Abstract
Fluorescent derivatives of σ2 high affinity ligand 1-cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazine 1 (PB28) were synthesized. NBD or Dansyl fluorescent tags were connected through a 5- or 6-atoms linker in two diverse positions of 1 structure. Good σ2 affinities were obtained when the fluorescent tag was linked to 5-methoxytetralin nucleus replacing the methyl function. NBD-bearing compound 16 displayed high σ2 affinity (Ki = 10.8 nM) and optimal fluorescent properties. Its uptake in pancreatic tumor cells was evaluated by flow cytometry showing that it partially occurs through endocytosis. In proliferating cells the uptake was higher supporting that σ2 receptors are markers of cell proliferation and that the higher is the proliferation, the stronger is the antiproliferative effect of σ2 agonists. Colocalization of 16 with subcellular organelles was studied by confocal microscopy: the greatest was in endoplasmic reticulum and lysosomes. Fluorescent σ2 ligands show their potential in clarifying the mechanisms of action of σ2 receptors.
Introduction
After their first discovery in 1976, sigma (σ) receptor research met a renovated enthusiasm in the early 1990’s when the two subtypes, σ1 and σ2, were identified.1 The σ1 subtype was soon thereafter isolated and cloned from different sources,2 and it has been recently classified as a receptor chaperone at the endoplasmic reticulum (ER) membrane that regulates ER-mitochondrial Ca2+ signalling and cell survival.3 Though their mechanism of action is still unclear, σ proteins receive much interest because of their potential applications as drug targets for a wide range of diseases. σ1 Receptor ligands display neuroprotective and neuroregulative functions and are under evaluation for the treatment of a number of neurological disorders4 such as depression,5 schizophrenia,6 Alzheimer’s and Parkinson’s diseases7-9 and for drug abuse (e.g., cocaine).10 The high therapeutic potential of σ2 receptors comes from the evidence that this subtype is overexpressed in a wide variety of cancer tissues, and activation of σ2 receptors lead tumor cells to death through different apoptotic pathways.11-13 Therefore, a number of σ2 receptor ligands are under investigation for cancer treatment and diagnosis.14-16 Nevertheless, the σ2 subtype is not as well as characterized as the σ1. It has not yet been cloned and attempted characterization from homogenate of σ2-overexpressing tumor cells led to isolation of histone proteins by affinity chromatography.17 The σ2 selector used was a derivative of 1-cyclohexyl-4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)-n-propyl]piperazine (1, PB28), one of the highest affinity σ2 receptor ligands known18,19 concluding that either σ2 receptors may be histones or histone binding proteins or compound 1 binds such proteins as well as σ2 receptors, with modeling studies conducted to rationalize these hypotheses.20 Such results were in disagreement with findings from fluorescence microscopy, which localizes σ2 subtypes in several organelles except the nucleus through the use of fluorescent σ2 ligands.21,22 Besides the intracellular localization, there are other ambiguities related to the σ2 receptors: evidence shows that σ2 ligands activate different apoptotic pathways in diverse tumor cells.11-13,23 With the aim of helping to clarify some of these ambiguities, we synthesized a small series of fluorescent derivatives of compound 1 to be used in microscopy studies for the purpose of localizing σ2 receptors subcellularly within cancer cells.
Intrinsically fluorescent compound 1 analogues have been synthesized in the past, with appreciable σ receptor affinity but with maximum excitation and emission wavelength (λexc and λem) inappropriate for use in living cells for fluorescence microscopy.24 Therefore, we followed a common approach to overcome this limitation: dansyl (λexc ~315 nm) and 7-nitro-2,1,3-benzoxadiazole (NBD) (λexc ~420 nm) moieties were alternatively inserted in two different positions on compound 1 structure through a 5- or 6-atoms linker. Such separation between the pharmacophore and the fluorescent tag should prevent the loss of affinity leaving the fluorescent properties of the fluorescent moieties almost unchanged. Compound 16, with the best fluorescence/pharmacological properties, was used for preliminary fluorescence microscopy analyses in murine and human pancreatic tumor cells which have been previously shown to overexpress σ2 receptors.11 Human pancreatic tumor cells (BxPC3) were selected for more extensive studies of compound 16 whose internalization and colocalization by confocal microscopy with subcellular organelles were evaluated.
Results and Discussion
Chemistry
The synthetic pathways for final compounds 7, 8, 16-19 are depicted in Scheme 1 and 2. Key intermediate 6 was prepared starting from commercial piperidin-4-one ethylene ketal (1,4-dioxa-8-aza-spiro[4.5]decane) which was alkylated with 6-bromohexanenitrile affording intermediate 2. Upon reduction with LiAlH4 to the corresponding amine and subsequent amine-protection through acetylation, compound 2 provided derivative 3. Acidic deprotection of the ethylene-acetal function with HCl led to intermediate 4 which underwent reductive amination with piperazine 525 providing the acetyl derivative of 6, which was deacetylated affording key amine 6. Reaction of this latter alternatively with NBD-chloride or dansyl chloride afforded the fluorescent compounds 7 and 8 respectively (Scheme 1).
Scheme 1.
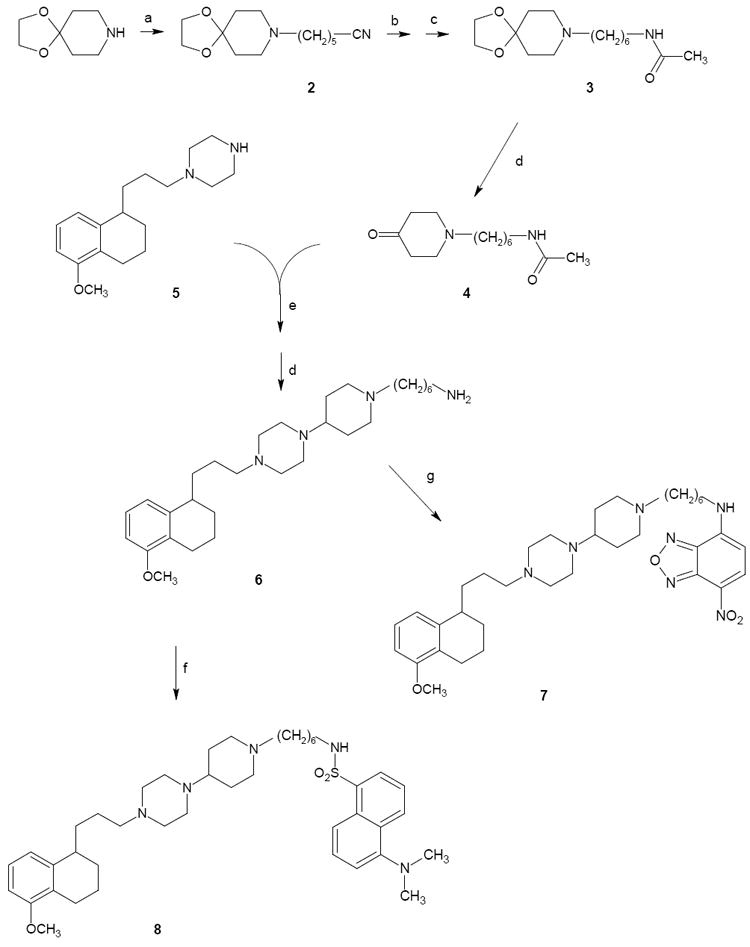
Synthesis of Fluorescent Compound 1 Analogues: fluorescent tag at the piperazine.a
aReagents: (a) 6-Br(CH2)5CN; (b) LiAlH4; (c) CH3COCl; (d) HCl; (e) ZnCl2, NaCNBH3; (f) Dansyl chloride; (g) NBD-chloride.
Scheme 2.
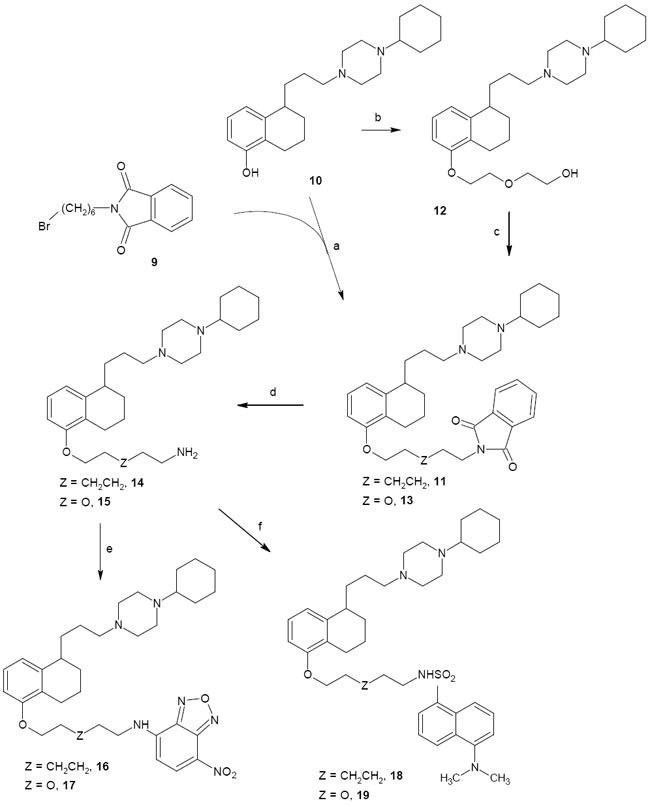
Synthesis of Fluorescent Compound 1 Analogues: fluorescent tag at the tetralin nucleus.a
aReagents: (a) K2CO3; (b) ClCH2CH2OCH2CH2OH, K2CO3; (c) Ph3P, Phthlimide, DIAD; (d) hydrazine hydrate; (e) NBD-chloride; (f) Dansyl chloride.
