Abstract
Immunotherapy and chemotherapy are generally effective against small tumors in animal models of cancer. However, these treatment regimens are generally ineffective against large, bulky tumors. We have found that a multimodality treatment regimen using DNA vaccination in combination with chemotherapeutic agent epigallocatechin-3-gallate (EGCG), a compound found in green tea, is effective in inhibiting large tumor growth. EGCG was found to induce tumor cellular apoptosis in a dose-dependent manner. The combination of EGCG and DNA vaccination led to an enhanced tumor-specific T-cell immune response and enhanced antitumor effects, resulting in a higher cure rate than either immunotherapy or EGCG alone. In addition, combined DNA vaccination and oral EGCG treatment provided long-term antitumor protection in cured mice. Cured animals rejected a challenge of E7-expressing tumors, such as TC-1 and B16E7, but not a challenge of B16 7 weeks after the combined treatment, showing antigen-specific immune responses. These results suggest that multimodality treatment strategies, such as combining immunotherapy with a tumor-killing cancer drug, may be a more effective anticancer strategy than single-modality treatments.
Introduction
Multimodality treatments that combine conventional cancer therapies with immunotherapy, such as DNA vaccines, have emerged as a potentially plausible approach in the fight against cancer (for reviews, see refs. 1, 2). We have developed DNA vaccine systems for human papillomavirus (HPV)–associated cervical neoplasia and HPV-associated head and neck cancers (for review, see refs. 3, 4). Cervical cancer can serve as a model of how a viral infection can progress through a multistep process from initial infection to premalignant dysplasia, called cervical intraepithelial neoplasia, to invasive cancer. HPV, particularly HPV-16, is associated with a majority of cervical cancers and a subset of head and neck cancers (for review, see ref. 5). HPV-16 E7, one of its oncoproteins, is essential for the induction and maintenance of cellular transformation (5). Thus, HPV-16 E7 is an ideal target for developing vaccine and immunotherapeutic strategies for the control of HPV infections and HPV-associated lesions (for review, see ref. 6). However, the antigen-specific immune responses and antitumor effects generated by DNA vaccine encoding wild-type E7 is weak and not enough to be effective in controlling tumor growth. To overcome the weak antigenicity of E7, we have previously created a DNA vaccine encoding HPV-16 E7 linked to the sorting signal of the lysosome-associated membrane protein 1 (LAMP-1; refs. 7–9). The encoded chimeric protein (Sig/E7/LAMP-1) also includes the signal peptide derived from LAMP-1 protein. Vaccination with Sig/E7/LAMP-1 DNA led to a significantly enhanced E7-specific CD4+ and CD8+ T cell–mediated immune responses, resulting in potent antitumor effects against E7-expressing tumors in vaccinated mice (7–9).
Although antigen-specific DNA vaccines may be effective against small tumors in preclinical models, many tumors can grow rapidly resulting in bulky tumors, which present a challenge to immuno-therapeutic strategies alone. We hypothesize that this challenge may be overcome by multimodality treatment regimens, which combine immunotherapy, such as DNA vaccination, with an apoptosis-inducing chemotherapeutic drug, such as epigallocate-chin-3-gallate (EGCG), a compound found in green tea.
EGCG is the major polyphenol component found in green tea (for reviews, see refs. 10–13). EGCG has shown antitumor effects in various human and animal models, including cancers of the breast, prostate, stomach, esophagus, colon, pancreas, skin, lung, and other sites (for reviews, see refs. 10, 14, 15). EGCG has been shown to act on different pathways to regulate cancer cell growth, survival, angiogenesis, and metastasis (for reviews, see refs. 10, 11, 16). For example, some studies suggest that EGCG protects against cancer by causing cell cycle arrest and inducing apoptosis (17). It is also reported that telomerase inhibition might be one of the major mechanisms underlying the anticancer effects of EGCG (18). In comparison with commonly used antitumor agents, including retinoids and doxorubicin, EGCG has a relatively low toxicity and is convenient to give due to its oral bioavailability (19, 20). Thus, EGCG has been used in clinical trials (21) and seems to be a potentially ideal antitumor agent (22, 23).
In the current study, we investigate whether immunotherapy with a DNA vaccine encoding Sig/E7/LAMP-1 acts synergistically with EGCG. The rationale was that EGCG might aid DNA vaccine–mediated antitumor immunity by inhibiting tumor growth, thereby allowing time for the antitumor immune response to develop. We observed that chemotherapy with EGCG led to increased apoptotic tumor cell death. Our data showed a dramatic increase in E7-specific CD8+ and CD4+ T cell–mediated immune responses after combining Sig/E7/LAMP-1 DNA vaccination with oral administration of EGCG in drinking water. Thus, combination of cancer immunotherapy with a tumor-killing cancer drug may be a plausible approach for the control of bulky tumors. The clinical implications of our study are discussed.
Materials and Methods
Mice
Six- to 8-week-old female C57BL/6 mice were purchased from Daehan Biolink (Chungbuk, Korea). All animal procedures were done according to approved protocols and in accordance with recommendations for the proper use and care of laboratory animals.
Tumor models
Three cell lines of H-2b background (TC-1, B16, and B16E7) were used as murine tumor models. The HPV-16 E7-expressing murine tumor model (TC-1) has been described previously (24). In brief, HPV-16 E6, E7, and ras oncogene were used to transform primary C57BL/6 mice lung epithelial cells to generate the TC-1 cell line. The generation of a B16 melanoma cell line expressing HPV-16 E7 antigen, referred to as B16E7, has been previously described (25). These cell lines were cultured in vitro in RPMI 1640 supplemented with 10% fetal bovine serum, 50 units/mL penicillin/streptomycin, 2 mmol/L L-glutamine, 1 mmol/L sodium pyruvate, and 2 mmol/L nonessential amino acids and grown at 37°C with 5% CO2.
DNA vaccination
The generation and purification of pcDNA3-Sig/E7/LAMP-1 has been described previously (8). DNA-coated gold particles were prepared according to a previously described protocol (26). DNA-coated gold particles were delivered to the shaved abdominal region of mice using a helium-driven gene gun (Bio-Rad, Hercules, CA) with a discharge pressure of 400 p.s.i. C57BL/6 mice were immunized with 2 μg of a plasmid encoding Sig/E7/LAMP-1 or a control plasmid with no insert. The mice received a booster with the same dose 7 days later.
Determination of apoptotic cells in tumors
C57BL/6 mice (five per group) were injected s.c. in the right leg with 5 × 105 TC-1 tumor cells per mouse. Ten days later, EGCG (Sigma Chemical Co., St. Louis, MO) was given in the drinking water at a concentration of 0, 0.1, 0.5, or 2.5 mg/mL for 5 days. After emulsifying the isolated tumors into single-cell preparations, detection of apoptotic cells was done using phycoerythrin-conjugated rabbit anti-active caspase-3 antibody (BD Biosciences, San Diego, CA) according to the manufacturer’s instructions. To characterize the expression of HPV-16 E7 in TC-1 cells, single-cell suspensions of isolated tumors were stained with E7-specific monoclonal antibody, which was kindly provided by Dr. Ju-Hong Jeon (Seoul National University College of Medicine; ref. 27). The percentage of apoptotic cells was analyzed using flow cytometry.
Activation of an E7-specific CD8+ T-cell line by CD11c+-enriched cells from vaccinated mice
Ten days after tumor inoculation, tumor-bearing mice were given EGCG in their drinking water at a concentration of 0 or 0.5 mg/mL for 5 days. Inguinal lymph nodes were then harvested from treated mice, and CD11c+ cells were enriched from a single-cell suspension of isolated inguinal lymph nodes using CD11c (N418) microbe-ads (Miltenyi Biotec, Auburn, CA). Enriched CD11c+ cells were analyzed by forward and side scatter and gated around a population of cells with size and granular characteristics of dendritic cells. The isolated CD11c+ dendritic cells (2 × 104) were incubated with 2 × 106 E7-specific CD8+ T cells for 16 h. Cells were then stained for both surface CD8 and intracellular IFN-γ and analyzed by flow cytometry (8).
