Abstract
Systemic lupus erythematosus (SLE) and its preclinical lupus-prone mouse models are autoimmune disorders involving the production of pathogenic autoantibodies. Genetic predisposition to SLE results in B cell hyperactivity, survival of self-reactive B cells, and differentiation to autoantibody-secreting plasma cells (PC). These corrupt B cell responses are, in part, controlled by excess levels of the cytokine B cell activation factor from the TNF family (BAFF) that normally maintains B cell homeostasis and self-tolerance through limited production. B cell maturation antigen (BCMA) is a receptor for BAFF that, under nonautoimmune conditions, is important for sustaining enduring antibody protection by mediating survival of long-lived PCs, but is not required for B cell maturation and homeostasis. Through analysis of two different lupus-prone mouse models deficient in BCMA, we identify BCMA as an important factor in regulating peripheral B cell expansion, differentiation, and survival. We demonstrate that a BCMA deficiency combined with the lpr mutation or the murine lupus susceptibility locus Nba2 cause dramatic B cell and PC lymphoproliferation, accelerated autoantibody production, and early lethality. This study unexpectedly reveals that BCMA works to control B cell homeostasis and self-tolerance in systemic autoimmunity.
Introduction
The development of a protective humoral immune response involves multiple checkpoints to minimize the possibility of B cells with self-reactivity from responding to self Ags and, in turn, produce autoantibodies that are harmful to the host. These checkpoints include elimination of self-reactive B cells during their development and anergy, suppression, or apoptosis for self-reactive B cells escaping to the periphery (1). A breakdown in more than one of these checkpoints, resulting in the loss of B cell tolerance, hyperactivity, and autoantibody production, is generally required for disease pathogenesis in autoimmune disorders such as rheumatoid arthritis and SLE (2). Thus, defects in factors that restrain B cell homeostasis, differentiation, and survival promote autoimmunity.
The BAFF cytokine is a key regulator of mature naïve B cell homeostasis, controlling the overall numbers of peripheral B cells through binding its receptor, BAFF-R, first expressed at the transitional B cell developmental stage and on mature B cells (3–5). BAFF also plays a critical role in maintaining B cell self-tolerance by balancing the need to stringently eliminate autoreactive B cells while promoting survival of mature nonautoreactive B cells. This balance is achieved normally through competition for limited available BAFF. However, in circumstances where availability of BAFF is unusually high such as transgenic expression of BAFF in mice (6), lupus-prone animals (7), and in patients with rheumatoid arthritis and SLE (8), the stringency for removal of autoreactive B cells is relaxed, resulting in increased numbers of peripheral self-reactive B cells, heightened B cell activation, and elevated autoantibody production. How excess BAFF dysregulates the properties of B cells, however, is difficult to interpret since BAFF interacts not only with BAFF-R but with two additional receptors expressed on B cells, B cell maturation antigen (BCMA) and transmembrane activator and calcium modulator ligand interactor (TACI) (8).
Studies examining the therapeutic benefit of neutralizing BAFF in lupus-prone mice demonstrated reductions in peripheral B cell numbers as well as reduction in the frequency of PCs when both BAFF and it closely related homologue APRIL were blocked (9, 10). This finding suggests that, in addition to dysregulating peripheral B cell homeostasis, increased expression of BAFF contributes to disease activity by affecting PC differentiation and/or survival. This is thought to be achieved through the binding of BAFF to BCMA, which is predominantly expressed on murine PCs and is critical for survival of healthy long-lived bone marrow PCs (11, 12).
In lupus-prone mice, long-lived PCs can secrete pathogenic autoantibodies and accumulate not only in the bone marrow but also in secondary lymphoid organs and inflamed tissues (13, 14). Thus, we reasoned that a deficiency in BCMA would prevent BAFF/APRIL-mediated survival of long-lived PCs in lupus-prone mice and, in so doing, reduce pathogenic autoantibody production. B6 lpr mice carry a naturally occurring Faslpr mutation and develop a benign, slow-progressing lupus-like disease, including elevated levels of anti-DNA autoantibodies, mild lymphadenopathy, and moderate late onset immune-complex mediated glomerulonephritis (GN) (15). In this study, we unexpectedly find that B6 lpr mice with a BCMA deficiency develop both accelerated autoimmunity and a fatal lymphoproliferative disorder compared with single-mutant mice. In contrast to reduced numbers of long-lived PCs anticipated, lymphoproliferation within secondary lymphoid organs of BCMA-deficient B6 lpr mice was populated by significantly increased numbers of PCs displaying features consistent with both short- and long-lived PCs. To determine the mechanisms accounting for the BCMA-deficient B6 lpr phenotype, we demonstrated that CD4+ T cells contribute to autoantibody production but not hyperproliferation of B cells and differentiation into PCs when B6 lpr mice lack BCMA. Finally, we demonstrated that the BCMA deficiency exacerbates the development of lymphoproliferation and autoimmunity when combined with other murine lupus susceptibility genes using congenic mice expressing the locus Nba2 that is associated with increased autoantibody production (16, 17). We infer from these studies that BCMA serves an important role in controlling disease susceptibility in lupus-prone mice.
Materials and Methods
Mice
The generation of BCMA−/− mice was previously described (18). These mice were on the mixed (129 × B6) genetic background and determined to have impaired survival of long-lived PCs within bone marrow (12). We subsequently backcrossed BCMA−/− mice onto the C57BL/6 (B6) background for 12 generations. Genotyping was performed using a panel of polymorphic microsatellite markers distributed across the entire genome to confirm B6 genetic background of BCMA−/− mice (Supplemental Table I; obtainable at the Mouse Genome Database at www.informatics.jax.org). The PCR products of all markers were separated on 4% agarose gels. Representative markers distributed across chromosomes 1–6 are shown in Supplemental Fig. 1. Congenic B6.Nba2 mice were previously described (16). B6.BCMA−/− mice were bred to B6.MRL-Faslpr/J (The Jackson Laboratory) and B6.Nba2 mice, respectively. B6.Nba2 mice were intercrossed with B6.BCMA−/− mice (Nba2.BCMA−/−) and F2 generations of the correct genotype were selected by PCR (Supplemental Table I). Age-matched WT B6 mice were purchased from Charles River Laboratories. All experiments were performed using 4-mo-old female mice unless indicated. Mice were housed in a specific pathogen-free animal facility at the University of Virginia. All animal procedures were conducted in compliance with the National Institutes of Health guidelines and are approved by the Institutional Animal Care and Use Committee of the University of Virginia.
Immunohistochemistry
Tissue for Hematoxylin and Eosin staining was fixed in 10% formalin (Thermo Fisher Scientific) for 24 hrs, embedded in paraffin, sectioned, and stained. For immunofluorescence staining, tissue was snap frozen, embedded in OCT and cut into 5-µm sections. Slides were fixed in acetone, blocked with rat or goat serum in 3% BSA/PBS and incubated directly with FITC conjugated antibodies specific for IgG (eBiosciences) and C3 (R&D Systems). Images were captured using an OLYMPUS BX51 fluorescent microscope.
ELISA and ELISPOT assays
Serum autoantibody IgG levels to chromatin, total histones, ssDNA and dsDNA were determined by ELISA as previously described (17). RF IgM was measured using mouse IgG1 or IgG2a for capture and biotin-conjugated anti-mouse IgM F(ab’)2 fragment, µ-chain specific antibody for detection (Southern Biotech). Serum titers of BAFF were measured using the mouse BAFF detection set according to the manufacture’s protocol (Enzo Life Sciences). Serum was diluted 1:300 and serially titrated in twofold increments. Pooled serum from 9-mo-old B6.lpr mice or mAbs toward DNA and histones were used as positive controls to calculate units per milliliter. IgM- and IgG secreting cells were quantified by ELISPOT as previously described (19). Serial dilutions of cells were made with an initial cell concentration of 150,000 per well in duplicates. The number of antibody-secreting spots was quantified using a dual-axis light dissecting microscope.
Flow cytometry
Single cell suspensions were prepared from spleen and pooled LN. Red blood cells were depleted by ammonium chloride-Tris lysis. 2–3 × 106 cells were stained with mAbs and the Live/Dead Fixable Violet Dye (Invitrogen) according to the manufacturer’s instructions, followed by fixation in 1% formaldehyde/PBS. Nonspecific staining was reduced by the addition of rat serum Ig or FcR block during staining. For intracellular staining of BrdU and BCMA, Cytofix/Cytoperm reagent (BD Biosciences) was used, following the manufacturer’s protocol. Data were collected using a DakoCytomation CyAN ADP LX and analyzed by FlowJo software (Tree Star, Inc.). Profiles are presented as 5% probability contours with gating based on fluorescence-minus-one (FMO) and isotype controls. Cell sorting was performed using a FACSVantage SE TurboSort (BD Biosciences), yielding >98% purity. Antibodies were conjugated to FITC, PE, PE-Texas red, PE-Cy5.5, PE-Cy7, APC, or APC-Cy7. Antibodies specific for the following mouse antigens were used: CD45R (B220), CD138, IgD, GL7, AA4.1, CD23, IgM, CD21, CD3, CD4, CD8, CD44, CD62L, CD69, FoxP3, CD11c, MHCII, NK1.1, F4/80, CD11b, TCRβ, CD80, and CD95 (BD Biosciences); BCMA, TACI, BAFF-R, BAFF (R&D Systems).
In vivo BrdU incorporation assay
5-bromo-2-deoxyuridine (BrdU; Sigma-Aldrich) was added at 1 mg/ml to the drinking water of 2-mo-old and 4-mo-old mice for a period of 10 days. Single-cell suspensions were prepared from spleens of mice and stained for membrane-expressed antigens. Cells were subsequently stained for BrdU using the FITC BrdU Flow Kit as per manufacturer’s instructions (BD Biosciences) and analyzed by flow cytometry.
In vivo CD4+ T cell depletion
10-wk-old mice received i.p. injections of 0.5 mg GK1.5 (anti-CD4) mAb or control rat IgG, twice weekly for 6 wks. Following treatment, cells from peripheral blood, spleen, and cervical LN were prepared and analyzed for efficiency of CD4+ T cell depletion. Serum and cells were also analyzed for antibody titers and numbers of B cells and PC.
Immunoblotting
Spleen B cells (B220+ CD138−) were isolated by removing CD138+ cells through negative selection using PE-conjugated anti-CD138 mAb (BD Biosciences), followed by depletion using MACS anti-PE magnetic beads (Miltenyi Biotec) according to the manufacturer’s protocol. B cells were then positively selected using MACS anti-B220 beads (Miltenyi Biotec). The purity of B220+ CD4− CD138− cells was >90% as measured by flow cytometry. B cells were cultured with medium only, anti-IgM B7.6 antibody (20 µg/ml), murine BAFF (0.1 µg/ml; R&D Systems), B7.6 + BAFF, or anti-CD40 FGK.45 antibody (10 µg/ml). After 24 hr, cells were collected and lysed using NE-PER nuclear and cytoplasmic extraction kit (Thermo Scientific). Protein extracts were loaded on 10% Tris-HCl gel (Bio-Rad) and transferred onto nitrocellulose membranes. Membranes were probed with antibodies against p100/p52, p65, HDAC1 (Cell Signaling Technology), and HRP-conjugated β-actin (Sigma-Aldrich). ECL anti-rabbit IgG-HRP (GE Healthcare) was used as a secondary antibody. Data was scanned by Bio-Rad GS-800 Calibrated Densitometer and the band intensities from immunoblots were analyzed using ImageQuant TL software (GE Healthcare). Protein expression was quantified by first normalizing the densities of each target protein to its loading control protein, β-actin or HDAC1, for cytoplasmic and nuclear extracts, respectively. Fold changes of p100 and p52 in stimulated B cells of all genotypes were then calculated relative to steady-state, nonstimulated levels in B cells from B6 mice. Fold changes of p65 in stimulated B cells of each genotype were calculated relative to the steady-state, nonstimulated amount of same genotype.