The final compounds 16-19 were synthesized as outlined in Scheme 2. The reaction between potassium phthalimide and 1,6-dibromohexane gave intermediate 926 which was used to alkylate the key phenolic intermediate 1019 affording phthalimide 11. Alkylation of the phenolic intermediate 9 with 2-(2-chloroethoxy)ethanol afforded the corresponding alcohol 12 that underwent Mitsunobu condensation with phthalimide in the presence of triphenylphosphine and diisopropylazodicarboxylate (DIAD) to yield compound 13. Phthalimide derivatives 11 and 13 underwent hydrazinolysis to afford intermediate primary amines 14 and 15 respectively. Reaction of 14 with NBD-chloride or dansyl chloride afforded the fluorescent compounds 16 and 18 respectively. Reaction of 15 with NBD-chloride or dansyl chloride afforded respectively the fluorescent final compounds 17 and 19. All the final compounds were converted to the corresponding hydrochloride salts with gaseous HCl.
Radioligand Binding and σ1 and σ2 Receptor Affinities
Results from binding assays are expressed as inhibition constants (Ki values) in Table 1. The introduction of the fluorescent tag and the linker produced a decrease in the affinity at both σ receptors with respect to lead compound 1. The most dramatic drop in the affinity at both σ receptors was observed with compounds 7 (Ki = 2570 nM for σ1 and Ki = 1720 nM for σ2 receptor), and 8 (Ki > 5000 nM for σ1 and Ki = 5020 nM for σ2 receptor) which reached micromolar values. In such compounds the piperidine ring replacing the cyclohexyl ring was functionalized with the fluorescent tag through a six-methylene chain. The drop in the affinity was independent from the nature of the fluorescent tag indicating that functionalization (at least with a six-atom linker) in that position of the pharmacophore was not tolerated by the σ2 receptors, in accordance with a previous study in which substitution of the cyclohexyl with more hindered substituents led to reduced σ affinities.18,27 On the other hand, fluorescent final compounds obtained through insertion of the alkyl fluorescent tag on the 5-methoxy-tetralin ring in place of the methyl group, displayed nanomolar affinities at both σ receptors (compounds 16-19). Previous SAfiR studies demonstrated how the methoxy substituent was unessential for σ2 receptor binding indeed.28 NBD or Dansyl moieties were tolerated at the σ2 receptor with the highest affinity displayed by the NBD-bearing compound 16 (Kis = 10.8 nM). The presence of the NBD, but not of the dansyl moiety, appeared to be detrimental (Ki = 78.8 nM for 16 and Ki = 96.2 nM for 17) for σ1 receptor binding. With σ1 and σ2 affinities in the same range, dansyl-bearing ligands 18 and 19 did not show any σ2 versus σ1 selectivity, whereas compounds bearing NBD (16 and 17) displayed a moderate σ2 selectivity (8-fold and 2.5-fold respectively). Selectivity was missing in lead compound 1 when binding assays were performed on animal tissues according to literature protocols.18 Results obtained with this small series of compounds demonstrated that a fluorescent tag spaced out from the tetralinoxy moiety by a 5- or 6-atom linker leads to molecules with good σ receptor affinities useful for in living cells visualization of σ2 receptors, with compound 16 displaying the best pharmacological properties for further investigation.
Table 1.
Receptor Affinities and Fluorescence Properties of Final Compounds
 | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ki nMa | CHCl3 | EtOHb | PBSb,c | |||||||||||
| comp | X | Y | Z | σ1 | σ2 | λexc nm | λem nm | Φ | λexc nm | λem nm | Φ | λexc nm | λem nm | εd |
| 1e | CH3 | CH2 | 0.38±0.10 | 0.68±0.20 | ||||||||||
| 7 | CH3 | A | CH2CH2 | 2570f | 1720±160 | 450 | 515 | 0.17 | 476 | 520 | 0.08 | 480 | 535 | 14391 |
| 8 | CH3 | B | CH2CH2 | >5000f | 5020±180 | 346 | 490 | 0.32 | 335 | 507 | 0.29 | 340 | 510 | 2600 |
| 16 | A | CH2 | CH2CH2 | 78.7±18.2 | 10.8±3.0 | 451 | 514 | 0.20 | 467 | 520 | 0.05 | 460 | 520 | 11300 |
| 17 | A | CH2 | O | 96.2f | 39.3±11.8 | 450 | 512 | 0.18 | 465 | 520 | 0.04 | 460 | 520 | 6544 |
| 18 | B | CH2 | CH2CH2 | 9.08±1.32 | 20.8±1.5 | 340 | 490 | 0.30 | 335 | 507 | 0.20 | 345 | 485 | 4000 |
| 19 | B | CH2 | O | 19.8±8.7 | 25.7±4.7 | 345 | 490 | 0.48 | 335 | 507 | 0.23 | 343 | 510 | 2741 |
|
| ||||||||||||||
| (+)-pentazocine | 2.62±0.25 | |||||||||||||
| DTG | 24.6±2.2 | |||||||||||||
Values are the means of n ≥ 2 separate experiments.
Fluorescence properties herein reported were evaluated on compounds as free bases, but they were also evaluated on their corresponding hydrochloride salts in EtOH and PBS solutions. A maximum of 5 nm shift was observed in the excitation and emission wavelengths when compared to the excitation and emission wavelengths from the corresponding free bases.
All compounds solubilized in PBS gave Φ̣ value very close to 0 and therefore they are not reported.
From EtOH solutions of compounds in EtOH.
From Ref 18 where results from binding on human cells, in which compound 1 displays about 40-fold σ2 versus σ1 selectivity, are also reported.
From a unique experiment.
Fluorescent Ligand Studies
The fluorescent properties of final compounds are listed in Table 1. The excitation and emission spectra were obtained from solution of the final compounds in organic solvents (EtOH and CHCl3) and in aqueous solution (PBS buffer). The NBD-bearing compounds (8, 16, 17) displayed excitation peaks at two different wavelengths (~ 335 nm and ~450 nm) and for both the wavelenghts, the corresponding λem was ~520 nm. The λexc selected to perform the assays in living cells was 450 nm to avoid cells autofluorescence phenomena. Dansyl-bearing compounds (7, 18, 19) displayed a λexc more shifted toward the UV region (~340 nm). All of the compounds showed an important difference between λexc and λem (Stokes shift). Quantum yields (Φ) were determined in the above mentioned solvents to probe the environment affecting the sensitivity of the final fluorescent ligands, since the fluorophores selected (Dansyl and NBD) are endowed with environment sensitivity properties, (i.e. low quantum yield in aqueous solution but high fluorescence in nonpolar solvents or when bound to a hydrophobic sites). All tested compounds exhibited very low fluorescence in PBS buffer but became fluorescent in the organic solvents. The highest quantum yields were those recorded in CHCl3 for all the final compounds: Φ values were 2- or 4-fold higher in CHCl3 than in EtOH for NBD-bearing compounds (8, 16, 17) and several fold higher than in PBS buffer. Dansyl-bearing compounds (7, 18, 19) showed a less pronounced increase in Φ values from EtOH to CHCl3 solutions, although the highest Φ was shown by compound 19 (Φ = 0.48). Molar extinction coefficients (ε) were determined for all final compounds (in EtOH), with the lowest values displayed by the dansyl-bearing compounds (2600-4000 L/mol·cm) and the highest values displayed by the NBD-bearing compounds (6544-14391 L/mol·cm) indicating that the fluorescence intensity of the latter compounds is stronger. Preliminary fluorescence microscopy experiments were conducted with compound 16 which displayed the best combination between pharmacological (σ2 receptor affinity and selectivity) and fluorescence properties (convenient excitation and emission wavelengths and high ε value) and promising results were obtained in different tumor pancreatic cells which were previously shown to overexpress σ2 receptors.11 Therefore, compound 16 was evaluated in more details in in vitro internalization studies and cellular colocalization by confocal microscopy in human pancreatic tumor cells (BxPC3).
