SUMMARY
Itch, the unpleasant sensation that evokes a desire to scratch, accompanies numerous skin and nervous system disorders. In many cases, pathological itch is insensitive to antihistamine treatment. Recent studies have identified members of the Mas-related GPCR (Mrgpr) family that are activated by mast cell mediators and promote histamine-independent itch. MrgprA3 and MrgprC11 act as receptors for the pruritogens chloroquine and BAM8–22, respectively. However, the signaling pathways and transduction channels activated downstream of these pruritogens are largely unknown. We found that TRPA1 is the downstream target of both MrgprA3 and MrgprC11, in cultured sensory neurons and heterologous cells. TRPA1 is required for Mrgpr-mediated signaling, as sensory neurons from TRPA1-deficient mice exhibited profoundly diminished responses to chloroquine and BAM8–22. Likewise, TRPA1-deficient mice displayed little to no scratching in response to these pruritogens. Our findings demonstrate that TRPA1 is an essential component of the signaling pathways that promote histamine-independent itch.
INTRODUCTION
Acute pruritus, or itch, serves an important protective function by warning against harmful agents in the environment such as insects, toxic plants or other irritants. Itch also promotes scratching, which aids in clearing pruritogens and attenuates itch sensations. In contrast, pruritus can also be a debilitating condition that accompanies numerous skin, systemic, and nervous system disorders1. While many forms of itch are mediated by histamine signaling, there are clearly other key neural pathways. For example, a side effect of the antimalaria drug chloroquine (CQ) is antihistamine-resistant, intolerable itch2. Likewise, spicules from the plant Mucuna pruriens produce intense itch via a histamine-independent pathway3–5. Moreover, immune cells release a variety of pruritogens that mediate allergy-evoked itch, psoriasis and eczema, and antihistamines are not effective in treating the full spectrum of allergic disorders6, 7. Finally, most pathophysiological itch conditions are insensitive to antihistamine treatment and therapeutic targets have yet to be identified8–11.
While the molecular and cellular mechanisms of itch have yet to be fully elucidated, recent studies have begun to delineate the basic characteristics of the itch circuitry. There is now evidence implicating dedicated neuronal pathways for itch, separate from pain12, 13. Mice lacking gastrin-releasing peptide receptor (GRPR)-positive cells in dorsal horn of the spinal cord display reduced itch behaviors, but normal pain behaviors14. Distinct subsets of primary afferent neurons mediating itch have also been identified. Approximately 5–20% of primary afferent C-fibers are activated by endogenous itch-producing compounds released by non-neuronal cells in the skin (e.g., mast cells), as well as by exogenous pruritogens, such as chloroquine1, 15, 16.
Itch-sensitive C-fibers can be divided into multiple subgroups based on pruritogen-sensitivity. A subset of primary afferent C-fibers that express the capsaicin receptor, TRPV1, can be divided into three groups based on receptor expression and pruritogen sensitivity. The first group expresses the 5-hydroxytryptamine receptor 3 and the H1 histamine receptor, and mediates itch-evoked responses to serotonin and histamine15. A second group expresses Mas-related GPCR A3 (MrgprA3) that mediates itch-evoked responses to CQ. The third group expresses both MrgprA3 and MrgprC11, the receptor for the endogenous pruritogen, BAM8–22 (BAM)16. MrgprA3 and MrgprC11 are members of the newly identified, sensory neuron-specific Mas-related G protein-coupled receptor family. While the function of most Mrgprs remains unknown, MrgprA3 and MrgprC11 have been shown to play key roles in histamine-independent pruritus. MrgprC11 is targeted by mast cell pruritogens released during allergic inflammation17. MrgprA3 is activated by the antimalaria drug CQ, which causes acute itch in rodents and intolerable itch in some patients.
The signaling mechanisms by which pruritogen-evoked activation of MrgprA3 and MrgprC11 leads to neuronal excitation remain unknown. MrgprA3 and MrgprC11 are expressed in a subset of TRPV1 positive afferents. In addition, MrgprA3-evoked excitation is inhibited by ruthenium red, a blocker of TRPA1 and TRPV1 channels16. While TRPV1-expressing afferents mediate responses to a variety of pruritogens, mice lacking functional TRPV1 channels display reduced responses to histamine, but normal responses to serotonin and endothelin-115. These data imply that other ion channels are also activated by pruritogens in TRPV1-expressing afferents. These findings suggest that both TRPV1 and TRPA1 are candidate transduction channels in the Mrgpr-pruritic pathways.
The irritant receptor TRPA1 is highly expressed in a subset of TRPV1-positive neurons. TRPA1 is activated by a number of pain producing compounds such as isothiocyanates, the pungent compounds present in mustard oil and other Brassica plants, cinnamon oil, and cannabinoids. Additionally, TRPA1 is activated downstream of G protein-coupled receptors, including the pro-algesic bradykinin receptor18, 19. Histamine, serotonin, chloroquine and BAM8–22 all evoke itch by acting on G protein-coupled receptors16, 20, 21. Thus, TRPA1 is a key candidate transduction channel for itch.
Here we show that TRPA1 is an essential player in the transduction of Mrgpr-mediated itch. Cultured sensory neurons from TRPA1-deficient mice exhibit profoundly diminished responses to both chloroquine and BAM8–22. The functional coupling between MrgprA3 and TRPA1 is attenuated by disruption of Gβγ signaling, while coupling between MrgprC11 and TRPA1 requires PLC signaling. TRPA1 is required for Mrgpr-evoked itch in vivo, as mice lacking TRPA1 do not display the chloroquine- or BAM8–22-evoked itch behaviors typical of wild type animals. Our findings support an emerging role for TRP channels in the transduction of pruritic stimuli.
RESULTS
BAM8–22 and CQ activate TRPA1 and TRPV1-expressing neurons
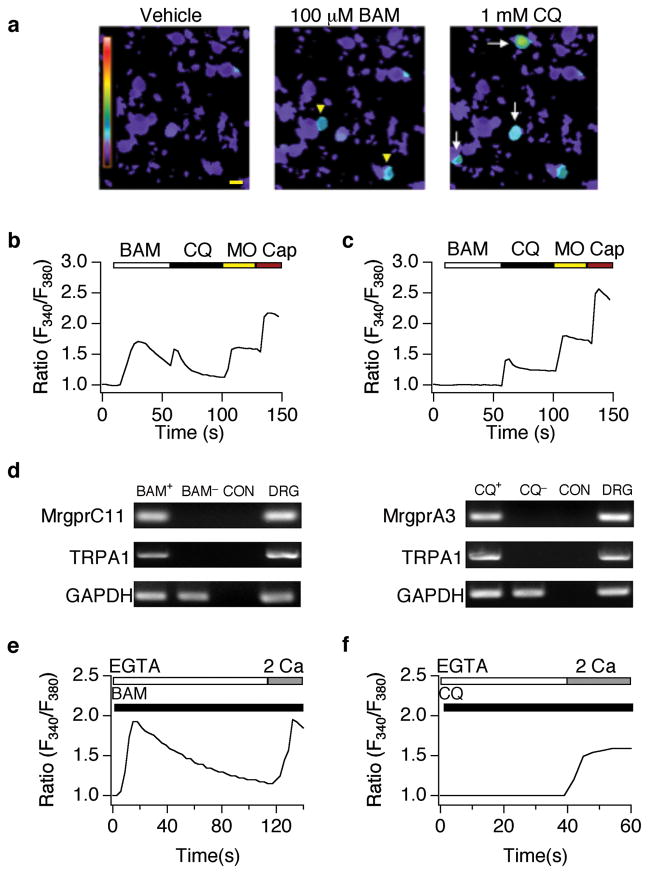
The endogenous pruritogen BAM8–22 and the pruritic antimalaria drug chloroquine activate a subset of TRPV1-positive neurons16. To determine whether these pruritogens activate the subset of TRPV1-positive neurons that also express TRPA1, we used ratiometric calcium (Ca2+) imaging to examine overlap between BAM- and CQ-sensitivity, and sensitivity to the TRPA1 agonist, allyl isothiocyanate (mustard oil; Fig. 1). We found that 9.8±1.2% of dorsal root ganglia (DRG) neurons and 16.1±2.3% of trigeminal (TG) neurons (Fig. 1a–c; n≥1050 neurons) showed robust increases in intracellular Ca2+ following CQ (1 mM) application, while only 6.2±1.2% of DRG and 5.4±0.9% of TG neurons were responsive to both CQ and BAM (100 μM; Fig. 1a–c; n≥390 neurons). Subsequent exposure to mustard oil (MO; 200 μM) or capsaicin (Cap; 1 μM) produced further increases in Ca2+ levels in all CQ- and BAM-positive cells (Fig. 1b–c). These results suggest that BAM and CQ activate a subset of TRPV1-positive sensory neurons that also express the ion channel TRPA1. To further test this, we used PCR to correlate TRPA1 gene expression with CQ and BAM sensitivity in individual sensory neurons, as determined by calcium imaging. Cells were subjected to RT-PCR using MrgprA3, MrgprC11 and TRPA1-specific primers. As previously reported, BAM- and CQ-sensitive neurons showed amplification of MrgprA3 and MrgprC11, respectively (Fig. 1d; Supplementary Figure 1, online). Likewise, all BAM-sensitive neurons also expressed MrgprA3 (7 of 7) consistent with previous studies22, and our imaging data (Fig. 1b). In addition, the TRPA1 was amplified from all CQ-sensitive neurons (CQ+; n=7) and BAM-responsive (BAM+; n=7) neurons (Fig. 1d; Supplementary Figure 1, online). In contrast, BAM-, CQ-, and MO-insensitive cells did not display MrgprA3, MrgprC11 or TRPA1 expression (BAM− and CQ−; Fig. 1d; Supplementary Figure 1, online). These results clearly show that CQ activates a subset of sensory neurons that express TRPA1 and TRPV1; BAM, in turn, activates a subset of CQ-sensitive cells.
