Summary
Purpose
We undertook a systematic review of the evidence on disparities in epilepsy with a focus on North American data (Canada, United States, and the English-speaking Caribbean).
Methods
We identified and evaluated: access to and outcomes following medical and surgical treatment, disability, incidence and prevalence, and knowledge and attitudes. An exhaustive search (1965–2007) was done, including: (1) disparities by socioeconomic status (SES), race/ethnicity, age, or education of subgroups of the epilepsy population; or (2) disparities between people with epilepsy (PWE) and healthy people or with other chronic illnesses.
Results
From 1,455 citations, 278 eligible abstracts were identified and 44 articles were reviewed. Comparative research data were scarce in all areas. PWE have been shown to have lower education and employment status; among PWE, differences in access to surgery have been shown by racial/ethnic groups. Aboriginals, women, and children have been shown to differ in use of health resources. Poor compliance has been shown to be associated with lower SES, insufficient insurance, poor relationship with treating clinicians, and not having regular responsibilities.
Discussion
Comprehensive, comparative research on all aspects of disparities in epilepsy is needed to understand the causes of disparities and the development of any policies aimed at addressing health disparities and minimizing their impact.
Keywords: Epilepsy, Disparity, North America
Over the last two decades, the North American population has become more diverse, and geographic areas that were exclusively monoethnic are becoming multiethnic. The needs of these emerging ethnic groups are different and, in some cases, challenging to physicians who are looking for the best evidence to guide the care of these patients.
Among the various aspects of inequalities in health, disparities in health care carry a special sense of immediacy and relevance. There is ongoing debate about the pros and cons of, and the best approach to collecting and publishing data regarding disparities in health care, particularly with respect to racial and ethnic differences in health (Krieger, 2000; LaVeist, 2000). Because journal articles contribute importantly to setting policies on research and health care, some journals have made specific recommendations regarding the publication of data about disparities involving racial and ethnic aspects (Kaplan & Bennett, 2003). However, disparities in health care involve more than racial/ethnic differences. Educational level, socioeconomics, age, gender, and place of residence are some of the variables that may play a role in inequalities of health care for various conditions. To our knowledge, no systematic evaluation or recommendations have been made regarding the analysis and publication of these types of data in epilepsy.
Kaplan and Bennett’s approach may be applicable to disparities beyond racial and ethnic issues, because it has heuristic value in avoiding harmful consequences when writing about health disparities (Kaplan & Bennett, 2003). First, these authors suggest that commentators should account for the limitations of available data. The lack of consistency across studies and data sets and in the selection and definition of categories, outcome measures, and measurement itself, makes it difficult to generalize and may also detract from the validity of the data. Race and ethnicity are particularly susceptible to problems of definition and categorization. For example, Boehmer et al. have documented extensive discordance in race/ethnicity classifications (Boehmer et al., 2002), and these categorizations should not be assumed as reliable, valid, or comprehensive. Second, it is important to distinguish between risk factors and risk markers for disparities in health. That is, statistically significant associations between disparities in health related to a specific characteristic or belonging to a specific group, do not necessarily imply causation. Furthermore, the likelihood of finding inequalities in health may vary considerably among members of the group, and those who are actually at risk may share relevant characteristics with people in other groups. Variables are also often closely interrelated, as illustrated by race/ethnicity and socioeconomic status (SES). One must be aware that unknown factors may underpin the observed associations, and that the variables we observe are simply markers of other, unexplored domains. The third recommendation pertains to taking care not to contribute adversely to social stigma and separation. Language influences people’s views and their notion of reality, and authors must be mindful of the plausible interpretation and consequences of their statements.
An initial effort to address health disparities in epilepsy took place in Rockville, MD, United States, on November 13th of 2002. A panel of investigators in epilepsy concluded that additional information on disparities in epilepsy is important for the development of effective intervention programs or campaigns (NIH, 2002). Subsequently, two nonsystematic reviews were published focusing on disparities in epilepsy. Both arrived at similar conclusions: There is scarce information about disparities in epilepsy, and research on the area is needed (Szaflarski et al., 2006; Theodore et al., 2006). Furthermore, a study assessing the reporting of race/ethnicity in epilepsy clinical trials in high-impact journals specialized in the areas of neurology and epilepsy, found that only 6.6% of clinical trials reported race/ethnicity of study participants, and only 1.9% attempted to analyze possible differences between participants (Burneo & Martin, 2004).
A recent initiative from the North American Commission (NAC) of the International League Against Epilepsy (ILAE) assembled a task force to systematically evaluate what is known about disparities in epilepsy. The task force embarked on a systematic review of the literature to identify and evaluate what is known, knowledge gaps, and important areas for research efforts. These are the results.
Methods
Objectives and definitions
Our main objective was to identify disparities in the following areas of epilepsy: access to medical and surgical care, knowledge and attitudes, disability in employment and education, epidemiology of epilepsy, and outcomes following medical and surgical treatment.
The scope of this article is centered in North America and the Caribbean, a geographic region that included Canada, the United States, and the English-speaking countries of the Caribbean region.
Disparities were defined as differences in incidence or prevalence, knowledge and attitudes, health care use, or outcomes. We assessed the association of disparities with differences in SES, race/ethnicity, and other sociodemographic or health care system factors (Carter-Pokras & Baquet, 2002).
To identify the disparity literature we followed the Rockefeller Foundation recommendations (Rockefeller Foundation, 2002), which include: (1) defining which aspects of epilepsy to measure; (2) indentifying the relevant population groups across which to compare differences; and (3) choosing reference groups for comparison, which may be internal (within the population of interest) or external (from a different population).
Data sources
A medical librarian undertook a comprehensive literature search of Medline (via PubMed), EMBASE, and The Cochrane database (see Appendix S1). In addition, we searched through bibliographies of pertinent original articles, reviews, abstracts, and book chapters, to include all literature up to and including 2007.
Study selection
Five aspects of disparities were considered: (1) access to medical and surgical care, (2) knowledge and attitudes, (3) disability in employment and education, (4) epidemiology, and (5) outcomes following medical and surgical treatment. We included articles describing at least one of the following variables: SES, race/ethnicity, age, education, and psychiatric and somatic comorbidities. In addition, studies had to contain internal comparisons within subgroups of people with epilepsy (PWE) or a comparison between PWE and other chronic illnesses.
Exclusion criteria were: case reports, sample size <20, letters, and nonsystematic reviews (except for consulting cited references).
We focused on studies dealing with the North America region, as defined by the ILAE (Canada, United States, and English-speaking Caribbean countries).
Data gathering
All authors contributed to the review. They independently selected any abstracts that could be potentially eligible. Full text articles were distributed to four groups of two reviewers each, who agreed on the selection of the final set of articles for each area of interest (see preceding).
