Abstract
Objective
To evaluate the effect of an 8-week water-based exercise program (experimental group) over an upper extremity function program (control group) to increase cardiovascular fitness within a community setting for individuals with stroke.
Design
Single-blind randomized controlled trial
Setting
Public community centre
Participants
12 community-dwelling individuals who have had a stroke with mild to moderate motor deficits; volunteer sample
Intervention
Experimental and control groups participated in group exercise programs undertaken in one hour sessions, three times per week for 8 weeks. The experimental group undertook chest deep water exercises at targeted heart rates. The control group performed arm and hand exercises while sitting.
Main Outcome Measures
The primary outcome measure was cardiovascular fitness (VO2max). Secondary measures were maximal workload, muscle strength, gait speed, and the Berg Balance Score.
Results
The experimental group attained significant improvements over the control group in cardiovascular fitness, maximal workload, gait speed, and paretic lower extremity muscle strength. The relatively short program (8 weeks) of water-based exercise resulted in a large improvement (22%) in cardiovascular fitness in a small group of individuals with stroke with relatively high function.
Conclusions
A water-based exercise program can be undertaken in the community as a group program and may be an effective means to promote fitness in individuals with stroke.
Keywords: endurance, stroke, randomized trial, exercise, cardiovascular fitness, clinical trial
Introduction
Increasing exercise capacity following a stroke can help prevent deterioration to the cardiovascular system. Such effects are important given that cardiovascular disease is the leading prospective cause of death in individuals with stroke1,2. Furthermore, increasing exercise capacity in persons with stroke may improve the ability to perform functions of daily living, which might be limited by weakness and fatigue secondary to the stroke. Recommendations for physical activity levels outlined by the American College of Sports Medicine (ACSM)indicate that adults should engage in moderate levels of physical activity for a total of 30 minutes on most days to maintain health3. To see improvements in cardiovascular fitness, moderate to high intensity exercise needs to be performed at least 3 times per week for 30 minutes per day4. Given these recommendations, it is important to develop a variety of exercise protocols that can accommodate the diverse levels of function that are found in individuals with stroke.
Cardiovascular fitness has been improved (as measured by increases in VO2max, workload, exercise time, or gait efficiency) in individuals with stroke using treadmill training5,6, modified cycle ergometer training7, and circuit training8. A water-based exercise program (WBE) is potentially another viable exercise medium given that water is 700 times the density of air9 allowing for increased energy expenditure per work done10, reduction in joint impact loading and partial weight support provided by the buoyancy of water, and ability to undertake the exercise in local recreation centers without expensive equipment.
Eight to twelve weeks of WBE have been shown to improve cardiovascular fitness and strength in healthy older adults11,12 and individuals with rheumatoid arthritis13. No studies have previously examined WBE in individuals with stroke and it is not known whether impairments might limit the ability to undertake intensive exercise in the water. It was the purpose of this study to determine the effectiveness of an 8-week WBE in improving cardiovascular fitness and functional mobility in individuals with chronic stroke.
Methods
Study Participants
We recruited individuals on a volunteer basis who were community-dwelling and had residual unilateral weakness secondary to stroke. The study was approved by the local university and hospital research ethics committees and participants provided their informed consent. The participants and the inclusion/exclusion criteria have been described previously14 and are briefly described here. The initial screening criteria was queried with a telephone interview: 1) have a history of only one cerebrovascular accident of at least one year post stroke, 2) independent in walking (with or without assistive device), 3) medically stable (i.e., exclusion criteria were uncontrolled hypertension, arrhythmia, or unstable cardiovascular status, 4) no previous myocardial infarction, and 5) no significant musculoskeletal problems from conditions other than stroke. Participants then attained written permission to participate in the study from a primary care physician who was informed about the screening criteria and the study protocol. Participants were then assessed in the lab to meet the next screening criteria which was to pedal a cycle ergometer and reach 60% of their age-predicted heart rate maximum (220 beats/min minus their age).
Research Design
Individuals with chronic stroke were randomized to one of two eight-week group programs: a) water-based exercise program focused on leg exercises to improve cardiovascular fitness (experimental group) and b) arm exercise group (control group). Participants were only aware that they were involved in either a leg or arm training programs (partial subject blinding since they did not know that cardiovascular fitness was the main outcome measure). For group assignment, stratified randomization was undertaken. First, subjects were identified for two levels of two strata (stratum1=VO2max: less than group median/greater than or equal to the group median, stratum2=age: 50–59 years/60+ years). Then, subjects were randomly assigned such that there were equal numbers of subjects for each level of stratum in the experimental and control groups15,16. One researcher undertook all randomization procedures while assessments were undertaken by testers who had no knowledge of the participants’ grouping (researcher blinding).
