Abstract
BACKGROUND
The Vulnerable Elders-13 Survey (VES-13) is a short tool that predicts functional decline and mortality over a 1–2 year follow-up interval. Prognosis over longer intervals is often needed in clinical care.
OBJECTIVE
To test the predictive properties of the VES-13 over a 5-year interval.
DESIGN
Longitudinal evaluation with mean follow-up of 4.5 years.
SETTING
Two managed-care organizations.
PARTICIPANTS
649 community-dwelling elders (age ≥75 and older) enrolled in the Assessing Care of Vulnerable Elders (ACOVE) observational study who screened positively for symptoms of fear of falling/falls, bothersome urinary incontinence, or memory problems.
MEASUREMENTS
VES-13 score (range 1–10, higher indicates worse prognosis); functional decline (defined as decline in count of 5 activities of daily living or nursing home entry); deaths.
RESULTS
Greater VES-13 scores are associated with greater predicted probability of death and decline among older patients over a mean observation period of 4.5 years. For each additional VES-13 point, the odds of the combined outcome of functional decline or death was 1.37 (95% CI 1.25–1.50), and the area under the receiver operating curve (AUC) was 0.75 (95% CI .71–.80). In the Cox proportional hazards model predicting time to death, the hazard ratio was 1.23 (95% CI 1.19–1.27) per additional VES-13 point.
CONCLUSION
This study extends the utility of the VES-13 to clinical decisions that require longer-term prognostic estimates of functional status and survival.
Keywords: vulnerable elder, functional decline, survival
INTRODUCTION
In response to an increasingly older population with growing needs to treat and prevent disability,1, 2 screening tools designed to target older populations at risk for functional decline and death have been developed.3, 4 The Vulnerable Elders Survey-13 (VES-13) is a simple screening tool that can be administered by non-medical personnel in approximately 4 minutes in person or over the telephone. Respondents need not understand detailed medical information (i.e., medical co-morbidities, medications, or lab values); rather, respondents describe their ability to perform daily tasks. Using a cutoff of three or more points, the VES-13 can identify elders who have 4.2 times the odds of functional decline or death over the next 2 years in comparison to those with scores of 2 or less.3 Greater VES-13 scores have been shown to prospectively predict death and functional decline over a 1-year follow up.4 The effect of VES-13 scores on outcomes is independent of gender and number of co-morbidities. The tool performed equally well when proxy respondents were used.
The VES-13 has been used to assess vulnerability among elderly patients in various clinical and research settings.5–8 However, determining whether cancer screening or preventive services are likely to be beneficial9–13 often involves predicting a patient's health and function over the next 5 or more years, rather than the relatively short 1–2 year time frame over which the VES-13 has been studied. Practice guidelines targeted to appropriate treatment of older patients, such as the Clinical Glidepaths,13 American Geriatric Society's diabetes care guidelines for elderly14 and Assessing the Care of Vulnerable Elders (ACOVE) quality indicators advocate consideration of prognosis before applying evidence-based guidelines derived from studies of younger patients,15 e.g., strict blood sugar control.16 Such guidelines encourage providers to consider whether or not implementation of the guideline will result in clinical benefit that fits within the horizon of an individual's life expectancy, especially for complex patients with functional impairments and multiple medical co-morbidities.
Our aim was to test whether the VES-13 could predict functional decline and death over an observation time of 5 years among older ambulatory patients with common geriatric conditions. To answer this research question, we conducted a longitudinal observational study of the Assessing Care of Vulnerable Elders-2 (ACOVE) cohort.17
METHODS
Study Sample and Data
The ACOVE-2 study sample was recruited from two large multi-site medical groups in the western United States during a 13-month baseline study period in 2002–3. ACOVE-2 was a controlled intervention targeted at the level of the medical practice to improve recommended care for three geriatric conditions: falls/fear of falling, bothersome symptoms of urinary incontinence, and memory impairment/dementia.17 Eligibility criteria consisted of advanced age (age ≥75 years), and one or more positive screening questions for the study conditions. Screening included three falls questions (“Have you fallen two or more times in the past year?” and “Have you fallen and hurt yourself or needed to see the doctor because of a fall in the past year?” and “Are you afraid that you would fall due to balance or walking problems?”), one urinary incontinence question (“Have you had a problem with urinary incontinence (or your bladder) that is bothersome enough that you would like to know more about how it could be treated?”), and either a three-item recall test for subject respondents or a yes/no question to the proxy pertaining to increasing trouble with memory. Study personnel at intervention and control sites identified potential participants based on age and performed screening and enrollment over the telephone prior to their office visits.