Intracellular cytokine staining and flow cytometry analysis
Splenocytes were harvested from the Sig/E7/LAMP-1 DNA and/or EGCG-treated mice (five per group) 7 days after the last vaccination. Before intracellular cytokine staining, 4 × 106 pooled splenocytes from each vaccination group were incubated overnight with 1 μg/mL of E7 peptide containing either an MHC class I epitope (amino acids 49–57) for detecting E7-specific CD8+ T-cell precursors, or 5 μg/mL of E7 peptide containing an MHC class II epitope (amino acids 30–67) for detecting E7-specific CD4+ T-cell precursors (7). Intracellular interleukin-4 (IL-4) and IFN-γ staining and flow cytometric analysis were done as described previously (26). Analyses were done on a Becton-Dickinson FACScan with CELLQuest software (Becton Dickinson Immunocytometry System, Mountain View, CA).
In vivo tumor growth experiments
In vivo tumor growth experiments were done in tumor-challenged mice treated with EGCG at various concentrations. C57BL/6 mice (five per group) were injected s.c. in the right leg with 5 × 105 TC-1 tumor cells per mouse. Ten days after tumor inoculation, EGCG was given in the drinking water at a concentration of 0, 0.1, 0.5, or 2.5 mg/mL for 5 days. The TC-1 tumor–challenged mice were characterized for tumor growth by measuring the tumor volume 1 week after the termination of EGCG treatment.
For in vivo tumor protection experiments, C57BL/6 mice (five per group) were vaccinated and received a booster with the Sig/E7/LAMP-1 DNA or control DNA via gene gun and challenged with 5 × 105 TC-1 tumor cells per mouse s.c. in the right leg 3 days after the initial vaccination. EGCG (Sigma Chemical) was given in the drinking water of animals at various concentrations (0, 0.02, 0.1, 0.5, or 2.5 mg/mL) at the time of tumor challenge and continued for 11 days. Mice were monitored for evidence of tumor growth by measuring the tumor volume at 14 days after tumor challenge. In another set of tumor protection experiments, EGCG was given in the drinking water of animals at the concentration of 0.5 mg/mL at the time of tumor challenge and continued for 11 days. Treated mice were monitored for evidence of tumor growth by inspection and palpation twice a week.
For the characterization of the subsets of lymphocytes important for the antitumor effects, C57BL/6 mice (five per group) were vaccinated and received a booster with the Sig/E7/LAMP-1 DNA via gene gun and were subsequently challenged with TC-1 tumor cells 3 days after initial vaccination. EGCG was provided in the drinking water at a concentration of 0.5 mg/mL at the time of tumor challenge and continued for 11 days. Antibody depletion of subsets of lymphocytes was initiated 1 week after the last immunization using the methods described previously (24). MAb GK1.5 was used for CD4 depletion; MAb 2.43 was used for CD8 depletion; and MAb PK136 was used for NK1.1 depletion. Depletion was terminated on day 40 after tumor challenge. Mice were monitored for evidence of tumor growth by inspection and palpation twice a week.
For long-term tumor protection experiments, C57BL/6 mice (five per group) were vaccinated and received a booster with Sig/E7/LAMP-1 DNA via gene gun. Three days after the initial vaccination, the mice were s.c. challenged with 5 × 105 TC-1 tumor cells per mouse in the right leg. EGCG (Sigma Chemical) was given in the drinking water of animals at a dose of 0.5 mg/mL at the time of tumor challenge and continued for 11 days. Seven weeks after the last vaccination, the mice were injected with TC-1, B-16, or B16-E7 at a dose of 5 × 104 tumor cells per mouse via tail vein to simulate hematogenous spread of tumors and evaluate long-term protection. Mice were sacrificed 24 days after tumor challenge and assayed for tumor growth in the lung.
For the tumor treatment experiments, mice were challenged with 1 × 104 TC-1 tumor cells per mouse s.c. Three days later, the mice were vaccinated with Sig/E7/LAMP-1 DNA and received a booster with the same DNA via gene gun 1 week later. EGCG was given in the drinking water at a concentration of 0.5 mg/mL at the time of initial DNA treatment and continued for 14 days. Tumor volumes were measured and recorded twice a week for 78 days following tumor challenge. In vivo tumor experiments were done thrice to generate reproducible data.
Statistical analysis
All data are expressed as means ± SD and are representative of at least two separate experiments. Results for intracellular cytokine staining with flow cytometry analysis, and tumor treatment experiments were evaluated by ANOVA. Comparisons between individual data points were made using Student’s t test. In the tumor protection experiments, the principal outcome measure was time to tumor development. The event time distributions for different mice were compared using the Kaplan and Meier method and the log-rank statistic. All Ps < 0.05 were considered significant.
Results
Treatment with EGCG induced apoptotic cell death of tumors, generated HPV-16 E7-specific CD8+ T cells, and inhibited tumor growth of E7-expressing tumors
We measured the percentage of apoptotic tumor cells and antigen presentation in the draining lymph nodes after EGCG administration in mice with established tumors. Mice were s.c. inoculated with 5 × 105 TC-1 tumor cells per mouse. TC-1 is a previously described E7-expressing tumor model (24). Ten days after tumor inoculation, EGCG was given for 5 days in the drinking water at a concentration of 0, 0.1, 0.5, or 2.5 mg/mL. After preparation of single-cell suspensions of isolated tumors, detection of apoptotic cells was done using phycoerythrin-conjugated rabbit anti-active caspase-3 antibody, according to the manufacturer’s instructions. To identify TC-1 cells, single-cell suspensions of the tumor were also stained with E7-specific monoclonal antibody. The percentage of apoptotic tumor cells was analyzed using flow cytometry. As shown in Fig. 1A and B, tumors of mice treated with EGCG showed dose-dependent apoptosis. There was an increased percentage of tumor cell apoptosis in a dose-dependent manner of given EGCG. In fact, there was a >11-fold increase in the percentage of apoptosis in TC-1 tumors in mice treated with 2.5 mg/mL EGCG in the drinking water compared with mice treated with 0 mg/mL EGCG (3.41% versus 0.29%). To determine whether EGCG-induced apoptosis leads to a decrease in the tumor volume, tumor-bearing mice were treated with EGCG as described above, and tumor volume was measured 1 week after the termination of ECGC treatment. As shown in Fig. 1C, there was a correlative decrease in tumor volume as EGCG concentrations increased from 0 to 0.5 mg/mL. However, at the highest dose of EGCG (2.5 mg/mL), there was a relative increase in tumor volume compared with the 0.5 mg/mL dose. Furthermore, we measured the E7-specific CD8+ T-cell immune response in tumor-bearing mice treated with various concentrations of EGCG. As shown in Fig. 1D, there was an observed increase in the number of E7-specific CD8+ T cells in a dose-dependent manner of EGCG given at doses ranging from 0 to 0.5 mg/mL. However, the number of E7-specific CD8+ T cells decreased when EGCG was given at a concentration of 2.5 mg/mL, which correlated with the increased tumor volume observed at this concentration as shown in Fig. 1C. These results indicate that tumor cell apoptosis occurs in a linear relationship with the dose of EGCG given. Furthermore, immune cell responses and antitumor effects correlate with increasing doses of EGCG given at a certain dose range (0–0.5 mg/mL). However, when EGCG is given at the highest dose of 2.5 mg/mL, there seems to be a decrease in E7-specific immune responses and a decrease in the observed antitumor effect. Our data suggest that at higher doses of EGCG, the enhancement of antigen-specific CD8+ T-cell immune responses mediated by induced tumor cell apoptosis may be countered by the potential immunosuppressive effects of EGCG on the immune system.
