Abstract
Protein phosphatase-2A (PP2A) activity, which is compromised in Alzheimer disease brain, is regulated by two endogenous inhibitors, one of them being I2PP2A, a 277 amino acid long protein also known as SET. Here we report that both the amino terminal fragment (I2NTF; aa 1–175) and the carboxy terminal fragment (I2CTF; aa 176–277) of I2PP2A inhibit PP2A by binding to its catalytic subunit PP2Ac and cause hyperphosphorylation of tau. The C-terminal acidic region in I2CTF and Val 92 in I2NTF are essential for their association with PP2Ac and inhibition of the phosphatase activity.
1. Introduction
Abnormal hyperphosphorylation and aggregation of microtubule associated protein tau into paired helical filaments/neurofibrillary tangles is a hallmark of Alzheimer disease (AD) and related neurodegenerative disorders, called tauopathies [1–3]. The hyperphosphorylated tau sequesters normal tau, MAP1 and MAP2, which results in the breakdown of the microtubule network and, probably in a progressive retrograde degeneration of the affected neurons [4].
Protein phosphatase 2A (PP2A) is a heterotrimeric enzyme, consisting of one catalytic C subunit, one scaffolding A subunit, and one of several structurally distinct, regulatory B subunits [5]. The phosphorylation of tau that suppresses its microtubule binding and assembly activities in adult mammalian brain is primarily regulated by PP2A [6]. PP2A regulates tau phosphorylation, both directly as tau phosphatase and indirectly by regulating the activities of several tau kinases which include CaM Kinase II, PKA, MAP kinase kinase (MEK 1/2), extracellular regulated kinase (ERK 1/2), GSK-3β, and P70S6 kinase [7]. PP2A activity in AD brain is decreased [8].
The activity of PP2A is regulated by an inhibitor protein, I2PP2A [9–11]; This protein, also known as SETα, TAF-1β, and PHAPII protein [12–14] was discovered as a translocated gene fused to the CAN gene in a patient with acute undifferentiated leukemia [14]. I2PP2A is a 277 amino acid long polypeptide with an apparent molecular weight of 39 kDa in SDS-PAGE. This protein is widely expressed in various tissues and localizes primarily in the nucleus [12,15,16] where it has been reported to block DNase activity and acetylation of histones [17]. Previously, we showed that I2PP2A is selectively cleaved at N175 into I2NTF (N-terminal fragment) and I2CTF (C-terminal fragment) and translocated from the neuronal nucleus to the cytoplasm, co-localized with neurofibrillary tangles in the affected areas of AD brain [18]. However, the exact molecular mechanism of the inhibition of PP2A activity and generation of abnormal hyperphosphorylation of tau by I2PP2A in the cell was not understood. The present study shows that I2PP2A inhibits PP2A activity dose-dependently and cleavage of this inhibitor into I2NTF and I2CTF not only results in its translocation from the cell nucleus to the cytoplasm but also potentiates its inhibitory activity, for each of these two fragments can interact with the PP2A catalytic subunit PP2Ac. I2CTF interacts with PP2Ac through its highly acidic C-terminal domain. Interaction of I2NTF with PP2Ac and its ability to inhibit the phosphatase activity is completely inhibited when valine 92 in the inhibitor is mutated to alanine.
2. Materials and Methods
For generation of I2PP2A and its deletion mutant plasmids, GST pull-down assays, coimmunoprecipitation, PP2A activity assays, and immunocytochemical staining, see Supplementary Data.
3. Results
3.1 I2PP2A interaction with PP2Ac subunit
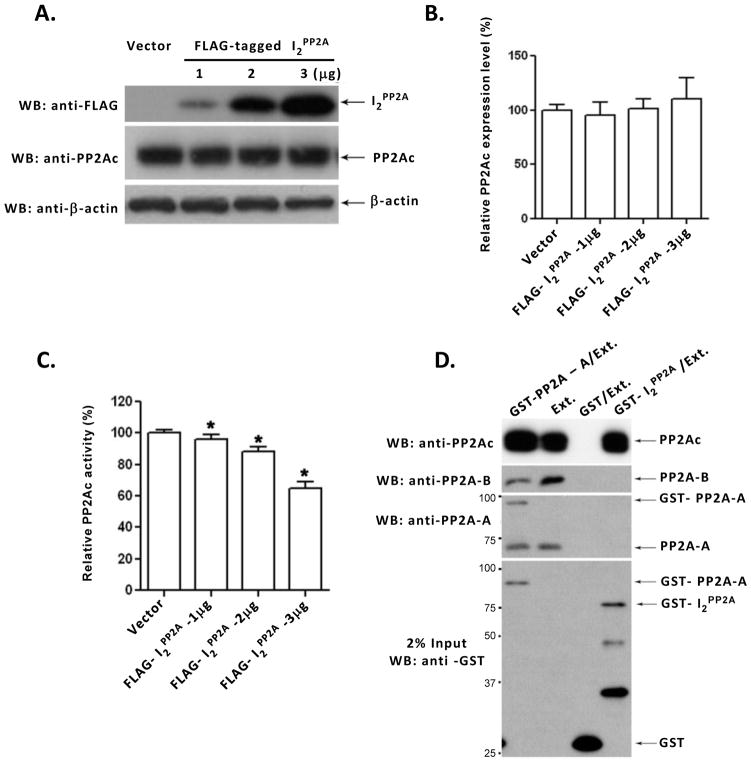
I2PP2A was previously shown to inhibit PP2A activity in vitro [9,11]. To date, it is not clear whether I2PP2A can decrease PP2A activity in cells and whether this reduction involves inhibition of the PP2A expression level. To address to this question, we carried out transient transfection of NIH3T3 cells with different amounts of pCMV2B-I2PP2A. After 48 hours transfection the cell lysates were collected and one half of the sample was employed for Western blots (Fig. 1A, B), whereas the other half was subjected to immunoprecipitation with anti-PP2Ac, followed by PP2A activity assay (Fig. 1C). The FLAG-tagged I2PP2A expression in transfected cells increased with the amount of DNA used for transfection (Fig. 1A). The expression of PP2Ac, however, was not altered (Fig. 1B), suggesting that the decrease in PP2A activity in transfected cells (Fig. 1C) was due to PP2A inhibition and not to a reduction of the PP2A expression level.