In vitro Internalization Studies
Uptake of compound 16 in BxPC3 pancreatic cancer cells was analyzed immediately following treatment (25 nM) and the mean fluorescence recorded over time (Figure 1A). The T1/2 of maximum fluorescence at 60 min was 7.8 ± 1.5 min (mean ± SEM). We further studied uptake for the purpose of better understanding the involvement of endocytosis of these compounds and the receptor. Caveolin-mediated (lipid rafts-mediated) endocytotis can be inhibited by Filipin III29 and clathrin-mediated endocytosis by phenylarsine oxide (PAO).30 BxPC3 cells were pretreated with Filipin III (5 μg/mL) or PAO (10 mM) for 30 min prior to treatment with compound 16 (25 nM) and mean fluorescence was collected over 60 min. The rate of uptake was decreased from 7.8 ± 1.5 min to 6.8 ± 0.8 min for Filipin III and 4.9 ± 2.1 min for PAO. As well, the overall uptake at 60 min was decreased to 69% and 50% for Filipin III and PAO respectively. Taken together, the T1/2 in the range of min and the decreased uptake in the presence of endocytosis inhibitors, suggest that the internalization of 16 occurs, in part, through the caveolin- and clathrin-mediated endocytotic pathways in addition to simple membrane diffusion. Uptake by the clathrin-dependent pathway has been described previously with other σ2 receptor ligands,21,22 but uptake by the caveolin-dependent/lipid raft pathway has not been previously reported. Interaction and endocytosis of compound 16 through lipid rafts is of note considering that σ2 receptor agonists were initially found to bind to protein constituents of the lipid rafts,31,32 cholesterol-rich domains in the cell membrane. They form flask shaped invaginations called caveolae, for the caveolin protein that coats them, and act as platforms for glycophosphatidylinositol-linked protein mediated signaling pathways and internalization of cholesterol.33 Interest has grown in targeting this pathway in cancer cells, which may have disrupted lipid rafts contributing to aberrances in pathways implicated in chemoresistance such as the epidermal growth factor receptor and tumor necrosis factor alpha receptor.34
Figure 1.
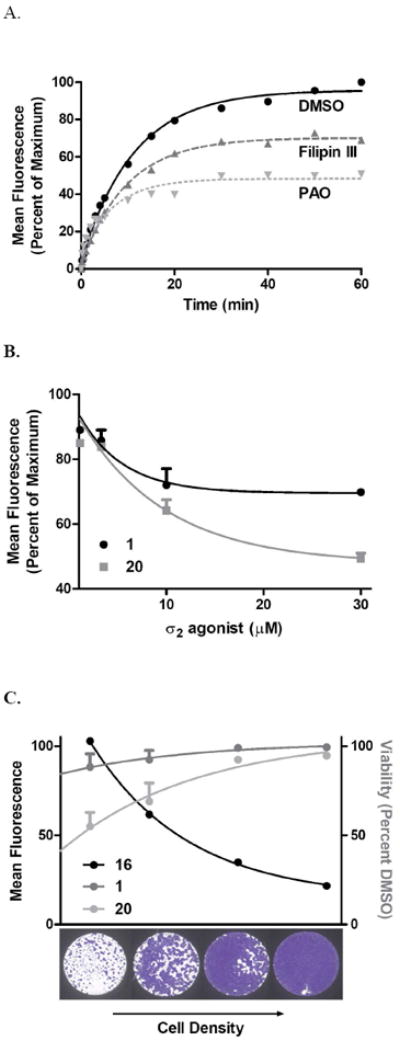
Cellular Internalization of the Fluorescent σ2 Receptor Ligand 16.
A) Kinetic uptake of compound 16: BxPC3 pancreatic cancer cells were treated for 1 h with the endocytosis inhibitors Filipin III (5 μg/mL) or phenylarsine oxide (PAO, 10 μM) or DMSO vehicle for 60 min prior to kinetic uptake analysis of 16 (25 nM) by flow cytometry. Compound 16 uptake represents the percentage of the mean fluorescence of maximum signal intensity.
B) Competition of compound 16 by σ2 agonist structural analogs. BxPC3 cells were treated with increasing doses of compounds 1 or 20 for 45 min prior to replacement with compound 16 (25 nM) for 45 min and fluorescence intensity quantified by flow cytometry.
C) Cell density dependence σ2 agonist uptake and cell death. BxPC3 cells were seeded at increasing densities to achieve a range of dividing, subconfluent and quiescent, confluent cultures. The following day, cells were treated with compound 16 (25 nM) and fluorescence intensity quantitated by flow cytometry. Alternatively, cell were treated with compound 1 or 20 (100 μM) for 24 hours and viability compared to DMSO control.
Therefore, implication of lipid raft disruption in oncology signaling and response to chemotherapy together with the presence of σ2 proteins in lipid rafts suggest that σ2 receptor mediated toxicity and chemoresistance overcome (which has been shown with different σ2 receptor agonists),23,35 likely involve lipid rafts.
In vitro competition was performed to quantify interaction of compound 16 with σ2 agonists in the live cell. Preloading with 1 or the recently produced σ2 agonist cis-1-cyclohexyl-4-[4-(2,6-difluorophenyl)cyclohexyl]piperazine36 (20) for 45 min prior to addition of compound 16 for 45 min decreased the mean fluorescence intensity of 16 with increasing concentrations of σ2 agonist (Figure 1B). This indicates that the fluorescent compound functionally competes for localization in the same plane as the parent and analog compound.
The correlation between the proliferative status of tumors and the expression of the σ2 receptors has been widely demonstrated in different cancer cell lines,22,37,38 and we have further detailed the expression and apoptosis response in pancreatic cancer cells.11,39 In this study, we further evaluated the impact of proliferation on σ2 agonist uptake and sensitivity (Figure 1C). In order to maintain proliferating versus quiescent cell cultures, subcultured cells were seeded at increasing densities in order to achieve subconfluent and confluent cultures respectively. Compound 16 mean fluorescence at 30 min decreased as cell density increased, in accordance with earlier findings that σ2 receptors are markers of cell proliferation.40 In addition, the decreased uptake was associated with decreased cell death as the density increased, so that the reduction of the antiproliferative activity of σ2 agonists 1 and 20 was likely due to the σ2 reduced presence in non proliferating cells. Together, these findings show that compound 16 performs biologically as expected for a σ2 ligand, and that increased uptake of σ2 agonists by proliferating cells is a critical step for mediating cell death.
Cellular colocalization studies of these fluorescent analogs of 1 were initially screened by epifluorescent microscopy (supplementary information), and confocal microscopy results were in accordance with those findings (Figure 2). BxPC3 cells were incubated with fluorescent ligand 16 and MitoTracker Red, ERTracker Red, LysoTracker Red, or Vybrant cholera-toxin B subunit (CT-B), at 37 °C for 30 min prior to fixation and nuclear staining with TO-PRO-3. Compound 16 is found in the membrane fractions of the cell, and colocalizes greatest in the endoplasmic reticulum and lysosomes, with moderate colocalization in the mitochondria and the plasma membrane (CT-B). Colocalization in the nucleus was not observed with TO-PRO-3.
Figure 2.
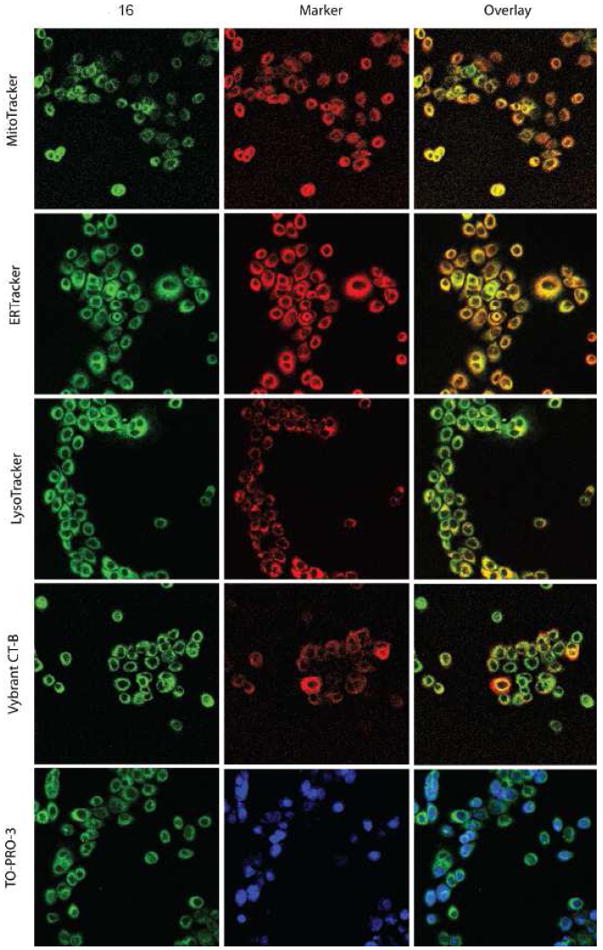
Cellular Colocalization of 16 with Subcellular Organelles. BxPC3 pancreatic cancer cells were incubated with 16 and subcellular markers, as described in the Materials and Methods, and imaged by confocal microscopy. 16 Is presented as green, organelle markers in red, and overlays in yellow.
