Abstract
Adolescence is a period marked by changes in motivational and cognitive brain systems. However, the development of the interactions between reward and cognitive control processing are just beginning to be understood. Using event-related functional neuroimaging and an incentive modulated antisaccade task, we compared blood-oxygen level dependent activity underlying motivated response inhibition in children, adolescents, and adults. Behaviorally, children and adolescents performed significantly worse than adults during neutral trials. However, children and adolescents showed significant performance increases during reward trials. Adults showed no performance changes across conditions. fMRI results demonstrated that all groups recruited a similar circuitry to support task performance, including regions typically associated with rewards (striatum and orbital frontal cortex), and regions known to be involved in inhibitory control (putative frontal and supplementary eye fields, and posterior parietal cortex, and prefrontal loci). During rewarded trials adolescents showed increased activity in striatal regions, while adults demonstrated heightened activation in the OFC relative to children and adolescents. Children showed greater reliance on prefrontal executive regions that may be related to increased effort in inhibiting responses. Overall, these results indicate that response inhibition is enhanced with reward contingencies over development. Adolescents’ heightened response in striatal regions may be one factor contributing to reward-biased decision making and perhaps risk taking behavior.
Keywords: Adolescence, Reward, Inhibitory control, Antisaccade, fMRI
1. Introduction
Adolescence is a unique period of development characterized by immature reward processing and inconsistencies in inhibitory control, an important, fundamental component of the cognitive control of behavior. Adolescent behavior is distinct from childhood and adulthood, as evidenced by heightened incidents of sub-optimal or immature decision-making. Although adolescents have improved decision making skills compared to children, unique immaturities exist that often result in a peak in risk taking behavior. We define adulthood as the model system and describe functions and behavior by younger individuals that deviate from this system as “immature” (for review see Luna et al., 2010). While important work has been done to delineate adolescent immaturities underlying reward processing (Bjork et al., 2004, Cohen et al., 2010, Ernst et al., 2005, Ernst et al., 2006, Eshel et al., 2007, Galvan, 2010, May et al., 2004, van Leijenhorst et al., 2009), and cognitive control (Bunge et al., 2002, Levin et al., 1991, Liston et al., 2006, Luna et al., 2004, Paus et al., 1990, Ridderinkhof et al., 1999, Ridderinkhof and van der Molen, 1997, Williams et al., 1999; for review see Luna, 2009), the influence of incentives on components of cognitive control within a developmental context is relatively understudied (for review see Geier and Luna, 2009). Furthermore, in order to better understand immature processes that are unique to the adolescent period, adolescent processes must be contrasted with the preceding stage of childhood and the following mature stage of adulthood.
Behavioral evidence clearly indicates that adolescents can demonstrate mature levels of inhibitory control, but do so inconsistently compared to adults (Bedard et al., 2002, Luna et al., 2004, Ridderinkhof et al., 1999, Van den Wildenberg and van der Molen, 2004, Velanova et al., 2009, Wise et al., 1975). Furthermore, neuroimaging studies have demonstrated that adolescents performing tasks of inhibitory control exhibit a distinct neurofunctional profile, likely reflecting continued brain immaturities (Luna et al., 2001, Rubia et al., 2007, Velanova et al., 2008, Velanova et al., 2009). During adolescence, key reward processing and control regions including the striatum and prefrontal cortex demonstrate continued gray matter thinning (Giedd et al., 1996, Gogtay et al., 2004, Sowell et al., 1999, Toga et al., 2006). Similarly, white matter connections between these regions strengthen, indicating increased fidelity/speed of distal neuronal transmission, which may support the functional integration necessary for complex behavior (Asato et al., 2010). The transition to mature behavior coupled with still-immature neural function may be reflected in these maturational processes and in functional neuroimaging studies that have demonstrated that in the absence of performance differences, adolescents demonstrate differences in recruitment of key brain regions. For example, adolescents who demonstrate adult-levels of mature behavior (i.e. no performance differences in laboratory tasks of cognition), demonstrate increased activity of prefrontal cortex, suggesting increased effort required to perform the task at equivalent levels (Luna et al., 2001; for review see Luna, 2009).
One particularly robust and reliable assay of developmental changes in inhibitory control behavior and the neural systems that support it is the antisaccade (AS) task (Hallett, 1978). The AS task, which requires a participant to inhibit the reflexive tendency to look toward a sudden presentation of a peripheral stimulus and instead make an eye movement (saccade) to its mirror location, has extensively been used to characterize the neural basis of inhibitory control in both humans and non-human primates (Brown et al., 2007, Butler et al., 1999, Cherkasova et al., 2002, Everling and Fischer, 1998, Fischer and Weber, 1996, Matsuda et al., 2004, Munoz et al., 1998, Munoz and Everling, 2004, Schlag-Rey et al., 1997). Work in humans and non-human primates have delineated a widely distributed circuitry that supports AS performance including the frontal, supplementary, and parietal eye fields (FEF, SEF, PEF respectively), as well as prefrontal cortex (PFC) and various subcortical structures such as striatum, thalamus, and cerebellum (Brown et al., 2006, Luna and Sweeney, 1999, Matsuda et al., 2004). Neuroimaging studies suggest that brain function underlying AS performance continues to demonstrate immaturities (Luna et al., 2001, Velanova et al., 2008, Velanova et al., 2009), despite behavioral evidence suggesting that the rate of inhibitory AS errors begins to reach adult levels in mid adolescence (Fischer et al., 1997, Klein and Foerster, 2001, Luna et al., 2004, Munoz et al., 1998). These functional immaturities include the recruitment of brain processes that support AS error processing (Velanova et al., 2008), and the ability to retain an inhibitory response state (Velanova et al., 2009), which continue to improve into young adulthood.
Immaturities in reward processing are also evident during adolescence. Converging lines of evidence from single-cell recording, lesion and neuroimaging studies have delineated a circuitry related to reward processing that originates in the ventral tegmental area of the midbrain, extending through the ventral striatum (VS) (including the nucleus accumbens), and projecting out to medial and ventral regions of the PFC (including the orbital frontal cortex (OFC)), and the anterior cingulate cortex (ACC) (Apicella et al., 1991, Bjork et al., 2004, Breiter et al., 2001, Chambers et al., 2003, Delgado et al., 2000, Delgado et al., 2003, Elliott et al., 2003, Hikosaka and Watanabe, 2000, Knutson et al., 2000, Roesch and Olson, 2003, Roesch and Olson, 2004, Schultz et al., 2000, Thut et al., 1997, van Leijenhorst et al., 2009, Wise, 2002). Developmental fMRI studies on reward processing have found age related differences in the magnitude of recruitment of striatal and prefrontal regions (Bjork et al., 2004, Ernst et al., 2005, Galvan et al., 2006, Guyer et al., 2006, May et al., 2004, van Leijenhorst et al., 2009). In some studies, adolescents were found to exhibit a relative decrease of VS, OFC and mesial PFC recruitment during reward cue and anticipation (Bjork et al., 2004, Bjork et al., 2007, Bjork et al., 2010). In contrast, other work has suggested that adolescents demonstrate increased activity of VS primarily during reward receipt (Ernst et al., 2005, Galvan et al., 2006, van Leijenhorst et al., 2009, van Leijenhorst et al., 2010). Our previous work has provided evidence indicating that adolescents demonstrate an initial decrease in recruitment of the VS during incentive cue assessment but markedly increased VS activity during reward anticipation relative to adults (Geier et al., 2010). Although these results indicate that immaturities are present during adolescence in reward processing, it remains to be seen whether such immaturities are also present in childhood. Moreover, studies that have considered childhood to adulthood have focused on reward reactivity exclusively but not on its effects on cognitive control (Cohen et al., 2010, Galvan et al., 2006, van Leijenhorst et al., 2009), Recently, van Leijenhorst et al. (2010) using a gambling task designed to assess the neural correlates of high-risk and low-risk monetary gambles, demonstrated that reward related activity peaked in adolescence compared to children and adults whereas cognitive control related activity followed a linear trajectory. This finding suggests that an over-reactive reward system coupled with a still developing cognitive system may account for unique influences of rewards on decision making.
In the present study, we aimed at studying the effects of cognitive control on reward processing in childhood, adolescence and adulthood. We hypothesized that adolescents would show enhanced activity in key reward related regions relative to adults (Cohen et al., 2010, Galvan et al., 2006, van Leijenhorst et al., 2009, van Leijenhorst et al., 2010), Moreover, we expected a similarly distinct adolescent response when compared to children. Given our prior finding that rewards improve AS performance (Geier et al., 2010) and enhance activity in oculomotor control regions, we hypothesized that improved AS performance would be accompanied by increased recruitment of oculomotor control regions known to support antisaccade processing (Luna et al., 2001, Luna et al., 2004). Finally, we predicted that children would demonstrate increased recruitment of prefrontal cognitive control regions (such as the anterior cingulate cortex and dorsolateral prefrontal cortex) in line with previous work demonstrating immature over-reliance on prefrontal systems in children when performing cognitive tasks (Luna et al., 2001).