Figure 1.
Tumor treated with EGCG induced apoptosis, generated HPV-16 E7-specific CD8+ T cells, and inhibited tumor growth of E7-expressing tumors. C57BL/6 mice (five per group) were injected s.c. in the right leg with 5 × l05 TC-1 tumor cells per mouse. Ten days after tumor inoculation, EGCG was given in the drinking water at a concentration of 0, 0.1, 0.5, or 2.5 mg/mL for 5 d. To characterize the expression of HPV-16 E7 protein in TC-1 tumor cells, single-cell suspensions of isolated tumor were prepared and stained with E7-specific monoclonal antibody. Detection of apoptotic cells was done using phycoerythrin-conjugated rabbit anti-active caspase-3, a marker of apoptosis. The TC-1 tumor–challenged mice were characterized for tumor growth by measuring the tumor volume. The HPV-16 E7-specific CD8+ T-cell immune responses in treated mice were characterized by intracellular cytokine staining for IFN-γ followed by flow cytometry analysis of splenocytes. Characterization of tumor volume and the number of E7-specific CD8+ T cells was done 1 wk after the termination of ECGC treatment. A, representative flow cytometry data. B, columns, mean percentage of apoptotic cells observed in TC-1 tumors; bars, SD. C, columns, mean volume of TC-1 tumors; bars, SD. D, columns, mean number of IFN-γ–secreting E7-specific CD8+ T cells per 3 × 105 splenocytes; bars, SD.
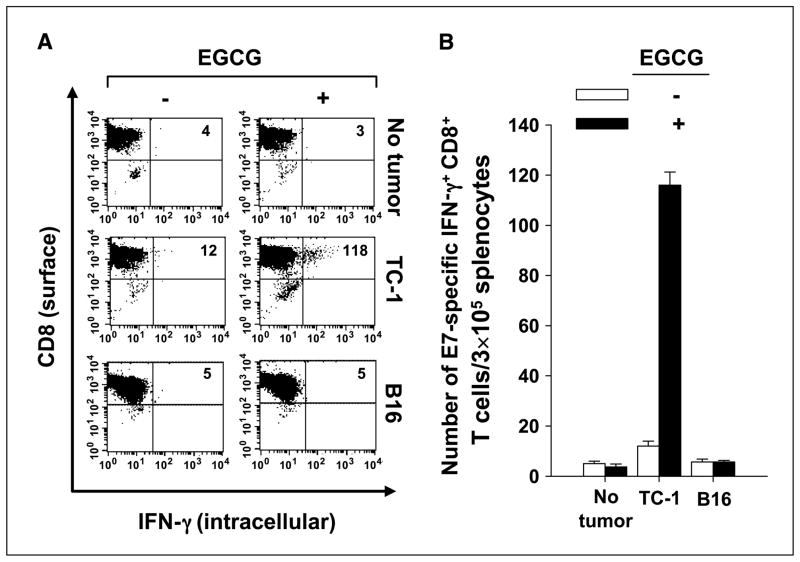
Treatment with EGCG generated higher levels of antigen-loaded dendritic cells in the draining lymph nodes of tumor-bearing mice
To determine whether apoptosis increased antigen cross-presentation in draining lymph nodes, tumor-bearing mice were treated with EGCG in the drinking water at a concentration of 0.5 mg/mL, as described in Fig. 1A and B. The selection of the EGCG dose at the concentration of 0.5 mg/mL was based on the observed findings from Fig. 1C and D. After EGCG treatment, inguinal lymph nodes were harvested. CD11c+ cells were enriched from a single-cell suspension of isolated inguinal lymph nodes and then incubated for 16 h with an E7-specific CD8+ T-cell line. Cells were then stained for both surface CD8 and intracellular IFN-γ and analyzed by flow cytometry to measure in vitro activation of E7-specific CD8+ T cells (8). As shown in Fig. 2, CD11c+-enriched cells isolated from mice treated with 0.5 mg/mL EGCG were more effective in stimulating E7-specific CD8+ T cells to secrete IFN-γ, when compared with CD11c+-enriched cells from mice not treated with EGCG. These effects are antigen specific as shown by the lack of response observed in mice bearing a non–E7-expressing tumor B16. These results show that tumor-bearing mice treated with EGCG generate higher levels of antigen-loaded dendritic cells in draining lymph nodes, which are able to activate antigen-specific CD8+ T-cell immune responses.
Figure 2.
TC-1 tumor treated with EGCG generated higher levels of E7 peptide–loaded dendritic cells in the draining lymph nodes of tumor-bearing mice. Ten days after tumor inoculation, tumor-bearing mice were given EGCG in their drinking water at a concentration of 0.5 mg/mL for 5 d. Inguinal lymph nodes were then harvested from the mice, and CD11c+ cells were enriched from a single-cell suspension of isolated inguinal lymph nodes using CD11c (N418) microbeads (Miltenyi Biotec). Enriched CD11c+ cells were analyzed by forward and side scatter and gated around a population of cells with size and granular characteristics of dendritic cells. isolated CD11c+ dendritic cells (2 × 104) were incubated for 16 h with 2 × 106 E7-specific CD8+ T cells. Cells were then stained for both surface CD8 and intracellular IFN-γ and analyzed by flow cytometry. A, representative flow cytometry data. B, columns, mean number of IFN-γ–secreting E7-specific CD8+ T cells per 3 × 105 cells; bars, SD. One representative experiment of three experiments done.
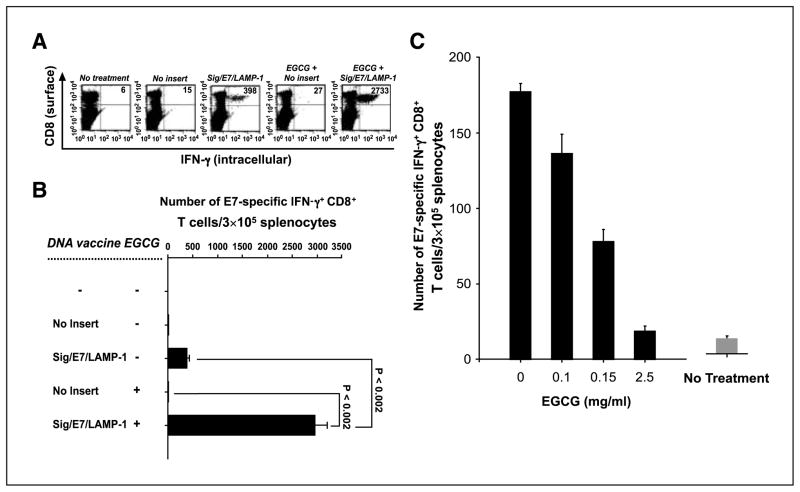
Combined DNA vaccination and EGCG treatment generated an enhanced E7-specific CD8+ T-cell immune response compared with monotherapy alone
We tested the ability of a combined strategy of DNA vaccination and EGCG treatment to generate E7-specific CD8+ T-cell immune responses. Mice were inoculated with 1 × 104 TC-1 tumor cells per mouse s.c. Three days later, the mice were vaccinated with Sig/E7/LAMP-1 DNA or a control DNA without any insert. EGCG was given in the drinking water at a concentration of 0.5 mg/mL at the time of vaccination and continued for 14 days. We assessed the E7-specific CD8+ T-cell immune response in the mice treated as described above. As shown in Fig. 3A and B, the combination treatment with Sig/E7/LAMP-1 DNA and EGCG resulted in a robust increase in the number of IFN-γ–secreting E7-specific CD8+ T-cell precursors compared with single therapy with Sig/E7/LAMP-1 DNA alone (at least a 6.5-fold increase) or EGCG treatment alone. Thus, our data show that a combination of Sig/E7/LAMP-1 DNA vaccine with orally given EGCG can significantly enhance tumor antigen-specific CD8+ T-cell immune responses.
