Abstract
There is now considerable interest in the intestinally derived soy isoflavone metabolite, equol, which occurs in the enantiomeric forms, S-(−)equol and R-(+)equol, both differing in biological actions. Little is known about effects of either enantiomer on reproductive development, yet such knowledge is fundamental because of the recent commercialization of S-(−)equol as a dietary supplement. S-(−)equol and R-(+)equol were therefore investigated to determine their effects on reproductive development and fertility in the Sprague–Dawley rat. Neither enantiomer affected fertility, number of litters produced, number of pups per litter, number of male and female pups born, birth weight, anogenital distance, testicular descent or vaginal opening. Histological analysis showed no major abnormalities in ovary, testis, prostate or seminal vesicle tissue with dietary exposure to S-(−)equol or R-(+)equol, but both enantiomers triggered hyperplasia of uterine tissue. With R-(+)equol this stimulatory effect subsided after exposure was discontinued, but the effect of S-(−)equol was prolonged.
Keywords: Equol, Enantiomer, Rodent model, Reproduction, Toxicology
1. Introduction
For more than two decades, soy and its constituent isoflavones have been associated with reduced risk of cancer, coronary vascular disease, osteoporosis and pathologies related to menopause and ageing [1,2]. More recently, the focus of research on isoflavones has shifted to the intestinally derived soy isoflavone metabolite [3–7], S-(−)equol, which is not produced by all adults consuming soy foods or isoflavones. Interest in equol was stimulated by the hypothesis that soy foods may have significantly greater efficacy in those people who are able to produce equol [3,8,9]. This represents only 20–30% of the adult Western population and 50–60% of the Asian population [5,6,10]. Differential effects between equol-producers and non-producers in dietary intervention studies of soy foods or isoflavone supplements have been reported in a number, although not all studies, with the former sub-group showing more favorable effects on bone density and bone markers [11–15] on breast density, a risk marker for breast cancer [16], on menopausal symptoms [17], on reducing serum LDL-cholesterol, and on improving endothelial function [18,19] and lowering blood pressure [18]. Case–control and epidemiological studies have also found that equol-producers have a reduced risk for prostate cancer when compared with non-equol producers [20–22] which may be explained by the anti-androgen effects of equol in binding dihydrotestosterone [23]. Such differences are perhaps not surprising because differential effects have been observed at the molecular level with significant differences between equol-producers and non-producers demonstrated for effects on gene expression of a number of estrogen-responsive genes in isolated fibroblasts from postmenopausal women administered a soy isoflavone supplement [24].
Equol has significantly greater biological activity than its precursor daidzein/daidzin [3,4,7], one of the predominant isoflavones in soy products [25,26]. Furthermore, it is unique in having a chiral carbon at position C-3 of the furan ring which results in two distinct enantiomeric forms, S-(−)equol and R-(+)equol (Fig. 1) [7], that differ significantly in their conformational structure and binding affinities for estrogen receptors [3,27–30].
Fig. 1.
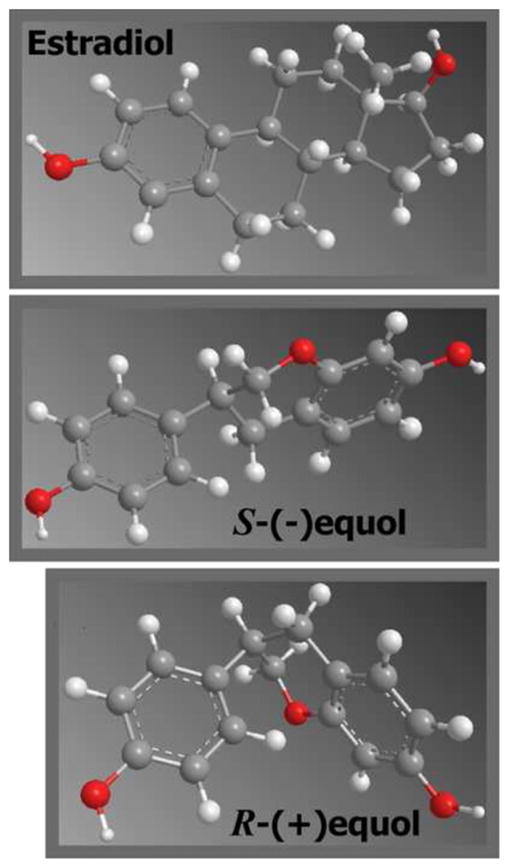
Structures.
Our development of a large-scale asymmetric chemical synthesis of enantiomeric pure S-(−)equol and R-(+)equol [31] was a major breakthrough that permitted, for the first time, animal studies investigating the chemopreventive properties of both enantiomers [32,33]. The soy isoflavones daidzein and genistein and the intestinally derived metabolite S-(−)equol all readily cross the placenta [34–36] and are secreted in mammalian milk [37]. In the 1940s, equol was discovered to be the cause of endometriosis and infertility in sheep grazing on isoflavone-rich clovers [38]. This condition, referred to as Clover disease was the result of chronic exposure to massive doses of isoflavones that were metabolized to S-(−)equol by rumenal bacteria. In view of the commercialization of S-(−)equol as a dietary supplement [39,40], and its development as a pharmaceutical for hormone-dependent conditions [41], it is important to gain as much information about its potential toxicity, particularly with regard to reproductive development. There is a paucity of data on the reproductive toxicology of S-(−)equol [32,42–44]. We now describe for the first time a comparison of the effects of dietary exposure to S-(−)equol or R-(+)equol during pregnancy and lactation, a period of fetal sensitivity to estrogenic compounds when development of reproductive tissues in male and female pups occur. While S-(−)equol is the natural enantiomer, the opportunity to study R-(+)equol, which we have shown is chemopreventive in the breast [32,33], permitted the novel comparison of the effects of two essentially identical molecules that differ significantly in their binding affinities for ERs.
2. Materials and methods
2.1. Animals
Sprague–Dawley rats, 50–60 days old, were purchased from Charles River Breeding Laboratories (Raleigh, North Carolina) and bred in house to obtain pups that had never been exposed to soy. All rats were housed in an AAALAC-accredited facility that met the Animal Welfare Act requirements, and the study protocol [5B09057] was approved by the Children’s Hospital Research Foundation’s Animal Use Committee.
2.2. Chemicals and supplies
S-(−)equol and R-(+)equol were synthesized using chiral chemistry by a patented methodology [31]. The chemical structure and purity of both equol enantiomers were confirmed by NMR and chiral HPLC–MS analysis. All chemicals and reagents were purchased from Sigma Chemicals (St. Louis, MO) unless otherwise specified. The 10% neutral buffered formalin was obtained from Fisher Scientific, Pittsburgh, PA. Isoflurane “Iso Flo” was obtained from Abbott Laboratories (Abbott Park, IL). Harris Hematoxylin stain was obtained from Polyscientific (Bay Shore, NY), and Eosin-Y Alcoholic stain from Thermo Scientific (Waltham, MA). The Periodic Acid-Schiff (PAS) stain was obtained from DAKO (Glostrup, Denmark).
2.3. Animal diets
The AIN-93G soy-free/isoflavone-free diet was purchased from Dyets Inc. (Bethlehem, PA) for use as a control diet. Treatment animals were fed AIN-93G diets containing S-(−)equol or R-(+)equol to a content of 250 mg equol enantiomer/kg diet also prepared by Dyets Inc. The equol composition of each batch was confirmed prior to use by a chiral HPLC method previously published [31].
2.4. Study design
Twenty-seven female Sprague–Dawley rats reared in house on a soy free diet, were randomly assigned to one of 3 groups, 9 rats/group, fed the AIN-93G control diet or AIN-93G containing 250 mg/kg S-(−)equol or R-(+)equol beginning 10 days prior to breeding, and continuing until pups were weaned at 21-days of age. Proven male Sprague–Dawley breeders were housed with the females, 1 male to 1 female/cage for 10 days, which allowed 2 estrus cycles for possible pregnancy to occur. All animals were housed in polycarbonate cages with hardwood (maple) chips. The number of dams producing litters and the total number of offspring was recorded. Within the first 24 h pups were weighed, sexed (noting the number of male and female pups per litter), and the anogenital distance recorded for all litters with the exception of 1 control litter of 4 male and 6 female pups where this information was not obtained. Once this information was recorded, larger litters were reduced to 10 pups, with equal numbers of males and females when possible. Pups were weighed weekly until sacrifice with CO2 inhalation at PND-21, 22 or 50.
A separate group of pups were reared and exposed as described and blood collected via cardiac stick at 21 days of age while they were under isoflurane anesthesia, then they were sacrificed by CO2 inhalation. Litter mates had equol exposure stopped at day 21 and were placed on a soy free AIN93G diet and sacrificed as described at day 35. The blood was allowed to sit for approximately 3 h, centrifuged at 3000 rpm for 10 min and the serum removed and stored at −20 °C until analyzed by GC–MS as described previously [33].
2.4.1. Females
Ten female pups/group (from a minimum of 4 litters) were sacrificed on PND-21. The remaining female pups (minimum 8 rats/group from 4 litters) were placed on the control diet on PND-21 and checked daily for vaginal opening. Beginning at 40–42 days of age the estrus cycle was monitored by daily vaginal lavage, using cytological criteria described by Schedin et al. [45], for a minimum of one full estrus cycle and rats were sacrificed during diestrus near day 50. At sacrifice the uterus, vagina, and ovaries were cleared of fat, and removed. The uterus and vagina were left intact and wet weight taken immediately then all tissues were placed in 10% neutral buffered formalin.
