Abstract
Formation of bacterial biofilms at solid-liquid interfaces creates numerous problems in both industrial and biomedical sciences. In this study, the susceptibility of Staphylococcus aureus biofilms to discharge gas generated from plasma was tested. It was found that despite distinct chemical/physical properties, discharge gases from oxygen, nitrogen, and argon demonstrated very potent and almost the same anti-biofilm activity. The bacterial cells in S. aureus biofilms were killed (>99.9%) by discharge gas within minutes of exposure. Under optimal experimental conditions, no bacteria and biofilm re-growth from discharge gas treated biofilms was found. Further studies revealed that the anti-biofilm activity of the discharge gas occurred by two distinct mechanisms: 1) killing bacteria in biofilms by causing severe cell membrane damage, and 2) damaging the extracellular polymeric matrix in the architecture of the biofilm to release biofilm from the surface of the solid substratum . Information gathered from this study provides an insight into the anti-biofilm mechanisms of plasma and confirms the applications of discharge gas in the treatment of biofilms and biofilm related bacterial infections.
Keywords: S. aureus, bacterial biofilms, plasma, discharge gas, susceptibility
Introduction
Bacteria prefer to attach to moist surfaces. The moist environment allows the bacteria to thrive, developing into biofilms [Costerton et al 1995]. The solid-liquid interface between body fluids (eg blood) and the surfaces of teeth, tissues, and implanted devices, provides an ideal environment for bacterial attachment and colonization [Cos et al 2010]. Conventional methods such as chemical (antibiotic and ethylene oxide) and physical (autoclave and gamma radiation) treatments are adequate for planktonic (free-living) bacteria; however, they are not effective enough for biofilm in term of completely killing bacteria in biofilms and removing biofilms from contaminated surfaces [Stewart and Chen 2000, Vickery et al 2000]. This is also true for biofilm -associated medical problems. One of the most important features of bacterial biofilms is their resistance to antibiotics and to the immune system of the [Cos et al 2010]. Bacteria living in biofilms can exhibit up to 1,000 times greater resistance to antibiotics than planktonic bacteria [Mah and O’Toole 2001]. Existing antibiotics are unlikely to be effective at typical dosages for the treatment of biofilm-related infections, largely because they only kill the susceptible biofilm population and do not affect the phenotypic variants inside the biofilm. Systemic antibiotic treatment of biofilm, clinically called maintenance chemotherapy, can only maintain biofilm growth but cannot eradicate it [Stoodley et al 2002; Patel 2005; Lewis 2008]. .
The resistant mechanism of bacteria in biofilms can be attributed to several factors [Stewart 2002]. Nutrient and oxygen depletion within the biofilm cause some bacteria to enter a nongrowing (ie, stationary) state, in which they are less susceptible to growth-dependent antimicrobial killing [Cos et al 2010]. A subpopulation of bacteria might differentiate into a phenotypically resistant state [Lewis 2008]. Some organisms in biofilms have been shown to express biofilm-specific antimicrobial resistance genes that are not required for biofilm formation [Patel 2005]. In addition, the presence of biofilm architectures serve as physical barriers to antimicrobial agents by blocking them from access to the bacteria in the deeper zone of the biofilm [Stewart and Costerton 2001; Stoodley et al. 2002; Lynch and Abbanat 2010 ]. However, the ability of the extracellular polymeric matrix of the biofilm to act as a physical barrier depends on the type of antibiotics used, the concentration employed, and the binding of the matrix to that specific antibacterial agent [Picioreanu et al 2000]. Strongly charged or chemically reactive agents fail to reach the bacteria in the biofilm due to the negatively charged extracellular polymeric matrix of biofilms which acts as an ion-exchange resin and removes these molecules from the environment [Costerton et al 1995]. However, some small molecules such as water and oxygen have been found to be able to travel freely throughout the biofilm by using channels with varying diameters [Dunne et al 1993; Jefferson et al 2005].
Plasma, a reactive cloud of ions, electrons, radicals, and neutral atomic particles, is considered as a fourth state of matter and is distinguishable from liquid, solid, or gas [Conrads and Schmidt 2000]. Plasma discharge gas is generated by supplying energy to natural gases to form small charge carriers, reactive species, and UV radiation [Conrads and Schmidt 2000; Hino et al 2004]. Plasma-mediated inactivation of bacteria has been extensively studied over the past decade and cold plasma has been successfully used in food and device sterilization [Niemira and Sites 2008; Morris et al 2009; Sureshkumar et al 2010]. The activity of plasma on bacteria and biofilms has been recently reported recently [Joaquin et al 2009; Joshi et al 2010]. It has been found that plasma can kill bacteria, including antibiotic resistant bacteria, in biofilms effectively and efficiently. However, the acting mechanism of discharges on biofilms has not been well studied and fully revealed. In this study, the effects of discharge gas on bacteria in biofilms, biofilm structures, and biofilm growth were studied. The reactive discharge gas generated from plasma was found to be very active against bacteria in biofilms and to demonstrate strong anti-biofilm activity. The anti-biofilm activity of the discharge gas is implicated by two distinct mechanisms, viz. killing bacteria in biofilms and releasing biofilm from the solid substrata .
Materials and methods
Bacterial strains and medium
Staphylococcus aureus (penicillin resistant, ATCC 29213) was purchased from the American Type Culture Collection (ATCC, Manassas, VA). Tryptic soy broth supplemented with 0.2 % glucose (TSBG) was purchased from Sigma (St Louis, MO).
Reagents and Solutions
A LIVE/DEAD staining kit was purchased from Invitrogen Life Technologies (Carlsbad, CA) for the staining of biofilms. It comprised a solution of 5% MTT (methylthiazolyldiphenyl-tetrazolium bromide, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide) in PBS. Crystal violet (CV) and other reagents were purchased from the Sigma Chemical Laboratory (St Louis, MO).