Figure 1. Chloroquine and BAM8–22 activate a subset of TRPA1-positive sensory neurons.
(a) BAM8–22- (BAM; 100 μM; yellow arrowheads) and chloroquine (CQ; 1 mM; white arrows)-evoked responses in cultured dorsal root ganglia neurons (representative Fura-2 ratiometric images). Scale bar=10 μm. (b) Representative BAM- and CQ-responsive cell. Fura-2 ratio in response to BAM (100 μM), CQ (1 mM), allyl isothiocyanate (mustard oil: MO; 200 μM), and capsaicin (Cap; 1 μM). (c) Representative CQ-sensitive, BAM-insensitive cell. Fura-2 ratio in response to BAM (100 μM), CQ (1 mM), MO (200 μM), and Cap (1 μM). (d) PCR analysis of MrgprA3, MrgprC11, and TRPA1 expression in CQ-positive, BAM-positive and CQ/BAM/MO-negative large diameter sensory neurons. MrgprC11 and TRPA1 were amplified in BAM-sensitive (BAM+), but not BAM-negative (BAM−) or no-RT control (CON) cells (right). MrgprA3 and TRPA1 were amplified in chloroquine-positive cells (CQ+), but not chloroquine-negative (CQ−), or no-RT control (CON) cells (left). MrgprA3, MrgprC11 and TRPA1 were all amplified from DRG cDNA (DRG). Note the presence of control GAPDH product in all samples. (e) Representative trace showing Ca2+ response to BAM (100 μM) in the absence (1mM EGTA), and presence (2 mM Ca2+) of extracellular calcium. (f) Representative response to CQ (1 mM) in the absence (1 mM EGTA), and presence (2 mM) of extracellular calcium.
Histamine and other phospholipase C (PLC)-coupled receptor agonists promote the release of Ca2+ from intracellular stores and subsequent activation of TRP channels. Consistent with a previous study showing that the BAM receptor, MrgprC11, couples to PLC23, BAM application evokes Ca2+ release from intracellular stores in the absence of extracellular Ca2+ (Ca2+EXT; Fig. 1e). Subsequent addition of Ca2+EXT triggers a rise intracellular Ca2+due to influx (Fig. 1e). Unlike BAM, CQ application in the absence of Ca2+EXT fails to mobilize Ca2+ release from stores. However, CQ application in extracellular Ca2+ triggers influx across the plasma membrane (Fig. 1f). This demonstrates that both BAM and CQ trigger the influx of Ca2+ through transduction channels in the plasma membrane. TRPV1 and TRPA1 are likely candidate transducers because they are expressed in CQ- and BAM-sensitive cells (Fig. 1) and are inhibited by ruthenium red, which abolishes CQ-evoked signaling16. We thus asked whether BAM- and CQ-evoked excitation is attenuated by pharmacological or genetic knockdown of TRPV1 or TRPA1 channels.
TRPV1 is not required for BAM or CQ signaling
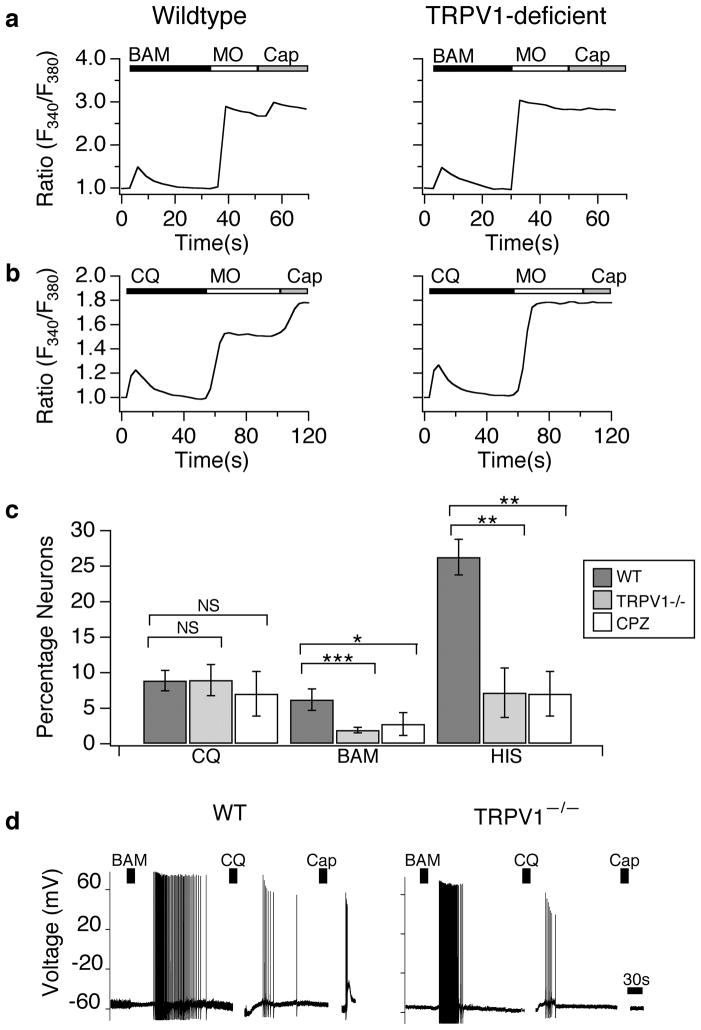
We first compared BAM- and CQ-evoked Ca2+ signals in neurons isolated from TRPV1-deficient mice to those isolated from wild type littermates (Fig. 2a–c). Cultured neurons isolated from TRPV1-deficient mice showed a decrease in the proportion of BAM-sensitive neurons (Fig. 2a,c) but no change in the magnitude of the Ca2+ signal in the responsive cells, as compared to wild type (WT peak=1.38±0.11; V1−/− peak=1.52±0.16; p=0.59). Similar results were observed in wild type neurons treated with the TRPV1 antagonist, capsazepine (Fig. 2c). In contrast, no significant differences in the amplitude (WT peak=1.57±0.18; V1−/− peak=1.62±0.21) or prevalence (Fig. 2a,c) of CQ-evoked signals were observed. Wild type neurons treated with capsazepine displayed normal CQ-evoked signals (Fig. 2c).
Figure 2. TRPV1 is not required for chloroquine or BAM8–22-evoked excitation of neurons.