A practice-based quality improvement intervention was implemented in one site at each medical group. The intervention provided clinicians with a condition-specific clinic visit note template; an in-service on recommended care for the three geriatric syndromes, and availability of condition-specific patient educational materials.18 Control sites employed identical screening questions but were not exposed to the intervention. The intervention was shown to improve quality of care for falls and urinary incontinence17; however, overall quality of care for dementia and non-intervention conditions (i.e., coronary artery disease, diabetes) was not affected.17, 19
Upon enrollment into the ACOVE-2 study, all participants were asked all items from the VES-133 over the telephone. As a part of the VES-13, five functional activities of daily living, the short functional survey (SFS)20 were assessed. The SFS has been previously shown using nationally-representative cross-sectional data to identify 93% of elders who have difficulty with any of 11 activities of daily living.20 Detailed sociodemographic data (e.g., living alone, marital status, income) were collected in a separate telephone interview in 2003. Proxy respondents answered surveys for study subjects who were unable.
Patients were recontacted by telephone to collect functional status at approximately 5 years after enrollment (2006–7). The follow-up telephone interviews repeated the SFS that had been asked at baseline. Respondents (or their proxies) were also asked if their living situation had changed (i.e., admitted to long-term nursing home since baseline).
We obtained mortality data from the Social Security Death Index (SSDI) for dates of death up until the last day of the follow-up functional status interviews (August 27, 2007). Dates of deaths for deceased individuals were obtained by searching on first and last names, birth dates (self-reported with confirmation by medical records) and last known residences provided by the two medical groups in 2006. This was approved by the RAND Institutional Review Board.
Measures
The outcomes of interest were functional decline and death between the baseline assessment and the follow-up assessment. Functional decline was defined as a change in number of activities of daily living abilities on the 5-item SFS, with negative scores representing functional decline. The change in SFS score is highly correlated with the change in number of abilities determined by a longer survey of 12 functional abilities.21 A patient who was living at home at baseline but in a nursing home at follow-up was also considered to have functionally declined. Deaths were considered either as a separate outcome from functional decline in a trichotomous outcome (no decline versus functional decline versus death) or in a combined outcome with functional decline (no decline versus functional decline OR death). Time to death was calculated as the number of days between enrollment and death.
Our main predictor of interest was the VES-13 score (range between 0 and 10), with higher scores representing higher predicted risk of poor health outcome. Scores were calculated based on age (75–84 = 1 point, ≥85 = 3 points), self-rated health (fair or poor = 1 point), limitations in physical capability (difficulty with stooping, lifting 10 pounds, reaching, grasping small items, walking quarter of a mile, heavy housework; 1 point for each activity, maximum of 2 points), and functional limitations (“getting help with due to difficulty” or “not doing due to health” for shopping, managing money, walking across a room, light housework, bathing; 4 points for any of these 5 activities).5 Because the study only enrolled adults 75 and older, the lowest possible VES-13 score in this sample was 1.
Observation time was considered as a confounding variable in sensitivity analyses for functional decline because of variations in the time between enrollment screening and final follow-up.
Analysis
Baseline characteristics for the overall and subsamples based on positive screen condition (fear of falls/falls, urinary incontinence, memory problems/dementia) were calculated. Pairwise comparisons (t-tests for means, test of proportions for proportions) were performed using the largest subsample (falls) as the comparison group against the two smaller subsamples (incontinence and dementia). Because participants could screen positively for more than one condition, pairwise comparison t-tests did not include those who screened positively for both conditions being considered.
For the overall sample, mean baseline VES-13 scores were compared between elders with no decline, functional decline, or death (the trichotomous outcome). Mean baseline VES-13 scores were also compared among elders with no decline versus death or functional decline (the dichotomous outcome). Outcome categories were compared using pairwise two-sample t-tests and one-way analysis of variance.
Because the sample experienced loss-to-follow up with respect to the functional decline outcome (i.e., unable to contact by telephone for an interview), we developed and employed attrition weights for use in all regression analyses. These weights account for the possibility that those with poorer health would be more likely to undergo functional decline but also be more likely to be lost to follow-up. We assumed complete information regarding death from the Social Security Master Death Files. Weights were based on the inverse probability of obtaining follow-up functional status data, using a model that included baseline VES-13 score, gender, age, low income, living alone, intervention versus control site, and geographical location.5, 7 The probability of inclusion in the follow-up sample for those who died before the day of the last follow-up interview was assumed to be 1. Additional details regarding the development of these weights in a similarly structured dataset has been previously described4.