Descriptive Measures
Descriptive measures of the subjects were taken: age, duration of injury, type of stroke, severity of lower extremity physical impairment, and the American Heart Association Stroke Functional Classification17. Chedoke-McMaster Stroke Assessment was measured to determine the severity of lower limb physical impairment and has been shown to be valid and reliable in individuals with stroke18. The lower extremity score of the Chedoke-McMaster Assessment (maximum 14) was evaluated from a structured physical assessment of the subject and consists of a leg score (1=no active movement and maximum 7=can complete normal age appropriate complex movements like rapid stepping) and foot score (1=no active movement and maximum 7=can complete normal age appropriate complex movements like rise on toes) 18.
Outcome Measures
The primary outcome measure was VO2max which is the gold standard criterion of cardiovascular fitness19 and was measured during a symptom-limited graded cycle ergometer test. Secondary measures were 1) maximal workload (watts) assessed during the symptom-limited ergometer test, 2) self-selected gait speed (m/s) over 8 m, 3) 14-item Berg Balance Scale20,21 and 4) muscle strength (N/kg). All assessments were performed over 3 days, with at least 2 days rest between each test session. We have shown that measurements of gait speed and muscle strength have excellent reliability in individuals with stroke and their protocols have been described in detail previously22,23. Gait speed was measured with subjects using their usual assistive device (see Table 1). Self-paced gait speed was calculated from the mean of three walking trials. The cumulative distance and time of consecutive strides (i.e., foot contact to the next foot contact of the same leg) were recorded by infrared emitting diodesa attached to the foot during the middle section (i.e., approximately a 4 m section representative of constant gait speed) of the 8-meter walkway. The Berg Balance Test consists of 14 tasks which challenge balance while sitting, standing or stepping (minimum score = 0 and maximum score = 56 with higher scores indicating better balance performance) and has been shown to be a valid and reliable measure20,21.
Table 1.
Subject Characteristics (N= 12)
| Experimental Group | Control Group | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | Mean or # | Std | Range | Mean or # | Std | Range |
| Gender (M/F) | 6/1 | 5/0 | ||||
| Type of Stroke (ischemic/hemorrhage) | 3/4 | 5/0 | ||||
| Paretic Side (L/R) | 3/4 | 2/3 | ||||
| AHASFC* II/III | 5/2 | 3/2 | ||||
| Mobility Aids (walker/cane/brace/none) |
1/0/2/4 | 0/3/2/2 | ||||
| Chedoke Score (/14)† | 10.0 | 2.5 | 7–12 | 8.6 | 2.6 | 6–12 |
| Age (years) | 61.9 | 9.4 | 51–70 | 63.4 | 8.4 | 51–72 |
| Time Since Stroke (years) | 3.0 | 2.0 | 1–7 | 4.2 | 2.1 | 1–6 |
| Mass (kg) | 83.5 | 15.1 | 64.7–113.8 | 85.3 | 13.3 | 71.7–106.6 |
| Height (cm) | 175.9 | 7.7 | 164.2–185.7 | 171.6 | 5.8 | 164.8–178.8 |
| Body Mass Index (kg/m2) | 26.9 | 3.6 | 22.7–33.0 | 28.9 | 3.1 | 24.6–33.3 |
abbreviation for American Heart Association Stroke Functional Classification
Maximum Chedoke-McMaster Lower Extremity Score is 14
None of the subject characteristics were significantly different between groups (p>0.2)
For the measurement of VO2max, all subjects were required to refrain from alcohol and strenuous exercise 24 hours and caffeine and food intake 4 hours prior to the test. For the maximal cycle test, subjects began pedaling on a stationary bikeb between 50–70 rpm at 0 watts with workload increments of 20 watts/min and oxygen consumption (VO2) was measured using a metabolic cartc and cardiovascular stability measured using a 12-lead ECGd.. All subjects were familiarized with Borg’s 16-point Rating of Perceived Exertion Scale (RPE)24 to assess how hard they felt they were working. Blood pressure was monitored before and immediately after the test session. Determination of whether maximal effort was achieved during this test followed the criteria outlined by the ACSM4: 1) respiratory exchange ratio ≥ 1.15, 2) no increase in HR following increases in workload, 3) a plateau of VO2 defined by < 1.5ml/kg/min increase in VO2 with workload increases, or 4) volitional fatigue. Our VO2max found test-retest intraclass correlations over 0.90 for these same subjects which was reported previously14.