Conclusions
Fluorescent σ2 ligands were obtained linking dansyl or NBD moieties in two different positions of compound 1 structure. High affinity σ2 ligands were obtained when the fluorescent tag was attached on the tetralin ring through an alkyl linker replacing the methyl in the methoxy function. On the other hand, the approach of attaching a fluorescent tag at the compound 1 cyclohexyl moiety -replaced by a piperidine ring- was unsuccessful, and a dramatic drop in the affinity was recorded. NBD-bearing compounds displayed better fluorescent properties (more convenient λexc and λem and high fluorescence intensity) than dansyl-bearing compounds, and among them, compound 16 displayed high σ2 receptor affinity and moderate σ1/σ2 selectivity. Therefore, compound 16 internalization was studied by flow cytometry and by confocal microscopy for colocalization with subcellular organelles in BxPC3 pancreatic tumor cells. The uptake of fluorescent ligand 16 decreased in the presence of non-fluorescent σ2 ligands showing that fluorescent and non-fluorescent compounds compete for localization in the same plane. Endocytosis inhibitors decreased the uptake of compound 16 showing that internalization occurs, in part, through endocytotic pathways besides simple membrane diffusion, and that interaction with lipid rafts, which may be able to influence the membrane composition and downstream signalling, takes place, so that σ2 receptor mediated toxicity and chemoresistance overcome likely involve lipid rafts. The influence of proliferation, with cell density as a surrogate, on σ2 agonist uptake and sensitivity was studied and showed that the uptake is higher in proliferating cells, supporting that σ2 receptors are markers of cell proliferation.37,38 Furthermore, the sensitivity of BxPC3 cells for σ2 agonists decreased together with the uptake, as the density (i.e. quiescent cells) increased, suggesting that uptake is a critical step for mediating σ2 agonists-dependent cell death. Compound 16 colocalized to the greatest extent in the endoplasmic reticulum and lysosomes, with moderate colocalization in the mitochondria and the plasma membrane, but no colocalization in the nucleus was observed in disagreement with the histone hypothesis which will have to be further analyzed.
All in all, it was demonstrated that the use of fluorescent σ2 ligands may help in clarifying the mechanisms of action of the still enigmatic σ2 receptors. The use of such compounds in different cell lines, may contribute to understand the different cell type apoptotic pathways activated by σ2 ligands. Furthermore, a scaffold which appears optimal for inferring high σ receptor affinities was herein produced, and it can be further exploited for obtaining fluorescent molecules with λexc and λem more shifted towards the Near-Infrared (NIR) region of the spectrum for a wider application of σ receptor fluorescent ligands in optical molecular imaging techniques.
Materials and Methods
Chemistry
Both column chromatography and flash column chromatography were performed with 60 Å pore size silica gel as the stationary phase (1:30 w/w, 63–200 μm particle size, from ICN and 1:15 w/w, 15-40 μm particle size, from Merck respectively). Melting points were determined in open capillaries on a Gallenkamp electrothermal apparatus. Purity of tested compounds was established by combustion analysis, confirming a purity ≥ 95%. Elemental analyses (C, H, N) were performed on an Eurovector Euro EA 3000 analyzer; the analytical results were within ± 0.4% of the theoretical values unless otherwise indicated. 1H NMR spectra were recorded on a Mercury Varian 300 MHz using CDCl3 as solvent. The following data were reported: chemical shift (δ) in ppm, multiplicity (s = singlet, d = doublet, t = triplet, m = multiplet), integration and coupling constant(s) in Hertz. Recording of mass spectra was done on an Agilent 6890-5973 MSD gas chromatograph/mass spectrometer and on an Agilent 1100 series LC-MSD trap system VL mass spectrometer; only significant m/z peaks, with their percentage of relative intensity in parentheses, are reported. Chemicals were from Aldrich and Across and were used without any further purification.
6-(1,4-dioxa-8-azaspiro[4.5]dec-8-yl)hexanenitrile (2)
A mixture of 1,4-dioxa-8-azaspiro[4.5]decane (0.72 mL, 5.6 mmol), triethylamine (0.78 mL, 5.6 mmol) and 6-bromohexanenitrile (0.74 mL, 5.6 mmol) in CH2Cl2 was stirred at room temperature. The reaction mixture was washed with H2O and the separated organic layer was concentrated under reduced pressure to give a crude mixture, which was purified by column chromatography with CH2Cl2/MeOH (95:5) as eluent, to afford the target compound as a pale yellow oil (1.16 g, 87% yield): 1H NMR δ 1.42-1.56 (m, 2H), 1.64-1.84 (m, 4H), 1.86-2.10 (m, 4H), 2.36 (t, 2H, J = 7.2 Hz), 2.68 (t, 2H, J = 8 Hz), 2.80-3.00 (m, 4H), 4.00 (s, 4H); GC-MS m/z 239 (M++1, 1), 238 (M+, 3), 156 (100).
N-[6-(1,4-dioxa-8-aza-spiro[4.5]dec-8-yl)hexyl]acetamide (3)
A solution of the nitrile 2 (1.0 g, 4.2 mmol) in anhydrous Et2O (25 mL) was added in a dropwise manner to a suspension of LiAlH4 (0.32 g, 8.4 mmol) in the same solvent kept under N2 at 0 °C. The mixture was stirred at 0 °C for 45 min and then at room temperature overnight. H2O was carefully added into the reaction pot, and the obtained mixture was filtered on Celite pad, and the filtrate evaporated under reduced pressure to give the corresponding amine as a pale yellow gummy solid (1.0 g, 99% yield); GC-MS m/z 242 (M+, 1), 156 (100). Such amine (1.0 g, 4.2 mmol) was dissolved in anhydrous CH2Cl2 (40 mL) and added with triethylamine (1.2 mL, 8.6 mmol). Acetyl chloride (0.45 ml, 6.4 mmol) was then dropped at 0 °C, under N2. The mixture was stirred at room temperature for 3 h, then treated with NaHCO3 (sat. solution 20 mL), and extracted with CH2Cl2 (3 × 20 mL). The organic layers collected were dried (Na2SO4), and concentrated under reduced pressure to give a crude mixture which was purified by column chromatography with CH2Cl2/MeOH (9:1) as eluent to yield title compound as a pale yellow oil (0.78 g, 66% yield); GC-MS m/z 284 (M+, 1), 156 (100).
N-[6-(4-oxopiperidino)hexyl]acetamide (4)
To a solution of amide 3 (0.32 g, 1.13 mmol) in acetone, 2 N HCl (37 mL) was added and the mixture was heated at reflux for 1 h followed by 1 h at room temperature. The solvent was removed under reduced pressure and conc. NaOH was added to obtain an alkaline pH. The acqueous phase was extracted with AcOEt (3 × 15 mL), the organic layers collected and dried (Na2SO4) and evaporated under reduced pressure to afford the target compound as a yellow oil (0.17 g, 63% yield) which was used for the next step without further purification; 1H NMR δ 1.42-1.84 (m, 8H), 2.10-2.68 (s+m, 13H), 2.90-3.10 (m, 2H), 5.10 (broad s, 1H); GC-MS m/z 240 (M+, 1), 112 (100).
6-[4-[4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazin-1-yl]piperidino]hexylamine (6)
The piperidine 4 (0.16 g, 0.67 mmol) and piperazine 5 (0.19 g, 0.68 mmol) were reacted with ZnCl2 (0.05 g, 0.39 mmol) and NaCNBH3 (0.044 g, 0.70 mmol) in 2-propanol (20 mL). The mixture was stirred for 48 h at room temperature. Then, the reaction mixture was evaporated to dryness and the residue was diluted with 2 N NaOH and extracted with AcOEt. The organic layers were washed with brine, dried (Na2SO4) and then concentrated under reduced pressure to give a crude residue which was purified by column chromatography with CH2Cl2/MeOH (8:2) as eluent to afford the intermediate N-acetyl derivative of compound 6 as a white solid (0.13 g, 39% yield): 1H NMR 1.20-2.00 (m, 23H), 2.20-2.40 (m, 6H), 2.45-3.00 (m, 14H), 3.20 (m, 2H), 3.80 (s, 3H), 5.45 (broad s, 1H), 6.65 (d, 1H, J = 7.7 Hz), 6.80 (d, 1H, J = 7.7 Hz), 7.08 (t, 1H, J = 7.9 Hz). LC-MS (ESI+) m/z 513 [M+H]+, 535 [M+Na]+. Such intermediate acetamide (0.22 g, 0.44 mmol) was refluxed in 3N HCl (6.5 mL) for 4 h. After cooling, the mixture was made alkaline with K2CO3 (sat. solution, 10 mL) and extracted with CH2Cl2 (3 × 10 mL). The collected organic layers were dried (Na2SO4), and the solvent was evaporated to produce a brown semisolid (0.20 g, 99% yield) which was used for the next step without any further purification. 1H NMR 1.20-2.00 (m, 20H), 2.20-2.80 (m, 19H), 2.85-3.05 (m, 3H), 3.80 (s, 3H), 5.45 (broad s, 2H, D2O exchanged), 6.65 (d, 1H, J = 7.7 Hz), 6.80 (d, 1H, J = 7.7 Hz), 7.08 (t, 1H, J = 7.9 Hz). LC-MS (ESI+) m/z 471 [M+H]+.