Fig. 1. Inhibition of PP2A activity by I2PP2A and its interaction with PP2Ac.
(A) NIH3T3 cells were transfected with different amounts of pCMV2B-I2PP2A to express FLAG-tagged I2PP2A or, as a control, with vector alone. After 48 hours transfection, cells were lysed and expression of PP2Ac, I2PP2A, and, as a loading control, β-actin were determined by Western blots. (B) The relative PP2Ac expression level was normalized by the expression of β-actin. PP2Ac expression level did not show any significant difference among different amounts of expression of I2PP2A in NIH3T3 cells. One way ANOVA, p = 0.1369; t-test, vector vs. 1 μg, p = 0.2189; vector vs. 2 μg, p = 0.5031; vector vs. 3 μg cDNA, p = 0.1992. (C) PP2A was immunoprecipitated from cell lysates with anti-PP2Ac and the phosphatase activity assayed colorimetrically using pNPP as a substrate. I2PP2A inhibited the PP2A activity in a pCMV2B-I2PP2A dose-dependent fashion. *p<0.05 compared with control. (D) GST-pull down assay. Rat brain extract (Ext.), used as a source of PP2A holoenzyme, was incubated with Sepharose 4B beads bearing GST, GST-I2PP2A, or GST-PP2A-A. After washing, bound PP2Ac, PP2A-B, and PP2A-A were detected by Western blots. The GST, GST-I2PP2A and GST-PP2A-A used in pull down assay are shown in the lowest panel. GST-I2PP2A pulled down PP2Ac (PP2A catalytic subunit) but not PR55α (PP2A Bα regulatory subunit) or PR65α (PP2A A regulatory subunit). From left to right, Lane 2 (Ext.) is input, and Lane 3 (GST/Ext.) is a negative control. Positions of protein size markers are indicated on the left of the panel. Error bars in panels B and C indicate means ± SE; n=3.
I2PP2A was previously shown to inhibit PP2A1 (the ABC complex), PP2A2 (the AC dimer), and PP2Ac (the C subunit) in a non-competitive manner [9,19]. However, no direct evidence was reported to confirm this association. To study the interaction between PP2A and I2PP2A, in vitro pull-down assays were carried out using bacterially expressed GST alone (negative control), GST-PP2A-A (positive control), or GST-I2PP2A. Rat brain extract as the source of PP2A holoenzyme was added to purified GST-PP2A-A, GST-I2PP2A, or GST alone, on glutathione Sepharose 4B beads and the interactions were detected by Western blots developed with antibodies to PP2A subunits A, B, or C. We found that I2PP2A could pull down PP2Ac but not PP2A-A or PP2A-B subunits (Fig. 1D). These findings suggest that I2PP2A interacts with the PP2A catalytic subunit rather than the PP2A regulatory subunits PP2A-A or PP2A-B.
I2FL, I2NTF and I2CTF localize differently in HEK293FT cells
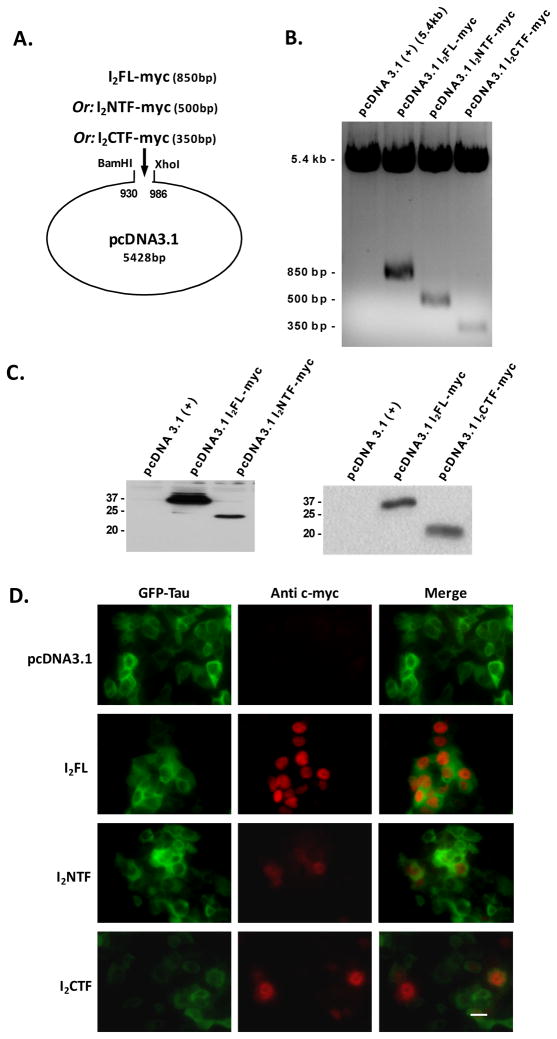
Previously we reported that in AD brain I2PP2A is selectively cleaved at N175 and is translocated from the neuronal nucleus to the cytoplasm [18]. To test whether the N-terminal fragment (I2NTF, aa1–175) or C-terminal fragment (I2CTF, aa176–277) or both can translocate from the cell nucleus to the cytoplasm, we generated three constructs corresponding to I2PP2A full length (I2FL), I2NTF, and I2CTF, each tagged with myc, and inserted them in pcDNA3.1 vector (Fig. 2A) for expression in eukaryotic cells. To confirm the integrity of the constructs, the plasmids were multiplied in dH5α E. coli, purified and digested by BamHI and Xhol restriction enzymes. The resulting DNA fragments were separated and extracted from 1% agarose gel (Fig. 2B). The accuracy of the DNA fragments was attested with subsequent sequencing. Finally, the plasmids were transfected either in HEK293FT cells or NIH3T3 cells and by Western blot analysis we confirmed the expression of myc-tagged I2FL, I2NTF, and I2CTF, at 39 kDA, 22 kDa, and 20 kDa apparent molecular weight, respectively (Fig. 2C).