Ex vivo proliferation assay
Purified spleen B cells were prepared as described above and labeled with 2 µM CFSE. 0.5 × 106 cells were added to wells of a 96-well plate and cultured in complete RPMI with various stimuli as follows: medium only, IL-4 (10 ng/ml; Peprotech), anti-IgM B7.6 antibody (20 µg/ml), murine BAFF (0.1 µg/ml), anti-IgM + BAFF, and anti-CD40 FGK.45 antibody (10 µg/ml). Cells were harvested 24–72 hrs later, stained for B220, CD138, CD69, CD80, and CD95, and analyzed by flow cytometry. The % divided B cells was determined using FlowJo software.
Adoptive B cell transfers
Donor B cells (B220+ CD138− CD3−) were purified from single-cell suspensions of spleens from 2-mo-old B6, B6 lpr, or BCMA−/− lpr mice (all CD45.2+) using a two-step magnetic bead separation protocol according to manufacturer’s instructions (Miltenyi Biotec). CD3+ and CD138+ cells were first removed from spleen cells by negative selection, followed by positive selection of B220+ B cells. After purification, an aliquot of donor B cells for each genotype was analyzed by flow cytometry and confirmed to be >95% pure. Donor B cells were labeled with CFSE and injected i.v. (15×106 cells in 0.5 ml of PBS) into CD45.1+ B6 recipients. Three days after transfer, the spleens of recipient mice were harvested and the percentages of donor B cells (CD45.2+ B220+) that diluted CFSE and acquired CD138 expression were measured by flow cytometry.
Real-time RT-PCR
Total RNA was isolated from whole splenocytes or freshly purified splenic B cells using the RNeasy System (Qiagen, Valenica, CA), and was treated with DNase I before cDNA synthesis. BCMA and APRIL transcripts were analyzed by real-time PCR using the SYBR Green PCR Core Kit (Applied Biosystems, Foster City, CA) on an iCycler iQ instrument (Bio-Rad) and normalized to the transcript levels of the housekeeping gene HPRT. Primers used were as follows: BCMA: forward, 5’-ATCTTCTTGGGGCTGACCTT-3’ and reverse, 5’-CTTTGAGGCTGGTCCTTCAG-3’; APRIL: forward, 5’-TTTCACAATGGGTCAGGTGGTA-3’ and reverse, 5’-AGGCATACTTCTGATACATCGGAAT-3’.
Statistical analysis
Comparisons between study groups were analyzed with an unpaired two-tailed Student t test. p values <0.05 were considered significant and are noted in the figure legends.
Online supplemental material
Supplemental Fig. 1 confirms B6 genetic background of BCMA−/− lpr mice across chromosomes 1–6. Supplemental Fig. 2 demonstrates the number of PCs secreting IgG subtypes. Supplemental Fig. 3 shows membrane expression of BAFF-R and TACI on B cells, GC B cells, and PCs from all genotypes. Supplemental Fig. 4 shows quantified protein levels of NF-κB transcription factors from B cells after BCR and BAFF stimulation. Supplemental Fig. 5 compares BAFF- and APRIL-mediated proliferation of B cells. Supplemental Table I lists the PCR primers used for genotyping the Nba2 congenic region when crossed onto BCMA−/− background and the polymorphic microsatellite markers used for genotyping B6:129 background. Supplemental Table II lists the absolute numbers of lymphocyte and myeloid subsets from spleen and LN of all genotypes.
Results
BCMA−/− lpr mice develop a fatal lymphoproliferative disorder with autoimmune symptoms
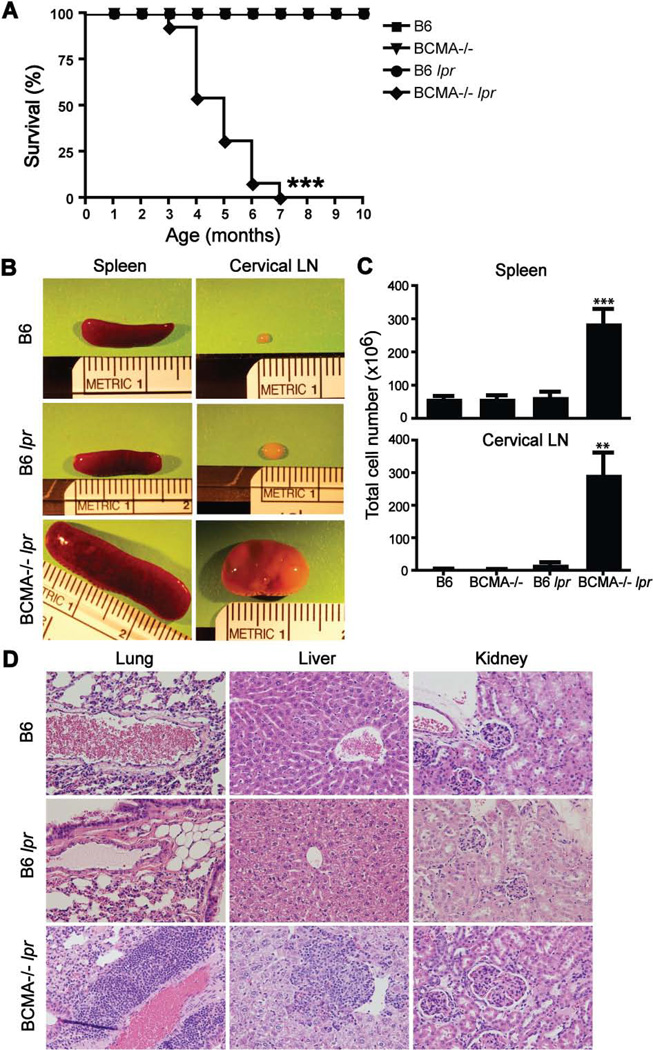
To determine the role of BCMA in the development of autoimmunity, we generated Faslpr/lpr mice lacking BCMA. Faslpr/lpr mice on C57BL/6 background were intercrossed with BCMA−/− mice on C57BL/6 background, which are normally resistant to the development of autoimmunity and have normal B-cell physiology (18). By 3 mo of age, BCMA−/− lpr mice showed increased mortality, reaching 100% lethality by 7 mo of age, compared to mice from wild-type and single-mutant backgrounds (Fig. 1A). BCMA−/− lpr mice rapidly developed severe splenomegaly and lymphadenopathy with 100% penetrance in both males and females (Fig. 1B and 1C). Histologic examination of lungs, liver, and kidneys at 4 mo of age demonstrated significant lymphocyte infiltration within these non-lymphoid organs of BCMA−/− lpr mice (Fig. 1D), as well as salivary gland, lacrimal gland, pancreas, and, to a lesser extent, the heart (data not shown). Perivascular lymphoid infiltration into lungs and accumulation of lymphocytes around the central vein within liver were frequently observed in BCMA−/− lpr tissue. Although kidneys from BCMA−/− lpr mice showed a proliferative GN phenotype with enlarged and hypercellular glomeruli, kidney damage, as measured by proteinuria, was not detected (data not shown). Together, these are features characteristic of a pronounced lymphoproliferative disease.
Figure 1. BCMA−/− lpr mice develop a fatal lymphoproliferative disorder.
(A) Kaplan-Meier survival curve is shown from aging cohorts of mice. n = 10–13 mice per group. ***, p < 0.0001 compared with other strains. (B) Enlarged spleens and cervical LNs from 4-mo-old B6, B6 lpr, and BCMA−/− lpr mice are shown. (C) Total cell numbers were quantified from 4-mo-old mice. n = 7–9 mice per group. **, p < 0.002; ***, p < 0.0005 compared to B6 and single-mutant strains. (D) Representative H&E staining of lung, liver, and kidney sections showed increased cellular infiltration and enlarged glomeruli from BCMA−/− lpr mice compared to control mice, analyzed in panel C. Images were taken at 20× magnification.
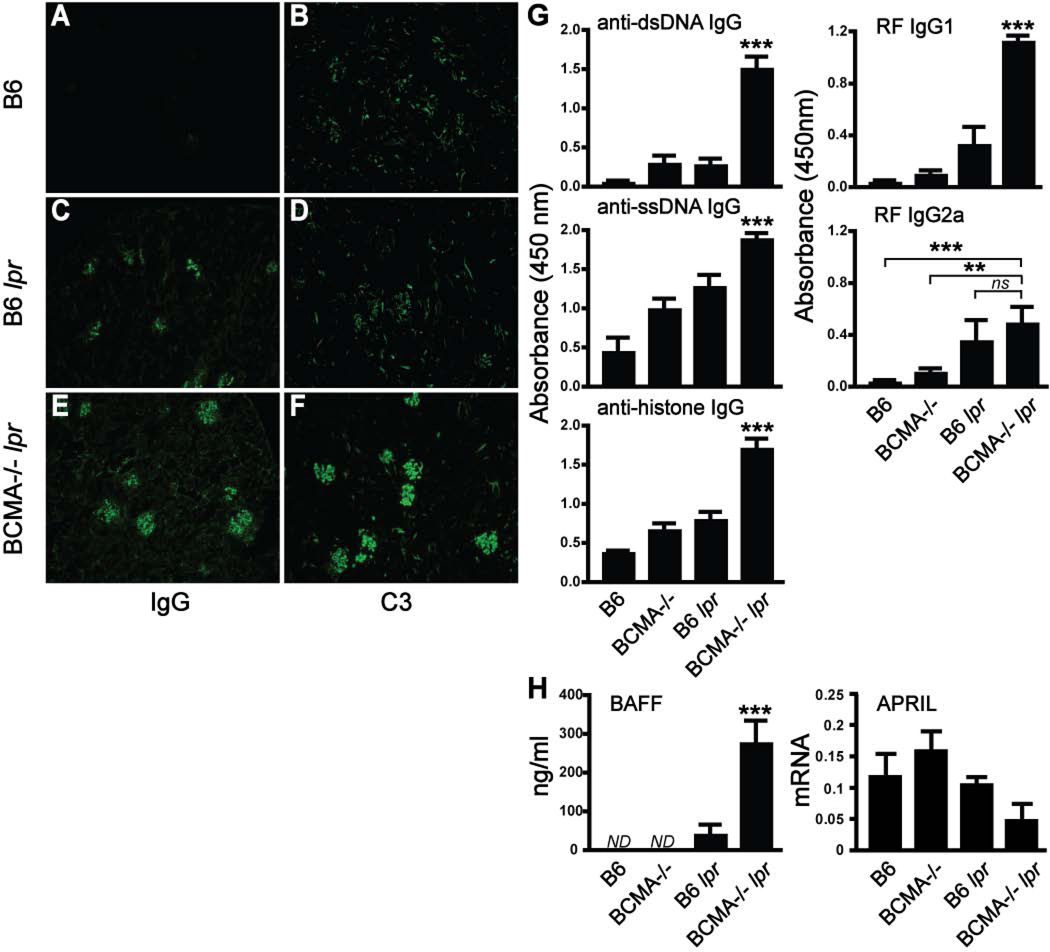
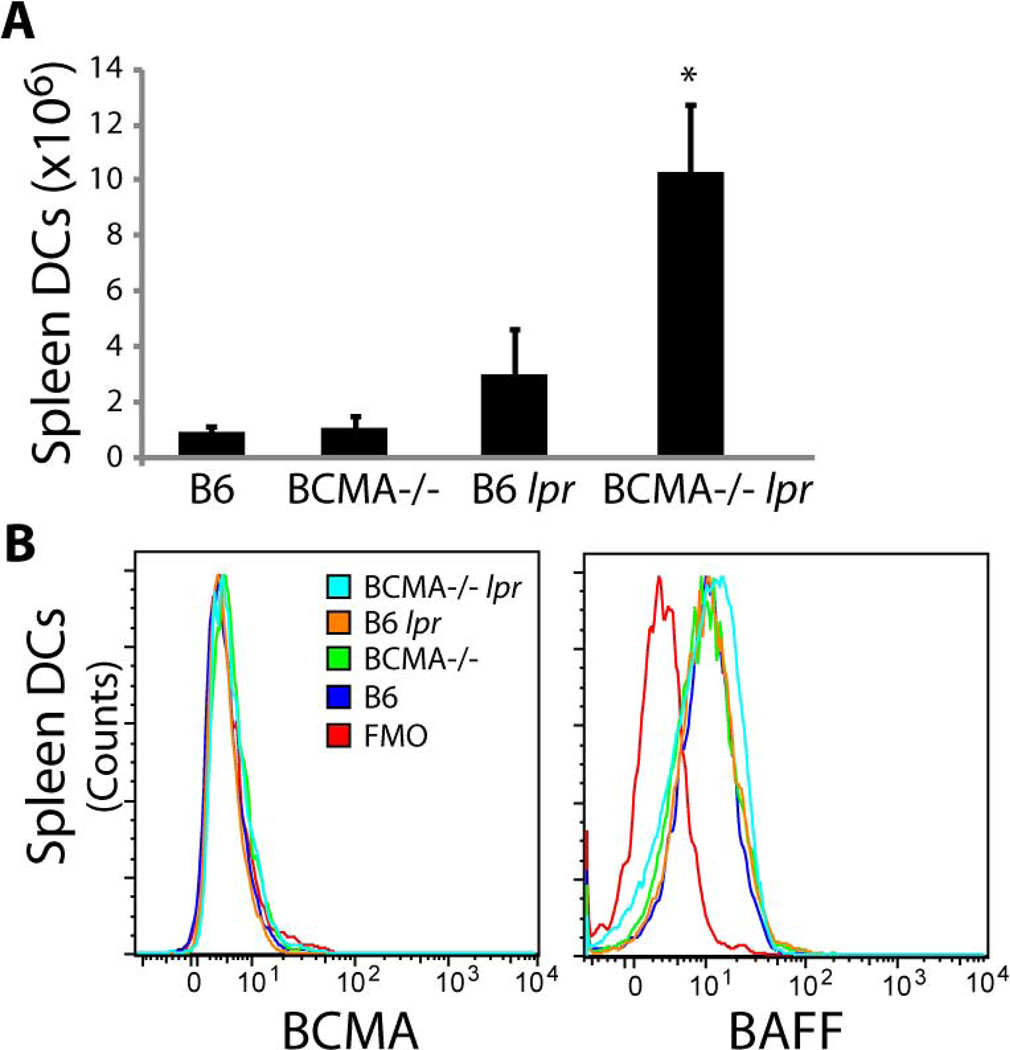
