Abstract
T cells play an important role in cancer immunosurveillance and tumor destruction. However, tumor cells alter immune responses by modulating immune cells through antigen stimulation and immunoregulatory cytokines. A better understanding of the interplay between tumor cells and T cells might provide new strategies to enhance anti-tumor immunity. Through an antigen-screening approach using colorectal tumor-reactive T cells, we identified a HLA-DR11-restricted T-cell epitope encoded by KIAA0040 as well as MHC-unrestricted human galectin-3 (Gal-3) expressed by tumor cells. Although the biological function of KIAA0040 remains to be determined, we found that galectin-3 functioned as an immune regulator for direct T cell activation and function. T cell activation induced by galectin-3 resulted in T cell apoptosis. We showed that a high level of expression of galectin-3 promoted tumor growth in vitro and in vivo. Using a mouse tumor model, we demonstrated that delivery of high doses of galectin-3 inhibited tumor-reactive T cells and promoted tumor growth in mice receiving tumor-reactive CD8+ T cells. These findings suggest that galectin-3 may function as an immune regulator to inhibit T cell immune responses and promote tumor growth, thus providing a new mechanism for tumor immune tolerance.
Keywords: Galectin-3, Tumor-reactive T cells, Tumor microenvironment, Immune regulation
Introduction
Increasing evidence from both preclinical tumor models and human clinical trials indicates the importance of T cells in the control and destruction of tumor cells (1). The identification of many tumor antigens recognized by T cells has set the stage for the development of effective cancer vaccines (2). Many clinical trials with molecularly defined cancer antigens or in combination with dendritic cells (DCs) demonstrate that antigen-specific immune responses can be readily induced, but the clinical response rate remains relatively low (3). Hence, these studies suggest that immune suppression in the tumor microenvironment is a major obstacle for the development of effective cancer immunotherapy.
Recent studies show that regulatory T cells play a detrimental role in cancer immunotherapy because these cells accumulate in the tumor microenvironment and suppress immune responses (4, 5). Moreover, we recently demonstrated the presence of tumor-specific CD4+, CD8+ and γδ Treg cells in several types of tumors, suggesting that they can induce antigen-specific, local immune tolerance at tumor sites (6–8). Tumor-associated macrophages and myeloid-derived suppressor cells (MSCs) could also play an important role in inhibiting immune responses and chronic inflammation (9), which has been linked to cancer development and progression (10). Both tumor-associated macrophages/DCs and MSCs promote tumor growth by either secreting immunosuppressive cytokines, including IL-10, TGF-β and IL-1β, or by inducing regulatory T cell differentiation (11). More importantly, tumor cells have been shown to express inhibitory factors (IL-10, TGF-β and IDO) to alter T cell function (4, 12). Immunosuppressive factors, such as FasL and TGF-β expressed by tumor cells, may directly inhibit tumor-reactive T cell expansion or induce T cell apoptosis (13). A recent study suggests that tumor-associated galectin-1, a member of the animal lectin family, contributes to tumor-immune escapes by inhibiting the function of tumor-reactive T cells (14). Therefore, tumor cells constantly modulate T cell responses by presenting tumor antigens and secreting immunoregulatory cytokines. Understanding the interplay between tumor cells and immune cells in the tumor microenvironment is essential for the development of effective cancer immunotherapy.
In this study we describe the identification of HLA-DR11-restricted T cell epitope encoded by the KIAA0040 gene, which can stimulate the cytokine production of a colorectal tumor-reactive T cell line. Interestingly, we also identified human galectin-3 (Gal-3) expressed by tumor cells as an immune regulator of T cells using the same screening system. Although galectin-3 can activate antigen-experienced T cells, it induces T cell apoptosis at a relatively high concentration. Using a mouse tumor model, we demonstrated that a high dose of galectin-3 treatment abrogates the efficacy of tumor-reactive T cells and promotes tumor growth. Since galectin-3 is highly expressed in various types of tumor cells (15, 16), our study may provide a new mechanism by which tumor cells escape the attack of tumor-reactive T cells. These new findings provide a better understanding of interplay between immune cells and cancer cells and identify a new mechanism by which tumor cells induce immune tolerance.
Materials and Methods
Cell lines and antibodies
The tumor-reactive T cell lines used in this study were previously generated in our lab as described (6). The CT28 T cell line was established from a colon cancer patient; TIL586, TIL108, TIL162, TIL1333 and TIL102 T cell lines came from five melanoma patients; TILBT16 and TILBT29 came from two breast cancer patients; TIL194 came from a prostate cancer patient. The melanoma cell line, 586mel, and the colon carcinoma cell line, CC28, were established from fresh tumor samples. 293IMDR11 cells were established by transfecting plasmid DNA encoding DRB1*1101 cDNA into 293 ECII cells as described (17). The expression of DR11 in 293 ECII cells was verified by FACS.
To determine whether T cell recognition could be blocked by specific antibodies, we measured T cell activity in the absence or presence of various antibodies, as previously described (18).
Tumor cDNA library screening
The generation and screening of the cDNA library of CC28 tumor cells was similar to that described previously (19).
Peptides synthesis and T cell epitopes
The candidates of antigenic peptides for CT28 T cell epitopes were predicted by the SYFPEITHI T cell epitopes prediction tool (http://www.syfpeithi.de/scripts/MHCServer.dll/home.htm). The peptides were synthesized by a solid-phase method using a peptide synthesizer (model Apex396; AApptec., Inc., Louisville, KY). The synthesized peptides were dissolved in DMSO in 10 mg/ml. HLA-DR11 expressing 1558 EBV transformed B cells (1558 LCLs) were pulsed with these peptides at the concentration of 5 μg/ml for 3 hours in RPMI 1640. The pulsed LCLs were cocultured with CT28 T cells overnight. To determine the reactivity of CT28 to these peptides, IFN-γ concentrations in the supernatants were measured by ELISA.
Cell proliferation assay
To evaluate T cell proliferation, galectin-3 or a control protein were diluted to the indicated concentrations with PBS and coated at 100 μl/well to flat bottom 96-well plates at 4°C overnight. The plates were washed twice with PBS to remove unbound proteins. Cell proliferation assays were performed as described (6). All experiments were performed in triplicate.
Detection of galectin-3 binding on tumor-reactive T cells
According to the manufacturer’s instructions, galectin-3 and a control protein, PDEF, were labeled with FITC (Invtitogen, Inc). 5×105 cells were stained for 30 minutes by FITC-labeled galectin-3 or labeled control protein (5 μg/ml) with or without 50 mM lactose on ice and analyzed by flow cytometry (BD Bioscience, San Jose, CA). For immunofluorescence microscopy analysis, 1×106 cells were fixed with paraformaldehyde (2%) for 30 min and stained with FITC-labeled galectin-3 and PE-labeled cholera toxin subunit B (Invitrogen) for 30 min. The cells were washed, dropped on glass microscope slides and observed under a fluorescence microscope.
Cell apoptosis analysis
Tumor-reactive T cells were stimulated with soluble galectin-3 or a control protein for the indicated time. Treated cells were washed twice using PBS and stained with propidium iodide (PI) and FITC-Annexin V (BD Bioscience) according to the manufacturer’s instruction. Stained cells were analyzed on a FACSCalibur (BD Bioscience).
Measure of galectin-3 in cell culture supernatants
10×106 cells were cultured in 15 ml RPMI 1640 with 10% FBS for 24 hours. The concentrations of galectin-3 in the supernatants were determined by ELISA using standard methods. The paired antibodies for galectin-3 ELISA assay are anti-human galectin-3 (AF1154) and Biotin conjugated anti-human galectin-3 (BAF1154) from R&D system. Each reported value is the mean of triplicate assays.