Fig. 2. Intracellular localization of I2FL, I2NTF, and I2CTF with reference to tau.
(A) I2FL-myc, I2NTF-myc, and I2CTF-myc cDNA were generated using pEGFP-N3/I2PP2A (wt) as a template. The PCR inserts as well as pcDNA3.1 were digested with BamHl and Xhol. The products were subsequently ligated into pcDNA3.1 vector and transformed into E. Coli DH4-α. (B) After amplification, the purified plasmids were digested with BamHl and Xhol, separated by electrophoresis, and sequenced. (C) Left panel: Western blots specific for the C-terminal myc tag of each protein confirmed the expression of I2FL and I2NTF. Right panel: Western blots specific for the C-terminal myc tag of each protein confirmed the expression of I2FL and I2CTF. (D) Immunocytochemical staining of HEK293FT cells transfected with GFP-Tau and pcDNA3.1 or pcDNA3.1-I2FL, -I2NTF, or -I2CTF. I2FL tagged with c-myc was localized exclusively in the nucleus of HEK293FT cells. I2NTF tagged with c-myc was diffused all over the HEK293FT cells. I2CTF tagged with c-myc, although concentrated in the nucleus, was also found distributed within the cytoplasm. Magnification bar = 10 μm.
To assess the intracellular localization, we co-transfected HEK293FT cells with pcDNA3.1-I2FL and its fragments with an exclusively cytoplasmic GFP fused tau protein. Then, 72 hours post-transfection, the HEK293FT cells were fixed with 4% paraformaldehyde solution and analyzed by immunocytochemistry. The antibody to myc recognized the exogenously expressed I2PP2A. While as expected I2FL was found within the nucleus, I2NTF and I2CTF were found diffused both in the nucleus and the cytoplasm (Fig 2D).
3.2 PP2Ac co-immunoprecipitates with I2FL, I2NTF, and I2CTF
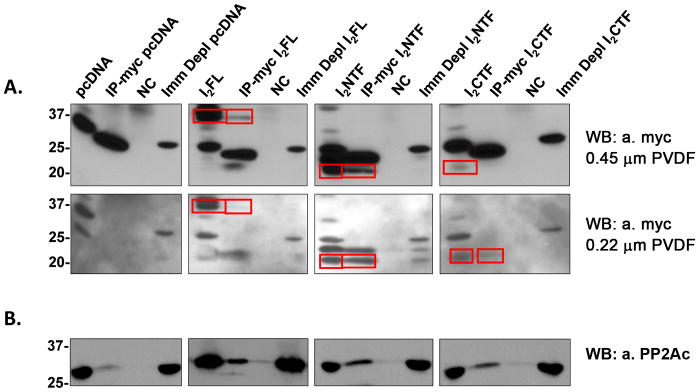
Employing GST-pull down assays we showed above (Fig. 1D) that in vitro I2FL could interact with PP2Ac but not with PP2A-A or PP2A-B. To confirm this interaction in cells, HEK293FT cells were transfected with myc-tagged I2FL, I2NTF, or I2CTF for 72 hours, followed by immunoprecipitation with anti-myc from these cell lysates and analysis by Western blots. PP2Ac co-immunoprecipitated with I2FL as well as I2NTF and I2CTF (Figs. 3A, B). These findings suggested that cleavage of I2PP2A into I2NTF and I2CTF could result in an increased inhibition of PP2A activity.
Fig. 3. Co-immunoprecipitation of PP2Ac with I2FL, I2NTF, or I2CTF.
HEK293FT cells transfected to overexpress c-myc-tagged I2FL, I2NTF or I2CTF were lysed and c-myc tag antibody was used for immunoprecipitation. The pulled down proteins were separated on SDS-polyacrylamide gels, then transferred onto two stacked PVDF membranes of decreasing pore size and analyzed by Western blots. (A) Upper row: Western blots on the 0.45 μm PVDF membrane developed with c-myc tag antibody. Lower row: Western blots on the 0.22 μm PVDF membrane developed with c-myc tag antibody. I2FL, I2NTF, and I2CTF were all immunoprecipitated with the c-myc antibody (see framed bands). (B) Western blots developed with anti-PP2Ac. NC=negative control, i.e. non-treated with the vector. Positions of protein molecular markers are shown on the left of the blots.