(a) Cultured sensory neurons isolated from wild type and TRPV1-deficient mice were exposed to BAM8–22 (BAM; 100 μM), followed by allyl isothiocyanate (mustard oil: MO; 200 μM), and capsaicin (Cap; 1 μM) and analyzed by Fura-2 ratiometric calcium imaging (representative responses). (b) Cultured sensory neurons isolated from wild type and TRPV1-deficient mice were exposed to chloroquine (CQ; 1 μM), followed by allyl isothiocyanate (mustard oil: MO; 200 μM), and capsaicin (Cap; 1 μM) and analyzed by Fura-2 ratiometric calcium imaging (representative responses). (c) The prevalence of CQ-sensitivity was similar in wild type (black), TRPV1-deficient (grey), and capsazepine treated (CPZ; 20 μM; white) neurons (p>0.5; one-way ANOVA). In contrast, the prevalence of BAM-sensitivity was reduced in TRPV1-deficient (grey; p<0.01; one-way ANOVA) and CPZ treated neurons (white, p <0.05; one-way ANOVA) relative to wild type neurons (black). The prevalence of histamine (HIS)-sensitivity was also reduced in TRPV1-deficient (grey; p<0.05; one-way ANOVA) and CPZ treated neurons (white, p<0.05; one–way ANOVA; n=3 animals per genotype; n≥500 neurons per genotype) relative to wild type neurons (black). (d) TRPV1 is not required for CQ- or BAM-evoked action potential firing. Representative current-clamp recording shows that wild type and TRPV1-deficient neurons fire similar numbers of action potentials in response to BAM8–22 (BAM; 100 μM; p=0.728; one-way ANOVA) and chloroquine (CQ; 1 mM; p=0.739; one-way ANOVA). No responses to capsaicin (Cap; 1 μM) were observed in TRPV1-deficient neurons. Error bars represent s.e.m. n=5–13 cells/genotype. (*p<0.05, **p<0.01 ***p<0.001).
To further probe the role of TRPV1 in CQ and BAM signaling, we next performed current-clamp recording of CQ-and BAM-evoked action potential firing in wild type and TRPV1-deficient neurons (Fig. 2d). No significant differences in action potential firing were observed between wild type and TRPV1-deficient neurons following application of BAM (WT: 39.1±10.5; Trpv1−/−: 46.0 ±15.0; p=0.73; Fig. 2d) or CQ (WT: 8.0±1.8; Trpv1−/−: 7.2±1.6; p=0.74; Fig. 2d). Taken together, these results demonstrate that functional TRPV1 channels are not required for BAM- or CQ-evoked excitation.
TRPA1 is required for BAM and CQ-evoked neuronal excitation
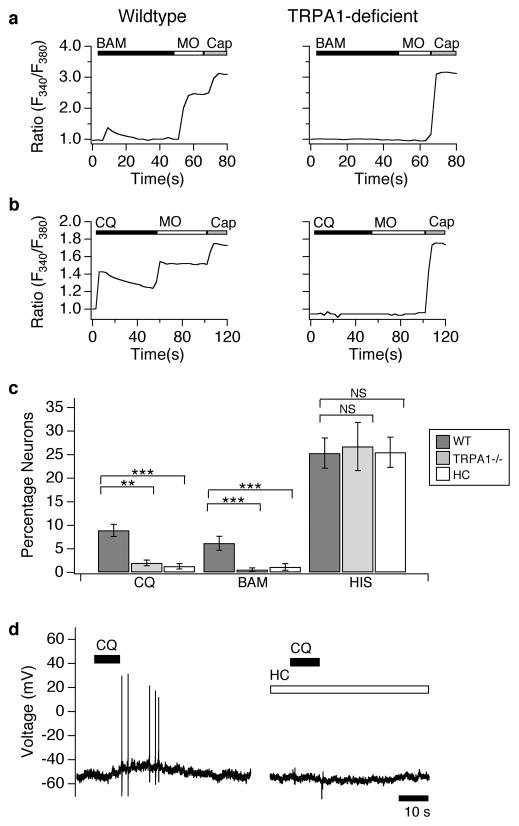
We next asked whether deficiencies in TRPA1 would alter neuronal CQ and BAM sensitivity. Unlike TRPV1-deficient neurons that display a partial attenuation of BAM responses (Fig. 2), BAM-evoked Ca2+ signaling is ablated in TRPA1-deficient neurons (Fig. 3a,c). Similarly, pharmacological inhibition of TRPA1 with the selective antagonist HC-030031 (HC; 100μM)24–26 significantly decreased neuronal sensitivity to BAM (Fig. 3c; Trpa1+/+: 6.18±1.49; Trpa1−/−: 0.57±0.36; HC-treated: 1.12±0.71).
Figure 3. TRPA1-deficient neurons display a loss of chloroquine and BAM8–22 sensitivity.
(a) Cultured sensory neurons isolated from wild type and TRPA1-deficient mice were exposed to BAM8–22 (BAM; 100 μM), followed by allyl isothiocyanate (mustard oil: MO; 200 μM), and capsaicin (Cap; 1 μM) and responses measured by Fura-2 ratiometric calcium imaging (representative response). (b) Cultured sensory neurons isolated from wild type and TRPA1-deficient mice were exposed to chloroquine (CQ; 1 μM), followed by allyl isothiocyanate (mustard oil: MO; 200 μM), and capsaicin (Cap; 1 μM) and responses measured by Fura-2 ratiometric calcium imaging (representative response). (c) The prevalence of CQ sensitivity was significantly reduced in TRPA1-deficient (grey; p>0.5; one-way ANOVA) and HC-03001 treated (HC; 100 μM; white) neurons (p>0.1; one-way ANOVA), relative to wild type neurons (black). Similarly, the prevalence of BAM sensitivity was reduced in TRPA1-deficient (grey; p<0.01; one-way ANOVA) and HC treated neurons (white, p<0.01; one-way ANOVA), relative to wild type (black). In contrast, the prevalence of histamine sensitivity was similar in wild type (black), TRPA1-deficient (grey; p=.73; one-way ANOVA), and HC treated neurons (white, p=0.61; one-way ANOVA, n=3 animals per genotype; n≥500 neurons per genotype. (d) TRPA1 is required for CQ-evoked action potential firing. Representative current clamp recording shows that HC-03001 (HC; 100 μM) significantly blocks CQ-evoked action potential firing relative to vehicle (CQ: p<0.01; one-way ANOVA; n≥5 cells/compound). Error bars represent s.e.m. (*p<0.05, **p<0.01 ***p<0.001)
We also examined the role of TRPA1 in CQ-evoked neuronal activation. CQ-evoked Ca2+ signals were significantly attenuated in TRPA1-deficient neurons (Fig. 3b,c) as compared to wild type neurons (Fig. 3b). Consistent with previous studies, MO-evoked responses were also attenuated in TRPA1-deficient neurons (Fig. 3a,b). Likewise, pharmacological inhibition of TRPA1 with HC-030031 (HC; 100 μM) significantly decreased neuronal sensitivity to CQ (Fig. 3c). Importantly, the prevalence of capsaicin-responsive cells was similar in wild type, mutant, and HC-treated neurons (Trpa1+/+: 52.1±5.17%; Trpa1−/−: 56.7±6.9%; HC-treated: 55.5±6.1%).
Finally, we used current-clamp recording to probe the role of TRPA1 in CQ- and BAM-evoked neuronal excitation. CQ- and BAM-evoked action potential firing was compared in DRG neurons treated with vehicle versus HC-030031 (Fig. 3d). TRPA1 inhibition significantly attenuated CQ-evoked action potential firing (CQ+ vehicle=6.2±1.2; 100μM HC-030031=0.25±0.1; p=0.003; Fig. 3d) and BAM-evoked firing (BAM+ vehicle=21.3±4.2; 100 μM HC-030031=2.40±0.98; p=0.002; Fig. 3d). Together, our results clearly show that functional TRPA1 channels are required for CQ and BAM-evoked neuronal excitability.
While TRPA1 is required for CQ and BAM signaling, it does not mediate all forms of itch. Neurons isolated from TRPA1-deficient animals (Fig. 3c) or treated with HC-030031 (100μM; Fig. 3c) display normal histamine-evoked responses. These findings are consistent with previous studies showing that TRPV1, but not TRPA1, is required for histamine signaling in sensory neurons15, 27, 28.
