Abstract
Aging is associated with bone loss and degenerative joint diseases, in which the aging of bone marrow-derived mesenchymal stem cell (bmMSC) may play an important role. In this study, we analyzed the gene expression profiles of bmMSC from 14 donors between 36 and 74 years old, and obtained age-associated genes (in the background of osteoarthritis) and osteoarthritis-associated genes (in the background of old age). Pathway analysis of these genes suggests that alterations in glycobiology might play an important role in the aging of human bmMSC. On the other hand, antigen presentation and signaling of immune cells were the top pathways enriched by osteoarthritis-associated genes, suggesting that alteration in immunology of bmMSC might be involved in the pathogenesis of osteoarthritis. Most intriguingly, we found significant age-associated differential expression of HEXA, HEXB, CTSK, SULF1, ADAMTS5, SPP1, COL8A2, GPNMB, TNFAIP6, and RPL29; those genes have been implicated in the bone loss and the pathology of osteoporosis and osteoarthritis in aging. Collectively, our results suggest a pathological role of bmMSC in aging-related skeletal diseases, and suggest the possibility that alteration in the immunology of bmMSC might also play an important role in the etiology of adult-onset osteoarthritis.
Keywords: human bone marrow mesenchymal stem cell, aging, osteoporosis, osteoarthritis
INTRODUCTION
Adult skeleton constantly undergoes bone remodeling to replace old/damaged bones by new bones, which is required for the maintenance of bone shape and strength of adult skeleton. However, bone mass decreases and bone fragility increases with age in both men and women. Although an increase in bone resorption rate associated with menopause is the primary cause of low bone mass in postmenopausal women, a decline in bone formation rate may also contribute to the loss of bone mass in both postmenopausal and age-related osteoporosis.
In bone marrow, mesenchymal stem cell (bmMSC) is a small population of multipotent cells that are capable of self-renewal proliferation and differentiating into several cell lineages such as osteoblast, chondrocyte, and adipocyte [1]. Thus, bmMSC play a critical role in bone formation that occurs in the skeletal development and growth, in the maintenance of fully grown skeleton, and in fracture repair [2]. It is reasonable to assume that the osteogenic potential of bmMSC may decrease with age. Indeed, animal studies have shown that bmMSCs from aged rats are less responsive to growth factors than cells from adult rats, and that bmMSCs from old bone are defective in bone induction potential [3-6]. However, the effect of aging on human bmMSC is not clear, and the pathological role of bmMSC in the aging-related bone defects is still under debate. An understanding of the changes in gene expression of bmMSC with age may provide clues and give insights into the basic cause of bone defects during aging.
In this study, using Illumina bead chip expression microarray, we analyzed the gene expression profiles of bmMSC derived from 14 donors between 36 and 74 years old, including many patients with osteoarthritis (OA). We identified genes whose change in expression was highly associated with age or OA. Putative biological functions and molecular pathways corresponding to those identified genes were retrieved by bioinformatics analysis, through which a possible pathological role of bmMSC in the development of skeletal diseases in aging was proposed.
Patients and Methods
Isolation of bmMSC from bone marrow
Human bone marrows were either collected from osteoarthritis patients undergoing total knee replacement, or from the femurs of healthy donors receiving bone surgery because of trauma. Informed consent was obtained from each donor. The use of human bmMSCs in this study was approved by both the Institutional Review Board at National Health Research Institutes and Miaoli General Hospital. Bone marrows were subjected to ficoll density fractionation to collect the plastic-adherent mononuclear cells as described previously [7]. These cells were maintained in low glucose Dulbecco's modified Eagle's medium (GIBCO-BRL, California, USA) containing 20% fetal bovine serum (FBS) (Hyclone, Utah, USA), glutamine (GIBCO-BRL, California, USA), penicillin and streptomycin (GIBCO-BRL, California, USA), and maintained in a humidified atmosphere containing 5% CO2 at 37°C. After 10-14 days, colonies were dispersed and seeded at a density of 1.3 x 103 cells/cm2 (passage 1), and were passaged at 70%-80% confluence afterward. Cells of the fourth passage were maintained in medium containing 10% FBS for 4-7 days and subjected to flow cytometric analysis. Cultures that were CD31- and CD45-negative, but CD90- and CD105-positive were defined as bmMSCs and used for RNA extraction [7].
RNA extraction, microarray experiments, and RT-qPCR validation
Total RNA was isolated from cells using Trizol (Invitrogen, Califonia, USA) according to the protocol provided by the manufacturer. Methods for sample labeling, array experiments, and TaqMan probe-based RT-qPCR experiments, were as described elsewhere [8]. The probes and primers used in RT-qPCR are as listed in Supplemental Table SIV.
Statistical analysis
(1) Data normalization. To avoid possible unwanted technical variation between samples or batches of array, raw data from microarray experiments were subject to either quantile normalization [9] using preprocessCore package in the Bioconductor, or global normalization that linearly adjust signal intensity according to signal of those spike-in control probes in the Illumina arrays (see supplemental methods). Normalized data were subjected to the following statistical analysis.
(2) Neighborhood analysis. This analysis, developed by Golub et al. [10], was used to detect if there were genes, in our array data, showing strong correlation in their expression with age or OA. This analysis was performed as described in supplemental methods.
(3) Multiple regression/ criteria of probes selection. Multivariate linear regression was used to model the expression level of a gene and its relation with age, gender, or the presence of OA of each subject. This model is described as follows:
For probe, j = 1,…,J, let Yij be expression data of ith individual. For i = 1,…,N, let Ai, Gi and Di be the age, gender and OA respectively. In particular, Gi = 1 if and only if the ith individual is male; Di if and only if the ith individual has OA. Assume the gene expression
where αA, αD, αG and μj are the model parameters and εij is the error. Finally, a filter was applied to remove probes/genes having (i) average expression level < 300 arbitrary units or (ii) p>0.05 in multiple regression analysis, which then generated age-or OA-associated gene list. Since two normalization methods were applied, qualified probes/genes from either method that meet the above criteria were included in the candidate gene lists.
Measurement of DNA synthesis
DNA synthesis was assessed by measuring the incorporation of 5-bromo-2-deoxyuridine (BrdU) into DNA using BrdU Cell Proliferation Assay kit based on the protocol provided by manufacturer (Millipore, MA, USA). Briefly, cells were treated with BrdU or phosphate buffer saline (PBS, as background control) for 24 h. Cells were then fixed and DNAs were denatured using Fixing Solution. The BrdU label was detected by an anti-BrdU monoclonal antibody, and the signals were quantitated with a spectrophotometer microplate reader set at wavelength of 450/550 nm.