To determine whether the combination of BCMA deficiency and the lpr mutation modified the development of lupus traits, double-mutant mice were evaluated for immune complex deposits within kidneys, serum anti-DNA IgG titers, and serum BAFF levels. Deposition of IgG and C3 within glomeruli was detected at significantly higher levels in BCMA−/− lpr mice compared to control B6 lpr animals (Fig. 2C–F). Immune complex deposition was undetectable in kidneys from both WT B6 (Fig. 2A and 2B) and BCMA−/− single-mutant mice (data not shown). It has been previously reported that, in 4-mo-old B6 lpr single-mutant mice, anti-DNA IgG titers are low and only at 9 mo of age do they develop dsDNA antibodies (20). By 4 mo of age, BCMA−/− lpr mice produced significantly higher titers of IgG autoantibodies specific to dsDNA, ssDNA, histones, and rheumatoid factor (RF) compared to WT and single-mutant strains (Fig. 2G). Given that overexpression of BAFF leads to lupus-like traits, we evaluated mouse serum for circulating BAFF and found a significant increase in the levels of BAFF in BCMA−/− lpr mice compared to the other strains (Fig. 2H). In contrast, no difference in mRNA transcripts encoding APRIL in splenocytes was measured among the strains. Our observation that BAFF levels are increased in BCMA−/− lpr mice prompted us to compare the frequencies of myeloid cells that can produce BAFF in secondary lymphoid organs of these mice and other strains. Analysis of dendritic cell and macrophage populations in spleen and lymph nodes demonstrated a significant increase of both populations in BCMA−/− lpr mice compared to other strains (Fig. 3A; Supplemental Table II). We further determined by flow cytometric analysis whether membrane and intracellular expression of BAFF in dendritic cells was different between BCMA−/− lpr mice and other strains. Results demonstrated that dendritic cells from all the strains expressed equivalent levels of BAFF (Fig. 3B). These observations suggest that the heightened levels of circulating BAFF in BCMA−/− lpr mice comes from elevated numbers of BAFF-producing cells, such as dendritic cells, rather than BAFF production being increased at the individual cell level. Given that increased numbers of dendritic cells in B6 lpr mice inversely correlated with the BCMA deficiency, we explored whether dendritic cells normally express BCMA. As shown in Fig. 3B, no BCMA expression (membrane plus intracellular staining) was detected on dendritic cells from WT and B6 lpr mice compared to BCMA-deficient animals. In summary, the above data indicated that the BCMA deficiency in B6 lpr mice induces autoantibody production, immune complex deposition, and BAFF protein levels.
Figure 2. Loss of BCMA and the lpr mutation increases the severity of autoimmune traits.
(A–F) Immune complex deposition of IgG (panels A, C, and E) and C3 (panels B, D, and F) was measured by immunostaining of frozen kidney sections. Positive IgG and C3 depositions are shown from 4-mo-old BCMA−/− lpr mice compared to B6 and B6 lpr mice. Images were taken at 10× magnification and are representative of >6 mice for each strain. (G) Sera from a minimum of six 4-mo-old mice were analyzed for IgG autoantibodies to dsDNA, ssDNA, histones, and rheumatoid factor (RF) by ELISA. (H) Sera in panel G were analyzed for BAFF levels by ELISA and APRIL mRNA expression in total spleen was measured by real-time PCR relative to HPRT. ND, below detection level; ns, not significant; **, p < 0.005; ***, p < 0.001 compared to wild-type and single-mutant mice.
Figure 3. Increased BAFF production correlates with elevated numbers of DCs in BCMA−/− lpr mice.
(A) Total numbers of conventional DCs (CD11c+ MHCII+) in spleens of 4-mo-old mice were quantified. n = 5–6 mice per group. *, p < 0.05 compared to B6 lpr mice. (B) Representative FACS plots demonstrating no BCMA expression on DCs and equivalent levels of BAFF among DCs in all mouse strains. Data are representative of 6–8 mice for each genotype. FMO, fluorescence-minus-one control.
PCs are increased within secondary lymphoid organs of BCMA−/− lpr mice
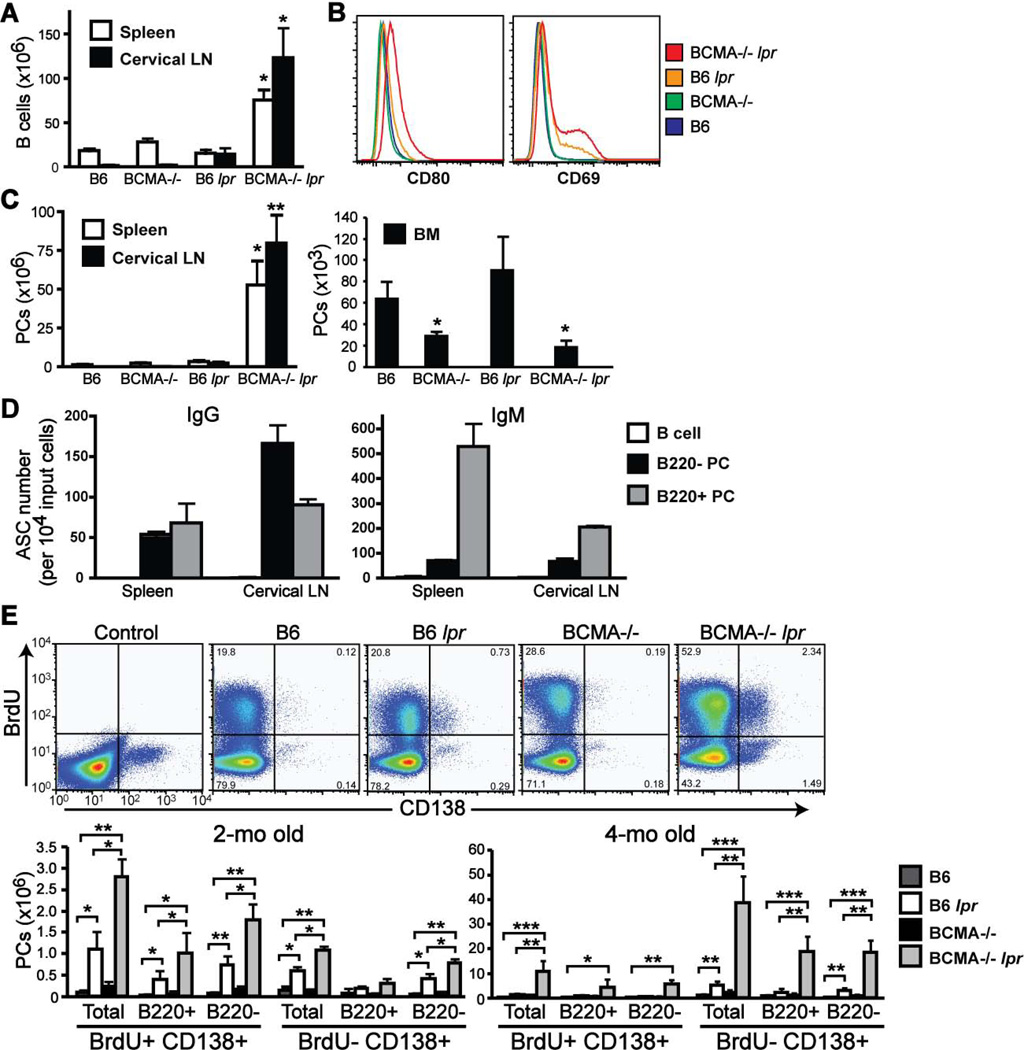
Because signaling through Fas on B cells is important for both maintaining homeostasis of B cell numbers and triggering apoptosis of autoreactive B cells, we examined the effect of BCMA deficiency and lpr on steady-state levels of peripheral B cell populations. Total B220+ CD138− B cell numbers were markedly increased in both spleen and cervical lymph nodes (LN) of 4-mo-old BCMA−/− lpr mice (Fig. 4A). Spontaneous B cell activation, as measured by CD80 and CD69 expression, was apparent in BCMA−/− lpr mice compared to age-matched single-mutant strains (Fig. 4B). Furthermore, B cells terminally differentiating into CD138+ PCs were significantly increased in spleen and LNs of BCMA−/− lpr mice (Fig. 4C). This result was consistent with increased numbers of spontaneous B220+ IgD−GL7+ germinal center (GC) B cells (Supplemental Table II), suggesting that PCs may develop from GC B cells, and thus, be partly comprised of long-lived PCs. Given that BCMA expression on long-lived bone marrow PCs normally promotes their survival (12), we determined whether the BCMA deficiency in B6 lpr mice controls survival of bone marrow PCs. Consistent with BCMA−/− mice, BCMA−/− lpr mice had significantly less numbers of bone marrow PCs as compared to B6 and B6 lpr strains (Fig. 4C). These observations suggested that most PCs spontaneously generated in BCMA−/− lpr mice can persist within the secondary lymphoid organs but not bone marrow.