Results
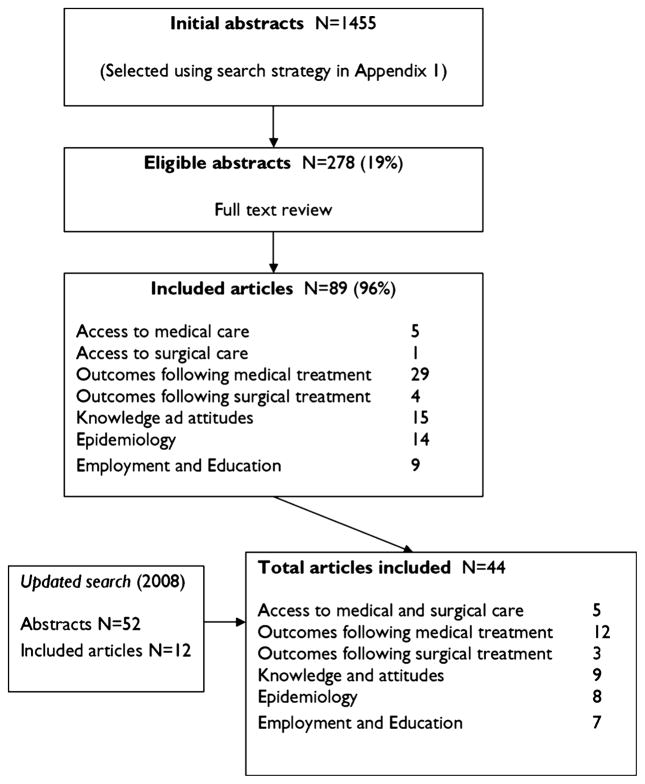
A total of 1,455 abstracts were initially selected, but only 278 were considered eligible for full review after inclusion and exclusion criteria were applied (Table S1). Only 44 articles fulfilled the eligibility criteria (Fig. 1). Of these, five articles focused on access to medical and surgical care, 12 on outcomes following medical treatment and 3 following surgical treatment, 9 covered knowledge and attitudes, 8 dealt with epidemiology, and 7 with disability in employment and education. No articles from the English-speaking Caribbean region were found.
Figure 1.
Literature search strategy used in the systematic review.
Epilepsia © ILAE
General concepts
Disparities in epilepsy care occur when sociodemographic, geographic, and health system factors are more associated with variations in the aspects of epilepsy than the personal biological condition of patients (Andersen & Aday, 1978; Institute-of-Medicine-Committee-on-the-Consequences-of-Uninsurance, 2003). For example, in the United States where health insurance coverage is not universal, the positive relationship between health insurance coverage and the rate of specialist physician visits would suggest an insurance-related disparity. There is a fairly extensive and growing literature on the factors associated with epilepsy care in different countries, although much less is known about the situation in developed than in developing countries. These studies, which have largely been conducted in the United States and Canada have shown that racial/ethnic disparities exist with respect to access to medical and surgical care, use of antiepileptic drugs (AEDs), and the likelihood of receiving treatment in hospital emergency departments.
Disparities in access to surgical and medical care in epilepsy
There is a profound lack of evidence regarding the important theme of disparities in health care in epilepsy. Although our extensive literature search using the broad terms “disparities” and “health care” revealed 2,449 publications in diverse areas of medicine, only five studies (involving 2,169 patients) in the area of epilepsy met eligibility criteria for inclusion in this analysis (Dodrill et al., 1987; Wiebe et al., 1999; Ott et al., 2003; Burneo et al., 2005a; Jette et al., 2008). Of these studies, three were from the United States (Dodrill et al., 1987; Ott et al., 2003; Burneo et al., 2005a) and two were from Canada (Wiebe et al., 1999; Jette et al., 2008). Seventy-five percent of the patients were contributed by the two Canadian studies (n = 1,744), in particular by the study of (Jette et al., 2008) (n = 1,431). The studies varied substantially in methodology, definitions, populations, and outcomes. Only the Canadian studies were population based, whereas other studies referred to specific populations, referral centers, or local/regional registries, and ranged in sample size from 30 to 122.
Disparities in rates of epilepsy surgery
The one study dealing with surgical care retrospectively explored rates of temporal lobe epilepsy surgery in consecutive patients undergoing presurgical evaluation for temporal lobe epilepsy at one center in the United States (Burneo et al., 2005a). The assessment of disparities focused on the comparison between African American (n = 30) and non-Hispanic whites (n = 70), but there is no description on how race/ethnicity was ascertained. In unadjusted univariate analyses, lower rates of epilepsy surgery were associated with being African-American [odds ratio (OR) 0.3], receiving Medicare (OR 0.2), and having a lower SES (OR 6.0), although the latter was not significant. However, on multivariate analyses, adjusting for all of these variables in addition to education, bitemporal epileptogenesis (as a marker of ineligibility for surgery), and gender, none of the differences between the two ethnic/racial groups were statistically significant. The lack of significance on multivariate analysis may reflect a lack of power due to the small sample, or the fact that race/ethnicity is a marker but not the cause of the disparity, or both.
It is of note that this study did not explore access to surgical care as such, but rather, whether surgery was performed. In fact these patients all underwent presurgical evaluation, they were deemed candidates for surgical intervention at similar rates in both groups, and they had sufficient health insurance to cover the surgical intervention. The reasons for a lower rate of surgery in the African-American group are unclear, and the authors speculate that it may be due to differences in health risk perceptions, communication issues between patients and clinicians, and attitudes toward surgery.
Disparities in medical care
Four studies dealt with aspects of medical care (Dodrill et al., 1987; Wiebe et al., 1999; Ott et al., 2003; Jette et al., 2008). Because their design, scope, and focus vary highly, it is not possible to derive aggregate estimates; instead, we present a description under several aspects of medical treatment.
Compliance with AEDs
One study dealt with disparities in adherence to AEDs (Dodrill et al., 1987). Dodrill et al. assessed the impact of cognitive function, SES, psychological aspects, and social function in a cohort of compliers (n = 80) and noncompliers (n = 42) assembled prospectively from a regional epilepsy center in the United States involving adults with all types of epilepsy. Compliance was defined by physicians’ ratings at two extremes of a scale (definitely compliant and noncompliant), based on a single AED blood level taken at the time of neuropsychological assessment. More patients among the noncomplier group reported perceived financial distress (p < 0.05), difficulty interacting with their physician (p < 0.02), having Medicaid as opposed to private insurance (p = 0.05), and not having regular daily responsibilities (p = 0.01) (Table 1). No differences emerged in other variables.
Table 1.
Factors associated with rate of use or access to care
| Epilepsy surgery |
| Lower in African Americans—but not significant |
| Medical therapy |
| AED compliance was lower if |
| Lower SES |
| Fewer financial responsibilities |
| Medicaid or no insurance |
| No daily responsibilities |
| Poor communication with clinicians |
| Non-Caucasian |
| Mental health care in children was lower if |
| Lower parental education |
| Older age |
| More than one psychiatric diagnosis |
| AED polytherapy |
| Higher verbal IQ |
AED, antiepileptic drug; IQ, intelligence quotient; SES, socioeconomic status.