3.3 Inhibition of PP2A activity and generation of hyperphosphorylated tau by I2FL, I2NTF, and I2CTF
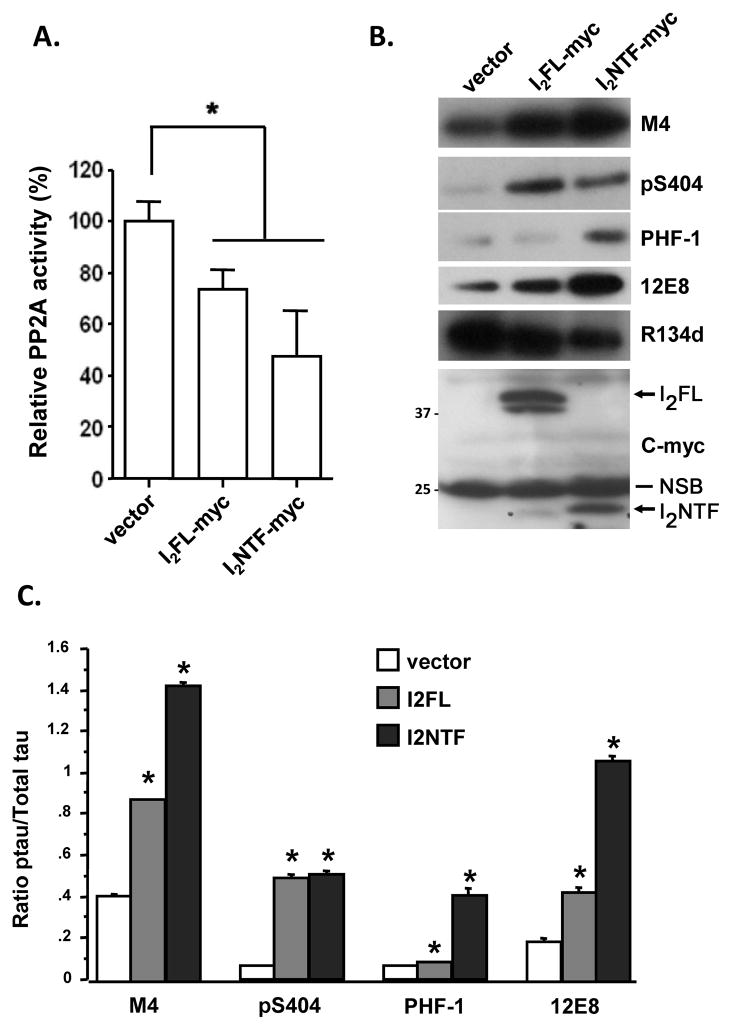
Next we proceeded to assess the effect of interactions of I2FL, I2NTF and I2CTF with PP2A on the phosphatase activity and on phosphorylation of tau. A previous study had reported that I2PP2A inhibits PP2A through its amino terminal region [12]. We, therefore, first examined whether I2NTF could inhibit PP2A activity. We transfected HEK293FT cells with pcDNA3.1-I2FL, -I2NTF, or vector alone for 72 hours, and then assayed okadaic acid sensitive PP2A activity towards pSer199 tau [20] in the cell lysates. We found that PP2A activity was reduced to ~70% and ~50% in I2FL and I2NTF transfected cells, respectively (Fig. 4A). In order to study the effect of this PP2A inhibition on abnormal hyperphosphorylation of tau, we expressed in tau441-stably transfected PC12 cells [21] I2FL or I2NTF for 72 hours. We then examined the phosphorylation of tau at multiple known pathological sites by Western blots of the cell lysates developed with various phospho-specific antibodies. We found both in I2FL and in I2NTF transfected cells abnormal hyperphosphorylation of tau at M4 (Thr231/Ser235), pS404 (Ser404), PHF-1 (Ser396/404), and 12E8 (Ser262/356) sites. The tau hyperphosphorylation at M4, PHF-1, and 12E8 sites, which are believed to be major sites in Alzheimer-type neurofibrillary degeneration, was quite robust in cells transfected with I2NTF (Fig. 4B,C).
Fig. 4. Inhibition of PP2A activity by I2FL and I2NTF.
(A) Relative PP2A activity in transiently transfected HEK293FT cells with N-terminal myc tagged I2FL and I2NTF. I2FL and I2NTF reduced PP2A activity to ~70% and ~50%, respectively, in HEK293FT cells. *p<0.05 compared with the vector transfection control. (B) Tau441 stably transfected PC12 cells were transiently transfected to express myc tagged I2FL or I2NTF and tau phosphorylation at M4 (pT231/pS235), pS404, PHF-1 (pS396/404) and 12E8 (pS262/356), total tau (R134d), as well as transfected I2PP2A were detected by Western blots from same amounts of lysates. The myc-tagged proteins were detectedwith anti-myc monoclonal 9E10 (1:100 in 5% defatted milk and 0.1% Tween-20) from Millipore/Chemicon. (C) The level of phosphorylated tau was quantified by densitometry and normalized to the amount of total tau. *p<0.05 compared with the vector transfection control. Positions of protein size markers are indicated on the left of the blots. Positions of I2FL and I2NTF bands are labeled with arrows. NSB = non-specific band.
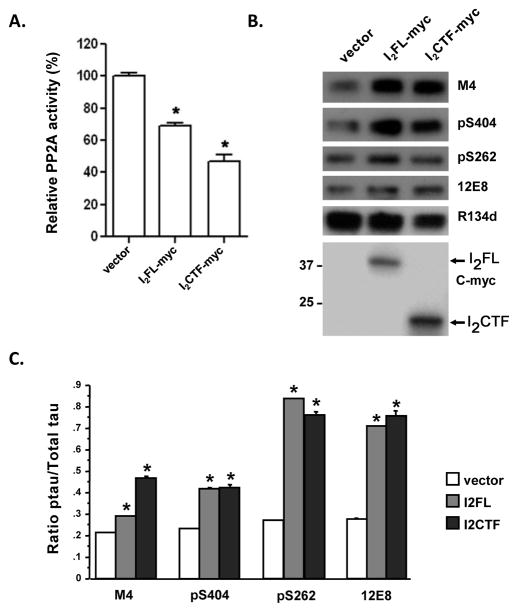
Since both in the GST-pull down assays as well as the co-immunoprecipitation assays we had found that not only I2NTF but also I2CTF interacted with PP2Ac (see above), we studied the effect of I2CTF in comparison to I2FL on PP2A activity and hyperphosphorylation of tau in cells. In lysates of NIH3T3 cells which were transfected with I2FL, I2CTF, or vector alone, we found a ~31% and ~53% reduction in PP2A activity, respectively (Fig. 5A). Tau441 stably transfected PC12 cells transfected with pcDNA3.1-I2FL or -I2CTF showed a marked increase in abnormal hyperphosphorylation of tau at M4 (Thr231/Ser235), pS404 (Ser404), pS262 (Ser262), and 12E8 (Ser262/346) sites (Fig. 5B,C).
Fig. 5. Inhibition of PP2Ac activity by I2FL and I2CTF.