2. Materials and methods
2.1. Participants
We recruited 34 participants for this study. Four children were excluded due to non-compliance with the task instructions. We thus report on thirty healthy, right-handed participants, ten adults (ages 18–25, mean = 20.6 (±2.2 st dev); six females), ten adolescents (ages 14–17; mean = 15.8 (±1.2 st dev), six females), and ten children (ages 8–13 years, mean = 11.1 (±1.5 st dev), six females). Age groups were defined based on previous behavioral studies indicating differential cognitive performance on the AS task (Luna et al., 2004). Participants were native English speakers with no personal or first-degree relative history of neurological disease, brain injury, or psychiatric illness as determined by interview. Vision was normal or corrected to normal using MRI compatible glasses or contact lenses. Full scale IQ scores determined using the WASI (Wechsler Abbreviated Scale of Intelligence) were above 85 and there were no significant differences in IQ across age groups (Children: mean = 112.4 (±13.8 st. dev), Adolescents: mean = 108.6 (±7.5 st. dev), Adults: 116.7 (±10.2 st. dev), p = .263). Immediately prior to scanning, participants were given explicit verbal instructions and trained on the antisaccade (AS) and visually guided saccades (VGS) tasks in a separate behavioral testing room till they became comfortable performing the task (corresponded to 4–5 trials each on average). Participants also spent approximately 15 min in a mock scanner to acclimate them to the MR environment (Rosenberg et al., 1997). Experimental procedures for this study complied with the Code of Ethics of the World Medical Association (1964 Declaration of Helsinki) and the Institutional Review Board at the University of Pittsburgh. Participants were paid for their participation in the study with a chance to win extra money during the fMRI task.
2.2. Behavioral paradigm
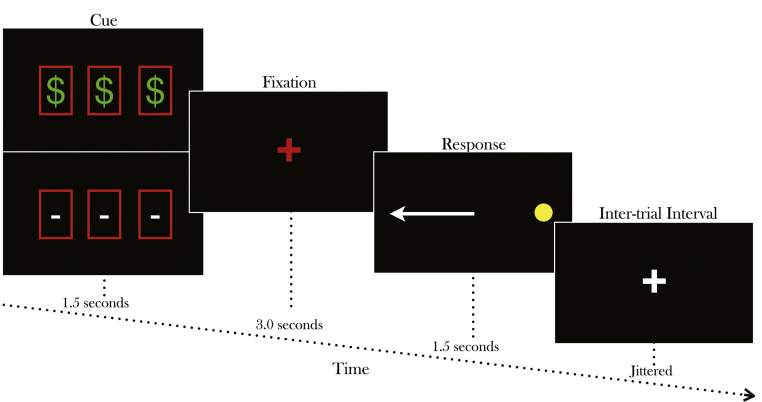
At the onset of each AS trial, participants were first presented with one of two incentive cues (1500 ms) (Fig. 1). For rewarded trials, the cue consisted of three rectangles containing dollar signs ($ $ $), indicating that money could be earned on that trial if correctly performed. Participants were told that they could win up to US $25 based on their performance during the task. However, they did not know how much they could win on any given trial in order to prevent them from keeping a running tally of their earnings and invoking processes (i.e. working memory) separate from inhibitory control and reward processing. For neutral trials, the three consecutive rectangles each contained a dash (– – –), which indicated that no monetary gain was at stake for that trial. After the initial cue, a central red fixation cross subtending ∼0.7° of visual angle appeared (3000 ms), instructing participants to prepare for the target stimulus. The red central fixation then disappeared and a horizontally peripheral target stimulus (yellow spot, subtending ∼0.5°) appeared (1500 ms) at an unpredictable location on the horizontal meridian (±3°, 6°, or 9°). Participants were instructed to refrain from looking at the stimulus when it appeared but instead move their eyes to its mirror location. Target location was randomized within each run. During the VGS trials, participants were presented with a green fixation cross (1500 ms) which instructed them to look toward the peripheral stimulus when it appeared. No incentive cue was provided for VGS trials. The VGS trials were randomly interspersed between the AS trials to minimize the possibility that participants would establish an inhibitory response set (Velanova et al., 2009), but were not further analyzed. As indicated in previous studies, (Ollinger et al., 2001b, Ollinger et al., 2001a), the inter-trial fixation period was jittered between intervals of 1.5, 3, or 4.5 s (uniformly distributed) and consisted of participants simply fixating a central white cross on a black background. Participants performed three functional runs of the task (5 min 2 s each in duration) for a total of 30 reward AS trials, 30 neutral AS trials and 15 VGS trials.
Fig. 1.
Rewarded antisaccade task schematic. A row of three dollar signs indicated that participant could win money contingent on their performance in the upcoming trial (reward trial). A row of three horizontal dashed lines indicated that no money could be won in the upcoming trial (neutral trial). Incentive cues were presented for 1.5 s. Following that, a red fixation cross appeared for 3 s. A peripheral light appeared for 1.5 s during which, participants were instructed to generate a saccade to its mirror location.
2.3. Eye tracking
Eye movement measurements were obtained in the MR environment using a long-range optics eye-tracking system (Model R-LRO6, Applied Science Laboratories, Bedford, MA). Simultaneous video monitoring was also used to assure task compliance. Nine-point calibrations were performed at the beginning of the session and between runs as necessary. Stimuli were presented using E-Prime (Psychology Software Tools, Inc., Pittsburgh, PA), projected onto a flat screen positioned behind the magnet. Participants viewed the screen using a mirror mounted on the RF head coil. Eye-movement data were analyzed and scored offline using ILAB (Gitelman, 2002) in conjunction with an in-house scoring suite. Variables of interest included latencies for correct AS trials and error rate (the number of inhibitory failures/total number of scorable trials) during rewarded and neutral trials. A correct response in the AS task was one in which the first eye movement during the saccade response epoch with velocity greater than or equal to 30°/s (Gitelman, 2002) was made toward the mirror location of the peripheral cue, and extended beyond a 2.5°/visual angle central fixation zone. AS errors (also often referred to as prosaccades) occurred when the first saccade during the saccade response epoch was directed toward the suddenly appearing peripheral stimulus and exceeded the 2.5°/visual angle central fixation zone. Participants usually corrected inhibitory errors indicating that they understood the instruction but were unable to stop the initial reflexive gaze to the visual stimulus.
2.4. fMRI
2.4.1. Image acquisition and preprocessing
Imaging data were acquired using a Siemens 3-Tesla MAGNETOM Allegra (Erlangen, Germany) system with a standard radiofrequency (RF) head coil at the Brain Imaging Research Center, University of Pittsburgh, Pittsburgh, PA. Structural images were acquired using a sagittal magnetization prepared rapid gradient echo (MPRAGE) T1-weighted pulse sequence with 224 slices with 0.7825 mm slice thickness. Functional images were acquired using a gradient echo echo-planar (EPI) sequence sensitive to blood-oxygen-dependent (BOLD) contrast (T2*) (TR = 1.5 s, TE = 25 ms, flip angle = 70°, voxel size = 3.125 × 3.125 × 4 mm in-plane resolution, 216 volumes). Twenty-nine slices per volume were collected with no gap and aligned to the anterior and posterior commissure (AC–PC) plane. The first four volumes in each run were discarded to allow stabilization of longitudinal magnetization.
Imaging data were preprocessed using FSL (FMRIB Software Library; Smith et al., 2004). Briefly, our preprocessing procedures included the following: First, slice-timing correction was performed, adjusting for interleaved slice acquisition. Images were rigid-body motion corrected by aligning all volumes with the volume acquired in the middle of the fMRI session. Rotational and translational head movement estimates were calculated. Following brain extraction (using FSL's brain extraction tool, BET) (Smith, 2002), functional images were affine registered and warped to structural MPRAGE images in Talairach space (Talairach and Tournoux, 1988), using both the FLIRT and FNIRT tools in FSL (Jenkinson and Smith, 2001). No participants were excluded due to motion, instead the temporal derivative of the relative displacement from the middle volume for each run was calculated for each volume in the x, y and z directions. Magnitude of the velocity was then calculated by taking the square root of the sum of squares of the x, y and z components for each volume. Volumes with a velocity (in mm per TR) of over 1.2 mm were removed (censored) from subsequent analyses. Participant groups did not differ in number of volumes removed due to excessive motion (censored 3 volumes from two children and 1 volume from two adolescents). Images were then spatially smoothed with a 5 mm full-width at half maximum (FWHM) Gaussian smoothing kernel and high-pass filtered (sigma = 30 s) to remove low frequency drift. Data from each run were then scaled to a mean of one hundred and multiple runs were concatenated.