2-(6-[5-[3-(4-cyclohexylpiperazin-1-yl)propyl]-5,6,7,8-tetrahydronaphthalen-1-yloxy]hexyl)isoindole-1,3-dione (11)
A grain of NaI, K2CO3 (0.14 g, 1.0 mmol) and phthalimide 9 (0.29 g, 1.0 mmol) were added to a solution of phenol 10 (0.26 g, 0.74 mmol) in DMF (5 mL), and the reaction mixture was heated at 100 °C for 18 h. After cooling, the solvent was removed under reduced pressure, then H2O (5 mL) was added to the residue and the mixture was extracted with AcOEt (3 × 10 mL). The crude was purified by flash chromatography with ethyl acetate/CH2Cl2 (6:4) as eluent to give compound 11 as a yellow oil (0.094g, 16% yield); 1H NMR δ 1.21-1.97 (m, 26H), 2.10-2.95 (m, 14H), 3.69 (t, 2H, J = 7.2 Hz), 3.90 (m, 2H), 6.57-7.07 (m, 3H), 7.60-7.86 (m, 4H). LC-MS (ESI+) m/z 586 [M+H]+, 608 [M+Na]+.
2-[2-[5-[3-(4-Cyclohexylpiperazin-1-yl)propyl]-5,6,7,8-tetrahydronaphthalen-1-yloxy]ethoxy]ethanol (12)
To a solution of phenol 10 (0.14 g, 0.4 mmol) in DMF (5 mL), a grain of NaI, K2CO3 (0.06 g, 0.5 mmol) and 2-(2-chloroethoxy)ethanol (0.05 mL, 0.5 mmol) were added, and the reaction mixture was heated at 120 °C for 18 h. After cooling, the solvent was evaporated under reduced pressure, and then water was added to the residue. The mixture was extracted with AcOEt (3 × 5 mL), and the collected organic layers collected were dried (Na2SO4) and evaporated to afford a crude which was purified by flash chromathography with AcOEt/CH2Cl2 (7:3) as eluent to give the target compound 12 as a yellow oil (0.11 g, 62% yield); 1H NMR δ 1.00-1.40 (m, 6H), 1.40-2.00 (m, 12H), 2.40-3.20 (m, 15H), 3.65-3.80 (m, 4H), 3.86-3.90 (m, 2H), 4.06-4.12 (m, 2H), 6.65-7.05 (m, 3H). GC-MS m/z 445 (M++1, 5), 444 (M+, 22), 181 (100); LC-MS (ESI+) m/z 445 [M+H]+, 467 [M+Na]+.
2-[2-[2-[5-[3-(4-Cyclohexylpiperazin-1-yl)propyl]-5,6,7,8-tetrahydronaphthalen-1-yloxy]ethoxy)]ethyl]isoindole-1,3-dione (13)
To a stirred solution of alcohol 12 (0.12 g, 0.27 mmol) in dry THF (10 mL) kept under N2, triphenylphosphine (0.12 g, 0.46 mmol), phthalimide (0.069 g, 0.47 mmol) and DIAD (0.12 ml, 0.60 mmol) were added and the mixture was stirred at room temperature for 18 h. Then the solvent was evaporated under reduced pressure, the resulting residue was treated with H2O and the aqueous layer was extracted with AcOEt (3 × 20 mL). The combined organic layers were dried (Na2SO4) and concentrated under reduced pressure to give a crude residue which was purified by column chromatography using CH2Cl2/MeOH (95:5) as eluent, to afford the target compound as a yellow oil (0.10 g, 65% yield); LC-MS (ESI+) m/z 574 [M+H]+, 596 [M+Na]+.
General Procedure for the Synthesis of 6-[1-[3-(4-cyclohexylpiperazin-1-yl)-propyl]-1,2,3,4-tetrahydronaphthalen-5-yloxy]hexylamine (14) and 2-[2-[1-[3-(4-cyclohexylpiperazin-1-yl)propyl]-1,2,3,4-tetrahydronaphthalen-5-yloxy]ethoxy]ethylamine (15)
Hydrazine hydrate 50% (0.085 mL, 0.85 mmol) was added to a solution of either 11 or 13 (0.29 mmol) in methanol (3 mL), and the reaction mixture was stirred at room temperature for 30 min 1.5 N HCl (1.2 mL) was then added and the mixture was stirred for further 12 h. Then 3 N HCl was added until a pH < 2 was obtained, and the mixture was heated at reflux for 30 min After cooling down to room temperature, the mixture was filtered, the solid residue was washed with cold MeOH and with Et2O and dried under vacuum. The white solid obtained was made free base with alkaline tratment to afford the target compound as a pale yellow oil (70% yield).
6-[1-[3-(4-Cyclohexylpiperazin-1-yl)propyl]-1,2,3,4-tetrahydronaphthalen-5-yloxy]hexylamine (14)
1H NMR δ 1.00-1.30 (m, 5H), 1.40-2.00 (m, 23H), 2.20-2.80 (m, 16H), 3.90 (t, 2H, J = 6.0 Hz), 6.60 (d, 1H, J = 7.9 Hz), 6.75 (d, 1H, J = 7.7 Hz), 7.05 (t, 1H, J = 7.9 Hz); LC-MS (ESI+) m/z 456 [M+H]+.
2-[2-[1-[3-(4-Cyclohexylpiperazin-1-yl)propyl]-1,2,3,4-tetrahydronaphthalen-5-yloxy]ethoxy]ethylamine (15)
1H NMR δ 1.05-1.50 (m, 6H), 1.60-2.10 (m, 12H), 2.40-3.20 (m, 18H), 3.65-3.90 (m, 6H), 6.65-7.05 (m, 3H). LC-MS (ESI+) m/z 444 [M+H]+, 466 [M+Na]+.
General Procedure for the Synthesis of Final Compounds 7, 16, 17
4-Chloro-7-nitro-2,1,3-benzoxadiazole (NBD-Cl, 1.0 mmol) was dissolved in absolute EtOH (15 mL) and added in a dropwise manner to one among amines 6, 14 or 15 (1.0 mmol) dissolved in the same solvent (15 mL). The mixture was stirred for 1.5 h at room temperature. Then, the reaction mixture was filtered and the filtrate evaporated under reduced pressure.
6-[4-[4-[3-(5-Methoxy-1,2,3,4-tetrahydronaphthalen-1-yl]propyl]piperazin-1-yl]piperidino]-N-(7-nitro-2,1,3-benzoxadiazol-4-yl)hexanamine (7)
The crude semisolid was purified by column chromatography with AcOEt/MeOH (7:3) as eluent to give the final compound 7 as an orange semisolid (0.26 g, 42% yield); 1H NMR 1.20-2.20 (m, 21H), 2.25-2.35 (m, 8H), 2.45-2.80 (m, 11H), 3.00-3.10 (m, 2H), 3.20-3.40 (broad s, 1H), 3.80 (s, 3H), 6.18 (d, 1H, J = 8.5 Hz), 6.60 (d, 1H, J = 7.7 Hz), 6.80 (d, 1H, J = 7.7 Hz), 7.05 (t, 1H, J = 7.9 Hz), 8.50 (d, 1H, J = 8.5 Hz); LC-MS (ESI+) m/z 634 [M+H]+, 656 [M+Na]+. Anal. (C35H51N7O4·3.8HCl) C, H, N.
6-[5-[3-(4-Cyclohexylpiperazin-1-yl)propyl]-5,6,7,8-tetrahydronaphthalen-5-yloxy]-N-(7-nitro-2,1,3-benzoxadiazol-4-yl)hexanamine (16)
The crude semisolid was purified by column chromatography using CH2Cl2/MeOH (99:1) as eluent to give the target compound 16 as a brown oil (0.37 g, 60% yield); 1H NMR 1.00-1.40 (m, 10H), 1.50-2.00 (m, 16H), 2.18-2.80 (m, 13H), 3.45-3.55 (m, 3H), 3.94 (t, 2H, J = 6.0 Hz), 6.17 (d, 1H, J = 8.5 Hz), 6.20-6.30 (broad s, 1H, D2O exchanged), 6.60 (d, 1H, J = 7.7 Hz), 6.78 (d, 1H, J = 7.7 Hz), 7.05 (t, 1H, J = 7.9 Hz), 8.50 (d, 1H, J = 8.5 Hz). LC-MS (ESI-) m/z 617 [M-H]−. Anal. (C35H50N6O4·3HCl·5/4H2O) C, H, N.