2.4.2. Males
All male pups were weaned at 22 days of age and placed on the control diet; they were monitored for testis descent beginning on PND-21. Ten male pups/group (from a minimum of 4 litters/group) were sacrificed on PND-22, and the remaining males, 10/group from a minimum of 4 litters/group, were sacrificed on PND 50. For rats sacrificed at 22 and 50 days, testis, epididymus, ventral (VP) and dorso-lateral (DLP) prostate, and seminal vesicles (SV) were removed. For 22-day old males, DLP and SV were left intact to avoid tissue damage during dissection. Each testis was weighed immediately after dissection and all tissues fixed in 10% neutral buffered formalin.
2.4.3. Histological preparation
All tissues were fixed for a minimum of 4 days, processed, and embedded in paraffin. Ovaries were serially sectioned at 5 μm, retaining the 1st tissue section and every 10th section thereafter. For all other tissues, 5 μm mid-sagittal sections were taken. Testes and epididymus were stained with PAS and hematoxylin; other tissue sections were stained with hematoxylin and eosin.
2.4.4. Statistical methods
Analysis of variance (AOV) was performed to compare mean values of different end-points among three diets (AIN-93G, S-(−)equol, R-(+)equol) for female and male rates at days 21, 22 (males), and 50. For uterine and testes analyses, repeated measurements of each parameter were analyzed. Post hoc t-tests were performed, and were adjusted for multiple comparisons by Tukey’s method. For the analyses of stromal infiltration, the number of affected areas were categorized into two groups (<6 ≥6 infiltrates). Logistic regressions were performed and odds ratios were calculated, comparing the odds of acini counts <6 to ≥6 for the S-(−) equol diet to the control diet, and the R-(+) equol diet to the control diet. Separate analyses were performed to analyze stromal infiltration grading for ventral prostate at days 22 and 50 and dorso-lateral prostate at day 50. A p-value <0.05 was used to judge statistical significance. The analysis was performed using SAS for Windows, version 9.2, SAS Institute, Cary NC.
3. Tissue analysis
3.1. Females
3.1.1. Ovaries
The ovaries of 8 animals/group for 21 and 50-day old rats were evaluated in a blinded fashion. Follicles were classified as healthy or atretic, and according to the stage of development–primordial, primary, secondary, antral, preovulatory, or corpora leutea as appropriate, using criteria described by Hsueh et al. [46].
3.1.2. Uterine tissue and estrous cycle
Uterine sections from 8 to 10 animals/group were blinded and evaluated for 21 and 50-day old rats. When possible mid-sagittal sections from both horns were evaluated; if this could not be obtained in a single slide, a mid-sagittal section from one horn was used. Cell, compartment, and gland parameters were determined using Image J software (available from NIH). To measure luminal epithelial cell height, photomicrographs were taken of 4 fields (400×), and 5 luminal epithelial cells were measured/field. To measure stromal and myometrium thickness, photomicrographs were taken of both sides of the lumen at any one location, since at optimal magnification the screen field did not span the entire uterine horn width. These paired pictures were at 100× for 21-day old rats, and at 40× for 50-day old rats, and 5 measurements were taken of both stroma and myometrium in each picture. The number of uterine glands and uterine gland circumference was measured in 4 photomicrographs (200×), measuring up to 5 glands per picture.
The estrus cycle was monitored on control (n = 7), S-(−)equol (n = 12) and R-(+)equol (n = 12) rats beginning days 40–42 and lavages were taken through one complete cycle until the beginning of the next cycle.
3.2. Males
3.2.1. Testes
To determine the degree of sperm maturation, testis sections from a minimum of 9 animals/group were evaluated in a blinded fashion. Testis sections from 22-day old rats were examined for spermatocytes through the pachytene stage. Testis sections from 50-day old rats were examined for the presence of spermatogonia, spermatocyte, and spermatid stages, as described previously [47,48]. Also, seminiferous tubule diameter was measured in 5 of the most circular tubules near the outside edge of the structure. To measure tubule diameter, photomicrographs were taken at 200× and 100× for 22 and 50-day sections, respectively, and measurements were completed using the Image J software. For tubules that were slightly oblong, the diameter was taken across the narrower dimension.
3.2.2. Epididymus
The epididymal caput and cauda regions from 10 animals from AIN-93G control, S-(−)equol, and R-(+)equol fed groups were examined at PND-50 for the presence of mature sperm, and the apical surface of tubule epithelium for stereocilia.
3.2.3. Ventral prostate
Ventral prostate tissue sections from 10 rats/group for 22-and 50-day old animals were blinded and evaluated for abnormal histology by a licensed pathologist assisted by a research technician. The extent of flattened acinar epithelium, leucocyte stromal infiltration and the number of acini having flattened epithelium was determined for the entire VP tissue section. Additionally, thirty areas of the stroma – defined as the space between 2 or 3 acini (depending on acinar arrangement) – throughout the entire tissue section were evaluated to determine the mean severity grade for leucocyte infiltration, defined as grade 1, 2, or 3 (Grade 1 = 0–5, Grade 2 = 6–10, and Grade 3 = 11 or more cellular infiltrates present). Sections where acini were too tightly associated to see stroma or where the space between acini was so large that it was impossible to designate “areas” were by-passed.
3.2.4. Dorsolateral prostate
Dorsolateral prostate sections from 8 to 10 rats/group of 22 and 50-day old animals were examined for histological differences between groups, focusing particularly on the number of areas that had acini with flattened epithelium and indications of stromal infiltration by leukocytes. The analysis of the dorsolateral prostate was completed as described above for the 22-day old and 50-day old ventral prostate sections.
3.2.5. Seminal vesicles and coagulating gland
Seminal vesicles sections from 10 AIN-93G control, S-(−)equol, and R-(+)equol fed pups/groups were examined at 22 and 50-days of age. Measurements were taken of 5 major septa that extended into the lumen of the seminal vesicle utilizing photomicrographs as described above. Histological evaluation included the size of acini, epithelial cell height, cell shape and nuclear placement, lumen size, shape and the presence of eosinophilic secretions, presence of leucocyte infiltration and the extent of surrounding musculature [49].
4. Results
4.1. Breeding
There were 8 successful pregnancies/group for the 9 females that were bred/group resulting in 102, 96 and 105 pups born to the control, S-(−)equol, and R-(+)equol groups, respectively. The ninth control dam became pregnant and she birthed 3 live and 1 dead pup; however within 24 h she cannibalized 2 of the living pups, and upon her sacrifice, it was discovered that she was reabsorbing about 10 fetuses. No sex, weight or anogenital distance data was collected from this litter. One S-(−)equol-fed dam cannibalized 3 of her pups for unknown reasons. One S-(−)equol-fed dam, near the end of her pregnancy, jumped from the cage during weekly weighing and fell about 4 feet hitting the floor. Shortly thereafter, she delivered 12 pups, 3 were dead, 3 were immediately sacrificed due to their bruised condition and of the remaining pups, 4 were sacrificed, fixed in formalin and submitted to a pathologist for histological examination. The pathologist reported that no histopathological or gross abnormalities could be detected in these pups. In summary, there were no significant differences in the number of dams that conceived pups, the total number of pups/dam, or the numbers and ratio of males/females among the 3 groups (Table 1).
Table 1.
Breeding results.
| # of Dams bred | Mean # of days to gestation | # of successful pregnancies | Total # of pups | # of m/f pups | |
|---|---|---|---|---|---|
| Controls | 9 | 2.9 ± 0.9 | 9a | 102 | 49m/53f |
| S-(−)equol | 9 | 3.3 ± 1.3 | 8 | 96b | 46m/47f |
| R-(+)equol | 9 | 3.4 ± 1.1 | 8 | 105 | 52m/53f |
One control dam became pregnant, but delivery was not complete and she began to cannibalize the live pups just delivered, upon sacrifice it was discovered that she was reabsorbing about 10 fetuses. No sex, weight or anogenital distance data was collected from this litter.
The sex of the 3 pups partially cannibalized by the Dam could not be determined.
4.2. Pup birth weights and anogenital distances
Table 2 summarizes birth weight and anogenital distance data for all pups from control (n = 7), S-(−)equol (n = 7) and R-(+)equol (n = 8) treated litters. Male and female pups born to S-(−)equol-fed dams were significantly heavier (p = 0.008 and p = 0.004), respectively, than those born to control animals. They also had significantly longer absolute anogenital distances for males (p = 0.010) and females (p = 0.011) than the pups from the control group, however this significance was lost when the anogenital distance was normalized for birth weight. Comparing the two treatment groups, both male and female S-(−)equol exposed pups were significantly heavier than R-(+)equol exposed pups (p = 0.002 and p = 0.001) respectively, yet there were no differences in either absolute anogenital distance length or anogenital distance normalized for body weight between the enantiomers.
Table 2.
Male and female pup birth weights and anogenital distances (mean ± SD).
| Treatment group | n | Birth weight (g) | Anogenital distance (mm) [distance normalized for body weight] | |
|---|---|---|---|---|
| Male pups | ||||
| Control | 45 | 6.1 ± 0.7 | 3.68 ± 0.30 | [0.61 ± 0.08] |
| S-(−)equol | 40 | 6.6 ± 1.0a,b | 3.87 ± 0.37c | [0.59 ± 0.06] |
| R-(+)equol | 52 | 6.0 ± 0.7 | 3.75 ± 0.33 | [0.63 ± 0.10] |
| Female pups | ||||
| Control | 47 | 5.8 ± 0.9 | 1.67 ± 0.26 | [0.29 ± 0.05] |
| S-(−)equol | 44 | 6.3 ± 0.8a,b | 1.81 ± 0.25c | [0.29 ± 0.04] |
| R-(+)equol | 53 | 5.8 ± 0.6 | 1.74 ± 0.21 | [0.30 ± 0.05] |
p ≤ 0.008 S-(−)equol-treated pups were heavier than control pups.
p ≤ 0.002 S-(−)equol-treated pups were heavier than R-(+)equol-treated pups.
p ≤ 0.011 S-(−)equol-treated pups had longer anogenital distances than control pups, this significance was lost when normalized for body weight.