Next, the unadjusted association of the VES-13 with the dichotomous and trichotomous outcomes were estimated using logistic and multinomial logistic regression, respectively. Stratified analyses were also performed within subsamples defined by condition. To test whether the effect of the VES-13 varied between the condition-based subsamples, we tested fully interacted models on the full analytic sample (i.e., including multiplicative terms between VES-13 score and the three conditions). Sensitivity tests assessed the extent to which variation in follow-up time and intervention status confounded the association between VES-13 score and outcomes at follow-up (functional decline versus no decline only for variation in follow-up).
Next, we performed survival analysis using the time to death variable. We selected a cutoff score for better versus poorer prognosis to compare Kaplan-Meier survival curves between the two groups. The VES score was then entered into the Cox proportional hazards model as a continuous variable to evaluate the hazard of each point on death versus survival throughout the 5-year study period. For this analysis we considered the overall sample as well as subsamples for the three geriatric conditions and a fully interacted model (VES-13 score with three conditions) on the full sample.
Patients who saw more than one clinician during the ACOVE study were assigned to a modal primary care provider. Thirty-nine clinicians were identified as primary care providers at the two practices. They cared for 1 to 46 (mean 17) patients in our study. We adjusted all analyses for clustering by primary care provider. All analyses were conducted using Stata 10 (Stata Corp., College Station, TX) statistical software.
RESULTS
Of the 3010 elders age ≥ 75 who had scheduled primary care visits during the ACOVE-2 study, 2671 (88.7%) were screened for the three geriatric syndromes, 57 (1.9%) could not be screened due to language barrier, 50 (1.7%) had no available proxy, 78 (2.6%) refused to participate, and 154 (5.1%) could not be reached by telephone. Of those who were screened, 784 (29.3%) screened positively for one or more of the geriatric conditions (602 [22.5%] for fear of fall/falls, 279 [10.4%] for urinary incontinence, and 104 [3.9%] for memory impairment/dementia). Those who screened positively for one or more conditions were older (mean 82 versus 81, p<.001), less often male (32.8% versus 45.3%, p<.001), and more likely to participate by proxy respondent (19.2% versus 4.7%, p<.001) than those who screened negative for all three conditions. Of those who screened positively, 649 (82.8%) consented to participate in the study. Those who consented did not differ significantly (p<.05) from those who declined with respect to age, gender, or need for proxy respondent.
There were 501 who screened positively for falls, 233 who screened positively for UI, and 78 who screened positively for dementia. Individuals could screen positive for more than one condition: 115 screened positively for both falls and UI, 44 for both falls and dementia, 16 for both UI and dementia, and 11 for all three conditions. At baseline the mean age was 81 for the original ACOVE-2 sample as well as for the falls and incontinence subsamples. The dementia subsample was slightly older at mean 84 years (p=.10 compared to falls subsample). The overall sample was 37.2% male, with fewer males in the incontinence sample (24.9% versus 40.0% for the falls subsample, p<.001). A proxy respondent was used for 19.9% of participants, with fewer proxies in the UI group (13.6%, p= .03) and exclusively proxy respondents for the dementia group (p<.001). The baseline count of daily activity abilities (Short Functional Survey Score, range 0–5) averaged 4.0 for the overall sample, but the UI group was slightly more independent (4.2 abilities, p=.008) and the dementia group was less independent (2.6 abilities, p=.005) than the falls subsample (3.9 abilities). While the overall sample baseline VES-13 score averaged 4.7, the incontinence subsample had a slightly lower average (4.3, p=<.001), whereas the dementia subsample had a higher mean (6.9, p=.004) compared to the falls subsample (4.9) (Table 1).
Table 1.