Figure 3.
Combined DNA vaccination and EGCG treatment in the presence of tumor generated an enhanced E7-specific CD8+ T-cell immune response compared with monotherapy alone. C57BL/6 mice (five per group) were inoculated with TC-1 tumor cells (A and B) or 1× PBS (C) s.c. Three days later, the mice were vaccinated with either the Sig/E7/LAMP-1 DNA vaccine or a control DNA containing no insert. Mice received a booster of Sig/E7/LAMP-1 DNA vaccine 7 d after the first vaccination. A and B, in the presence of tumor, oral EGCG treatment (0.5 mg/mL) was initiated at the time of vaccination and continued for 14 d. C, in the absence of tumor, EGCG treatment was given at various concentrations (0, 0.1, 0.5, or 2.5 mg/mL) was initiated at the time of vaccination and continued for 14 d. Intracellular cytokine staining for IFN-γ was done followed by flow cytometry analysis to characterize HPV-16 E7-specific CD8+ T-cell immune responses in treated mice. A, representative set of the flow cytometry data. B and C, columns, mean number of E7-specific IFN-γ–secreting CD8+ T cells per 3 × 105 splenocytes; bars, SD. One representative experiment of three experiments done.
To determine whether EGCG treatment affects the generation of E7-specific CD8+ T cell–mediated immunity in DNA-vaccinated mice in the absence of tumor, we vaccinated C57BL/6 mice with the Sig/E7/LAMP-1 DNA intradermally and received a booster with the same DNA vaccine at the same dose via gene gun 1 week later. EGCG was given in the drinking water at various concentrations ranging from 0, 0.1, 0.5, or 2.5 mg/mL at the time of vaccination and continued for 14 days. HPV-16 E7-specific CD8+ T-cell immune responses in treated mice were characterized by intracellular cytokine staining followed by flow cytometry analysis 14 days after DNA vaccination. As shown in Fig. 3C, in the absence of tumor, the HPV-16 E7-specific CD8+ T-cell immune responses in vaccinated mice continued to decrease with the increasing amount of EGCG given orally. Taken together, these data indicated that the enhanced antigen-specific CD8+ T-cell immune responses observed by the DNA vaccine in combination with EGCG are only observed in the presence of tumor and are likely due to increased tumor cell apoptosis mediated by EGCG.
Levels of E7-specific CD8+ T-cell immune responses and antitumor effects against E7-expressing tumors are related to the dose of EGCG given
We further determined if the doses of EGCG treatment affects the generation of E7-specific CD8+ T cell–mediated immunity and antitumor effects in tumor-challenged mice. C57BL/6 mice were vaccinated and received a booster with the Sig/E7/LAMP-1 DNA or a DNA vector without insert and were subsequently challenged with TC-1 tumor cells 3 days after initial vaccination. EGCG was provided at various concentrations, specifically 0, 0.02, 0.1, 0.5, or 2.5 mg/mL, at the time of tumor challenge and continued for 11 days. Antigen-specific immune responses and tumor volume were measured 14 days after TC-1 challenge. As shown in Fig. 4A, the E7-specific CD8+ T-cell immune responses increased in a dose-dependent manner with the concentration of EGCG, at a dose range of 0 to 0.5 mg/mL in mice immunized with Sig/E7/LAMP-1 DNA vaccine. However, EGCG treatment at 2.5 mg/mL dramatically decreased the number of E7-specific CD8+ T cells compared with mice treated with EGCG at a dose of 0.5 mg/mL. Mice immunized with a DNA containing no insert failed to generate any significant levels of E7-specific CD8+ T-cell immunity at any of the tested concentrations. Similarly, tumor volume decreased in a dose-dependent manner with the concentration of EGCG in mice vaccinated with Sig/E7/LAMP-1 DNA (Fig. 4B). However, the tumor volume of the DNA-vaccinated mice treated with 2.5 mg/mL of EGCG was significantly larger than those mice treated with 0.5 mg/mL of EGCG. Taken together, in the presence of tumor, the antigen-specific immune responses and antitumor effects in DNA-vaccinated, EGCG-treated mice were enhanced at certain dose ranges of EGCG, and at higher doses of EGCG, the benefits of its antitumor effects may be countered by the potential immunosuppressive effects of EGCG on the immune system.
Figure 4.
Characterization of E7-specific CD8+ T-cell immune responses and antitumor effects generated by the Sig/E7/LAMP-1 DNA vaccine combined with EGCG. C57BL/6 mice (five per group for all of the studies) were vaccinated and received a booster with the Sig/E7/LAMP-1 DNA (solid columns) or a control DNA containing no insert (open columns) and were subsequently challenged with TC-1 tumor cells s.c. 3 d after initial vaccination. A and B, EGCG of various concentrations was provided in the drinking water, ranging from 0 to 2.5 mg/mL at the time of tumor challenge and continued for 11 d. C and D, EGCG was provided in the drinking water at the concentration of 0.5 mg/mL at the time of tumor challenge and continued for 11 d. A, intracellular cytokine staining for IFN-γ followed by flow cytometry analysis was done to characterize HPV-16 E7-specific CD8+ T-cell immune responses in treated mice. Columns, mean number of E7-specific IFN-γ–secreting CD8+ T-cell precursors per 3 × 105 splenocytes; bars, SD. B, in vivo tumor growth experiments. TC-1 tumor–challenged mice were evaluated for tumor growth by measuring the tumor volume 14 d after TC-1 tumor challenge. C, in vivo tumor growth experiments. Tumor growth was monitored by inspection and palpation twice a week following s.c. TC-1 tumor challenge. D, in vivo antibody depletion experiment to characterize the subsets of lymphocytes important for the antitumor effects. Antibody depletion was initiated 1 wk following the last immunization. Tumor growth was monitored by inspection and palpation twice a week.
Antibody depletion experiments showed that CD8+ T cells were important for the antitumor effects generated by the combined therapy
We also characterized the antitumor effects generated by immunization with the Sig/E7/LAMP-1 DNA vaccine or an empty DNA vector in the presence or absence of EGCG administration at a concentration of 0.5 mg/mL. Mice were vaccinated with the DNA vaccine and were subsequently challenged 3 days later with TC-1 tumor cells. Mice were then given plain drinking water or drinking water containing EGCG at the time of tumor challenge and continued for 11 days. Tumor growth was monitored twice a week by inspection and palpation. As shown in Fig. 4C, only the mice receiving the combined therapy with DNA vaccine and EGCG had tumor regression within 20 days after tumor challenge. All of the mice receiving Sig/E7/LAMP-1 DNA in combination with EGCG remained tumor-free 42 days after TC-1 tumor challenge. In contrast, all of the mice treated with Sig/E7/LAMP-1 or EGCG alone continued to show tumor growth.
To determine the subset of lymphocytes that are important for the antitumor effects generated by combined therapy, we did in vivo antibody depletion experiments in mice that were challenged with TC-1 tumors and treated with Sig/E7/LAMP-1 DNA vaccine in combination with EGCG at a concentration of 0.5 mg/mL. As shown in Fig. 4D, all of the mice depleted of CD8+ T cells did not show tumor regression. In comparison, all of the mice depleted of natural killer cells showed tumor regression similar to mice without antibody depletion. Eighty percent of mice depleted of CD4 cells showed tumor regression. These data suggest that CD8+ T cells are essential for the antitumor effects generated by the combined therapy.