Figure 4. Lymphoproliferation in BCMA−/− lpr mice is accompanied by increased numbers of both short- and long-lived PCs in spleen and cervical LNs.
(A, C) Total numbers of B cells (B220+ CD138−) and PCs (B220+/− CD138+) in spleen, cervical LNs, and bone marrow (BM) of 4-mo-old mice were quantified. n = 5–6 mice per group. *, p < 0.05; **, p < 0.01 compared to wild-type and single-mutant strains. (B) Histograms comparing expression of CD80 and CD69 on B220+ CD138− gated B cells. All data are representative of 3–5 mice per genotype. (D) B cells, B220+ PCs, and B220− PCs were sort-purified from spleen and cervical LNs of individual 4-mo-old BCMA−/− lpr mice, and were analyzed for the number of IgG and IgM antibody secreting cells (ASC) by ELISPOT. Data are expressed as the mean ± SEM from 4–6 mice per group and is representative of three independent experiments. (E) Numbers of BrdU− PCs resident in spleens of BCMA−/− lpr mice increase with age. Groups of 2-mo- and 4-mo-old mice were fed BrdU in the drinking water at 1 mg/ml for 10 days. Spleens were isolated and analyzed for BrdU uptake within total CD138+ PCs, B220+ PCs, and B220− PCs. Representative FACS plots for each genotype at 2-mo of age demonstrate both BrdU− and BrdU+ CD138+ PCs are present in spleens. The ratio of cells within each quadrant is shown. Total numbers of BrdU+ and BrdU− CD138+ PCs were quantified. In addition, the numbers of BrdU+ and BrdU− CD138+ PCs that expressed B220 or not, relative to FMO control, were quantified. Data shown represent mean ± SEM for 5 mice per group. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared to B6 or B6 lpr mice, as indicated.
To further characterize the PCs resident in secondary lymphoid organs of BCMA−/− lpr mice, we analyzed their phenotype, antibody production, and proliferation. Splenic CD138+ PCs that express the B cell marker B220 are characteristic of short-lived PCs and early PCs that terminally differentiate into mature long-lived PCs, which express low levels of B220 and produce mainly class-switched antibodies (21). The capacity of purified B220+ CD138+ and B220− CD138+ PCs to secrete IgM and IgG was measured by ELISPOT (Fig. 4D). Results demonstrated that of resident spleen and cervical LN B220− CD138+ PCs, the majority produced IgG compared to IgM, suggesting that long-lived PCs reside within this population. In contrast, the resident spleen and cervical LN B220+ CD138+ populations were comprised of both IgM- and IgG-secreting PCs, suggesting that both short- and long-lived PCs reside within this population. Undetectable numbers of antibody-secreting cells were measured from mature B cells and served as a negative control. Further evaluation of IgG-secreting PCs isolated from spleens and LNs of BCMA−/− lpr mice revealed that these PCs produced mainly IgG2a, IgG2b, and IgG3 isotypes, in contrast to IgG1, suggesting a role for pro-inflammatory cytokines in influencing antibody isotype switching (Supplemental Fig. 2).
We then used the thymidine analog BrdU, which is incorporated into the DNA of dividing cells (22), to distinguish between proliferating (short-lived) and nonproliferating (long-lived) PCs. Groups of 2-mo and 4-mo old mice were administered BrdU over a 10 day period and incorporation of BrdU by CD138+ PCs within spleens of individual mice was measured by flow cytometric analysis. Total numbers of BrdU+ and BrdU− CD138+ PCs were quantified (Fig. 4E). Results demonstrated that at 2-mo of age the overall numbers of spontaneously generated PCs in B6 lpr and BCMA−/− lpr mice were low compared to 4-mo of age. Of the resident spleen PCs analyzed in 2-mo-old mice, BCMA−/− lpr mice had significantly increased numbers BrdU+ and BrdU− PCs compared to B6 lpr mice, yet both B6 lpr and BCMA−/− lpr mice had increased numbers of BrdU+ and BrdU− PCs compared to B6 and BCMA−/− strains. In addition, the BrdU+ CD138+ population in 2-mo-old B6 lpr and BCMA−/− lpr mice was comprised of B220+ and B220− PCs in contrast to the BrdU− CD138+ population that contained more B220− PCs. These findings indicate that both short-lived and long-lived PCs are present in spleens of 2-mo-old B6 lpr and BCMA−/− lpr mice, but more total CD138+ PCs when mice lack BCMA. At 4-mo of age, BCMA−/− lpr mice also had significantly increased numbers of BrdU− PCs and BrdU+ PCs, regardless of B220 expression, compared to B6 lpr mice, even though B6 lpr mice had increased numbers of BrdU− PCs compared to B6 mice. These results indicate that with age the numbers of both short-lived and long-lived PCs are increased in B6 lpr mice when BCMA is absent. Together, these results suggest that the BCMA deficiency and lpr mutation cooperate to induce B lymphocyte activation, expansion, and terminal differentiation into PCs within secondary lymphoid organs.
Autoantibody production but not B lymphocyte expansion and differentiation into PCs is reduced when CD4+ T cell help is blocked in BCMA−/− lpr mice
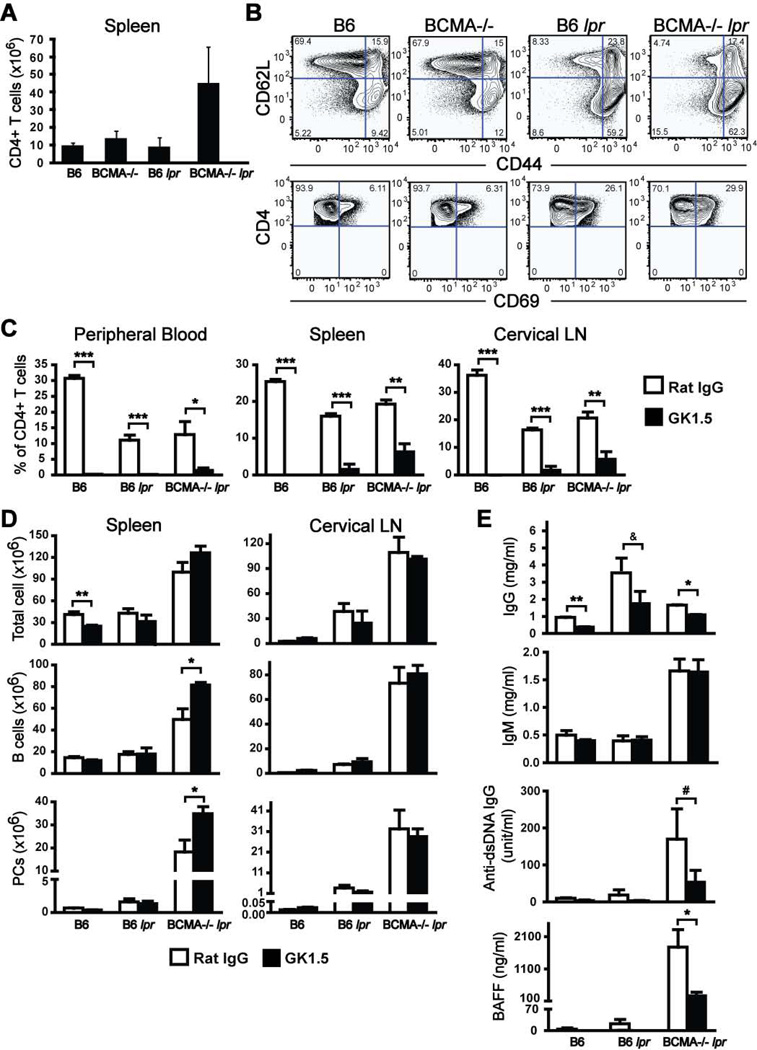
Given that the numbers of B cells, GC B cells, and PCs were increased in spleen and LNs of BCMA−/− lpr mice, we examined the numbers of CD4+ T cells within secondary lymphoid organs. By 4 mo of age, BCMA−/− lpr mice displayed elevated numbers of CD4+ T cells within spleen and LNs compared with other strains (Fig. 5A; Supplemental Table II). Coincident with the increase in CD4+ T cells, the percentage of activated (CD69+) and effector (CD62L−CD44high) CD4+ T cells in the spleens of BCMA−/− lpr mice also increased (Fig. 5B). An increase in CD4+ T cell activation was also found in the spleens of B6 lpr mice even though the overall numbers of cells were within normal range. Together, these initial findings suggested that there was a correlation between an increase in B cell lymphoproliferation and differentiation into PCs, T cell activation, and autoimmune traits in BCMA−/− lpr mice.
Figure 5. In vivo depletion of CD4+ T cells within BCMA−/− lpr mice does not affect lymphoproliferation but significantly reduces autoimmune traits.
(A) Total numbers of CD4+ T cells in spleens of 4-mo-old mice were quantified. (B) Representative FACS plots demonstrating CD4+ T cell activation in 4-mo-old mice by analysis of the expression of CD69, CD62L, and CD44 are shown. Data are representative of 6–8 mice for each genotype. (C–E) 10-wk-old B6, B6 lpr, and BCMA−/− lpr mice were i.p. injected twice weekly with 0.5 mg GK1.5 mAb or control rat IgG for 6 wks. Following treatment, sera, spleen cells, and cervical LN cells from individual mice were analyzed. Shown are the mean ± SEM CD4+ T cell percentages after GK1.5 treatment confirming CD4+ T cell depletion. Total cellularity, B cells, and CD138+ PCs from spleens and cervical LNs of mice were determined by flow cytometry. Sera from individual mice were analyzed for titers of total IgG, total IgM, anti-dsDNA IgG, and BAFF. Data shown represent mean ± SEM for 6 mice per group. *, p < 0.05; **, p < 0.01; ***, p < 0.001; &, p < 0.06; #, p < 0.08 compared to rat IgG treated mice of same genotype.
To examine the contribution of endogenous CD4+ T cells in BCMA−/− lpr mice to B cell expansion and differentiation into PCs, we administered the CD4-depleting GK1.5 mAb to animals, beginning at 10 wks of age when no signs of lymphoproliferation are present and survival is 100%. Age-matched B6 and B6 lpr mice served as controls. Analysis of the CD4+ T cell percentage within peripheral blood, spleen, and cervical LN of mice treated with GK1.5 over a period of 6 wks indicated that the numbers of CD4+ T cells were significantly reduced compared to animals treated with rat IgG isotype control antibody (Fig. 5C). Results demonstrated that depletion of CD4+ T cells did not affect splenomegaly, lymphadenopathy, expansion of mature B cells, or the development of CD138+ PCs in BCMA−/− lpr mice (Fig. 5D). Analysis of serum IgM titers from mice demonstrated that production of IgM was unaffected upon depletion of CD4+ T cells (Fig. 5E). These findings are consistent with previous reports demonstrating that B cells terminally differentiate into IgM-secreting cells even in the absence of T cell help (23). In contrast, serum IgG titers of mice were reduced after T cell depletion (Fig. 5E), suggesting that some IgG production developed from spontaneous T cell-dependent GC B cell responses and may contribute to disease. CD4+ T cells are known to play an important role in the development of autoimmunity in B6 lpr mice (15); indeed, the development of autoimmune traits, like elevated serum titers of BAFF and dsDNA-specific IgG, were significantly reduced in BCMA−/− lpr mice upon GK1.5 treatment (Fig. 5E). These data demonstrate that a reduction of T cell hyperactivity in BCMA−/− lpr mice, achieved through GK1.5 treatment, leads to reduced serum levels of total IgG, dsDNA IgG, and BAFF. In contrast, reduced T cell hyperactivity in BCMA−/− lpr mice did not diminish the numbers of B cells and PCs, suggesting that the development of B cell lymphoproliferation and differentiation into PCs is caused by non-T cell signals.