Baseline Characteristics
| Overall Sample | Fear of Falls/Falls Subsample* | Urinary Incontinence Subsample* | Memory Problem/Dementia Subsample* | |
|---|---|---|---|---|
| N | 508 | 395 | 169 | 63 |
| Age (mean) | 81.3 | 81.4 | 81.3 | 83.8† |
| Male (%) | 37% | 40% | 25%§ | 46% |
| Use of proxy respondent (%) | 20% | 18% | 14%§ | 100%§ |
| Baseline Short Functional Survey Score (Range 0–5)** | 4.0 | 4.0 | 4.2† | 2.6† |
| MeanVES-1 score (range 1–10) | 4.7 | 4.9 | 4.3† | 7.0† |
Participants could screen into more than one subsample
p<.05 for comparison of means using two-sample t-test, comparison group = falls subsample.
T-tests between falls and UI subsamples omitted 115 individuals who were in both groups; t-tests between falls and dementia omitted 44 individuals who were in both groups.
p<.05 for comparison of two proportions, comparison group = falls subsample. Tests of proportions between falls and UI subsamples omitted 115 individuals who were in both groups;tests of proportions between falls and dementia omitted 44 individuals who were in both groups.
Number of functional abilities among 5 in the Short Functional Survey (shopping, doing light housework, bathing, managing finances, and walking)
Among the full ACOVE-2 sample (n=649), 222 (34..2% died during the study period, with date of death ranging from 7 days to 5.8 years after study enrollment. Perfect matches (exact date of birth, same full name, and same city of death) were found for 195 (87.8%), a different city of death (but exact birth date and name) was listed for 21 (9.5%), initials for first name were found on Social Security records instead of full first name (but same date of birth and city of death was same as city of residence) for 3 individuals (1.4%), and date of birth differed by one digit (but same full name and city of residence) for 6 (2.7%).
We interviewed 295 of the surviving cohort (69.1%) to obtain their follow-up functional status. Those who survived but were lost to follow-up interview (n = 141) did not differ significantly on the basis of mean age (age 81 for both groups, p=.78) or low income (28.4% versus 26.0%, p=.6) compared to those for whom who we had follow-up information (interview or mortality data, n=508). However, those lost to follow up were more likely to be female (37.2% versus 22.0%, p=.007) and have a lower baseline VES-13 score (mean 4.2 versus 4.7, p=.04) compared to our analytic sample. Mean time to interview was 4.5 years (3.8–5.3 years, standard deviation = .35 years).
Of those interviewed (n=295), 116 (39.3%) experienced functional decline. Seven participants who underwent functional decline also died after their interview but within the study period (range = 38 to 214 days after their interviews). We classified these individuals as deaths for the trichotomous (no decline versus decline versus death) outcome. Therefore, among our analytic sample (n=508), 177 (34.8%) survived and did not decline, 109 underwent functional decline (21.5%), and 222 (43.7%) died.
The mean baseline VES-13 score for the trichotomous outcome categories were 3.1 for those who did not decline, 4.8 for those who declined, and 6.0 for those who died (p<.0001). The mean baseline VES-13 score was 5.6 for those who declined or died (vs. 3.1 for those who did not, p<.0001).
The VES-13 score (multinomial logistic regression unadjusted for covariates but weighted for non-response and adjusted for clustering) was associated with greater odds of death versus no decline (OR 1.45 per point, 95% CI 1.33–1.58) and decline versus no decline (OR 1.28. 95% CI 1.14–1.42) (Table 2). An elder with a baseline VES-13 score of 1 (age ≥ 75 only) had a predicted probability of death of 5% and a 66% chance of surviving without functional decline or death over the 5-year follow up period. In contrast, an elder with a baseline VES-13 score of 10 had a 64% predicted risk of dying and a 10% chance of surviving without functional decline over the 5-year follow up period (Figure 1).
Table 2.