Isokinetic flexor and extensor muscle strength of the paretic and non-paretic knee and hip joints were measured on a KinCom strength dynamometere at 60°/sec as these muscle groups are thought be largely activated during water running9 and improvements have been reported following water-based training programs in adults12. The exact strength testing protocol is reported in Eng et al.22 Muscle torques normalized to body mass were summed over each body side to generate a composite muscle strength score for the paretic and non-paretic side. Composite lower extremity strength scores have been reported to be sensitive to muscle strengthening and physical conditioning programs in persons with stroke.25,26
Experimental group: Water-based endurance program
The main objective of this program was to improve cardiovascular fitness in individuals with stroke following eight weeks (three days/week, one hr/session) of intensive water-based exercises in chest level water at a local community centre swimming pool (water temperature between 26–28°C). Participants wore a heart rate monitorf and either a flotation beltg or lifejacketh. The exercise intervention consisted of: 10 minutes of land-based stretching, 5 minutes of light aerobic warm-up in the water (marching on the spot, single and double legged hopping holding onto the pool edge), 30 minutes of moderate to high aerobic activities (shallow water walking, running, side stepping) at the target heart rate prescribed for that week, 5 minutes of a light cool down (marching on the spot), and 10 minutes of gentle stretching in the water. Target heart rates were set at 50–70%, 75%, and 80% heart rate reserve ± 5 beats/min (as determined by the initial maximal exercise test) for weeks 1–2, 3–5, and 6–8, respectively. Subjects were instructed to stop or reduce their exercise workload if the following occurred: 1) lightheadedness or dizziness, 2) chest discomfort/pain, or 3) RPE value >15. All exercise sessions were supervised by one physical therapist and two exercise physiologists.
Control group: Arm function program
Participants in the control group attended the same amount of time as the experimental group (eight weeks, three days/week, one hr/session). The main objective of this program was to improve upper extremity function. Each session commenced with a five minute warm-up of active upper extremity movements. A six station circuit then focused on gross upper limb movement (e.g., reaching), fine motor movement (e.g., adjusting small screws/bolts) and muscle strengthening of the shoulder, elbow, wrist, and fingers (e.g., exercises using hand putty, theraband and weights). Participants focused primarily on the paretic upper extremity unless there was remaining time within each of the 7-minute stations. Participants were seated at each station, but had to stand and walk a few steps to the next station. The program ended with a 5 minute cool-down of active upper extremity exercises. All exercise sessions were supervised by 1 occupational therapist and exercise physiologist.
Statistical Analysis
Independent T-tests compared baseline measures between the experimental and control group. A two-way ANOVA compared differences between groups over time (pre-test/post-test). The alpha value was 0.05.
Results
Twenty-seven individuals with stroke met the initial telephone screen criteria. Fourteen subjects were then excluded for a remaining 13 participants. The reasons for exclusion were: written permission from the family physician was not acquired because the subject’s physician found their patient to be medically unstable (n=2), unable to position leg on the cycle ergometer due to leg adductor spasticity, muscle weakness or reduced joint range (n=5), inability to generate sufficient leg force during cycling to elicit a HR of at least 60% age-predicted heart rate maximum (n=5), dysphagia which caused frequent coughing and disruption to the subject’s performance (n=1), and unable to complete all exercise tests due to time commitments (n=1). The remaining 13 subjects then performed the maximal cycle test of which none were excluded as all reached maximal effort as defined by the ACSM guidelines4 and satisfied a minimum of three of the four criteria: 1) respiratory exchange ratio ≥ 1.15, 2) failure of HR to increase with further increases in exercise intensity, 3) a plateau in VO2 or < 1.5 ml/kg/min increase in VO2 following workload increases, or 4) volitional fatigue. Subjects reached a mean 95% of their age-predicted HR maximum during the symptom-limited graded exercise test and all but one subject’s actual HR maximum was within 10% of their age-predicted HR maximum. These thirteen subjects were then randomized into an experimental (n=7) and control group (n=6). One subject withdrew from the control group after week 2 as she found the program too fatiguing. Other then this one subject, no other adverse effects occurred during the testing or training sessions. Subject characteristics for the remaining 12 participants are presented in Table 1. Subjects were currently taking prescribed medications to control for hypertension (peripheral vasodilators, n=1; diuretics, n=1; ACE inhibitors, n=8; and depression, n=5).