Formation of S. aureus biofilms
For each experiment, an isolated single bacterial colony was picked from an agar plate, transferred to 5 ~ 10 ml of TSBG medium, and then incubated under orbital agitation (100 ~150 rpm) at 37°C for 18-24 h . An vernight culture of S. aureus was diluted in TSBG to ~ 2 × 106 cells ml−1, and then inoculated in 6-well flat bottom cell culture plates (polystyrene). Cells were cultured 37°C for up to 7 days with medium change every day. At the end of incubation, the supernatant was removed and the formed biofilms were washed with PBS to remove planktonic and loosely attached bacteria.
Generation of discharge gas
Discharge gas was produced using a Plasma Prep III device (SPI Supplies) (Figure 1). This plasma device operated under vacuum and contained a reactor comprising of a pyrex glass chamber (10.45 nm in diameter) and a pair of electrodes (an upper and lower electrode). A radio frequency generator was supplied with a frequency of 13.56 MHz and had an output of up to 100W. The system was evacuated to 300 mtorr, respectively and the dry gas from gas cylinder was introduced to the chamber at a flow rate of 2.4 ft3 h−1 and the chamber pressure was maintained at 460 mtorr , respectively. Subsequently, discharge gas was generated at an electric power of between 0-100W for a desired period of time. Nitrogen, argon and oxygen bottled gases were all purchased from Praxair (Keasbey NJ) and were prepared by Cryogenic Air separation which led to a purity of > 99.9%.
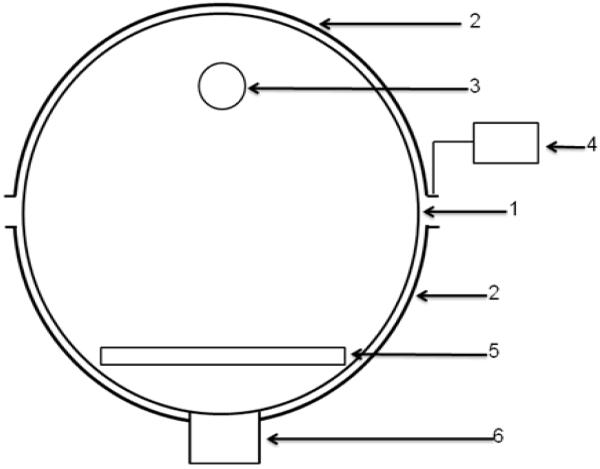
Figure 1.
Schematic diagram of the reaction chamber. (1) Quartz reaction chamber; (2) semitubular electrode; (3) gas outlet; (4) RF power supply; (5) sample holder; (6) vacuum connection. The distance between the gas outlet and the sample holder is about 12 cm.
Treatment of biofilms with discharge gases
After the washing step, biofilms grown on 6-well plates were placed in the center of the chamber. Plasma power, gas, gas flow rate, and exposure time were adjusted according to the requirements of the experiment.
Biofilm susceptibility assays
Crystal violet (CV) staining and a validated MTT cell viability assay were used to assess biofilm susceptibility to each discharge gas. Unlike the widely used CV staining method, the MTT assay does not stain polysaccharides, DNA, proteins, and other biological molecules within the biofilm. Only live bacteria in the biofilms are counted in the MTT assay by measuring the metabolic activity of each individual bacterial cell. There is an excellent correlation between formazan concentrations (absorbance at OD570nm) and CFU counting (Kharidia and Liang 2011). Thus, CV staining was used for the quantification of biofilm formation (biomass) while the MTT assay was utilized to evaluate the viability of bacteria in biofilms. In CV staining, biofilms in 6-well plates were stained with 0.1% (w/v) CV for 10 min . The excess dye was removed by thoroughly rinsing the plate with water and PBS. CV dye associated with biofilms was then extracted by 33% glacial acetic acid and quantified using a microplate reader by measuring solution absorbance values at 570 nm. In the MTT assay, biofilms were incubated with MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide, a yellow tetrazole) (0.5 mg ml−1 ) at 37°C for 10 min . After washing, the purple formazan that formed inside the bacterial cells was dissolved by SDS and then measured using a microplate reader by setting the detecting and reference wavelengths at 570 nm and 630 nm, respectively.
Staining of live and dead bacteria in biofilms
Live and dead bacterial distributions in biofilms were studied by confocal laser scanning microscopy using a LIVE/DEAD staining kit. Briefly, S. aureus biofilms were grown on LabTek 8-well cover-glass chambers. After washing , the biofilms formed were treated with discharged gases under the experimental conditions indicated. At the end of treatment, biofilms were washed extensively with PBS and then stained with LIVE/DEAD staining kit according to the manufacturer’s protocol. A Zeiss confocal laser scanning microscope (CLSM 510) was used to visualize stained bacteria in biofilms. Images were analyzed using the Zeiss LSM Image Browser.
Staining of bacterial cell membranes
S. aureus biofilms were grown and treated as described in the previous section. At the end of treatment, S. aureus cells in biofilms were stained with fluorescent (FITC) labeled wheat germ agglutinin (WGA) in the dark for 10 min. Biofilm images were taken using a Zeiss LSM 510 confocal microscope by setting the excitation and emission wavelengths at 488 nm and 530 nm, respectively
Bacterial chromosomal DNA isolation and analysis
S. aureus biofilms grown in 6-well plates were treated with lysis solution (ZR Fungal/Bacterial DNA MiniPrep ™, Zymo Research). Bacterial chromosomal DNA was isolated and purified according to the standard manufacturer’s protocol. Bacterial chromosomal DNA isolated from planktonic S. aureus was used as a control. Isolated DNA samples were loaded on 1.0 % agarose gel, run for 30 min at 100 volts, and visualized with ethidium bromide staining.
Results and discussion
Activity of eischarge gas to bacteria in biofilms
A good biofilm forming S. aureus strain (ATCC 29213) was used in this study. This S. aureus can grow into biofilms of 15~20 μm thickness in TSBG after 24 h incubation. These 1-day old biofilms had typical biofilm structures (Kharidia and Liang, 2011) and developed antibiotic resistance (Kharidia and Liang, 2011). One-day old S. aureus biofilms were used throughout this study unless indicated otherwise. Six-well plates were used in this study to ensure uniform exposure of biofilms to the discharge gas, subsequently, reducing the standard deviations (SDs) in all the experiments.