Association between Vulnerable Elders-13 Survey and 5-year Functional Status and Survival Outcomes
| Trichotomous outcome | Dichotomous outcome | |||
|---|---|---|---|---|
| Sample | OR of Functional Decline versus No Decline per additional point on VES-13* (95% CI) | OR of Death versus No Decline OR per additional point on VES-13*(95% CI) | OR of Functional Decline OR Death versus No Decline per additional point on VES-13* (95% CI) | AUC (95% CI) |
| Overall (n=508) | 1.28 (1.14–1.42) | 1.45 (1.33–1.58) | 1.37 (1.25–1.50) | .75 (.71–.80) |
| Fear of Falls/Falls Subsample (n=395) | 1.20 (1.07–1.34) | 1.40 (1.27–1.55) | 1.30 (1.18–1.44) | .72 (.67–.77) |
| Urinary Incontinence Subsample (n=169) | 1.38 (1.16–1.64) | 1.49 (1.28–1.74) | 1.43 (1.21–1.69) | .79 (.72–.86) |
| Memory Problem/Dementia Subsample (n=63) | 1.71 (1.15–2.55) | 1.72 (1.16–2.57) | 1.72 (1.16–2.55) | .83 (.62–1.0) |
AUC: area under the receiver operating characteristic curve; OR: odds ratio; VES-13: Vulnerable Elders-13 Survey
VES-13 score (possible range = 0–10) is calculated as sum of: age (1 point for age 75–84, 3 points for age ≥85 ), self-rated health (1 point for fair or poor), limitations in physical capability (difficulty with stooping, lifting 10 pounds, reaching, grasping small items, walking quarter of a mile, heavy housework; one point for each up to 2 points), and functional limitations (getting help with shopping, managing money, walking across a room, light housework, bathing; four points for any difficulty requiring help or not done due to health)
The difference in effect of the VES-13 by screened conditions was not significant in a pooled sample (p=.37, .27 joint test of interaction terms [VES-13 score with each condition] in the models using trichotomous and dichotomous outcomes, respectively).
Figure 1.
 Predicted probability of death
Predicted probability of death
 Combined probability of functional decline + death
Combined probability of functional decline + death
VES-13 = Vulnerable Elders-13 Survey
Predicted probabilities of death (dotted line) or the additive probability of functional decline and death (solid line) were calculated based on a multinomial logistic regression with cluster adjustment for primary care clinician and weighted for loss to follow up.
Using clustered logistic regression, with sample weighting for non-response, each 1 point increase in baseline VES-13 was associated with a 1.37 times higher odds of death or decline (95% CI 1.25–1.50; Table 2). The area under the receiver operating characteristic curve was 0.75 (95% CI .71–.80) (Table 2). The sensitivities and specificities for cutoff values from 2 to 10 for this sample of patients age 75 and older who screened positive for a geriatric condition appear in Table 3. A cutoff VES-13 score of 5 results in a sensitivity and specificity of .69. Other cut-points can be selected based on desired sensitivity or specificity.
Table 3.
Unadjusted Sensitivity and Specificities for Predicting Poor Health Outcomes At Different Cutoff Values of the Vulnerable Elders-13 Score
| Outcome: Death or Functional Status Decline | ||
|---|---|---|
| Cutoff value | Sensitivity | Specificity |
| ≥2 | 92% | 37% |
| ≥3 | 86% | 54% |
| ≥4 | 69% | 69% |
| ≥5 | 60% | 75% |
| ≥6 | 51% | 80% |
| ≥7 | 45% | 81% |
| ≥8 | 32% | 91% |
| ≥9 | 17% | 99% |
| 10 | 7% | 99% |
| Outcome: Death Only | ||
|---|---|---|
| Cutoff value | Sensitivity | Specificity |
| ≥2 | 92% | 25% |
| ≥3 | 87% | 37% |
| ≥4 | 74% | 56% |
| ≥5 | 66% | 66% |
| ≥6 | 58% | 71% |
| ≥7 | 50% | 74% |
| ≥8 | 39% | 85% |
| ≥9 | 21% | 95% |
| 10 | 9% | 98% |
Adding length of follow-up time or an indicator of intervention status as a predictor in the regression models did not result in substantial changes in the odds ratios associated with VES-13 scores.
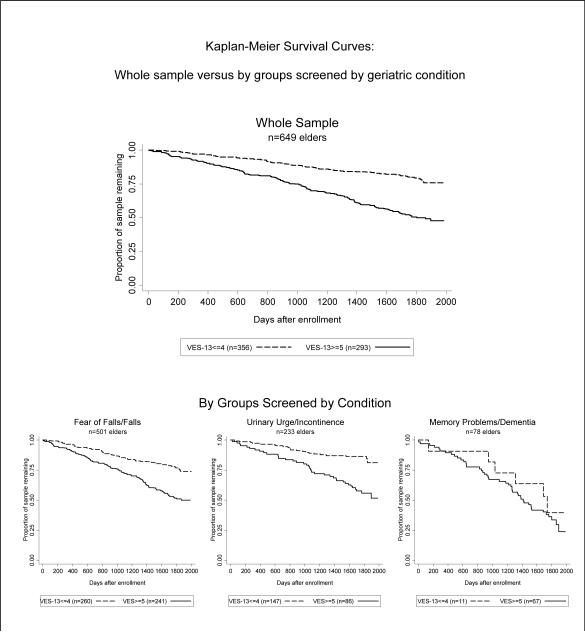
The mean number of years until death among decedents was 2.9 (range 0.02 to 5.8). The sample was divided into better versus poorer prognosis (VES-13 score of ≤ 4 versus ≥ 5). Kaplan-Meier curves appear in Figure 2. The Cox proportional hazard ratio associated with each additional VES-13 point was 1.23 (95% CI 1.19–1.27).