Combined DNA vaccination and EGCG treatment generated an enhanced Th1 E7-specific CD4+ T-cell immune response
The ability of the Sig/E7/LAMP-1 targeting strategy to enhance antigen presentation to CD4+ T lymphocytes is achieved by targeting the expressed antigen to endosomal/lysosomal compartments and subsequently to the MHC class II antigen presentation pathway. To determine the nature of the E7-specific CD4+ T-cell response to the combined treatment with Sig/E7/LAMP-1 DNA vaccination and oral EGCG administration, we did intracellular cytokine staining for IFN-γ (secreted by Th1 cells) or IL-4 (secreted by Th2 cells) using flow cytometry analysis. C57BL/6 mice (five per group) were inoculated with TC-1 tumor cells s.c. Three days later, the mice were treated with either the Sig/E7/LAMP-1 DNA vaccine or a control DNA containing no insert. Mice received a booster of Sig/E7/LAMP-1 DNA or control DNA 7 days after the first vaccination. Oral EGCG treatment (0.5 mg/mL) was initiated at the time of DNA vaccination and continued for 14 days. Vaccination with Sig/E7/LAMP-1 DNA combined with EGCG administration generated significantly higher levels of E7-specific Th1 CD4+ T lymphocytes than vaccination with Sig/E7/LAMP-1 alone or EGCG treatment alone. In contrast, there was only a slight increase in E7-specific Th2 CD4+ T lymphocytes (Supplementary Fig. S1). These data suggest that the combination of Sig/E7/LAMP-1 DNA vaccination with oral EGCG treatment may contribute to an enhanced E7-specific CD4+ Th1 cell response.
Combined DNA vaccination and EGCG treatment generated significant long-term immune response and antitumor protection in treated mice
Ideally, a successful cancer treatment must be capable of generating effective long-term protection. Therefore, we assessed the ability of our combined therapy to generate long-term E7-specific CD8+ T-cell immune responses and protective antitumor effects. Intracellular cytokine staining was followed by flow cytometry analysis to identify E7-specific CD8+ T cells 1 and 7 weeks after the last immunization of the mice, which did not had evidence of tumor growth following the TC-1 tumor challenge. As shown in Fig. 5A and B, significant levels of the E7-specific IFN-γ CD8+ T lymphocyte response generated by the combined therapy were still present up to 7 weeks after immunization. All of the mice remained tumor-free.
Figure 5.
Combined DNA vaccination and oral EGCG treatment generated a significant long-term immune response and antitumor protection in cured mice. C57BL/6 mice (five per group) were vaccinated and received a booster with the Sig/E7/LAMP-1 DNA vaccine and subsequently challenged with TC-1 tumor cells 3 d after initial vaccination. Mice were treated with EGCG provided in the drinking water at a dose of 0.5 mg/mL at the time of tumor challenge and continued for 11 d. Intracellular cytokine staining followed by flow cytometric analysis was done at week 1 and week 7 after the last vaccination to characterize the levels of E7-specific CD8+ T cells generated in treated mice. A, representative set of the flow cytometric analysis data. One representative experiment of three experiments done. B, columns, mean number of E7-specific IFN-γ–secreting CD8+ T-cell precursors per 3 × 105 in splenocytes; bars, SD. C, long-term in vivo tumor protection experiments using TC-1, B-16 or B-16E7 tumor cells. To determine the long-term tumor protection ability of our vaccination strategy, we re-challenged the tumor-free mice with 5 × 104 tumor cells per mouse of TC-1, B16 or B16E7 7 wks after the last immunization.
To determine the long-term tumor protective ability of our vaccination strategy, we re-challenged the tumor-free mice i.v. with 5 × 104 TC-1 tumor cells 7 weeks after the final immunization. As shown in Fig. 5C, the naive mice exhibited 151.6 ± 42.3 tumor nodules 42 days after TC-1 challenge, whereas the mice treated with the Sig/E7/LAMP-1 DNA vaccine and oral EGCG treatment exhibited no pulmonary tumor nodules. Thus, in a tumor protection experiment, the combined therapy successfully prevented tumor nodule formation up to 7 weeks after vaccination. This long-term antitumor immunity was highly E7 specific because vaccinated mice were not protected from a non–E7-expressing tumor model B16. In comparison, an E7 antigen-expressing B16 tumor cell line (B16E7) failed to form a high number of tumor nodules in the vaccinated mice. Taken together, these data indicate that DNA vaccination combined with oral EGCG treatment generates a strong long-term antigen-specific CD8+ T-cell immune response with excellent long-term protective antitumor effects.
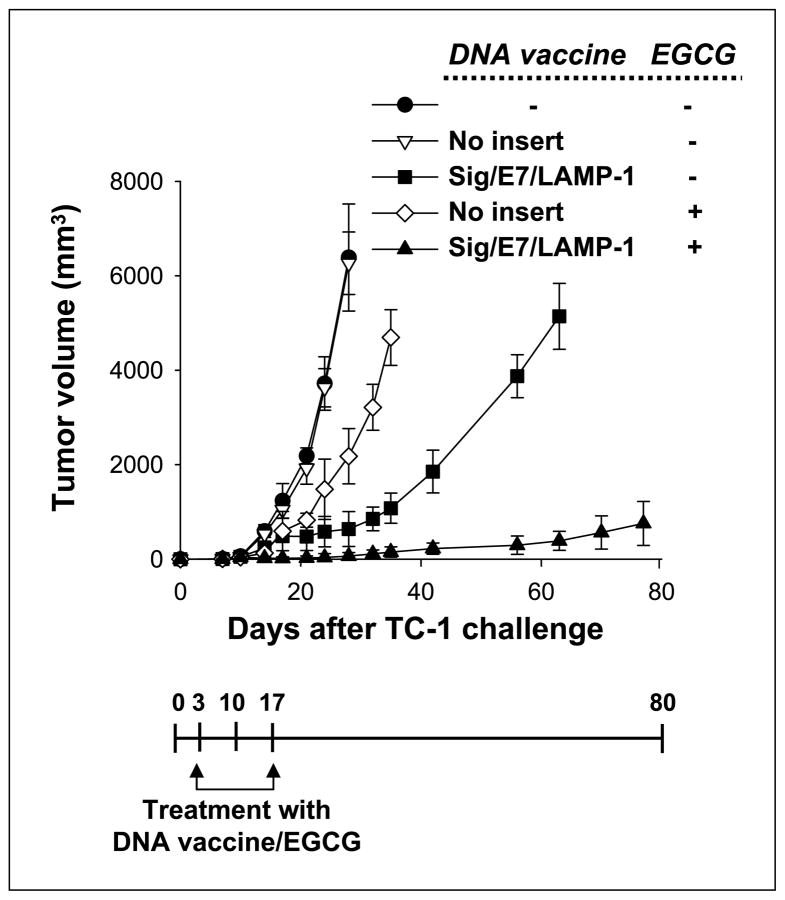
Combined DNA vaccination and EGCG treatment generated synergistic antitumor therapeutic effects than monother-apy alone
For the tumor treatment experiments, mice were inoculated with 1 × 104 TC-1 tumor cells per mouse s.c. Three days later, mice were vaccinated with Sig/E7/LAMP-1 DNA. EGCG was given in the drinking water at a concentration of 0.5 mg/mL at the start of the vaccination and continued for 14 days. Tumor volumes were measured and recorded twice per week for 8 weeks following immunization. We found that the tumors in mice treated with the combined cancer therapy remained the smallest in size (Fig. 6). This indicates that the combined strategy of DNA vaccination and oral EGCG treatment results in greater locoregional control of tumor than monotherapy alone in the TC-1 model.