Subjects attended 157 out of a possible 168 classes for the experiment group and 111 out of a possible 120 classes for the control group. There were no significant group differences for the descriptive characteristics or baseline outcome measures (Table 2). A significant time × group interaction showed greater improvements of VO2max, self selected gait speed, and composite paretic muscle strength for the experimental group compared to the control group following the 8 week intervention. The observed power when the alternative hypothesis was set based on the observed values was greater than 0.80 for the primary outcome measure, VO2max. A 22% improvement in VO2max and 19% in gait speed was observed in the experimental group following 8 weeks of water-based exercise, while these measures did not change for the control group (Table 2). The time × group interaction for the Berg Balance Score approached significance (0.094) with a greater improvement for the control group following the intervention.
Table 2.
Outcome measures: Baseline and post-test (N= 12)
| Variable | Exercise Group | Control Group | ||
|---|---|---|---|---|
| Baseline. | Post-test | Baseline | Post-test. | |
| VO2max (ml/kg/min)* | 17.3 (3.0) | 21.2(2.3) | 17.1(3.2) | 17.6(4.7) |
| Maximal Workload (watts)* | 137.7(37.9) | 150.3(44.8) | 127.6(29.9) | 111.2(39.0) |
| Berg Balance Score (max=56) | 51.6(4.7) | 52.0(3.3) | 49.8(3.9) | 52.2(3.6) |
| Self selected gait speed (m/s)* | 0.99(0.33) | 1.18(0.44) | 1.01(0.29) | 1.04(0.40) |
| Strength (composite) | ||||
| More affected side (Nm/kg)* | 2.75(0.86) | 2.97(0.91) | 2.12(0.41) | 1.96(0.59) |
| Less affected side (Nm/kg) | 3.79(0.72) | 3.82(0.65) | 3.29(0.44) | 3.24(0.37) |
Means (standard deviations)
None of the baseline measures were significantly different between groups (p>0.40)
significant time × group interaction, p<0.05
Discussion
Despite the relatively mild impairments of these subjects, their baseline cardiovascular fitness is considered poor for their gender and age27 but comparable to studies reporting cardiovascular fitness in individuals with chronic stroke5,7,8,28. Although exercise capacity was low in this sample, exercise tolerance was excellent, as all subjects in the water-based program were able to exercise at 70–80% of their baseline age-predicted HR maximum without discomfort. Adherence was also very good for both programs with over a 92% attendance rate. Surprisingly, the only subject to withdraw from the program was from the control group and she felt that the exercises were too fatiguing. Three instructors were required for the first week of the water-based program as one subject required one-on-one supervision due to his fear in the water, in addition to his low functional status. However, even one instructor would have been sufficient for the seven water-based program participants following that first week
The findings from this study demonstrated that a relatively short program (8 weeks) of water-based exercise improved cardiovascular fitness (VO2max) by 22% in chronic stroke survivors. These findings have important clinical implications for stroke rehabilitation as it has been well documented that improvements in VO2max are related to improvements in risk factors associated with cardiovascular disease29. The improvement in cardiovascular fitness found in our study is much greater than the 8 to 14% improvements reported previously for individuals with stroke following cycle-ergometer, treadmill and circuit-based protocols5,7,8. The combination of a higher prescribed exercise intensity and greater resistance (compared to land-based programs) likely contributed to the enhanced cardiovascular improvements found in this study. In addition, the lower the initial level of cardiovascular fitness, the greater the potential gain in VO2max31.
Our outcome measures did not allow us to partition factors which contributed to the improvements in VO2max resulting from peripheral muscle mechanisms (e.g., muscle oxidative capacity) or central cardiovascular mechanisms (e.g., cardiac output). We found only a modest improvement (9%) in maximal workload compared to others who have reported between 28 to 44% increase in maximal workload5,7,8. These studies used the same task to assess and train cardiovascular fitness and thus, task-specific motor learning might account for these large improvements in maximal workload. Our subjects demonstrated more modest improvements in maximal workload which can be attributed to the fact that we used different tasks to assess (cycle ergometer) and train (water-based exercise) cardiovascular fitness. Thus, our subjects did not experience the cycling practice which might have improved their ability to pedal more efficiently in light of the well known impairments in motor coordination in stroke32, the task-specificity of the muscular responses33 and the neural changes (e.g., motor unit recruitment and rate coding) that take place with practice34 which might enhance force output. It would be interesting to compare a land-based versus water-based exercise program on cardiovascular fitness when exercising at similar intensities.