Establishment of a tumor cell line expressing shRNA to galectin-3
The expression of galectin-3 in tumor cells was inhibited by green fluorescent protein (GFP)-expressing lentivtius-based shRNAs for human galectin-3 (20). Two galectin-3 specific shRNAs targeting 5′-GAGAGTCATTGTTTGCAATA and 5′-GCTCACTTGTTGCAGTACA, respectively, were constructed and tested the knockdown efficiency. Irrelevant gene ORF3 shRNA served as a control with the target sequence of 5′-GCCCTTCATTGTAGATCTGA. The generation of tumor cell lines expressing galectin-3 shRNA or control shRNA was performed as described previously (21). Infected tumor cells were collected on day 10 and the GFP+ expressing cells were sorted with FACSARIA (BD Bioscience).
In vivo animal experiments
For subcutaneous tumor challenge, 6–8 week old Rag2γC-deficient mice (Taconic, Inc. Hudson, NY) were injected with 5x106 586mel tumor cells/mouse with or without galectin-3 expression. The growth of tumor cells was monitored every three days. For adoptive T cell transfer, Rag2γC-deficient mice were injected with 5×106 cells/mouse (control shRNA expressing 586mel tumor cells or galectin-3 shRNA expressing 586mel tumor cells) and the tumors were allowed to grow for two days. The mice were given an intravenous injection of 20×106 TIL586 T cells/mouse. Treatment with galectin-3 (0.5 mg/ml) was delivered into the mice through intravenous injection.
Statistical analysis
Unless indicated otherwise, data are expressed as mean+/−SD. The significance of difference between groups was determined by Student’ t test or the two-way ANOVA.
Results
Establishment of tumor-reactive CD4+ T cells and tumor library screening
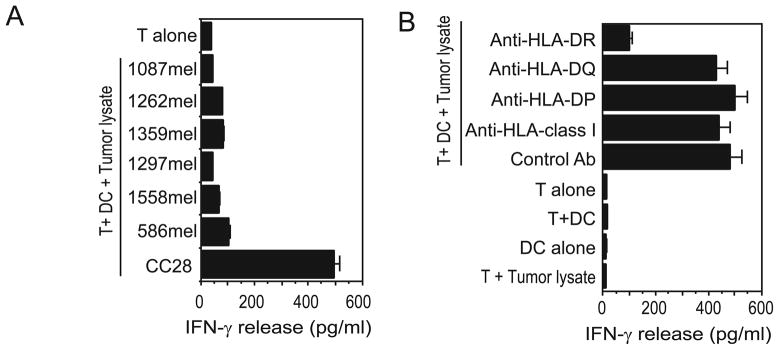
Although many tumor antigens have been identified from melanoma, very few colorectal cancer antigens are identified thus far. We have recently established a colorectal cancer-reactive CD4+ T cell line, CT28, from a fresh colon carcinoma sample. This T cell line recognized autologous DC pulsed with tumor cell lysate, but not DC pulsed with lysates from other melanoma lines (Figure 1A). Recognition of CC28 by CT28 could be specifically blocked by a monoclonal antibody against HLA-DR, but not by anti-HLA-DP, anti-HLA-DQ or anti-MHC class I molecules (Figure 1B). HLA typing analysis indicated that the patient was homozygous for HLA-DR11, suggesting that CT28 T cells recognize a tumor antigen in the context of the HLA-DR11 molecule. Therefore, HLA-DR11 was selectedas the restriction element for the initial cDNA library screening.
Figure 1.
The tumor reactivity of colorectal tumor-reactive CD4+ CT28 T cells. (A) Recognition of autologous tumor cells by CT28 T cells. CT28 T cells were tested for the ability to recognize cell lysates from various target cells. CT28 T cells specifically responded to autologous DC pulsed with autologous tumor cells (CC28), but did not recognize autologous DC pulsed with other cell lines tested. (B) HLA restriction of CT28 T cell recognition. Autologous DCs pulsed with CC28 tumor lysates were cocultured with CT28 T cells in the presence or absence of various anti-HLA antibodies.
To isolate proteins that stimulate tumor-reactive CT28 T cells, we used a genetic targeting expression system developed in our lab that has been successfully used to identify several MHC class II–restricted tumor antigens (6, 22, 23). After screening a total of 2×105 cDNA clones, we identified one positive cDNA pool that was recognized by CT28 cells when transfected into 293IMDR11 cells (Supplementary Figure 1A). From this positive pool, two positive clones, clone 2C6 containing 700 bp insert and clone 1E5 including a 1 Kb cDNA, were identified(Supplementary Figure 1B). These two clones were used as representative clones for further experiments.
Two positive clones are capable of activating CD4+ T cells in different manners
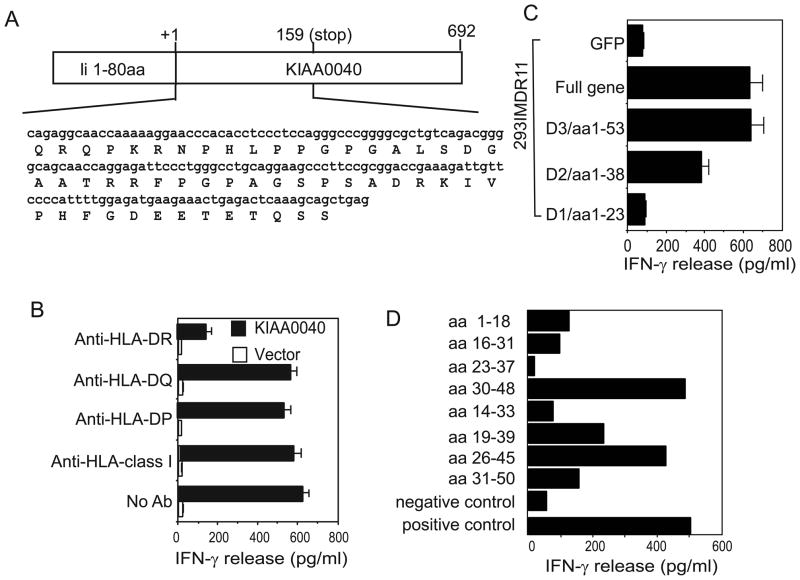
DNA sequence analysis revealed that these two clones contain two different genes. The cDNA insert of clone 2C6 shares 100% similarity with the human KIAA0040 clone (BC020789.1) in the gene database, which is a novel gene that has been mapped to chromosome 1 (Figure 2A). The function of KIAA0040 is not known. T cell reactivity of KIAA0040 by CT28 could be specifically blocked by a monoclonal antibody against HLA-DR, but not by anti-HLA-DP, anti-HLA-DQ or anti-MHC class I molecules (Figure 2B). This result suggests that this novel gene encodes an HLA-DR11-restricted tumor antigen recognized by colon cancer-reactive T cells. To identify the DNA fragment of KIAA0040 that encodes the T cell epitope for CT28 T cells, we made several deletions of KIAA0040 and tested their ability to stimulate T cells. We found that the first 158 bp fragment can stimulate CT28 to produce the same amount of cytokine as the full KIAA0040 gene, while further deletions of KIAA0040 either partially or fully lost their capacity to stimulate CT28 T cells (Figure 2C). To identify the epitope from KIAA0040 recognized by CT28 T cells, eight overlapping peptides were synthesized based on the predicted amino acid sequence and were tested for T cell recognition by pulsing these peptides onto HLA-DR11-expressing LCLs. CT28 T cells were found to respond to stimulation from the two overlapping peptides, indicating that the T cell epitope of KIAA0040 for the recognition of CT28 T cells is AGSPSADRKIVPHFGD (Figure 2D).
Figure 2.
Identification of T cell epitopes of KIAA0040 recognized by T cells in the context of HLA-DR11 molecules. (A) Schematic presentation of the DNA and the predicted amino acid sequence of human KIAA0040 cDNA. (B) Determination of the requirement of the HLA restriction element for recognition of KIAA0040 by CT28 T cells. 293IMDR11 cells were transfected with KIAA0040 or vector only, and then cocultured with T cells in the presence or absence of various anti-HLA antibodies. (C) Identification of the DNA fragment of KIAA0040 with T cell reactivity. A series of 3′ end deletions of KIAA0040 were generated by PCR and cloned into the pTSX vector. Truncated fragments were transfected into 293IMDR11 cells and tested for their ability to stimulate CT28 T cells. (D) Specific recognition of two peptides by CD4+ CT28 T cells. Eight overlapping synthetic peptides were pulsed onto HLA-DR11-expressing 1558 EBV-transformed B cells (1558 LCLs) for 3 hours. After three washes, T cells were added and incubated for 18 hours. T cell reactivity was determined by ELISA. 293IMDR11 cells transfected with KIAA40 served as a positive control. Similar results in (B)–(D) were obtained in repeated experiments.