(A) Relative PP2A activity in transiently transfected cells with C-terminal myc tagged constructs was detected as described in Fig. 1C. I2FL reduced PP2A activity to ~69% whereas I2CTF inhibited PP2A activity to ~47%. *p<0.05 compared with the vector transfection control. (B) Tau phosphorylation at M4 (pT231/pS235), pS404, pS262, 12E8 (pS262/356), total tau (R1334D) and I2PP2A were detected by Western blots from same amounts of lysates from I2FL and I2CTF transfected cells. The myc-tagged proteins were detected with anti-myc monoclonal 9B11 (1:1,000 in 5% defatted milk and 0.1% Tween-20). (C) The level of phosphorylated tau was quantified by densitometry and normalized using the amount of total tau. *p<0.05 compared with the vector transfection control. The rest of the details are the same as in Figure 4.
3.4 Domains of I2PP2Ainvolved in its binding to PP2A catalytic subunit
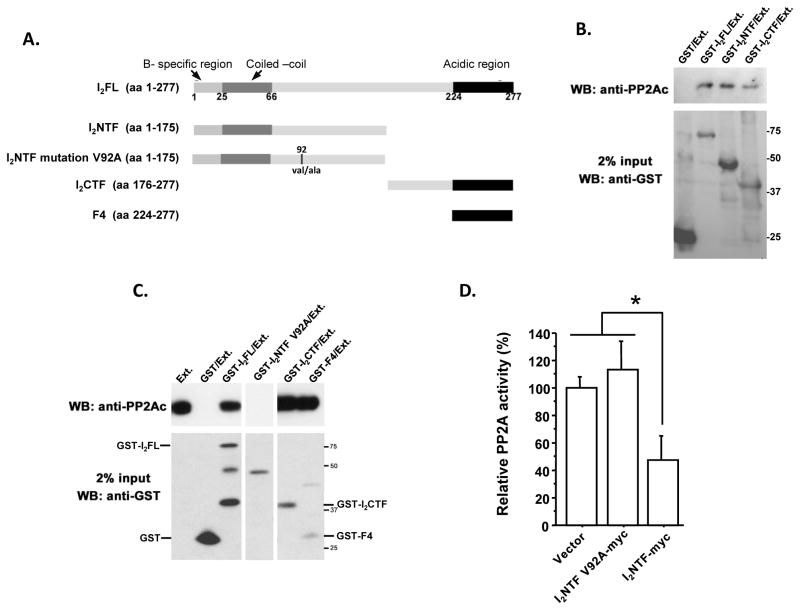
Finally, based on the structural features of I2PP2A (TAF-Iβ) characterized by a subtype specific N-terminal region, a coiled-coil region involved in dimerization of TAF-I, a putative nuclear localization signal, and a long stretch of acidic amino acids at C-terminal ends [12,22], we attempted to define the PP2Ac binding domain in I2PP2A. For this purpose, based on the structure, a series of the deletion mutants of I2PP2A, I2NTF, I2CTF and F4 corresponding to the C-terminal acidic region were generated (Fig. 6A). During these studies Val 92 in I2NTF was accidently mutated to Ala. While we corrected this mutation by mutagenesis, we also decided to study the effect of this point mutation on interaction of I2NTF with PP2A. In vitro pull-down assays were carried out with bacterially expressed GST fusion I2PP2A full length protein or its mutants. Rat brain extract was added to purified GST fusion proteins or GST alone on glutathione Sepharose 4B beads and PP2A catalytic subunit that was pulled down by these beads was detected by Western blots. The results showed that I2PP2A and its mutants containing C-terminal acidic region (I2PP2AF4 and I2CTF) had the ability of binding with PP2Ac, whereas, the mutation of valine 92 to alanine in the leucine rich region of I2NTF lost its PP2Ac binding ability (Fig. 6B and C). Thus, the minimal region of I2CTF required for the binding of PP2Ac is localized at the C-terminal acidic region (amino acid 225–277), and Val 92 in I2NTF is not only necessary for the binding but also for its inhibitory activity (Fig 6D).
Fig. 6. Identification of minimal and critical regions of I2PP2A required for interaction with PP2Ac.
(A) Schematic diagram of the constructs of functional domains of I2PP2A employed for the interaction studies. (B, C) GST-pull down assays with rat brain extract as a source of PP2A holoenzyme. The pull down assays were carried out using 0.5 μg of GST-fusion protein per mg brain extract, except in cases of GST-I2CTF (0.1 μg was employed), and GST alone (double the amount, i.e. 1 μg was employed). I2CTF, F4, and I2NTF but not I2NTFV92A interacted with PP2Ac. (D) Relative PP2A activity in transiently transfected NIH3T3 cells with C-terminal myc tagged constructs I2NTF-myc can reduce PP2A activity and mutation of V92 to A in I2NTF results in a complete loss of this inhibitory activity. *p<0.05.