2-[2-[5-[3-(4-Cyclohexylpiperazin-1-yl)propyl]-5,6,7,8-tetrahydronaphthalen-1-yloxy]ethoxy]-N-(7-nitro-2,1,3-benzoxadiazol-4-yl)ethanamine (17)
The crude semisolid was purified by column chromatography using CH2Cl2/MeOH (99:1) as eluent to give the final compound 17 as a brown oil (0.37 g, 62% yield); 1H NMR 1.40-2.20 (m, 18H), 2.25-2.80 (m, 14H), 3.50-3.75 (m, 3H), 3.85-4.00 (m, 4H), 4.08-4.15 (m, 2H), 6.17 (d, 1H, J = 8.5 Hz), 6.60 (d, 1H, J = 7.7 Hz), 6.80 (d, 1H, J = 7.7 Hz), 7.05 (t, 1H, J = 7.9 Hz), 8.45 (d, 1H, J = 8.5 Hz). LC-MS (ESI+) m/z 607 [M + H]+. Anal. (C33H46N6O5·3HCl) C, H, N.
General Procedure for the Synthesis of Final Compounds 8, 18, 19
A solution of 5-(dimethylao)naphathalene-1-sulfonyl chloride (dansyl chloride) (0.21 g, 0.8 mmol) in anhydrous CH2Cl2 (15 mL) was added in a dropwise manner to one among amines 6, 14 or 15 (1.0 mmol) dissolved in the same solvent (15 mL). The mixture was stirred at room temperature for 18 h. Then, the reaction mixture was washed with H2O (2 × 30 mL) and the organic phases were collected, dried (Na2SO4) and evaporated under reduced pressure to afford a crude residue which was purified as described below.
5-Dimethylaonaphthalene-1-sulfonic acid 6-[4-[4-[3-(5-methoxy-1,2,3,4-tetrahydronaphthalen-1-yl)propyl]piperazin-1-yl]piperidino]hexanamide (8)
The crude semisolid was purified by column chromatography using CHCl3/MeOH (9:1) as eluent to give the target compound as a green oil (0.29 g, 42% yield); 1H NMR 1.04-1.45 (m, 8H), 1.50-2.10 (m, 12H), 2.20-2.40 (m, 6H), 2.45-2.80 (m, 11H), 2.92-3.02 (m, 11H), 3.80 (s, 3H), 4.90-5.00 (m, 1H D2O exchanged), 6.63 (d, 1H, J = 7.7 Hz), 6.78 (d, 1H, J = 7.7 Hz), 7.08 (t, 1H, J = 7.7 Hz), 7.18 (d, 1H, J = 7.4 Hz), 7.50-7.60 (m, 2H), 8.22 (d, 1H, J = 7.4Hz), 8.30 (d, 1H, J = 7.4 Hz), 8.55 (d, 1H, J = 7.4 Hz); LC-MS (ESI+) m/z 704 [M+H]+. Anal. (C41H61N5O3S·4HCl·2H2O) C, H, N.
5-Dimethylaonaphthalene-1-sulfonic acid 6-[5-[3-(4-cyclohexylpiperazin-1-yl)propyl]-5,6,7,8-tetrahydronaphthalen-1-yloxy]hexanamide (18)
The crude semisolid was purified by column chromatography using CH2Cl2/MeOH (97:3) as eluent to give the target compound 18 as a pale green oil (0.33 g, 48% yield). 1H NMR 1.00-1.90 (m, 24H), 2.00-2.20 (m, 2H), 2.40-3.10 (m, 22H), 3.80 (t, 2H, J = 6 Hz), 4.70-4.80 (m, 1H, D2O exchanged), 6.55 (d, 1H, J = 7.9 Hz), 6.73 (d, 1H, J = 7.7 Hz), 7.00-7.05 (m, 1H), 7.20 (d, 1H, J = 7.9 Hz), 7.50-7.60 (m, 2H), 8.20-8.40 (m, 2H), 8.51 (d, 1H, J = 8.5 Hz); LC-MS (ESI+) m/z 689 [M + H]+. Anal. (C41H60N4O3S·3HCl·3/2H2O) C, H, N.
5-Dimethylaonaphthalene-1-sulfonic acid 2-[2-[5-[3-(4-cyclohexylpiperazin-1-yl)propyl]-5,6,7,8-tetrahydronaphthalen-5-yloxy]ethoxy]ethanamide (19)
The crude semisolid was purified by column chromatography using CH2Cl2/MeOH (98:2) as eluent to give the target compound as a pale green oil (0.47 g, 70% yield); 1H NMR 1.00-2.30 (m, 18H), 2.50-3.40 (m, 22H), 3.45 (t, 2H, J = 5 Hz), 3.55 (t, 2H, J = 6 Hz), 3.90 (t, 2H, J = 6 Hz), 5.20-5.30 (m, 1H, D2O exchanged), 6.55 (d, 1H, J = 7.9 Hz), 6.76 (d, 1H, J = 7.7 Hz), 7.00-7.10 (m, 1H), 7.14 (d, 1H, J = 7.9 Hz), 7.40-7.54 (m, 2H), 8.20-8.26 (m, 2H), 8.51 (d, 1H, J = 8.5 Hz); LC-MS (ESI+) m/z 677 [M + H]+. Anal. (C39H56N4O4S·3HCl) C, H, N.
Fluorescence Spectroscopy and Molar Extinction Coefficient
Emission spectra of compounds 7, 8, 16-19 were determined in EtOH, CHCl3 and in PBS buffer solution. In all experiments the excitation and the emission bandpass was set at 10 nm. The emission spectra were obtained from 300 to 700 nm with excitation set at the appropriate excitation wavelength. The excitation spectra of compounds 8, 18, 19 were obtained from 250 to 450 nm with the emission being recorded at the appropriate wavelenght. The excitation spectra of compounds 7, 16, 17 were obtained from 300 to 550 nm with the emission being recorded at the appropriate wavelenght. Fluorescence quantum yields were calculated with respect to quinine sulfate (Fluka) in 0.5 M H2SO4 as a standard (Φ = 0.546).41 Solutions of both the sample and the reference were prepared from original solutions diluted with the appropriate solvent so that absorbance was below 0.2 at the same excitation wavelength (347 nm). Fluorescence measurements were carried out for each solution with the same instrument parameters, and the fluorescence spectra were corrected for instrumental response before integration. The quantum yield for each sample was calculated according the following equation:42
where Φ is the emission quantum yield, A is the absorbance at the excitation wavelength, F is the area under the corrected emission curve, n is the refractive index of the solvent for the sample (X) and the standard (S). Absorption spectra were recorded with a PerkinElmer UV-Vis-NIR spectrophotometer, fluorescence spectra were obtained with a PerkinElmer LS55 spectrofluorometer. Molar extinction coefficients (ε) were determined for each final compound (7, 8, 16-19) dissolved in EtOH with concentration ranging from 1 μM to 100 μM and absorbance spectra recorded from 200 nm to 600 nm in standard quartz cuvettes. ε Values were determined by fitting the Beer’s law: A = ε × c × d where (A) is the absorbance at the λexc; (c) is the molar concentration of the solution, and (d) was the optical path length (d = 1 cm). Measurements were repeated twice.
Biological Methods and Materials: Radioligand Binding Assays
All the procedures for the binding assays were previously described. σ1 And σ2 receptor binding were carried out according to Matsumoto et al.43 [3H]-DTG (30 Ci/mmol) and (+)-[3H]-pentazocine (34 Ci/mmol) were purchased from PerkinElmer Life Sciences (Zavantem, Belgium). DTG was purchased from Tocris Cookson Ltd., U.K. (+)-Pentazocine was obtained from Sigma-Aldrich-RBI s.r.l. (Milan, Italy). Male Dunkin guinea-pigs and Wistar Hannover rats (250-300 g) were from Harlan, Italy. The specific radioligands and tissue sources were respectively: (a) σ1 receptor, (+)-[3H]-pentazocine (+)-[2S-(2α,6α,11R)]-1,2,3,4,5,6-hexahydro-6,11-dimethyl-3-(3-methyl-2-butenyl)-2,6-methano-3-benzazocine-8-ol), guinea-pig brain membranes without cerebellum; (b) σ2 receptor, [3H]DTG in the presence of 1 μM (+)-pentazocine to mask σ1 receptors, rat liver membranes. The following compounds were used to define the specific binding reported in parentheses: (a) (+)-pentazocine (73-87%), (b) DTG (85-96%). Concentrations required to inhibit 50% of radioligand specific binding (IC50) were determined by using six to nine different concentrations of the drug studied in two or three experiments with samples in duplicate. Scatchard parameters (Kd and Bmax) and apparent inhibition constants (Ki) values were determined by nonlinear curve fitting, using the Prism, version 3.0, GraphPad software.44
Cell Culture
BxPC3 pancreatic cancer cells were maintained in Roswell Park Memorial Institute (RPMI) media (GIBCO) supplemented with L-glutamine (2 mM), 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES) (1 mM), pyruvate (1 mM), sodium bicarbonate (0.075% w/v), penicillin and streptomycin (100 IU/mL), amphotericin (0.25 μg/mL), and 10% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA). Cells were seeded at a density of 2 × 105/mL unless otherwise stated and maintained in a humidified atmosphere of 5% CO2 at 37°C.