Mental health care in children
Ott et al. assessed the impact of age, intelligence, clinical factors, and parental education on mental health care in 114 children with complex partial and generalized seizures, recruited from tertiary and primary care settings in the United States. Epilepsy and psychiatric diagnoses were carefully documented. The prevalence of psychiatric diagnoses was 61% of the total sample, but only half received mental health care. A multivariate analysis revealed significantly more parents with no education in the group that did not receive mental health care (OR 5.6). In addition, absence of mental health care was associated with older age (OR 1.3), presence of more than one psychiatric diagnosis (OR 1.9), being on AED polytherapy (OR 2.8), and higher verbal IQ (OR 0.96). For every 10-point decrease in verbal IQ, the child was 2.5 times less likely to receive mental care (Table 1). The authors speculate on a number of reasons for these associations, but none have empirical support.
Type of health care received and access to care (Table 2)
Table 2.
Comparisons of type of health care received and access to care
| As compared to those without epilepsy, PWE have |
| Higher use of |
| family doctors, specialists, nurses, and psychologists/counselors |
| emergency room, hospital admissions, telephone advice |
| Lower use of |
| dentist, physiotherapist, or chiropractor |
| Among PWEa |
| Children have |
| Higher use of neurologists, emergency room, hospital admissions |
| Females have |
| Higher use of neurologists |
| Lower use of family physician |
| Aboriginals have |
| Higher use of emergency room and hospital admissions |
| Lower use of neurologists |
These were independent of SES or urban/rural residence.
Two population-based Canadian studies have explored disparities in the type of health care (health resource utilization) that patients with epilepsy receive (Wiebe et al., 1999; Jette et al., 2008). One study used an omnibus general population health survey utilizing validated instruments and probability sampling of the entire population of a Canadian Province (population 8.1 million) (Wiebe et al., 1999). Medical conditions were identified by self-report. The other study used administrative data from an entire health region, which captured all medical care provided to a population of 1.3 million in that region (Jette et al., 2008). This could be considered population based because the health facilities are the sole health care provider in the geographic region. Authors utilized standard methods for case identification using validated ICD codes for epilepsy. Wiebe et al., 1999 assessed the 12-month use of health resources by 313 patients with epilepsy of all ages, and compared it with the general population and with people with other common chronic conditions (e.g., such as asthma, cancer, cardiac disease, and diabetes) in 1990. They found that people with epilepsy had 10–25% higher use than the general population of family doctors, specialists, nurses, and psychologists/counselors. The difference was smaller, but still significant when resource use was compared with people with other chronic conditions (4–9%). On the other hand, fewer people with epilepsy saw a dentist, physiotherapist, or chiropractor. People with epilepsy visited the emergency room (ER), were admitted to hospital, or received telephone advice 8–10% more frequently than those with other chronic conditions.
Jette et al. compared types of health resources used by 1,431 Canadian Aboriginal and non-Aboriginal patients of all ages with all types of epilepsy in 2001–2002 (Jette et al., 2008). Lower SES was assessed by the reception of Welfare support. Aboriginal status was defined based on the boriginal indicator (First Nations Flag) in the universal health care plan, which classifies registrants as aboriginal if they have a Band number and/or a First Nations group number. The term “status Aboriginal” refers to a person who is registered under the Indian Act (Government of Canada, 1996). The Indian Act criteria have to be met for individuals to be eligible for such registration. With regard to disparities between Aboriginals and non-Aboriginals, the findings are in keeping with numerous publications in other health conditions.
In a multivariate analysis, children were more likely than adults to see a neurologist [OR = 1.7, 95% confidence interval (CI) 1.3–2.3], visit the ER (OR = 4.9, 95% CI 3.2–7.4), or be hospitalized (OR = 2.9, 95% CI 2.0–4.3). Females were less likely to see a general practitioner but more likely to see a neurologist. Aboriginals were more likely than non-Aboriginals to visit the ER (OR = 2.3, 95% CI 1.1–5.0) or be hospitalized (OR = 2.8, 95% CI 1.5–5.1) but less likely to see a neurologist (OR = 0.3, 95% CI 0.2–0.6). Lower SES and residence location (urban versus rural) were not associated with level of health resource use. The authors discussed possible causes of disparities between Aboriginals and non-Aboriginals, such as difficulties with communication, cultural differences, geographic remoteness (more Aboriginals had rural addresses) and SES. However, it is worth noting that neither lower SES nor rural residence was an independent indicator of differences in health resource use. Wiebe et al., 1999 also explored the influence of geographic residence on health resource use. They found that people with epilepsy living in less densely populated areas used ER services more frequently and dental services less frequently than those in more heavily populated regions. On the other hand, people with epilepsy dwelling in urban areas experienced significantly more barriers to health care and were seen more frequently by psychologists/counselors. Wiebe et al., 1999 also found a significantly higher rate of hospitalization among females than males with epilepsy.
Knowledge and attitudes
Studies concerning disparities in knowledge and attitudes toward epilepsy range from those that focus on the general population to those that study PWE (Caveness et al., 1969, 1974; Long et al., 2000; Shore et al., 2002; Young et al., 2002; Swarztrauber et al., 2003; Tellez-Zenteno et al., 2004; Lu et al., 2005; Szaflarski et al., 2006). One difficulty in interpreting the data is that almost none of the studies includes a disease comparison group. However, several researchers were able to stratify their samples by socioeconomic or geographic factors, or to show secular trends.
General population studies
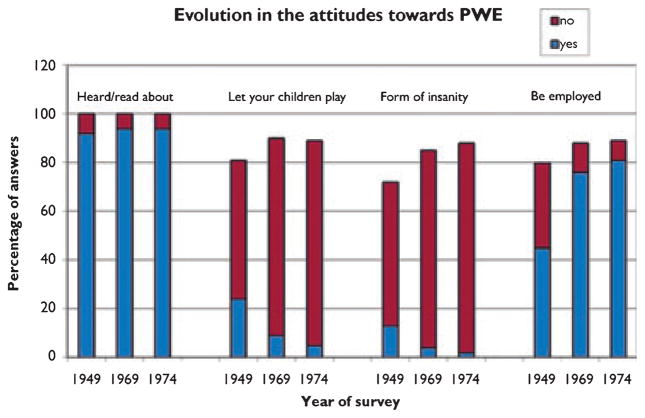
A series of studies (Fig. 2) described attitudes toward epilepsy in a large U.S. sample over three decades (Caveness et al., 1969, 1974). There was no change over time in the proportion (90–95%) who had heard of epilepsy. However, people living in the Southern United States and those with only grade school education were least informed. The number who would object to their child having a friend with epilepsy declined from 24% in 1949 to 5% in 1974. Only 22% in 1947, compared with 81% in 1974 thought PWE should be employed like others. Thirteen percent in 1947 and 2% in 1974 thought epilepsy was a form of insanity. Men, residents of the South, and those with only grade school education had more negative attitudes. The authors attributed improvement in social attitudes over time to an overall change in attitudes toward diseases like tuberculosis, syphilis, and cancer, improved seizure control, and professional and lay educational efforts.
Figure 2.