4. Discussion
I2PP2A/SET is a multifunctional protein. It is believed to have a nuclear localization signal between amino acid residues 168–181 and is imported in the cell nucleus mainly by importin alpha-3 [23]. In its primary location in the cell nucleus it protects cells both by inhibiting DNAse [24] and acetylation of histones [17]. Translocation of I2PP2A from the nucleus to the cytoplasm has been reported to be cytotoxic and exacerbates DNA damage [23]. Generation of intracytoplasmic juxtamembrane domains of APP and APLP2 by caspase has also been shown to lead to the translocation of I2PP2A from the nucleus to the cytoplasm and cell death [25]. In vitro studies had shown that I2PP2A was also a potent inhibitor of PP2A [19] and that this inhibitory activity was localized in its amino terminal region [26]. Previously, we discovered that in AD brain I2FL is selectively cleaved at N175 into I2NTF and I2CTF, and translocated from the neuronal nucleus to the cytoplasm where it colocalizes with abnormally hyperphosphorylated tau/neurofibrillary tangles [18]. A subsequent study showed that the cleavage of I2PP2A at N175 can be catalyzed by a lysosomal/endosomal enzyme, asparaginyl endopeptidase (also known as legumain) which occurs during cerebral acidosis caused by ischemic stroke or seizures [27]. However, neither the exact molecular mechanism by which I2PP2A inhibits PP2A activity nor how its cleavage into I2NTF and I2CTF contributes to abnormal hyperphosphorylation of tau were understood. The present study for the first time shows that I2PP2A decreases PP2A activity in the cell by directly interacting with and inhibiting, and not by affecting the expression of PP2Ac. Furthermore, while I2FL localizes in the cell nucleus, both I2NTF and I2CTF are diffused into the cytoplasm where they interact with PP2A through PP2Ac and inhibit the phosphatase activity. I2NTF and I2CTF appear to be quite robust in inhibiting PP2A activity and causing abnormal hyperphosphorylation of tau. I2CTF interacts with PP2Ac through its carboxy terminal acidic region. In I2NTF valine 92 is required for its interaction with PP2Ac and inhibition of the phosphatase activity. The inhibition of PP2A activity by I2FL, INTF, and I2CTF was observed in all three cell lines, i.e. NIH3T3, HEK293FT, and PC12 cells, studied.
Because of the small sizes of I2NTF and I2CTF as compared with I2FL, these proteins transferred to the PVDF membrane at quite variable rates and, therefore, it was not possible to normalize the inhibition of PP2A activity or hyperphosphorylation of tau from the intensity of the protein bands seen in Western blots. However, hyperphosphorylation of tau at various sites observed in I2NTF or I2CTF as compared with I2FL-transfected cells indicated that the two fragments of I2PP2A probably inhibited PP2A activity towards phosphotau in a substrate-specific manner. Relative to I2FL, the expression of I2NTF caused a high level of hyperphosphorylation of tau at Thr231/Ser235 (M4 site), Ser262/356 (12E8 site), and Ser396/404 (PHF-1 site) but not at Ser404, and I2CTF at Thr231/Ser235 (M4 site). Previously, in an in vivo study in rat, we showed that I2CTF caused hyperphosphorylation of tau at Ser396 [28].
In AD brain the activity of PP2A is compromised [8] and tau is abnormally hyperphosphorylated [1,3]. The AD abnormally hyperphosphorylated tau probably disrupts the microtubule network and leads to neurodegeneration by sequestration of normal tau as well as microtubule associated proteins MAP1 and MAP2 [4]. In the present study, the PP2A inhibition by I2PP2A, especially I2NTF and I2CTF, resulted in abnormal hyperphosphorylation of tau at Thr231/Ser235, Ser262/356, and Ser396/404 in cultured cells. These phosphorylation sites are known to inhibit binding of tau to microtubules [7] and cause neurodegeneration, and cognitive impairment [29]. Since the inhibition of PP2A by the carboxy terminal region of I2PP2A was not previously known, based on the present study, we expressed I2CTF in the brain and found inhibition of PP2A activity, neurodegeneration associated with abnormal hyperphosphorylation of tau, and intraneuronal accumulation of Aβ and impairment in spatial learning and memory in rats [28]. These in vivo findings on the PP2A inhibitory activity of I2CTF are in agreement with the present study.
PP2A, which accounts for ~70% of the total phosphoseryl/phosphothreonyl protein phosphatase activity in human brain, is the major tau phosphatase [30]. PP2A regulates phosphorylation of tau both directly and by regulating the activities of several tau protein kinases which include PKA, CaMKII, MEK1/2, ERK1/2, and p70S6 kinase [7]. I2PP2A binds the neuronal cdk5 activator p35 and enhances the ckd5/p35 activity [31]. Knockdown and overexpression of I2PP2A in the brain have been found to decrease and increase GSK-3β activity, respectively [28,32]. Thus, a dysregulation of I2PP2A can have a profound effect on affected neurons, both through activation of several protein kinases and abnormal hyperphosphorylation of tau.
Besides I2PP2A, PP2A activity is also regulated by methylation and phosphotyrosinylation of PP2Ac and by I1PP2A, also known as PHAPI (putative histocompatibility leukocyte antigen class II associated protein-1), mapmodulin, pp32, and LANP [9]. Both mRNA and protein expressions of I1PP2A are elevated and the inhibitor is co-localized with neurofibrillary tangles in AD brain [18]. Demethylation of PP2Ac, which decreases its activity, was reported in AD brain [33,34]. Phosphorylation of PP2Ac at tyrosine 307, which inhibits its activity, was found to be elevated in AD brain [35]. Microarray analyses showed an upregulation of the expression of SET genes in AD hippocampus [36]. While the mRNA and protein expressions of I2PP2A are elevated in AD brain, reported by us previously [18], the dual action of I2NTF and I2CTF in inhibiting PP2A activity shown in the present study shows how the cleavage and translocation of this inhibitor could work as the last straw which broke the camel’s back in producing neurofibrillary degeneration in AD.
Supplementary Material
Highlights.
Molecular mechanism of protein phosphatase-2A activity by I2PP2A/SET
Role of cleavage of I2PP2A into I2NTF and I2CTF in inhibition of PP2A activity
Acknowledgments
We thank Dr. Dale Schenk, Elan Pharmaceuticals, San Francisco, CA, for 12E8 antibody; Dr. Peter Davies, Albert Einstein College of Medicine, Bronx, NY, for PHF-1 antibody; Dr. Yasuo Ihara, Tokyo University, Tokyo, Japan, for M4 antibody; Dr. Guohua Fan from our laboratory for detecting Valine 92 alanine mutation in I2NTF; and Dr. Ezzat El-Akkad from our AudioVisual Department, GRAMS, for the preparation of the figures. Janet Murphy provided secretarial assistance including preparation of this manuscript.