Lack of BCMA signaling in lpr mice renders B cells hyperresponsive despite normal NF-κB activation by BAFF and BCR stimulation
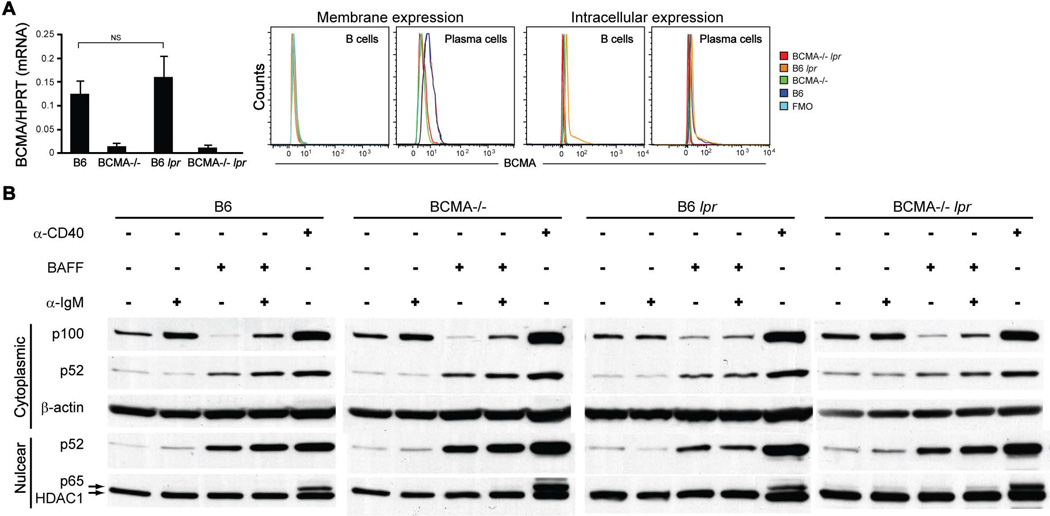
In normal adult humans and mice, mature B cells do not express detectable levels of membrane-anchored BCMA protein (11, 24, 25), suggesting that BCMA does not play a major role in the survival of peripheral B cells. Consistent with these reports, we found that mature B cells from B6 and B6 lpr mice expressed low levels of BCMA mRNA transcripts and no detectable membrane expression of BCMA compared to BCMA−/− and BCMA−/− lpr strains (Fig. 6A); however, as B cells differentiate into PCs the expression of BCMA is upregulated as previously reported (11, 24). Previous immunofluorescence studies revealed that BCMA is located in the Golgi at high levels in PCs (26); therefore, we measured intracellular expression levels of BCMA. Results demonstrated higher expression levels of BCMA within B cells from B6 lpr mice but not in B cells from other strains and, as anticipated, higher expression levels of BCMA within PCs from both B6 and B6 lpr mice (Fig. 6A). BAFF-R and TACI were positively expressed on total B cells and negatively expressed on GC B cells at equivalent levels among all four strains (Supplemental Fig. 3), suggesting that BCMA deficiency does not lead to alterations in other BAFF receptors on these B cell subsets.
Figure 6. B cells from BCMA−/− lpr mice have normal BCR- and BAFF-mediated NF-κB activation.
(A) BCMA mRNA expression levels relative to HPRT in purified splenic B cells and representative FACS plots of BCMA protein expression by B cells and PCs in the spleens of 4-mo-old mice are shown. NS, not significant. n = 5–6 mice. (B) Immunoblot analysis of cytoplasmic extracts prepared from 24 hr stimulated cells was performed using antibodies against p100, p52, and β-actin. Nuclear extracts were probed with antibodies recognizing p52, p65, and HDAC1. Data shown are representative of three individual experiments.
Given that continuous signaling through both BCR and BAFF is necessary for homeostasis of peripheral B cells, we hypothesized that BCMA−/− lpr B cells might be hyperresponsive to these prosurvival signals through activation of NF-κB transcription factors and this could, in part, explain the increased B cell numbers within secondary lymphoid organs. To test this hypothesis, we analyzed the induction and localization of both classical and nonclassical NF-κB substrates in B cells stimulated through the BCR and BAFF. It has been reported that BCR signaling induces production of the classical NF-κB pathway substrate p100 (27), which is required for BAFF-mediated processing to generate sustained levels of the nonclassical NF-κB substrate p52 for B cell survival (28, 29). Results demonstrated that, as previously reported (29), mature B cells isolated from spleens of all four genotypes expressed steady-state levels (non-stimulated) of p100 in cytoplasm, with little processing to cytoplasmic and nuclear p52 (Fig. 6B and quantified in Supplemental Fig. 4).
The expression of cytoplasmic p100 in B cells isolated from all mice increased after IgM crosslinking, likely through the classical NF-κB pathway (29, 30), since processing of p100 to the active p52 form was not observed (Fig. 6B). Without BCR-induced synthesis of p100, we found that all B cells stimulated with BAFF, which promotes the nonclassical NF-κB pathway (28, 29), significantly depleted p100 stores, generating increased levels of p52 that localized to the nucleus. Finally, stimulation through both BCR and BAFF of B cells from all mice demonstrated that p100 expression can be sustained and processed, leading to nuclear localization of p52. BCR and BAFF costimulation of B cells from B6 lpr and BCMA−/− lpr mice resulted in less nuclear p52 expression compared to B cells from B6 and BCMA−/− mice, but were not statistically significant. B cells stimulated through CD40, which activates both classical and nonclassical NF-κB pathways (29), confirmed expression of p100 and p52 as well as nuclear expression of the classical NF-κB substrate p65. Together, these results suggest that the BCMA deficiency with the lpr mutation does not dysregulate BCR- and BAFF-mediated B cell proliferation and prosurvival signals through activation of NF-κB transcription factors.
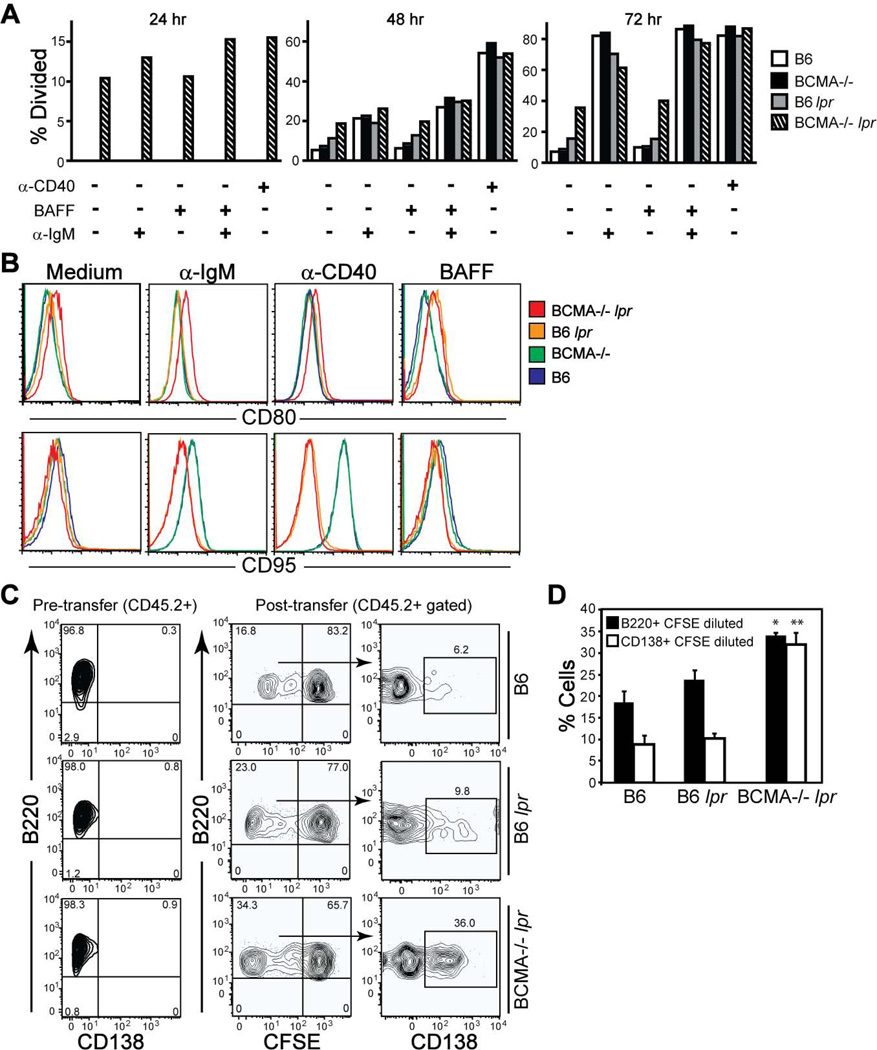
We further analyzed in vitro proliferation of B cells among the four genotypes of mice stimulated through the BCR and BAFF. Results demonstrated that the lpr mutation combined with the BCMA deficiency conferred marked intrinsic proliferation of nonstimulated B cells (~10%) over the preceding 24 hrs in vitro, increasing to ~40% dividing B cells after 72 hrs in culture (Fig. 7A). In contrast, nonstimulated B cells divided <10% from WT and BCMA−/− mice and <20% from B6 lpr mice after 72 hrs in vitro. The capacity of B cells to proliferate in response to IgM crosslinking alone or when combined with BAFF was equivalent among all genotypes after 72 hrs (Fig. 7A), consistent with normal NF-κB activation. No differences in B cell proliferation were found when stimulated with APRIL (Supplemental Fig. 5). To test whether the BCMA deficiency directly regulates B cell responses, we measured CD80 and CD95 expression levels on B cells stimulated with BAFF and to agonistic anti-IgM and anti-CD40 mAb. BCMA−/− lpr B cells expressed higher amounts of CD80 after stimulation compared to other B cell genotypes (Fig. 7B). CD95 was not induced on stimulated B cells from B6 lpr and BCMA−/− lpr mice compared to B6 and BCMA−/− mice, serving as an internal control.
Figure 7. B cells from BCMA−/− lpr mice are hyperproliferative in vitro and in vivo.
(A) B cells (B220+ CD138−) isolated from spleens of 4-mo-old mice were labeled with CFSE and cultured 24–72 hrs in medium ± the indicated stimuli. Cells were recovered at each time point and the percent divided B cells were determined by flow cytometry. (B) Flow cytometric analyses of B cells analyzed in panel A after stimulation with the indicated stimuli for 72 hrs. Histograms of CD80 and CD95 expression levels are representative of 3–5 mice per genotype. (C) Donor B cells (B220+ CD138− CD3−) were purified from splenocytes of 2-mo-old B6, B6 lpr, and BCMA−/− lpr mice (all CD45.2+). Representative FACS plots demonstrating that the purity of donor B cells before transfer was >95% are shown. Cells were CFSE-labeled and 15 × 106 were injected i.v. into CD45.1+ wild-type recipients. 3 d after transfer, the percentage of dividing CD45.2+ B220+ B cells and differentiation into CD138+ PCs in the spleens of recipient mice were measured by flow cytometry. (D) Data are expressed as means ± SEM (n = 6 recipients per donor group). *, p < 0.05; **, p < 0.01 compared to B6 and B6 lpr donor groups.