Improvements in strength and gait speed suggest that the water-based exercise can improve lower extremity muscle function and functional mobility. The gains in muscle strength may have contributed to the improvements in gait speed. The trend towards balance improvement in the control group may have resulted from the mobility required between the stations or from improved trunk and leg weight-bearing function from the reaching movements. For example, Dean et al.30 demonstrated improved leg weight-bearing and sit-to-stand ability with the practice of seated reaching tasks. The lack of balance improvement for the water-based exercise group was not a surprise considering that balance training was not a focus in this study. Furthermore, the buoyancy characteristic of the water, combined with the flotation device, may not have allowed balance to be challenged during the water-based program.
The findings of this pilot study are promising considering the short length and large magnitude of improvement in cardiovascular fitness and functional mobility. This study demonstrates that feasibility of the protocol in that subjects could tolerate moderate to high intensity exercise programs using a water-based program. A water-based program can be undertaken in the community as a group program and is therefore a cost-effective and convenient means to promote fitness in individuals with stroke. This study is the first to evaluate a water-based community program for individuals with stroke. Given the small sample size and relatively high function of this sample, caution needs to be taken when generalizing these results to the wider stroke population.
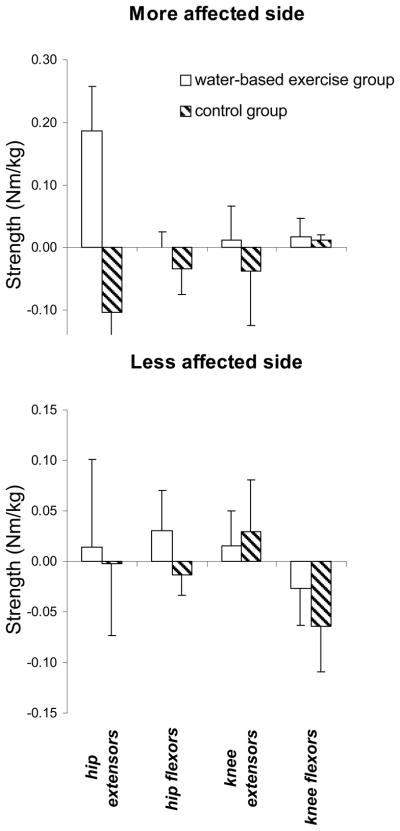
Figure 1.
Muscle strength changes in Nm/kg (plus 1 standard error) for individual muscles (+ve=improvement)
Acknowledgments
Funding sources: This study was supported by a grant-in-aid from the Heart and Stroke Foundation of BC and Yukon and a career scientist award to JJE from the Canadian Institute of Health Research (MSH-63617) and the Michael Smith Foundation for Health Research.
We thank Dr. Don McKenzie and Ms. Diana Jespersen for their expertise and use of the Allan McGavin Sports Medicine Centre.
Footnotes
Optotrak, Northern Digital, 103 Randall Drive, Waterloo, ON, Canada, N2V 1C5.
Excalibre bike, Lode Medical Technology, Zernikepark 16, 9747 AN Groningen, The Netherlands.
CPX-D Metabolic System, Medical Graphics Corporation, 350 Oak Grove Parkway, St. Paul, Minnesota, USA, 55127.
Schiller AT10i, Schiller AG, Altgasse 68, PO Box 1052, CH-6341 Baar, Switzerland.
Chattanooga Group Inc., 4717 Adams Road, Hixson, TN, USA, 37343.
Polar Electro Inc., 370 Crossways Park Drive, Woodbury, NY, USA, 11797-2050.
Aquam Inc., 1320 Route 9, Champlain NY, USA, 12919.
Mustang Survival Canada, 3810 Jacombs Road, Richmond, BC, Canada, V6V 1Y6.