Bioinformatic analysis
We perform gene ontology and pathway analysis using Ingenuity Pathway Analysis 9.0 for enrichment of biological functions and pathways in which our selected genes of interest were involved.
RESULTS
Age-dependent differences in the growth rate of primary cultures of bone marrow-derived plastic-adherent cells
We analyzed the gene expression profiles of bmMSC derived from 14 human donors. Donors 1, 5, and 7 showed no signs of bone diseases and were designated as healthy donors, whereas the other 11 donors were osteoarthritis (OA) patients (Table I).
Table I. Demography of bone marrow donors.
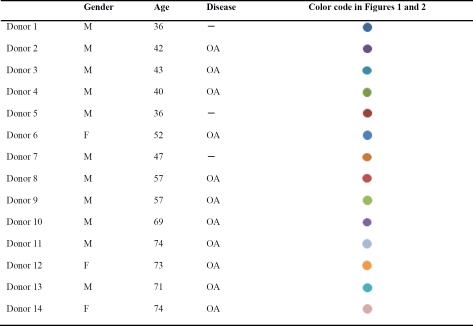 |
— means there is no sign of skeletal diseases
While expanding stem cell populations from each bone marrow sample, we counted the plastic-adherent cells after three and six days post-seeding at passage one, calculated the fold increase in cell number for each line of bone marrow-derived cells, and correlated the data with the age of their donors. As shown in the left panel of Figure 1A, after three days post-seeding, the fold increase in cell numbers was negatively correlated with the age of donors, although this was not significant (r=−0.50, p=0.069). Nevertheless, a rather significant correlation (r=−0.85, p=0.001) was found after six days post-seeding (right panel, Figure 1A). Subsequently, these cells were characterized as bmMSC by flow cytometric analyses (Figure 1B).
Figure 1. Growth rate of human bone marrow-derived plastic-adherent cells.
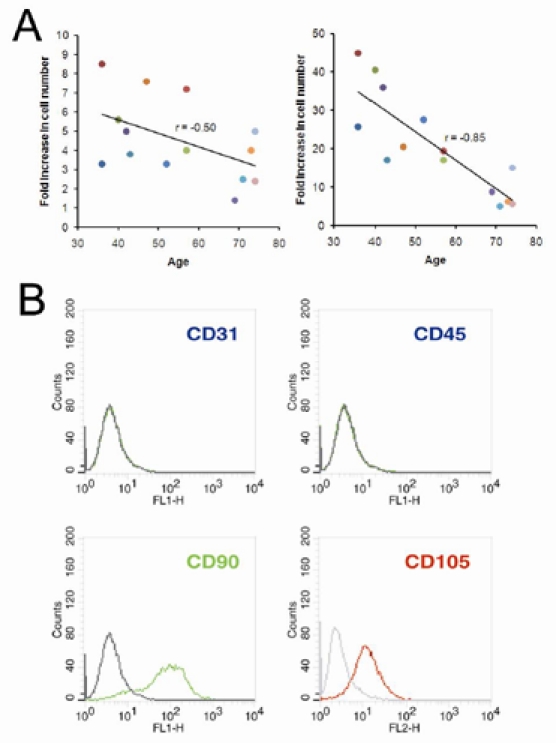
(A) Plastic-adherent cells harvested separately from bone marrows of 14 donors were seeded at a density of 1.3 × 103 cells/cm2 (passage 1). Cells were counted after 3 days (left panel) and 6 days (right panel) post-seeding using a hemocytometer, and the fold increase in cell number was calculated. Donors are color-coded as shown in Table I. (B) Cells harvested 6 days post-seeding were subjected to flow cytometric analyses. A representative result is shown.
Quality assessment of genome-wide microarray data and generation of age- and OA-associated genes
We noted that under in vitro culture condition, the gene expression profile of bmMSC might change with serial passaging. Indeed, it has been shown that in vitro aging has a similar effect as in vivo aging on human stem cells in terms of gene expression profiling [11]. Therefore, in this study, all the bmMSC cultures used for the genome-wide cDNA microarray analysis were harvested at the same passage (passage 4). It should be noted that clonogenic bmMSC is a heterogeneous mix of cells containing the multipotent stem cells and their progenitors. For convenience, these stem and progenitor cells were both named as bmMSC in this study.
The microarray analysis was performed using Illumina gene expression chips. Considering that the numbers of female donor as well as OA-free donor were low in this study, we thought that it would be more proper for us to focus on the age-related genes. However, because OA is an aging-associated disease, we also examined the gene expression profile associated with OA. First, we performed neighborhood analysis applying t-statistics (see Methods) to know if there exist genes that are associated with age or the presence of OA. The results showed that the expression level of many genes did have strong correlation with either age or the presence of OA (supplemental information, Figure S1-A and -B). Subsequently, we analyzed the microarray data with a multivariate linear regression model considering age, gender, and disease status (with or without OA) as covariates. Through such analysis, out of 48804 probes, the expression of 574 probes that contain 497 genes was found significantly correlated with age (p<0.05), as represented by 20 selected genes including HEXA, HEXB, CTSK, SULF1, ADAMTS5, SPP1, COL8A2, GPNMB, RPL29, TNFAIP6, CDKN2B, etc. (Figure 2, see Discussion). These genes were named age-associated genes (supplemental Table SI). On the other hand, there were 112 probes containing 92 genes correlated with OA, which were named OA-associated genes (supplemental Table SII). There was a moderate overlap of only 38 probes (29 genes) between age- and OA-associated genes (supplemental Table SIII).
Figure 2. Representative plots of donor age versus normalized mRNA expression level for selective age-associated genes.
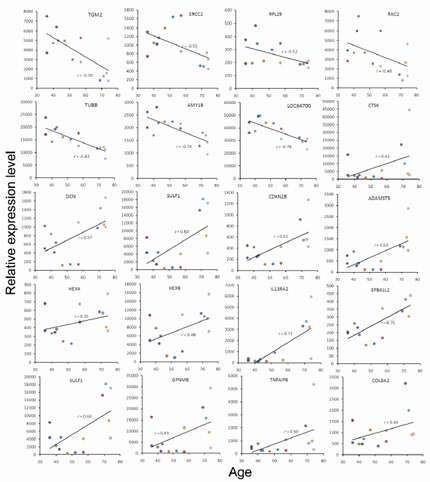
Each solid dot represented a bone marrow donor. The regression lines (solid line) and correlation coefficients (r) showed trend of change in gene expression with increasing donor age. Donors are color-coded as shown in Table I.