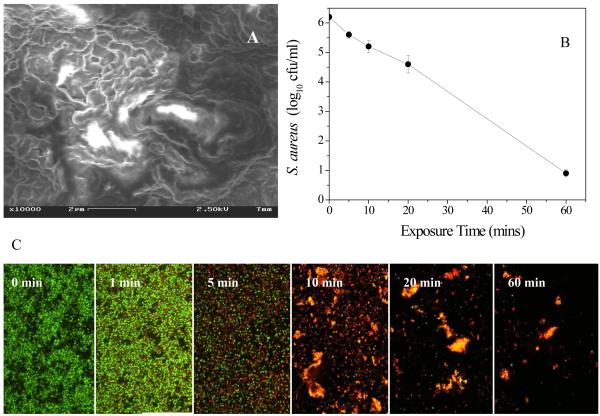
The killing activities of discharge gases to the bacteria in the biofilms were first evaluated through the MTT assay. As shown in Figure 2A, all three of the tested discharge gases (oxygen, nitrogen, and argon) demonstrated strong antibacterial activity to S. aureus in biofilms. Nearly all S. aureus cells (>99.9%) in the biofilms were killed within 5 min by discharge gas generated at 100W. Although there was a slight temperature increase inside the plasma-generating chamber during the treatment (from room temperature to 30~50 °C), it had no measurable effect on the viability of S. aureus in the biofilms as determined by treating biofilms at 50 °C for 20 min (data not shown). Throughout this study, control groups were subjected to an electric field, but no gas supply and > 98 % of these S. aureus in biofilms were still alive (data not shown). These results confirm that the anti-biofilm activity of discharge gas was dependent solely on the various reactive species and UV radiation associated the with plasma treatment.
Figure 2.
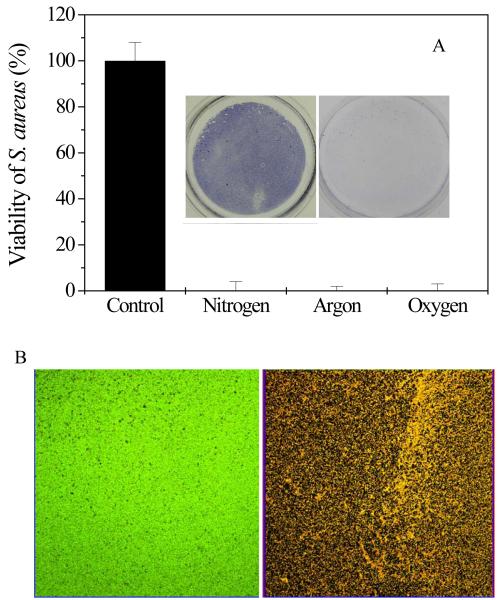
A) The killing activity of discharged gas to bacteria in 1-day old S. aureus biofilms as measured using the MTT assay. Insert: images of MTT stained bacteria in biofilms grown on 6-well plates before (left) and after (right) discharge argon treatment. B) Confocol microscope images of live and dead bacteria in biofilms before (left) and after (right) treatment with discharge argon. Experimental conditions: power: 100W; exposure time: 5 min ; gas flow rate: 2.4 ft3 h−1 . Data represent the mean and SD of at least three samples.
In addition to the MTT method, another cell viability assay using a Live/Dead staining kit was also used to evaluate the anti-biofilm activity of the discharge gas. The SYTO 9 dye, a nucleic acid probe with green fluorescence color, is permeable to healthy cell membranes and thus bacteria with intact (live) cell membranes are stained green. Propidium iodide, a nucleic acid probe with red fluorescence color, is cell membrane impermeable and thus only stains dead bacteria with damaged cell membranes. As shown in Figure 2B, almost all S. aureus in the treated biofilms were stained red, indicating that nearly all S. aureus cells in the biofilms were killed by the discharge gas. This is consistent with the results obtained in the MTT assays (Figure 2A) and confirms the antibacterial activity of the discharge gas to S. aureus cells in biofilms. The power supplied in plasma generation (Figure 3A) and the exposure time (Figure 3B) greatly affected the antibacterial activity of the discharge gas to S. aureus in biofilms. Higher power and longer exposure time were associated with improved effectiveness of the discharge gases in killing bacteria in biofilms. This is consistent with the results from antibacterial activity studies of discharge gas performed on planktonic bacteria [Hong et al 2009].
Figure 3.
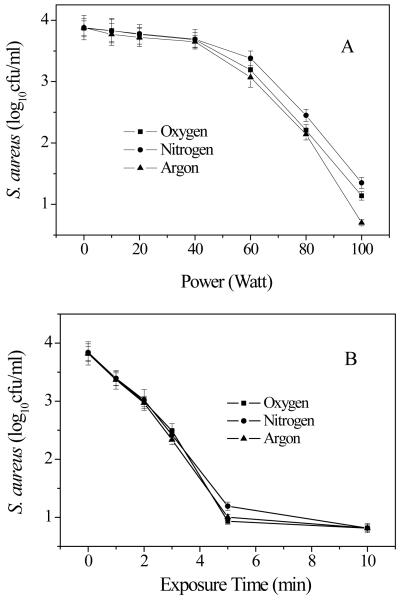
Plasma power and exposure time affected antibacterial activity of discharged gases on S. aureus in 1-day old biofilms. Experimental conditions: A) gas flow rate: 2.4 ft3 h−1 ; exposure time: 5 min ; B) Power: 100W; gas flow rate: 2.4 ft3 h−1 . Data represent the mean and SD of at least three samples. *, p<0.01.