Figure 6.
Combined DNA vaccination and oral EGCG treatment generated synergistic antitumor therapeutic effects compared with monotherapy alone. For the tumor treatment experiments, C57BL/6 mice (five per group) were inoculated s.c. with 1 × 104 TC-1 tumor cells per mouse. Three days after tumor inoculation, mice were vaccinated with Sig/E7/LAMP-1 DNA. Mice received a booster of Sig/E7/LAMP-1 DNA vaccine with the same dose and regimen 7 d after the first vaccination. EGCG was given in the drinking water at a concentration of 0.5 mg/mL at the start of the vaccination and continued for 14 d. Tumor volumes were measured and recorded twice per week for 8 wks following immunization. Tumor treatment experiments were done thrice to generate reproducible data.
Discussion
Administration of highly cytotoxic cancer drugs has severe adverse side effects and causes discomfort for cancer patients. These highly toxic drugs also limit host immune reactions against cancers. In this study, we have shown that oral administration of a low-toxic cancer drug EGCG resulted in complete tumor regression in mice vaccinated with Sig/E7/LAMP-1 DNA vaccine, without any severe systemic toxicity, such as loss of hair, weight, or lymphopenia. Importantly, this combined therapeutic strategy generated stronger tumor-specific cytotoxic T-cell immune responses, when compared with mice immunized with DNA vaccine alone. In addition, combined DNA vaccination and oral EGCG treatment generated a significant long-term immune response and protected mice from tumor growth upon repeated tumor challenges.
Immunotherapy and chemotherapy are generally rarely curative, even in small animal models of cancer, because many of these tumors rapidly grow to become large, bulky tumors, which present a challenge to either treatment regimen alone. At the start of this study, we expected that EGCG might aid DNA vaccine–mediated antitumor effects by inhibiting tumor growth, thereby allowing time for a curative immune response to develop. Unexpectedly, however, we observed a dramatic increase in E7-specific CD8+ T-cell immunity after combining DNA vaccination with oral administration of EGCG. This does not seem to be a direct adjuvant effect of EGCG on induction of E7-specific CD8+ T-cell immunity because oral administration of EGCG alone failed to increase the number of E7-specific CD8+ T cells generated by Sig/E7/LAMP-1 DNA vaccine in mice not bearing TC-1 tumors (see Fig. 3C). From these data, we propose that EGCG treatment may augment the antitumor immunity induced by genetic vaccination through enhanced tumor cell death, resulting in increased uptake of tumor antigens by antigen processing cells, such as dendritic cells, and enhanced antigen presentation in draining lymph nodes, which can then activate CD8+ T cells (for review, see refs. 28, 29). There is increasing evidence that the tumor antigens phagocytosed by bone marrow–derived dendritic cells are introduced not only into the MHC class II but also into the class I processing pathway to cross-prime naive T cells for development of potent immunity (30–32). Our data are consistent with this notion. Oral EGCG administration increased the percentage of apoptotic tumor cells and tumor-specific CD8+ T-cell immunity in a dose-dependent manner up to certain level of EGCG concentration (0.5 mg/mL). Thus, these data provide direct evidence of how, after chemotherapy, the increased number of dying tumor cells led to more tumor antigen-loaded CD11c+ dendritic cells in draining lymph nodes, resulting in increased tumor antigen-specific CD8+ T cells through cross-presentation.
Chemotherapy and immunotherapy have often been regarded as mutually exclusive. One of the reasons that contribute to this is lymphopenia, a common side effect of most cancer drugs, which has been implicated as being detrimental to the antitumor immune response. We observed that a high dose (2.5 mg/mL) of EGCG failed to enhance E7-specific CD8+ T-cell immunity in mice with or without TC-1 tumors (see Fig. 3C and Fig. 4A) and, on the contrary, even decreased the antitumor effect in TC-1 tumor–bearing mice (see Fig. 1C and Fig. 4B). This immune suppression may be related to an immune suppressive effect on T cells (33) and/or monocyte apoptosis (34) caused by high doses of EGCG, as has been reported by another group. Thus, in the presence of tumor, the antigen-specific immune responses and antitumor effects generated by EGCG was only observed at certain dose ranges (0.1–0.5 mg/mL). However, at higher doses of EGCG (2.5 mg/mL), the benefits of its antitumor effects may be countered by the potential immunosuppressive effects of EGCG on the immune system.
Another possible reason that chemotherapy and immunotherapy have often been regarded as mutually exclusive is that chemotherapy-induced apoptosis of cancer cells has been regarded as nonimmunogenic, or even tolerogenic, in the absence of inflammatory molecules, called “danger signals,” which are necessary for the maturation of antigen-presenting cells, such as dendritic cells. The apoptotic death of a tumor cell, in the absence of inflammation, might seem as normal tissue turnover and generate immune ignorance or tolerance against a tumor cell (for review, see refs. 35–37). However, there is now increasing evidence that in appropriate immunologic settings, cancer drug–induced apoptotic death of tumor cells can trigger the generation of effective antitumor immune responses (38–40). One such successful demonstration has been done with cyclophosphamide. It is known that appropriate doses of cyclophosphamide help to generate strong immune priming after immunotherapy by depleting regulatory T cells from animals bearing tolerogenic tumors (41, 42).
Although sufficient numbers of tumor antigens are present within apoptotic tumor cells, their ability to induce a CTL response in the host may not be sufficient to cause rejection of the tumor as observed in our study using EGCG alone as a cancer drug. Under our experimental conditions, only weak E7-specific T-cell immunity was shown in mice bearing tumors that were treated with only EGCG, and dramatic regressions of the tumors did not occur (see Fig. 1). Only in the setting of combined DNA vaccination with EGCG treatment did we observe enhanced E7-specific immune responses and antitumor therapeutic effects. One possible explanation for this observation is that EGCG induces tumor apoptosis, resulting in uptake of tumor antigen by professional antigen-presenting cells, such as dendritic cells, and cross-presentation in tumor-bearing mice. Dendritic cells play a critical role in priming and boosting adaptive immune responses. A number of investigators have shown that dendritic cells pulsed with tumor antigens induced cytokine production, enhanced proliferation of T cells in lymphoid tissues, and increased tumor infiltration by activated T cells (43–45). However, these strategies require ex vivo manipulation of dendritic cell and thus often are time and labor intensive. The combined therapy we propose in this study might be a promising approach for providing tumor-specific antigens to dendritic cells in draining lymph nodes for the enhancement of immune responses induced by vaccination.
We strongly believe that the results in the present study have great clinical implications. Because there are well-established effective chemotherapy protocols for controlling the rate of tumor growth and causing tumor cells to undergo apoptosis, immuno-therapy might be used synergistically with chemotherapy for enhancing antitumor activity. Based on the fact that complete tumor regression and long-lasting tumor immunity were observed in this present study, we suggest that this same strategy could be applied to the treatment of other tumors using various immuno-therapy models combined with effective cancer drugs. We have also tested a classic cytotoxic agent, such as cisplatin, in conjunction with DNA vaccination and have found that the combination of DNA vaccines with cisplatin also generated therapeutic effects in the control of TC-1 tumors compared with monotherapy alone.9 The efficacy of immuno-chemotherapy for cancer has often been limited by the toxicity of the cancer drugs. We predict that local treatment of tumors using other efficient cancer treatments, such as radiotherapy (for review, see ref. 46), antiangiogenesis agents (for review, see ref. 47), prodrug (for review, see ref. 48) strategies, or the use of drug delivery systems such as hydrogel-based systems (49), may be made more effective by increasing local toxic effects against tumors with minimal damage to host immune systems. Before undertaking such treatments, the routes and doses of drugs need to be optimized.