MrgprA3 and MrgprC11 functionally couple to TRPA1
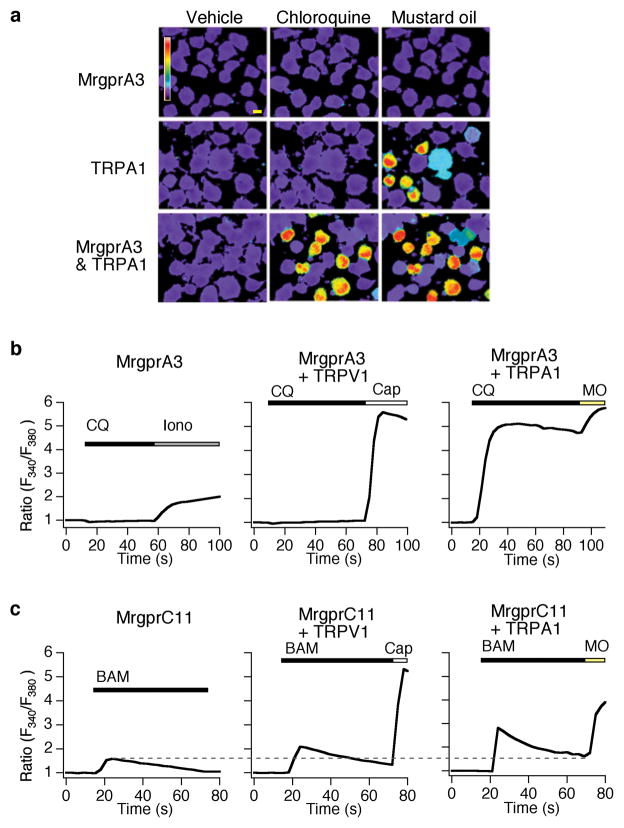
The GPCRs MrgprA3 and MrgprC11 are required for CQ and BAM signaling in sensory neurons, respectively16. In addition to being activated directly by endogenous and exogenous irritants, TRPA1 is a receptor-operated channel that can be activated by bradykinin, or other GPCR-coupled inflammatory mediators29, 30. We therefore asked whether CQ or BAM could activate heterologous TRPA1 channels expressed in the CQ- and BAM-insensitive neuroblastoma cell line, NG108. CQ and BAM fail to trigger Ca2+ influx into TRPA1-transfected cells (Fig. 4a). However, these cells responded robustly to application of MO (200μM; Fig. 4a), confirming the presence of functional TRPA1 channels. CQ-evoked Ca2+ signals were not observed in NG108 cells transfected with Mrgpra3 alone (Fig. 4ab). Consistent with our findings in TRPV1-deficient neurons (Fig. 2), CQ failed to trigger Ca2+ signals in cells expressing Mrgpra3 and TRPV1 (Fig. 4b). In contrast, NG108 cells transfected with both TRPA1 and Mrgpra3 (A1/A3) displayed robust increases in intracellular Ca2+ following CQ application (Fig. 4a–b); these responses were attenuated by HC-030031 (100μM; not shown). Thus, both MrgprA3 and TRPA1 receptors are required to confer CQ-sensitivity to NG108 cells.
Figure 4. MrgprA3 and MrgprC11 signal via TRPA1 in neuronal cell lines.
(a) Chloroquine-evoked (CQ; 1 mM) calcium response in NG108 cells cotransfected with TRPA1 and Mrgpra3 (bottom), Mrgpra3 alone (top) and TRPA1 alone (middle). TRPA1 expression was assessed by application of mustard oil (MO; 100 μM). Scale bar=10 μm. (b) Chloroquine-evoked Fura-2 ratiometric responses (average traces) in NG108 cells transfected with Mrgpra3 alone (left), with TRPV1 (middle) and with TRPA1 (right). Ionomycin (1 μM) was used to show that the Mrgpra3 transfected cells were healthy and loaded with Fura-2. Capsaicin (Cap; 1 μM) and mustard oil (MO; 200 μM) were used to activate TRPV1 and TRPA1 channels, respectively. MrgprA3 expression was assessed by GFP fluorescence (not shown). (c) BAM8–22-evoked Fura-2 ratiometric responses (average traces) in NG108 cells transfected with Mrgprc11 alone (1.58±0.16; left), with TRPV1 (2.1±0.3; middle) and with TRPA1 (2.8±0.3; right). Values are shown as peak ± s.e.m. (Mrgprc11 alone versus Mrgprc11 + TRPV1: p=0.005; Mrgprc11 alone versus Mrgprc11 + TRPA1: p=0.0001; Mrgprc11 + TRPA1 versus Mrgprc11 + TRPV1: p=0.004). Capsaicin (Cap; 1 μM) and mustard oil (MO; 200 μM) were used to activate TRPV1 and TRPA1 channels, respectively. MrgprC11 expression was assessed by GFP fluorescence (not shown).
BAM-evoked Ca2+ signals were observed in NG108 cells transfected with Mrgprc11 alone, but not cells transfected with TRPA1, TRPV1 or vector alone (Fig. 4c). This is consistent with our data showing that MrgprC11 activation leads to Ca2+-release from stores (Fig. 1e), and previous studies linking MrgprC11 and PLC31. Co-transfection of TRPV1 with Mrgprc11 caused an increase in the amplitude of the BAM response (30.1% increase; p=.005 Fig. 4c, middle). However, co-expression of TRPA1 with Mrgprc11 led to an even more robust increase in intracellular Ca2+, 81% higher than with Mrgprc11 alone (p=.0001; Fig. 4c). These data suggest that both TRPV1 and TRPA1 couple to MrgprC11, consistent with our findings that both channels contribute to BAM-evoked Ca2+ responses in neurons (Figs. 2c and 3c).
MrgprA3 and MrgprC11 couple to TRPA1 via distinct mechanisms
We next examined the mechanisms by which MrgprA3 preferentially activates TRPA1, but not TRPV1. Because many TRP channels are activated or modulated by PLC-coupled receptors32, and many pruritogens and members of the Mrgpr family activate PLC signaling23, we first tested the role of PLC in CQ-evoked signaling. The PLC inhibitor, U73122, had no effect on the amplitude (Fig. 5a) or prevalence (Fig. 5b) of CQ-evoked Ca2+ signals in cultured neurons or A1/A3 NG108 cells (not shown). BAM activation of MrgprC11 has been previously demonstrated to act through PLC23. Consistent with these findings, U73122 significantly reduced both the amplitude of BAM-evoked Ca2+ signals in cultured neurons (Fig. 5b) and the prevalence of BAM-sensitive neurons (Fig. 5c). Likewise, U73122 significantly attenuated histamine signaling in neurons (Fig. 5c). These data show that while PLC signaling is required for BAM- and histamine-evoked signaling, it is not required for MrgprA3 mediated activation of TRPA1.
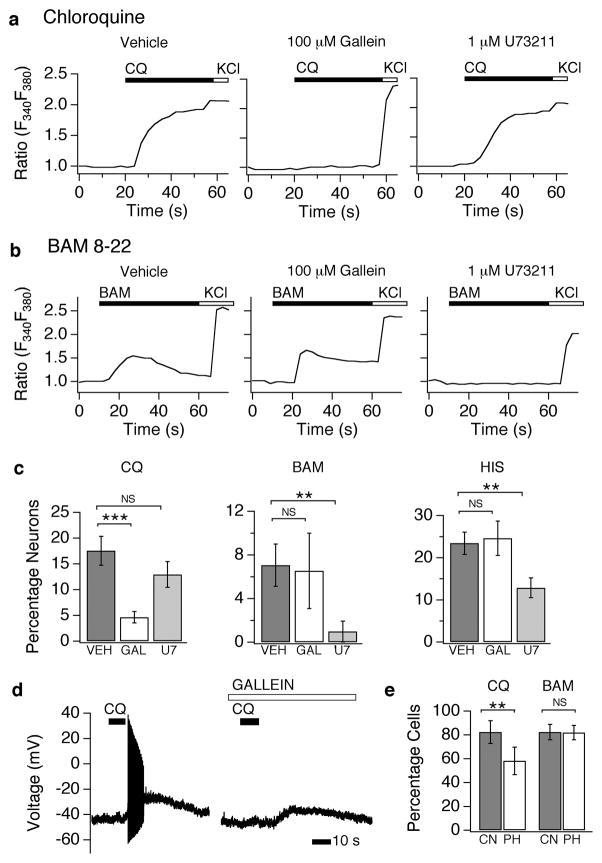
Figure 5. MrgprA3 and MrgprC11 utilize distinct signaling pathways to activate TRPA1.