References
- 1.Roth EJ. Heart disease in patients with stroke: incidence, impact, and implications for rehabilitation. Part I: classification and prevalence. Arch Phys Med Rehabil. 1993;74:752–60. doi: 10.1016/0003-9993(93)90038-c. [DOI] [PubMed] [Google Scholar]
- 2.Sacco RL, Wolf PA, Kannel WB, McNamara PM. Survival and recurrence following stroke: the Framingham Study. Stroke. 1982;13:290–5. doi: 10.1161/01.str.13.3.290. [DOI] [PubMed] [Google Scholar]
- 3.Pate RR, Pratt M, Blair SN, Haskell WL, Macera CA, Bouchard C, et al. Physical activity and public health: A reommendation from the centres for disease control and prevention and the American collect of sports medigin. JAMA. 1995;273:402–7. doi: 10.1001/jama.273.5.402. [DOI] [PubMed] [Google Scholar]
- 4.American College of Sports Medicine. Guidelines for Exercise Testing and Prescription. 6. Baltimore: Williams and Wilkins; 2000. pp. 104–117. [Google Scholar]
- 5.Macko RF, Smith GV, Dobrovolney CL, Sorkin JD, Goldberg AP, Silver KH. Treadmill training improves fitness reserve in chronic stroke patients. Arch Phys Med Rehabil. 2001;82:879–84. doi: 10.1053/apmr.2001.23853. [DOI] [PubMed] [Google Scholar]
- 6.Macko RF, DeSouza CA, Tretter LD, Silver KH, Smith GV, Anderson PA, et al. Treadmill aerobic exercise training reduces the energy expenditure and cardiovascular demands of hemiparetic gait in chronic stroke patients: a preliminary report. Stroke. 1997;28:326–30. doi: 10.1161/01.str.28.2.326. [DOI] [PubMed] [Google Scholar]
- 7.Potempa K, Lopez M, Braun LT, Szidon JP, Fogg L, Tincknell T. Physiological outcomes of aerobic exercise training in hemiparetic stroke patients. Stroke. 1995;26:101–5. doi: 10.1161/01.str.26.1.101. [DOI] [PubMed] [Google Scholar]
- 8.Rimmer JH, Riley B, Creviston T, Nicola T. Exercise training in a predominantly African-American group of stroke survivors. Med Sci Sports Exer. 2000;32:1990–6. doi: 10.1097/00005768-200012000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Moening D, Scheidt A, Shepardson L, Davies GJ. Biomechanical comparison of water running and treadmill running. Iso Exer Sci. 1993;3:207–15. [Google Scholar]
- 10.Costill DL. Energy requirements during exercise in water. J Sports Med Phys Fitness. 1971;11:87–92. [PubMed] [Google Scholar]
- 11.Taunton JE, Rhodes EC, Wolski LA, Donelly M, Warren J, Elliot J, et al. Effect of land-based and water-based fitness programs on the cardiovascular fitness, strength and flexibility of women aged 65–75 years. Gerontology. 1996;42:204–10. doi: 10.1159/000213794. [DOI] [PubMed] [Google Scholar]
- 12.Takeshima N, Rogers ME, Watanabe E, Brechue WF, Okada A, Yamada T, et al. Water-based exercise improves health-related aspects of fitness in older women. Med Sci Sports Exer. 2002;33:544–51. doi: 10.1097/00005768-200203000-00024. [DOI] [PubMed] [Google Scholar]
- 13.Danneskiold-Samsøe B, Lyngberg K, Risum T, Telling M. The effect of water exercise therapy given to patients with rheumatoid arthritis. Scand J Rehabil Med. 1987;19:31–5. [PubMed] [Google Scholar]
- 14.Eng JJ, Dawson AS, Chu KS. Submaximal exercise in individuals with stroke: Test-retest reliability and concurrent validity with VO2max. Arch Phys Med Rehabil. doi: 10.1016/s0003-9993(03)00436-2. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domholdt E. Physical Therapy Research: Principles and Applications. 1. Philadelphia: W.B. Saunders Company; 1993. [Google Scholar]
- 16.Tate DG, Findley TJ, Dijkers M, Nobunaga AI, Karunas RB. Randomized clinical trials in medical rehabilitation research. Am J Phys Med Rehabil. 1999;78:486–99. doi: 10.1097/00002060-199909000-00016. [DOI] [PubMed] [Google Scholar]