Figure 2.
Poorer (higher) VES-13 scores are associated with greater risk of dying throughout the 4.5 year follow up period for the overall whole sample, the falls subset, and the urinary incontinence subset. The dementia subset experienced similar mortality rates above and below VES-13 cutoff scores of 5. The hazard ratios associated with each 1-point increase on the VES-13 were: (whole sample) HR 1.23 (95% CI 1.19–1.27); (falls subset) HR 1.21 (95% CI 1.16–1.25); (urinary incontinence subset) HR 1.22 (95% CI 1.14–1.31); (dementia subset) HR1.07 (95% CI 0.95–1.22). The difference in effect of the VES-13 by screened conditions was not significant in a pooled model (p=.29 for joint test of interaction terms [VES-13 score with each condition]).
VES-13: Vulnerable Elders-13 Survey
The direction of the effect of the VES-13 scores on trichotomous and dichotomous outcomes was similar within the three condition subgroups (Table 2). Each additional point on the VES-13 appears to have had a greater effect on poor outcomes for the dementia subgroup, but the confidence intervals were wider due to smaller subsample. Upon examination of graphical representation of the multinomial logistic regressions and survival analyses by subsample (Figures 1 and 2), VES score consistently predicted increasingly poor outcomes in all subsamples. The difference in effect of the VES-13 by screened conditions was not significant in a pooled sample (p=.37, .27, .29 for joint test of interaction terms in the models using trichotomous, dichotomous, and death-only outcomes, respectively).
DISCUSSION
In this prospective study of functional status and survival outcomes among older ambulatory care patients, we found that the VES-13 is an excellent predictor of health outcomes over a 5-year period. Increasing scores on the VES-13 are linearly related to the odds of death and functional decline. Predicted probabilities can be translated into information that can readily be used to make clinical decisions.
This study extends previous work testing the VES-13, a simple survey that can be obtained over the telephone in 4 minutes with patients or their proxies, and does not require patient knowledge of their medical diagnoses, laboratory values, or medications.5, 7 The predictions from the current study are based on 5-year, rather than 1–2 year outcomes, thus greatly expanding the clinical utility of the tool. Possible applications include informing decisions regarding screening tests and aggressive chronic disease therapies that might result in benefit if an individual has a better 5-year prognosis,9 for example, screening colonoscopy12 or mammography22. A prognostic tool available at the time of the clinical encounter can enable proposed strategies to guide recommendations concerning preventive care and application of evidence-based guidelines to older patients.9, 10, 13, 14, 16
These results can also be used to select a VES-13 cutoff score to screen for appropriateness of interventions. The choice of higher (e.g., VES-13 score of 7, which yields a relatively higher specificity) versus lower cutoff (e.g., VES-13 score of 3 which yields a relatively higher sensitivity) would depend on the goal of the screening. For example, a more sensitive cutoff score may be instrumental in targeting those at risk for death or decline for enrollment into a low cost intervention, while better specificity may be more important when deciding to withhold an intervention because likelihood of survival to realize benefit is low.
The strength of this study was that it was a prospectively-designed longitudinal study of both functional decline and survival. The two outcomes studied are valued by older patients, caregivers, and clinicians. The cohort was a “real-world” sample of older patients suffering from early to late symptoms of geriatric conditions, recruited from primary care clinics. They represent a vulnerable cohort of elders who sustained substantial mortality and functional decline over the 5-year follow-up period.
The main limitation of this study is that the sample is limited to patients with three baseline conditions (falls, incontinence, cognitive impairment), as well as an older age criteria than prior studies. The resulting restricted range of baseline health status may have understated the association of the VES-13 scores with outcomes. In addition, because the overall health of our sample may be worse than the general older population due to their screened conditions, the predicted rates of death and decline may be slightly higher in comparison to an individual of similar age, functional status, and (non-screened) co-morbid conditions.