2.4.2. Data analyses
AFNI (Analysis and Visualization of Functional Neuroimages) software (Cox, 1996) was used for individual subject deconvolution as well as subsequent group analyses. Deconvolution methods followed steps delineated previously (Ward, 1998). Briefly, our model consisted of two orthogonal regressors of interest for reward and neutral correct AS trials, as well as regressors for incorrect AS trials and all VGS trials. Linear and non-linear trends and six motion parameters were also included as nuisance regressors. A unique estimated impulse response function (i.e. hemodynamic response function) for each regressor of interest (correct reward and neutral AS trials) was determined by a weighted linear sum of eight sine basis functions multiplied by data determined least squares estimated beta weights. The estimated impulse response function reflects the estimated BOLD response to a type of trial (reward AS trial) after controlling for variations in the BOLD signal due to other regressors. We made no assumptions about the shape of the function. We specified the duration of the estimated response from the trial onset (0 s) to 24 s (17 TRs) post trial onset, a sufficient time window for the hemodynamic response to peak and return to baseline, which was defined as the jittered fixation periods between trials.
For group analyses, impulse response function values associated with correct reward and neutral AS trials from each participant were entered into a voxel-wise linear mixed effects model, with ‘subjects’ as a random factor and time (0–16 TRs) and ‘incentive’ (reward, neutral) as within-group factors, and ‘age-group’ (children, adolescent, adult) as between-group fixed factors. The ‘main effect of time’ image that resulted from this model was used as a base image from which functional regions of interest (ROIs), were defined (see below) because it shows all regions that demonstrate a significant modulation from baseline across all groups and conditions, making it unbiased with respect to all effects of interest and has been reliable in delineating the basic circuitry recruited in our study (Geier et al., 2010, Velanova et al., 2008).
Functionally defined regions of interest were determined using methods already established in the literature (Wheeler et al., 2005). First, the main effect of time map was corrected for multiple comparisons using a combination of cluster size and individual voxel probabilities and parameters determined following a Monte Carlo simulation using AFNI's AlphaSim program. This analysis specified that 23 contiguous voxels along with a single-voxel threshold of p < 0.001 was required to achieve a corrected, cluster-level alpha value of 0.05.
Second, peak voxels in the corrected main effect of time map were identified using an automatic search algorithm. Twelve-millimeter diameter spheres were centered on these peak voxels, resulting in a ‘sphere map’. Finally, a conjunction of the ‘sphere map’ and the corrected main effect of time map yielded a functional ROI map, which was used as a mask for subsequent analyses in order to extract time course values for each participant. Due to the relatively small size of the VS, a ten millimeter diameter sphere (encompassing approximately 20 voxels) was manually traced around peak voxels that fell within the region (as defined by the Talairach and Tourneaux atlas (Talairach and Tournoux, 1988)) in both hemispheres.
We focused our subsequent analyses on these functionally defined clusters that fell within the boundaries of several a priori anatomical regions of interest purportedly involved in oculomotor control and reward processing. These included the paracentral sulcus, which is considered to represent the SEF, the superior aspect of the precentral sulcus, which is considered to represent the FEF (Curtis and Connolly, 2008, Luna et al., 1998), and the SPL, which is considered to be the parietal eye field (Curtis and Connolly, 2008, Luna et al., 1998), the dorsal and ventral striatum, the ACC, and the OFC.
Mean estimated time courses from each participant were extracted from the voxels constituting each corrected sphere mask across both reward and neutral incentives. Mean time course values at each time point (0–16 TRs) were entered into a repeated measures ANOVA using age group as the between subjects factor and time and incentive type as within subjects factors. Below, we report regions that demonstrated an age-group by time, incentive-condition by time and/or an age-group by incentive-condition by time interaction across the modeled window of 17 TRs. While it is crucial that effects be determined based on the entire modeled timecourse, extended timecourses can often incur noise, especially at the tail-end of the window, which can undermine the ability to assess magnitude differences. Therefore, we also analyzed regions across the first half of the modeled response (8 TRs), which encompassed the rise and peak of the hemodynamic response.
3. Results
3.1. Behavior
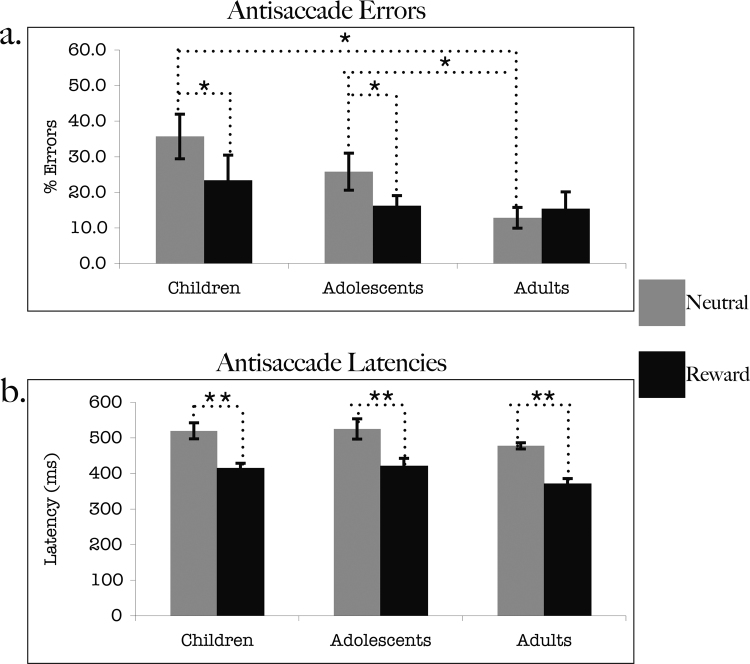
Behavioral results showed a main effect of incentive type for AS error rate, (F(1,27) = 8.357, p < 0.01) with more errors occurring in the neutral vs. reward conditions. There was a trend for a main effect of age group on rate of AS errors (p = .094). Simple effects of age group during the neutral trials were evident with children (t(18) = 3.287, p < .005) and adolescents (t(18) = 2.172, p < .05) demonstrating worse performance during neutral trials relative to adults. There were no differences between children and adolescents during neutral trials (p = 0.242).
There was an incentive type by age group interaction (F(2,27) = 4.884, p < .05). There was no effect of age group during rewarded trials. Within each age group, children (t(9) = −4.71, p < .001) and adolescents (t(9) = −2.24, p < .05), but not adults (p = .46), generated fewer errors during rewarded trials compared to neutral. Post-hoc comparisons indicated that all three groups demonstrated equivalent performance on reward trials (Fig. 2a). There were no differences in the number of dropped trials (i.e. participant did not attempt to perform the task) between rewarded and neutral conditions across age groups.
Fig. 2.
Behavioral results. (A) Antisaccade error rate (% errors) for children (left), adolescents (middle) and adults (right) for both rewarded (gray bars) and neutral (black bars) trials. (B) Latencies (ms) of correct antisaccades for children (left), adolescents (middle) and adults (right) for both rewarded (gray bars) and neutral (black bars) trials. Single asterisk (*) indicates significance at the 0.05 alpha level. Double asterisks (**) indicate significance at the .001 alpha level.
The latency of correct antisaccades showed a main effect of incentive type (F(1,27) = 209.618, p < .0001) but no main effect of age group (p = .138) or age group by incentive type interaction (p = .975). All three age-groups generated significantly faster correct anti-saccade responses during reward trials compared to neutral (children: t(9) = 2.26, p < .0001, adolescents: t(9) = 2.26, p < .0001, adults: t(9) = 2.26, p < .0001) (Fig. 2b).
3.2. Imaging
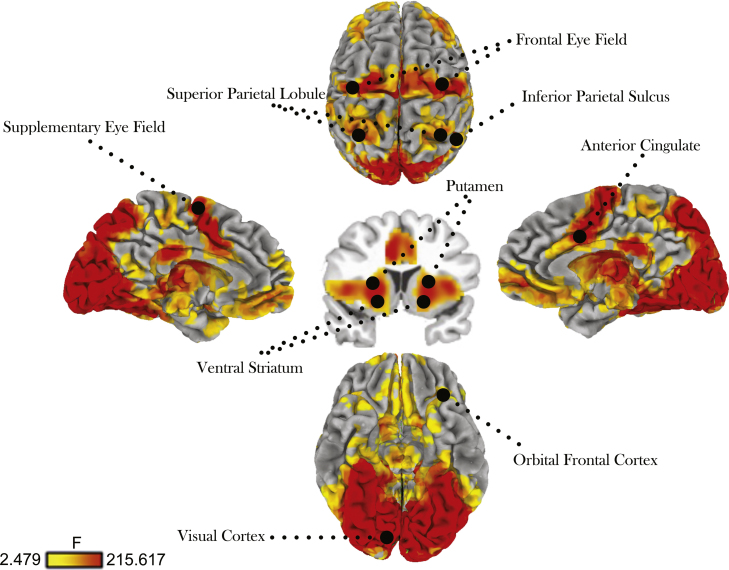
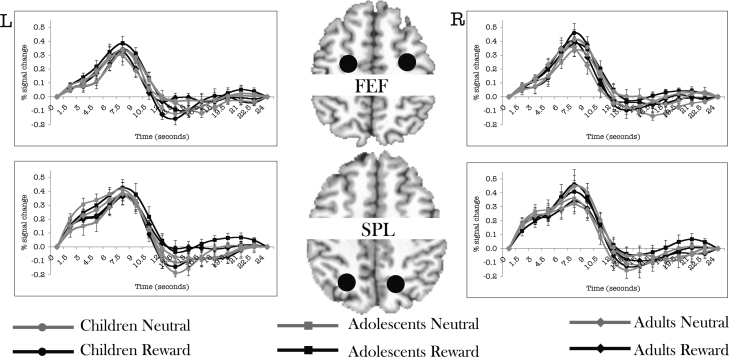
Table 1 provides a summary of all regions of interest that demonstrated a main effect of time. Main effect of time effects across conditions and age groups demonstrated robust recruitment of a distributed circuitry including frontal, supplementary, posterior parietal cortex, basal ganglia, PFC, VS and OFC (Fig. 3). Within these regions, bilateral FEF, and superior parietal cortex, did not demonstrate any age or incentive interactions with time (Fig. 4).