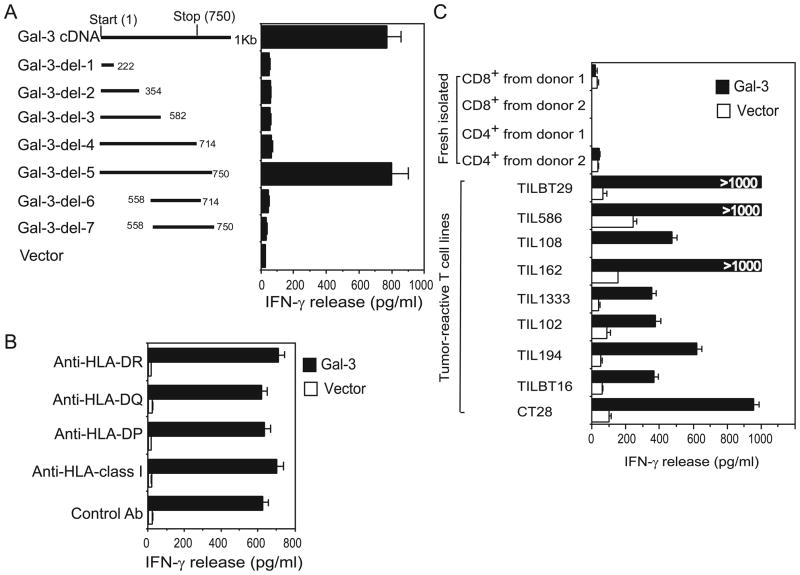
In contrast, clone 1E5 encodes the full-length protein of human galectin-3, which belongs to a growing family of animal lectins defined by their high affinity for β-galactoside and the presence of at least one conserved carbohydrate-recognition domain (CRD)(15). No mutation was detected in clon1E5 when compared to the galectin-3 sequence from the databases (data not shown). To our surprise, CT28 T cells could be activated only by the full-length form of galectin-3, but not by any of the truncated forms, suggesting that the full-length protein of galectin-3 is required for the activation of CT28 (Figure 3A). Moreover, all anti-MHC molecules antibodies failed to block the reactivity of CT28 T cells to galectin-3 (Figure 3B). Unlike the T cell recognition of KIAA0040, CT28 T cells can produce IFN-γ when exposed to galectin-3 presented by 293 cells with or without matched MHC class II molecule expression (Supplementary Figure 1C). These results indicate that galectin-3 can directly activate CT28 T cells without the requirement of MHC class II molecules.
Figure 3.
Activation of CT28 CD4+ T cells and other antigen-experienced T cells by galectin-3 without the involvement of MHC class II molecules. (A) Requirement of full-length of galectin-3 for CT28 T cell reactivity. Truncated galectin-3 fragments were transfected into 293IMDR11 cells and tested for their ability to stimulate CT28 T cells based on IFN-γ release. (B) Failure of anti-HLA antibodies to inhibit the activity of CT28 T cells by galectin-3 presented by 293IMDR11 cells. (C) The response of naïve T cells and tumor-reactive T cell lines to human galectin-3. 293 cells expressing galectin-3 were cocultured with various T cells, and IFN-γ release in the supernatant was measured by ELISA. TILBT29 and TILBT16 cell lines were generated from two breast cancer patients; TIL 194 T cell line was from a prostate cancer patient. TIL586, TIL108, TIL162, TIL1333, and TIL102 cell lines were obtained from different melanoma patients. Naïve CD4+ and CD8+ T cells were purified from the PBMC of healthy donors. Similar results in (A)–(C) were obtained in three repeat experiments.
We next tested whether galectin-3 could activate other tumor-reactive or antigen-experienced T cells. Five melanoma-reactive T cell lines, one prostate cancer-derived T cell line and two breast cancer-derived T cell lines were selected and cocultured with 293 cells expressing galectin-3 for 12–16 h. We found that galectin-3-expressing 293 cells activated all of these T cell lines to secrete IFN-γ, but failed to activate naive CD4+ and CD8+ T cells purified from PBMC of healthy donors (Figure 3C), suggesting that naïve T cell activation requires a strong TCR-mediated activation, while tumor-reactive T cells can be readily activated by galectin-3. We also evaluated the cytokine profiles of CT28 T cells upon galectin-3 stimulation. Galectin-3 induced a high level of IFN-γ, GM-CSF and low to middle levels of IL-4, which is similar to cytokine production induced by anti-CD3 (OKT3) stimulation (Supplementary Figure 2). Although the cytokine profiles were different among the tumor-reactive T cell lines tested, there was no difference between galectin-3-and anti-CD3-stimulation for each tumor-reactive T cell line (data not shown).
Cytokine production and proliferation of tumor-reactive T cells by recombinant galectin-3
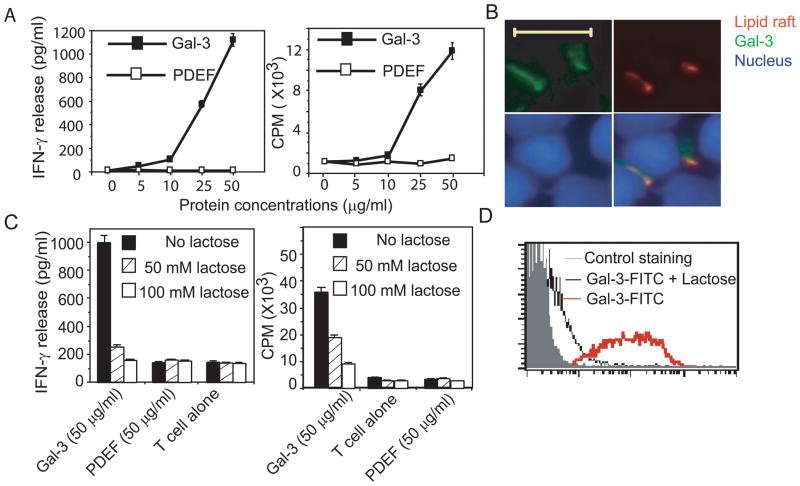
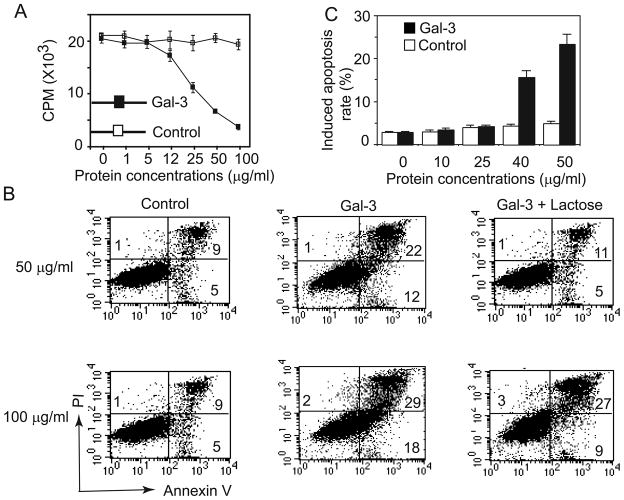
To further assess the stimulatory effect of soluble galectin-3 on T cells, we expressed and purified the full-length galectin-3 using a His-tag purification system. The purity of the recombinant galectin-3 was analyzed by SDS-PAGE and was more than 95% (data not shown). The identity of recombinant human galectin-3 was also confirmed by Western blotting analysis using anti-galectin-3 and anti-Hisantibodies, showing a band with 30 kDa molecular weight (Supplementary Figure 3A, B). An irrelevant protein, prostate epithelium-derived ets transcription factor (PDEF), was also purified by the same way as a control protein for further experiments. We observed that tumor-reactive T cells developed homotypic aggregation as a sign of activation 8 hours after culturing with 50 μg/ml of soluble galectin-3 (Supplementary Figure 3C). However, tumor-reactive T cells treated with the control protein did not show such an aggregation, suggesting that the soluble galectin-3 protein can stimulate tumor-reactive T cells. Titration experiments revealed that galectin-3 induced cytokine production from tumor-reactive T cells was in a dose-dependent manner and that 25 μg/ml of galectin-3 was required to stimulate detectable T cell response (Figure 4A). We next tested whether galectin-3 can enhance the proliferation of tumor-reactive T cells. Although tumor-reactive T cells did not display appreciable proliferation at a 25 μg/ml concentration of soluble galectin-3 (data not shown), a galectin-3 coated-plate of the same concentration induced potent proliferation of tumor-reactive T cells (Figure 4A), suggesting that galectin-3 immobilization is necessary to induce the proliferation of tumor-reactive T cells.