There are other potential limitations to this study. The ACOVE-2 intervention to improve care for three geriatric conditions was implemented in half of the sample. Although the intervention had no effect on non-intervention conditions,19 and therefore we did not suspect that it would have an effect on long-term outcomes, we assessed possible effects in sensitivity analysis. In addition, we had substantial loss to follow-up for the functional decline outcome of surviving members of the sample, which we attempted to address by including sampling weights in all analyses. Finally, the small size of the memory impairment/dementia subsample limits our ability to draw conclusions specific to this subpopulation and our results need to be repeated among larger sample specifically diagnosed with cognitive impairment or dementia.
In conclusion, this prospective study confirmed that the VES-13 is a useful prognostic tool over a 5-year follow-up period among vulnerable older primary care patients. This simple survey may be helpful in clinical decisions that depend on prognostic assessments during this time horizon.
ACKNOWLEDGEMENTS
Dr. Min is supported by grants from the NIH (R21 HS017621-01) and NIA-UCLA (K12 AG001004). Mr. Yoon was a NIA/AFAR & Lillian R. Gleitsman Medical Student Training in Aging Research (MSTAR) Program Scholar in 2008. Dr. Mariano was supported by a Faculty Development to Advance Geriatric Education (FD-AGE) award from the Donald W. Reynolds Foundation. This project also received support from the UCLA Older Americans Independence Center (Pepper Center). This paper was presented as an abstract at the 2009 American Geriatric Society Scientific Meeting.
Sponsor's Role: Pfizer Inc supported the recruitment of the original ACOVE-2 cohort, but had no role in obtaining outcomes data, data analysis, or preparation of this manuscript. The UCLA Older Americans Independence Center (Pepper Center) provided support for statistical analysis.
Footnotes
Author Contributions: Lillian Min: concept and design, acquisition of subjects and/or data, analysis and interpretation of data, and preparation of manuscript.
William Yoon: acquisition of subjects and/or data, analysis and interpretation of data, and preparation of manuscript.
Jeff Mariano: concept and design, acquisition of subjects and/or data, and preparation of manuscript.
Neil S. Wenger: concept and design, acquisition of subjects and/or data, analysis and interpretation of data, and preparation of manuscript.
Marc Elliot: concept and design, analysis and interpretation of data, and preparation of manuscript.
Caren Kamberg: concept and design, acquisition of subjects and/or data.
Debra Saliba: concept and design, acquisition of subjects and/or data, analysis and interpretation of data, and preparation of manuscript.
REFERENCES
- 1.Covinsky KE, Eng C, Lui LY, Sands LP, Yaffe K. The last 2 years of life: functional trajectories of frail older people. J Am Geriatr Soc. 2003;51:492–498. doi: 10.1046/j.1532-5415.2003.51157.x. [DOI] [PubMed] [Google Scholar]
- 2.Guralnik JM, LaCroix AZ, Branch LG, Kasl SV, Wallace RB. Morbidity and disability in older persons in the years prior to death. Am J Public Health. 1991;81:443–447. doi: 10.2105/ajph.81.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Saliba D, Elliott M, Rubenstein LZ, et al. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2001;49:1691–1699. doi: 10.1046/j.1532-5415.2001.49281.x. [DOI] [PubMed] [Google Scholar]
- 4.Min LC, Elliott MN, Wenger NS, Saliba D. Higher vulnerable elders survey scores predict death and functional decline in vulnerable older people. J Am Geriatr Soc. 2006;54:507–511. doi: 10.1111/j.1532-5415.2005.00615.x. [DOI] [PubMed] [Google Scholar]
- 5.Arora VM, Johnson M, Olson J, et al. Using assessing care of vulnerable elders quality indicators to measure quality of hospital care for vulnerable elders. J Am Geriatr Soc. 2007;55:1705–1711. doi: 10.1111/j.1532-5415.2007.01444.x. [DOI] [PubMed] [Google Scholar]