- 17.Kelly-Hayes M, Robertson JT, Broderick JP, Duncan PW, Hersey LA, Roth EJ, et al. The American Heart Association Stroke Outcome Classification: executive summary. Circulation. 1998;97:2474–8. doi: 10.1161/01.cir.97.24.2474. [DOI] [PubMed] [Google Scholar]
- 18.Gowland C, Stratford P, Ward M, Moreland J, Torresin W, Van Hullenaar S, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- 19.Franklin BA. ASCM’s Resource Manual for Guidelines for Exercise Testing and Prescription. 4. Philadelphia: 2001. Normal Cardiorespiratory Responses to Acute Aerobic Exercise; p. 145. [Google Scholar]
- 20.Berg K, Wood-Dauphinee S, William JI, Gayton D. Measuring balance in the elderly: preliminary development of an instrument. Physiother Can. 1989;41:304–11. [Google Scholar]
- 21.Berg K, Maki BE, Williams JI, Holliday J, Wood-Dauphinee SL. Clinical and laboratory measures of postural balance in an elderly population. Arch Phys Med Rehabil. 1992;73:1073–80. [PubMed] [Google Scholar]
- 22.Eng JJ, Kim CM, MacIntyre DL. Reliability of lower extremity strength measures in persons with chronic stroke. Arch Phys Med Rehabil. 2002;83:322–8. doi: 10.1053/apmr.2002.29622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eng JJ, Chu KS, Dawson AS, Kim CM, Hepburn K. Functional walk test in individuals with stroke: relationship to perceived exertion and myocardial exertion. Stroke. 2002;33:756–61. doi: 10.1161/hs0302.104195. [DOI] [PubMed] [Google Scholar]
- 24.Borg G. Perceived exertion as an indicator of somatic stress. Scand J Rehabil Med. 1970;2:92–8. [PubMed] [Google Scholar]
- 25.Teixeira-Salmela LF, Olney SJ, Nadeau S, Brouwer B. Muscle strengthening and physical conditioning to reduce impairment and disability on chronic stroke survivors. Arch Phys Med Rehabil. 1999;80:211–8. doi: 10.1016/s0003-9993(99)90018-7. [DOI] [PubMed] [Google Scholar]
- 26.Kim CM, Eng JJ, MacIntyre DL, Dawson AS. Effects of isokinetic strength training on walking in persons with stroke: a double-blind controlled pilot study. J Stroke Cerebrovasc Dis. 2001;10:265–73. doi: 10.1053/jscd.2001.123775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brooks GA, Fahey TD, White TP. Exercise Physiology: Human Bioenergetics and It Applications. 2. Mountain View: Mayfair Publishing; 1996. p. 589. [Google Scholar]
- 28.Ryan AS, Dobrovolny CL, Silver KH, Smith GV, Macko RF. Cardiovascular fitness after stroke: role of muscle mass and gait deficit severity. J Stroke Cerebrovasc Dis. 2000;9:185–91. doi: 10.1053/jscd.2000.7237. [DOI] [PubMed] [Google Scholar]
- 29.Rogers MA, Yammamoto C, Hagberg JM, Holloszy JO, Ehsani AA. The effect of 7 years of intense exercise training on patients with coronary artery disease. J Am Coll Cardiol. 1987;10:321–6. doi: 10.1016/s0735-1097(87)80014-1. [DOI] [PubMed] [Google Scholar]
- 30.Dean CM, Shepherd RB. Task-related training improves performance of seated reaching tasks after stroke. A randomized controlled trial. Stroke. 1997;28:722–8. doi: 10.1161/01.str.28.4.722. [DOI] [PubMed] [Google Scholar]
- 31.Blomqvist G, Saltin B. Cardiovascular adaptations to physical training. Annu Rev Phys. 1983;45:169–89. doi: 10.1146/annurev.ph.45.030183.001125. [DOI] [PubMed] [Google Scholar]
- 32.Duncan PW, Badke MB, editors. Stroke Rehabilitation: the recovery of motor control. Chicago: Year Book Medical Publishers; 1987. [Google Scholar]
- 33.Rutherford OM. Muscular coordination and strength training. Implications for injury rehabilitation. Sports Med. 1988;5:196–202. doi: 10.2165/00007256-198805030-00006. [DOI] [PubMed] [Google Scholar]
- 34.McComas AJ. Human neuromuscular adaptations that accompany changes in activity. Med Sci Sports Exerc. 1994;26:1498–509. [PubMed] [Google Scholar]