Figure 4.
Galectin-3 binds and activates tumor-reactive T cells through carbohydrate-specific interaction. (A) Dose-dependent responses of the cytokine production and proliferation of the tumor-reactive CT28 T cells to human galectin-3. For cytokine production (left subpanel), CT28 T cells were cultured in the presence of human galectin-3 or control protein at indicated concentrations for 18 hours. The concentration of IFN-γ in the supernatant was measured by ELISA. For proliferation assay (right subpanel), 96-well plates were coated with galectin-3 or a control protein for 18 hours and followed by three washes with PBS. CT28 cells were cultured in presence of coated galectin-3 and the proliferation measured by adding [3H] thymidine during the last 12–16 h of culture. (B) The interaction of galectin-3 and lipid rafts on tumor-reactive T cells. CT28 T cells were fixed and stained with nuclear dye, DAPI (Violet), FITC-labeled galectin-3 (Green) and PE-labeled cholera toxin subunit B (Red), which recognizes ganglioside enriched in lipid rafts. The cells were subjected to fluorescence microscopic analysis. The colocalization of galectin-3 and lipid rafts was shown in the overlay image in the low-right subpanel. The length of included scale bar is 10 μM. (C) Lactose inhibits galectin-3 induced cytokine production and proliferation of tumor-reactive T cells. For cytokine production (left subpanel), CT28 cells were cultured with 50 μg/ml recombinant galectin-3 in the presence or absence of lactose for 18 hours at 37°C. The IFN-γ production of CT28 cells was measured by ELISA. For proliferation assay (right subpanel), CT28 T cells were cultured in the galectin-3 coated plates with or without lactose for 72 hours and used to measure the uptaking of [3H] thymidine. Each experiment was performed in triplicate. (D) The binding of galectin-3 to CT28 cells is completely blocked by lactose. CT28 cells stained by FITC-conjugated galectin-3 were analyzed for fluorescence intensity using flow cytometry. The data are representative of three individual experiments.
Given that galectin-3 mediated T cell activation does not require MHC class II molecules, we postulated that galectin-3 might directly interact with the immunological synapse (IS) for T cell activation. To test this possibility, we analyzed the co-localization of galectin-3 with lipid rafts, which locate in the center of the IS, by fluorescence microscopy. Tumor-reactive CT28 T cells were stained with the nuclear staining dye DAPI (Violet), FITC-labeled galectin-3 (Green) and PE-labeled cholera toxin (Red), which recognizes gangliosides enriched in lipid rafts. We found that galectin-3 can bind to the lipid raft-accumulated regions on the surface of tumor-reactive T cells (Figure 4B). These results indicate that galectin-3 has the ability to bind the IS on tumor-reactive T cells, which might contribute to the activation of tumor-reactive T cells.
Lactose inhibits the activation of tumor-reactive T cells induced by galectin-3
Because galectin-3 is a carbohydrate-binding protein, it is important to determine whether its effect on tumor-reactive T cells is dependent on protein-protein interactions or protein-sugar interactions. Given the essential role of the CRD domain for the interaction of galectin-3 with sugars, we next investigated whether the effect of galectin-3 on tumor-effective T cells was dependent on the CRD domain. Lactose is a simple disaccharide with a high affinity to the CRD domain of galectin-3 and can specifically block the interaction of galectin-3 and glycosylated proteins. We found that galectin-3-induced cytokine production and proliferation of tumor-reactive T cells were inhibited by lactose in a dose-dependent manner (Figure 4C). Lactose treatment alone had no effect on the cytokine production and proliferation of tumor-reactive T cells. The binding of galectin-3 on the surface of tumor-reactive T cells was also strongly inhibited by lactose at a 50 μM concentration (Figure 4D). Taken together, these results indicate that the activation of tumor-reactive T cells by galectin-3 requires the interaction between a lectin of carbohydrate molecules and the CRD domain of galectin-3.
Induction of tumor-reactive T cell apoptosis by galectin-3
Several members of the galectin protein family have been shown to induce the apoptosis of T cells(24). We next tested whether galectin-3 has the ability to induce the apoptosis of tumor-reactive T cells and found that the proliferation of tumor-reactive T cells treated with both an anti-CD3 antibody and galectin-3 was lower than that of T cells treated with anti-CD3 alone (Figure 5A), suggesting that galectin-3 can induce T cell activation-induced apoptosis. To directly determine the ability of galectin-3 to induce tumor-reactive T cell apoptosis, galectin-3 was added to CT28 cells for 24 hours and the rates of Annex V+ cells, which includes cells in early stage of apoptosis (Annex V+/PI−) and late stage of apoptosis (Annex V+/PI+), were determined. Increased cell apoptosis rate of CT28 cells was observed in presence of the galectin-3, but not with a control protein (Figure 5B). Furthermore, lactose could effectively block T cell apoptosis induced by galectin-3. We also observed that galectin-3-induced T cell apoptosis increased with increasing concentrations of galectin-3 (Figure 5C), while galectin-3 induced T cell apoptosis was negligible when the concentration of soluble galectin-3 was lower than 25 μg/ml. These results suggested that the induction of tumor-reactive T cell apoptosis by galectin-3 is dose-dependent.
Figure 5.
Galectin-3 induces the apoptosis of tumor-reactive T cell. (A) Proliferation of tumor-reactive T cells after stimulation with anti-CD3 and galectin-3. 96-well plates were coated with CD3 (3 μg/ml) at 4°C overnight. CT28 cells were cultured in anti-CD3 coated plates with soluble galectin-3 or control protein for 72 hours, and used to measure the uptaking of [3H] thymidine. (B) Galectin-3 induces T cell apoptosis in a dose-dependent way. Tumor-reactive T cells were cultured in T cell growth medium in the presence of galectin-3 or a control protein with indicated concentrations for 16 hours. Apoptosis was determined by Annexin V-FITC and PI staining. Shown are mean percentage of Annexin V+ cells, which include the cells in early-stage (Annexin V+/PI−cells) and late-stage (Annexin V+/PI+ cells) apoptosis. Similar results were obtained in repeated experiments. (C) CT28 T cells were treated with galectin-3 or a control protein with or without lactose for 24 hours. The numbers in the subpanels indicate percentages of cells in each quadrant. Similar results were obtained in repeated experiments.