We performed real-time quantitative PCR (RT-qPCR) analyses on 18 genes of interest selected from the abovementioned age- or OA-associated genes using cDNA prepared from selected adult and aged donors, and compared the results with array data. We calculated the correlation coefficients (r) between array and RT-qPCR data, and found that 11 of them had r >0.9 and 16 of them had r >0.8 (Table II). These results indicated that, in general, the results of RT-qPCR measurements were highly comparable with the array data.
Table II. Analysis of correlation between microarray data and RT-qPCR measurements.
| Gene | r | p | Gene | r | p |
|---|---|---|---|---|---|
| S100A4 | 0.98 | 0.0006 | TGM2 | 0.954 | 0.003094 |
| GPNMB | 0.998 | 4.78E-06 | STC1 | 0.951 | 0.003594 |
| CDH6 | 0.948 | 0.004 | NEFM | 0.371 | 0.469 |
| RRAGD | 0.888 | 0.017977 | TPI1 | 0.869 | 0.024519 |
| CD55 | 0.904 | 0.013265 | ADAM19 | 0.935 | 0.006163 |
| CDKN2B | 0.971 | 0.00126 | RAC2 | 0.859 | 0.028484 |
| NBL1 | 0.928 | 0.0008 | AVPI1 | 0.528 | 0.282 |
| SULF1 | 0.859 | 0.028523 | KRT19 | 0.948 | 0.004054 |
| PPFIBP2 | 0.888 | 0.018161 | PDE1A | 0.966 | 0.001742 |
Gene Ontology/pathways analysis of age- or OA associated gene expression changes
To know what cellular functions or molecular pathways in which the age- and OA-associated genes were involved, we categorized these genes using the Ingenuity Pathway Analysis (IPA). Table III summarized the enriched top functions and canonical pathways. As shown, among the top ranked Gene Ontology function terms enriched by age-associated genes were cell growth and proliferation (p = 2.7×10−5~1.9×10−2), cellular movement (p = 2.94×10−5~2.07×10−2), cell cycle (p = 6.2×10−5~2.2×10−2), and cell morphology (p = 8.2×10−5~1.7×10−2). These results suggested that these cellular functions are most likely to change with age in human bmMSC. In spite of these results, there was no significant enrichment of specific cellular location (data not shown), indicating that the aging-related cellular activities might take place in various cellular compartments, rather than specific locations. Most intriguingly, our results showed that pathways for degradation of N-glycans (p = 8.9×10−8, Figure 3), degradation of glycosaminoglycans (GAGs, p = 5.7×10−4), and biosynthesis of glycosphingolipids (p = 5.7×10−3) were the top canonical pathways enriched by age-associated genes. The ratio of enrichment of genes in a specific pathway was 9/31, 6/71, and 4/45, respectively.
Table III. Top GO function terms and canonical pathways enriched by IPA.
| Top functions | p value | Top pathways | p value | |
|---|---|---|---|---|
| Age-associated | Genetic Disorder | 2.91E-06-2.01E-02 | N-Glycan Degradation | 8.91251E-08 |
| Metabolic Disease | 1.1E-05-1.64E-02 | Glycosaminoglycan Degradation | 0.00057544 | |
| Cellular Growth and Proliferation | 2.66E-05-1.9E-02 | Glycosphingolipid Biosynthesis - Globoseries | 0.005754399 | |
| Cellular Movement | 2.94E-05-2.07E-02 | Hepatic Fibrosis / Hepatic Stellate Cell Activation | 0.00724436 | |
| Cell Cycle | 6.62E-05-2.15E-02 | PTEN Signaling | 0.011481536 | |
| Cell Morphology | 8.24E-05-1.68E-02 | Glycosphingolipid Biosynthesis - Ganglioseries | 0.012302688 | |
| Cellular Development | 8.24E-05-1.96E-02 | Agrin Interactions at Neuromuscular Junction | 0.013803843 | |
| Skeletal and Muscular System Development and Function | 1.18E-04-1.96E-02 | Aminophosphonate Metabolism | 0.013803843 | |
| OA-associated | Nucleic Acid Metabolism | 5.26E-04-4E-02 | Antigen Presentation Pathway | 1.28825E-06 |
| Small Molecule Biochemistry | 5.26E-04-4E-02 | Crosstalk between Dendritic Cells and Natural Killer Cells | 8.70964E-05 | |
| Cardiovascular System Development and Function | 8.96E-04-3.51E-02 | Allograft Rejection Signaling | 0.000933254 | |
| Cell Morphology | 8.96E-04-4.48E-02 | Cytotoxic T Lymphocyte-mediated Apoptosis of Target Cells | 0.001348963 | |
| Cell-To-Cell Signaling and Interaction | 8.96E-04-4.97E-02 | OX40 Signaling Pathway | 0.001862087 | |
| Cellular Development | 8.96E-04-4.97E-02 | Cdc42 Signaling | 0.003388442 | |
| Nervous System Development and Function | 8.96E-04-4.97E-02 | Communication between Innate and Adaptive Immune Cells | 0.006025596 | |
| Cellular Assembly and Organization | 2.23E-03-4.97E-02 | Dendritic Cell Maturation | 0.006309573 |
Figure 3. N-glycan degradation pathway enriched by pathway analysis.
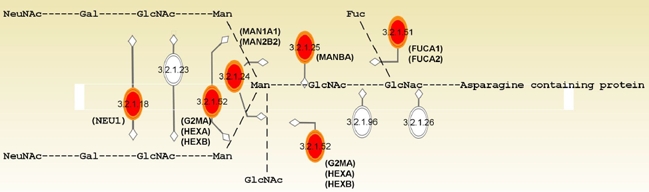
Enzymes (in EC number) with their names of coding genes and the corresponding sites of actions in the modification of N-glycan are indicated. Genes (n=9) that are differentially expressed with age and enriched by IPA analysis are marked in red.