Confocal Microscopy
For sub-cellular compartmentalization, cells grown on glass cover slips were incubated with compound 16 (100 nM) and either ERTracker Red (1 μM), MitoTracker Red (100 nM), or LysoTracker Red (50 nM) for 30 min at 37 °C. The plasma membrane was visualized using the Vybrant Alexa Fluor 594 Lipid Raft Labeling Kit as directed by the manufacturer. All reagents were obtained from Molecular Probes. Cells were washed with PBS and fixed in 2% paraformaldehyde for 30 min at 37 °C prior to additional washing and mounting to a slide with ProLong Gold antifade reagent. Imaging was performed on a Carl Zeiss Axiovert 100 inverted microscope, fitted with LSM 510 laser scanning microscope camera and software. Images were collected with filter bandwidths corresponding to 505–530 nm for green, 560–615 nm for red, and > 650 nm for far red, with 4 scans over 11.8 sec.
Internalization of Compound 16 by Flow Cytometry
To quantify internalization of compound 16, cells were pretreated with the endocytosis inhibitors phenylarsine oxide (10 μM) or Filipin III (5 μg/mL) for 60 min at 37 °C prior to washing and resuspension in cell media. Compound 16 (25 nM) was added and the mean fluorescence (FL1) quantified with a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA) with kinetic readings over a time period of 60 min. To detect competition of compound 16 with parent and analogous compounds, BxPC3 cells were treated with compounds 1 or 20 at increasing concentrations for 45 min prior to replacement with compound 16 (25 nM) for 45 min at 37 °C and fluorescence intensity quantified by flow cytometry. To detere the influence of cell proliferation by increasing the cell seeding density, BxPC3 cells were seeded at increasing concentrations and the following day, cells were treated with compound 16 (25 nM) for 30 min at 37 °C and fluorescence intensity quantified by flow cytometry.
Cell Viability
Sub-cultured BxPC3 cells were seeded at increasing densities from 1 × 105 to 9 × 105 into 96 well clear bottom plates 24 h prior to treatment with compounds 1 or 20 (100 μM). Eighteen h later cells were washed with PBS, fixed with 4% paraformaldehyde for 30 min at 37°C, and stained with crystal violet for 15 min at 37 °C. Cells were then washed with PBS and cell density detected with a Bio-Rad Laboratories ChemiDoc XRS+ Imager and quantified with Quantity One software. Viability is represented as the percent density of σ2 agonist treated cells compared to those treated with DMSO vehicle.
Supplementary Material
Acknowledgments
This project was partially financed by Veterans Adistration Merit Award (Project 1136919) (WGH), American Cancer Society (Project MRSG08019-01CDD) (WGH), National Institute of Health T32 Training Grant (Project 5T32CA009621-22) (JRH).
Abbreviations
- NBD
7-nitro-1,2,3-benzoxadiazole
- ER
endoplasmic reticulum
- DIAD
diisopropylazodicarboxylate
- SAfiR
Structure–Affinity Relationship
- PBS
phosphate buffered saline
- PAO
phenylarsine oxide
- UV
Ultra-Violet
- NIR
Near-Infrared
- DMSO
dimethyl sulfoxide
Footnotes
Supporting Information Available: Elemental analyses of the novel end products; Formulas, melting points of hydrochloride salts; fluorescence microscopy images taken with compound 16 and subcellular organelles trackers. This material is available free of charge via the Internet at http://pubs.acs.org.
References
- 1.Quirion R, Bowen WD, Itzhak Y, Junien JL, Musachio JM, Rothman RB, Su T-P, Tam SW, Taylor DP. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci. 1992;13:85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- 2.Hanner M, Moebius FF, Flandorfer A, Knaus HG, Striessnig JM, Kempner E, Glossmann H. Purification, molecular cloning, and expression of the mammalian sigma1-binding site. Proc Natl Acad Sci U S A. 1996;93:8072–8077. doi: 10.1073/pnas.93.15.8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tsai S-Y, Hayashi T, Mori T, Su T-P. Sigma-1 receptor chaperones and diseases. Cent Nerv Syst Agents Med Chem. 2009;9:184–189. doi: 10.2174/1871524910909030184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cobos EJ, Entrena JM, Nieto FR, Cendan CM, Del Pozo E. Pharmacology and therapeutic potential of sigma1 receptor ligands. Curr Neuropharmacol. 2008;6:344–366. doi: 10.2174/157015908787386113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Skuza G. Potential antidepressant activity of sigma ligands. Pol J Pharm. 2003;55:923–934. [PubMed] [Google Scholar]
- 6.Skuza G, Rogoz Z. Effect of BD1047, a sigma1 receptor antagonist, in the animal models predictive of antipsychotic activity. Pharmacol Rep. 2006;58:626–635. [PubMed] [Google Scholar]
- 7.Jansen KL, Faull RL, Storey P, Leslie RA. Loss of sigma binding sites in the CA1 area of the anterior hippocampus in Alzheimer’s disease correlates with CA1 pyramidal cell loss. Brain Res. 1993;623:299–302. doi: 10.1016/0006-8993(93)91441-t. [DOI] [PubMed] [Google Scholar]
- 8.Mishina M, Ishiwata K, Ishii K, Kitamura S, Kimura Y, Kawamura K, Oda K, Sasaki T, Sakayori O, Hamamoto M, Kobayashi S, Katayama Y. Function of sigma1 receptors in Parkinson’s disease. Acta Neurol Scand. 2005;112:103–107. doi: 10.1111/j.1600-0404.2005.00432.x. [DOI] [PubMed] [Google Scholar]
- 9.Mishina M, Ohyama M, Ishii K, Kitamura S, Kimura Y, Oda K, Kawamura K, Sasaki T, Kobayashi S, Katayama Y, Ishiwata K. Low density of sigma1 receptors in early Alzheimer’s disease. Ann Nucl Med. 2008;22:151–56. doi: 10.1007/s12149-007-0094-z. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto RR, Gilmore DL, Pouw B, Bowen WD, Williams W, Kausar A, Coop A. Novel analogs of the σ receptor ligand BD1008 attenuate cocaine induced toxicity in mice. Eur J Pharmacol. 2004;492:21–26. doi: 10.1016/j.ejphar.2004.03.037. [DOI] [PubMed] [Google Scholar]
- 11.Kashiwagi H, McDunn JE, Simon PO, Goedegebuure PS, Xu J, Jones L, Chang K, Johnston F, Trinkaus K, Hotchkiss RS, Mach RH, Hawkins WG. Selective sigma-2 ligands preferentially bind to pancreatic adenocarcinomas: applications in diagnostic imaging and therapy. Mol Cancer. 2007;6:48. doi: 10.1186/1476-4598-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Crawford KW, Bowen WD. Sigma-2 receptor agonists activate a novel apoptotic pathway and potentiate antineoplastic drugs in breast tumor cell lines. Cancer Res. 2002;62:313–322. [PubMed] [Google Scholar]
- 13.Ostenfeld MS, Fehrenbacher N, Hover-Hansen M, Thomsen C, Farkas T, Jaattela M. Effective tumor cell death by sigma-2 receptor ligand siramesine involves lysosomal leakage and oxidative stress. Cancer Res. 2005;65:8975–8983. doi: 10.1158/0008-5472.CAN-05-0269. [DOI] [PubMed] [Google Scholar]
- 14.van Waarde A, Rybczynska AA, Ramakrishnan N, Ishiwata K, Elsinga PH, Dierckx RA. Sigma receptors in oncology: therapeutic and diagnostic applications of sigma ligands. Curr Pharm Des. 2010;16:3519–3537. doi: 10.2174/138161210793563365. [DOI] [PubMed] [Google Scholar]
- 15.Megalizzi V, Le Mercier M, Decaestecker C. Sigma receptors and their ligands in cancer biology: overview and new perspectives for cancer therapy. Med Res Rev. 2011;31 doi: 10.1002/med.20218. n/a. [DOI] [PubMed] [Google Scholar]
- 16.Phase I Clinical Trial. ClinicalTrials.gov Identifier: NCT00968656. Assessment of Cellular Proliferation in Tumors by Positron Emission Tomography (PET) Using [18F]ISO-1 (FISO PET/CT) [Google Scholar]