The role of galectin-3 expression in tumor growth
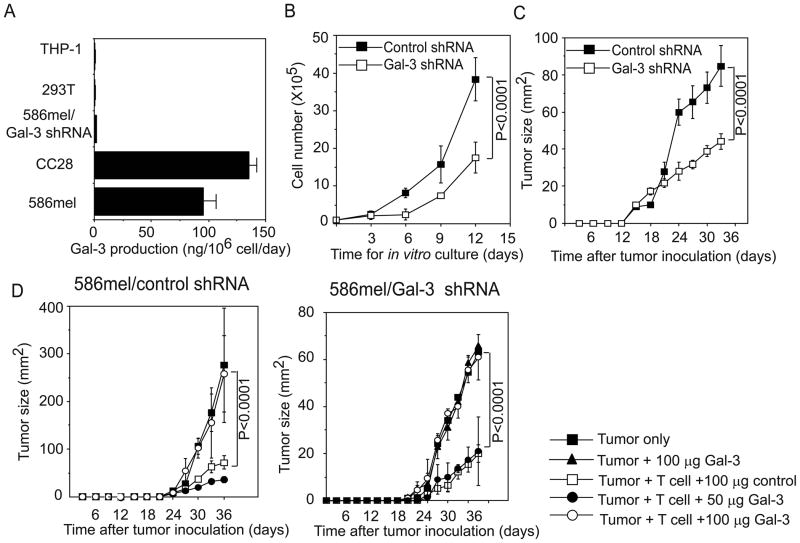
The expression of galectin-3 appears to be significantly upregulated in tumor cells and in the serum of cancer patients (16, 25, 26). Moreover, our results showed that galectin-3 expressed by tumor cells can be secreted into extracellular compartments (Supplementary Figure 4B). As shown in Figure 6A, both melanoma tumor cells and colorectal tumor cells released around 100 ng of galectin-3 per million cells in 24 hour in vitro culture. It is conceivable that the expression of galectin-3 by tumor cells might contribute to tumor progression through regulating tumor cell growth and T cell-mediated anti-tumor immunity. In our previous study, we found that 586mel tumor cells can progressively grow in immunodeficient mice, but were inhibited when co-transferred with autologous tumor-reactive TIL586 cells (21). To test the role of galectin-3 in tumor development both in vitro culture and in this mouse tumor model, we constructed galectin-3-specific lentiviral-based shRNA to knock down endogenous galectin-3 expression in 586mel tumor cells. 586mel cells transduced with galectin-3 shRNAs effectively reduced the levels of galectin-3 expression compared with 586mel cells transduced with a control shRNA (Supplementary Figure 4A). Galectin-3 could be detected in the cell medium of 586mel cells transduced with control shRNA, but not from galectin-3-silencing 586mel cells (Supplementary Figure 4B). We next determined whether there is any difference in the growth rate in vitro between galectin-3-silencing 586mel and control shRNA-expressing 586mel cells. As shown in Figure 6B, the growth rate of galectin-3-silencing 586mel tumor cells was slower than that of control shRNA-expressing 586mel cells under in vitro culture condition. 586mel cells expressing another independent galectin-3 specific shRNA were also shown decreased growth rate, compared with control tumor cells (Supplementary Figure 4C). To determine their growth properties in vivo, we inoculated Rag2γC-deficient mice with control shRNA-expressing 586mel and galectin-3-silencing 586mel tumor cells and found that galectin-3-silencing 586mel cells grew much slower than the control shRNA-expressing tumor cells (Figure 6C). These results demonstrate that galectin-3 promotes tumor growth, while the knockdown of galectin-3 reduces tumor growth both in vitro and in vivo.
Figure 6.
Effects of intracellular and extracellular galectin-3 on tumor growth. (A) Secretion of galectin-3 by tumor cells. 10×106 indicated cells were cultured in 15 ml culture medium for 24 hours. The concentrations of galectin-3 in the supernatants were determined by ELISA. Shown are converted amounts of galectin-3 released by indicated cells (ng/106 cell/day). (B) Effect of galectin-3 knockdown on tumor growth in vitro. Control shRNA-expressing 586mel tumor cells and galectin-3-silencing 586mel tumor cells were cultured and their cell numbers were determined. (C) Galectin-3-silencing tumor cells reduce the growth rate in Rag2γC-deficient mice compared with control shRNA-expressing 586mel tumor cells. (D) The effect of galectin-3 treatment on T cell mediated anti-tumor immune response and tumor growth. Control shRNA-expressing (left subpanel) or galectin-3-silencing 586mel tumor cells (right subpanel) were subcutaneously injected into Rag2γC-deficient mice and followed by intravenously injecting tumor-reactive TIL586 T cells. Tumor growth rates were monitored every three days and plotted as means ± SD. P values in panel D was determined by two-way ANOVA.
We next tested whether galectin-3 secreted by tumor cells can modulate the function or apoptosis of tumor-reactive T cells, thus inhibiting or promoting tumor growth in vivo. We first injected Rag2γC-deficient mice with control shRNA-expressing 586mel tumor cells and then intravenously injected tumor-reactive CD8+ TIL586 T cells two days later. The formation and size of tumors in these mice was monitored every three days. As shown in Figure 6D (left subpanel), tumor growth was inhibited by TIL586 cells compared with tumor cells alone, but no significant difference in tumor growth was observed in a group of mice treated with TIL586 plus a control protein or in a group of mice treated TIL586 plus a low dose (50 μg/mouse) of galectin-3. However, treatment with galectin-3 at a high concentration (100 μg/mouse) completely abrogated the ability of TIL586 to inhibit tumor growth. Similar results were obtained from Rag2γC-deficient mice challenged with 586mel tumor cells expressing galtectin-3 shRNA (Figure 6D, right subpanel). Notably, tumor cells with a high concentration of soluble galectin-3 alone did not change tumor growth. Taken together, these results demonstrate that soluble galectin-3 can induce T cell activation and apoptosis, thus inhibiting the ability of T cells to kill tumor cells at a relatively high concentration. Inhibition of galectin-3 expression may serve as a therapeutic target for cancer therapy.
Discussion
Using the tumor-reactive T cell line (CT28), we identified two tumor-associated antigens by screening a colorectal cancer-derived Ii-fusion cDNA library. One of the positive clones is the gene product encoded by a nonmutated gene, KIAA0040, mapping to chromosome 1. Recognition of the KIAA0040 gene product by CT28 cells is restricted by HLA-DR11 molecules, suggesting that the KIAA0040 gene product may represent one of the tumor antigens expressed on colorectal cancer cells and recognized by CT28. Like many tumor antigens, such as NY-ESO-1, the biological function of this protein remains to be determined. The second gene we identified is human galectin-3. However, galectin-3 does not serve as a true antigen for T cell recognition because T cell activation does not require the involvement of MHC class II molecules. Similar observations have been reported in the earlier studies showing that MUC1 is one of the first tumor antigens recognized by human tumor-reactive T cells, but is independent of MHC molecules. The tandem repeat of the extracellular domain of tumor MUC1 is required for the MHC-unrestricted T cell recognition(27). Subsequently, MHC class I and class II restricted T cell epitopes of MUC1 were identified (28). Compared with MUC1, our data demonstrated that MHC-unrestricted recognition of galecetin-3 is dependent on the CRD of galectin-3. Unlike MUC1, activation of tumor-reactive T cells by galectin-3 induces cell apoptosis in a dose dependent fashion. Therefore, galectin-3 functions as an immune regulator of tumor-reactive T cells
Galectins are a family of animal lectins, which includes fifteen mammalian galectins. Based on the number of CRD, the members of this family are divided into three types. Galectin-3 is unique one-CRD galectin which contains non-lectin domain fused with the CRD (15). The overall homology of human intergalectins is about 20%, but their CRDs are relatively conserved (29). In the last few years it has been demonstrated that the members of the galectin family proteins are closely associated with immune regulation in various diseases (24). The galectin protein family plays important roles in various biological processes including modulation of the function of immune effector cells, such as T cells (15). For example, galectin-1 induces the apoptosis of thymocytes and activated peripheral T cells (30). Administration of human galectin-1 in mice contributes to the suppression of intestinal inflammation induced by IFN-γ production from Th1 cells (31). Treatment of recombinant galectin-1 is also reported to suppress Th1-mediated experimental autoimmune retinal disease in mouse model by inducing CD4+ regulatory T cells (32). The targeted inhibition of galectin-1 gene expression in tumor cells promotes T cell-mediated tumor rejection in vivo (14). Galectin-2 and 9 have been reported to induce apoptosis of activated T cell lines (29, 33). Galectin-4 acts as a stimulator of mucosal CD4+ T cells, by specifically inducing IL-6 production in mice with inflammatory bowel disease, and contributes to the exacerbation of intestinal inflammation (34).
