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd., Portland, OR 97239, USA; Department of Neurology, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd., Portland, OR 97201, USA.
Halina Offner, Department of Anesthesiology and Perioperative Medicine, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd., Portland, OR 97239, USA; Neuroimmunology Research, Portland VA Medical Center, R&D-31, 3710 SW US Veterans Hospital Rd., Portland, OR 97239, USA; Department of Neurology, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd., Portland, OR 97201, USA.
Paco S. Herson, Email: hersonp@ohsu.edu, Department of Anesthesiology and Perioperative Medicine, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd., Portland, OR 97239, USA.
Patricia D. Hurn, Department of Anesthesiology and Perioperative Medicine, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd., Portland, OR 97239, USA Department of Neurology, Oregon Health & Science University, 3181 SW Sam Jackson Park Rd., Portland, OR 97201, USA.
References
- 1.Ahmad M, Graham SH. Inflammation after stroke: mechanisms and therapeutic approaches. Transl Stroke Res. 2010;1:74–84. doi: 10.1007/s12975-010-0023-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Burrows GG, Chang JW, Bachinger HP, Bourdette DN, Offner H, Vandenbark AA. Design, engineering and production of functional single-chain T cell receptor ligands. Protein Eng. 1999;12:771–778. doi: 10.1093/protein/12.9.771. [DOI] [PubMed] [Google Scholar]
- 3.Emsley HC, Smith CJ, Georgiou RF, Vail A, Tyrrell PJ, Barberan EM, et al. Correlation of systemic inflammatory response with infarct volume in acute ischemic stroke patients. Stroke. 2005;36:228–229. doi: 10.1161/01.STR.0000155197.88944.ac. [DOI] [PubMed] [Google Scholar]
- 4.Finn TP, Jones RE, Rich C, Dahan R, Link J, David CS, et al. HLA-DRB1*1501 risk association in multiple sclerosis may not be related to presentation of myelin epitopes. J Neurosci Res. 2004;78:100–114. doi: 10.1002/jnr.20227. [DOI] [PubMed] [Google Scholar]
- 5.Gelderblom M, Leypoldt F, Steinbach K, Behrens D, Choe CU, Siler DA, et al. Temporal and spatial dynamics of cerebral immune cell accumulation in stroke. Stroke. 2009;40:1849–1857. doi: 10.1161/STROKEAHA.108.534503. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Gay MA, Zanelli E, Khare SD, Krco CJ, Zhou P, Inoko H, et al. Human leukocyte antigen-DRB1*1502 (DR2Dw12) transgene reduces incidence and severity of arthritis in mice. Hum Immunol. 1996;50:54–60. doi: 10.1016/0198-8859(96)00123-1. [DOI] [PubMed] [Google Scholar]
- 7.Herson PS, Palmateer J, Hurn PD, DeVries AC. Evaluating behavioral outcomes from ischemic brain injury. In: Raber J, editor. Translational animal models of behavioral analysis. New York: Humana; 2010. [Google Scholar]
- 8.Huan J, Kaler LJ, Mooney JL, Subramanian S, Hopke C, Vandenbark AA, et al. MHC class II derived recombinant T cell receptor ligands protect DBA/1LacJ mice from collagen-induced arthritis. J Immunol. 2008;180:1249–1257. doi: 10.4049/jimmunol.180.2.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kleinschnitz C, Schwab N, Kraft P, Hagedorn I, Dreykluft A, Schwarz T, et al. Early detrimental T-cell effects in experimental cerebral ischemia are neither related to adaptive immunity nor thrombus formation. Blood. 2010;115:3835–3842. doi: 10.1182/blood-2009-10-249078. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Blizzard KK, Zeng Z, DeVries AC, Hurn PD, McCullough LD. Chronic behavioral testing after focal ischemia in the mouse: functional recovery and the effects of gender. Exp Neurol. 2004;187:94–104. doi: 10.1016/j.expneurol.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 11.Liesz A, Suri-Payer E, Veltkamp C, Doerr H, Sommer C, Rivest S, et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15:192–199. doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 12.Lloyd-Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De SG, et al. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010;121:e46–e215. doi: 10.1161/CIRCULATIONAHA.109.192667. [DOI] [PubMed] [Google Scholar]
- 13.Maeda K, Hata R, Hossmann KA. Differences in the cerebrovascular anatomy of C57black/6 and SV129 mice. Neuroreport. 1998;9:1317–1319. doi: 10.1097/00001756-199805110-00012. [DOI] [PubMed] [Google Scholar]
- 14.Noskin O, Krakauer JW, Lazar RM, Festa JR, Handy C, O'Brien KA, et al. Ipsilateral motor dysfunction from unilateral stroke: implications for the functional neuroanatomy of hemiparesis. J Neurol Neurosurg Psychiatry. 2008;79:401–406. doi: 10.1136/jnnp.2007.118463. [DOI] [PubMed] [Google Scholar]
- 15.Offner H, Subramanian S, Parker SM, Afentoulis ME, Vandenbark AA, Hurn PD. Experimental stroke induces massive, rapid activation of the peripheral immune system. J Cereb Blood Flow Metab. 2006;26:654–665. doi: 10.1038/sj.jcbfm.9600217. [DOI] [PubMed] [Google Scholar]
- 16.Offner H, Sinha S, Burrows GG, Ferro AJ, Vandenbark AA. RTL therapy for multiple sclerosis: a phase I clinical study. J Neuroimmunol. 2010;231(1–2):7–14. doi: 10.1016/j.jneuroim.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schaefer SY, Haaland KY, Sainburg RL. Ipsilesional motor deficits following stroke reflect hemispheric specializations for movement control. Brain. 2007;130:2146–2158. doi: 10.1093/brain/awm145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sinha S, Subramanian S, Proctor TM, Kaler LJ, Grafe M, Dahan R, et al. A promising therapeutic approach for multiple sclerosis: recombinant T-cell receptor ligands modulate experimental autoimmune encephalomyelitis by reducing interleukin-17 production and inhibiting migration of encephalitogenic cells into the CNS. J Neurosci. 2007;27:12531–12539. doi: 10.1523/JNEUROSCI.3599-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sinha S, Subramanian S, Emerson-Webber A, Lindner M, Burrows GG, Grafe M, et al. Recombinant TCR ligand reverses clinical signs and CNS damage of EAE induced by recombinant human MOG. J Neuroimmune Pharmacol. 2010;5:231–239. doi: 10.1007/s11481-009-9175-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith CJ, Emsley HC, Gavin CM, Georgiou RF, Vail A, Barberan EM, et al. Peak plasma interleukin-6 and other peripheral markers of inflammation in the first week of ischaemic stroke correlate with brain infarct volume, stroke severity and long-term outcome. BMC Neurol. 2004;4:2. doi: 10.1186/1471-2377-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Subramanian S, Zhang B, Kosaka Y, Burrows GG, Grafe MR, Vandenbark AA, et al. Recombinant T cell receptor ligand treats experimental stroke. Stroke. 2009;40:2539–2545. doi: 10.1161/STROKEAHA.108.543991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Uchida M, Palmateer JM, Herson PS, DeVries AC, Cheng J, Hurn PD. Dose-dependent effects of androgens on outcome after focal cerebral ischemia in adult male mice. J Cereb Blood Flow Metab. 2009;29:1454–1462. doi: 10.1038/jcbfm.2009.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu W, Wang L, Zhang L, Palmateer JM, Libal NL, Hurn PD, et al. Isoflurane preconditioning neuroprotection in experimental focal stroke is androgen-dependent in male mice. Neuroscience. 2010;169:758–769. doi: 10.1016/j.neuroscience.2010.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]