The figure shows the evolution of answers from respondents to surveys in 1949, 1969, and 1974 (Caveness et al., 1969, 1974) to the question: have you ever heard or read about the disease called epilepsy, or convulsive seizures? Would you object to having any of your children in school or at play associate with persons who sometimes had seizures? Do you think epilepsy is a form of insanity or not? Do you think people with epilepsy should be employed in jobs like others? PWE, people with epilepsy.
Epilepsia © ILAE
Another study observed that the overall knowledge of Canadian college students about epilepsy was limited, although no comparison with other disorders was performed (Young et al., 2002). Five percent objected to a future child associating with a child with epilepsy, and 4% objected to a relative marrying a PWE. Eleven percent thought that PWE should not have children, and 14% thought they should not have usual jobs. However, a subsequent brief educative intervention on epilepsy improved their knowledge.
In a study of 175 PWE in the United States, knowledge did not correlate with age, years of education, or epilepsy duration (Long et al., 2000). Thirty percent believed that epilepsy is a mental contagious disorder and 41% believed it is appropriate to place an object in a patient’s mouth during a seizure to prevent injury. There was poor knowledge of legal issues related to driving and employment, as only 14% and 48% of respondents answered correctly, respectively.
A structured interview of 84 families of children with epilepsy in Canada, found that families of children with medically intractable epilepsy and parents with more education consulted more information sources (Lu et al., 2005). Clinic-recommended Internet sites (100%), clinic nurse (97%), and neurologist (93%) had the highest perception of accuracy, followed by other Internet sites or family members within the medical profession (85% for both) and lay organizations (84%) (Fig. 3). Friends within the medical profession, other families, and complementary health care providers also ranked highly in terms of reliability of medical information.
Figure 3.
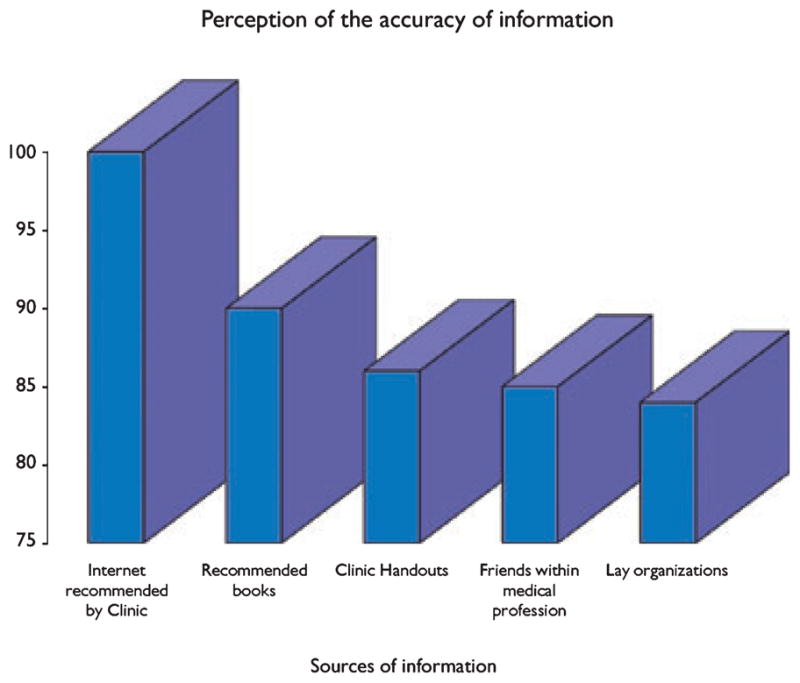
The figure shows the differences in how families interviewed in a Canadian epilepsy clinic felt about the sources of information they accessed and their perceived accuracy of this information (Lu et al., 2005).
Epilepsia © ILAE
A small urban U.S. study showed that patients with intractable epilepsy exaggerated potential risks of surgery (Swarztrauber et al., 2003). In this study, African-American participants also described distrust in their health care providers.
Finally, workplace discrimination is subject to legal limitations as well as definitions. Several recent Supreme Court Decisions were perceived initially as placing potential limitations on the Americans with Disabilities Act (West et al., 2006). However, a study by the Equal Employment Opportunities Commission found that the percentage of claims by PWE found to be “with merit” actually increased from 21.6% to 30.4% after the relevant cases.
Specific subgroups
Specific ethnocultural groups may have attitudes toward epilepsy conditioned by traditional beliefs. Some Native Americans attribute epilepsy to spiritual causes; this may affect willingness to access nontraditional treatment (see Szaflarski et al., 2006 for review). In a study of Spanish speaking and non-Hispanic adults in seven large U.S. Hispanic metropolitan areas, significantly more Hispanics (21%, p = 0.001) compared to the non-Hispanic sample in the study, reported no familiarity with epilepsy; and a one-third of Hispanics believed that PWE were “dangerous to others” (Sirven et al., 2005). Some Hispanics thought seizures are caused by sins (8%, p = 0.001), and that “exorcism” would be a good remedy, up to 10% in those with less than high school education.
Attitudes in the workplace
Harden et al. used clinical vignettes to compare attitudes toward epilepsy, multiple sclerosis (MS), and depression in urban charity workers (Harden et al., 2004). Workers were more worried about having a coworker with epilepsy than with MS, but not depression. They did feel more comfortable about providing first aid for either MS or depression than epilepsy. Higher status employees had less discomfort related to epilepsy. Workers thought they were more likely to get depression than epilepsy or MS.
Employment and education
Seven studies focusing on employment and education fulfilled eligibility criteria (Wiebe et al., 1999; Gehlert et al., 2000; Kobau & DiIorio, 2003; Kobau et al., 2004; Oyegbile et al., 2004; West et al., 2006; McNelis et al., 2007; Tse et al., 2007).
Socioeconomic disparities in epilepsy were also identified between those with and those without epilepsy. Lower household income, higher unemployment rate, and lower educational attainment were seen in those with epilepsy compared to their matched peers without epilepsy in Georgia and Tennessee (Kobau et al., 2004).
In the United States, children with epilepsy have shown to have a decline in their academic performance when they have recurrent seizures as compared to those without seizure recurrence (McNelis et al., 2007). They also have poorer social skills and are less assertive than their siblings without seizures (Tse et al., 2007).