The observation that nonstimulated B cells from BCMA−/− lpr mice proliferated in vitro prompted us to compare steady-state levels of B cell proliferation and differentiation into PCs when placed into a normal environment. To determine whether B cells hyperproliferate in vivo, we adoptively transferred CFSE-labeled B cells purified from spleens of 2-mo-old B6, B6 lpr, and BCMA−/− lpr mice (all CD45.2+) into wild-type CD45.1+ recipients. Analysis of donor B cells before transfer demonstrated that they were >95% pure (Fig. 7C). We found that by day 3 after transfer, >34% of BCMA−/− lpr B cells had divided compared to B6 (~17%) and B6 lpr (~23%) donor B cells of total cells recovered in recipient spleens (Fig. 7C; quantified in Fig. 7D). Moreover, of the B cells that had diluted CFSE, a significant percentage of BCMA−/− lpr B cells (>30%) acquired expression of CD138 compared to B6 and B6 lpr B cells. These data indicate that when B6 lpr mice lack BCMA, donor B cells have increased proliferation and differentiation in a normal environment. This could be achieved through a B cell intrinsic defect or the presence of pre-plasmablasts that lack CD138 expression, within the donor B cell population, which are already programmed to proliferate and differentiate.
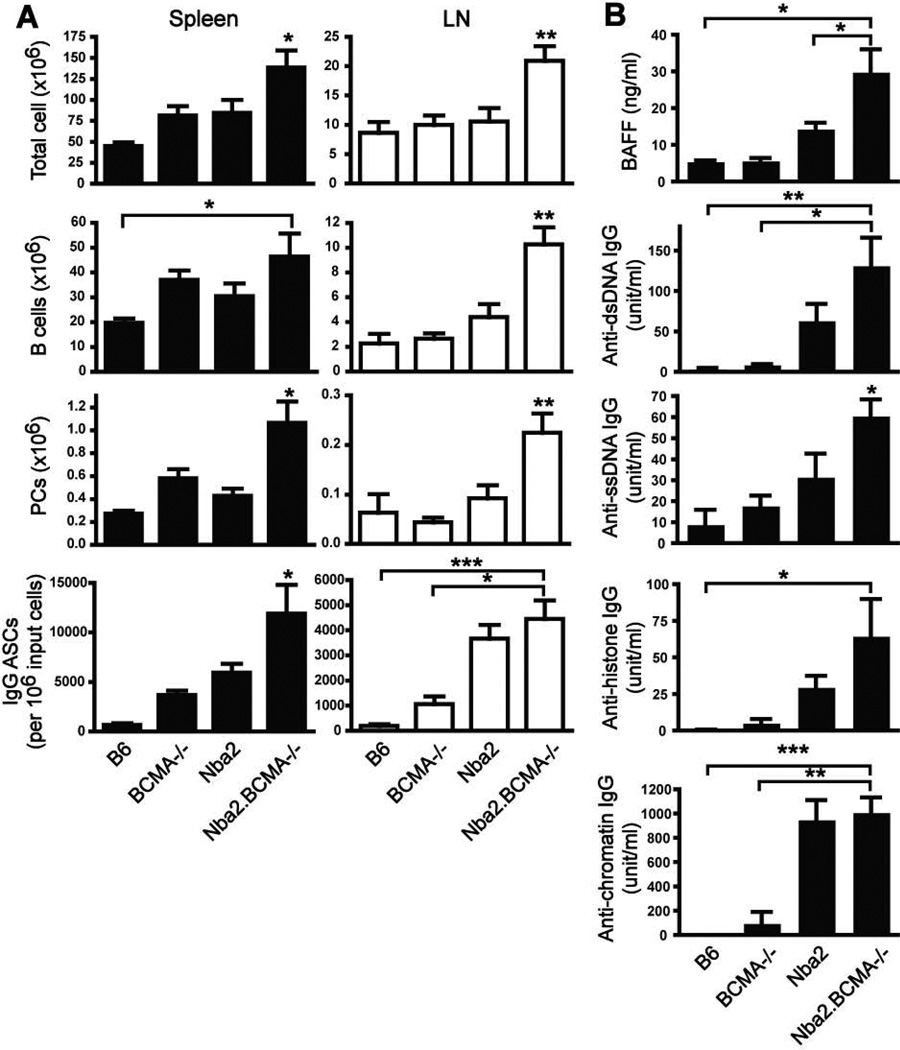
BCMA deficiency and expression of the murine lupus susceptibility locus Nba2 act cooperatively to enhance lymphoproliferation and autoimmune traits
To determine whether BCMA deficiency could worsen the development of lymphoproliferation and autoimmunity when combined with other lupus susceptibility genes, we generated BCMA−/− mice that express the Nba2 lupus susceptibility locus (Nba2.BCMA−/−). Congenic B6.Nba2 female mice produce elevated levels of IgG autoantibodies, mild splenomegaly, but no kidney damage by 7 mo of age (16, 17). Results demonstrated that Nba2.BCMA−/− mice developed increased splenomegaly and lymphadenopathy compared to parental B6.Nba2 mice (Fig. 8A). Of total cell numbers, B cells and PCs were significantly increased in both spleen and LN of Nba2.BCMA−/− mice compared to other genotypes. Nba2.BCMA−/− animals also spontaneously produced increased numbers of IgG-secreting PCs in spleen and LN compared to other genotypes. To determine whether the combination of BCMA deficiency and the Nba2 locus modified the development of lupus traits, Nba2.BCMA−/− mice were evaluated for serum titers of BAFF and IgG autoantibodies. Results demonstrated that Nba2.BCMA−/− mice produced significantly increased amounts of BAFF and IgG specific for dsDNA, ssDNA, and histones compared to other strains (Fig. 8B). Serum titers of chromatin-specific IgG in B6.Nba2 mice, normally found at high levels (16, 19), were equivalent to levels measured in Nba2.BCMA−/− animals. BCMA deficiency in B6.Nba2 mice did not promote kidney damage as measured by proteinuria levels (data not shown). The absence of GN in congenic Nba2 mice, even with BCMA deficiency, is consistent with the need for additional susceptibility genes to develop progressive GN (2). These data demonstrate that, using the congenic Nba2 lupus-prone mouse model, BCMA expression is also important for controlling B cell homeostasis and the development of certain autoimmune traits.
Figure 8. BCMA deficiency in congenic mice expressing the murine Nba2 autoimmune susceptibility locus enhances lymphoproliferation and autoimmune traits.
(A) Total cells, B cells, and PCs within spleens and LNs of 7-mo-old female mice were determined using flow cytometry. Numbers of IgG ASCs were determined by ELISPOT. (B) Serum samples were analyzed for BAFF and IgG specific to dsDNA, ssDNA, histones, and chromatin by ELISA. n = 6–9 mice per group. *, p < 0.05; **, p < 0.01; ***, p < 0.001 compared to B6, BCMA−/−, or Nba2 mice, as indicated.
Discussion
The BAFF binding receptors, BAFF-R and TACI, have been extensively studied and demonstrated to be critical for the development and homeostasis of mature B cells (31, 32). BAFF-dependent B cell maturation in mice is mediated through the BAFF-R (5); thus, mice deficient in BAFF-R or that express mutant BAFF-R exhibit a block in B cell maturation (33–35). Analysis of TACI-deficient mice, which exhibit splenomegaly and increased numbers of mature B cells (36, 37), has suggested that TACI plays an important role in negatively regulating mature B cell homeostasis. In contrast, our understanding of BCMA is limited, other than it is upregulated on both murine and human PCs and that it is critical for the survival of normal murine PCs residing in the bone marrow (11, 12, 24, 38).
In this study, we describe our analysis of BCMA in controlling survival of PCs and, in particular, its contribution to the development and survival of autoantibody-secreting PCs, in lupus-prone mice. Our results reveal a previously unappreciated role for BCMA in maintaining stability of mature B cells, regulating their differentiation into PCs, and providing stringency for survival of autoreactive B cells under autoimmune predisposing conditions. Specifically, our findings suggest for the first time that BCMA serves as a major determinant of B cell homeostasis and the development of autoantibody-mediated autoimmunity in mice bearing the lpr mutation and the Nba2 autoimmune susceptibility locus.
Autoreactive B cells are competitively eliminated due to their increased dependence on BAFF for survival, but can be rescued by overexpression of BAFF thought to act through binding BAFF-R that promotes cell survival (39–41). In collaboration with cytokines, TLR signaling, and co-stimulatory molecules, BAFF also promotes antibody production and Ig class switching of antigen-stimulated B cells (42, 43). Thus, BAFF transgenic mice and lupus-prone mice that overexpress BAFF have an increased number of mature B cells and PCs in secondary lymphoid organs that associate with high levels of Ig, RF, and anti-DNA autoantibodies in their serum (6, 7). In most lupus-prone mouse models, in vivo inhibition of BAFF (by BAFF-R-Ig) and BAFF/APRIL (by TACI-Ig) are both effective at delaying disease onset, likely a consequence of reduced B cell maturation and survival (10). However, in certain lupus-prone mice neither antagonist reduces autoantibody production and immune complex deposition within kidneys (10), suggesting that low levels of BAFF and APRIL can support the survival of autoantibody-secreting PCs. Given that terminal differentiation of B cells into PCs results in upregulated expression of BCMA, an alternative strategy to eliminate survival of autoantibody-producing PCs is through the loss of signaling through BCMA on PCs.
We hypothesized that knocking out the BCMA gene in B6 lpr mice and B6 congenic Nba2 lupus-prone mice would result in amelioration of autoantibodies as a consequence of reduced PC survival. Unexpectedly, we found dramatically increased numbers of mature B cells and PCs in secondary lymphoid organs of BCMA−/− lpr and Nba2.BCMA−/− mice (Figs. 4, 8). These observations corresponded with accelerated production of increased titers of serum autoantibodies, BAFF levels, and immune complex deposition in kidneys (Fig. 2). Expansion of mature B cells in these mice was surprising since the frequency of peripheral B cells in BCMA−/− mice is normal ((18); Fig. 2) and membrane expression of BCMA is undetectable on B cells from B6 and B6 lpr mice compared to their BCMA-deficient counterparts (Fig. 6). These initial findings suggested that BCMA expression on B cells does not play a major role in maintaining peripheral B cell homeostasis. However, intracellular expression of BCMA was detected at higher steady-state levels in B cells from B6 lpr mice compared to B cells from other strains. Interestingly, B cells from SLE patients have recently been shown to have increased levels of BCMA expression compared to B cells from healthy donors (44, 45). Expression of BCMA parallels several markers of B cell activation in both SLE patients and healthy controls and is increased following TLR9 stimulation (45). Furthermore, signaling through BCMA using an agonistic antibody enhances TLR9-mediated B cell proliferation and antibody production in both SLE and healthy B cells, including anti-dsDNA and ANA antibody secretion from SLE B cells (45). These findings suggest that increased BCMA expression levels on peripheral human B cells correlates with B cell activation and responsiveness to BAFF and APRIL that can contribute to autoantibody production from autoreactive B cells activated through TLR9. Therefore, it is possible that increased BCMA expression levels on B cells from B6 lpr mice represent B cells activated in vivo by pro-inflammatory cytokines or TLR9 stimulation by DNA-containing immune complexes, as demonstrated for human peripheral B cells (24, 44, 45). Despite the fact that in vitro analyses of BAFF/APRIL-mediated proliferation and NF-κB activation of murine B cells indicated there were no differences among the mouse strains (Figs. 6, 7; Supplemental Fig. 5), further work will be needed to determine whether BCMA+ B cells from lupus-prone mice have an activated phenotype compared to BCMA− B cells and if BCMA expression is upregulated on healthy murine B cells when activated through TLR9.