In Fig. 3A, “Power (Watt)” should read “Power (W)” and “(log10 cfu/ml)” should read “(log10 cfu ml−1)”
In Fig. 3B, “Exposure Time” should read “Exposure time” and “(log10 cfu/ml)” should read “(log10 cfu ml−1)”
It is known that charge carriers, reactive species, and UV radiation generated from gas-discharge plasma can all cause severe cell damage and cell death [Moisan et al. 2001; Park et al 2003]. Each discharge gas has different reactive species present [Laroussi and Leipold 2004]. The effectiveness of discharge gas on planktonic bacteria is determined by the composition of the discharge gas. For example, oxygen discharge gas contains radicals and excited molecules (atomic and singlet molecular oxygen), hydroxyl radicals, and ozone [Moisan et al. 2002; Halfmann et al. 2007; Boscariol et al. 2008]. These reactive species along with the intensity and wavelengths of the emitted UV radiation all partake in planktonic bacterial cell inactivation [Moisan et al 2002; Kamgang et al 2007]. Oxygen and nitrogen-based plasmas have been found to be much more effective than argon plasma because of the specific reactive molecular species generated in these two gases. However, it was interesting to find that the antibacterial activity of discharge gas to bacteria in biofilms was not consistent with that measured on planktonic bacteria [; Laroussi 1996, 2002; Kelly-Wintenberg et al 1998]. Despite the distinct chemical and physical properties among oxygen, nitrogen, and argon, all three discharge gases showed almost identical activity towards bacteria in biofilms under the experimental conditions in this study (Figures 2A and 3). It seems that some common reactive species existing in all three types of discharge gases might be responsible for the killing of bacteria in biofilms [Lerouge et al 2001]. It should be pointed out that the discharge argon was more stable in comparison with the discharge oxygen and nitrogen. Therefore, discharge argon might demonstrate slightly better anti-biofilm activity than oxygen and nitrogen at high power (100 watts, the highest power that can be generated by Plasma Prep III) (Figure 3A).
Biofilm inactivation mechanism: antibacterial activity vs etching effects
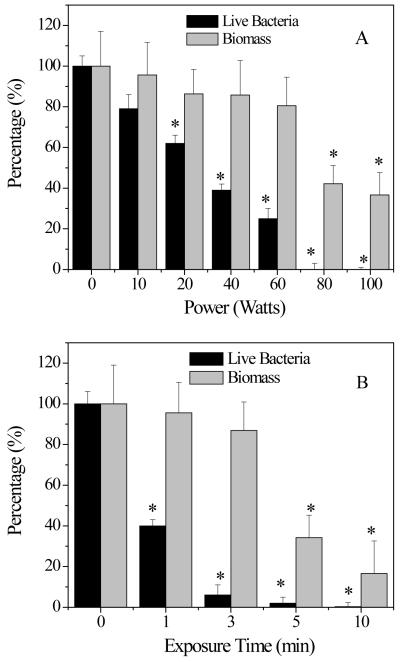
It should be noted that the power dependent inactivation of biofilms showed a biphasic behavior, ie a gradual viability decrease was followed by a rapid decline. Significant anti-biofilm activity increases were observed for discharge gas generated in the power range of 70 ~100W (Figure 3A). Unlike the power dependent inactivation of biofilms, time dependent inactivation showed a steady decrease in the number of viable bacterial cells (Figure 3B). There are two possible mechanisms involved in the discharge gas-mediated biofilm inactivation observed in this study. The first involves the diffusion and penetration of discharge gas into the biofilms which caused the erosion of bacterial cells and resulted in severe damages to the cell membrane or to the DNA. The second mechanism involves the etching effect associated with plasma generation. In this mechanism, the discharge gas causes the chemical break down of the extracellular polymeric matrix. Once the architecture of bioiflms is damaged, the extracelluar polymeric matrix becomes unable to hold the content and bacteria (both dead and live) are released into the bulk solution. As the damage becomes more severe, detachment of biofilms from the solid substratum surface takes place . If biofilms were inactivated through the first mechanism, the CV staining results should indicate no significant biofilm biomass change before and after discharge gas treatment. However, if the biofilms were inactivated through the second mechanism, a significant biomass loss in biofilms associated with discharge gas treatments would be seen. The results from kinetic studies revealed that the inactivation of biofilm by discharge gas was through a more complex process which involved the two mechanisms (Figure 4A and 4B). Bacterial damage at the cellular level happened even when low discharge power and short exposure time were applied. In contrast, the etching effect was only observed at high discharge power and extended exposure time. Therefore, the rapid viability decline associated with the second phase of biofilm inactivation (Figure 3A) was mainly attributed to the etching effect of the discharge gases.
Figure 4.
Discharge gases caused bacterial death (A) and biomass loss (B) in 1-day old S. aureus biofilms. Total biomass and bacterial viability in biofilms were determined by CV and MTT staining, respectively. Experimental conditions: A) discharge gas: argon; exposure time: 5 min; power: 0-100W; gas flow rate: 2.4 ft3 h−1 . B) discharge gas: argon; exposure time: 0-10 min; power: 100W; flow rate: 2.4 ft3 h−1 .
In Fig. 4A, “Power (Watts)” should read “Power (W)” and “Live Bacteria” should read “Live bacteria”
In Fig. 4B, “Exposure Time” should read “Exposure time” and “Live Bacteria” should read “Live bacteria”
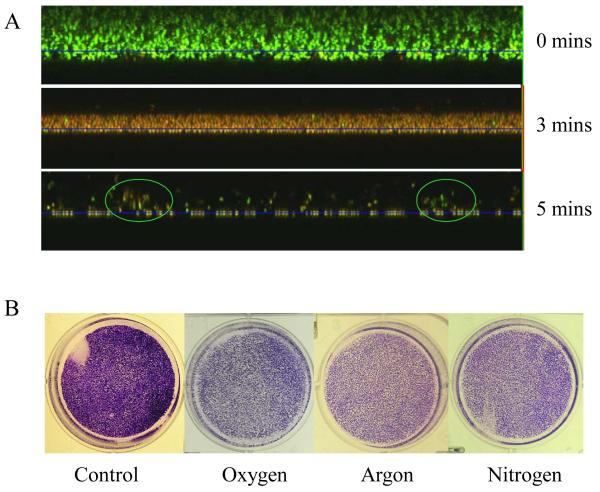
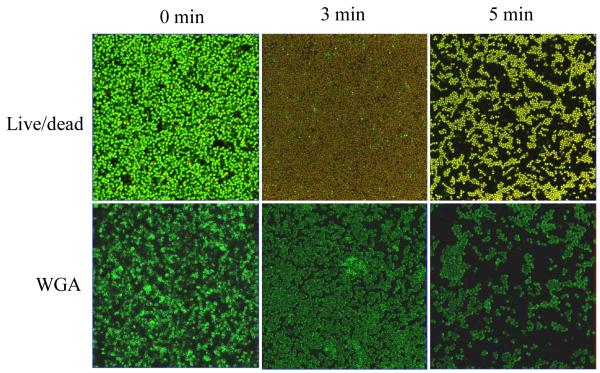
Confocol microscope technology provided an opportunity to visualize and quantify the contributions of the anti-bacterial activity and the etching effect of the discharge gas in biofilm inactivation (Figure 5A). Untreated 1-day old S. aureus biofilms were about 15 μm in thickness and were filled with live bacteria (stained green). The thickness of the same biofilm was reduced to ~ 6 μm after treatment with discharge argon for 3 min . It should be noted that 99.9% of the bacteria in the remaining biofilms were killed (stained red), indicating that discharge gases were able to travel readily or freely throughout the biofilm. Only loosely packed bacterial clusters with very few live bacteria (circled areas) were observed on the substratum surfaces (Figure 5A). The loosely packed bacterial clusters represent the bacteria that remained in biofilms with severely damaged architectures. When the exposure time was extended beyond 5 min complete bacterial death and inoculation of the solid substratum was observed.