Table 1.
Regions of interest demonstrating a main effect of time.
| Region (Broadmann area) | Coordinatea |
Peak F | n voxels | Effect | F effect | Group effectb | F group effect | ||
|---|---|---|---|---|---|---|---|---|---|
| x | y | z | |||||||
| Left Frontal Eye Field (6) | −22 | −8 | 48 | 86.84 | 33 | Time | 87.171 | None | n/a |
| Right Frontal Eye Field (6) | 29 | −11 | 46 | 82.77 | 33 | Time | 83.314 | None | n/a |
| Left Superior Parietal Lobule (7) | −25 | −59 | 43 | 75.46 | 33 | Time | 83.003 | None | n/a |
| Right Superior Parietal Lobule (7) | 26 | −62 | 43 | 81.96 | 33 | Time | 80.311 | None | n/a |
| Right Inferior Parietal Sulcus (40) | 41 | −44 | 40 | 24.78 | 33 | Age × Incentive × Time | 2.730** | TR > TN | 4.894*** |
| Supplementary Eye Field (6) | 2 | −2 | 52 | 61.70 | 33 | Age × Time | 1.940* | CN > (TN = AN) | 2.232** |
| Right Dorsal Anterior Cingulate (24) | 8 | 7 | 34 | 63.53 | 33 | Age × Time | 2.484* | C > (T = A) | 2.484* |
| Left Putamen | −22 | 4 | 1 | 51.76 | 33 | Incentive × Time | 2.857* | TR > TN | 5.008*** |
| Right Putamen | 20 | 7 | 4 | 54.21 | 33 | Incentive × Time | 2.589* | TR > TN | 3.735* |
| Left VS | −10 | 8 | −4 | 9.12 | 19 | Incentive × Time | 2.343* | TR > TN | 4.805*** |
| Right VS | 14 | 8 | −4 | 19.91 | 19 | Incentive × Time | 2.501* | TR > TN | 3.735* |
| OFC (47) | 35 | 28 | −11 | 9.83 | 28 | Age × Incentive × Time | 2.935* | AR > AN | 2.283** |
Taliarach.
C = children, T = teens, A = adults.
p < .05.
p < .001.
p < .0001.
Fig. 3.
Activation maps for main effect of time collapsed across incentive conditions and age groups. Threshold set at p < 0.001 (corrected). Right side of image = right brain.
Fig. 4.
Time courses showing key oculomotor control regions that showed no group differences. See materials and methods for how time courses were extracted. Error bars represent ±1 standard error of the mean at each time point. For visualization purposes only, filled black circles indicating location of the masks are schematically shown above slices of the AFNI Talairach atlas, drawn using AFNI. The circles do not reflect the actual shape of the mask. R = Right side. L = Left Side.
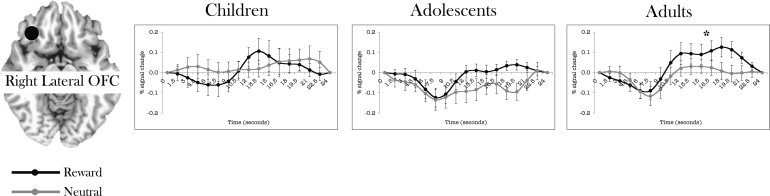
Across the entire modeled response (17 TRs), in right lateral OFC, there was a significant age-group by incentive by time interaction (F(8,108) = 2.935, p < .05). However this was due to a late increased peak in adults during rewarded relative to neutral trials (F(16,144) = 2.283, p < .005) (Fig. 5).
Fig. 5.
Time courses separated by age group in left lateral OFC. See materials and methods for how time courses were extracted. Error bars represent ±1 standard error of the mean at each time point. For visualization purposes only, filled black circles indicating location of the masks are schematically shown above slices of the AFNI Talairach atlas, drawn using AFNI. The circles do not reflect the actual shape of the mask.
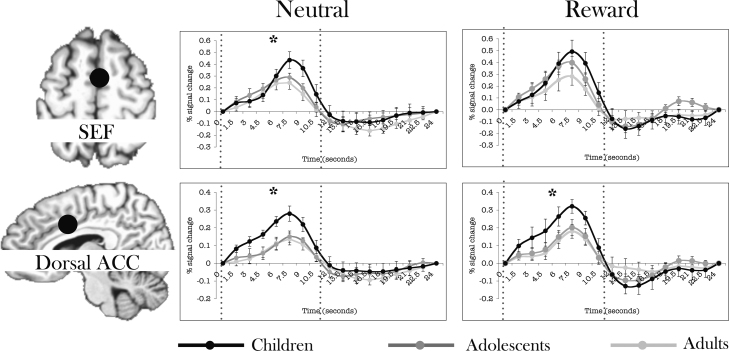
Significant group differences across the first half of the modeled response (8 TRs) were noted in the SEF and dorsal ACC. In SEF, there was a significant age-group by time interaction (F(14,189) = 1.940, p < .05). Post-hoc tests indicated that children demonstrated increased activity relative to adults and adolescents during neutral (time by age: F(14,189) = 2.232, p < .01) but not rewarded trials (p = .11) (Fig. 6a). In the dorsal ACC, children demonstrated increased activity during both rewarded and neutral trials relative to adults (age by time: F(7,126) = 2.484, p < .05) (Fig. 6b).
Fig. 6.
Time courses separated by incentive condition of regions where children demonstrated increased activity relative to other two groups. See materials and methods for how time courses were extracted. Error bars represent ±1 standard error of the mean at each time point. Vertical dotted lines represent the first half of the modeled response (8 TRs) that showed a significant interaction effect. For visualization purposes only, filled black circles indicating location of the masks are schematically shown above slices of the AFNI Talairach atlas, drawn using AFNI. The circles do not reflect the actual shape of the mask.
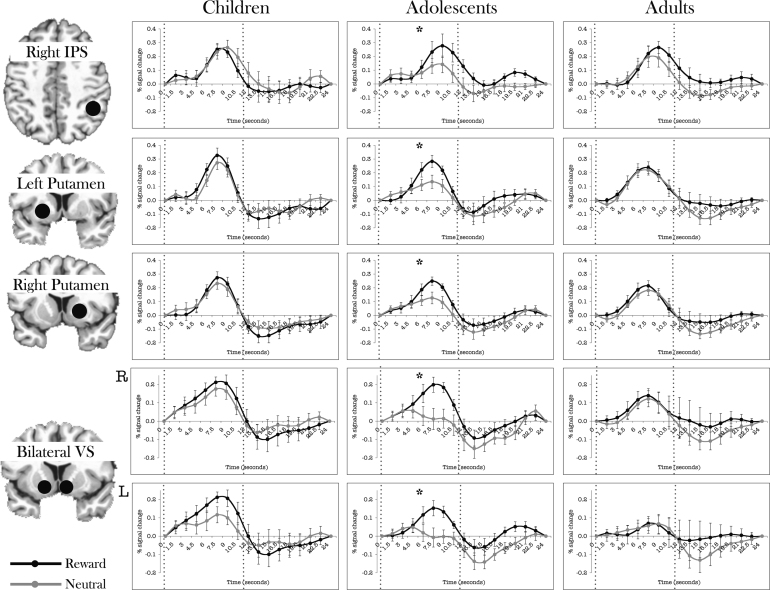
Across a range of regions including IPS, putamen, and VS, only adolescents demonstrated greater activity for rewarded relative to neutral trials. A significant age-group by incentive by time effect was found in right IPS (F(14,189) = 2.730, p < .001). Post-hoc comparisons indicated that only adolescents (incentive by time: F(7,63) = 4.894, p < .0001) demonstrated a significant condition by time interaction, increasing activity in response to reward trials relative to neutral (Fig. 7a).