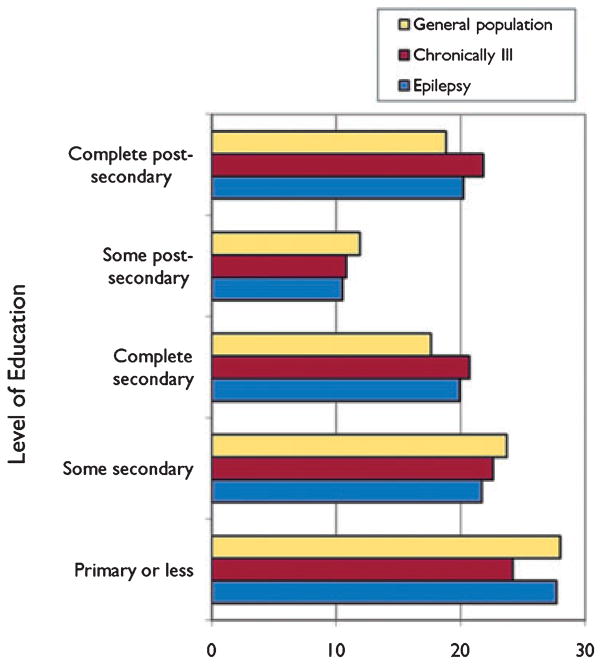
In The Ontario Health Survey (Wiebe et al., 1999), the authors found that more PWE (22.1%) had a low annual income (<12,000 Canadian dollars regardless of family size, $12,000–$19,999 if household size was 2 or more, or $20,000–$29,999 if household was 4 or more) than those with other chronic problems (14.6%), the general population (13.6%), or healthy subjects (12%) (p < 0.001 for all comparisons). In terms of employment, fewer PWE (9.5%) were in the service or transport industry, as compared with the chronically ill or the general population (16% each). In terms of education, PWE achieved an academic status that was similar to that of other groups (28% obtained only some or complete primary education, 41% initiated secondary instruction, and 31% engaged in postsecondary education). However, PWE were less likely than the general population to complete secondary or postsecondary education (OR = 0.8 at each level) (Fig. 4).
Figure 4.
Taken from the Ontario Health Survey (Wiebe et al., 1999), the figure shows the different levels of education (weighted proportions) achieved by the different groups that were analyzed in the study.
Epilepsia © ILAE
Disparities found in epidemiologic studies of epilepsy
A total of eight studies fulfilled the inclusion/exclusion criteria for disparities in epidemiologic studies (Murphy et al., 1995; Annegers et al., 1999; Wiebe et al., 1999; Kobau et al., 2004; Kozyrskyj & Prasad, 2004; Strine et al., 2005; Hussain et al., 2006; Whitehead et al., 2006). All were population-based, with two cohort (Hussain et al., 2006; Whitehead et al., 2006), one case–control (Annegers et al., 1999), and four cross-sectional studies (Wiebe et al., 1999; Kobau et al., 2004; Kozyrskyj & Prasad, 2004; Murphy et al., 1995; Strine et al., 2005).
Three studies pertained to the Canadian population and five were done in the United States. Five studies included only children (Murphy et al., 1995; Kozyrskyj & Prasad, 2004; Reading et al., 2006; Whitehead et al., 2006; Hamdy et al., 2007), three only adults (Kobau et al., 2004; Strine et al., 2005; Hussain et al., 2006), and the remaining included subjects of all ages. Lifetime prevalence ranged from 1.4 to 9.2, whereas active prevalence ranged from 3.6 to 6.4 per 1,000 people. Incidence ranged from 31.5 to 66 per 100,000 people per year.
In the United States, most studies found no differences in the rates of epilepsy between non-Hispanic Caucasians and African-Americans (Murphy et al., 1995; Annegers et al., 1999; Kobau et al., 2004; Strine et al., 2005; Hussain et al., 2006), with few exceptions: (1) a higher rate of generalized epilepsy in African-Americans compared to non-Hispanic Caucasians in Atlanta (Murphy et al., 1995), (2) a higher seizure rate in African-Americans older than 60 compared to their Caucasians counterparts in New York (Hussain et al., 2006), and (3) a higher rate of epilepsy in young African-Americans and Hispanics compared with their Caucasians counterparts in Texas (Annegers et al., 1999).
The U.S. National Health Interview Survey found that PWE are more likely to be single or divorced and unemployed or formerly worked, compared with their peers without epilepsy (Strine et al., 2005).
In Canada, a population-based study on the burden of seizures in Manitoba children found a prevalence of seizures of 4.7 per 1,000 children in that province (Kozyrskyj & Prasad, 2004), with a higher prevalence rate in the oldest group (15–19 years of age), and in the lower income quintile groups. The Ontario Health Survey (Wiebe et al., 1999), which included all age groups, found a prevalence of 5.8 per 1,000 people. In this study the authors found a lower annual income in those with epilepsy compared to the general population, and they were less likely to complete secondary and postsecondary education (OR = 0.8 at each level).
Finally, a study of children in Nova Scotia found an incidence of epilepsy of 63 per 100,000 children (Whitehead et al., 2006). A higher rate of parents of children with epilepsy were not married (OR = 1.4, 95% CI = 1.2–1.7), were smokers (OR = 1.2, 95% CI = 1.1–1.5), and previously had infants with low birth weight (OR = 1.8, 95% CI = 1.3–2.5) compared to the population without epilepsy.
Disparities in outcomes following medical treatment of epilepsy
Only 12 studies were selected for review (Green & Hartlage, 1971; Dansky et al., 1980; Mitchell et al., 1994; Wiebe et al., 1999; Mitchell et al., 2000; Snodgrass et al., 2001; Williams et al., 2001; Getz et al., 2003; Ott et al., 2003; Williams et al., 2003; Oyegbile et al., 2004; Elliott et al., 2006), using the inclusion and exclusion criteria mentioned previously. Additional criteria for inclusion required studies to: (1) describe medical treatment(s), which could be considered as predictive factors; (2) define disparity risk factor(s), including one or more of SES/education, race/ethnicity, sex, age, or co-morbidities; and (3) describe and measure medical outcomes in terms of seizure control (e.g., seizure frequency or severity or a proxy measure) or quality-of-life.
Among the North American studies, none described treatment as a predictor or examined medical outcomes per se (e.g., seizure frequency or severity). Two studies examined children evidently receiving standard care (not analyzed as a variable) and compared outcomes as they varied by race or some aspect of SES. In a case–control study of a pediatric epilepsy clinic population at the University of Mississippi, Snodgrass et al. (2001) found that non-Caucasians were more likely than Caucasians to be noncompliant with prescribed medication, as determined by nondetectable blood levels. From their published data, an odds ratio of 7.7 (95% CI 3.0–20.3) can be calculated; however, these results are not adjusted for differences in SES. The authors also found an association between lack of insurance and lack of compliance. In a Los Angeles pediatric epilepsy clinic, Mitchell et al., 2000 examined “acculturative risk” (based on mother’s education, duration of mother’s residence in the United States, child’s nationality, and primary language) as predictors of visit adherence and medication adherence. A wide range of other medical, behavioral, and environmental factors were also taken into account. Contrary to their expectations, they found (using multivariate structural equation modeling) that higher acculturative risk was associated with greater adherence with visits (partial standardized regression coefficient r = 0.35, p < 0.01) and medication (r = 0.24, p < 0.05). No other studies met our inclusion criteria.
Disparities in outcomes following surgical treatment
Only three eligible articles were found in the literature, all from the United States. One study assessed marital status in relation to gender and age of epilepsy onset and compared marital rates in epilepsy with rates in the U.S. population. The authors found that more than 4 years after epilepsy surgery, patients who were seizure-free were more likely to change marital status than those who had recurrent seizures. Seizure-free women were more likely to divorce than were seizure-free men. They also found that most men who married were employed, whereas women who divorced were usually unemployed (Carran et al., 1999).