Figure 5.
Etching effects of discharge gases on 1-day old biofilms. A) Z-stack confocal images of stained (Live/Dead kit) S. aureus biofilms after exposure to discharge argon for the indicated time; B) microscope images of CV stained S. aureus biofilms treated with discharge gases for 5 min at 100W. Data represent the mean and SD of at least three samples.
In Fig. 5, “mins” (x3) should read “min”
Plasma-mediated inactivation of bacteria in biofilms has been reported in two recent publications [Joaquin et al 2009; Joshi et al 2010]. However, the potential contribution of etching effects to the anti-biofilm activity of the discharge gases were not studied or discussed. In both studies, discharge gases were generated from high power plasma devices. The present authors believe that the etching effect may contribute greatly to the measured anti-biofilm activity of discharge gases in these studies.
Since aged biofilms contain more extracellular polymeric matrix and have more packed biofilm structures, the etching effect might be expected to play a more critical role in the anti-biofilm activity of discharge gas against these biofilms. For this reason, the anti-biofilm activity of discharge argon was tested on 7-day old biofilms. Unlike the 1-day old biofilms, S. aureus cells in 7-day old biofilms were completely buried inside the extracellular polymeric matrix and no single bacterium could be identified in SEM biofilm images (Figure 6A). As shown in Figure 6B, only 5% of S. aureus cells in 7-day old biofilms were killed after treatment for 5 min with discharge argon. Extended treatment time beyond 10 min did not substantially reduce the number of viable bacteria in the 7-day old biofilms. Instead, the formation of large aggregates from partially detached biofilm debris was observed (Figure 6C). This was different from what was observed for 1-day old biofilms (Figure 3A). Bacteria that were wrapped inside these aggregates with a rich extracellular polymeric matrix became more resistant to the discharge gas and consequently more difficult to treat . Obviously, the impact of the etching effect on the anti-biofilm activity of discharge gases can be positive (eg 1-day old biofilms, Figure 3) or negative (eg 7-day biofilms, Figure 6), depending on maturation and possibly the type of biofilm, the power supply, and exposure time. The results from this study clearly show that the etching effect of discharge gases should be seriously considered in any attempts to apply discharge gases for biofilm inactivation and related treatments.
Figure 6.
A) SEM image of 7 -day old S. aureus biofilms; B) confocol images of stained (Live/Dead kit) 7 -day old S. aureus biofilms treated with discharge argon (power: 100W; gas flow rate: 2.4 ft3 h−1 ). (Exposure time is indicated on each image.) C) Viability of S. aureus (as measured by MTT assay) in 7 -day old biofilms treated with discharge argon under the same experimental conditions as described in Figure 6B.
In Fig. 6B, “Exposure Time” should read “Exposure time” and “cfu/ml” should read “cfu ml−1”
Bacteria and biofilm re-growth after treatment with discharge gases
Sterilization by gas-discharge plasma has proven to be as effective as or even more effective than other methods which use a single killing agent [Moisan et al. 2002; Kamgang et al. 2007]. It may therefore be asked whether discharge gases are effective enough for sterilization of biofilms? A simple bacteria and biofilm re-growth experiment was conducted on treated biofilms to evaluate the sterilization abilities of the discharge gas on biofilms. In these experiments, biofilms were treated with discharge gases under the optimal experimental conditions as described above. After treatment, biofilms were directly fed with fresh TSBG (without washing) and incubated for an additional 48 h . Bacterial growth from treated biofilms was measured by monitoring the bacterial concentration increase in the culture medium while biofilm re-growth was estimated by measuring biomass changes in biofilms using CV staining.