Fig. 7.
Time courses separated by age group of regions where adolescents demonstrated a modulation by incentive condition. See materials and methods for how time courses were extracted. Error bars represent ±1 standard error of the mean at each time point. Vertical dotted lines represent the first half of the modeled response (8 TRs) that showed a significant interaction effect. For visualization purposes only, filled black circles indicating location of the masks are schematically shown above slices of the AFNI Talairach atlas template brain, drawn using AFNI. The circles do not reflect the actual shape of the mask. R = Right hemisphere of the brain. L = Left hemisphere of the brain.
In the right putamen, a significant incentive by time interaction was observed (F(7,189) = 2.589, p < .05). Adolescents demonstrated increased activity to rewarded relative to neutral trials (incentive by time: F(7,63) = 3.735, p < .005) whereas adults and children did not. The left putamen showed a similar pattern of activity, with a significant incentive by time interaction (F(7,189) = 2.857, p < .05), with only adolescents showing increased activity for rewarded relative to neutral trials (incentive by time: F = (7,63) = 5.008, p < .0001) (Fig. 7b and c).
In right ventral striatum, there was a significant incentive by time interaction (F(7,189) = 2.501, p < .05) and a trend for a age-group by incentive by time interaction (p = .08). Adolescents demonstrated significantly increased activity for rewarded trials relative to neutral (incentive by time F(7,63) = 3.735, p < .005), but children and adults did not. In left ventral striatum, similar to the contra-lateral region, a significant incentive by time interaction was observed (F(7,189) = 2.343, p < .05). As before, adolescents increased activity during rewarded trials relative to neutral (incentive by time: F(7,63) = 4.805, p < .0001) whereas adults and children did not (Fig. 7d and e).
4. Discussion
The purpose of this study was to better understand processes underlying the influence of rewards on inhibitory control in adolescence by including child and adult groups. Behavioral results indicated that rewards enhanced task performance (i.e. reduced latencies and error rates) across ages. Imaging results indicated that heightened VS activation during rewarded relative to neutral trials was specific to adolescence, following a non-linear trajectory from childhood. Importantly, results also demonstrated rewards-enhanced activity in regions associated with oculomotor and inhibitory control in adolescence, providing further insight on the possible processes underlying reward-modulated cognitive control during this developmental period.
4.1. Rewards enhance inhibitory control behavior
Consistent with previous developmental studies of inhibitory control (without an incentive) using the AS task (Fischer et al., 1997, Klein and Foerster, 2001, Luna et al., 2004, Munoz et al., 1998), there were differences in performance in children and adolescents relative to adults on neutral trials. However, this was not observed in the reward condition, where children and adolescents’ performance increased to adult levels. This result suggests that younger participants have the ability to perform like adults when provided with an incentive to do so, reflecting a heightened relative motivation and a particular sensitivity to rewards.
Adults showed consistent inhibitory error rates (10–20%) across incentives suggesting that their cognitive control is more stable and less prone to external influences and may already be optimal, at ceiling levels. However, similar to younger participants, adults showed faster latencies for correct rewarded AS trials relative to correct AS neutral trials supporting the notion that incentives influence the generation of voluntary saccades, consistent with previous work (Hikosaka et al., 2006, Geier et al., 2010). Developmental results are consistent with previous findings demonstrating improved cognitive performance and decreased latencies with the presentation of a monetary incentive in adolescents (Duka and Lupp, 1997, Geier et al., 2010, Hardin et al., 2007, Jazbec et al., 2005, Jazbec et al., 2006). The decrease in latencies and error rate during rewarded trials suggest optimization of behavior that leads to the receipt of a reward. Younger participants demonstrate that they improve performance in tasks that have known immaturities within the context of a potential reward, suggesting an enhancement in motivation that may be required to achieve adult levels of performance. Similarly, immaturities in reward processing may enhance behaviors that appear to lead to a reward such as sensation seeking and risk-taking, which can at times be suboptimal (Steinberg, 2004).
4.2. Reward incentives enhance brain activity in adolescents
The brain regions supporting the generation of voluntary saccadic eye movements as well as the processing of rewards are well-delineated (Apicella et al., 1991, Breiter et al., 2001, Brown et al., 2006, Delgado et al., 2000, Delgado et al., 2003, Elliott et al., 2003, Hikosaka and Watanabe, 2000, Knutson et al., 2000, Luna and Sweeney, 1999, Matsuda et al., 2004, Munoz and Everling, 2004, Roesch and Olson, 2003, Roesch and Olson, 2004, Schultz, 2000, Schultz et al., 2000, Thut et al., 1997). In the present study, all three age groups robustly engaged key oculomotor control regions bilaterally across incentives including the FEF, SEF, inferior parietal sulcus (IPS), superior parietal lobule (SPL), putamen, and the dorsal ACC. Across ages, reward related regions were also recruited including VS, OFC and ACC. These results suggest that the basic circuitry supporting inhibitory control and reward processing is in place by childhood.
Results indicated several age-related differences in the magnitude of recruitment of this circuitry suggesting unique developmental profiles of reward processing and its influence on cognitive control in adolescence. Only adolescents showed a modulation of rewards on activity in right IPS, bilateral putamen, and bilateral VS. The IPS and putamen have both been associated with response planning, oculomotor control (Everling and Munoz, 2000), reward prediction (Peck et al., 2009) and outcome (Delgado et al., 2003). Increased activity of these key regions may support improved performance during rewarded trials. The VS is a region that has been consistently associated with all phases of the processing of rewards, including detection, anticipation, and outcome (Bjork et al., 2004, Dreher et al., 2006, Galvan et al., 2006, Knutson et al., 2001, Schultz et al., 1992) and may underlie bias for immediate over future rewards (McClure et al., 2004). In this region, we observed a modulation of incentive condition in adolescents and a lack of differentiation by incentive type children and adults.
Age-related differences in incentive processing have been observed in other studies, with some studies demonstrating a relative under-activity during different stages of reward processing such as cue detection (Geier et al., 2010) and reward anticipation (Bjork et al., 2004, Bjork et al., 2010) in VS and over-activity during reward receipt (Ernst et al., 2005, van Leijenhorst et al., 2009, van Leijenhorst et al., 2010) and response preparation (Geier et al., 2010), as well as across an entire reward trial (Galvan et al., 2006). However, in our study, age-related differences were determined by the relative attenuated response to neutral trials in adolescents, a differentiation that was not present in children and adults. Neutral trials in the context of an incentive task may be perceived as a relative loss of a reward, rather than simply lacking in reward value. Furthermore, this relative difference between rewarded and neutral trials observed in adolescents may indicate an increased sensitivity to incentives that is not present in children or adults. DA neurons that heighten responses to reward contingencies in primary reward regions (such as VS), may contribute to enhanced signaling of oculomotor control neurons in regions such as the IPS, that may underlie enhanced performance. The IPS in particular has been found to be involved in antisaccade preparation (Curtis and Connolly, 2008). Enhanced VS activity in the adolescent may result in increases in regions supporting the specific behavior that leads to rewards such as the IPS and its role in antisaccade performance (Brown et al., 2007, Curtis and Connolly, 2008).
Although children displayed the same behavioral pattern as adolescents, their brain function in VS, putamen and IPS mimicked those of adults (i.e. did not differentiate by incentive condition). This finding is similar to other studies that demonstrated an “inverted U” in brain function across development, and highlights the peak in reward sensitivity in adolescence (Cohen et al., 2010, Somerville et al., 2010, van Leijenhorst et al., 2009, van Leijenhorst et al., 2010). Furthermore, children demonstrated increased activity in SEF for neutral trials and in the dorsal ACC for both reward and neutral trials relative to the older groups. Increased reliance on oculomotor and prefrontal control regions during correct AS trails suggests that children may have relative increased difficulty in performing the antisaccade at optimal levels and may require greater engagement of critical regions to perform the task (Luna et al., 2001, Luna et al., 2004) diminishing potential differences between reward and neutral trials. Alternatively, children may have recruited regions outside of our a priori functionally defined brain regions to support better performance in rewarded trials. Overall, children showed a distinct profile from adolescence and adulthood, reflecting dependence on medial prefrontal structures to perform the task regardless of incentives and not relying on oculomotor control or reward related regions to support improved performance during rewarded trials.
Finally, similar to previous findings (Galvan et al., 2006, Geier et al., 2010, van Leijenhorst et al., 2009) only adults recruited the OFC during rewarded trials. The lateral OFC has been previously implicated in many aspects of reward processing especially in coding representations of valence and magnitude of reward and punishment and is highly connected to the basal ganglia (Breiter et al., 2001, Delgado et al., 2000, Hikosaka and Watanabe, 2000, Knutson et al., 2000, O’Doherty et al., 2004, Roesch and Olson, 2003, Roesch and Olson, 2004, Schultz et al., 2000, Wise, 2002). The OFC may support the executive processing of rewards. This more executive component of reward processing may still be immature in adolescence.