Although we cannot exclude a potential role for BCMA stimulation to enhance murine B cell activation, our results demonstrate that B cell homeostasis and survival is abnormally controlled in lupus-prone mice when BCMA is absent, suggesting that BCMA receptor engagement on B cells from lupus-prone mice helps restrain activation. This could result from differences in the ability of lupus-prone B cells to respond to BAFF and APRIL through BAFF-R and TACI when BCMA is absent. Alternatively, BCMA stimulation of murine lupus-prone B cells may curb intrinsic hyperproliferation and increased production of pre-plasmablasts and differentiation to PCs in contrast to BCMA stimulation of B cells from SLE patients. Interestingly, previous studies using chimeric strategies have demonstrated that lpr B cells are intrinsically prone to produce autoantibodies (46–48). This unknown B cell-intrinsic defect, leading to hyperactivity and antibody production, could potentially be exacerbated in the absence of BCMA by extrinsic signals. Indirect evidence to support either of these possibilities is provided by adoptive transfer of purified B cells from BCMA−/− lpr mice into healthy recipients, demonstrating that lupus-prone B cells deficient in BCMA expand and differentiate into PCs at significantly higher frequencies in vivo compared to control and B6 lpr B cells (Fig. 7). It will be interesting to determine whether BCMA deficiency confers hypersensitivity of TACI signaling on B cells and/or PCs from BCMA−/− lupus-prone mice. Both human and mouse B cells and PCs can express TACI (24, 49–51; Supplemental Fig. 3). Therefore, signaling through TACI by BAFF and/or APRIL may lead to increased expansion, differentiation, and survival of B cells. Our data does not exclude the possibility that BAFF-R and/or TACI signaling in vivo contributes to the lymphoproliferation and autoimmune manifestations in BCMA−/− lupus-prone mice. Higher-order BAFF and APRIL oligomers, which are efficient TACI agonists, can support the survival of activated B cells and antibody-secreting cells (52). Interestingly, TLR4 stimulation of B cells upregulates TACI and BAFF-R that, upon signaling by BAFF, induces Fas expression and causes susceptibility to FasL-mediated apoptosis (51). The upregulation of Fas expression on B cells was proposed to be through BAFF-R signaling; however, TACI signaling could also play an important role in this process. A model proposed by Mackay and Schneider (32) suggests that TLR4/TACI signaling may regulate B cell activation and plasmablast survival via induction of susceptibility to Fas killing. This proposed mechanism might also involve BCMA signaling in autoimmune conditions and would be inactive in mice with the lpr mutation. Further work is in progress to test this possibility.
Increased intrinsic hyperactivity of B cells from BCMA−/− lpr mice could also be achieved through T cell help via cognate interactions or cytokines. CD4+ T cells are known to play an important role in the development of autoimmunity in the B6 lpr mouse model (15, 53) as well as the MRL-lpr/lpr model of murine lupus that develop a severely accelerated form of lupus (54, 55). The notion that CD4+ T cells help autoantibody production is consistent with our findings that the development of elevated serum titers of dsDNA-specific IgG was significantly reduced in BCMA−/− lpr mice upon GK1.5 treatment (Fig. 5). In contrast, expansion of peripheral B cells and differentiation into PCs were unaffected when T cell help was reduced, suggesting that these B cell responses are driven by B-cell intrinsic defects and/or non-CD4+ T cell signals.
BCMA may also control B cell homeostasis indirectly by regulating circulating BAFF levels. Both B6 lpr and B6.Nba2 mice deficient in BCMA produced significantly increased levels of serum BAFF compared to B6 BCMA−/− mice and parental lupus-prone strains. Given that B6 BCMA−/− mice do not exhibit heightened levels of circulating BAFF and that membrane BCMA expression is undetectable on mature B cells from all strains, it seems unlikely that removing BCMA alleviates a natural sink for BAFF consumption. Alternatively, elevated BAFF levels in BCMA−/− lupus-prone animals could result from increased numbers of BAFF-producing cells or increased levels of BAFF produced by antigen presenting cells such as dendritic cells and macrophages. Our data indicate that both dendritic cells and macrophages are significantly increased in secondary lymphoid organs of BCMA−/− lpr mice (Fig. 3, Supplemental Table II). Splenic dendritic cells from all strains expressed BAFF at equivalent levels, suggesting that the increased circulating BAFF levels in BCMA−/− lupus-prone mice arises from increased numbers of BAFF-producing cells. This finding indicates that BCMA signaling contributes to the homeostasis of BAFF-producing cells by some unknown mechanism. We did not find detectable BCMA expression by splenic dendritic cells (Fig. 3B), suggesting that BCMA signaling within this cell population is not directly involved in regulating homeostasis. Alternatively, increased numbers of BAFF-producing cells could be a consequence of interactions with B cells or T cells. It has been previously demonstrated that dendritic cells mature through membrane-bound BAFF binding to TACI expressed on activated B cells, leading to amplification of mature dendritic cells and BAFF production (56). Overproduction of BAFF by hyperactive myeloid cells in lupus-prone lyn−/− mice was shown to directly activate T cells, promoting IFN-ɣ production that further activates myeloid cells to secrete more BAFF (57). Thus, in lyn−/− mice, T cells contribute to an inflammatory cytokine loop that exacerbates autoimmunity through excess BAFF. This effect, however, has not been observed in other lupus-prone mouse models such as MRL-lpr/lpr mice that continue to exhibit T cell activation after BAFF inhibition with TACI-Ig treatment (58). Our data does not exclude the possibility that increased levels of BAFF in BCMA−/− lupus-prone mice arise from interactions of myeloid cells with B cells and/or T cells. Further work will be needed to test this interesting possibility. In sum, these studies underscore the heterogeneity of the mechanisms controlling BAFF production in lupus-prone mouse models and suggest that BCMA is an important factor in some of these mechanisms.
Supplementary Material
Acknowledgments
We thank Joanne Lannigan and Michael Solga of the Flow Cytometry Core for expert technical assistance. We thank Dr. Marcia J. McDuffie for primers to polymorphic microsatellite markers.
Footnotes
This work was supported by the Lupus Research Institute and by the Arthritis Foundation (to L.D.E).
References
- 1.Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28:18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 2.Li L, Mohan C. Genetic basis of murine lupus nephritis. Semin. Nephrol. 2007;27:12–21. doi: 10.1016/j.semnephrol.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 3.Miller DJ, Hayes CE. Phenotypic and genetic characterization of a unique B lymphocyte deficiency in strain A/WySnJ mice. Eur. J. Immunol. 1991;21:1123–1130. doi: 10.1002/eji.1830210506. [DOI] [PubMed] [Google Scholar]
- 4.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 5.Thompson JS, Bixler SA, Qian F, Vora K, Scott ML, Cachero TG, Hession C, Schneider P, Sizing ID, Mullen C, Strauch K, Zafari M, Benjamin CD, Tschopp J, Browning JL, Ambrose C. BAFF-R, a newly identified TNF receptor that specifically interacts with BAFF. Science. 2001;293:2012–2013. doi: 10.1126/science.1061965. [DOI] [PubMed] [Google Scholar]
- 6.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J. Exp. Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gross JA, Johnston J, Mudri S, Enselman R, Dillon SR, Madden K, Xu W, Parrish-Novak J, Foster D, Lofton-Day C, Moore M, Littau A, Grossman A, Haugen H, Foley K, Blumberg H, Harrison K, Kindsvogel W, Clegg CH. TACI and BCMA are receptors for a TNF homologue implicated in B-cell autoimmune disease. Nature. 2000;404:995–999. doi: 10.1038/35010115. [DOI] [PubMed] [Google Scholar]
- 8.Mackay F, Silveira PA, Brink R. B cells and the BAFF/APRIL axis: fast-forward on autoimmunity and signaling. Curr. Opin. Immunol. 2007;19:327–336. doi: 10.1016/j.coi.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 9.Ramanujam M, Wang X, Huang W, Schiffer L, Grimaldi C, Akkerman A, Diamond B, Madaio MP, Davidson A. Mechanism of action of transmembrane activator and calcium modulator ligand interactor-Ig in murine systemic lupus erythematosus. J. Immunol. 2004;173:3524–3534. doi: 10.4049/jimmunol.173.5.3524. [DOI] [PubMed] [Google Scholar]
- 10.Ramanujam M, Wang X, Huang W, Liu Z, Schiffer L, Tao H, Frank D, Rice J, Diamond B, Yu KO, Porcelli S, Davidson A. Similarities and differences between selective and nonselective BAFF blockade in murine SLE. J. Clin. Invest. 2006;116:724–734. doi: 10.1172/JCI26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benson MJ, Dillon SR, Castigli E, Geha RS, Xu S, Lam KP, Noelle RJ. Cutting Edge: The dependence of plasma cells and independence of memory B cells on BAFF and APRIL. J. Immunol. 2008;180:3655–3659. doi: 10.4049/jimmunol.180.6.3655. [DOI] [PubMed] [Google Scholar]
- 12.O'Connor BP, Raman VS, Erickson LD, Cook WJ, Weaver LK, Ahonen C, Lin LL, Mantchev GT, Bram RJ, Noelle RJ. BCMA is essential for the survival of long-lived bone marrow plasma cells. J. Exp. Med. 2004;199:91–98. doi: 10.1084/jem.20031330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho F, Lortan JE, MacLennan IC, Khan M. Distinct short-lived and long-lived antibody-producing cell populations. Eur. J. Immunol. 1986;16:1297–1301. doi: 10.1002/eji.1830161018. [DOI] [PubMed] [Google Scholar]
- 14.Hoyer BF, Moser K, Hauser AE, Peddinghaus A, Voigt C, Eilat D, Radbruch A, Hiepe F, Manz RA. Short-lived plasmablasts and long-lived plasma cells contribute to chronic humoral autoimmunity in NZB/W mice. J. Exp. Med. 2004;199:1577–1584. doi: 10.1084/jem.20040168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cohen PL, Eisenberg RA. Lpr and gld: single gene models of systemic autoimmunity and lymphoproliferative disease. Annu. Rev. Immunol. 1991;9:243–269. doi: 10.1146/annurev.iy.09.040191.001331. [DOI] [PubMed] [Google Scholar]
- 16.Rozzo SJ, Allard JD, Choubey D, Vyse TJ, Izui S, Peltz G, Kotzin BL. Evidence for an interferon-inducible gene, Ifi202, in the susceptibility to systemic lupus. Immunity. 2001;15:435–443. doi: 10.1016/s1074-7613(01)00196-0. [DOI] [PubMed] [Google Scholar]
- 17.Vyse TJ, Rozzo SJ, Drake CG, Izui S, Kotzin BL. Control of multiple autoantibodies linked with a lupus nephritis susceptibility locus in New Zealand black mice. J. Immunol. 1997;158:5566–5574. [PubMed] [Google Scholar]