In addition, we found that nucleic acid metabolism (p = 5.3×10−4~4.0×10−2), small molecule biochemistry (p = 5.3×10−4~4.0×10−2), cell morphology (p = 9.0×10−4~4.5×10−2), and cell-to-cell signaling and interaction (p = 9.0×10−4~5.0×10−2) were the top ranked Gene Ontology function terms, while antigen presentation pathway (p = 1.3×10−6), cross talk between dendritic cells and natural killer cells (p = 8.7×10−5), and allograft rejection signaling (p = 9.3×10−4) were the top canonical pathways enriched by the OA-associated genes (Table III). The ratio of enrichment for each corresponding pathway was 5/43, 5/98, and 3/97, respectively. These results indicated a significant association between OA and bmMSC with altered immunological functions. Moreover, analyses showed that cell-mediated immune response, immunological disease, cell growth and proliferation, and cell cycle were among the top networks enriched by OA-associated genes (data not shown). Therefore, like increasing age, OA might also associate with alteration in the proliferative capacity of bmMSC. On the other hand, we found none of Gene Ontology function terms and pathways was enriched significantly by those 29 overlapping genes.
Analysis of the effect of sodium sulfate on the DNA synthesis in bmMSC from adult and aged donors
As shown in supplemental Table SI, the expression of several sulfatase-encoding genes such as SULF1, ARSB, IDS in bmMSC were up-regulated with donor age. Sulfatases can desulfate the sulfated proteoglycans at the cell surface, affecting membrane metabolism, signal transduction, proliferation, etc, whereas, sodium sulfate (Na2SO4) increases sulfation of proteoglycans [12]. Since elevated levels of various types of sulfatases were found in aged donors and aged bmMSCs grew slower in culture, we tested whether attenuation in desulfation would be able to increase DNA synthesis differentially in bmMSCs from aged versus adult donors. Therefore, we treated adult (donors 1, 3) and aged (donors 10, 12, 13) bmMSCs with Na2SO4 of various doses and measured DNA synthetic activity by BrdU incorporation assays (Figure 4). For bmMSCs from donor 1, 12 mM Na2SO4 induced approximately 20% (p<0.01) increase in DNA synthesis, whereas 16 mM Na2SO4 did not further increase DNA synthesis. For bmMSCs from donor 3, Na2SO4 seemed not to induce DNA synthesis. In the aged group, for bmMSCs from donor 10, 12 mM Na2SO4 induced approximately 70% (p=0.013) increase in DNA synthesis. For bmMSCs from donor 12, 16 mM Na2SO4 induced approximately 60% (p=0.01) increase in DNA synthesis, whereas the increase induced by lower Na2SO4 concentration did not reach to statistical significance. For bmMSCs from donor 13, 8, 12, and 16 mM Na2SO4 induced approximately 30% (p=0.041), 40% (p=0.0014), and 50% (p=0.0013) increase in DNA synthesis, respectively. Moreover, the induction in DNA synthesis in bmMSCs from 2 out of 3 aged donors was significantly stronger than that in cells from 2 adult donors at higher Na2SO4 concentration (12 and 16 mM), which coincided with our findings that several sulfatase-encoding genes in bmMSC were up-regulated with donor age. Taken together, these results indicated that aged bmMSCs were more responsive to Na2SO4 treatment than adult bmMSCs in terms of induction in DNA synthesis, which supported the notion that elevated expression of sulfatases might cause a deleterious effect to the proliferative activity of bmMSCs from aged donors.
Figure 4. DNA synthesis measured by BrdU incorporation assays.
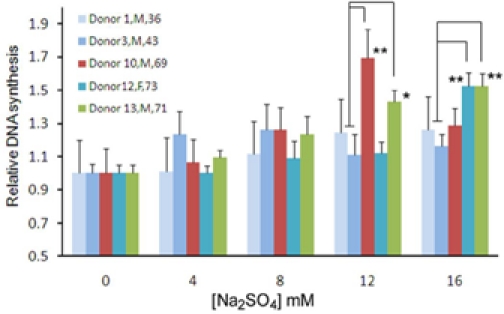
BmMSC from donors 1, 3, 10, 12, and 13 were seeded into 96-well culture plate (1.2 × 102 cells/well). Cells were either left untreated or treated with 4, 8, 12, and 16 mM Na2SO4 (Merck, Darmstadt, Germany) for 48 h. Then, either BrdU or PBS was added in medium, and cells were incubated for 24 h. Subsequently, cells were subjected to detection of the BrdU signals. The fold increase in DNA synthesis was calculated by comparing the BrdU signals of Na2SO4 treated cells to that of untreated cells (to which a value of 1 was assigned). The gender and age of donors are as indicated in the figure. Data represent the mean ± S.D. from three duplicate analyses. **, p<0.05; *, p=0.051 (comparison of individual aged sample to the average of adult samples, t-test).
DISCUSSION
Aged bones are featured by decreased bone mass and increased fragility compared with young bones. Inadequate bone formation following excessive bone resorption is a major cause of age-related bone loss. Besides, aged skeleton is also commonly accompanied by inflammatory disease such as OA, as represented by the bone marrow donors in this study. Given that bmMSCs give rise to bone-forming cells, it is likely that the aging of bmMSC play an important role in the aging of skeleton, and may be even involved in the development of aging-associated skeletal diseases such as osteoporosis (OP) and OA. Unfortunately, molecular evidence that can support the above conjecture and link aging of bmMSC to OP/OA is still lacking. It is thus of great interest to understand the age-associated gene expression change to know the role of bmMSC in the pathogenesis of aging-related skeletal diseases. In this regard, we examined the transcriptome-wide changes of genes of bmMSCs derived from 14 donors of various age, and analyzed the array data by exploiting a multivariate linear regression model considering all known variables, i.e., age, presence of OA, and gender. This analytic platform allowed us to correct effects of the OA- and/or gender-related change of gene expression, to obtain a list of age-dependent genes (in the background of OA), and also a list of OA-associated genes (in the background of old age). As far as the age-associated genes are concerned, our data are in agreement with the results reported by Wagner et al. who demonstrated related effects of aging and replicative senescence on the gene expression profiles of human bmMSC/progenitors [11]. However, there is an overlap of only 8 genes between our and their data. These include the age-associated up-regulation of EPB41L3 and TCEAL7 involved in regulating cell proliferation; IL13RA2 involved in the signaling of transforming growth factor β1-mediated fibrosis; MFAP5 encoding a microfibrillar associated protein; ROBO1 involved in axon guidance; S100A4 encoding a calcium binding protein; STEAP3 involved in iron metabolism; and UBE2E2 involved in protein degradation. The little overlap might be due in part to the differences in the populations studied, cell culture conditions, array data processing, or analytical platform used. In addition to the above mentioned genes, we report novel findings about the age- and OA-associated changes in the gene expression profiles of bmMSC.