(a) Chloroquine (CQ, 1mM)-evoked calcium signals (representative traces) in cultured sensory neurons following pre-treatment (5 min) with vehicle (VEH; left), the Gβγ inhibitor, gallein (middle; 100 μM), or the PLC inhibitor, U73211 (right; 1 μM) as measured by Fura-2 ratiometric calcium imaging. (b) BAM8–22 (BAM, 100 μM)-evoked calcium signals (representative traces) in cultured sensory neurons following pre-treatment (5 min) with vehicle (VEH; left), the Gβγ inhibitor, gallein (middle; 100 μM), or the PLC inhibitor, U73211 (right; 1 μM) as measured by Fura-2 ratiometric calcium imaging. (c) Quantification of the percentage of CQ-, BAM-, and HIS-sensitive neurons following treatment with vehicle (VEH; black), gallein (GAL; white), or U73122 (U7; grey). (d) Gallein inhibits chloroquine-evoked action potential firing. Representative current clamp recording shows that gallein (100 μM) blocks CQ-evoked action potential firing. All error bars represent s.e.m. (*p<0.05, **p<0.01 ***p<0.001; one way ANOVA)
GPCR signaling leads to the dissociation of both Gα and Gβγ subunits. In addition, Gβγ signaling has been shown to directly open ion channels33. We thus asked whether Gβγ signaling is required for MrgprA3-evoked activation of TRPA1. Pre-treatment of neurons with gallein, a small molecule inhibitor of Gβγ dramatically reduced both the amplitude of CQ-evoked Ca2+ signals (Fig. 5a), and the number of CQ-sensitive cells (vehicle: 17.6±1.1%; gallein: 4.6±1.1%; Fig. 5c). Gallein does not act directly on TRPA1, as mustard oil-evoked activation of TRPA1 is not altered by this inhibitor (not shown). Likewise, gallein has no effect on histamine-evoked signaling in neurons (Fig. 5c). We also probed the role of Gβγ in CQ-evoked neuronal excitation using current-clamp recording. Gallein significantly attenuated membrane depolarization and action potential firing caused by CQ application (vehicle: 17.00±13.24; 100 μM gallein: 1.33±1.53; Fig. 5d). Finally, we explored the role of Gβγ in the coupling between MrgprA3 and TRPA1 in heterologous cells. Co-expression of phosducin (Pdc), a Gβγ chelating peptide34, or treatment with gallein, significantly attenuates CQ responses in NG108 cells (Fig. 5e; control=82.34±9.61; phosducin=58.21±11.61; p=0.003; and data not shown). These experiments suggest that Gβγ signaling is required for MrgprA3 coupling to TRPA1.
Gβγ signaling has also been shown to open channels via PLC35, 36. Thus, we asked whether Gβγ signaling is also required for the PLC-dependent coupling between MrgprC11 and TRPA1. Pre-treatment of neurons with gallein had no significant effect on the amplitude of BAM-evoked Ca2+ signals (Fig. 5b), or the fraction of BAM-sensitive cells (Fig. 5c; vehicle (VEH): 7.06±1.94%; U73122 (U7): 0.98±0.95%; gallein (GAL): 6.54±3.46%). Similarly, overexpression of Pdc in TRPA1/Mrgprc11 NG108 cells fails to attenuate BAM-evoked responses (Fig. 5e; control=82.37±6.55; phosducin=81.95±6.11; p=0.887). These experiments provide evidence that PLC signaling through Gαq is required for MrgprC11 evoked neuronal activation, and may explain why MrgprC11 can couple to both TRPA1 and TRPV1, similar to the bradykinin receptor19, 29.
TRPA1 is required for CQ- and BAM-evoked itch
Given the requirement for TRPA1 in the cellular actions of CQ and BAM, we asked whether TRPA1-deficient mice also exhibit behavioral deficits in CQ- and BAM-evoked itch. We examined scratching following injection of these pruritogens into the nape of the neck. CQ and BAM evoked robust scratching behaviors in wild type mice (Fig. 6a). The time spent scratching was significantly attenuated in TRPA1-deficient littermates (Fig. 6a) to levels similar to vehicle injection (Fig. 6a). In contrast, no differences between wild type mice and TRPV1-deficient littermates were observed for either CQ or BAM injection (Fig. 6a). These results suggest that while TRPV1 partially contributes to the cellular responses to BAM in culture, the residual BAM-sensitivity in the TRPV1-deficient neurons drives BAM-evoked itch behaviors and requires functional TRPA1 channels.
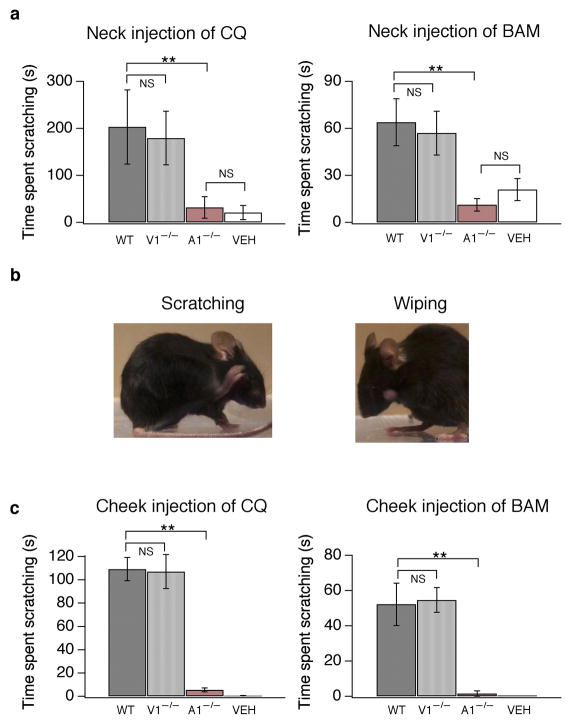
Figure 6. TRPA1-deficient mice are insensitive to chloroquine and BAM8–22.
(a) Itch-evoked scratching was measured in wild type (WT; black), TRPV1-deficient (V1−/−; grey) and TRPA1-deficient (A1−/−; red) mice following subcutaneous injection of chloroquine (CQ, 200 mg/50 μl, 8 mM) or BAM8–22 (60 μg/10 μl, 3.5 mM) into the nape of the neck. The total time spent scratching was quantified for 20 minutes after injection (p<0.01; one-way ANOVA). Injection of vehicle (PBS, 50 μL) elicited some scratching in wild type mice (VEH; white). (b) In the cheek model of itch, subcutaneous injection of a pruritogen into the cheek (chloroquine, 200 μg/10 μL, 40 mM) elicits scratching of the cheek with the hindpaw (left). In contrast, injection of an irritant, mustard oil (MO, 1 mM), evokes wiping with one of the forelimbs (right). (c) Itch-evoked scratching was measured in wild type (WT; black), Trpv1−/− (V1−/−; grey) and Trpa1−/− (A1−/−; red) mice following CQ (200 μg/10 μL, 40 mM) or BAM8–22 (60 μg/10 μl, 3.5 mM) injection in the cheek. The total time spent scratching was quantified for 20 minutes after injection. Injection of vehicle (PBS, 10μL) failed to elicit scratching or wiping (VEH; white; p<0.01; one-way ANOVA). All error bars represent s.e.m. n≥8 mice/genotype. (*p<0.05, **p<0.01 ***p<0.001)
To distinguish between CQ- and BAM-evoked itch and pain behavior, we used the “cheek” model of itch, where an irritant is injected into the cheek, rather than the neck37. Injection of CQ or BAM evokes robust scratching of the cheek with the hindlimb (Fig. 6b–c; Supplementary Movie 1). In contrast, injection of an irritant, such as mustard oil (1 mM), evokes wiping of the cheek with one of the forelimbs (Fig. 6b). Standard grooming behaviors always involve rubbing the head or face with both forelimbs (not shown). Wiping was never observed following injection of CQ or BAM. Thus we used this model to better examine the in vivo role of TRPA1 in CQ- and BAM-evoked itch. Using the cheek assay, CQ and BAM evoked prolonged periods of scratching in wild type mice. No significant differences were observed between Trpa1+/+ mice and Trpv1+/+ mice (BAM: A1-WT=49.2 s; V1-WT=50.5 s; p=0.90; one-way ANOVA; CQ: A1-WT=111 s; V1-WT=104.8 s; p=0.94; one-way ANOVA), thus data from these animals were combined (Fig. 6c). Similarly, no significant differences in BAM- or CQ-evoked scratching were observed between wild type and TRPV1-deficient mice (Fig. 6c). In contrast, this scratching behavior was never observed in TRPA1-deficient mice (Fig. 6c). TRPA1-deficient mice were not generally incapable of scratching, or insensitive to all pruritogens, as cheek injection of alpha-methyl-serotonin (2μM) evoked robust scratching (WT=48.3±10.8 s; Trpa1−/− =51.0±10.4 s; p=0.87; n=11/genotype). These experiments demonstrate that TRPA1 is required for both CQ and BAM-evoked itch.