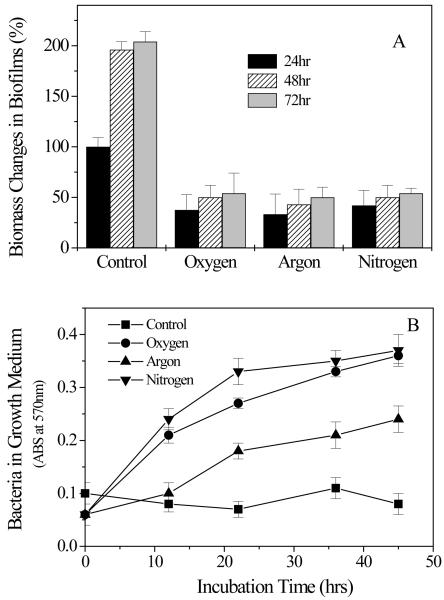
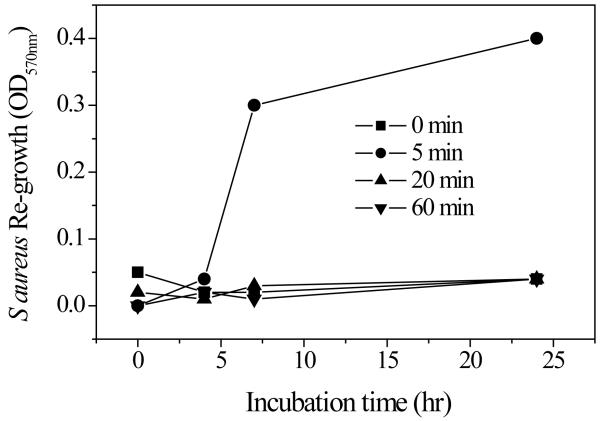
Untreated S. aureus biofilms grew rapidly in the first 24 h of incubation and the total biomass of biofilms doubled after incubation for an additional 24 h (Figure 7A). On the contrary, no significant re-growth could be observed in S. aureus biofilms treated with the discharge gas even after incubation for 48 h . This is consistent with the results from the MTT assays and confocol microscope analysis (Figure 2-4) and confirms the effectiveness of discharge gases in biofilm inactivation. Interestingly, bacterial growth in supernatants was only found in the biofilms treated by discharge gases (Figure 7B). A reasonable explanation of this phenomenon can be directly related to the etching effects of discharge gases [Kim et al 2003]. In the control group, biofilms with intact architecture could hold bacteria tightly and thus the release of bacteria from the untreated biofilm was not observed. However, the etching effect of discharge gas damaged the architecture of biofilms and in doing so a number of bacterial cells were released into the supernatant. Bacterial re-growth in the medium reflected the release of live bacteria which were not killed in situ under the specific experimental conditions (100W power and treatment for 5 min ). The lowest bacterial re-growth rate was found in argon treated biofilms (Figure 7B). This result may reflect the strong etching effect of discharge nitrogen and oxygen compared to argon. However, it should also be noted that discharge argon is better than discharge nitrogen and oxygen at killing bacteria in biofilms when high discharge power was applied (Figure 3A, only compare the points at 100 watts). Complete inhibition of bacteria and biofilm re-growth could be achieved by extending the exposure time of biofilms to each discharge gas (Figure 8).
Figure 7.
Biofilm (A) and S. aureus (B) re-growth from discharge argon treated biofilms. Experimental conditions: power: 100W; gas flow rate: 2.4 ft3 h−1 ; exposure time: 5 min . After treatment, biofilms were washed and fed with fresh TSBG medium and incubated for another 24h (labeled as 48 h ) and 48 h (labeled as 72 h ). Biofilm biomass was measured by CV staining while bacterial re-growth was estimated by measuring absorbance changes of culture supernatant at OD570 nm. Data represent the mean and SD of at least three samples.
In Fig. 7A, “hr” (x3) should read “h” and “Biomass Changes in Biofilms” should read “Biofilm biomass changes”
In Fig. 7B, “Bacteria in Growth Medium” should read “Bacteria in growth medium” and “Incubation Time (hrs)” should read “Incubation time (h)”
Figure 8.
S. aureus growth from discharge argon (100W and 2.4 ft3 h−1 ) treated biofilms was measured by monitoring the absorbance change in the culture supernatant at OD 570 nm.
In Fig. 8, “S. aureus Re-growth” should read “S. aureus re-growth” and “Incubation time (hr)” should read “Incubation time (h)”
Bacterial killing mechanisms of discharge gas
As already mentioned, charge carriers, reactive species, and UV radiation generated from gas-discharge plasma are all well known to cause cell damage and/or cell death [Laroussi 1996, 2002; Kelly-Wintenberg et al 1998]. In this regard, gas composition must be the determining factor for the effectiveness of discharge gas in biofilm inactivation. It has been found that oxygen discharge gas contains radicals and excited molecules (atomic and singlet molecular oxygen), hydroxyl radicals, and ozone [Moisan et al 2002; Halfmann et al 2007; Boscariol et al 2008] and thus is much more effective than nitrogen and argon plasma in bacteria inactivation [Kamgang et al 2007]. Interestingly, under the experimental conditions used in this study, plasma generated from oxygen, nitrogen, and argon gases with distinct chemical and physical properties showed very similar acting-profiles and demonstrated almost the same anti-biofilm activity (Figures 2 and 3), suggesting that some common reactive species existing in all three discharge gases may be directly correlated to the bacteria killing activity of the plasma. It should be pointed out that plasma induced breakdown of the extracellular polymeric matrix will be associated with the generation of many reactive chemical species. Therefore, potential contributions of secondary radiations from these reactive chemical species to the bacteria killing activity of discharge gas should not be excluded.
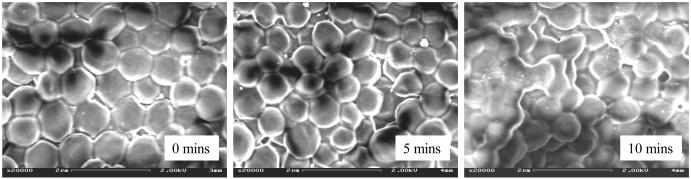
Studies on 1-day old S. aureus biofilms confirmed that all bacteria treated with discharge gas underwent a reduction in size (Figure 9). This shrinking of the bacteria was observed at a very early stage of the treatment ( < 1 min after exposure to the discharge gases). The same phenomenon was not seen in the control group (data not shown) where the biofilms were subjected to the same experimental conditions but without plasma power. Therefore, shrunken bacteria can be explained by gas flow induced dehydration of the cells. . In order to further understand the effects of discharge gases on the bacterial cell membranes, fluorescent (FITC) labeled wheat germ agglutinin (WGA), a probe specific for cell membrane glycans , was used to visualize cell membrane structure changes during the process. It was also interesting to find that discharge gas treatments induced cell membrane fusion in S. aureus cells in biofilms (Figure 9). SEM results revealed that bacterial size reduction and biofilm architectural damage occurred shortly after the biofilms were exposed to the discharge gas. However, cell membrane fusion occurred in the later stages of treatment and was associated with cell lysis (Figure 10).
Figure 9.
Confocal images of S. aureus in 1-day old biofilms treated with discharge argon (power: 100W; gas flow rate: 2.4 ft3 h−1 ). Reduced bacterial sizes and cell membrane damage were observed within 3 min of treatment.
Figure 10.