- 6.McGee HM, O'Hanlon A, Barker M, et al. Vulnerable older people in the community: relationship between the Vulnerable Elders Survey and health service use. J Am Geriatr Soc. 2008;56:8–15. doi: 10.1111/j.1532-5415.2007.01540.x. [DOI] [PubMed] [Google Scholar]
- 7.Sternberg SA. The Vulnerable Elders Survey: a tool for identifying vulnerable older people in the community. J Am Geriatr Soc. 2003;51:139–140. doi: 10.1034/j.1601-5215.2002.51030.x. [DOI] [PubMed] [Google Scholar]
- 8.Mohile SG, Bylow K, Dale W, et al. A pilot study of the vulnerable elders survey-13 compared with the comprehensive geriatric assessment for identifying disability in older patients with prostate cancer who receive androgen ablation. Cancer. 2007;109:802–810. doi: 10.1002/cncr.22495. [DOI] [PubMed] [Google Scholar]
- 9.Walter LC, Covinsky KE. Cancer screening in elderly patients: a framework for individualized decision making. Jama. 2001;285:2750–2756. doi: 10.1001/jama.285.21.2750. [DOI] [PubMed] [Google Scholar]
- 10.O'Connor PJ. Adding value to evidence-based clinical guidelines. Jama. 2005;294:741–743. doi: 10.1001/jama.294.6.741. [DOI] [PubMed] [Google Scholar]
- 11.Mehr DR, Tatum PE., 3rd Primary prevention of disease of old age. Clin Geriatr Med. 2002;18:407–430. doi: 10.1016/s0749-0690(02)00019-8. [DOI] [PubMed] [Google Scholar]
- 12.Lin OS, Kozarek RA, Schembre DB, et al. Screening colonoscopy in very elderly patients: prevalence of neoplasia and estimated impact on life expectancy. Jama. 2006;295:2357–2365. doi: 10.1001/jama.295.20.2357. [DOI] [PubMed] [Google Scholar]
- 13.Flaherty JH, Morley JE, Murphy DJ, Wasserman MR. The development of outpatient Clinical Glidepaths. J Am Geriatr Soc. 2002;50:1886–1901. doi: 10.1046/j.1532-5415.2002.50521.x. [DOI] [PubMed] [Google Scholar]
- 14.Brown AF, Mangione CM, Saliba D, Sarkisian CA. Guidelines for improving the care of the older person with diabetes mellitus. J Am Geriatr Soc. 2003;51:S265–280. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 15.Solomon DH, Wenger NS, Saliba D, et al. Appropriateness of quality indicators for older patients with advanced dementia and poor prognosis. J Am Geriatr Soc. 2003;51:902–907. doi: 10.1046/j.1365-2389.2003.513331.x. [DOI] [PubMed] [Google Scholar]
- 16.Shekelle P, Vijan S. Quality indicators for the care of diabetes mellitus in vulnerable elders. J Am Geriatr Soc. 2007;55(Suppl 2):S312–317. doi: 10.1111/j.1532-5415.2007.01337.x. [DOI] [PubMed] [Google Scholar]
- 17.Wenger NS, Roth CP, Shekelle PG, et al. A practice-based intervention to improve primary care for falls, urinary incontinence, and dementia. J Am Geriatr Soc. 2009;57:547–555. doi: 10.1111/j.1532-5415.2008.02128.x. [DOI] [PubMed] [Google Scholar]
- 18.Reuben DB, Roth C, Kamberg C, Wenger NS. Restructuring primary care practices to manage geriatric syndromes: the ACOVE-2 intervention. J Am Geriatr Soc. 2003;51:1787–1793. doi: 10.1046/j.1532-5415.2003.51565.x. [DOI] [PubMed] [Google Scholar]
- 19.Ganz DA, Wenger NS, Roth CP, et al. The effect of a quality improvement initiative on the quality of other aspects of health care: the law of unintended consequences? Med Care. 2007;45:8–18. doi: 10.1097/01.mlr.0000241115.31531.15. [DOI] [PubMed] [Google Scholar]
- 20.Saliba D, Orlando M, Wenger NS, Hays RD, Rubenstein LZ. Identifying a short functional disability screen for older persons. J Gerontol A Biol Sci Med Sci. 2000;55:M750–756. doi: 10.1093/gerona/55.12.m750. [DOI] [PubMed] [Google Scholar]
- 21.Min LC, Wenger NS, Reuben DB, Saliba D. A short functional survey is responsive to changes in functional status in vulnerable older people. J Am Geriatr Soc. 2008;56:1932–1936. doi: 10.1111/j.1532-5415.2008.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Badgwell BD, Giordano SH, Duan ZZ, et al. Mammography before diagnosis among women age 80 years and older with breast cancer. J Clin Oncol. 2008;26:2482–2488. doi: 10.1200/JCO.2007.12.8058. [DOI] [PubMed] [Google Scholar]