Two articles by Burneo et al. looked into possible racial differences in outcome following temporal lobectomy for the treatment of temporal lobe epilepsy due to hippocampal sclerosis (Burneo et al., 2006, 2005b). Risk adjustment was performed for socioeconomic status, age, gender, duration of epilepsy, education, history of febrile seizures, handedness, number of antiepileptic drugs, and lateralization of epileptogenic focus. The first study included a small population of patients from the state of Alabama (64 patients), and found that African-Americans appeared to be more likely than non-Hispanic Caucasians to have seizure recurrence after surgery, although this difference was not statistically significant (OR = 2.1, 95% CI = 0.6–8.0) (Burneo et al., 2005b). The second study included 252 patients from two epilepsy surgery centers in Alabama and New York, and included a third group of Hispanic patients in the analysis. No differences in surgical outcomes were found between racial/ethnic groups (Burneo et al., 2006).
Discussion
This array of studies provides ample evidence that minorities with epilepsy may be receiving lower levels of care than the nonminority white population in North America. This is a concern for advocates and policy makers seeking to eliminate inequalities in national health systems and for those interested in improving health in high-risk populations. None of these studies have attempted to explain the source of racial and ethnic disparities in care, however. Some of the key causal questions that arise include: are observed disparities due to overt or institutional discrimination or bias in health care systems design and availability? Do they result from cultural misunderstandings on the part of health care providers or patients? Or do minority patients receive different levels of care based on the settings where they seek care?
Although there is a suggestion that African-Americans have higher rates of hospitalizations and ER visits, and lower rates of epilepsy surgery, because insurance status and low SES also had an association in some of the studies, it is not clear whether racial/ethnic factors causally relate to rates of care. In Canada, Aboriginals with epilepsy were less likely to see a neurologist and more likely to visit the ER, a finding which could not be explained by lower SES or rural residence. Other disparities in population-based studies include more ER and fewer counseling visits in people living in less densely populated and remote areas, and more barriers to care in those living in urban areas. Women and children are more likely to see a neurologist than men and adults. In two studies with different methods, cultures, and populations, poor compliance with AEDs was associated with external factors such as lower income, insufficient insurance, and poor relationship with treating clinicians; as well as with internal factors such as not having regular responsibilities. Clinical, demographic, and cognitive factors were not associated with compliance with medication. Disparities in mental health care have been documented in people with epilepsy. Lower rates of mental health treatment in children with epilepsy are associated with older age, lower parental education, higher verbal IQ, and AED polytherapy.
Due to the absence of comparative studies, it is impossible to know how much and in what dimensions knowledge of or attitudes toward epilepsy differ from feelings concerning other chronic disorders. Community attitudes appear to have improved over time, and education can be a positive factor. Specific ethnocultural beliefs would need to be addressed. The lack of knowledge of PWE themselves is of concern.
There also appear to be discrepancies between different studies in term of employment status in patients with epilepsy when compared with those without it. However, there appears to be a lower educational level attained in those with epilepsy compared to the general population. More studies are needed to clarify these discrepancies.
Furthermore, there is little research on the role of SES, gender, race/ethnicity, age, education, and comorbidities in relation to outcomes following medical treatment for people with epilepsy. The published data are not sufficient to reach conclusions regarding the relationships between these factors.
Finally, there is scarce information about possible disparities following the surgical treatment of epilepsy. More studies, particularly with a large sample size, are needed.
Because of the limited number of racial and ethnic groups studied, small sample sizes, the limited number of other patient factors examined, the limited number of provider sites examined, and the large potential for confounding among the factors, more studies are needed. Care is required in the selection of diverse study populations across multiple institutional settings to gain a deeper understanding of the wide range of possible patient-related and provider-related factors involved. In order to isolate the underlying factors that may be causing disparities, studies must be carefully designed and attention given to a broad set of measures in data collection and analysis. Future research should address the reasons for disparities and inform programs and policies to reduce and/or eliminate disparities.
In the United States where health insurance coverage is not universal, there is ample evidence of disparities in epilepsy care associated with race/ethnicity and SES. Disparities are still found even in a system in which there is coexistence of private and public care, as in Canada, where health care delivery is based on the premise of universality, but where reports in other areas of medicine have found that important sex and gender disparities are present (Fowler et al., 2007). Those raise the question on how equitable is the Canadian Health Care system (Baxter, 2007), something that is still largely unknown for epilepsy.
In conclusion, studies on disparities in epilepsy care are scarce. More studies are needed involving large and selected populations of patients. Comparisons are needed between rural and urban, as well as from different geographic areas. No studies from the English-speaking Caribbean exist, and few describe native communities in North America.
Acknowledgments
We wish to thank Sylvia Katzer (Librarian, London Health Sciences Center) for assistance with the search strategy and the obtainment of initial abstracts.
Footnotes
Disclosures: The authors have nothing to disclose. The findings and conclusions in this report are those of the authors and do not necessarily reflect the official views of the Centers for Disease Control and Prevention.