The HPV DNA vaccine described in the current study is mainly for therapeutic purpose. The recently Food and Drug Administration (FDA)–approved HPV vaccine is a preventive HPV vaccine using HPV virus-like particles (VLP). Although the HPV VLP vaccine is highly effective, it only includes four types of HPVs (HPV-6, HPV-11, HPV-16, and HPV-18). Thus, the current preventive HPV vaccine can only prevent up to 70% of all cervical cancer (for review, see ref. 50). Furthermore, the preventive HPV vaccine cannot control existing HPV infections or HPV-associated lesions. A significant population of patients is currently suffering from HPV-associated morbidity or mortality. Thus, development of therapeutic vaccines such as the one reported here represents an important endeavor to complement the limitation of the FDA-approved preventive HPV vaccine.
In summary, our present study shows that combined treatment with immune-modulating doses of chemotherapy can enhance the tumor-specific immune responses and antitumor effects induced by DNA vaccines. These data provide an immunologic rationale for testing various combinations of tumor vaccines with chemotherapy in patients with cancer. Many vaccine strategies and chemical drugs have been developed to control cancer. Considering that there are a multitude of possible combinations, a great deal of work could be forthcoming to evaluate combined therapy of tumor vaccines and chemotherapy for enhancing therapeutic effectiveness.
Supplementary Material
Acknowledgments
Grant support: National Cancer Institute Specialized Programs of Research Excellence in Cervical Cancer grant P50 CA098252 and Science Research Center/Engineering Research Center program of the Korea Science and Engineering Foundation/Ministry of Science and Technology grant R11-2005-017-03003-0.
We thank Dr. Richard Roden for his critical review of the article and Roanne Calizo and Archana Monie for assistance in preparation of the article.
Footnotes
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
Hung et al., personal communication.
References
- 1.Moniz M, Yeatermeyer J, Wu TC. Control of cancers by combining antiangiogenesis and cancer immunotherapy. Drugs Today (Barc) 2005;41:471–94. doi: 10.1358/dot.2005.41.7.893623. [DOI] [PubMed] [Google Scholar]
- 2.Boyd D, Hung CF, Wu TC. DNA vaccines for cancer. IDrugs. 2003;6:1155–64. [PubMed] [Google Scholar]
- 3.Devaraj K, Gillison ML, Wu TC. Development of HPV vaccines for HPV-associated head and neck squamous cell carcinoma. Crit Rev Oral Biol Med. 2003;14:345–62. doi: 10.1177/154411130301400505. [DOI] [PubMed] [Google Scholar]
- 4.Hung CF, Wu TC. Improving DNA vaccine potency via modification of professional antigen presenting cells. Curr Opin Mol Ther. 2003;5:20–4. [PubMed] [Google Scholar]
- 5.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2:342–50. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 6.Roden RB, Ling M, Wu TC. Vaccination to prevent and treat cervical cancer. Hum Pathol. 2004;35:971–82. doi: 10.1016/j.humpath.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 7.Ji H, Wang TL, Chen CH, et al. Targeting human papillomavirus type 16 E7 to the endosomal/lysosomal compartment enhances the antitumor immunity of DNA vaccines against murine human papillomavirus type 16 E7-expressing tumors. Hum Gene Ther. 1999;10:2727–40. doi: 10.1089/10430349950016474. [DOI] [PubMed] [Google Scholar]
- 8.Kim TW, Hung CF, Ling M, et al. Enhancing DNA vaccine potency by coadministration of DNA encoding antiapoptotic proteins. J Clin Invest. 2003;112:109–17. doi: 10.1172/JCI17293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim TW, Hung CF, Boyd D, et al. Enhancing DNA vaccine potency by combining a strategy to prolong dendritic cell life with intracellular targeting strategies. J Immunol. 2003;171:2970–6. doi: 10.4049/jimmunol.171.6.2970. [DOI] [PubMed] [Google Scholar]
- 10.Beltz LA, Bayer DK, Moss AL, Simet IM. Mechanisms of cancer prevention by green and black tea polyphenols. Anticancer Agents Med Chem. 2006;6:389–406. doi: 10.2174/187152006778226468. [DOI] [PubMed] [Google Scholar]
- 11.Khan N, Afaq F, Saleem M, Ahmad N, Mukhtar H. Targeting multiple signaling pathways by green tea polyphenol (−)-epigallocatechin-3-gallate. Cancer Res. 2006;66:2500–5. doi: 10.1158/0008-5472.CAN-05-3636. [DOI] [PubMed] [Google Scholar]
- 12.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 13.Zaveri NT. Green tea and its polyphenolic catechins: medicinal uses in cancer and noncancer applications. Life Sci. 2006;78:2073–80. doi: 10.1016/j.lfs.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 14.Mukhtar H, Ahmad N. Tea polyphenols: prevention of cancer and optimizing health. Am J Clin Nutr. 2000;71:1698–702S. doi: 10.1093/ajcn/71.6.1698S. discussion 703–4S. [DOI] [PubMed] [Google Scholar]
- 15.Yang CS, Maliakal P, Meng X. Inhibition of carcinogenesis by tea. Annu Rev Pharmacol Toxicol. 2002;42:25–54. doi: 10.1146/annurev.pharmtox.42.082101.154309. [DOI] [PubMed] [Google Scholar]
- 16.Chen D, Daniel KG, Kuhn DJ, et al. Green tea and tea polyphenols in cancer prevention. Front Biosci. 2004;9:2618–31. doi: 10.2741/1421. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad N, Feyes DK, Nieminen AL, Agarwal R, Mukhtar H. Green tea constituent epigallocatechin-3-gallate and induction of apoptosis and cell cycle arrest in human carcinoma cells. J Natl Cancer Inst. 1997;89:1881–6. doi: 10.1093/jnci/89.24.1881. [DOI] [PubMed] [Google Scholar]
- 18.Naasani I, Oh-Hashi F, Oh-Hara T, et al. Blocking telomerase by dietary polyphenols is a major mechanism for limiting the growth of human cancer cells in vitro and in vivo. Cancer Res. 2003;63:824–30. [PubMed] [Google Scholar]
- 19.Chow HH, Cai Y, Hakim IA, et al. Pharmacokinetics and safety of green tea polyphenols after multiple-dose administration of epigallocatechin gallate and polyphenon E in healthy individuals. Clin Cancer Res. 2003;9:3312–9. [PubMed] [Google Scholar]
- 20.Chow HH, Hakim IA, Vining DR, et al. Effects of dosing condition on the oral bioavailability of green tea catechins after single-dose administration of polyphenon E in healthy individuals. Clin Cancer Res. 2005;11:4627–33. doi: 10.1158/1078-0432.CCR-04-2549. [DOI] [PubMed] [Google Scholar]
- 21.Ahn WS, Yoo J, Huh SW, et al. Protective effects of green tea extracts (polyphenon E and EGCG) on human cervical lesions. Eur J Cancer Prev. 2003;12:383–90. doi: 10.1097/00008469-200310000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Bettuzzi S, Brausi M, Rizzi F, Castagnetti G, Peracchia G, Corti A. Chemoprevention of human prostate cancer by oral administration of green tea catechins in volunteers with high-grade prostate intraepithelial neoplasia: a preliminary report from a one-year proof-of-principle study. Cancer Res. 2006;66:1234–40. doi: 10.1158/0008-5472.CAN-05-1145. [DOI] [PubMed] [Google Scholar]