4.3. Testes descent, vaginal opening and estrus cycle
Testis descent in the rat normally occurs on or about day 21 [50] and was not affected by dietary exposure to either equol enantiomer (Table 3). The average age of vaginal opening was 33 days for female pups of all 3 groups, which falls in the normal pubertal age range, 32–38 days [51], and there were no differences among the groups.
Table 3.
21-day and 50-day mean (±SE) healthy ovarian follicular counts.
| Treatment | Age | Primordial follicles | # Primary | # Secondary | # Antral | # Mature | Corpus luteum |
|---|---|---|---|---|---|---|---|
| Control | (21d) | n.c | 28.0 ± 3.7 | 63.3 ± 6.3 | 111.1 ± 8.8 | 2.5 ± 0.6 | n.a. |
| S-(−)equol | (21 d) | n.c. | 35.0 ± 5.4 | 62.1 ± 6.4 | 109.4 ± 6.0 | 2.9 ± 1.1 | n.a. |
| R-(+)equol | (21 d) | n.c. | 32.8 ± 3.7 | 59.1 ± 7.3 | 142.1 ± 10.9a | 3.3 ± 0.5 | n.a. |
| Control | (50 d) | 162.1 ± 41.1 | 50.4 ± 12 | 77.0 ± 7.6 | 71.9 ± 15.6 | 8.3 ± 0.8 | 25.5 ± 2.5 |
| S-(−)equol | (50 d) | 186.8 ± 50.1 | 45.3 ± 8.1 | 73.9 ± 9.1 | 66.6 ± 5.7 | 9.9 ± 1.3 | 31.9 ± 2.7 |
| R-(−)equol | (50 d) | 303.8 ± 40.0b | 56.5 ± 10.4 | 73.2 ± 10.6 | 72.2 ± 10.3 | 14.8 ± 4.7 | 26.8 ± 2.7 |
n.c.: not counted and n.a.: not applicable.
p = < 0.05 R-(+)equol-treated pups had more healthy antral follicles than control or S-(−)equol-treated pups.
p = 0.05 R-(+)equol-treated pups had more healthy primordial follicles than controls.
The estrus cycle lengths means ± SD were 4.1 ± 0.4 days, 4.3 ± 0.6 days and 4.0 ± 0.5 days for control, S-(−)equol and R-(+)equol, respectively. There were no significant differences between the control and the treatment groups in cycle length or stages and all treated animals cycled.
4.4. Equol concentration and body weight at sacrifice
The concentrations (mean ± SEM) of S-(−)equol and R(+)equol in the blood of 21 day old nursing pups was 203.7 ± 2.2 ng/mL and 187.5 ± 2.4 ng/mL, respectively. Once dietary exposure was discontinued, blood levels of both enantiomer dropped quickly and at 35 of age the levels of S-(−)equol and R(+)equol were at baseline values.
The mean body weight (±SD) for 21-day old female pups at time of sacrifice was 49.4 ± 3.6 g, 47.8 ± 3.8 g, and 49.8 ± 3.8 g for control, S-(−)equol, and R-(+)equol fed groups, respectively. The mean body weight (±SD) for 50-day old female pups at time of sacrifice was 201.0 ± 15.8 g, 188.1 ± 19.3 g, and 196.9 ± 14.6 g for control, S-(−)equol, and R-(+)equol fed groups, respectively. There were no significant differences in the sacrifice weights for 21-day old or 50-day old female pups comparing the 3 groups.
The mean body weight (±SD) for 22-day old male pups at time of sacrifice was 51.8 ± 2.1 g, 52.3 ± 8.2 g, and 57.3 ± 5.0 g for control, S-(−)equol, and R-(+)equol groups, respectively. R-(+)equol pups were significantly heavier than the AIN-93G fed controls (p = 0.005). The mean body weight (±SD) for 50-day old male pups at time of sacrifice was 276.3 ± 20.8 g, 277.2 ± 15.0 g, and 273.8 ± 31.1 g for control, S-(−)equol, and R-(+)equol fed groups, respectively, and these differences were not significant.
4.5. Female toxicology
4.5.1. Ovaries
The results of histological analysis of the PND-21 and 50 ovaries are summarized in Table 3. The ovaries from 21-day old R-(+)equol fed rats had greater numbers of healthy antral follicles than those fed either AIN-93G (p = 0.04), or S-(−)equol (p = 0.02). There were no differences among the groups in the numbers of atretic follicles in ovaries from 21-day old pups. In 50-day old ovaries of animals fed R-(+)equol there was a marginal increase (p = 0.052) in the number of primordial follicles compared with controls but again there were no significant differences in numbers of atretic follicles.
4.5.2. Uterus
Uterine weights (mean ± SD) from 21-day old pups (n = 10/group) were 55.9 ± 14.8, 61.3 ± 7.6, and 65.5 ± 8.3 mg for control, S-(−)equol and R-(+)equol fed groups, respectively. Both the absolute uterine weight and uterine weight adjusted for body weight of R-(+)equol exposed pups was significantly greater than that of control pups (p = 0.005 and p = 0.02, respectively). A significant difference was evident for S-(−)equol treated pups only when uterine weight was adjusted for body weight (p = 0.005). Uterine weights (mean ± SD) for 50-day old rats (n = 8/group) were 441.5 ± 21.6, 464.6 ± 26.0, and 450.8 ± 24.4 mg for control, S-(−)equol and R-(+)equol fed animals, respectively. There were no differences for either treatment group when absolute weight was compared to controls, and only a modest indication of S-(−)equol treated rats continuing to have greater uterine weight (p = 0.04) when adjusted for body weight.
The findings from the histological analysis of the 21-day and 50-day uteri are summarized in Table 4 and show that S-(−)equol and R-(+)equol both stimulate the uterus, producing significant stromal thickening (p < 0.0001) by PND-21. Once treatment was discontinued (PND-21), the effect of both enantiomers on the uterus continued through PND-50, but was less pronounced for R-(+)equol (p = 0.04) than for S-(−)equol (p < 0.0006) fed animals. There was also an increase in the thickness of the myometrial compartment in only the R-(+)equol fed animals. At PND-21 (p = < 0.00001), this was very significant, but once exposure to R-(+)equol was discontinued the effect began to subside, so that by PND-50 the myometrial compartment thickness was significantly less at a p value = 0.01 compared to the controls. R-(+)equol exposure led to a transient increase in luminal epithelial cell height at PND-21 compared to control tissue (p = 0.02) this difference disappeared by PND-50. S-(−)equol exposed rats did not show the same early response in luminal cell height (PND-21), but a significant increase was observed at PND-50 (p = 0.004). A slight decrease in uterine gland count was observed only for S-(−)equol, and seen only at the end of treatment (PND-21) and did not continue on to day 50. Both S-(−)equol (p > 0.001) and R-(+)equol (p > 0.01) fed animals had greater gland circumference than controls with early exposure to the enantiomers, but this difference was not evident later after discontinuing exposure.
Table 4.
21-day and 50-day (mean ± SD) uterine morphometric data.
| Treatment group | Luminal epithelial (LE) cell height (μm) (n = 200 values/group) | Stromal thickness (μm) (n = 140–180 values/group) | Myometrial thickness (μm) (n = 140–180 values/group) | Glandular circumference (μm) (n = 162–181 values/group) | Gland count in 200× field (n = 40 values/group) |
|---|---|---|---|---|---|
| Control (21) | 13.6 ± 1.9 | 173.4 ± 55.9 | 123.2 ± 28.5 | 109.7 ± 18.5 | 14.4 ± 4.4 |
| S-(−)equol (21) | 13.5 ± 1.6 | 213.8 ± 67.0c | 123.1 ± 38.9 | 116.6 ± 18.6b | 12.2 ± 3.9d |
| R-(+)equol (21) | 14.0 ± 1.8a | 227.5 ± 63.3c | 136.4 ± 32.3c | 115.2 ± 17.7a | 13.7 ± 5.0 |
| Control (50) | 20.0 ± 3.3 | 620.7 ± 169 | 305.6 ± 88 | 169.3 ± 31 | 21.8 ± 7.6 |
| S-(−)equol (50) | 21.2 ± 4.2b | 690.1 ± 187c | 310.0 ± 91 | 173.0 ± 29 | 23.2 ± 7.2 |
| R-(+)equol (50) | 19.9 ± 2.8 | 662.0 ± 187d | 280.9 ± 78a | 173.8 ± 30 | 22.3 ± 6.8 |
p ≤ 0.02 R-(+)equol increased LE height and gland circumference, reduced myometrium compared to control.
p ≤ 0.004 S-(+)equol increased LE height and gland circumference compared to controls.
p < 0.0001 S-(−)equol/R-(+)equol treated pups had greater stromal or myometrial thickness than control pups.
p ≤ 0.05 R-(+)equol greater stromal thickness, S-(−)equol lower uterine gland count than control pups.
4.6. Male toxicology
4.6.1. Testes
Testis weights (mean ± SD) from 22-day old pups were 133.3 ± 16.4, 129.1 ± 23.7, and 151.9 ± 20.1 mg for control, S-(−)equol and R-(+)equol fed groups respectively, and the testis weight of R-(+)equol exposed pups was significantly greater than that of control (p = 0.003), or S-(−)equol (p = 0.002) pups. However, when adjusted for body weight there were no significant differences among the groups. Testis weights for 50-day old pups were 1.297 ± 0.1, 1.286 ± 0.06, and 1.231 ± 0.10 g for control, S-(−)equol and R-(+)equol fed groups, respectively. The marginally lower weight resulting from R-(+)equol treatment was not evident when testis weights were adjusted for body weight.