Abbreviations
- AD
Alzheimer disease
- PP2A
protein phosphatase 2A
- MEK1/2
MAP kinase kinase
- ERK 1/2
extracellular regulated kinase
- PHAP1
putative histocompatibility leukocyte antigen class II associated protein-1
Footnotes
Structured summary of protein interactions
I2PP2A physically interacts with PP2A-C by anti tag coimmunoprecipitation (View Interaction 1, 2)
I2PP2A physically interacts with PP2A-C by pull down (View Interaction 1, 2)
PP2A-A physically interacts with PP2A-B and PP2A-C by pull down (View interaction)
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau. A component of Alzheimer paired helical filaments. J Biol Chem. 1986a;261:6084–9. [PubMed] [Google Scholar]
- 2.Grundke-Iqbal I, Iqbal K, Tung YC, Quinlan M, Wisniewski HM, Binder LI. Abnormal phosphorylation of the microtubule-associated protein tau (tau) in Alzheimer cytoskeletal pathology. Proc Natl Acad Sci USA. 1986b;83:4913–7. doi: 10.1073/pnas.83.13.4913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee VM, Balin BJ, Otvos L, Jr, Trojanowski JQ. A68: a major subunit of paired helical filaments and derivatized forms of normal Tau. Science. 1991;251:675–8. doi: 10.1126/science.1899488. [DOI] [PubMed] [Google Scholar]
- 4.Alonso AD, Li B, Grundke-Iqbal I, Iqbal K. Polymerization of hyperphosphorylated tau into filaments eliminates its inhibitory activity. Proc Natl Acad Sci USA. 2006;23:8864–8869. doi: 10.1073/pnas.0603214103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Virshup DM. Protein phosphatase 2A: a panoply of enzymes. Curr Opin Cell Biol. 2000;12:180–5. doi: 10.1016/s0955-0674(99)00074-5. [DOI] [PubMed] [Google Scholar]
- 6.Goedert M, Jakes R, Qi Z, Wang JH, Cohen P. Protein phosphatase 2A is the major enzyme in brain that dephosphorylates tau protein phosphorylated by proline-directed protein kinases or cyclic AMP-dependent protein kinase. J Neurochem. 1995;65:2804–7. doi: 10.1046/j.1471-4159.1995.65062804.x. [DOI] [PubMed] [Google Scholar]
- 7.Iqbal K, Liu F, Gong CX, Alonso Adel C, Grundke-Iqbal I. Mechanisms of tau-induced neurodegeneration. Acta Neuropathol. 2009;118:53–69. doi: 10.1007/s00401-009-0486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gong CX, Singh TJ, Grundke-Iqbal I, Iqbal K. Phosphoprotein phosphatase activities in Alzheimer disease brain. J Neurochem. 1993;61:921–7. doi: 10.1111/j.1471-4159.1993.tb03603.x. [DOI] [PubMed] [Google Scholar]
- 9.Li M, Guo H, Damuni Z. Purification and characterization of two potent heat-stable protein inhibitors of protein phosphatase 2A from bovine kidney. Biochemistry. 1995;34:1988–96. doi: 10.1021/bi00006a020. [DOI] [PubMed] [Google Scholar]
- 10.Li M, Makkinje A, Damuni Z. Molecular identification of I1PP2A, a novel potent heat-stable inhibitor protein of protein phosphatase 2A. Biochemistry. 1996;35:6998–7002. doi: 10.1021/bi960581y. [DOI] [PubMed] [Google Scholar]
- 11.Tsujio I, Zaidi T, Xu J, Kotula L, Grundke-Iqbal I, Iqbal K. Inhibitors of protein phosphatase-2A from human brain structures, immunocytological localization and activities towards dephosphorylation of the Alzheimer type hyperphosphorylated tau. FEBS Lett. 2005;579:363–72. doi: 10.1016/j.febslet.2004.11.097. [DOI] [PubMed] [Google Scholar]
- 12.Nagata K, et al. Replication factor encoded by a putative oncogene, set, associated with myeloid leukemogenesis. Proc Natl Acad Sci U S A. 1995;92:4279–83. doi: 10.1073/pnas.92.10.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vaesen M, Barnikol-Watanabe S, Gotz H, Awni LA, Cole T, Zimmermann B, Kratzin HD, Hilschmann N. Purification and characterization of two putative HLA class II associated proteins: PHAPI and PHAPII. Biol Chem Hoppe Seyler. 1994;375:113–26. doi: 10.1515/bchm3.1994.375.2.113. [DOI] [PubMed] [Google Scholar]
- 14.von Lindern M, van Baal S, Wiegant J, Raap A, Hagemeijer A, Grosveld G. Can, a putative oncogene associated with myeloid leukemogenesis, may be activated by fusion of its 3′ half to different genes: characterization of the set gene. Mol Cell Biol. 1992;12:3346–55. doi: 10.1128/mcb.12.8.3346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adachi Y, Pavlakis GN, Copeland TD. Identification of in vivo phosphorylation sites of SET, a nuclear phosphoprotein encoded by the translocation breakpoint in acute undifferentiated leukemia. FEBS Lett. 1994;340:231–5. doi: 10.1016/0014-5793(94)80144-4. [DOI] [PubMed] [Google Scholar]
- 16.Adachi Y, Pavlakis GN, Copeland TD. Identification and characterization of SET, a nuclear phosphoprotein encoded by the translocation break point in acute undifferentiated leukemia. J Biol Chem. 1994;269:2258–62. [PubMed] [Google Scholar]
- 17.Seo SB, McNamara P, Heo S, Turner A, Lane WS, Chakravarti D. Regulation of histone acetylation and transcription by INHAT, a human cellular complex containing the set oncoprotein. Cell. 2001;104:119–30. doi: 10.1016/s0092-8674(01)00196-9. [DOI] [PubMed] [Google Scholar]