DISCUSSION
Itch is mediated by both histamine-dependent and independent pathways. Chronic itch associated with skin and systemic diseases is insensitive to antihistamine treatment, and even allergic itch is only marginally inhibited by histamine receptor antagonists38. However, little is known about the mechanisms underlying histamine-independent itch. The GPCRs MrgprA3 and MrgprC11 are receptors for CQ and BAM8–22, respectively, two pruritogens that elicit robust antihistamine-insensitive itch16, 39. Our results clearly demonstrate that TRPA1 is activated downstream of both MrgprA3 and MrgprC11, and is the primary transduction channel mediating CQ- and BAM-evoked signaling and itch behaviors.
Most Mrgprs are orphan GPCRs and their underlying mechanisms of signal transduction are largely unknown. However, MrgprC11 has been shown to couple to the Gαq/11 pathway and activate PLC in heterologous cells23. Consistent with these findings, we show that MrgprC11-evoked excitation requires functional PLC signaling in neurons. Most TRP channels are activated or modulated by PLC, making them likely downstream targets of MrgprC11. Indeed, MrgprC11-positive BAM-sensitive neurons express both TRPA1 and TRPV1. Thus, it is not surprising that BAM activates both TRPA1 and TRPV1 in heterologous cells or that both channels contribute to BAM-evoked calcium signals in neurons. It is surprising, however, that TRPA1, but not TRPV1, is required for BAM-evoked itch behaviors. This finding is similar to bradykinin-evoked signaling whereby PLC activation robustly activates TRPA1, and weakly activates TPRV1 to promote calcium influx; because calcium also activates TRPA140, 41, calcium permeation through TRPV1 opens additional TRPA1 channels, leading ultimately to mechanical and thermal hypersensitivity. Similar to BAM, loss of TRPV1 or TRPA1 leads to diminished bradykinin-evoked calcium signaling in vitro, but only the loss of TRPA1 leads to attenuation of inflammatory behavioral responses. Thus TRPA1 plays a dominant role in both bradykinin and BAM signaling in vivo.
Unlike BAM, pharmacological inhibition of PLC does not alter CQ-evoked activation of TRPA1 in sensory neurons or transfected cell lines. These findings are consistent with a previous study showing that CQ-evoked itch is normal in mice lacking PLCβ15. In addition, CQ-evoked signaling does not require functional TRPV1 channels in neurons, and MrgprA3 fails to couple to TRPV1 in heterologous cells. What signaling pathway mediates the functional coupling of MrgprA3 to TRPA1, but not TRPV1? In somatosensation, Gβγ is required for morphine-evoked analgesia and directly activates N- and P/Q-type calcium channels in cultured dorsal root ganglia neurons42, 43. Here we show that Gβγ may be yet another signaling molecule capable of modulating the activity of a TRP channel. Gallein, a small molecule inhibitor of Gβγ and the Gβγ chelating peptide of phosducin specifically attenuate CQ-evoked signaling, with no effects on histamine or BAM signaling. Taken together, these data indicate that Gβγ is a likely candidate for mediating the specific coupling of MrgprA3 and TRPA1. Gβγ modulates several ion channels via direct binding, including members of the G protein-coupled inwardly-rectifying potassium channel and voltage-gated calcium channel families33. Future studies will elucidate whether Gβγ opens TRPA1 channels directly, or via another signaling intermediate.
Our findings support the hypothesis that TRP channels are key mediators of both pain and itch. Previous studies have shown that TRPV1 is a primary transducer of histamine-evoked itch15, 28. However, only a subset of TRPV1-positive neurons expresses histamine receptors and transduce itch. Likewise, only a subset of TRPA1-positive neurons co-express MrgprA3 and respond to CQ, and an even smaller subset of these cells also express MrgprC11 and respond to BAM. The molecularly distinct subsets of TRPA1-positive neurons that transduce BAM and CQ itch signals support the labeled line theory of itch, whereby distinct pruritogens use a dedicated pathway to transduce itch signals. In contrast, the identification of TRPA1 as a key transducer of itch and pain also supports the spatial contrast theory of itch, whereby itch is triggered by the activation of a small number of pain fibers within a receptive field, and pain is initiated when a larger cohort of cells are activated44. Like TRPA1 and TRPV1, MrgprC11 has been proposed to play a role not only in itch, but also in hyperlagesia45. In addition, several studies describe the inhibition of itch by painful chemical or mechanical stimuli1, 46, 47. Strong support of both itch theories has led to a modified “selectivity” theory of itch1, that incorporates aspects of both itch models. The recent discovery of itch specific spinal cord neurons suggests that central circuits may generate the specificity observed in itch signaling47, 48. However, the relationship between itch and pain remains a pressing question in somatosensation. Understanding the molecular mechanisms underlying both itch and pain is a first step towards understanding this complex relationship.
Our results reveal a novel role for TRPA1 in CQ-evoked itch. A major side effect of the MrgprA3 agonist and anti-malarial drug, CQ, is intolerable itch. CQ is cheap, easy to administer, and highly effective in both treating and preventing malaria. Indeed, the demand for CQ is on the rise, as recent studies have shown a decrease in CQ-resistant Plasmodium falciparum49. However, CQ-evoked itch, which is especially prevalent among dark-skinned Africans (up to 70%), is a major cause of poor compliance or treatment defaulting2. Differences in pruritic response to CQ may result from polymorphic differences in the Mrgpr signaling pathway or in TRPA1, as in Familial Episodic Pain Syndrome, recently linked to gain of function mutations in TRPA150. In such cases, improved therapeutics employing inhibition of MrgprA3 or TRPA1 aimed at alleviating chloroquine-induced itch may enable CQ to remain a useful and relevant treatment in Africa.
Aside from CQ, chronic itch results from skin diseases and systemic conditions, such as eczema, cirrhosis and some cancers, diabetes, as well as neurological disorders including multiple sclerosis, post-herpetic neuralgia and perhaps the most prevalent, allergic inflammation. Mast cell-neuronal interactions are known to play key roles in all of these pruritic conditions. Mast cells are in close association to peripheral nerves and release a variety of pruritic factors that act on sensory neurons. MrgprA4 and MrgprC11 are both activated by neuropeptide FF, a pruritogen released from mast cells during allergy-induced mast cell degranulation16, 17. These findings show that endogenous pruritogens target members of the Mrgpr family and demonstrate an essential role for MrgprC11, and therefore TRPA1, in allergic mast cell-mediated inflammation.
Perhaps most importantly, our findings demonstrate that TRPA1 is a downstream transduction channel onto which multiple histamine-independent itch pathways converge. BAM and CQ lead to TRPA1 excitation via two distinct signaling pathways. Our behavioral studies show a dramatic loss of itch-evoked behaviors in TRPA1-deficient animals in response to both of these pruritogens. As such, TRPA1 antagonists may be useful for the selective attenuation of antihistamine-insensitive itch, a problem that is especially relevant to pathological itch conditions. Whether MrgprA3, MrgprC11, and TRPA1 signaling contribute to chronic forms of itch is unknown. Mrgpr and TRPA1-deficient mice now provide a genetic model with which to assess the mechanisms of intractable itch.
EXPERIMENTAL PROCEDURES
Neuronal cell culture
For all experiments shown, trigeminal or dorsal root ganglion neurons were isolated from P0–P14 mouse pups. However, all results were also confirmed using neuronal cultures from adult mice. Preparation of neurons and ratiometric calcium imaging were carried out as previously described 29. Briefly, neurons from sensory ganglia were dissected and incubated for 10 minutes in 1.4 mg/ml Collagenase P (Roche) in Hanks Calcium-Free Balanced Salt Solution. Neurons were then incubated in 0.25% Standard Trypsin, Versene-EDTA solution (STV) for 3 minutes with gentle agitation. Cells were washed then triturated and plated in media (MEM Eagle’s with Earle’s BSS medium, supplemented with 10% horse serum, MEM vitamins, penicillin/streptomycin, and L-glutamine). Neurons were plated onto glass coverslips and used within 20 hours. All media and cell culture supplements were purchased from the UCSF Cell Culture Facility.
NG108 cell culture
NG108 cells were cultured on poly-D-lysine-coated chamberslides (Nalgene-Nunc). Cells were transfected with Lipofectamine 2000 (Invitrogen) using 150 ng human TRPA1, 150 ng human TRPV1, 500 ng human HRH1, 500 ng mouse Mrgprc11, and/or 500 ng mouse Mgrpra3 plasmids. 16 hours after transfection, cells were replated onto glass coverslides and used for calcium imaging.