Similar to other members of the galectin family, several studies demonstrate the role of galectin-3 in regulating the function and apoptosis of human Jurkat T cells (35–38). However, very little is known about the role of galectin-3 in the regulation of tumor-reactive T cells in the tumor microenvironment. Our studies demonstrate that galectin-3 directly interacts with the immune synapse on the surface of tumor-reactive T cells and activates T cells for apoptosis, depending upon the status of T cells and its protein concentrations. The activation of freshly isolated naïve T cells by galectin-3 is very weak or negligible; however, T cells become responsive to galectin-3 stimulation once they are treated with anti-CD3 and then rested for 10 days (data not shown). Consistent with this observation, we also found that antigen-experienced effector T cells can be readily activated by galectin-3. These results are consistent with studies using resting leukemic T cell lines (38). It appears that the initial TCR activation might change the glycosylation status of the surface of T cells and render these cells more sensitive to galectin-3 stimulation. An alternative explanation is that antigen-experienced T cells require a lower threshold for their activation by galectin-3. Moreover, recent studies show that the sensitivity of T cells to stimulation from galectin family members is influenced by different T cell subsets generated at a special condition. For example, only CD4+ T cells that are derived from inflammatory conditions can respond to galectin-4 stimulation (34). The susceptibility of Th1, Th2, and Th17 to galectin-1-induced apoptosis is different, suggesting that the glycosylation status of different subsets of T cells is different (39). Taken together, we believe that galectin-3 secreted by tumor cells may effectively or preferentially activate antigen-experienced or tumor reactive T cells to produce cytokines and induce them for apoptosis at a high level of concentration, thus inducing immune tolerance at tumor sites.
Like other members of galectin protein family, galectin-3 are highly expressed in many types of cancer cells (40–42). Besides its regulatory role in T cell activation and immune tolerance, galectin-3 may be associated with tumor growth as well as the aggressive phenotype of tumors (15, 16). Intracellular galectin-3 promotes tumor growth, survival and metastasis. We show that the knockdown of galectin-3 by shRNA inhibits tumor growth in vitro and in vivo, which is consistent with the published data obtained from different tumor cells, such as breast cancer (43, 44). However, we found that the soluble form of galectin-3 did not affect the growth of tumor cells, instead it modulated the tumor-reactive T cell function by inducing T cell activation and apoptosis in vitro. More importantly, we demonstrate that soluble galectin-3 inhibits T cell responses and promotes tumor growth in vivo, but this effect requires a relatively high concentration of galectin-3. This is in agreement with the data published by other groups, showing that the minimal concentration of galectin-3 required for the induction of apoptosis of a human T cell line is 3 μM (approximately 100 μg/ml) (36). Given the elevated expression of galectin-3 in tumor cells as well as in the serum of cancer patients (16, 26, 45), we postulate that galectin-3 may accumulate in the local tumor environment, creating the high concentrations necessary for the above observed effects. The high concentrations of galectin-3 in the local tumor environments may eventually drive tumor-reactive T cell apoptosis and thereby the loss of their anti-tumor effector functions. Supporting this premise, one study using immunohistochemical staining demonstrates that the expression of galectin-3 in human melanoma biopsies correlates with T cell apoptosis (46). Treatment of mice with galectin-3 inhibitor reduces the growth rate of human cancer cells in vivo (47–49). Taken together, our own findings along with those of other groups clearly indicate that galectin-3 inhibits antitumor immunity and promote tumor growth through two distinct mechanisms. Inhibition or knockdown of galectin-3 not only reduces tumor growth, but also improves the therapeutic potential of cancer immunotherapy.
Supplementary Material
Two positive clones were identified by screening the colorectal tumor cDNA library utilizing colorectal tumor-reactive CD4+ CT28 T cells. (A) Identification of a positive cDNA pool (no.29) capable of stimulating IFN-γ production from a tumor-reactive CT28 T cell line. Optical density reading at a 450 nm wavelength revealed a single positive pool in one of the 96-well plates. The data are representative of twenty 96-well plate screenings. (B) Isolation of two independent genes capable of stimulating CT28 T cell activation. All positive clones were digested by Not I and Nhe I restriction enzymes to determine their insert size. Clone 1E5 contained a 1 kb cDNA insert, while clone 2C6 had a 0.7 kb cDNA insert. (C) CT28 T cell reactivity to KIAA0040 or galectin-3 presented by various kinds of 293 cell lines. 293 cells expressing HLA-DR11 or DR4 and 293T cells without DR expression were transfected with KIAA0040, galectin-3 or vector only. The following day, the CT28 T cell activity was tested by ELISA. Each experiment was performed in triplicate.
Cytokine profile of CT28 T cells upon galectin-3 stimulation. 293 cells were transfected with either galectin-3 or vector only and cocultured with CT28 T cells. The following day, cytokine production from CT28 T cells was measured by ELISA. CT28 T cells stimulated with coated anti-CD3 (3 μg/ml) were used as a positive control.
Generation of human recombinant galectin-3. (A, B) Western blot analysis of recombinant galectin-3 was performed by anti-galectin-3 antibody (A) or anti-His6 antibody (B). PDEF was purified and served as control protein in the following experiments. (C) The morphology of tumor-reactive T cells treated with recombinant galectin-3 protein and control protein for 8 hours. (D, E) The interaction of galectin-3 with tumor-reactive T cells. CT28 T cells were stained with FITC labeled galectin-3 and a control protein. The stained cells were subjected to flow cytometry analysis (D) and fluorescence microscopic analysis (E). The overlay images from fluorescent microscopy are shown.
Knock down of galectin-3 in tumor cells. (A) Galectin-3 specific knockdown in 586 tumor cells by galectin-3 shRNA. Green fluorescent protein (GFP)-expressing lentivtius-based shRNAs for human galectin-3 were generated as described previously. 586mel cells were transduced with galectin-3 specific shRNAs or control shRNA. After sorting, GFP+ tumor cells were lysed and performed the western blotting with anti-human galectin-3 antibody. (B) Western blot detection of galectin-3 in concentrated SFCM from tumor cell lines generated from 586mel cells transduced with galectin-3 specific shRNA or control shRNA. 10×106 tumor cells were cultured in 10 ml RPMI for 24 h. Supernatants were collected and precipitated by acidic acetone. The precipitated proteins were resuspended in 1ml PBS. Samples were loaded with the indicated amount. Indicated amounts of recombinant his6-tagged galectin-3 were used as positive control. (C) Proliferation of 586mel cells with or without galectin-3 expression. 1×104 586mel cells expressing galectin-3 shRNA-2 or control shRNA were seeded into 96-well. After 24 or 72 h, cells were subjected to the MTT assay. Shown are the mean absorbances at a wavelength of 550 nm.
Acknowledgments
Grant support: Baylor College of Medicine and NIH grants R01 CA94237 and R01 CA90327 (R-F. W). We thank Dr. Dorothy Lewis and Cristina Palcu for critically reading the manuscript.
Footnotes
No potential conflicts of interest.