Histological analysis of testis tissue in young males (22-days) showed the seminiferous tubule diameter (mean ± SEM) to be significantly greater in both S-(−)equol (110.7 ± 1.0 μm p = 0.009) and R-(+)equol fed pups (113.2 ± 0.9 μm p < 0.001) than in controls (106.3 ± 1.2 μm). These differences remained even when diameter measurements were adjusted for differences in testis weight. However this difference was no longer evident at 50-day old of age, with seminiferous tubule diameter being 266.5 ± 2.9, 268.1 ± 2.5, and 269.7 ± 2.2 μm for control, S-(−)equol and R-(+)equol fed pups, respectively. This anatomical difference did not appear to negatively impact sperm development, and there was no loss of germinal epithelium, or other degenerative changes evident. Tissue analysis from 22-day old rats verified that controls and both equol-fed groups had spermatocytes through the pachytene stage, and tissue of 50-day old rats had tubule stages I–XIV as described by Leblond and Clermont [47] with appropriate spermatogonia, spermatocytes, and mature spermatids.
4.6.2. Epididymis
The caput and cauda regions of the epididymis were evaluated for each animal at 22- and 50-days of age and evidenced the range of differing tubular sizes, cell diameter and cell heights expected for these areas [52]. The presence of stereocilia were noted on the apical surface of the epithelium in all animals, and in 50-day old rats, mature sperm were found in the caput and caudate regions in control and both equol-fed groups. There were no histological abnormalities noted in the architecture of the cells or tubules for any of the groups.
4.6.3. Ventral prostate
The number of areas containing acini that had flattened epithelium, and the number of acini with flattened epithelium were counted. The number of ventral prostate areas containing acini having some flattened epithelium were (mean ± SE) 0.40 ± 0.22, 0.6 ± 0.22 and 0.5 ± 0.27 for 22-day old for control, S-(−)equol and R-(+)equol fed rat pups. The average number of acini with flattened epithelium per area was 0.4 ± 0.2, 0.7 ± 0.3 and 1.7 ± 1.1. There were no differences among groups for either criterion. An analysis of 30 fields (200×) for each prostate for the number of white blood cell infiltrates, a sign of possible tissue distress or degeneration, resulted in a grade of 1.05 ± 0.01, 1.04 ± 0.01 and 1.05 ± 0.01 for control, S-(−)equol and R-(+)equol fed animals and these values were not significantly different among groups.
In 50-day old rats, the number of ventral prostate areas containing acini having some flattened epithelium was (mean ± SE) 5.8 ± 1.1, 8.3 ± 2.1 and 5.2 ± 1.2 for control, S-(−)equol and R-(+)equol fed animals. Correspondingly, the average number of acini with flattened epithelium per area was 15.2 ± 3.8, 30.0 ± 7.9 and 23.8 ± 9.8, respectively. There were no differences among the groups for either criterion, and there was no evidence of pathology in the tissues. An analysis as described for cellular infiltrates resulted in a grade of 1.17 ± 0.023, 1.18 ± 0.024 and 1.23 ± 0.028 for control, S-(−)equol and R-(+)equol fed animals, and these values were not significantly different among the groups.
4.6.4. Dorsolateral prostate
The dorsolateral prostate of 50-day old rats in control and treated animals showed a great deal of variability in acinar architecture, but flattened epithelia were not present. An analysis of acini for white blood cell infiltrates resulted in a grade of 1.02 ± 0.01, 1.04 ± 0.01 and 1.02 ± 0.01 for control, S-(−)equol and R-(+)equol exposed animals with no difference between the groups.
4.6.5. Seminal vesicles and coagulating gland
The elongated curved seminal vesicles were similar in length and width in animals from all three groups, and the main chamber running the length of the gland was filled with homogenous eosinophilic secretion. The inner chamber is partially partitioned as numerous short fibro-muscular branches of the outer sheath extending inward. The main cavity and the extensions are covered by a single layer of epithelial cells which ranged in size from cuboidal to columnar. The columnar epithelial cells provide a sensitive indicator of testicular hormone production by the presence or absence of secretory vesicles which can fill the apical region of the cells [49]. Such secretory vesicles were prolific in columnar epithelial cells of control rats, and both S-(−)equol and R-(+)equol fed animals. The coagulating glands, laying along the concave side of the seminal vesicles, showed typical histology with the columnar epithelium arranged in branching papillary projections [53] in all three groups. The tubular spaces were fluid-filled and stained more lightly than the surrounding seminal vesicle main chamber, with densely staining nuclei midway in the cells between the apex and the basement membrane which is characteristic of normal tissue.
5. Discussion and conclusion
We describe for the first time a comprehensive analysis of the reproductive toxicology of the two enantiomeric forms of the intestinally derived soy isoflavone equol. This was possible because of our ability to synthesize in bulk, enantiomeric pure S-(−)equol, the natural enantiomer, and R-(+)equol its diastereoisomer [31], which could then be incorporated into the diet. These studies are timely in light of the recent commercialization of the S-(−)equol as a dietary supplement [29,39,54,55] and the continuing concerns over the safety and reproductive toxicity, especially the uterotrophic actions, of isoflavones in general [2,37,40,56–59]. There have been no previous in-depth reports of the toxicology of the individual enantiomers of equol, although several studies have reported on aspects of the reproductive toxicology of a racemic mixture of equol [42,60,61], or on uterine effects in adult animals [62,63]. Recently S-(−)equol only, was reported to be without genotoxicity or mutagenicity [40,64]. Given the contrasting ER binding affinities of the two enantiomers [27–30,58], and the recent finding that the racemic mixture displays different pharmacokinetic behavior to that of the individual pure enantiomers [55], findings from studies using the racemate may not necessarily apply to the individual pure enantiomers. In contrast, there is a large literature on adverse reproductive effects of soy isoflavones, most notably genistein, in animal species [65–67], although the value of the findings to humans remains debatable because in most studies the route of administration was by subcutaneous injection, which bypasses first-pass metabolism and changes the pharmacodynamics and thus the biological potency of the compounds. Route of administration and dose are crucial considerations in evaluating the clinical significance of any demonstrable reproductive effects. In this regard, and of greater relevance, we have examined the effects of early-life dietary exposure to the equol enantiomers, and the dose selected for testing, 250 mg/kg diet, was chosen to be consistent with our previous studies where differential chemopreventive effects of these enantiomers were reported in an animal model of chemically induced mammary cancer [32,33]. Furthermore, at this level of dietary intake, equol is metabolically handled similarly to that in humans consuming soy isoflavones [4,7], and to S-(−)equol when administered as a supplement [55], or as a pure compound [41,68]. The rat pups were exposed to equol enantiomers throughout pregnancy and during lactation. The transplacental passage of isoflavones is well established in both rodents [35,36] and humans [69,70], and the efficient transfer of isoflavones to the offspring via the dam’s milk during suckling has been confirmed from previous studies of rats fed soy-containing diets [71], or administered pure isoflavones [32,37,72]. In response to exposure to 250 mg/kg diet, relatively high steady-state plasma concentrations of S-(−)equol and R-(+)equol are evident on PND-21 of life [32]. These concentrations are in a similar range to those reported in humans consuming soy foods [4], or rodents fed soy-containing commercial rodent diets [34]. These plasma equol concentrations were also consistent with those reported by Lamartiniere et al. for animals fed daidzein early in life [37], which is very efficiently converted to S-(−)equol by rodents [30]; and based on the known pharmacokinetics of equol enantiomers, were within the plasma concentration range for adults consuming S-(−)equol as either a pure compound [41,68], or as a supplement [55].
The most significant concerns over the safety of isoflavones have been the issue of whether soy isoflavone exposure in females increases the risk of breast cancer in high-risk groups and whether it adversely affects the uterus [2,73,74]. The findings from the athymic mouse model of human breast cancer, where genistein was shown to stimulate the growth of transplanted human MCF-7 breast cancer cells [75], led to considerable concern over the consumption of soy foods or isoflavone exposure particularly by postmenopausal women and women at high risk for breast cancer. However, recent epidemiological studies of postmenopausal women now indicate these concerns are largely unfounded [76,77]. A large prospective follow-up of 5033 breast cancer survivors living in Shanghai, with a median follow-up of 3.9 years, found an inverse relationship between soy food intake and risk of mortality or recurrence, with women in the highest quartile of intake (>15.3 g/d soy protein, or >62.8 mg/d total isoflavones) being approximately 1/3 less likely to die from, or suffer breast cancer recurrence [77]. These findings were corroborated in data from a study of 1,954 Californian breast cancer survivors, diagnosed between 1997 and 2000, with a median follow-up of 6.2 years, which found a 60% reduction in breast cancer recurrence between highest and lowest intake of soy foods [76]. In neither of these studies was equol-producer status examined. However, we have shown previously that S-(−)equol does not stimulate tumor growth, and the unnatural enantiomer, R-(+)equol, in contrast is chemopreventive in the DMBA-induced animal model of breast cancer [33]. Interestingly, and in stark contrast to the effects of genistein [75], racemic equol was reported to have no stimulatory effect in the athymic mouse model of human breast cancer [78]. Thus, the body of evidence indicates that equol at physiologically relevant levels has no adverse effects on the breast.