SEM images of 1 -day old S. aureus biofilms before (A) and after treatment with discharge argon (power: 100W; gas flow rate: 2.4 ft3 h−1 ) for 5 min (B) and 10 min (C), respectively.
In Fig. 10, “mins) (x3) should read “min”
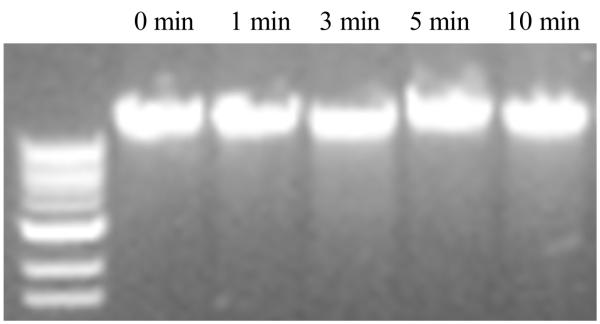
As is known, almost all reactive species generated in gas-discharge plasma are able to penetrate cell membranes and induce severe DNA damage [Laroussi 1996, 2002; Kelly-Wintenberg et al. 1998; Lerouge et al 2001]. In order to further understand the anti-biofilm mechanism of discharge gases, bacterial chromosomal DNA was isolated from biofilms treated with discharge argon for 0~10 min and analyzed by agarose electrophoresis. Interestingly, despite severely damaged cell membranes, no DNA fragmentation was observed (Figure 11). It seems that plasma induced death of S. aureus cells was mainly from cell membrane damage caused by the discharge gas. Further studies on the anti-biofilm mechanisms of discharge gases may enrich understanding of the biological functions of plasma which may in turn lead to the widespread application of plasma in biomedical sciences, especially in the treatment of biofilms and various biofilm related bacterial infections.
Figure 11.
DNA agarose gel of chromosomal DNA isolated from S. aureus in discharged argon (power: 100W; gas flow rate: 2.4 ft3 h−1 ) treated biofilms at different time points.
In summary, this study has confirmed the anti-biofilm ability of discharge gases on S. aureus biofilms in which antibiotic resistance has developed. Interestingly, all three of the tested gases, nitrogen, oxygen, and argon showed almost identical anti-biofilm activity despite their distinct chemical and physical properties. Since the cost of oxygen and nitrogen is substantially lower than that of argon, this finding could have major implications for the ways in which plasmas are generated and used in the future. It was also found that the etching effect will greatly affect the anti-biofilm activity of discharge gases. If bacteria in biofilms are not completely killed before the architecture of the biofilms is severely damaged by discharge gases, the release of live bacteria from biofilms may be problematic in the case of biomedical applications. The possibility of manipulating bacterial killing and the etching effects of discharge gases will be explored in future studies.
Acknowledgements
The authors would like to thank Long Chen and Jinwoo Park for acquiring confocal microscopy and SEM images, respectively, and Dr Natalya Voloshchuk for conducting the chromosomal DNA analysis. This work was supported by NIH grant AI079608.
Abbreviations
- MTT
methylthiazolyldiphenyl-tetrazolium bromide, 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide
- CV
crystal violet
- TSBG
tryptic soy broth supplemented with 0.2 % glucose
- CFU
colony forming unit
- SDS
sodium dodecyl-sulfate
References
- Boscariol MR, Moreira AJ, Mansano RD, Kikuchi IS, Pinto TJ. Sterilization by pure oxygen plasma and by oxygen-hydrogen peroxide plasma: an efficacy study. Int J Pharm. 2008;353:170–175. doi: 10.1016/j.ijpharm.2007.11.059. [DOI] [PubMed] [Google Scholar]
- Conrads H, Schmidt M. Plasma generation and plasma source. Plasma Sources Sci Technol. 2000;9:441–454. [Google Scholar]
- Cos P, Toté K, Horemans T, Maes L. Biofilms: an extra hurdle for effective antimicrobial therapy. Curr Pharm Des. 2010;16:2279–2295. doi: 10.2174/138161210791792868. [DOI] [PubMed] [Google Scholar]
- Costerton JW, Lewandowski Z, Caldwell DE, Korber DR, Lappin-Scott HM. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- Dunne WM, Mason EO, Kaplan SL. Diffusion of rifampin and vancomycin through a Staphylococcus epidermidis biofilm. Antimicrob Agents Chemother. 1993;37:2522–2526. doi: 10.1128/aac.37.12.2522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halfmann H, Bibinov N, Wunderlich J, Awakowicz P. A double inductively coupled plasma for sterilization of medical devices. J Phys D Appl Phys. 2007;40:4145–4154. [Google Scholar]
- Hino T, Yamauchi Y, Ono J, Hirohata Y. Enhancement of reactive species density in nitrogen plasma by mixture of helium and nitridation experiment for silicon. Vacuum. 2004;74:467–471. [Google Scholar]
- Hong YF, Kang JG, Lee HY, Uhm HS, Moon E, Park YH. Sterilization effect of atmospheric plasma on Escherichia coli and Bacillus subtilis endospores. Lett Appl Microbiol. 2009;48:33–37. doi: 10.1111/j.1472-765X.2008.02480.x. [DOI] [PubMed] [Google Scholar]
- Jefferson KK, Goldmann DA, Pier GB. Use of confocal microscopy to analyze the rate of vancomycin penetration through Staphylococcus aureus biofilms. Am Soc Microbiol. 2005;49:2467–2473. doi: 10.1128/AAC.49.6.2467-2473.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joaquin J, Kwan C, Abramzon N, Vandervoort K, Brelles-Marino G. Is gas discharge plasma a new solution to the old problem of biofilm inactivation? Microbiology. 2009;155:724–732. doi: 10.1099/mic.0.021501-0. [DOI] [PubMed] [Google Scholar]