- 23.Gupta S, Hastak K, Ahmad N, Lewin JS, Mukhtar H. Inhibition of prostate carcinogenesis in TRAMP mice by oral infusion of green tea polyphenols. Proc Natl Acad Sci U S A. 2001;98:10350–5. doi: 10.1073/pnas.171326098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin KY, Guarnieri FG, Staveley-O’Carroll KF, et al. Treatment of established tumors with a novel vaccine that enhances major histocompatibility class II presentation of tumor antigen. Cancer Res. 1996;56:21–6. [PubMed] [Google Scholar]
- 25.Chang EY, Chen CH, Ji H, et al. Antigen-specific cancer immunotherapy using a GM-CSF secreting allogeneic tumor cell-based vaccine. Int J Cancer. 2000;86:725–30. doi: 10.1002/(sici)1097-0215(20000601)86:5<725::aid-ijc19>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 26.Chen CH, Wang TL, Hung CF, et al. Enhancement of DNA vaccine potency by linkage of antigen gene to an HSP70 gene. Cancer Res. 2000;60:1035–42. [PubMed] [Google Scholar]
- 27.Jeon JH, Choi KH, Cho SY, et al. Transglutaminase 2 inhibits Rb binding of human papillomavirus E7 by incorporating polyamine. EMBO J. 2003;22:5273–82. doi: 10.1093/emboj/cdg495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yewdell JW, Norbury CC, Bennink JR. Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo: implications for generating CD8+ T cell responses to infectious agents, tumors, transplants, and vaccines. Adv Immunol. 1999;73:1–77. doi: 10.1016/s0065-2776(08)60785-3. [DOI] [PubMed] [Google Scholar]
- 29.Guermonprez P, Valladeau J, Zitvogel L, Thery C, Amigorena S. Antigen presentation and T cell stimulation by dendritic cells. Annu Rev Immunol. 2002;20:621–67. doi: 10.1146/annurev.immunol.20.100301.064828. [DOI] [PubMed] [Google Scholar]
- 30.Toes RE, Ossendorp F, Offringa R, Melief CJ. CD4 T cells and their role in antitumor immune responses. J Exp Med. 1999;189:753–6. doi: 10.1084/jem.189.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marzo AL, Kinnear BF, Lake RA, et al. Tumor-specific CD4+ T cells have a major “post-licensing” role in CTL mediated anti-tumor immunity. J Immunol. 2000;165:6047–55. doi: 10.4049/jimmunol.165.11.6047. [DOI] [PubMed] [Google Scholar]
- 32.Stumbles PA, Himbeck R, Frelinger JA, Collins EJ, Lake RA, Robinson BW. Cutting edge: tumor-specific CTL are constitutively cross-armed in draining lymph nodes and transiently disseminate to mediate tumor regression following systemic CD40 activation. J Immunol. 2004;173:5923–8. doi: 10.4049/jimmunol.173.10.5923. [DOI] [PubMed] [Google Scholar]
- 33.Kawai K, Tsuno NH, Kitayama J, et al. Epigallocatechin gallate attenuates adhesion and migration of CD8+ T cells by binding to CD11b. J Allergy Clin Immunol. 2004;113:1211–7. doi: 10.1016/j.jaci.2004.02.044. [DOI] [PubMed] [Google Scholar]
- 34.Kawai K, Tsuno NH, Kitayama J, et al. Epigallocatechin gallate induces apoptosis of monocytes. J Allergy Clin Immunol. 2005;115:186–91. doi: 10.1016/j.jaci.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 35.Matzinger P. Tolerance, danger, and the extended family. Annu Rev Immunol. 1994;12:991–1045. doi: 10.1146/annurev.iy.12.040194.005015. [DOI] [PubMed] [Google Scholar]
- 36.Medzhitov R, Janeway CA., Jr Decoding the patterns of self and nonself by the innate immune system. Science. 2002;296:298–300. doi: 10.1126/science.1068883. [DOI] [PubMed] [Google Scholar]
- 37.Sauter B, Albert ML, Francisco L, Larsson M, Somersan S, Bhardwaj N. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J Exp Med. 2000;191:423–34. doi: 10.1084/jem.191.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowak AK, Robinson BW, Lake RA. Synergy between chemotherapy and immunotherapy in the treatment of established murine solid tumors. Cancer Res. 2003;63:4490–6. [PubMed] [Google Scholar]
- 39.Nowak AK, Lake RA, Marzo AL, et al. Induction of tumor cell apoptosis in vivo increases tumor antigen cross-presentation, cross-priming rather than cross-tolerizing host tumor-specific CD8 T cells. J Immunol. 2003;170:4905–13. doi: 10.4049/jimmunol.170.10.4905. [DOI] [PubMed] [Google Scholar]
- 40.Correale P, Cusi MG, Del Vecchio MT, et al. Dendritic cell-mediated cross-presentation of antigens derived from colon carcinoma cells exposed to a highly cytotoxic multidrug regimen with gemcitabine, oxaliplatin, 5-fluorouracil, and leucovorin, elicits a powerful human antigen-specific CTL response with antitumor activity in vitro. J Immunol. 2005;175:820–8. doi: 10.4049/jimmunol.175.2.820. [DOI] [PubMed] [Google Scholar]
- 41.Ghiringhelli F, Larmonier N, Schmitt E, et al. CD4+CD25+ regulatory T cells suppress tumor immunity but are sensitive to cyclophosphamide which allows immunotherapy of established tumors to be curative. Eur J Immunol. 2004;34:336–44. doi: 10.1002/eji.200324181. [DOI] [PubMed] [Google Scholar]
- 42.Polak L, Turk JL. Reversal of immunological tolerance by cyclophosphamide through inhibition of suppressor cell activity. Nature. 1974;249:654–6. doi: 10.1038/249654a0. [DOI] [PubMed] [Google Scholar]
- 43.Schnurr M, Galambos P, Scholz C, et al. Tumor cell lysate-pulsed human dendritic cells induce a T-cell response against pancreatic carcinoma cells: an in vitro model for the assessment of tumor vaccines. Cancer Res. 2001;61:6445–50. [PubMed] [Google Scholar]
- 44.Lou Y, Wang G, Lizee G, et al. Dendritic cells strongly boost the antitumor activity of adoptively transferred T cells in vivo. Cancer Res. 2004;64:6783–90. doi: 10.1158/0008-5472.CAN-04-1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nestle FO, Alijagic S, Gilliet M, et al. Vaccination of melanoma patients with peptide- or tumor lysate-pulsed dendritic cells. Nat Med. 1998;4:328–32. doi: 10.1038/nm0398-328. [DOI] [PubMed] [Google Scholar]
- 46.Barcellos-Hoff MH, Park C, Wright EG. Radiation and the microenvironment-tumorigenesis and therapy. Nat Rev Cancer. 2005;5:867–75. doi: 10.1038/nrc1735. [DOI] [PubMed] [Google Scholar]
- 47.Kerbel R, Folkman J. Clinical translation of angiogenesis inhibitors. Nat Rev Cancer. 2002;2:727–39. doi: 10.1038/nrc905. [DOI] [PubMed] [Google Scholar]
- 48.Denny WA. Tumor-activated prodrugs-a new approach to cancer therapy. Cancer Invest. 2004;22:604–19. doi: 10.1081/cnv-200027148. [DOI] [PubMed] [Google Scholar]
- 49.Konishi M, Tabata Y, Kariya M, et al. In vivo anti-tumor effect through the controlled release of cisplatin from biodegradable gelatin hydrogel. J Control Release. 2003;92:301–13. doi: 10.1016/s0168-3659(03)00364-x. [DOI] [PubMed] [Google Scholar]
- 50.Roden R, Wu TC. How will HPV vaccines affect cervical cancer? Nat Rev Cancer. 2006;6:753–63. doi: 10.1038/nrc1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.