Equol, has been shown to adversely affect the uterus in several animal species [38,79–81]. The very high exposure to S-(−)equol derived from isoflavones, naturally abundant in red clover, devastated the sheep breeding industry in regions of SW Australia [38], while infertility and liver disease in captive cheetah were shown to be caused by dietary soy isoflavones, an effect that was exacerbated by the inability of cheetah to metabolize isoflavones by glucuronidation [79], the major metabolic pathway in humans [7]. To our knowledge there are no published data on the effects of equol in women. Legette et al. found that dietary racemic equol, while having a modest benefit on bone in the ovariectomized rat, was mildly uterotrophic [60]. On the other hand, racemic equol, when injected on postnatal days 1–5 as opposed to given in the diet, resulted in an initial non-significant increase in uterine weight followed later by a significant decrease by PND-20 and PND-25 [42]. Also, racemic equol injected at 10, 100, and 1000 μg/animal on PND-10 to PND-14 caused a dose-dependent decrease in the uterine gland count when animals were sacrificed at PND-14. These findings illustrate the importance of considering the route of administration when drawing conclusions and extrapolations to humans.
We previously reported that chronic feeding of both R-(+)equol and S-(−)equol at the dose used here had no significant impact on the absolute uterine weight of intact adult Sprague–Dawley rats [33]. However, this animal is relatively insensitive to estrogens, when compared with some other species of rodent, or when compared to the ovariectomized, or immature animal [82]. In data we present here, both dietary R-(+)equol and S-(−)equol exposure in early life, a period sensitive to hormonal changes, caused a significant increase in uterine weight that was also evidenced histologically by highly significant increases in stromal thickness on PND-21, an effect that was sustained at PND-50. S-(−)equol was not immediately effective in influencing luminal epithelial cell height and myometrial thickness on PND-21, but uterine hyperplasia was evident on PND-50, despite an approximate 30-day lapse in which there had been no exposure to S-(−)equol. These findings are consistent with a mild estrogen effect, also shown in ovariectomized rats with a 400 mg/kg diet of racemic equol [61], and are perhaps expected based on the low affinity for the ER. By contrast, exposure to R-(+)equol caused an early transient and significant increase in cell height and stromal and myometrial thickness seen on PND-21, but this hyperplastic effect was not sustained once exposure was withdrawn. By PND-50 myometrial thickness of rats exposed to R-(+)equol was actually significantly smaller than controls [Table 4]. Thus, while both enantiomers influence the uterus during development, the differential effects would suggest this possibly occurs by different mechanisms, which might be anticipated as R-(+)equol and S-(−)equol have different affinities for the ER subtypes [4,27–29].
Overall, prenatal/postnatal exposure to S-(−)equol or R-(+)equol had no adverse effect on the in-utero development and reproductive anatomy and physiology of the offspring. The breeding efficiency was similar among groups, as was the ratio of female/male offspring delivered [Table 1]. Rats fed commercial rodent diets containing soy isoflavones show an accelerated time of vaginal opening (VO), and there is an inverse correlation between the total daidzein and genistein content of the diet and the day of vaginal opening. The significance of VO timing in rats as it relates to the human is unclear, but it is considered a highly sensitive marker of exposure to environmental estrogens [83]. In our studies, the timing of vaginal opening in the offspring was unaffected by S-(−)equol or R-(+)equol at the exposure level of 250 mg/kg diet. Whether, higher doses would have invoked a change is uncertain, but these findings suggest a lack of profound estrogen effect of S-(−)equol or R-(+)equol at physiological levels of exposure. Similarly, anogenital distances did not differ significantly from the values of control animals.
The effect of S-(−)equol and R-(+)equol on the ovaries was unremarkable and indicative of a lack of overt toxicity. Histological analysis of the ovaries on PND-21 and PND-50 showed normal follicular development. This is perhaps not surprising, at least for S-(−)equol, because most rodents are reared on commercial soy-containing diets and are naturally exposed to very high circulating concentrations of S-(−)equol [34], which is formed by intestinal bacteria from daidzein/daidzein in the diet [30]. This finding is consistent with a previous study reporting pure daidzein fed during the prenatal/postnatal period and up to PND-50 did not adversely affect ovarian follicular development [37].
The potential for soy isoflavones to have adverse effects on male reproductive development and reproduction has been hotly debated [84]. Masculinization of the developing male fetus occurs early and testicular differentiation during the fetal period is critical for development of maleness [85]. Since we fed S-(−)equol and R-(+)equol throughout pregnancy the male fetus was exposed to equol from conception up to PND-21 and male reproductive structures could have been impacted at any stage of development.
Our data on the effects of S-(−)equol and R-(+)equol in male rats indicate a lack of overt toxicity on the male reproductive system as evidenced by typical anogenital distance when normalized to body weight and no significant effect on the timing of testicular descent. However, S-(−)equol and R-(+)equol seemed to trigger early development of the seminiferous tubules as seen by greater tubular diameter during treatment exposure, but no long-term effects once treatment was discontinued. The testes are structures that continue to increase in size well past a point when other structures have reached full maturity, for example the seminiferous tubular length and diameter increases up to PND-108 [86]. This early tubular development did not adversely affect sperm production as both control and treatment groups had appropriate stages of sperm on PND-22, and normal mature sperm were present in the seminiferous tubules of all groups on PND-50. The tortuous epididymal ducts, which store sperm in transit from the testis to the vas deferens, also evidenced no signs of abnormality as would have been indicated by the presence of cellular debris, immature germ cells or epithelium characterized by vacuoles and basophilic cytoplasmic staining [52]. But in keeping with the integrity of the seminiferous tubules in the testis we observed normal epididymal cell types, sizes and stereo cilia in the head, body and tail of the epididymus of both treatment groups at PND-22 and PND-50. Our results here, taken together with the findings from older studies that red clover had no adverse effects on sperm development or fertility in the ram [87,88] suggest there should be no concern for the use of equol enantiomers in men. This is supported by a recent review that concluded there is little clinical evidence that soy or soy isoflavones have any deleterious effects on male reproduction [84].
Development and maintenance of accessory sex glands is hormone dependent, and chemicals that adversely impact the testis hormonal secretions also cause degeneration of the seminal vesicles and prostate. Normal development of the dorsolateral prostate, ventral prostate, seminal vesicles and coagulating gland attest to a lack of negative effect by either equol enantiomer. These effects of the equol enantiomers differ from those of dietary genistein, which when give in the diet at a similar dose (300 mg/kg diet) resulted in smaller anogenital distance and testis size, as well as delayed preputial separation and abnormal reproductive behavior male rats [89]. Again these comparisons show that all isoflavones cannot be considered to have identical biological effects.
Finally, we previously reported significant effects of postnatal and adult exposure to dietary equol on body weight gain in female Sprague–Dawley rats [32,33], although these earlier studies did not examine any effect of exposure during pregnancy. In this study, S-(−)equol exposure in-utero led to both male and female pups being heavier at birth when compared to controls, an effect that could be considered beneficial since higher birth weight correlates positively with newborn survival. This effect on birth weight was not seen with in utero exposure to R-(+)equol. However, once the pups began suckling and were exposed post-natally to S-(−)equol via the mother’s milk, the female pups showed a lower rate of weight gain (although not statistically significant) than that of the controls, and this lag was sustained beyond the period of S-(−)equol exposure, until final sacrifice at PND-50. This is in agreement with our previous data where S-(−)equol was fed post-natally [32,90]. R-(+)equol exposed female pups showed no significant differences in body weights compared to controls at birth, PND-21 or PND-50. In male animals, there were no significant postnatal effects of either enantiomer on the rate of weight gain. It is highly likely that had we continued feeding S-(−)equol and R-(+)equol beyond PND-21, and throughout adult life there would have been a significant effect on body weight. It is well recognized that soy-containing diets given chronically lead to lower body weight gains and reduced amounts of adipose tissue in female rats [90,91] and the effects have been attributed to the presence of isoflavones [37,92], although the mechanism of action is not fully understood. Taken in context with the other studies, we suggest that S-(−)equol and R-(+)equol may play a role in dictating early weight gain and consequently reduced risk of obesity later in life.
In conclusion, we found that consistent with the low binding affinity of equol enantiomers for the estrogen receptor, dietary exposure to equol enantiomers throughout pregnancy and in the postnatal period up to day 21 of life had no major effects on reproduction or on the development of the male and female reproductive organs of the offspring. Of the significant findings, the effect of S-(−)equol on increasing birth weight can be considered beneficial, while the transient effect of R-(+)equol on stimulating greater production of primordial follicles is unclear with regard to risk/benefit to human infants. The sustained effect of S-(−)equol and R-(+)equol on the uterus in increasing luminal epithelial cell height, and myometrial and stromal thickness is not unexpected given that these enantiomers have an affinity for ERs. After all, if equol is to have clinically significant estrogenic effects of benefit to women’s health then it should also be expected at some exposure level to influence the uterus. Whether the effects seen in neonatal rats would be observed in newborn infants of mothers consuming equol during pregnancy or lactation is unknown. However, since 50–60% of Asians consuming soy foods produce equol [5,6,10], and during pregnancy Asian women do not avoid soy foods, it could be inferred that exposure to equol must occur during human pregnancy because isoflavones and equol readily cross the placenta [34,69,70,93]. This evidence, and the prolific Asian population consuming soy leads us to suggest that equol exposure is unlikely to lead to adverse effects in the newborn infant, and might even be beneficial in the longer term. Finally, the mild uterotrophic effect on myometrial thickness from early exposure that was sustained by S-(−)equol but not by R-(+)equol may suggest that very high doses of these enantiomers could negatively influence the uterus of the newborn and S(−)-equol should perhaps be contraindicated for women during pregnancy. With regard to men’s health, it would appear that the effects of both enantiomers on the development of the testes and prostate are rather benign, and this is an important finding because both enantiomers have clinical potential for the prevention and treatment of prostate cancer and are likely to be tested in future clinical trials.