- Joshi SG, Paff M, Friedman G, Fridman G, Fridman A, Brooks AD. Control of methicillin resistant Staphylococcus aureus in planktonic form and biofilms: a biocidal efficacy study of nonthermal dielectric-barrier discharge plasma. Am J Infect Control. 2010;38:293–301. doi: 10.1016/j.ajic.2009.11.002. [DOI] [PubMed] [Google Scholar]
- Kamgang JO, Briandet R, Herry JM, Brisset JL, Natali M. Destruction of planktonic, adherent and biofilm cells of Staphylococcus epidermidis using a gliding discharge in humid air. J Appl Microbiol. 2007;103:621–628. doi: 10.1111/j.1365-2672.2007.03286.x. [DOI] [PubMed] [Google Scholar]
- Kelly-Wintenberg K, Montie TC, Brickman C, Roth JR, Tsai PPY. Room temperature sterilization of surfaces and fabrics with one atmosphere uniform glow discharge plasma. J Ind Microbiol Biotechnol. 1998;20:69–74. doi: 10.1038/sj.jim.2900482. [DOI] [PubMed] [Google Scholar]
- Kharidia R, Liang JF. The activity of a small lytic peptide PTP-7 on Staphylococcus aureus biofilms. J Microbial. doi: 10.1007/s12275-011-1013-5. Forthcoming. [DOI] [PubMed] [Google Scholar]
- Kim D, Economou DJ, Woodworth JR, Miller PA, Shul RJ, Aragon BP, Hamilton TW, Willison CG. Plasma molding over surface topography: simulation and measurement of ion fluxes, energies and angular distributions over trenches in RF high density plasmas. IEEE Trans Plasma Sci. 2003;31:691–702. [Google Scholar]
- Laroussi M. Sterilization of contaminated matter with an atmospheric pressure plasma. IEEE Trans Plasma Sci. 1996;24:1188–1191. [Google Scholar]
- Laroussi M, Leipold F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int J Mass Spectrom 7. 2004;233:81–86. [Google Scholar]
- Laroussi M. Nonthermal decontamination of biological media by atmospheric pressure plasmas review, analysis, and prospects. IEEE Trans Plasma Sci. 2002;30:1409–1415. [Google Scholar]
- Lerouge S, Wertheimer MR, Yahia LH. Plasma sterilization: a review of parameters, mechanisms, and limitations. Plasmas Polym. 6:175–188. 200. [Google Scholar]
- Lewis K. Multidrug tolerance of biofilms and persister cells. Curr Top Microbiol Immunol. 2008;322:107–131. doi: 10.1007/978-3-540-75418-3_6. [DOI] [PubMed] [Google Scholar]
- Lynch AS, Abbanat D. New antibiotic agents and approaches to treat biofilm-associated infections. Expert Opin Ther Pat. 2010;20:1373–87. doi: 10.1517/13543776.2010.505923. [DOI] [PubMed] [Google Scholar]
- Mah TFC, O’Toole GA. Mechanisms of biofilms resistance to antimicrobial agents. Trend Microbiol. 2001;9:34–39. doi: 10.1016/s0966-842x(00)01913-2. [DOI] [PubMed] [Google Scholar]
- Moisan M, Barbeau J, Moreau S, Pelletier J, Tabrizian M, Yahia LH. Low-temperature sterilization using gas plasmas: a review of the experiments and an analysis of the inactivation mechanisms. Int J Pharm. 2001;226:1–21. doi: 10.1016/s0378-5173(01)00752-9. [DOI] [PubMed] [Google Scholar]
- Moisan M, Barbeau J, Crevier MC, Pelletier J, Philip N, Saoudi B. Plasma sterilization. Methods and mechanisms. Pure Appl Chem. 2002;74:349–358. [Google Scholar]
- Morris AD, McCombs 1 GB, Akan T, Hynes W, Laroussi M, Tolle SL. Cold plasma technology: bactericidal effects on Geobacillus stearothermophilus and Bacillus cereus microorganisms. J Dent Hyg. 2009;83:55–61. [PubMed] [Google Scholar]
- Niemira BA, Sites J. Cold plasma inactivates Salmonella Stanley and Escherichia coli O157:H7 inoculated on golden delicious apples. J Food Prot. 2008;71:1357–1365. doi: 10.4315/0362-028x-71.7.1357. [DOI] [PubMed] [Google Scholar]
- Park BJ, Lee DH, Park JC, Lee JS, Lee KY, Chun MS, Chung KH. Sterilization using a microwave-induced argon plasma system at atmospheric pressure. Phys Plasmas. 2003;10:4539–4544. [Google Scholar]
- Patel R. Biofilms and antimicrobial resistance. Clin Orthop Relat Res. 2005;437:41–47. doi: 10.1097/01.blo.0000175714.68624.74. [DOI] [PubMed] [Google Scholar]
- Picioreanu C, van Loosdrecht MCM, Heijnen JJ. Modeling and predicting biofilm structure. In: Allison DG, Gilbert P, Lappin-Scott HM, Wilson M, editors. Community structure and co-operation in biofilms. Cambridge University Press; Cambridge (UK): 2000. pp. 129–166. [Google Scholar]
- Stewart PS. Mechanisms of antibiotic resistance in bacterial biofilms. Int J Med Microbiol. 2002;292:107–113. doi: 10.1078/1438-4221-00196. [DOI] [PubMed] [Google Scholar]
- Stewart PS, Chen X. Biofilm removal caused by chemical treatments. Water Res. 2000;34:4229–4233. [Google Scholar]
- Stewart PS, Costerton JW. Antibiotic resistance of bacteria in biofilms. Lancet. 2001;358:135–138. doi: 10.1016/s0140-6736(01)05321-1. [DOI] [PubMed] [Google Scholar]
- Stoodley P, Sauer K, Davies DG, Costerton JW. Biofilms as complex differentiated communities. Annu Rev Microbiol. 2002;56:187–209. doi: 10.1146/annurev.micro.56.012302.160705. [DOI] [PubMed] [Google Scholar]
- Sureshkumar A, Sankar R, Mandal M, Neogi S. Effective bacterial inactivation using low temperature radio frequency plasma. Int J Pharm. 2010;396:17–22. doi: 10.1016/j.ijpharm.2010.05.045. [DOI] [PubMed] [Google Scholar]
- Vickery K, Pajkos A, Cossart Y. Evaluation of the effectiveness of decontamination of dental syringes. Br Dent J. 2000;189:620–4. doi: 10.1038/sj.bdj.4800847. [DOI] [PubMed] [Google Scholar]