Relative increased sensitivity in the VS coupled with under-activity of the OFC in response to rewards in adolescence may result in a vulnerability to behavior that is directed by incentives when executive value has not been properly assessed. The unique circuitry recruited by adolescents may be associated with the known structural immaturities including continued gray matter thinning of the basal ganglia (Sowell et al., 2002) and OFC (Gogtay et al., 2004), and increases in dopamine transmission (Kalsbeek et al., 1988, Meng et al., 1999, Rosenberg and Lewis, 1994, Rosenberg and Lewis, 1995, Seeman et al., 1987). This may enhance reward effects and undermine the executive assessment of rewards. Risk-taking involves behavior that is guided by reward receipt with limitations in executive aspects of reward value and consequences. In this manner, these results suggest that the circuitry that supports executive assessment of rewards and modulation of motivation may still be immature in adolescence and contribute to the high rate of risk-taking in adolescence. That is, adolescents may be more influenced by limbic system control, which could override their ability to effectively utilize executive control systems (Spear, 2000).
4.3. Limitations
We note limitations in the present study in order to inform future studies. Our sample size of 10 participants per age group limited our ability to assess pubertal status, sex, and continuous age effects. With our present sample size we had the power to detect medium to large effects with 3-way (Age Group by Condition by Time) and a 2 way interactions (Age Group by Condition) (effect size: .201). We also note that this limitation in power indicates that there may be even more age related differences than the ones reported in the present study, especially with regards to the modest differences found in the child group. On the other hand, our power indicates that our findings regarding functional differences in adolescent brain function is a robust effect. Furthermore, this study used monetary incentives as an index of reward. It is not clear whether the incentive was considered “equal” across age groups. Future studies in our laboratory focus on equating incentives to better assess developmental effects.
5. Conclusion
Overall, our results speak to a key component of adolescent immaturity, which lies in the differences in motivationally driven behaviors. A motivated behavior refers to the ability of an organism to designate a motor output based on the value it places on a stimulus input (which is based on learned associations or prior experience with the stimulus), thereby acting to approach or avoid the stimulus (Ernst et al., 2009, Salamone and Correa, 2002). Critical to motivated behaviors are the brain systems (perceptual, cognitive, emotional) that allow for the processing of external cues or internal brain states, allowing for an optimal response to be made. Our results that adolescents showed increased activity in regions supporting performance that resulted in reward receipt reflect enhancements in motivation. It is possible that younger individuals require this added motivation to perform the task at optimal levels.
The current findings suggest that basic neural circuitry underlying response inhibition and incentive processing is established in childhood. However, immaturities in regions associated with reward reactivity and executive assessment appear to follow a non-linear developmental trajectory from childhood through adolescence into adulthood. Adolescents showed reward related increases in reward and cognitive control related regions while showing limitations in executive assessment of rewards. Our results support current models regarding adolescent immaturities in reward processing and cognitive control, suggesting that an overall over-reactive reward response may enhance engaging in behaviors that result in immediate rewards. Taken together, these findings indicate immaturities in the developing brain that could be especially vulnerable to risk taking and other suboptimal behaviors during adolescence.
Future investigations into the nature of motivated behaviors in adolescence should examine other types of incentives (i.e. social), that likely play a large role in determining behavior.
Grant
This study was supported by National Institute of Mental Health Grant MH080243.
Acknowledgements
We thank Robert Terwilliger for advice related to the methods and analysis conducted in this manuscript. We also thank Emi Yasui, Natalie Nawarawong and Alina Vaisleib for assistance with data collection and scoring of eye data. We also thank all participants and their families who volunteered for this study.
References
- Apicella P., Ljungberg T., Scarnati E., Schultz W. Responses to reward in monkey dorsal and ventral striatum. Experimental Brain Research. 1991;85:491–500. doi: 10.1007/BF00231732. [DOI] [PubMed] [Google Scholar]
- Asato M.R., Terwilliger R., Woo J., Luna B. White matter development in adolescents: a DTI study. Cerebral Cortex. 2010;20:2122–2131. doi: 10.1093/cercor/bhp282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedard A.C., Nichols S., Barbosa J.A., Schachar R., Logan G.D., Tannock R. The development of selective inhibitory control across the life span. Developmental Neuropsychology. 2002;21:93–111. doi: 10.1207/S15326942DN2101_5. [DOI] [PubMed] [Google Scholar]
- Bjork J.M., Knutson B., Fong G.W., Caggiano D.M., Bennett S.M., Hommer D.W. Incentive-elicited brain activation in adolescents: similarities and differences from young adults. Journal of Neuroscience. 2004;24:1793–1802. doi: 10.1523/JNEUROSCI.4862-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Chen G., Hommer D.W. Adolescents, adults and rewards: comparing motivational neurocircuitry recruitment using fMRI. PLoS One. 2010;5:e11440. doi: 10.1371/journal.pone.0011440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjork J.M., Smith A.R., Danube C.L., Hommer D.W. Developmental differences in posterior mesofrontal cortex recruitment by risky rewards. Journal of Neuroscience. 2007;27:4839–4849. doi: 10.1523/JNEUROSCI.5469-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breiter H.C., Aharon I., Kahneman D., Dale A., Shizgal P. Functional imaging of neural responses to expectancy and experience of monetary gains and losses. Neuron. 2001;30:619–639. doi: 10.1016/s0896-6273(01)00303-8. [DOI] [PubMed] [Google Scholar]
- Brown M.R., Goltz H.C., Vilis T., Ford K.A., Everling S. Inhibition and generation of saccades: rapid event-related fMRI of prosaccades, antisaccades, and nogo trials. NeuroImage. 2006;33:644–659. doi: 10.1016/j.neuroimage.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Brown M.R., Vilis T., Everling S. Frontoparietal activation with preparation for antisaccades. Journal of Neurophysiology. 2007;98:1751–1762. doi: 10.1152/jn.00460.2007. [DOI] [PubMed] [Google Scholar]
- Bunge S.A., Dudukovic N.M., Thomason M.E., Vaidya C.J., Gabrieli J.D. Immature frontal lobe contributions to cognitive control in children: evidence from fMRI. Neuron. 2002;33:301–311. doi: 10.1016/s0896-6273(01)00583-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler K.M., Zacks R.T., Henderson J.M. Suppression of reflexive saccades in younger and older adults: age comparisons on an antisaccade task. Memory & Cognition. 1999;27:584–591. doi: 10.3758/bf03211552. [DOI] [PubMed] [Google Scholar]
- Chambers R.A., Taylor J.R., Petenza M.N. Developmental neurocircuitry of motivation in adolescence: a critical period of addiction vulnerability. American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherkasova M.V., Manoach D.S., Intriligator J.M., Barton J.J.S. Antisaccades and task-switching: interactions in controlled processing. Experimental Brain Research. 2002;144:528–537. doi: 10.1007/s00221-002-1075-z. [DOI] [PubMed] [Google Scholar]
- Cohen J.R., Asarnow R.F., Sabb F.W., Bilder R.M., Bookheimer S.Y., Knowlton B.J. A unique adolescent response to reward prediction errors. Nature Neuroscience. 2010;13:669–671. doi: 10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox R.W. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Computers and Biomedical Research. 1996;29:162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- Curtis C.E, Connolly J.D. Saccade preparation signals in the human frontal and parietal cortices. Journal of Neurophysiology. 2008;99:133–145. doi: 10.1152/jn.00899.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado M.R., Locke H.M., Stenger V.A., Fiez J.A. Dorsal striatum responses to reward and punishment: effects of valence and magnitude manipulations. Cognitive, Affective & Behavioral Neuroscience. 2003;3:27–38. doi: 10.3758/cabn.3.1.27. [DOI] [PubMed] [Google Scholar]
- Delgado M.R., Nystrom L.E., Fissell C., Noll D.C., Fiez J.A. Tracking the hemodynamic responses to reward and punishment in the striatum. Journal of Neurophysiology. 2000;84:3072–3077. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- Dreher J.C., Kohn P., Berman K.F. Neural coding of distinct statistical properties of reward information in humans. Cerebral Cortex. 2006;16:561–573. doi: 10.1093/cercor/bhj004. [DOI] [PubMed] [Google Scholar]