Acknowledgments
These studies were funded by the National Institutes of Health (Grant # R01AT-003313) to KDRS.
Abbreviations
- NMR
nuclear magnetic resonance
- HPLC–MS
high pressure liquid chromatography–mass spectrometry
- VP
ventral prostate
- DLP
dorso-lateral prostate
- SV
seminal vesicles
- PND
post natal day
Footnotes
Contributors
KDRS (Principal Investigator) and NMB (Co-Investigator) conceived and designed these studies. SLL (Research Assistant) maintained the rat colony and treated the animals supervised by NMB. DW (Pathologist) and SLL performed the histological analysis of reproductive tissue. KDRS and NMB evaluated study results and directed the work. The manuscript was written by NMB and KDRS, and SLL and DW proof read the manuscript.
Conflict of interest statement
KDRS has intellectual property on equol enantiomers, including patents licensed by Cincinnati Children’s Hospital Medical Center, Cincinnati, Ohio to industry. None of the other authors have a conflict of interest, or anything to disclose, financially or otherwise.
Contributor Information
Nadine M. Brown, Email: nadine.brown@cchmc.org.
Kenneth D.R. Setchell, Email: kenneth.setchell@cchmc.org.
References
- 1.Messina M, Barnes S. The role of soy products in reducing risk of cancer. J Natl Cancer Inst. 1991;83:541–6. doi: 10.1093/jnci/83.8.541. [DOI] [PubMed] [Google Scholar]
- 2.Messina MJ, Loprinzi CL. Soy for breast cancer survivors: a critical review of the literature. J Nutr. 2001;131:3095S–108S. doi: 10.1093/jn/131.11.3095S. [DOI] [PubMed] [Google Scholar]
- 3.Setchell KDR, Brown NM, Lydeking-Olsen E. The clinical importance of the metabolite equol-a clue to the effectiveness of soy and its isoflavones. J Nutr. 2002;132:3577–84. doi: 10.1093/jn/132.12.3577. [DOI] [PubMed] [Google Scholar]
- 4.Setchell KDR, Clerici C. Equol: pharmacokinetics and biological actions. J Nutr. 2010;140:1363S–8S. doi: 10.3945/jn.109.119784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rowland IR, Wiseman H, Sanders TA, Adlercreutz H, Bowey EA. Interindividual variation in metabolism of soy isoflavones and lignans: influence of habitual diet on equol production by the gut microflora. Nutr Cancer. 2000;36:27–32. doi: 10.1207/S15327914NC3601_5. [DOI] [PubMed] [Google Scholar]
- 6.Lampe JW, Karr SC, Hutchins AM, Slavin JL. Urinary equol excretion with a soy challenge: influence of habitual diet. Proc Soc Exp Biol Med. 1998;217:335–9. doi: 10.3181/00379727-217-44241. [DOI] [PubMed] [Google Scholar]
- 7.Setchell KDR, Clerici C. Equol: history, chemistry, and formation. J Nutr. 2010;140:1355S–62S. doi: 10.3945/jn.109.119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lampe JW. Is equol the key to the efficacy of soy foods? Am J Clin Nutr. 2009;89:1664S–7S. doi: 10.3945/ajcn.2009.26736T. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Setchell KD, Borriello SP, Hulme P, Kirk DN, Axelson M. Nonsteroidal estrogens of dietary origin: possible roles in hormone-dependent disease. Am J Clin Nutr. 1984;40:569–78. doi: 10.1093/ajcn/40.3.569. [DOI] [PubMed] [Google Scholar]
- 10.Setchell KDR, Cole SJ. Method of defining equol-producer status and its frequency among vegetarians. J Nutr. 2006;136:2188–93. doi: 10.1093/jn/136.8.2188. [DOI] [PubMed] [Google Scholar]
- 11.Lydeking-Olsen E, Beck-Jensen JE, Setchell KD, Holm-Jensen T. Soymilk or progesterone for prevention of bone loss–a 2 year randomized, placebo-controlled trial. Eur J Nutr. 2004;43:246–57. doi: 10.1007/s00394-004-0497-8. [DOI] [PubMed] [Google Scholar]
- 12.Uesugi S, Watanabe S, Ishiwata N, Uehara M, Ouchi K. Effects of isoflavone supplements on bone metabolic markers and climacteric symptoms in Japanese women. Biofactors. 2004;22:221–8. doi: 10.1002/biof.5520220145. [DOI] [PubMed] [Google Scholar]
- 13.Ishimi Y. Dietary equol and bone metabolism in postmenopausal Japanese women and osteoporotic mice. J Nutr. 2010;140:1373S–6S. doi: 10.3945/jn.110.124842. [DOI] [PubMed] [Google Scholar]
- 14.Wu JQ, Guo CJ, Gu JF. Progress in research of equol: a metabolite of the isoflavone daidzein. Sheng Li Ke Xue Jin Zhan. 2006;37:359–61. [PubMed] [Google Scholar]
- 15.Wu J, Oka J, Ezaki J, Ohtomo T, Ueno T, Uchiyama S, et al. Possible role of equol status in the effects of isoflavone on bone and fat mass in postmenopausal Japanese women: a double-blind, randomized, controlled trial. Menopause. 2007;14:866–74. doi: 10.1097/gme.0b013e3180305299. [DOI] [PubMed] [Google Scholar]
- 16.Fuhrman BJ, Teter BE, Barba M, Byrne C, Cavalleri A, Grant BJ, et al. Equol status modifies the association of soy intake and mammographic density in a sample of postmenopausal women. Cancer Epidemiol Biomarkers Prev. 2008;17:33–42. doi: 10.1158/1055-9965.EPI-07-0193. [DOI] [PubMed] [Google Scholar]
- 17.Jou H-J, Wu S-C, Chang F-W, Ling P-Y, Chu K-S, Wu W-H. Effect of intestinal production of equol on menopausal symptoms in women treated with soy isoflavones. Int J Gynecol Obst. 2008;102:44–9. doi: 10.1016/j.ijgo.2008.01.028. [DOI] [PubMed] [Google Scholar]
- 18.Clerici C, Setchell KD, Battezzati PM, Pirro M, Giuliano V, Asciutti S, et al. Pasta naturally enriched with isoflavone aglycons from soy germ reduces serum lipids and improves markers of cardiovascular risk. J Nutr. 2007;137:2270–8. doi: 10.1093/jn/137.10.2270. [DOI] [PubMed] [Google Scholar]
- 19.Tormala R, Appt S, Clarkson TB, Groop PH, Ronnback M, Ylikorkala O, et al. Equol production capability is associated with favorable vascular function in postmenopausal women using tibolone; no effect with soy supplementation. Atherosclerosis. 2008;198:174–8. doi: 10.1016/j.atherosclerosis.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 20.Akaza H, Miyanaga N, Takashima N, Naito S, Hirao Y, Tsukamoto T, et al. Comparisons of percent equol producers between prostate cancer patients and controls: case-controlled studies of isoflavones in Japanese, Korean and American residents. Jpn J Clin Oncol. 2004;34:86–9. doi: 10.1093/jjco/hyh015. [DOI] [PubMed] [Google Scholar]
- 21.Ozasa K, Nakao M, Watanabe Y, Hayashi K, Miki T, Mikami K, et al. Serum phytoestrogens and prostate cancer risk in a nested case–control study among Japanese men. Cancer Sci. 2004;95:65–71. doi: 10.1111/j.1349-7006.2004.tb03172.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jackson MD, McFarlane-Anderson ND, Simon GA, Bennett FI, Walker SP. Urinary phytoestrogens and risk of prostate cancer in Jamaican men. Cancer Causes Control. 2010;21:2249–57. doi: 10.1007/s10552-010-9648-9. [DOI] [PubMed] [Google Scholar]
- 23.Lund TD, Munson DJ, Haldy ME, Setchell KD, Lephart ED, Handa RJ. Equol is a novel anti-androgen that inhibits prostate growth and hormone feedback. Biol Reprod. 2004;70:1188–95. doi: 10.1095/biolreprod.103.023713. [DOI] [PubMed] [Google Scholar]
- 24.Niculescu MD, Pop EA, Fischer LM, Zeisel SH. Dietary isoflavones differentially induce gene expression changes in lymphocytes from postmenopausal women who form equol as compared with those who do not. J Nutr Biochem. 2007;18:380–90. doi: 10.1016/j.jnutbio.2006.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coward L, Barnes NC, Setchell KDR, Barnes S. Genistein, daidzein, and their β-glycoside conjugates: antitumor isoflavones in soybean foods from American and Asian diets. J Agric Food Chem. 1993;41:1961–7. [Google Scholar]
- 26.Murphy PA. Phytoestrogen content of processed soybean products. Food Technol. 1982;43:60–4. [Google Scholar]
- 27.Kostelac D, Rechkemmer G, Briviba K. Phytoestrogens modulate binding response of estrogen receptors alpha and beta to the estrogen response element. J Agric Food Chem. 2003;51:7632–5. doi: 10.1021/jf034427b. [DOI] [PubMed] [Google Scholar]
- 28.Morito K, Hirose T, Kinjo J, Hirakawa T, Okawa M, Nohara T, et al. Interaction of phytoestrogens with estrogen receptors alpha and beta. Biol Pharm Bull. 2001;24:351–6. doi: 10.1248/bpb.24.351. [DOI] [PubMed] [Google Scholar]