Calcium Imaging
For calcium imaging experiments, cells were loaded for 1 hour with 10 μM Fura-2 AM (Invitrogen), supplemented with 0.01% Pluronic F-127 (Invitrogen), in a physiological Ringer solution containing (in mM) 140 NaCl, 5 KCl, 10 HEPES, 2 CaCl2, 2 MgCl2, 10 D-(+)-glucose, pH 7.4. All chemicals were purchased from Sigma. Acquired images were displayed as the ratio of 340 nm to 380 nm and aligned using MetaMorph software. Cells were identified as neurons by eliciting depolarization with high potassium solution (75 mM) at the end of each experiment. Neurons were deemed to be sensitive to an agonist if the average ratio during the 10 s after agonist application was ≥15% above baseline. Image analysis and statistics were performed using custom routines in Matlab and Igor Pro (WaveMetrics). Statistical significance was assessed by one-way analysis of variance (ANOVA), followed by Tukey’s HSD. All graphs displaying Fura-2 ratios have been normalized to the baseline ratio: Ratio F340/F380= (Ratio)/(Ratiot=0).
Electrophysiology
Primary mouse DRG neurons were assessed for CQ- and BAM-sensitivity using calcium imaging as described above. Cells displaying a >15% change in Fura-2 ratio following a 15 second application of CQ (1 mM) or BAM (100 μM) were chosen for whole-cell current-clamp recordings. Current clamp recordings were performed as previously described (Fujita et al, 2008). Electrode resistance ranged between 2–6MΩ. Internal solution contained (in mM): 140 mM KCl, 5 mM EGTA, 10 mM HEPES (pH 7.4 with KOH). The pipette potential was canceled before seal formation. Liquid junction potentials were <5 mV and were not corrected. Experiments were carried out only on cells with a series resistance of under 30MΩ. Resting membrane potential averaged −55±8.2 mV with a firing threshold of −44.5±7.0 mV. Data were collected at 5 kHz and filtered at 2 kHz (Axopatch 200B, PClamp software).
Mice and Behavior
Mice (20–35 g) were housed with 12 hr light-dark cycle at 21°C. For assessing chloroquine-evoked itch behaviors, mice received a subcutaneous injection into the cheek (10 μL) or neck (50 μL), with one of three solutions: 1) Ca2+ and Mg2+-free Phosphate buffered saline (PBS); 2) 10 μg BAM dissolved in PBS; or 3) 200 μg chloroquine dissolved in PBS. Mice were videotaped for 25 minutes following injection. The amount of time each mouse spent scratching, and the number of scratch bouts, were quantified over a 20-minute period. One bout of scratching was defined as an episode in which a mouse lifted its paw and scratched continuously for any length of time, until the paw was returned to the floor. Behavioral scoring was performed while blind to genotype and to the solution injected. All experiments were performed under the policies and recommendations of the International Association for the Study of Pain and approved by the University of California, Berkeley Animal Care and Use Committee.
PCR
RNA was isolated from individual sensory neurons. Cells were first examined for chloroquine or BAM8–22 sensitivity by calcium imaging, 3–4 cells in each category were aspirated into a large–diameter glass electrode filled with lysis buffer (50 mM Tris-Cl, pH 8.3, 75 mM KCl, 3 mM MgCl2, 5 U−1 RNasin (Promega) and were flash frozen. Reverse transcription was performed using murine Moloney leukemia virus and avian reverse transcriptases at 37 °C for 1 h. The product was diluted 1:10 and used as the template for PCR experiments. Primers for PCR were:
TRPA1
5–GATGCCTTCAGCACCCCATTGCTTTCCTTAATC–3
5–CTAAAAGTCCGGGTGGCTAATAGAACA–3
MrgC11
5–GCCTCTTGGGCTTTACTTGTT–3
5–GGGACCTATGCTTTCTATGCTG–3
MrgA3
5–CGACAATGACACCCACAACAA–3
5–GGAAGCCAAGGAGCCAGAAC–3
GAPDH
5–CCATGACAACTTTGGCATTG–3
5–CCTGCTTCACCACCTTCTTG–3.
Statistical analysis
Values are reported as the mean ± s.e.m. For comparison between two groups, a one–way ANOVA followed by a Turkey–Kramer post hoc test was used. To analyze a variable between two or moregroups over multiple measurements, a two–way ANOVA was used.
Supplementary Material
Acknowledgments
This study was supported by an NIH Innovator Award, the Pew Scholars Program, and the McKnight Scholars Fund (DB), the NSF (SW), and NIH grant to XD. We thank Dr. Marion Kollarik for advice on PCR, Dr. Rachel Brem for use of equipment, Takeshi Morita for technical support and Drs. Brem, Ngai, Sack, Tsunozaki, Aryal and Pellegrino for helpful discussions and critical reading of the manuscript.
Footnotes
COMPETING FIANCIAL INTERESTS
The authors declare no competing financial interests.
AUTHOR CONTRIBUTIONS
SW and KG designed and carried out cellular imaging, electrophysiology and PCR experiments; SW and ABF designed and implemented behavioral studies; QL and KNP contributed to cellular and behavioral studies. SW, KG, and DB wrote the manuscript; XD and DB provided advice and guidance throughout.
References
- 1.Ikoma A, Steinhoff M, Stander S, Yosipovitch G, Schmelz M. The neurobiology of itch. Nat Rev Neurosci. 2006;7:535–547. doi: 10.1038/nrn1950. [DOI] [PubMed] [Google Scholar]
- 2.Ajayi AA, Oluokun A, Sofowora O, Akinleye A, Ajayi AT. Epidemiology of antimalarial-induced pruritus in Africans. Eur J Clin Pharmacol. 1989;37:539–540. doi: 10.1007/BF00558141. [DOI] [PubMed] [Google Scholar]
- 3.Reddy VB, Iuga AO, Shimada SG, LaMotte RH, Lerner EA. Cowhage-evoked itch is mediated by a novel cysteine protease: a ligand of protease-activated receptors. J Neurosci. 2008;28:4331–4335. doi: 10.1523/JNEUROSCI.0716-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johanek LM, et al. A role for polymodal C-fiber afferents in nonhistaminergic itch. J Neurosci. 2008;28:7659–7669. doi: 10.1523/JNEUROSCI.1760-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Namer B, et al. Separate peripheral pathways for pruritus in man. J Neurophysiol. 2008;100:2062–2069. doi: 10.1152/jn.90482.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sardana N, Santos C, Lehman E, Craig T. A comparison of intranasal corticosteroid, leukotriene receptor antagonist, and topical antihistamine in reducing symptoms of perennial allergic rhinitis as assessed through the Rhinitis Severity Score. Allergy Asthma Proc. 2010;31:5–9. doi: 10.2500/aap.2010.31.3308. [DOI] [PubMed] [Google Scholar]
- 7.Nathan RA. Management of patients with allergic rhinitis and asthma: literature review. South Med J. 2009;102:935–941. doi: 10.1097/SMJ.0b013e3181b01c68. [DOI] [PubMed] [Google Scholar]
- 8.Steinhoff M, et al. Proteinase-activated receptor-2 mediates itch: a novel pathway for pruritus in human skin. J Neurosci. 2003;23:6176–6180. doi: 10.1523/JNEUROSCI.23-15-06176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rukwied R, Lischetzki G, McGlone F, Heyer G, Schmelz M. Mast cell mediators other than histamine induce pruritus in atopic dermatitis patients: a dermal microdialysis study. Br J Dermatol. 2000;142:1114–1120. doi: 10.1046/j.1365-2133.2000.03535.x. [DOI] [PubMed] [Google Scholar]
- 10.Tsujii K, Andoh T, Ui H, Lee JB, Kuraishi Y. Involvement of Tryptase and Proteinase-Activated Receptor-2 in Spontaneous Itch-Associated Response in Mice With Atopy-like Dermatitis. J Pharmacol Sci. 2009;109:388–395. doi: 10.1254/jphs.08332fp. [DOI] [PubMed] [Google Scholar]
- 11.Howarth PH, Salagean M, Dokic D. Allergic rhinitis: not purely a histamine-related disease. Allergy. 2000;55 (Suppl 64):7–16. doi: 10.1034/j.1398-9995.2000.00802.x. [DOI] [PubMed] [Google Scholar]
- 12.Davidson S, et al. The itch-producing agents histamine and cowhage activate separate populations of primate spinothalamic tract neurons. J Neurosci. 2007;27:10007–10014. doi: 10.1523/JNEUROSCI.2862-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schmelz M, et al. Chemical response pattern of different classes of C-nociceptors to pruritogens and algogens. J Neurophysiol. 2003;89:2441–2448. doi: 10.1152/jn.01139.2002. [DOI] [PubMed] [Google Scholar]