Our data point out that cell growth, proliferation, and migration are the cellular functions that most possibly change with age in human bmMSC. Among the genes involved in these cellular functions are the cell cycle regulators-encoding CCND2, CCNE1, and CDKN2B. The former two genes encode D- and E-type cyclins, whereas CDKN2B encodes a cyclin-dependent kinase (CDK) inhibitor p15INK4b which arrests cell cycle by inhibiting the D-type cyclin-dependent kinase CDK4 activity. We observed that CCND2 and CDKN2B are up-regulated, but CCNE1 is down-regulated with age. Given the findings that CDK inhibitors play an important role in regulating the renewal proliferation of mice hematopoietic stem cells [13-15], and that there is a strong link between p16INK4a and cellular aging [13-16], our results suggest that regulation of CDKN2B expression may play an important role in the renewal proliferation as well as aging of human bmMSC. Besides the cell cycle regulators, we found the up-regulation of DCN, PODN, TP53INP1, and DRAM1, and down-regulation of ERCC2 and TGM2 with age. DCN encodes a proteoglycan which down-regulates the proliferation and migration of mammalian cells [17]. PODN encodes an extracellular matrix (ECM) component which inhibits cell growth and migration [18]. ERCC2 encodes a nucleotide excision repair enzyme critical for removal of damaged DNA fragments, while TP53INP1 and DRAM1 participate in the DNA damage-triggered growth arrest and apoptosis [19, 20]. As for TGM2, it was found to enhance cell growth and survival through anti-apoptosis signaling [21]. Interestingly, TGFBR3, RPS6KA2, PTGER4, FBXO32, SULF1, DBC1, TCEAL7, and EPB41L3 are up-regulated with age (supplemental Table SI). These genes have been reported to negatively control cancer cell proliferation [22-29]. Thus, up-regulation of these ‘tumor suppressor genes’ is likely to decrease the proliferation rate of human bmMSC. Data described above might underlie the aging-associated decrease in the proliferation rate of bmMSCs, an aging phenotype of mammalian bmMSCs [3, 30, 31]. Since our data are in agreement with the current findings regarding the deleterious effect of aging to the proliferation of stem cells, it is conceivable that our results can also reveal the other important age-associated functional changes in human bmMSC. Among them, as revealed by our analyses, are those involved in glycobiology.
Glycosylation is a cellular process that links glycans to macromolecules such as proteins and lipids by different types of glycosidic bonds. N-linked glycans (N-glycans), for example, are the polysaccharides that link to the peptides or proteins by N-glycosidic bond. Mature glycoproteins and glycolipids not only form the architecture of cell membrane but also participate in cellular signaling. Our results show that several genes involved in the modification of glycan are up-regulated with age (Table IV and Figure 3). Since modification of glycan is a pivotal process in the synthesis and catabolism of glycoproteins and glycolipids, up-regulation of these genes with age suggests that aging of human bmMSC may be accompanied by alterations in membrane homeostasis and in the glycosylation of membrane components, which may result in the alteration in cellular signaling. For example, hexosaminidase has been implicated in local hydrolysis of glycosphingolipids at cell membranes [32]. Given that glycosphingolipid is the major component of lipid rafts which play an important role in a variety of cellular processes including signal transduction and cell proliferation, elevated expression of HEXA and HEXB might enhance the degradation of glycosphingolipids at aged bmMSC surface, impact the formation of lipid rafts, and affect signaling. For another example, sulfatase 1 is able to desulfate the sulfated proteoglycans at the cell membrane, inhibits their co-receptor functions in the signaling of several growth factors. Accordingly, elevated expression of SULF1 in aged bmMSC is likely to impair cellular response to growth factors. In fact, we show that Na2SO4 is able to induce DNA synthesis in bmMSCs from adult and aged donors, and the induction is stronger in bmMSCs from aged donors than in bmMSCs from adult donors (Figure 4). Based on these findings, we postulate that alterations in the cellular functions regulating membrane homeostasis and glycosylation of membrane components are very likely to alter the proliferative capacity of bmMSC, and play an important role in the aging of bmMSC.
Table IV. Genes involved in glycan modification.
| Gene symbol | Product | Function |
|---|---|---|
| GLT8D2 | Glycosyltransferase 8 domain containing 2 | glycosyltransferase |
| FUCA1 | Tissue alpha-L-fucosidase 1 | fucosidases |
| FUCA2 | Tissue alpha-L-fucosidase 2 | fucosidases |
| MAN1A1 | Mannosidase, alpha, class 1A, member 1 | mannosidases |
| MAN2B2 | Mannosidase, alpha, class 2A, member 2 | mannosidases |
| MANBA | Lysosomal mannosidase, beta A | mannosidases |
| NEU1 | Lysosomal sialidase 1 | Sialidase |
| HEXA | Hexosaminidase A (α-polypeptide) | hexosaminidase (glycosylhydrolase) |
| HEXB | Hexosaminidase B (β-polypeptide) | hexosaminidase (glycosylhydrolase) |
| GM2A | GM2 ganglioside activator | cofactor of hexosaminidase |
| ARSB | Arylsulfatase B | sulfatases |
| IDS | Iduronate 2-sulfatase | sulfatases |
| SULF1 | Sulfatase 1 | sulfatases |
In addition, our results have provided clues to address the involvement of bmMSC in aging-associated skeletal diseases. OA is an inflammatory disease featured by the degeneration of cartilage matrix, which is due in part to excessive degradation of the matrix components aggrecan, collagen II and GAG [33-35]. It has been reported that hexosaminidase and sulfatase 1 which are involved in the degradation of GAG are the dominant enzymes in the synovial fluid and cartilage of OA patients [36, 37]. Inhibition of hexosaminidase activity has been proposed for preventing or even reversing cartilage degradation in OA patients [34]. ADAMTS5, an aggrecanase, was also found highly expressed in human OA cartilages [38]. Deletion of active ADAMTS5 has been shown to prevent cartilage degradation in a murine OA model [39]. As to the degradation of collagen II, cathepsin K was found involved in the cleavage of collagen II in articular cartilages in certain OA patients, suggesting that it might play a role in OA pathology [33]. Our data show that genes encoding these enzymes in bmMSCs are all up-regulated with age. In addition, COL8A2 and GPNMB, two OA candidate genes [40], are also up-regulated with age in bmMSCs. Given that bmMSCs are the primary source of cartilage chondrocytes, our data suggest a pathological role of aged bmMSC in aging-associated OA.