References
- 1.Rosenberg SA. Progress in the development of immunotherapy for the treatment of patients with cancer. J Intern Med. 2001;250(6):462–75. doi: 10.1046/j.1365-2796.2001.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang RF. Human tumor antigens: implications for cancer vaccine development. J Mol Med. 1999;77(9):640–55. doi: 10.1007/s001099900042. [DOI] [PubMed] [Google Scholar]
- 3.Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2004;10(9):909–15. doi: 10.1038/nm1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer. 2005;5(4):263–74. doi: 10.1038/nrc1586. [DOI] [PubMed] [Google Scholar]
- 5.Wang HY, Wang RF. Regulatory T cells and cancer. Curr Opin Immunol. 2007;19(2):217–23. doi: 10.1016/j.coi.2007.02.004. [DOI] [PubMed] [Google Scholar]
- 6.Wang HY, Lee DA, Peng G, et al. Tumor-specific human CD4+ regulatory T cells and their ligands: implications for immunotherapy. Immunity. 2004;20(1):107–18. doi: 10.1016/s1074-7613(03)00359-5. [DOI] [PubMed] [Google Scholar]
- 7.Kiniwa Y, Miyahara Y, Wang HY, et al. CD8+ Foxp3+ regulatory T cells mediate immunosuppression in prostate cancer. Clin Cancer Res. 2007;13(23):6947–58. doi: 10.1158/1078-0432.CCR-07-0842. [DOI] [PubMed] [Google Scholar]
- 8.Peng G, Wang HY, Peng W, Kiniwa Y, Seo KH, Wang RF. Tumor-infiltrating gammadelta T cells suppress T and dendritic cell function via mechanisms controlled by a unique toll-like receptor signaling pathway. Immunity. 2007;27(2):334–48. doi: 10.1016/j.immuni.2007.05.020. [DOI] [PubMed] [Google Scholar]
- 9.Fogg DK, Sibon C, Miled C, et al. A clonogenic bone marrow progenitor specific for macrophages and dendritic cells. Science. 2006;311(5757):83–7. doi: 10.1126/science.1117729. [DOI] [PubMed] [Google Scholar]
- 10.Nelson D, Ganss R. Tumor growth or regression: powered by inflammation. J Leukoc Biol. 2006;80(4):685–90. doi: 10.1189/jlb.1105646. [DOI] [PubMed] [Google Scholar]
- 11.Zhu P, Baek SH, Bourk EM, et al. Macrophage/cancer cell interactions mediate hormone resistance by a nuclear receptor derepression pathway. Cell. 2006;124(3):615–29. doi: 10.1016/j.cell.2005.12.032. [DOI] [PubMed] [Google Scholar]
- 12.Gajewski TF, Meng Y, Harlin H. Immune suppression in the tumor microenvironment. J Immunother (1997) 2006;29(3):233–40. doi: 10.1097/01.cji.0000199193.29048.56. [DOI] [PubMed] [Google Scholar]
- 13.Pardoll D. Does the immune system see tumors as foreign or self? Annu Rev Immunol. 2003;21:807–39. doi: 10.1146/annurev.immunol.21.120601.141135. [DOI] [PubMed] [Google Scholar]
- 14.Rubinstein N, Alvarez M, Zwirner NW, et al. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer Cell. 2004;5(3):241–51. doi: 10.1016/s1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- 15.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5(1):29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 16.Califice S, Castronovo V, Van Den Brule F. Galectin-3 and cancer (Review) Int J Oncol. 2004;25(4):983–92. [PubMed] [Google Scholar]
- 17.Wang RF, Wang X, Atwood AC, Topalian SL, Rosenberg SA. Cloning genes encoding MHC class II-restricted antigens: mutated CDC27 as a tumor antigen. Science. 1999;284(5418):1351–4. doi: 10.1126/science.284.5418.1351. [DOI] [PubMed] [Google Scholar]
- 18.Zeng G, Wang X, Robbins PF, Rosenberg SA, Wang RF. CD4(+) T cell recognition of MHC class II-restricted epitopes from NY-ESO-1 presented by a prevalent HLA DP4 allele: association with NY-ESO-1 antibody production. Proc Natl Acad Sci U S A. 2001;98(7):3964–9. doi: 10.1073/pnas.061507398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Voo KS, Zeng G, Mu JB, Zhou J, Su XZ, Wang RF. CD4+ T-cell response to mitochondrial cytochrome B in human melanoma. Cancer Res. 2006;66(11):5919–26. doi: 10.1158/0008-5472.CAN-05-4574. [DOI] [PubMed] [Google Scholar]
- 20.Rubinson DA, Dillon CP, Kwiatkowski AV, et al. A lentivirus-based system to functionally silence genes in primary mammalian cells, stem cells and transgenic mice by RNA interference. Nat Genet. 2003;33(3):401–6. doi: 10.1038/ng1117. [DOI] [PubMed] [Google Scholar]
- 21.Peng G, Guo Z, Kiniwa Y, et al. Toll-like receptor 8-mediated reversal of CD4+ regulatory T cell function. Science. 2005;309(5739):1380–4. doi: 10.1126/science.1113401. [DOI] [PubMed] [Google Scholar]
- 22.Wang HY, Zhou J, Zhu K, Riker AI, Marincola FM, Wang RF. Identification of a mutated fibronectin as a tumor antigen recognized by CD4+ T cells: its role in extracellular matrix formation and tumor metastasis. J Exp Med. 2002;195(11):1397–406. doi: 10.1084/jem.20020141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang HY, Peng G, Guo Z, Shevach EM, Wang RF. Recognition of a new ARTC1 peptide ligand uniquely expressed in tumor cells by antigen-specific CD4+ regulatory T cells. J Immunol. 2005;174(5):2661–70. doi: 10.4049/jimmunol.174.5.2661. [DOI] [PubMed] [Google Scholar]
- 24.Rabinovich GA, Baum LG, Tinari N, et al. Galectins and their ligands: amplifiers, silencers or tuners of the inflammatory response? Trends Immunol. 2002;23(6):313–20. doi: 10.1016/s1471-4906(02)02232-9. [DOI] [PubMed] [Google Scholar]
- 25.Iurisci I, Tinari N, Natoli C, Angelucci D, Cianchetti E, Iacobelli S. Concentrations of galectin-3 in the sera of normal controls and cancer patients. Clin Cancer Res. 2000;6(4):1389–93. [PubMed] [Google Scholar]
- 26.Vereecken P, Zouaoui Boudjeltia K, Debray C, et al. High serum galectin-3 in advanced melanoma: preliminary results. Clin Exp Dermatol. 2006;31(1):105–9. doi: 10.1111/j.1365-2230.2005.01992.x. [DOI] [PubMed] [Google Scholar]
- 27.Barnd DL, Lan MS, Metzgar RS, Finn OJ. Specific, major histocompatibility complex-unrestricted recognition of tumor-associated mucins by human cytotoxic T cells. Proc Natl Acad Sci U S A. 1989;86(18):7159–63. doi: 10.1073/pnas.86.18.7159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vlad AM, Kettel JC, Alajez NM, Carlos CA, Finn OJ. MUC1 immunobiology: from discovery to clinical applications. Adv Immunol. 2004;82:249–93. doi: 10.1016/S0065-2776(04)82006-6. [DOI] [PubMed] [Google Scholar]
- 29.Sturm A, Lensch M, Andre S, et al. Human galectin-2: novel inducer of T cell apoptosis with distinct profile of caspase activation. J Immunol. 2004;173(6):3825–37. doi: 10.4049/jimmunol.173.6.3825. [DOI] [PubMed] [Google Scholar]
- 30.Hernandez JD, Nguyen JT, He J, et al. Galectin-1 binds different CD43 glycoforms to cluster CD43 and regulate T cell death. J Immunol. 2006;177(8):5328–36. doi: 10.4049/jimmunol.177.8.5328. [DOI] [PubMed] [Google Scholar]
- 31.Santucci L, Fiorucci S, Rubinstein N, et al. Galectin-1 suppresses experimental colitis in mice. Gastroenterology. 2003;124(5):1381–94. doi: 10.1016/s0016-5085(03)00267-1. [DOI] [PubMed] [Google Scholar]
- 32.Toscano MA, Commodaro AG, Ilarregui JM, et al. Galectin-1 suppresses autoimmune retinal disease by promoting concomitant Th2- and T regulatory-mediated anti-inflammatory responses. J Immunol. 2006;176(10):6323–32. doi: 10.4049/jimmunol.176.10.6323. [DOI] [PubMed] [Google Scholar]
- 33.Lu LH, Nakagawa R, Kashio Y, et al. Characterization of galectin-9-induced death of Jurkat T cells. J Biochem. 2007;141(2):157–72. doi: 10.1093/jb/mvm019. [DOI] [PubMed] [Google Scholar]