- 18.Xu S, Lam KP. B-cell maturation protein, which binds the tumor necrosis factor family members BAFF and APRIL, is dispensable for humoral immune responses. Mol. Cell. Biol. 2001;21:4067–4074. doi: 10.1128/MCB.21.12.4067-4074.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jorgensen TN, Alfaro J, Enriquez HL, Jiang C, Loo WM, Atencio S, Bupp MR, Mailloux CM, Metzger T, Flannery S, Rozzo SJ, Kotzin BL, Rosemblatt M, Bono MR, Erickson LD. Development of murine lupus involves the combined genetic contribution of the SLAM and FcgammaR intervals within the Nba2 autoimmune susceptibility locus. J. Immunol. 2010;184:775–786. doi: 10.4049/jimmunol.0901322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Theofilopoulos AN, Dixon FJ. Murine models of systemic lupus erythematosus. Adv. Immunol. 1985;37:269–390. doi: 10.1016/s0065-2776(08)60342-9. [DOI] [PubMed] [Google Scholar]
- 21.Radbruch A, Muehlinghaus G, Luger EO, Inamine A, Smith KG, Dorner T, Hiepe F. Competence and competition: the challenge of becoming a long-lived plasma cell. Nat. Rev. Immunol. 2006;6:741–750. doi: 10.1038/nri1886. [DOI] [PubMed] [Google Scholar]
- 22.Pelletier N, McHeyzer-Williams LJ, Wong KA, Urich E, Fazilleau N, McHeyzer-Williams MG. Plasma cells negatively regulate the follicular helper T cell program. Nat. Immunol. 2010;11:1110–1118. doi: 10.1038/ni.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mond JJ, Vos Q, Lees A, Snapper CM. T cell independent antigens. Curr. Opin. Immunol. 1995;7:349–354. doi: 10.1016/0952-7915(95)80109-x. [DOI] [PubMed] [Google Scholar]
- 24.Darce JR, Arendt BK, Wu X, Jelinek DF. Regulated expression of BAFF-binding receptors during human B cell differentiation. J. Immunol. 2007;179:7276–7286. doi: 10.4049/jimmunol.179.11.7276. [DOI] [PubMed] [Google Scholar]
- 25.Ng LG, Ng CH, Woehl B, Sutherland AP, Huo J, Xu S, Mackay F, Lam KP. BAFF costimulation of Toll-like receptor-activated B-1 cells. Eur. J. Immunol. 2006;36:1837–1846. doi: 10.1002/eji.200635956. [DOI] [PubMed] [Google Scholar]
- 26.Gras MP, Laabi Y, Linares-Cruz G, Blondel MO, Rigaut JP, Brouet JC, Leca G, Haguenauer-Tsapis R, Tsapis A. BCMAp: an integral membrane protein in the Golgi apparatus of human mature B lymphocytes. Int. Immunol. 1995;7:1093–1106. doi: 10.1093/intimm/7.7.1093. [DOI] [PubMed] [Google Scholar]
- 27.Schulze-Luehrmann J, Ghosh S. Antigen-receptor signaling to nuclear factor kappa B. Immunity. 2006;25:701–715. doi: 10.1016/j.immuni.2006.10.010. [DOI] [PubMed] [Google Scholar]
- 28.Hatada EN, Do RK, Orlofsky A, Liou HC, Prystowsky M, MacLennan IC, Caamano J, Chen-Kiang S. NF-kappa B1 p50 is required for BLyS attenuation of apoptosis but dispensable for processing of NF-kappa B2 p100 to p52 in quiescent mature B cells. J. Immunol. 2003;171:761–768. doi: 10.4049/jimmunol.171.2.761. [DOI] [PubMed] [Google Scholar]
- 29.Stadanlick JE, Kaileh M, Karnell FG, Scholz JL, Miller JP, Quinn WJ, 3rd, Brezski RJ, Treml LS, Jordan KA, Monroe JG, Sen R, Cancro MP. Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling. Nat. Immunol. 2008;9:1379–1387. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shinners NP, Carlesso G, Castro I, Hoek KL, Corn RA, Woodland RT, Scott ML, Wang D, Khan WN. Bruton's tyrosine kinase mediates NF-kappa B activation and B cell survival by B cell-activating factor receptor of the TNF-R family. J. Immunol. 2007;179:3872–3880. doi: 10.4049/jimmunol.179.6.3872. [DOI] [PubMed] [Google Scholar]
- 31.Mackay F, Schneider P. Cracking the BAFF code. Nat. Rev. Immunol. 2009;9:491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 32.Mackay F, Schneider P. TACI, an enigmatic BAFF/APRIL receptor, with new unappreciated biochemical and biological properties. Cytokine Growth Factor Rev. 2008;19:263–276. doi: 10.1016/j.cytogfr.2008.04.006. [DOI] [PubMed] [Google Scholar]
- 33.Harless SM, Lentz VM, Sah AP, Hsu BL, Clise-Dwyer K, Hilbert DM, Hayes CE, Cancro MP. Competition for BLyS-mediated signaling through Bcmd/BR3 regulates peripheral B lymphocyte numbers. Curr. Biol. 2001;11:1986–1989. doi: 10.1016/s0960-9822(01)00598-x. [DOI] [PubMed] [Google Scholar]
- 34.Yan M, Marsters SA, Grewal IS, Wang H, Ashkenazi A, Dixit VM. Identification of a receptor for BLyS demonstrates a crucial role in humoral immunity. Nat. Immunol. 2000;1:37–41. doi: 10.1038/76889. [DOI] [PubMed] [Google Scholar]
- 35.Sasaki Y, Casola S, Kutok JL, Rajewsky K, Schmidt-Supprian M. TNF family member B cell-activating factor (BAFF) receptor-dependent and -independent roles for BAFF in B cell physiology. J. Immunol. 2004;173:2245–2252. doi: 10.4049/jimmunol.173.4.2245. [DOI] [PubMed] [Google Scholar]
- 36.von Bulow GU, van Deursen JM, Bram RJ. Regulation of the T-independent humoral response by TACI. Immunity. 2001;14:573–582. doi: 10.1016/s1074-7613(01)00130-3. [DOI] [PubMed] [Google Scholar]
- 37.Seshasayee D, Valdez P, Yan M, Dixit VM, Tumas D, Grewal IS. Loss of TACI causes fatal lymphoproliferation and autoimmunity, establishing TACI as an inhibitory BLyS receptor. Immunity. 2003;18:279–288. doi: 10.1016/s1074-7613(03)00025-6. [DOI] [PubMed] [Google Scholar]
- 38.Darce JR, Arendt BK, Chang SK, Jelinek DF. Divergent effects of BAFF on human memory B cell differentiation into Ig-secreting cells. J. Immunol. 2007;178:5612–5622. doi: 10.4049/jimmunol.178.9.5612. [DOI] [PubMed] [Google Scholar]
- 39.Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, Cyster JG. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 40.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 41.Ait-Azzouzene D, Gavin AL, Skog P, Duong B, Nemazee D. Effect of cell:cell competition and BAFF expression on peripheral B cell tolerance and B-1 cell survival in transgenic mice expressing a low level of Igkappa-reactive macroself antigen. Eur. J. Immunol. 2006;36:985–996. doi: 10.1002/eji.200535581. [DOI] [PubMed] [Google Scholar]
- 42.He B, Qiao X, Cerutti A. CpG DNA induces IgG class switch DNA recombination by activating human B cells through an innate pathway that requires TLR9 and cooperates with IL-10. J. Immunol. 2004;173:4479–4491. doi: 10.4049/jimmunol.173.7.4479. [DOI] [PubMed] [Google Scholar]
- 43.Xu W, Santini PA, Matthews AJ, Chiu A, Plebani A, He B, Chen K, Cerutti A. Viral double-stranded RNA triggers Ig class switching by activating upper respiratory mucosa B cells through an innate TLR3 pathway involving BAFF. J. Immunol. 2008;181:276–287. doi: 10.4049/jimmunol.181.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koarada S, Tada Y, Sohma Y, Haruta Y, Suematsu R, Mitamura M, Inoue H, Ehara H, Tokoro Y, Ohta A, Nagasawa K. Autoantibody-producing RP105(−) B cells, from patients with systemic lupus erythematosus, showed more preferential expression of BCMA compared with BAFF-R than normal subjects. Rheumatol. 2010;49:662–670. doi: 10.1093/rheumatology/kep437. [DOI] [PubMed] [Google Scholar]
- 45.Kim J, Gross JA, Dillon SR, Min J, Elkon KB. Increased BCMA expression in lupus marks activated B cells, and BCMA receptor engagement enhances the response to TLR9 stimulation. AutoImmunity. 2011;44:69–81. doi: 10.3109/08916934.2010.509122. [DOI] [PubMed] [Google Scholar]
- 46.Perkins DL, Glaser RM, Mahon CA, Michaelson J, Marshak-Rothstein A. Evidence for an intrinsic B cell defect in lpr/lpr mice apparent in neonatal chimeras. J. Immunol. 1990;145:549–555. [PubMed] [Google Scholar]
- 47.Nemazee D, Guiet C, Buerki K, Marshak-Rothstein A. B lymphocytes from the autoimmune-prone mouse strain MLR/lpr manifest an intrinsic defect in tetraparental MRL/lpr in equilibrium DBA/2 chimeras. J. Immunol. 1991;147:2536–2539. [PubMed] [Google Scholar]
- 48.Sobel ES, Katagiri T, Katagiri K, Morris SC, Cohen PL, Eisenberg RA. An intrinsic B cell defect is required for the production of autoantibodies in the lpr model of murine systemic autoimmunity. J. Exp. Med. 1991;173:1441–1449. doi: 10.1084/jem.173.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Khuda SE, Loo WM, Janz S, Van Ness B, Erickson LD. Deregulation of c-Myc Confers distinct survival requirements for memory B cells, plasma cells, and their progenitors. J. Immunol. 2008;181:7537–7549. doi: 10.4049/jimmunol.181.11.7537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hondowicz BD, Alexander ST, Quinn WJ, 3rd, Pagan AJ, Metzgar MH, Cancro MP, Erikson J. The role of BLyS/BLyS receptors in anti-chromatin B cell regulation. Int. Immunol. 2007;19:465–475. doi: 10.1093/intimm/dxm011. [DOI] [PubMed] [Google Scholar]
- 51.Acosta-Rodriguez EV, Craxton A, Hendricks DW, Merino MC, Montes CL, Clark EA, Gruppi A. BAFF and LPS cooperate to induce B cells to become susceptible to CD95/Fas-mediated cell death. Eur. J. Immunol. 2007;37:990–1000. doi: 10.1002/eji.200636698. [DOI] [PubMed] [Google Scholar]
- 52.Bossen C, Cachero TG, Tardivel A, Ingold K, Willen L, Dobles M, Scott ML, Maquelin A, Belnoue E, Siegrist CA, Chevrier S, Acha-Orbea H, Leung H, Mackay F, Tschopp J, Schneider P. TACI, unlike BAFF-R, is solely activated by oligomeric BAFF and APRIL to support survival of activated B cells and plasmablasts. Blood. 2008;111:1004–1012. doi: 10.1182/blood-2007-09-110874. [DOI] [PubMed] [Google Scholar]
- 53.Mosbach-Ozmen L, Gaveriaux C, Montecino-Rodriguez E, Loor F. The C57B1/6 nu/nu, lpr/lpr mouse. III. Autoimmunity status. Thymus. 1986;8:59–75. [PubMed] [Google Scholar]
- 54.Peng SL, Madaio MP, Hughes DP, Crispe IN, Owen MJ, Wen L, Hayday AC, Craft J. Murine lupus in the absence of alpha beta T cells. J. Immunol. 1996;156:4041–4049. [PubMed] [Google Scholar]
- 55.Peng SL, Cappadona J, McNiff JM, Madaio MP, Owen MJ, Hayday AC, Craft J. Pathogenesis of autoimmunity in alphabeta T cell-deficient lupus-prone mice. Clin. Exp. Immunol. 1998;111:107–116. doi: 10.1046/j.1365-2249.1998.00424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Diaz-de-Durana Y, Mantchev GT, Bram RJ, Franco A. TACI-BLyS signaling via B-cell-dendritic cell cooperation is required for naive CD8+ T-cell priming in vivo. Blood. 2006;107:594–601. doi: 10.1182/blood-2004-12-4708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Scapini P, Hu Y, Chu CL, Migone TS, Defranco AL, Cassatella MA, Lowell CA. Myeloid cells, BAFF, and IFN-gamma establish an inflammatory loop that exacerbates autoimmunity in Lyn-deficient mice. J. Exp. Med. 207:1757–1773. doi: 10.1084/jem.20100086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu W, Szalai A, Zhao L, Liu D, Martin F, Kimberly RP, Zhou T, Carter RH. Control of spontaneous B lymphocyte autoimmunity with adenovirus-encoded soluble TACI. Arthritis Rheum. 2004;50:1884–1896. doi: 10.1002/art.20290. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.