- 14.Sun YG, et al. Cellular basis of itch sensation. Science. 2009;325:1531–1534. doi: 10.1126/science.1174868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Imamachi N, et al. TRPV1-expressing primary afferents generate behavioral responses to pruritogens via multiple mechanisms. Proc Natl Acad Sci U S A. 2009;106:11330–11335. doi: 10.1073/pnas.0905605106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu Q, et al. Sensory neuron-specific GPCR Mrgprs are itch receptors mediating chloroquine-induced pruritus. Cell. 2009;139:1353–1365. doi: 10.1016/j.cell.2009.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee MG, et al. Agonists of the MAS-related gene (Mrgs) orphan receptors as novel mediators of mast cell-sensory nerve interactions. J Immunol. 2008;180:2251–2255. doi: 10.4049/jimmunol.180.4.2251. [DOI] [PubMed] [Google Scholar]
- 18.Jordt SE, et al. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- 19.Bandell M, et al. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 20.Parsons MEGC. Histamine and its receptors. Brit J Pharmacol. 2006;147:S127–S135. doi: 10.1038/sj.bjp.0706440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Julius D, MacDermott AB, Axel R, Jessell TM. Molecular characterization of a functional cDNA encoding the serotonin 1c receptor. Science. 1988;241:558–564. doi: 10.1126/science.3399891. [DOI] [PubMed] [Google Scholar]
- 22.Zylka MJ, Dong X, Southwell AL, Anderson DJ. Atypical expansion in mice of the sensory neuron-specific Mrg G protein-coupled receptor family. Proc Natl Acad Sci U S A. 2003;100:10043–10048. doi: 10.1073/pnas.1732949100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Han SK, et al. Orphan G protein-coupled receptors MrgA1 and MrgC11 are distinctively activated by RF-amide-related peptides through the Galpha q/11 pathway. Proc Natl Acad Sci U S A. 2002;99:14740–14745. doi: 10.1073/pnas.192565799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McNamara CR, et al. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eid SR, et al. HC-030031, a TRPA1 selective antagonist, attenuates inflammatory- and neuropathy-induced mechanical hypersensitivity. Mol Pain. 2008;4:48. doi: 10.1186/1744-8069-4-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kerstein PC, del Camino D, Moran MM, Stucky CL. Pharmacological blockade of TRPA1 inhibits mechanical firing in nociceptors. Mol Pain. 2009;5:19. doi: 10.1186/1744-8069-5-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim BM, Lee SH, Shim WS, Oh U. Histamine-induced Ca(2+) influx via the PLA(2)/lipoxygenase/TRPV1 pathway in rat sensory neurons. Neurosci Lett. 2004;361:159–162. doi: 10.1016/j.neulet.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 28.Shim WS, et al. TRPV1 mediates histamine-induced itching via the activation of phospholipase A2 and 12-lipoxygenase. J Neurosci. 2007;27:2331–2337. doi: 10.1523/JNEUROSCI.4643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bautista DM, et al. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 30.Wang S, et al. Phospholipase C and protein kinase A mediate bradykinin sensitization of TRPA1: a molecular mechanism of inflammatory pain. Brain. 2008;131:1241–1251. doi: 10.1093/brain/awn060. [DOI] [PubMed] [Google Scholar]
- 31.Han SK, Mancino V, Simon MI. Phospholipase Cbeta 3 mediates the scratching response activated by the histamine H1 receptor on C-fiber nociceptive neurons. Neuron. 2006;52:691–703. doi: 10.1016/j.neuron.2006.09.036. [DOI] [PubMed] [Google Scholar]
- 32.Montell C. In search of the holy grail for Drosophila TRP. Neuron. 2008;58:825–827. doi: 10.1016/j.neuron.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 33.Dascal N. Ion-channel regulation by G proteins. Trends in Endocrinology and Metabolism. 2001;12:391–398. doi: 10.1016/s1043-2760(01)00475-1. [DOI] [PubMed] [Google Scholar]
- 34.Rishal I, Porozov Y, Yakubovich D, Varon D, Dascal N. Gbetagamma-dependent and Gbetagamma-independent basal activity of G protein-activated K+ channels. J Biol Chem. 2005;280:16685–16694. doi: 10.1074/jbc.M412196200. [DOI] [PubMed] [Google Scholar]
- 35.Zhou Y, Sondek J, Harden TK. Activation of human phospholipase C-eta2 by Gbetagamma. Biochemistry. 2008;47:4410–4417. doi: 10.1021/bi800044n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bianchi E, Norcini M, Smrcka A, Ghelardini C. Supraspinal G beta gamma-dependent stimulation of PLC beta(3) originating from G inhibitory protein-mu opioid receptor-coupling is necessary for morphine induced acute hyperalgesia. Journal of Neurochemistry. 2009;111:171–180. doi: 10.1111/j.1471-4159.2009.06308.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimada SG, LaMotte RH. Behavioral differentiation between itch and pain in mouse. Pain. 2008;139:681–687. doi: 10.1016/j.pain.2008.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yosipovitch G, Fleisher A. Itch asscociated with skin disease: Advances in pathophysiology and emerging therapies. Am J Clin Dermatol. 2003;4:617–622. doi: 10.2165/00128071-200304090-00004. [DOI] [PubMed] [Google Scholar]
- 39.Abila B, Ezeamuzie IC, Igbigbi PS, Ambakederemo AW, Asomugha L. Effects of two antihistamines on chloroquine and histamine induced weal and flare in healthy African volunteers. Afr J Med Med Sci. 1994;23:139–142. [PubMed] [Google Scholar]
- 40.Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]
- 41.Wang YY, Chang RB, Waters HN, McKemy DD, Liman ER. The nociceptor ion channel TRPA1 is potentiated and inactivated by permeating calcium ions. J Biol Chem. 2008;283:32691–32703. doi: 10.1074/jbc.M803568200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mathews JL, Smrcka AV, Bidlack JM. A Novel G beta gamma-Subunit Inhibitor Selectively Modulates mu-Opioid-Dependent Antinociception and Attenuates Acute Morphine-Induced Antinociceptive Tolerance and Dependence. Journal of Neuroscience. 2008;28:12183–12189. doi: 10.1523/JNEUROSCI.2326-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rousset M, Cens T, Gouin-Charnet A, Scamps F, Charnet P. Ca2+ and phosphatidylinositol 4,5-bisphosphate stabilize a G beta gamma-sensitive state of Ca(V)2 Ca2+ channels. Journal of Biological Chemistry. 2004;279:14619–14630. doi: 10.1074/jbc.M313284200. [DOI] [PubMed] [Google Scholar]
- 44.Ma Q. Labeled lines meet and talk: population coding of somatic sensations. J Clin Invest. 120:3773–3778. doi: 10.1172/JCI43426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guan Y, et al. Mas-related G-protein-coupled receptors inhibit pathological pain in mice. Proc Natl Acad Sci U S A. 2010;107:15933–15938. doi: 10.1073/pnas.1011221107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Davidson S, Zhang X, Khasabov SG, Simone DA, Giesler GJ., Jr Relief of itch by scratching: state-dependent inhibition of primate spinothalamic tract neurons. Nat Neurosci. 2009;12:544–546. doi: 10.1038/nn.2292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ross SE, et al. Loss of inhibitory interneurons in the dorsal spinal cord and elevated itch in Bhlhb5 mutant mice. Neuron. 2010;65:886–898. doi: 10.1016/j.neuron.2010.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sun YG, Chen ZF. A gastrin-releasing peptide receptor mediates the itch sensation in the spinal cord. Nature. 2007;448:700–703. doi: 10.1038/nature06029. [DOI] [PubMed] [Google Scholar]
- 49.Andriantsoanirina V, Menard D, Tuseo L, Durand R. History and current status of Plasmodium falciparum antimalarial drug resistance in Madagascar. Scand J Infect Dis. 2010;42:22–32. doi: 10.3109/00365540903289670. [DOI] [PubMed] [Google Scholar]
- 50.Kremeyer B, et al. A Gain-of-Function Mutation in TRPA1 Causes Familial Episodic Pain Syndrome. Neuron. 2010;66:671–680. doi: 10.1016/j.neuron.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.