- 34.Hokama A, Mizoguchi E, Sugimoto K, et al. Induced reactivity of intestinal CD4(+) T cells with an epithelial cell lectin, galectin-4, contributes to exacerbation of intestinal inflammation. Immunity. 2004;20(6):681–93. doi: 10.1016/j.immuni.2004.05.009. [DOI] [PubMed] [Google Scholar]
- 35.Dong S, Hughes RC. Galectin-3 stimulates uptake of extracellular Ca2+ in human Jurkat T-cells. FEBS Lett. 1996;395(2–3):165–9. doi: 10.1016/0014-5793(96)01031-9. [DOI] [PubMed] [Google Scholar]
- 36.Fukumori T, Takenaka Y, Yoshii T, et al. CD29 and CD7 mediate galectin-3-induced type II T-cell apoptosis. Cancer Res. 2003;63(23):8302–11. [PubMed] [Google Scholar]
- 37.Stillman BN, Hsu DK, Pang M, et al. Galectin-3 and galectin-1 bind distinct cell surface glycoprotein receptors to induce T cell death. J Immunol. 2006;176(2):778–89. doi: 10.4049/jimmunol.176.2.778. [DOI] [PubMed] [Google Scholar]
- 38.Stowell SR, Qian Y, Karmakar S, et al. Differential roles of galectin-1 and galectin-3 in regulating leukocyte viability and cytokine secretion. J Immunol. 2008;180(5):3091–102. doi: 10.4049/jimmunol.180.5.3091. [DOI] [PubMed] [Google Scholar]
- 39.Toscano MA, Bianco GA, Ilarregui JM, et al. Differential glycosylation of TH1, TH2 and TH-17 effector cells selectively regulates susceptibility to cell death. Nat Immunol. 2007;8(8):825–34. doi: 10.1038/ni1482. [DOI] [PubMed] [Google Scholar]
- 40.Lahm H, Andre S, Hoeflich A, et al. Comprehensive galectin fingerprinting in a panel of 61 human tumor cell lines by RT-PCR and its implications for diagnostic and therapeutic procedures. J Cancer Res Clin Oncol. 2001;127(6):375–86. doi: 10.1007/s004320000207. [DOI] [PubMed] [Google Scholar]
- 41.Lotan R, Matsushita Y, Ohannesian D, et al. Lactose-binding lectin expression in human colorectal carcinomas. Relation to tumor progression. Carbohydr Res. 1991;213:47–57. doi: 10.1016/s0008-6215(00)90597-4. [DOI] [PubMed] [Google Scholar]
- 42.Miyazaki J, Hokari R, Kato S, et al. Increased expression of galectin-3 in primary gastric cancer and the metastatic lymph nodes. Oncol Rep. 2002;9(6):1307–12. [PubMed] [Google Scholar]
- 43.Moon BK, Lee YJ, Battle P, Jessup JM, Raz A, Kim HR. Galectin-3 protects human breast carcinoma cells against nitric oxide-induced apoptosis: implication of galectin-3 function during metastasis. Am J Pathol. 2001;159(3):1055–60. doi: 10.1016/S0002-9440(10)61780-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Honjo Y, Nangia-Makker P, Inohara H, Raz A. Down-regulation of galectin-3 suppresses tumorigenicity of human breast carcinoma cells. Clin Cancer Res. 2001;7(3):661–8. [PubMed] [Google Scholar]
- 45.Vereecken P, Heenen M. Serum galectin-3 in advanced melanoma patients: a hypothesis on a possible role in melanoma progression and inflammation. J Int Med Res. 2006;34(1):119–20. doi: 10.1177/147323000603400116. [DOI] [PubMed] [Google Scholar]
- 46.Zubieta MR, Furman D, Barrio M, Bravo AI, Domenichini E, Mordoh J. Galectin-3 expression correlates with apoptosis of tumor-associated lymphocytes in human melanoma biopsies. Am J Pathol. 2006;168(5):1666–75. doi: 10.2353/ajpath.2006.050971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nangia-Makker P, Nakahara S, Hogan V, Raz A. Galectin-3 in apoptosis, a novel therapeutic target. J Bioenerg Biomembr. 2007;39(1):79–84. doi: 10.1007/s10863-006-9063-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nangia-Makker P, Hogan V, Honjo Y, et al. Inhibition of human cancer cell growth and metastasis in nude mice by oral intake of modified citrus pectin. J Natl Cancer Inst. 2002;94(24):1854–62. doi: 10.1093/jnci/94.24.1854. [DOI] [PubMed] [Google Scholar]
- 49.Johnson KD, Glinskii OV, Mossine VV, et al. Galectin-3 as a potential therapeutic target in tumors arising from malignant endothelia. Neoplasia. 2007;9(8):662–70. doi: 10.1593/neo.07433. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two positive clones were identified by screening the colorectal tumor cDNA library utilizing colorectal tumor-reactive CD4+ CT28 T cells. (A) Identification of a positive cDNA pool (no.29) capable of stimulating IFN-γ production from a tumor-reactive CT28 T cell line. Optical density reading at a 450 nm wavelength revealed a single positive pool in one of the 96-well plates. The data are representative of twenty 96-well plate screenings. (B) Isolation of two independent genes capable of stimulating CT28 T cell activation. All positive clones were digested by Not I and Nhe I restriction enzymes to determine their insert size. Clone 1E5 contained a 1 kb cDNA insert, while clone 2C6 had a 0.7 kb cDNA insert. (C) CT28 T cell reactivity to KIAA0040 or galectin-3 presented by various kinds of 293 cell lines. 293 cells expressing HLA-DR11 or DR4 and 293T cells without DR expression were transfected with KIAA0040, galectin-3 or vector only. The following day, the CT28 T cell activity was tested by ELISA. Each experiment was performed in triplicate.
Cytokine profile of CT28 T cells upon galectin-3 stimulation. 293 cells were transfected with either galectin-3 or vector only and cocultured with CT28 T cells. The following day, cytokine production from CT28 T cells was measured by ELISA. CT28 T cells stimulated with coated anti-CD3 (3 μg/ml) were used as a positive control.
Generation of human recombinant galectin-3. (A, B) Western blot analysis of recombinant galectin-3 was performed by anti-galectin-3 antibody (A) or anti-His6 antibody (B). PDEF was purified and served as control protein in the following experiments. (C) The morphology of tumor-reactive T cells treated with recombinant galectin-3 protein and control protein for 8 hours. (D, E) The interaction of galectin-3 with tumor-reactive T cells. CT28 T cells were stained with FITC labeled galectin-3 and a control protein. The stained cells were subjected to flow cytometry analysis (D) and fluorescence microscopic analysis (E). The overlay images from fluorescent microscopy are shown.
Knock down of galectin-3 in tumor cells. (A) Galectin-3 specific knockdown in 586 tumor cells by galectin-3 shRNA. Green fluorescent protein (GFP)-expressing lentivtius-based shRNAs for human galectin-3 were generated as described previously. 586mel cells were transduced with galectin-3 specific shRNAs or control shRNA. After sorting, GFP+ tumor cells were lysed and performed the western blotting with anti-human galectin-3 antibody. (B) Western blot detection of galectin-3 in concentrated SFCM from tumor cell lines generated from 586mel cells transduced with galectin-3 specific shRNA or control shRNA. 10×106 tumor cells were cultured in 10 ml RPMI for 24 h. Supernatants were collected and precipitated by acidic acetone. The precipitated proteins were resuspended in 1ml PBS. Samples were loaded with the indicated amount. Indicated amounts of recombinant his6-tagged galectin-3 were used as positive control. (C) Proliferation of 586mel cells with or without galectin-3 expression. 1×104 586mel cells expressing galectin-3 shRNA-2 or control shRNA were seeded into 96-well. After 24 or 72 h, cells were subjected to the MTT assay. Shown are the mean absorbances at a wavelength of 550 nm.