References
- Andersen R, Aday LA. Access to medical care in the U.S.: realized and potential. Med Care. 1978;16:533–546. doi: 10.1097/00005650-197807000-00001. [DOI] [PubMed] [Google Scholar]
- Annegers JF, Dubinsky S, Coan SP, Newmark ME, Roht L. The incidence of epilepsy and unprovoked seizures in multiethnic, urban health maintenance organizations. Epilepsia. 1999;40:502–506. doi: 10.1111/j.1528-1157.1999.tb00748.x. [DOI] [PubMed] [Google Scholar]
- Baxter NN. Equal for whom? Addressing disparities in the Canadian medical system must become a national priority. CMAJ. 2007;177:1522–1523. doi: 10.1503/cmaj.071578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehmer U, Kressin NR, Berlowitz DR, Christiansen CL, Kazis LE, Jones JA. Self-reported vs administrative race/ethnicity data and study results. Am J Public Health. 2002;92:1471–1472. doi: 10.2105/ajph.92.9.1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burneo JG, Martin R. Reporting race/ethnicity in epilepsy clinical trials. Epilepsy Behav. 2004;5:743–745. doi: 10.1016/j.yebeh.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Burneo JG, Black L, Knowlton RC, Faught E, Morawetz R, Kuzniecky RI. Racial disparities in the use of surgical treatment for intractable temporal lobe epilepsy. Neurology. 2005a;64:50–54. doi: 10.1212/01.WNL.0000150829.89586.25. [DOI] [PubMed] [Google Scholar]
- Burneo JG, Knowlton RC, Martin R, Faught RE, Kuzniecky RI. Race/ethnicity: a predictor of temporal lobe epilepsy surgery outcome? Epilepsy Behav. 2005b;7:486–490. doi: 10.1016/j.yebeh.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Burneo JG, Black L, Martin R, Devinsky O, Pacia S, Faught E, Vasquez B, Knowlton RC, Luciano D, Doyle W, Najjar S, Kuzniecky RI. Race/ethnicity, sex, and socioeconomic status as predictors of outcome after surgery for temporal lobe epilepsy. Arch Neurol. 2006;63:1106–1110. doi: 10.1001/archneur.63.8.1106. [DOI] [PubMed] [Google Scholar]
- Carran MA, Kohler CG, O’Connor MJ, Cloud B, Sperling MR. Marital status after epilepsy surgery. Epilepsia. 1999;40:1755–1760. doi: 10.1111/j.1528-1157.1999.tb01594.x. [DOI] [PubMed] [Google Scholar]
- Carter-Pokras O, Baquet C. What is a “health disparity”? Public Health Rep. 2002;117:426–434. doi: 10.1093/phr/117.5.426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caveness WF, Merritt HH, Gallup GH. A survey of public attitudes toward epilepsy in 1969 with an indication of trends over the past twenty years. Epilepsia. 1969;10:429–440. doi: 10.1111/j.1528-1157.1969.tb06139.x. [DOI] [PubMed] [Google Scholar]
- Caveness WF, Merritt HH, Gallup GH., Jr A survey of public attitudes toward epilepsy in 1974 with an indication of trends over the past twenty-five years. Epilepsia. 1974;15:523–536. doi: 10.1111/j.1528-1157.1974.tb04026.x. [DOI] [PubMed] [Google Scholar]
- Dansky LV, Andermann E, Andermann F. Marriage and fertility in epileptic patients. Epilepsia. 1980;21:261–271. doi: 10.1111/j.1528-1157.1980.tb04072.x. [DOI] [PubMed] [Google Scholar]
- Dodrill CB, Batzel LW, Wilensky AJ, Yerby MS. The role of psychosocial and financial factors in medication noncompliance in epilepsy. Int J Psychiatry Med. 1987;17:143–154. doi: 10.2190/4ny4-3p1r-e2r9-mhcy. [DOI] [PubMed] [Google Scholar]
- Elliott JO, Jacobson MP, Seals BF. Self-efficacy, knowledge, health beliefs, quality of life, and stigma in relation to osteoprotective behaviors in epilepsy. Epilepsy Behav. 2006;9:478–491. doi: 10.1016/j.yebeh.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Fowler RA, Sabur N, Li P, Juurlink DN, Pinto R, Hladunewich MA, Adhikari NK, Sibbald WJ, Martin CM. Sex-and age-based differences in the delivery and outcomes of critical care. CMAJ. 2007;177:1513–1519. doi: 10.1503/cmaj.071112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gehlert S, DiFrancesco A, Chang CH. Black-white differences in the psychosocial outcomes of epilepsy. Epilepsy Res. 2000;42:63–73. doi: 10.1016/s0920-1211(00)00161-3. [DOI] [PubMed] [Google Scholar]
- Getz K, Hermann B, Seidenberg M, Bell B, Dow C, Jones J, Woodard A. Negative symptoms and psychosocial status in temporal lobe epilepsy. Epilepsy Res. 2003;53:240–244. doi: 10.1016/s0920-1211(03)00033-0. [DOI] [PubMed] [Google Scholar]
- Green JB, Hartlage LC. Comparative performance of epileptic and nonepileptic children and adolescents. (On tests of academic, communicative and social skills) Dis Nerv Syst. 1971;32:418–421. [PubMed] [Google Scholar]
- Hamdy NA, Ginby D, Feltbower R, Ferrie CD. Ethnic differences in the incidence of seizure disorders in children from Bradford, United kingdom. Epilepsia. 2007;48:913–916. doi: 10.1111/j.1528-1167.2007.01097.x. [DOI] [PubMed] [Google Scholar]
- Harden CL, Kossoy A, Vera S, Nikolov B. Reaction to epilepsy in the workplace. Epilepsia. 2004;45:1134–1140. doi: 10.1111/j.0013-9580.2004.67003.x. [DOI] [PubMed] [Google Scholar]
- Hussain SA, Haut SR, Lipton RB, Derby C, Markowitz SY, Shinnar S. Incidence of epilepsy in a racially diverse, community-dwelling, elderly cohort: results from the Einstein aging study. Epilepsy Res. 2006;71:195–205. doi: 10.1016/j.eplepsyres.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Institute-of-Medicine-Committee-on-the-Consequences-of-Uninsurance. Hidden costs, value lost: uninsurance in America. National Academy Press; Washington, DC: 2003. [Google Scholar]
- Jette N, Quan H, Faris P, Dean S, Li B, Fong A, Wiebe S. Health resource use in epilepsy: significant disparities by age, gender, and aboriginal status. Epilepsia. 2008;49:586–593. doi: 10.1111/j.1528-1167.2007.01466.x. [DOI] [PubMed] [Google Scholar]
- Kaplan JB, Bennett T. Use of race and ethnicity in biomedical publication. JAMA. 2003;289:2709–2716. doi: 10.1001/jama.289.20.2709. [DOI] [PubMed] [Google Scholar]
- Kobau R, DiIorio C. Epilepsy self-management: a comparison of self-efficacy and outcome expectancy for medication adherence and lifestyle behaviors among people with epilepsy. Epilepsy Behav. 2003;4:217–225. doi: 10.1016/s1525-5050(03)00057-x. [DOI] [PubMed] [Google Scholar]
- Kobau R, DiIorio CA, Price PH, Thurman DJ, Martin LM, Ridings DL, Henry TR. Prevalence of epilepsy and health status of adults with epilepsy in Georgia and Tennessee: Behavioral Risk Factor Surveillance System, 2002. Epilepsy Behav. 2004;5:358–366. doi: 10.1016/j.yebeh.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Kozyrskyj AL, Prasad AN. The burden of seizures in Manitoba children: a population-based study. Can J Neurol Sci. 2004;31:48–52. doi: 10.1017/s0317167100002821. [DOI] [PubMed] [Google Scholar]