- 18.Tanimukai H, Grundke-Iqbal I, Iqbal K. Up-regulation of inhibitors of protein phosphatase-2A in Alzheimer’s disease. Am J Pathol. 2005;166:1761–71. doi: 10.1016/S0002-9440(10)62486-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li M, Makkinje A, Damuni Z. The myeloid leukemia-associated protein SET is a potent inhibitor of protein phosphatase 2A. J Biol Chem. 1996;271:11059–62. doi: 10.1074/jbc.271.19.11059. [DOI] [PubMed] [Google Scholar]
- 20.Chohan MO, Khatoon S, Iqbal IG, Iqbal K. Involvement of I2PP2A in the abnormal hyperphosphorylation of tau and its reversal by Memantine. FEBS Lett. 2006;580:3973–9. doi: 10.1016/j.febslet.2006.06.021. [DOI] [PubMed] [Google Scholar]
- 21.Gong CX, Liu F, Wu G, Rossie S, Wegiel J, Li L, Grundke-Iqbal I, Iqbal K. Dephosphorylation of microtubule-associated protein tau by protein phosphatase 5. J Neurochem. 2004;88:298–310. doi: 10.1111/j.1471-4159.2004.02147.x. [DOI] [PubMed] [Google Scholar]
- 22.Miyaji-Yamaguchi M, Okuwaki M, Nagata K. Coiled-coil structure-mediated dimerization of template activating factor-I is critical for its chromatin remodeling activity. J Mol Biol. 1999;290:547–57. doi: 10.1006/jmbi.1999.2898. [DOI] [PubMed] [Google Scholar]
- 23.Qu D, Zhang Y, Ma J, Guo K, Li R, Yin Y, Cao X, Park DS. The nuclear localization of SET mediated by impalpha3/impbeta attenuates its cytosolic toxicity in neurons. J Neurochem. 2007;103:408–22. doi: 10.1111/j.1471-4159.2007.04747.x. [DOI] [PubMed] [Google Scholar]
- 24.Fan Z, Beresford PJ, Oh DY, Zhang D, Lieberman J. Tumor suppressor NM23-H1 is a granzyme A-activated DNase during CTL-mediated apoptosis, and the nucleosome assembly protein SET is its inhibitor. Cell. 2003;112:659–72. doi: 10.1016/s0092-8674(03)00150-8. [DOI] [PubMed] [Google Scholar]
- 25.Briand S, Facchinetti P, Clamagirand C, Madeira A, Pommet JM, Pimplikar SW, Allinquant B. PAT1 induces cell death signal and SET mislocalization into the cytoplasm by increasing APP/APLP2 at the cell surface. Neurobiol Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 26.Nagata K, et al. Cellular localization and expression of template-activating factor I in different cell types. Exp Cell Res. 1998;240:274–81. doi: 10.1006/excr.1997.3930. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, et al. Neuroprotective actions of PIKE-L by inhibition of SET proteolytic degradation by asparagine endopeptidase. Mol Cell. 2008;29:665–78. doi: 10.1016/j.molcel.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang X, Blanchard J, Kohlbrenner E, Clement N, Linden RM, Radu A, Grundke-Iqbal I, Iqbal K. The carboxy-terminal fragment of inhibitor-2 of protein phosphatase-2A induces Alzheimer disease pathology and cognitive impairment. FASEB Journal. 2010;24:4420–32. doi: 10.1096/fj.10-158477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu SJ, et al. Tau becomes a more favorable substrate for GSK-3 when it is prephosphorylated by PKA in rat brain. J Biol Chem. 2004;279:50078–88. doi: 10.1074/jbc.M406109200. [DOI] [PubMed] [Google Scholar]
- 30.Liu F, Grundke-Iqbal I, Iqbal K, Gong CX. Contributions of protein phosphatases PP1, PP2A, PP2B and PP5 to the regulation of tau phosphorylation. Eur J Neurosci. 2005;22:1942–50. doi: 10.1111/j.1460-9568.2005.04391.x. [DOI] [PubMed] [Google Scholar]
- 31.Qu D, Li Q, Lim HY, Cheung NS, Li R, Wang JH, Qi RZ. The protein SET binds the neuronal Cdk5 activator p35nck5a and modulates Cdk5/p35nck5a activity. J Biol Chem. 2002;277:7324–32. doi: 10.1074/jbc.M107270200. [DOI] [PubMed] [Google Scholar]
- 32.Liu GP, Zhang Y, Yao XQ, Zhang CE, Fang J, Wang Q, Wang JZ. Activation of glycogen synthase kinase-3 inhibits protein phosphatase-2A and the underlying mechanisms. Neurobiol Aging. 2008;29:1348–58. doi: 10.1016/j.neurobiolaging.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 33.Sontag E, Luangpirom A, Hladik C, Mudrak I, Ogris E, Speciale S, White CL., 3rd Altered expression levels of the protein phosphatase 2A ABalphaC enzyme are associated with Alzheimer disease pathology. J Neuropathol Exp Neurol. 2004;63:287–301. doi: 10.1093/jnen/63.4.287. [DOI] [PubMed] [Google Scholar]
- 34.Zhou XW, Gustafsson JA, Tanila H, Bjorkdahl C, Liu R, Winblad B, Pei JJ. Tau hyperphosphorylation correlates with reduced methylation of protein phosphatase 2A. Neurobiol Dis. 2008;31:386–94. doi: 10.1016/j.nbd.2008.05.013. [DOI] [PubMed] [Google Scholar]
- 35.Liu R, et al. Phosphorylated PP2A (tyrosine 307) is associated with Alzheimer neurofibrillary pathology. J Cell Mol Med. 2008;12:241–57. doi: 10.1111/j.1582-4934.2008.00249.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blalock EM, Geddes JW, Chen KC, Porter NM, Markesbery WR, Landfield PW. Incipient Alzheimer’s disease: microarray correlation analyses reveal major transcriptional and tumor suppressor responses. Proc Natl Acad Sci U S A. 2004;101:2173–8. doi: 10.1073/pnas.0308512100. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.