- 17.Colabufo NA, Berardi F, Abate C, Contino M, Niso M, Perrone R. Is the σ2 receptor a histone binding protein? J Med Chem. 2006;49:4153–4158. doi: 10.1021/jm0600592. [DOI] [PubMed] [Google Scholar]
- 18.Berardi F, Abate C, Ferorelli S, Colabufo NA, Perrone R. 1-Cyclohexylpiperazine and 3,3-dimethylpiperidine derivatives as sigma-1 (σ1) and sigma-2 (σ2) receptor ligands: a review. Cent Nerv Syst Agents Med Chem. 2009;9:205–219. doi: 10.2174/1871524910909030205. [DOI] [PubMed] [Google Scholar]
- 19.Berardi F, Ferorelli S, Abate C, Colabufo NA, Contino M, Perrone R, Tortorella V. 4-(Tetralin-1-yl)- and 4-(naphthalen-1-yl)alkyl derivatives of 1-cyclohexylpiperazine as σ receptor ligands with agonist σ2 activity. J Med Chem. 2004;47:2308–2317. doi: 10.1021/jm031026e. [DOI] [PubMed] [Google Scholar]
- 20.Abate C, Elenewski J, Niso M, Berardi F, Colabufo NA, Azzariti A, Perrone R, Glennon RA. Interaction of the σ2 receptor ligand PB28 with the human nucleosome: computational and experimental probes of interaction with the H2A/H2B dimer. Chem Med Chem. 2010;5:268–273. doi: 10.1002/cmdc.200900402. [DOI] [PubMed] [Google Scholar]
- 21.Zeng C, Vangveravong S, Xu J, Chang KC, Hotchkiss RS, Wheeler KT, Shen D, Zhuang Z-P, Kung HF, Mach RH. Subcellular localization of sigma-2 receptors in breast cancer cells using two-photon and confocal microscopy. Cancer Res. 2007;67:6708–6716. doi: 10.1158/0008-5472.CAN-06-3803. [DOI] [PubMed] [Google Scholar]
- 22.Zeng C, Vangveravong S, Jones LA, Hyrc K, Chang KC, Xu J, Rothfuss JM, Goldberg MP, Hotchkiss RS, Mach RH. Characterization and evaluation of two novel fluorescent sigma-2 receptor ligands as proliferation probes. Molecular Imaging. 2011:1–14. [PMC free article] [PubMed] [Google Scholar]
- 23.Azzariti A, Colabufo NA, Berardi F, Porcelli L, Niso M, Simone MG, Perrone R, Paradiso A. Cyclohexylpiperazine derivative PB28, a σ2 agonist and σ1 antagonist receptor inhibits cell growth, modulates P-glycoprotein, and synergizes with anthracyclines in breast cancer. Mol Cancer Ther. 2006;5:1807–1816. doi: 10.1158/1535-7163.MCT-05-0402. [DOI] [PubMed] [Google Scholar]
- 24.Ferorelli S, Abate C, Colabufo NA, Niso M, Inglese C, Berardi F, Perrone R. Design and evaluation of naphthol- and carbazole-containing fluorescent sigma ligands as potential probes for receptor binding studies. J Med Chem. 2007;50:4648–4655. doi: 10.1021/jm070373b. [DOI] [PubMed] [Google Scholar]
- 25.Berardi F, Colabufo NA, Giudice G, Perrone R, Tortorella V, Govoni S, Lucchi L. New σ and 5-HT1A receptor ligands: ω-(tetralin-1-yl)-n-alkylamine derivatives. J Med Chem. 1996;39:176–182. doi: 10.1021/jm950409c. [DOI] [PubMed] [Google Scholar]
- 26.Elslager EF, Moore AM, Short FW, Sullivan JM, Tendick FH. Synthetic amebicides. II. 7-Dialkylaminoalkylaobenz[c]acridines and other 7-aminobenz[c]acridines. JACS. 1957;79:4699–4703. [Google Scholar]
- 27.Perrone R, Berardi F, Colabufo NA, Leopoldo M, Abate C, Tortorella V. N-Aryl- orN-alkylpiperazine derivatives: the role of N-substituent on σ1, σ2, 5-HT1A and D2 receptor affinity. Med Chem Res. 2000;10:201–207. [Google Scholar]
- 28.Abate C, Mosier PD, Berardi F, Glennon RA. A structure-affinity and comparative molecular field analysis of sigma-2 (σ2) receptor ligands. Cent Nerv Syst Agents Med Chem. 2009;9:246–257. doi: 10.2174/1871524910909030246. [DOI] [PubMed] [Google Scholar]
- 29.Schnitzer JE, Oh P, Pinney E, Allard J. Filipin-sensitive caveolae-mediated transport in endothelium: reduced transcytosis, scavenger endocytosis, and capillary permeability of select macromolecules. J Cell Biol. 1994;127:1217–1232. doi: 10.1083/jcb.127.5.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gibson AE, Noel RJ, Herlihy JT, Ward WF. Phenylarsine oxide inhibition of endocytosis: effects on asialofetuin internalization. Am J Physiol. 1989;257:182–184. doi: 10.1152/ajpcell.1989.257.2.C182. [DOI] [PubMed] [Google Scholar]
- 31.Gebreselassie D, Bowen WD. Sigma-2 receptors are specifically localized to lipid rafts in rat liver membranes. Eur J Pharmacol. 2004;493:19–28. doi: 10.1016/j.ejphar.2004.04.005. [DOI] [PubMed] [Google Scholar]
- 32.Torrence-Campbell C, Bowen WD. Differential solubilization of rat liver sigma-1 and sigma-2 receptors: retention of sigma-2 sites in particulate fractions. Eur J Pharmacol. 1996;304:201–210. doi: 10.1016/0014-2999(96)00109-4. [DOI] [PubMed] [Google Scholar]
- 33.Lajoie P, Nabi IR. Lipid rafts, caveolae, and their endocytosis. Int Rev Cell Mol Biol. 2010;282:135–163. doi: 10.1016/S1937-6448(10)82003-9. [DOI] [PubMed] [Google Scholar]
- 34.Patra SK. Dissecting lipid raft facilitated cell signaling pathways in cancer. Biochim Biophys Acta. 2008;1785:182–206. doi: 10.1016/j.bbcan.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 35.Kashiwagi H, McDunn JE, Simon PO, Goedegebuure PS, Vangveravong S, Chang K, Hotchkiss RS, Mach RH, Hawkins WG. Sigma-2 receptor ligands potentiate chemotherapies and improve survival in models of pancreatic adenocarcinoma. J Transl Med. 2009;7:24. doi: 10.1186/1479-5876-7-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abate C, Niso M, Contino M, Colabufo NA, Ferorelli S, Perrone R, Berardi F. 1-Cyclohexyl-4-(4-arylcyclohexyl)piperazines: mixed σ and human Δ8–Δ7 Sterol Isomerase ligands with antiproliferative and P-Glycoprotein inhibitory activity. Chem Med Chem. 2011;6:73–80. doi: 10.1002/cmdc.201000371. [DOI] [PubMed] [Google Scholar]
- 37.Wheeler KT, Wang LM, Wallen CA, Childers SR, Cline JM, Keng PC, Mach RH. Sigma-2 receptors as a biomarker of proliferation in solid tumours. Br J Cancer. 2000;82:1223–1232. doi: 10.1054/bjoc.1999.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Al-Nabulsi I, Mach RH, Wang LM, Wallen CA, Keng PC, Sten K, Childers SR, Wheeler KT. Effect of ploidy, recruitment, environmental factors, and tamoxifen treatment on the expression of sigma-2 receptors in proliferating and quiescent tumour cells. Br J Cancer. 1999;81:925–933. doi: 10.1038/sj.bjc.6690789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hornick JR, Xu J, Vangveravong S, Tu Z, Mitchem JB, Spitzer D, Goedegebuure P, Mach RH, Hawkins WG. The novel sigma-2 receptor ligand SW43 stabilizes pancreas cancer progression in combination with gemcitabine. Mol Cancer. 2010;9:298. doi: 10.1186/1476-4598-9-298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hou C, Tu Z, Mach R, Kung HF, Kung MP. Characterization of a novel iodinated sigma-2 receptor ligand as a cell proliferation marker. Nucl Med Biol. 2006;33:203–209. doi: 10.1016/j.nucmedbio.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 41.Meech SR, Phillips D. Photophysics of some common fluorescence standards. J Photochem. 1983;23:193–217. [Google Scholar]
- 42.Demas JN, Crosby GA. Measurement of photoluescence quantum yields. A review. J Phys Chem. 1971;75:991–1024. [Google Scholar]
- 43.Matsumoto RR, Bowen WD, Tom MA, Truong DD, De Costa BR. Characterization of two novel sigma receptor ligands: antidystonic effects in rats suggest sigma receptor antagonism. Eur J Pharmacol. 1995;280:301–310. doi: 10.1016/0014-2999(95)00208-3. [DOI] [PubMed] [Google Scholar]
- 44.Prism Software, version 3.0 for Windows. GraphPad Software, Inc.; San Diego, CA: 1998. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