- Krieger N. Refiguring “race”: epidemiology, racialized biology, and biological expressions of race relations. Int J Health Serv. 2000;30:211–216. doi: 10.2190/672J-1PPF-K6QT-9N7U. [DOI] [PubMed] [Google Scholar]
- LaVeist TA. On the study of race, racism, and health: a shift from description to explanation. Int J Health Serv. 2000;30:217–219. doi: 10.2190/LKDF-UJQ5-W1KU-GLR1. [DOI] [PubMed] [Google Scholar]
- Long L, Reeves AL, Moore JL, Roach J, Pickering CT. An assessment of epilepsy patients’ knowledge of their disorder. Epilepsia. 2000;41:727–731. doi: 10.1111/j.1528-1157.2000.tb00235.x. [DOI] [PubMed] [Google Scholar]
- Lu C, Wirrell E, Blackman M. Where do families of children with epilepsy obtain their information? J Child Neurol. 2005;20:905–910. doi: 10.1177/08830738050200110801. [DOI] [PubMed] [Google Scholar]
- McNelis AM, Dunn DW, Johnson CS, Austin JK, Perkins SM. Academic performance in children with new-onset seizures and asthma: a prospective study. Epilepsy Behav. 2007;10:311–318. doi: 10.1016/j.yebeh.2006.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell WG, Scheier LM, Baker SA. Psychosocial, behavioral, and medical outcomes in children with epilepsy: a developmental risk factor model using longitudinal data. Pediatrics. 1994;94:471–477. [PubMed] [Google Scholar]
- Mitchell WG, Scheier LM, Baker SA. Adherence to treatment in children with epilepsy: who follows “doctor’s orders”? Epilepsia. 2000;41:1616–1625. doi: 10.1111/j.1499-1654.2000.001616.x. [DOI] [PubMed] [Google Scholar]
- Murphy CC, Trevathan E, Yeargin-Allsopp M. Prevalence of epilepsy and epileptic seizures in 10-year-old children: results from the Metropolitan Atlanta Developmental Disabilities Study. Epilepsia. 1995;36:866–872. doi: 10.1111/j.1528-1157.1995.tb01629.x. [DOI] [PubMed] [Google Scholar]
- NIH. Health disparities in epilepsy panel. Rockville, MD: 2002. [Accessed December 16, 2008]. Available from http://www.ninds.nih.gov/news_and_events/proceedings/epilepsy_panel_2002.htm. [Google Scholar]
- Ott D, Siddarth P, Gurbani S, Koh S, Tournay A, Shields WD, Caplan R. Behavioral disorders in pediatric epilepsy: unmet psychiatric need. Epilepsia. 2003;44:591–597. doi: 10.1046/j.1528-1157.2003.25002.x. [DOI] [PubMed] [Google Scholar]
- Oyegbile TO, Dow C, Jones J, Bell B, Rutecki P, Sheth R, Seidenberg M, Hermann BP. The nature and course of neuropsychological morbidity in chronic temporal lobe epilepsy. Neurology. 2004;62:1736–1742. doi: 10.1212/01.wnl.0000125186.04867.34. [DOI] [PubMed] [Google Scholar]
- Reading R, Haynes R, Beach R. Deprivation and incidence of epilepsy in children. Seizure. 2006;15:190–193. doi: 10.1016/j.seizure.2006.01.002. [DOI] [PubMed] [Google Scholar]
- Rockefeller Foundation. [Accessed December 19, 2007];Challenging inequities in health: from ethics to action. 2002 Available from: http://www.rockfound.org.
- Shore CP, Austin JK, Huster GA, Dunn DW. Identifying risk factors for maternal depression in families of adolescents with epilepsy. J Spec Pediatr Nurs. 2002;7:71–80. doi: 10.1111/j.1744-6155.2002.tb00153.x. [DOI] [PubMed] [Google Scholar]
- Sirven JI, Lopez RA, Vazquez B, Van Haverbeke P. Que es la Epilepsia? Attitudes and knowledge of epilepsy by Spanish-speaking adults in the United States. Epilepsy Behav. 2005;7:259–265. doi: 10.1016/j.yebeh.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Snodgrass SR, Vedanarayanan VV, Parker CC, Parks BR. Pediatric patients with undetectable anticonvulsant blood levels: comparison with compliant patients. J Child Neurol. 2001;16:164–168. doi: 10.1177/088307380101600302. [DOI] [PubMed] [Google Scholar]
- Strine TW, Kobau R, Chapman DP, Thurman DJ, Price P, Balluz LS. Psychological distress, comorbidities, and health behaviors among U.S. adults with seizures: results from the 2002 National Health Interview Survey. Epilepsia. 2005;46:1133–1139. doi: 10.1111/j.1528-1167.2005.01605.x. [DOI] [PubMed] [Google Scholar]
- Swarztrauber K, Dewar S, Engel J., Jr Patient attitudes about treatments for intractable epilepsy. Epilepsy Behav. 2003;4:19–25. doi: 10.1016/s1525-5050(02)00687-x. [DOI] [PubMed] [Google Scholar]
- Szaflarski M, Szaflarski JP, Privitera MD, Ficker DM, Horner RD. Racial/ethnic disparities in the treatment of epilepsy: what do we know? What do we need to know? Epilepsy Behav. 2006;9:243–264. doi: 10.1016/j.yebeh.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Tellez-Zenteno JF, Pondal-Sordo M, Matijevic S, Wiebe S. National and regional prevalence of self-reported epilepsy in Canada. Epilepsia. 2004;45:1623–1629. doi: 10.1111/j.0013-9580.2004.24904.x. [DOI] [PubMed] [Google Scholar]
- Theodore WH, Spencer SS, Wiebe S, Langfitt JT, Ali A, Shafer PO, Berg AT, Vickrey BG. Epilepsy in North America: a report prepared under the auspices of the global campaign against epilepsy, the International Bureau for Epilepsy, the International League Against Epilepsy, and the World Health Organization. Epilepsia. 2006;47:1700–1722. doi: 10.1111/j.1528-1167.2006.00633.x. [DOI] [PubMed] [Google Scholar]
- Tse E, Hamiwka L, Sherman EM, Wirrell E. Social skills problems in children with epilepsy: prevalence, nature and predictors. Epilepsy Behav. 2007;11:499–505. doi: 10.1016/j.yebeh.2007.08.008. [DOI] [PubMed] [Google Scholar]
- West MD, Dye AN, McMahon BT. Epilepsy and workplace discrimination: population characteristics and trends. Epilepsy Behav. 2006;9:101–105. doi: 10.1016/j.yebeh.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Whitehead E, Dodds L, Joseph KS, Gordon KE, Wood E, Allen AC, Camfield P, Dooley JM. Relation of pregnancy and neonatal factors to subsequent development of childhood epilepsy: a population-based cohort study. Pediatrics. 2006;117:1298–1306. doi: 10.1542/peds.2005-1660. [DOI] [PubMed] [Google Scholar]
- Wiebe S, Bellhouse DR, Fallahay C, Eliasziw M. Burden of epilepsy: the Ontario Health Survey. Can J Neurol Sci. 1999;26:263–270. doi: 10.1017/s0317167100000354. [DOI] [PubMed] [Google Scholar]
- Williams J, Phillips T, Griebel ML, Sharp GB, Lange B, Edgar T, Simpson P. Factors associated with academic achievement in children with controlled epilepsy. Epilepsy Behav. 2001;2:217–223. doi: 10.1006/ebeh.2001.0166. [DOI] [PubMed] [Google Scholar]
- Williams J, Steel C, Sharp GB, DelosReyes E, Phillips T, Bates S, Lange B, Griebel ML. Anxiety in children with epilepsy. Epilepsy Behav. 2003;4:729–732. doi: 10.1016/j.yebeh.2003.08.032. [DOI] [PubMed] [Google Scholar]
- Young GB, Derry P, Hutchinson I, John V, Matijevic S, Parrent L, Wiebe S. An epilepsy questionnaire study of knowledge and attitudes in Canadian college students. Epilepsia. 2002;43:652–658. doi: 10.1046/j.1528-1157.2002.01002.x. [DOI] [PubMed] [Google Scholar]