Moreover, the age-associated genes also cover genes participating in regulating bone resorption and formation. Data show that RPL29 is down-regulated with age in human bmMSC. RPL29 encodes a ribosomal protein. Mice lacking this gene display a short stature phenotype and exhibit increased bone fragility, which is due to delayed entry of osteoprogenitors into cell cycle and altered matrix protein synthesis rates [40]. In addition, we show that TNFAIP6 is up-regulated with age. This gene has been found down-regulated during osteoblastic differentiation; overexpression of this gene inhibits osteoblastic differentiation of human MSCs [41]. Thus, down-regulation of RPL29 and up-regulation of TNFAIP6 with age may represent a mechanism underlying the aging-associated defects in bone formation and osteoblastic differentiation of human bmMSC. As mentioned above, cathepsin K may play a role in OA pathology, and is up-regulated with age. In fact, there are evidences showing that capthesin K is also implicated in the pathogenesis of OP: (i) cathepsin K has been considered as a target for the pharmacological treatment of OP, and (ii) overexpression of CTSK has been shown to cause spontaneous development of synovial hyperplasia and fibrosis, cartilage degeneration, and bone destruction in transgenic mice upon aging [42]. Therefore, it is conceivable that osteoprogenitors/osteoblasts with elevated expression of CTSK may jeopardize their osteogenic activity. With these in mind, it is not surprising to find that SPP1, an OP susceptibility gene [43], is up-regulated with age in bmMSC (supplemental Table SI). Thus, our findings provide compelling molecular evidences to suggest a role of aged bmMSC in the pathogenesis of OP. Meanwhile, it has to be mentioned that there is a moderate overlap between age- and OA-associated gene lists though, the age-associated genes discussed above are only present in the former gene list. Taken together, it is tempting to postulate that by associating with the forming of pathological gene expression profile described above, increase of age may act as an intrinsic promoting factor to the development of aging-associated skeletal diseases.
Our analyses of the OA-associated genes have shown interesting findings regarding the etiology of OA. Based on current theory, OA is the consequence of long term mechanical stress on the articular cartilage. In response, the cartilage chondrocytes produce inflammatory cytokines and matrix metalloproteinases, which eventually causes destruction of articular cartilage. But recently, a genome-wide association study (GWAS) identified two single nucleotide polymophisms (SNPs) which are in a region containing HLA class genes including HLA-DRB4, associated with susceptibility to knee OA [44]. This finding suggests that immunological mechanism may be implicated in the etiology of OA. Here, we show that antigen presentation and signaling of immune cells are the top pathways enriched by OA-associated genes, and that CD74 and a list of HLA class genes including HLA-DRB4 are down-regulated with OA (Table III). Thus, coinciding with that GWAS result, our results also suggest an immunological issue associated with OA. Accordingly, we propose that bmMSC with altered immunological property might play an important role in the etiology of OA. On the other hand, we found that DAXX which encodes a pro-apoptotic factor in primary cells [45] is up-regulated with OA. Oppositely, GAS6, SKI, and RAD51 are down-regulated with OA (supplemental Table SII). Gas6 can promote cell proliferation, survival, and migration [46]. Ski can bind to the histone deacetylase SIRT1 and inactivate p53 [47]. Rad51 is the major recombinase involved in the repair of DNA double strand breaks. So, our results suggest that the presence of OA might associate with deficient DNA repair, and decreased proliferation and survival of bmMSC.
In summary, we have reported novel findings regarding to the age- and OA-associated changes in the gene expression profiles of human bmMSC. We show that increase of age and the presence of OA may independently associate with changes in gene expression profile that may hinder the proliferation and survival of bmMSC. In particular, our results suggest a pathological role of aged bmMSC in the development of OP and/or aging-associated OA, and also suggest a role of bmMSC with altered immunological property in the etiology of ‘adult-onset’ OA.
SUPPLEMENTAL MATERIAL
The Supplemental Information is found in Full Text version of this manuscript.
Acknowledgments
This work was supported by National Science Council, Taiwan (NSC-99-3112-B-400-011, NSC 99-3112-B-400-012, and NSC-100-3112-B-400-002) and Department of Health, Taiwan (DOH100-TD-C-111-004), and NHRI (CS-098-PP08 and CS-099-PP07).