- Duka T., Lupp A. The effects of incentive on antisaccades: is a dopaminergic mechanism involved. Behavioural Pharmacology. 1997;8:373–382. [PubMed] [Google Scholar]
- Elliott R., Newman J.L., Longe O.A., Deakin J.F. Differential response patterns in the striatum and orbitofrontal cortex to financial reward in humans: a parametric functional magnetic resonance imaging study. The Journal of Neuroscience. 2003;23:303–307. doi: 10.1523/JNEUROSCI.23-01-00303.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Nelson E.E., Jazbec S., McClure E.B., Monk C.S., Leibenluft E. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M., Pine D.S., Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M., Romeo R.D., Andersen S.L. Neurobiology of the development of motivated behaviors in adolescence: a window into a neural systems model. Pharmacology Biochemistry and Behavior. 2009;93:199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- Eshel N., Nelson E.E., Blair R.J., Pine D.S., Ernst M. Neural substrates of choice selection in adults and adolescents: development of the ventrolateral prefrontal and anterior cingulate cortices. Neuropsychologia. 2007;45:1270–1279. doi: 10.1016/j.neuropsychologia.2006.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everling S., Fischer B. The antisaccade: a review of basic research and clinical studies. Neuropsychologia. 1998;36:885–899. doi: 10.1016/s0028-3932(98)00020-7. [DOI] [PubMed] [Google Scholar]
- Everling S., Munoz D.P. Neuronal correlates for preparatory set associated with pro-saccades and anti-saccades in the primate frontal eye field. Journal of Neuroscience. 2000;20:387–400. doi: 10.1523/JNEUROSCI.20-01-00387.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer B., Biscaldi M., Gezeck S. On the development of voluntary and reflexive components in human saccade generation. Brain Research. 1997;754:285–297. doi: 10.1016/s0006-8993(97)00094-2. [DOI] [PubMed] [Google Scholar]
- Fischer B., Weber H. Effects of procues on error rate and reaction times of antisaccades in human subjects. Experimental Brain Research. 1996;109:507–512. doi: 10.1007/BF00229636. [DOI] [PubMed] [Google Scholar]
- Galvan A. Adolescent development of the reward system. Frontiers in Human Neuroscience. 2010;4 doi: 10.3389/neuro.09.006.2010. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan A., Hare T.A., Parra C.E., Penn J., Voss H., Glover G. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C.F., Luna B. The maturation of incentive processing and cognitive control. Pharmacology Biochemistry and Behavior. 2009;93:212–221. doi: 10.1016/j.pbb.2009.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier C.F., Terwilliger R., Teslovich T., Velanova K., Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd J.N., Vaituzis A.C., Hamburger S.D., Lange N., Rajapakse J.C., Kaysen D. Quantitative MRI of the temporal lobe, amygdala, and hippocampus in normal human development: ages 4–18 years. The Journal of Comparative Neurology. 1996;366:223–230. doi: 10.1002/(SICI)1096-9861(19960304)366:2<223::AID-CNE3>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Gitelman D.R. ILAB: a program for postexperimental eye movement analysis. Behavior Research Methods, Instruments, & Computers: A Journal of the Psychonomic Society, Inc. 2002;34:605–612. doi: 10.3758/bf03195488. [DOI] [PubMed] [Google Scholar]
- Gogtay N., Giedd J.N., Lusk L., Hayashi K.M., Greenstein D., Vaituzis A.C. Dynamic mapping of human cortical development during childhood through early adulthood. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyer A.E., Nelson E.E., Perez-Edgar K., Hardin M.G., Roberson-Nay R., Monk C.S. Striatal functional alteration in adolescents characterized by early childhood behavioral inhibition. Journal of Neuroscience. 2006;26:6399–6405. doi: 10.1523/JNEUROSCI.0666-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallett P.E. Primary and secondary saccades to goals defined by instructions. Vision Research. 1978;18:1279–1296. doi: 10.1016/0042-6989(78)90218-3. [DOI] [PubMed] [Google Scholar]
- Hardin M.G., Schroth E., Pine D.S., Ernst M. Incentive-related modulation of cognitive control in healthy, anxious, and depressed adolescents: development and psychopathology related differences. Journal of Child Psychology and Psychiatry and Allied Disciplines. 2007;48:446–454. doi: 10.1111/j.1469-7610.2006.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hikosaka K., Watanabe M. Delay activity of orbital and lateral prefrontal neurons of the monkey varying with different rewards. Cerebral Cortex. 2000;10:263–271. doi: 10.1093/cercor/10.3.263. [DOI] [PubMed] [Google Scholar]
- Hikosaka O., Nakumura K., Nakahara H. Basal ganglia orient eyes to reward. Journal of Neurophysiology. 2006;95:567–584. doi: 10.1152/jn.00458.2005. [DOI] [PubMed] [Google Scholar]
- Jazbec S., Hardin M.G., Schroth E., McClure E., Pine D.S., Ernst M. Age-related influence of contingencies on a saccade task. Experimental Brain Research. 2006;174:754–762. doi: 10.1007/s00221-006-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jazbec S., McClure E., Hardin M., Pine D.S., Ernst M. Cognitive control under contingencies in anxious and depressed adolescents: an antisaccade task. Biological Psychiatry. 2005;58:632–639. doi: 10.1016/j.biopsych.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Kalsbeek A., Voorn P., Buijs R.M., Pool C.W., Uylings H.B. Development of the dopaminergic innervation in the prefrontal cortex of the rat. Journal of Comparative Neurology. 1988;269:58–72. doi: 10.1002/cne.902690105. [DOI] [PubMed] [Google Scholar]
- Klein C., Foerster F. Development of prosaccade and antisaccade task performance in participants aged 6 to 26 years. Psychophysiology. 2001;38:179–189. [PubMed] [Google Scholar]
- Knutson B., Adams C.M., Fong G.W., Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. The Journal of Neuroscience. 2001;21:RC159. doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B., Westdorp A., Kaiser E., Hommer D. FMRI visualization of brain activity during a monetary incentive delay task. NeuroImage. 2000;12:20–27. doi: 10.1006/nimg.2000.0593. [DOI] [PubMed] [Google Scholar]
- Levin H.S., Culhane K.A., Hartmann J., Evankovich K., Mattson A.J. Developmental changes in performance on tests of purported frontal lobe functioning. Developmental Neuropsychology. 1991;7:377–395. [Google Scholar]
- Liston C., Watts R., Tottenham N., Davidson M.C., Niogi S., Ulug A.M. Frontostriatal microstructure modulates efficient recruitment of cognitive control. Cerebral Cortex. 2006;16:553–560. doi: 10.1093/cercor/bhj003. [DOI] [PubMed] [Google Scholar]
- Luna B. The maturation of cognitive control and the adolescent brain. In: Aboitiz F., Cosmelli D., editors. From Attention to Goal-Directed Behavior: Neurodynamical, Methodological and Clinical Trends. Springer-Verlag; Berlin/Heidelberg: 2009. pp. 249–274. [Google Scholar]
- Luna B., Garver K.E., Urban T.A., Lazar N.A., Sweeney J.A. Maturation of cognitive processes from late childhood to adulthood. Child Development. 2004;75:1357–1372. doi: 10.1111/j.1467-8624.2004.00745.x. [DOI] [PubMed] [Google Scholar]
- Luna B., Padmanabhan A., O’Hearn K. What has fMRI told us about the development of cognitive control through adolescence? Brain and Cognition. 2010;72:101–113. doi: 10.1016/j.bandc.2009.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna B., Sweeney J.A. Cognitive functional magnetic resonance imaging at very-high-field: eye movement control. Topics in Magnetic Resonance Imaging. 1999;10:3–15. doi: 10.1097/00002142-199902000-00002. [DOI] [PubMed] [Google Scholar]
- Luna B., Thulborn K.R., Munoz D.P., Merriam E.P., Garver K.E., Minshew N.J. Maturation of widely distributed brain function subserves cognitive development. NeuroImage. 2001;13:786–793. doi: 10.1006/nimg.2000.0743. [DOI] [PubMed] [Google Scholar]
- Luna B., Thulborn K.R., Strojwas M.H., McCurtain B.J., Berman R.A., Genovese C.R. Dorsal cortical regions subserving visually-guided saccades in humans: an fMRI study. Cerebral Cortex. 1998;8:40–47. doi: 10.1093/cercor/8.1.40. [DOI] [PubMed] [Google Scholar]
- Matsuda T., Matsuura M., Ohkubo T., Ohkubo H., Matsushima E., Inoue K. Functional MRI mapping of brain activation during visually guided saccades and antisaccades: cortical and subcortical networks. Psychiatry Research. 2004;131:147–155. doi: 10.1016/j.pscychresns.2003.12.007. [DOI] [PubMed] [Google Scholar]
- May J.C., Delgado M.R., Dahl R.E., Stenger V.A., Ryan N.D., Fiez J.A. Event-related functional magnetic resonance imaging of reward-related brain circuitry in children and adolescents. Biological Psychiatry. 2004;55:359–366. doi: 10.1016/j.biopsych.2003.11.008. [DOI] [PubMed] [Google Scholar]
- McClure S.M., York M.K., Montague P.R. The neural substrates of reward processing in humans: the modern role of FMRI. Neuroscientist. 2004;10:260–268. doi: 10.1177/1073858404263526. [DOI] [PubMed] [Google Scholar]