- 29.Muthyala RS, Ju YH, Sheng S, Williams LD, Doerge DR, Katzenellenbogen BS, et al. Equol, a natural estrogenic metabolite from soy isoflavones: convenient preparation and resolution of R- and S-equols and their differing binding and biological activity through estrogen receptors alpha and beta. Bioorg Med Chem. 2004;12:1559–67. doi: 10.1016/j.bmc.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 30.Setchell KDR, Clerici C, Lephart ED, Cole SJ, Heenan C, Castellani D, et al. S-equol, a potent ligand for estrogen receptor beta, is the exclusive enantiomeric form of the soy isoflavone metabolite produced by human intestinal bacterial flora. Am J Clin Nutr. 2005;81:1072–9. doi: 10.1093/ajcn/81.5.1072. [DOI] [PubMed] [Google Scholar]
- 31.Setchell KDR, Sirokin VI Office UP. Method for the enantioseleactive hydrogenation of chromenes. USA: 2005. [Google Scholar]
- 32.Brown NM, Belles CA, Lindley SL, Zimmer-Nechemias L, Witte DP, Kim MO, et al. Mammary gland differentiation by early life exposure to enantiomers of the soy isoflavone metabolite equol. Food Chem Toxicol. 2010;48:3042–50. doi: 10.1016/j.fct.2010.07.042. [DOI] [PubMed] [Google Scholar]
- 33.Brown NM, Belles CA, Lindley S, Zimmer-Nechemias LD, Zhao X, Witte DP, et al. The chemopreventive action of equol enantiomers in a chemically induced animal model of breast cancer. Carcinogenesis. 2010;31:886–93. doi: 10.1093/carcin/bgq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown NM, Setchell KD. Animal models impacted by phytoestrogens in commercial chow: implications for pathways influenced by hormones. Lab Invest. 2001;81:735–47. doi: 10.1038/labinvest.3780282. [DOI] [PubMed] [Google Scholar]
- 35.Degen GH, Janning P, Diel P, Michna H, Bolt HM. Transplacental transfer of the phytoestrogen daidzein in DA/Han rats. Arch Toxicol. 2002;76:23–9. doi: 10.1007/s00204-001-0305-7. [DOI] [PubMed] [Google Scholar]
- 36.Doerge DR, Churchwell MI, Chang HC, Newbold RR, Delclos KB. Placental transfer of the soy isoflavone genistein following dietary and gavage administration to Sprague Dawley rats. Reprod Toxicol. 2001;15:105–10. doi: 10.1016/s0890-6238(01)00108-3. [DOI] [PubMed] [Google Scholar]
- 37.Lamartiniere CA, Wang J, Smith-Johnson M, Eltoum IE. Daidzein: bioavailability, potential for reproductive toxicity, and breast cancer chemoprevention in female rats. Toxicol Sci. 2002;65:228–38. doi: 10.1093/toxsci/65.2.228. [DOI] [PubMed] [Google Scholar]
- 38.Bennetts HW, Underwood EJ, Shier F. A specific breeding problem of sheep on subterranean clover pastures in Western Australia. Aust Vet J. 1946;22:2–12. doi: 10.1111/j.1751-0813.1946.tb15473.x. [DOI] [PubMed] [Google Scholar]
- 39.Ishiwata N, Melby MK, Mizuno S, Watanabe S. New equol supplement for relieving menopausal symptoms: randomized, placebo-controlled trial of Japanese women. Menopause. 2009;16:141–8. doi: 10.1097/gme.0b013e31818379fa. [DOI] [PubMed] [Google Scholar]
- 40.Matulka RA, Matsuura I, Uesugi T, Ueno T, Burdock G. Developmental and reproductive effects of SE5-OH: an equol-rich soy-based ingredient. J Toxicol. 2009;2009:307618. doi: 10.1155/2009/307618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jackson RL, Greiwe JS, Desai PB, Schwen RJ. Single-dose and steady-state pharmacokinetic studies of S-equol, a potent nonhormonal, estrogen receptor beta-agonist being developed for the treatment of menopausal symptoms. Menopause. 2011;18:185–93. [PubMed] [Google Scholar]
- 42.Medlock KL, Branham WS, Sheehan DM. Effects of coumestrol and equol on the developing reproductive tract of the rat. Proc Soc Exp Biol Med. 1995;208:67–71. doi: 10.3181/00379727-208-43833. [DOI] [PubMed] [Google Scholar]
- 43.Awoniyi CA, Roberts D, Veeramachaneni DN, Hurst BS, Tucker KE, Schlaff WD. Reproductive sequelae in female rats after in utero and neonatal exposure to the phytoestrogen genistein. Fertil Steril. 1998;70:440–7. doi: 10.1016/s0015-0282(98)00185-x. [DOI] [PubMed] [Google Scholar]
- 44.Nagata C. Factors to consider in the association between soy isoflavone intake and breast cancer risk. J Epidemiol. 2010;20:83–9. doi: 10.2188/jea.JE20090181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schedin P, Mitrenga T, Kaeck M. Estrous cycle regulation of mammary epithelial cell proliferation, differentiation, and death in the Sprague–Dawley rat: a model for investigating the role of estrous cycling in mammary carcinogenesis. J Mammary Gland Biol Neoplasia. 2000;5:211–25. doi: 10.1023/a:1026447506666. [DOI] [PubMed] [Google Scholar]
- 46.Hsueh AJ, Billig H, Tsafriri A. Ovarian follicle atresia: a hormonally controlled apoptotic process. Endocr Rev. 1994;15:707–24. doi: 10.1210/edrv-15-6-707. [DOI] [PubMed] [Google Scholar]
- 47.Leblond CP, Clermont Y. Definition of the stages of the cycle of the seminiferous epithelium in the rat. Ann N Y Acad Sci. 1952;55:548–73. doi: 10.1111/j.1749-6632.1952.tb26576.x. [DOI] [PubMed] [Google Scholar]
- 48.Leblond CP, Clermont Y. Spermiogenesis of rat, mouse, hamster and guinea pig as revealed by the periodic acid-fuchsin sulfurous acid technique. Am J Anat. 1952;90:167–215. doi: 10.1002/aja.1000900202. [DOI] [PubMed] [Google Scholar]
- 49.Moore C, Hughes W, Gallagher T. Rat seminal vesicle cytology as a testis-hormone indicator and the prevention of castration changes by testis extract injection. Am J Anat. 1930;45:109–35. [Google Scholar]
- 50.Frey HL, Peng S, Rajfer J. Synergy of abdominal pressure and androgens in testicular descent. Biol Reprod. 1983;29:1233–9. doi: 10.1095/biolreprod29.5.1233. [DOI] [PubMed] [Google Scholar]
- 51.Ojeda SR, Jameson HE. Developmental patterns of plasma and pituitary growth hormone (GH) in the female rat. Endocrinology. 1977;100:881–9. doi: 10.1210/endo-100-3-881. [DOI] [PubMed] [Google Scholar]
- 52.Reid B, Cleland K. The structure and function of the epididymis. Aust J Zool. 1957;5:223–52. [Google Scholar]
- 53.Boorman GA, Eustis SL, Elwell MR, MacKenzie WF, Montgomery C. Pathology of the fischer rat. 1990:1–556. [Google Scholar]
- 54.Yee S, Burdock GA, Kurata Y, Enomoto Y, Narumi K, Hamada S, et al. Acute and subchronic toxicity and genotoxicity of SE5-OH, an equol-rich product produced by Lactococcus garvieae. Food Chem Toxicol. 2008;46:2713–20. doi: 10.1016/j.fct.2008.04.026. [DOI] [PubMed] [Google Scholar]
- 55.Setchell KDR, Zhao X, Shoaf SE, Ragland K. The pharmacokinetics of S-(−)equol administered as SE5-OH tablets to healthy postmenopausal women. J Nutr. 2009;139:2037–43. doi: 10.3945/jn.109.110874. [DOI] [PubMed] [Google Scholar]
- 56.Medlock KL, Branham WS, Sheehan DM. The effects of phytoestrogens on neonatal rat uterine growth and development. Proc Soc Exp Biol Med. 1995;208:307–13. doi: 10.3181/00379727-208-43861. [DOI] [PubMed] [Google Scholar]
- 57.Messina M. The endometrial effects of isoflavones: a discussion paper. Complement Ther Clin Pract. 2008;14:212–4. doi: 10.1016/j.ctcp.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 58.Setchell KDR. Soy isoflavones–benefits and risks from nature’s selective estrogen receptor modulators (SERMs) J Am Coll Nutr. 2001;20:354S–62S. doi: 10.1080/07315724.2001.10719168. (discussion 381S–383S) [DOI] [PubMed] [Google Scholar]
- 59.Unfer V, Casini ML, Costabile L, Mignosa M, Gerli S, Di Renzo GC. Endometrial effects of long-term treatment with phytoestrogens: a randomized, double-blind, placebo-controlled study. Fertil Steril. 2004;82:145–8. doi: 10.1016/j.fertnstert.2003.11.041. (quiz 265) [DOI] [PubMed] [Google Scholar]
- 60.Legette LL, Martin BR, Shahnazari M, Lee WH, Helferich WG, Qian J, et al. Supplemental dietary racemic equol has modest benefits to bone but has mild uterotropic activity in ovariectomized rats. J Nutr. 2009;139:1908–13. doi: 10.3945/jn.109.108225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rachon D, Vortherms T, Seidlova-Wuttke D, Menche A, Wuttke W. Uterotropic effects of dietary equol administration in ovariectomized Sprague–Dawley rats. Climacteric. 2007;10:416–26. doi: 10.1080/13697130701624757. [DOI] [PubMed] [Google Scholar]