Abbreviations
- bmMSC
bone marrow-derived mesenchymal stem cell
- OA
osteoarthritis
- OP
osteoporosis
REFERENCES
- Friedenstein AJ. Precursor cells of mechanocytes. Int Rev Cytol. 1976;47:327–359. doi: 10.1016/s0074-7696(08)60092-3. [DOI] [PubMed] [Google Scholar]
- Yoo JU, Johnstone B. The role of osteochondral progenitor cells in fracture repair. Clin Orthop Relat Res. 1998:S73–81. doi: 10.1097/00003086-199810001-00009. [DOI] [PubMed] [Google Scholar]
- Quarto R, Thomas D, Liang CT. Bone progenitor cell deficits and the age-associated decline in bone repair capacity. Calcif Tissue Int. 1995;56:123–129. doi: 10.1007/BF00296343. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Liang CT. Effect of platelet-derived growth factor on DNA synthesis and gene expression in bone marrow stromal cells derived from adult and old rats. J Cell Physiol. 1995;164:367–375. doi: 10.1002/jcp.1041640217. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Liang CT. Mitogenic activity but not phenotype expression of rat osteoprogenitor cells in response to IGF-I is impaired in aged rats. Mech Ageing Dev. 1996;92:1–10. doi: 10.1016/s0047-6374(96)01793-9. [DOI] [PubMed] [Google Scholar]
- Tanaka H, Ogasa H, Barnes J, Liang CT. Actions of bFGF on mitogenic activity and lineage expression in rat osteoprogenitor cells: effect of age. Mol Cell Endocrinol. 1999;150:1–10. doi: 10.1016/s0303-7207(99)00046-5. [DOI] [PubMed] [Google Scholar]
- Lin JL, Wang MJ, Lee D, Liang CC, Lin S. Hypoxia-inducible factor-1alpha regulates matrix metalloproteinase-1 activity in human bone marrow-derived mesenchymal stem cells. FEBS Lett. 2008;582:2615–2619. doi: 10.1016/j.febslet.2008.06.033. [DOI] [PubMed] [Google Scholar]
- Jiang SS, Fang WT, Hou YH, Huang SF, Yen BL, Chang JL, Li SM, Liu HP, Liu YL, Huang CT, Li YW, Jang TH, Chan SH, et al. Upregulation of SOX9 in lung adenocarcinoma and its involvement in the regulation of cell growth and tumorigenicity. Clin Cancer Res. 2010;16:4363–4373. doi: 10.1158/1078-0432.CCR-10-0138. [DOI] [PubMed] [Google Scholar]
- Bolstad BM, Irizarry RA, Astrand M, Speed TP. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics. 2003;19:185–193. doi: 10.1093/bioinformatics/19.2.185. [DOI] [PubMed] [Google Scholar]
- Golub TR, Slonim DK, Tamayo P, Huard C, Gaasenbeek M, Mesirov JP, Coller H, Loh ML, Downing JR, Caligiuri MA, Bloomfield CD, Lander ES. Molecular classification of cancer: class discovery and class prediction by gene expression monitoring. Science. 1999;286:531–537. doi: 10.1126/science.286.5439.531. [DOI] [PubMed] [Google Scholar]
- Wagner W, Bork S, Horn P, Krunic D, Walenda T, Diehlmann A, Benes V, Blake J, Huber FX, Eckstein V, Boukamp P, Ho AD. Aging and replicative senescence have related effects on human stem and progenitor cells. PLoS One. 2009;4:e5846. doi: 10.1371/journal.pone.0005846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill R, Hitchins L, Fletcher F, Dhoot GK. Sulf1A and HGF regulate satellite-cell growth. J Cell Sci. 2010;123:1873–1883. doi: 10.1242/jcs.061242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, Dombkowski DM, Cheng T, DePinho RA, Sharpless NE, Scadden DT. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- Walkley CR, Fero ML, Chien WM, Purton LE, McArthur GA. Negative cell-cycle regulators cooperatively control self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2005;7:172–178. doi: 10.1038/ncb1214. [DOI] [PubMed] [Google Scholar]
- Yuan Y, Shen H, Franklin DS, Scadden DT, Cheng T. In vivo self-renewing divisions of haematopoietic stem cells are increased in the absence of the early G1-phase inhibitor, p18INK4C. Nat Cell Biol. 2004;6:436–442. doi: 10.1038/ncb1126. [DOI] [PubMed] [Google Scholar]
- Kim WY, Sharpless NE. The regulation of INK4/ARF in cancer and aging. Cell. 2006;127:265–275. doi: 10.1016/j.cell.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Ferdous Z, Peterson SB, Tseng H, Anderson DK, Iozzo RV, Grande-Allen KJ. A role for decorin in controlling proliferation, adhesion, and migration of murine embryonic fibroblasts. J Biomed Mater Res A. 2010;93:419–428. doi: 10.1002/jbm.a.32545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piao C, Jin M, Kim HB, Lee SM, Amatya PN, Hyun JW, Chang IY, You HJ. Ribonucleotide reductase small subunit p53R2 suppresses MEK-ERK activity by binding to ERK kinase 2. Oncogene. 2009;28:2173–2184. doi: 10.1038/onc.2009.84. [DOI] [PubMed] [Google Scholar]
- Tomasini R, Seux M, Nowak J, Bontemps C, Carrier A, Dagorn JC, Pebusque MJ, Iovanna JL, Dusetti NJ. TP53INP1 is a novel p73 target gene that induces cell cycle arrest and cell death by modulating p73 transcriptional activity. Oncogene. 2005;24:8093–8104. doi: 10.1038/sj.onc.1208951. [DOI] [PubMed] [Google Scholar]
- Zhang XD, Qin ZH, Wang J. The role of p53 in cell metabolism. Acta Pharmacol Sin. 2010;31:1208–1212. doi: 10.1038/aps.2010.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyoshi N, Ishii H, Mimori K, Tanaka F, Hitora T, Tei M, Sekimoto M, Doki Y, Mori M. TGM2 is a novel marker for prognosis and therapeutic target in colorectal cancer. Ann Surg Oncol. 2010;17:967–972. doi: 10.1245/s10434-009-0865-y. [DOI] [PubMed] [Google Scholar]
- Bignone PA, Lee KY, Liu Y, Emilion G, Finch J, Soosay AE, Charnock FM, Beck S, Dunham I, Mungall AJ, Ganesan TS. RPS6KA2, a putative tumour suppressor gene at 6q27 in sporadic epithelial ovarian cancer. Oncogene. 2007;26:683–700. doi: 10.1038/sj.onc.1209827. [DOI] [PubMed] [Google Scholar]
- Chien J, Staub J, Avula R, Zhang H, Liu W, Hartmann LC, Kaufmann SH, Smith DI, Shridhar V. Epigenetic silencing of TCEAL7 (Bex4) in ovarian cancer. Oncogene. 2005;24:5089–5100. doi: 10.1038/sj.onc.1208700. [DOI] [PubMed] [Google Scholar]