- Meng S.Z., Ozawa Y., Itoh M., Takashima S. Developmental and age-related changes of dopamine transporter, and dopamine D1 and D2 receptors in human basal ganglia. Brain Research. 1999;843:136–144. doi: 10.1016/s0006-8993(99)01933-2. [DOI] [PubMed] [Google Scholar]
- Munoz D.P., Broughton J.R., Goldring J.E., Armstrong I.T. Age-related performance of human subjects on saccadic eye movement tasks. Experimental Brain Research. 1998;121:391–400. doi: 10.1007/s002210050473. [DOI] [PubMed] [Google Scholar]
- Munoz D.P., Everling S. Look away: the anti-saccade task and the voluntary control of eye movement. Nature Reviews Neuroscience. 2004;5:218–228. doi: 10.1038/nrn1345. [DOI] [PubMed] [Google Scholar]
- O’Doherty J., Dayan P., Schultz J., Deichmann R., Friston K., Dolan R.J. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Ollinger J.M., Corbetta M., Shuldman G.L. Separating processes within a trial in event-related functional MRI: Part II. NeuroImage. 2001;13:218–229. doi: 10.1006/nimg.2000.0711. [DOI] [PubMed] [Google Scholar]
- Ollinger J.M., Shulman G.L., Corbetta M. Separating processes within a trial in event-related functional MRI: Part I. NeuroImage. 2001;13:210–217. doi: 10.1006/nimg.2000.0710. [DOI] [PubMed] [Google Scholar]
- Paus T., Babenko V., Radil T. Development of an ability to maintain verbally instructed central gaze fixation studied in 8- to 10-year-old children. International Journal of Psychophysiology. 1990;10:53–61. doi: 10.1016/0167-8760(90)90045-f. [DOI] [PubMed] [Google Scholar]
- Peck C.J., Jangraw D.C., Suzuki M., Efem R., Gottleib J. Reward modulates attention independently of action value in posterior parietal cortex. The Journal of Neuroscience. 2009;29:11182–11191. doi: 10.1523/JNEUROSCI.1929-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridderinkhof K.R., Band G.P.H., Logan G.D. A study of adaptive behavior: effects of age and irrelevant information on the ability to inhibit one's actions. Acta Psychologica. 1999;101:315–337. [Google Scholar]
- Ridderinkhof K.R., van der Molen M.W. Mental resources, processing speed, and inhibitory control: a developmental perspective. Biological Psychology. 1997;45:241–261. doi: 10.1016/s0301-0511(96)05230-1. [DOI] [PubMed] [Google Scholar]
- Roesch M.R., Olson C.R. Impact of expected reward on neuronal activity in prefrontal cortex, frontal and supplementary eye fields and premotor cortex. Journal of Neurophysiology. 2003;90:1766–1789. doi: 10.1152/jn.00019.2003. [DOI] [PubMed] [Google Scholar]
- Roesch M.R, Olson C.R. Neuronal activity related to reward value and motivation in primate frontal cortex. Science. 2004;304:307–310. doi: 10.1126/science.1093223. [DOI] [PubMed] [Google Scholar]
- Rosenberg D.R., Lewis D.A. Changes in the dopaminergic innervation of monkey prefrontal cortex during late postnatal development: a tyrosine hydroxylase immunohistochemical study. Biological Psychiatry. 1994;36:272–277. doi: 10.1016/0006-3223(94)90610-6. [DOI] [PubMed] [Google Scholar]
- Rosenberg D.R., Lewis D.A. Postnatal maturation of the dopaminergic innervation of monkey prefrontal and motor cortices: a tyrosine hydroxylase immunohistochemical analysis. Journal of Comparative Neurology. 1995;358:383–400. doi: 10.1002/cne.903580306. [DOI] [PubMed] [Google Scholar]
- Rosenberg D.R., Sweeney J.A., Gillen J., Kim J., Varenelli M., O’Hearn K. Magnetic resonance imaging of children without sedation: preparation with simulation. Journal of the American Academy of Child and Adolescent Psychiatry. 1997;36:853–859. doi: 10.1097/00004583-199706000-00024. [DOI] [PubMed] [Google Scholar]
- Rubia K., Smith A.B., Taylor E., Brammer M. Linear age-correlated functional development of right inferior fronto-striato-cerebellar networks during response inhibition and anterior cingulate during error-related processes. Human Brain Mapping. 2007;28:1163–1177. doi: 10.1002/hbm.20347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone J.D., Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behavioural Brain Research. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- Schlag-Rey M., Amador N., Sanchez H., Schlag J. Antisaccade performance predicted by neuronal activity in the supplementary eye field. Nature. 1997;390:398–401. doi: 10.1038/37114. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple reward signals in the brain. Nature Reviews Neuroscience. 2000;1:199–207. doi: 10.1038/35044563. [DOI] [PubMed] [Google Scholar]
- Schultz W., Apicella P., Scarnati E., Ljungberg T. Neuronal activity in monkey ventral striatum related to the expectation of reward. Journal of Neuroscience. 1992;12:4595–4610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W., Tremblay L., Hollerman J.R. Reward processing in primate orbitofrontal cortex and basal ganglia. Cerebral Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Seeman P., Bzowej N.H., Guan H.C., Bergeron C., Becker L.E., Reynolds G.P. Human brain dopamine receptors in children and aging adults. Synapse. 1987;1:399–404. doi: 10.1002/syn.890010503. [DOI] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 2004;23:S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Somerville L.H., Hare T., Casey B.J. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2010 doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell E.R., Thompson P.M., Holmes C.J., Jernigan T.L., Toga A.W. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2:859–861. doi: 10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Sowell E.R., Trauner D.A., Gamst A., Jernigan T.L. Development of cortical and subcortical brain structures in childhood and adolescence: a structural MRI study. Developmental Medicine and Child Neurology. 2002;44:4–16. doi: 10.1017/s0012162201001591. [DOI] [PubMed] [Google Scholar]
- Spear L.P. The adolescent brain and age-related behavioral manifestations. Neuroscience and Behavioral Reviews. 2000;24:417–463. doi: 10.1016/s0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- Steinberg L. Risk taking in adolescence: what changes, and why? Annals of the New York Academy of Sciences. 2004;1021:51–58. doi: 10.1196/annals.1308.005. [DOI] [PubMed] [Google Scholar]
- Talairach J., Tournoux P. Thieme Medical Publishers; New York: 1988. Co-Planar Stereotaxic Atlas of the Human Brain. [Google Scholar]
- Thut G., Schultz W., Roelcke U., Nienhusmeier M., Missimer J., Maguire R.P. Activation of the human brain by monetary reward. Neuroreport. 1997;8:1225–1228. doi: 10.1097/00001756-199703240-00033. [DOI] [PubMed] [Google Scholar]
- Toga A.W., Thompson P.M., Sowell E.R. Mapping brain maturation. Trends in Neurosciences. 2006;29:148–159. doi: 10.1016/j.tins.2006.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Wildenberg W.P.M., van der Molen M.W. Developmental trends in simple and selective inhibition of compatible and incompatible responses. Journal of Experimental Child Psychology. 2004;87:201–220. doi: 10.1016/j.jecp.2003.11.003. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L., Moor B.G., Op de Macks Z.A., Rombouts S.A., Westenberg P.M., Crone E.A. Adolescent risky decision-making: neurocognitive development of reward and control regions. NeuroImage. 2010;51(1):345–355. doi: 10.1016/j.neuroimage.2010.02.038. [DOI] [PubMed] [Google Scholar]
- van Leijenhorst L., Zanolie K., Van Meel C.S., Westenberg P.M., Rombouts S.A., Crone E.A. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2009 doi: 10.1093/cercor/bhp078. (Epub ahead of print) [DOI] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. Maturational changes in anterior cingulate and frontoparietal recruitment support the development of error processing and inhibitory control. Cerebral Cortex. 2008;18:2505–2522. doi: 10.1093/cercor/bhn012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velanova K., Wheeler M.E., Luna B. The maturation of task set-related activation supports late developmental improvements in inhibitory control. Journal of Neuroscience. 2009;29:12558–12567. doi: 10.1523/JNEUROSCI.1579-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, B.D., 1998. Deconvolution analysis of fMRI time series data: documentation for the AFNI software package. Unpublished work.
- Wheeler M.E., Shulman G.L., Buckner R.L., Miezin F.M., Velanova K., Petersen S.E. Evidence for separate perceptual reactivation and search processes during remembering. Cerebral Cortex. 2005;16:949–959. doi: 10.1093/cercor/bhj037. [DOI] [PubMed] [Google Scholar]
- Williams B.R., Ponesse J.S., Schachar R.J., Logan G.D., Tannock R. Development of inhibitory control across the life span. Developmental Psychology. 1999;35:205–213. doi: 10.1037//0012-1649.35.1.205. [DOI] [PubMed] [Google Scholar]
- Wise L.A., Sutton J.A., Gibbons P.D. Decrement in Stroop interference time with age. Perceptual and Motor Skills. 1975;41:149–150. [Google Scholar]
- Wise R.A. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36:229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]