- 62.Phrakonkham P, Chevalier J, Desmetz C, Pinnert MF, Berges R, Jover E, et al. Isoflavonoid-based bone-sparing treatments exert a low activity on reproductive organs and on hepatic metabolism of estradiol in ovariectomized rats. Toxicol Appl Pharmacol. 2007;224:105–15. doi: 10.1016/j.taap.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 63.Wood CE, Appt SE, Clarkson TB, Franke AA, Lees CJ, Doerge DR, et al. Effects of high-dose soy isoflavones and equol on reproductive tissues in female cynomolgus monkeys. Biol Reprod. 2006;75:477–86. doi: 10.1095/biolreprod.106.052142. [DOI] [PubMed] [Google Scholar]
- 64.Schwen R, Jackson R, Proudlock R. Genotoxicity assessment of S-equol in bacterial mutation, chromosomal aberration, and rodent bone marrow micronucleus tests. Food Chem Toxicol. 2010;48:3481–5. doi: 10.1016/j.fct.2010.09.027. [DOI] [PubMed] [Google Scholar]
- 65.Ferguson SA, Delclos KB, Newbold RR, Flynn KM. Few effects of multi-generational dietary exposure to genistein or nonylphenol on sodium solution intake in male and female Sprague–Dawley rats. Neurotoxicol Teratol. 2009;31:143–8. doi: 10.1016/j.ntt.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 66.Jefferson WN, Padilla-Banks E, Goulding EH, Lao SP, Newbold RR, Williams CJ. Neonatal exposure to genistein disrupts ability of female mouse reproductive tract to support preimplantation embryo development and implantation. Biol Reprod. 2009;80:425–31. doi: 10.1095/biolreprod.108.073171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Newbold RR, Banks EP, Bullock B, Jefferson WN. Uterine adenocarcinoma in mice treated neonatally with genistein. Cancer Res. 2001;61:4325–8. [PubMed] [Google Scholar]
- 68.Setchell KDR, Zhao X, Jha P, Heibi JE, Brown NM. The pharmacokinetic behavior of the soy isoflavone metabolite S-(−)equol and its diastereoisomer R-(+)equol in healthy adults determined by using stable-isotope-labeled tracers. Am J Clin Nutr. 2009;90:1029–37. doi: 10.3945/ajcn.2009.27981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Adlercreutz H, Yamada T, Wahala K, Watanabe S. Maternal and neonatal phytoestrogens in Japanese women during birth. Am J Obstet Gynecol. 1999;180:737–43. doi: 10.1016/s0002-9378(99)70281-4. [DOI] [PubMed] [Google Scholar]
- 70.Foster WG, Chan S, Platt L, Hughes CL., Jr Detection of phytoestrogens in samples of second trimester human amniotic fluid. Toxicol Lett. 2002;129:199–205. doi: 10.1016/s0378-4274(02)00018-8. [DOI] [PubMed] [Google Scholar]
- 71.Weber KS, Setchell KD, Lephart ED. Maternal and perinatal brain aromatase: effects of dietary soy phytoestrogens. Brain Res Dev Brain Res. 2001;126:217–21. doi: 10.1016/s0165-3806(00)00138-3. [DOI] [PubMed] [Google Scholar]
- 72.McClain RM, Wolz E, Davidovich A, Edwards J, Bausch J. Reproductive safety studies with genistein in rats. Food Chem Toxicol. 2007;45:1319–32. doi: 10.1016/j.fct.2007.01.009. [DOI] [PubMed] [Google Scholar]
- 73.Messina M. Resolving the soy-breast cancer controversy. J Am Diet Assoc. 2006;106:363–4. doi: 10.1016/j.jada.2006.01.015. [DOI] [PubMed] [Google Scholar]
- 74.Messina MJ, Persky V, Setchell KD, Barnes S. Soy intake and cancer risk: a review of the in vitro and in vivo data. Nutr Cancer. 1994;21:113–31. doi: 10.1080/01635589409514310. [DOI] [PubMed] [Google Scholar]
- 75.Ju YH, Allred KF, Allred CD, Helferich WG. Genistein stimulates growth of human breast cancer cells in a novel, postmenopausal animal model, with low plasma estradiol concentrations. Carcinogenesis. 2006;27:1292–9. doi: 10.1093/carcin/bgi370. [DOI] [PubMed] [Google Scholar]
- 76.Guha N, Kwan ML, Quesenberry CP, Jr, Weltzien EK, Castillo AL, Caan BJ. Soy isoflavones and risk of cancer recurrence in a cohort of breast cancer survivors: the Life After Cancer Epidemiology study. Breast Cancer Res Treat. 2009;118:395–405. doi: 10.1007/s10549-009-0321-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Shu XO, Zheng Y, Cai H, Gu K, Chen Z, Zheng W, et al. Soy food intake and breast cancer survival. JAMA. 2009;302:2437–43. doi: 10.1001/jama.2009.1783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ju YH, Fultz J, Allred KF, Doerge DR, Helferich WG. Effects of dietary daidzein and its metabolite, equol, at physiological concentrations on the growth of estrogen-dependent human breast cancer (MCF-7) tumors implanted in ovariectomized athymic mice. Carcinogenesis. 2006;27:856–63. doi: 10.1093/carcin/bgi320. [DOI] [PubMed] [Google Scholar]
- 79.Setchell KDR, Gosselin SJ, Welsh MB, Johnston JO, Balistreri WF, Kramer LW, et al. Dietary estrogens–a probable cause of infertility and liver disease in captive cheetahs. Gastroenterology. 1987;93:225–33. doi: 10.1016/0016-5085(87)91006-7. [DOI] [PubMed] [Google Scholar]
- 80.Nielsen TS, Norgaard JV, Purup S, Frette XC, Bonefeld-Jorgensen EC. Estrogenic activity of bovine milk high or low in equol using immature mouse uterotrophic responses and an estrogen receptor transactivation assay. Cancer Epidemiol. 2009;33:61–8. doi: 10.1016/j.canep.2009.04.003. [DOI] [PubMed] [Google Scholar]
- 81.Selvaraj V, Zakroczymski MA, Naaz A, Mukai M, Ju YH, Doerge DR, et al. Estrogenicity of the isoflavone metabolite equol on reproductive and non-reproductive organs in mice. Biol Reprod. 2004;71:966–72. doi: 10.1095/biolreprod.104.029512. [DOI] [PubMed] [Google Scholar]
- 82.Thigpen JE, Setchell KDR, Padilla-Banks E, Haseman JK, Saunders HE, Caviness GF. Variations in phytoestrogen content between different mill dates of the same diet produces significant differences in the time of vaginal opening in CD-1 mice and F344 rats but not in CD Sprague–Dawley rats. Environ Health Perspect. 2007;115:1717–26. doi: 10.1289/ehp.10165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Thigpen JE, Haseman JK, Saunders HE, Setchell KDR, Grant MG, Forsythe DB. Dietary phytoestrogens accelerate the time of vaginal opening in immature CD-1 mice. Comp Med. 2003;53:607–15. [PubMed] [Google Scholar]
- 84.Messina M. Soybean isoflavone exposure does not have feminizing effects on men: a critical examination of the clinical evidence. Fertil Steril. 2010;93:2095–104. doi: 10.1016/j.fertnstert.2010.03.002. [DOI] [PubMed] [Google Scholar]
- 85.Huhtaniemi I. Molecular aspects of the ontogeny of the pituitary-gonadal axis. Reprod Fertil Dev. 1995;7:1025–35. doi: 10.1071/rd9951025. [DOI] [PubMed] [Google Scholar]
- 86.Boorman G, Chapin R. Pathology of the fischer rat. San Diego, CA: Academic Press, Inc; 1990. [Google Scholar]
- 87.Shutt DA. The effects of plant oestrogens on animal reproduction. Endeavor. 1976;35:110–3. doi: 10.1016/0160-9327(76)90004-1. [DOI] [PubMed] [Google Scholar]
- 88.Pope GS. The importance of pasture plant oestrogens in the reproduction and lactation of grazing animals. Dairy Sci Abstr. 1954;16:334–55. [Google Scholar]
- 89.Wisniewski AB, Klein SL, Lakshmanan Y, Gearhart JP. Exposure to genistein during gestation and lactation demasculinizes the reproductive system in rats. J Urol. 2003;169:1582–6. doi: 10.1097/01.ju.0000046780.23389.e0. [DOI] [PubMed] [Google Scholar]
- 90.Rachon D, Vortherms T, Seidlova-Wuttke D, Wuttke W. Effects of dietary equol on body weight gain, intra-abdominal fat accumulation, plasma lipids, and glucose tolerance in ovariectomized Sprague–Dawley rats. Menopause. 2007;14:925–32. doi: 10.1097/GME.0b013e31802d979b. [DOI] [PubMed] [Google Scholar]
- 91.Lephart ED, Porter JP, Lund TD, Bu L, Setchell KD, Ramoz G. Dietary isoflavones alter regulatory behaviors, metabolic hormones and neuroendocrine function in Long–Evans male rats. Nutr Metab (Lond) 2004;1:16. doi: 10.1186/1743-7075-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kishida T, Mizushige T, Ohtsu Y, Ishikawa S, Nagamoto M, Izumi T, et al. Dietary soy isoflavone-aglycone lowers food intake in female rats with and without ovariectomy. Obesity. 2008 Silver Spring;16:290–7. doi: 10.1038/oby.2007.68. [DOI] [PubMed] [Google Scholar]
- 93.Engel SM, Levy B, Liu Z, Kaplan D, Wolff MS. Xenobiotic phenols in early pregnancy amniotic fluid. Reprod Toxicol. 2006;21:110–2. doi: 10.1016/j.reprotox.2005.07.007. [DOI] [PubMed] [Google Scholar]