- Chou JL, Su HY, Chen LY, Liao YP, Hartman-Frey C, Lai YH, Yang HW, Deatherage DE, Kuo CT, Huang YW, Yan PS, Hsiao SH, Tai CK, et al. Promoter hypermethylation of FBXO32, a novel TGF-beta/SMAD4 target gene and tumor suppressor, is associated with poor prognosis in human ovarian cancer. Lab Invest. 2010;90:414–425. doi: 10.1038/labinvest.2009.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dafou D, Grun B, Sinclair J, Lawrenson K, Benjamin EC, Hogdall E, Kruger-Kjaer S, Christensen L, Sowter HM, Al-Attar A, Edmondson R, Darby S, Berchuck A, et al. Microcell-mediated chromosome transfer identifies EPB41L3 as a functional suppressor of epithelial ovarian cancers. Neoplasia. 2010;12:579–589. doi: 10.1593/neo.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatza CE, Oh SY, Blobe GC. Roles for the type III TGF-beta receptor in human cancer. Cell Signal. 2010;22:1163–1174. doi: 10.1016/j.cellsig.2010.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JE, Chen J, Lou Z. DBC1 is a negative regulator of SIRT1. Nature. 2008;451:583–586. doi: 10.1038/nature06500. [DOI] [PubMed] [Google Scholar]
- Lai JP, Sandhu DS, Shire AM, Roberts LR. The tumor suppressor function of human sulfatase 1 (SULF1) in carcinogenesis. J Gastrointest Cancer. 2008;39:149–158. doi: 10.1007/s12029-009-9058-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murn J, Alibert O, Wu N, Tendil S, Gidrol X. Prostaglandin E2 regulates B cell proliferation through a candidate tumor suppressor, Ptger4. J Exp Med. 2008;205:3091–3103. doi: 10.1084/jem.20081163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes SC, Tibbe JM, Veenhof M, Bakker K, Both S, Platenburg PP, Oner FC, de Bruijn JD, van Blitterswijk CA. Bone tissue-engineered implants using human bone marrow stromal cells: effect of culture conditions and donor age. Tissue Eng. 2002;8:911–920. doi: 10.1089/107632702320934010. [DOI] [PubMed] [Google Scholar]
- Zhou S, Greenberger JS, Epperly MW, Goff JP, Adler C, Leboff MS, Glowacki J. Age-related intrinsic changes in human bone-marrow-derived mesenchymal stem cells and their differentiation to osteoblasts. Aging Cell. 2008;7:335–343. doi: 10.1111/j.1474-9726.2008.00377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prinetti A, Loberto N, Chigorno V, Sonnino S. Glycosphingolipid behaviour in complex membranes. Biochim Biophys Acta. 2009;1788:184–193. doi: 10.1016/j.bbamem.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Dejica VM, Mort JS, Laverty S, Percival MD, Antoniou J, Zukor DJ, Poole AR. Cleavage of type II collagen by cathepsin K in human osteoarthritic cartilage. Am J Pathol. 2008;173:161–169. doi: 10.2353/ajpath.2008.070494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Shikhman AR, Lotz MK, Wong CH. Hexosaminidase inhibitors as new drug candidates for the therapy of osteoarthritis. Chem Biol. 2001;8:701–711. doi: 10.1016/s1074-5521(01)00045-x. [DOI] [PubMed] [Google Scholar]
- Stewart MC, Fosang AJ, Bai Y, Osborn B, Plaas A, Sandy JD. ADAMTS5-mediated aggrecanolysis in murine epiphyseal chondrocyte cultures. Osteoarthritis Cartilage. 2006;14:392–402. doi: 10.1016/j.joca.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Otsuki S, Taniguchi N, Grogan SP, D'Lima D, Kinoshita M, Lotz M. Expression of novel extracellular sulfatases Sulf-1 and Sulf-2 in normal and osteoarthritic articular cartilage. Arthritis Res Ther. 2008;10:R61. doi: 10.1186/ar2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shikhman AR, Brinson DC, Lotz M. Profile of glycosaminoglycan-degrading glycosidases and glycoside sulfatases secreted by human articular chondrocytes in homeostasis and inflammation. Arthritis Rheum. 2000;43:1307–1314. doi: 10.1002/1529-0131(200006)43:6<1307::AID-ANR13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Plaas A, Osborn B, Yoshihara Y, Bai Y, Bloom T, Nelson F, Mikecz K, Sandy JD. Aggrecanolysis in human osteoarthritis: confocal localization and biochemical characterization of ADAMTS5-hyaluronan complexes in articular cartilages. Osteoarthritis Cartilage. 2007;15:719–734. doi: 10.1016/j.joca.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Glasson SS, Askew R, Sheppard B, Carito BA, Blanchet T, Ma HL, Flannery CR, Kanki K, Wang E, Peluso D, Yang Z, Majumdar MK, Morris EA. Characterization of and osteoarthritis susceptibility in ADAMTS-4-knockout mice. Arthritis Rheum. 2004;50:2547–2558. doi: 10.1002/art.20558. [DOI] [PubMed] [Google Scholar]
- Oristian DS, Sloofman LG, Zhou X, Wang L, Farach-Carson MC, Kirn-Safran CB. Ribosomal protein L29/HIP deficiency delays osteogenesis and increases fragility of adult bone in mice. J Orthop Res. 2009;27:28–35. doi: 10.1002/jor.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsukahara S, Ikeda R, Goto S, Yoshida K, Mitsumori R, Sakamoto Y, Tajima A, Yokoyama T, Toh S, Furukawa K, Inoue I. Tumour necrosis factor alpha-stimulated gene-6 inhibits osteoblastic differentiation of human mesenchymal stem cells induced by osteogenic differentiation medium and BMP-2. Biochem J. 2006;398:595–603. doi: 10.1042/BJ20060027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morko J, Kiviranta R, Joronen K, Saamanen AM, Vuorio E, Salminen-Mankonen H. Spontaneous development of synovitis and cartilage degeneration in transgenic mice overexpressing cathepsin K. Arthritis Rheum. 2005;52:3713–3717. doi: 10.1002/art.21423. [DOI] [PubMed] [Google Scholar]
- Li WF, Hou SX, Yu B, Li MM, Ferec C, Chen JM. Genetics of osteoporosis: accelerating pace in gene identification and validation. Hum Genet. 2010;127:249–285. doi: 10.1007/s00439-009-0773-z. [DOI] [PubMed] [Google Scholar]
- Nakajima M, Takahashi A, Kou I, Rodriguez-Fontenla C, Gomez-Reino JJ, Furuichi T, Dai J, Sudo A, Uchida A, Fukui N, Kubo M, Kamatani N, Tsunoda T, et al. New sequence variants in HLA class II/III region associated with susceptibility to knee osteoarthritis identified by genome-wide association study. PLoS One. 2010;5:e9723. doi: 10.1371/journal.pone.0009723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomoni P, Khelifi AF. Daxx: death or survival protein? Trends Cell Biol. 2006;16:97–104. doi: 10.1016/j.tcb.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Hafizi S, Dahlback B. Gas6 and protein S. Vitamin K-dependent ligands for the Axl receptor tyrosine kinase subfamily. FEBS J. 2006;273:5231–5244. doi: 10.1111/j.1742-4658.2006.05529.x. [DOI] [PubMed] [Google Scholar]
- Inoue Y, Imamura T. Regulation of TGF-beta family signaling by E3 ubiquitin ligases. Cancer Sci. 2008;99:2107–2112. doi: 10.1111/j.1349-7006.2008.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Supplemental Information is found in Full Text version of this manuscript.


