Abstract
Initial exposure to human immunodeficiency virus type 1 (HIV-1) during heterosexual transmission occurs in the genital tract. Although much of the literature on the immune response to HIV-1 infection is based on studies performed at the systemic level, our understanding of tissue-specific immunity is lacking. Levels of both genital mucosal and blood interleukin (IL)-2, IL-4, IL-6, IL-10, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ production were compared between 57 HIV-1-uninfected and 52 HIV-1-infected female commercial sex workers (CSWs) as well as 73 HIV-1-uninfected non-CSW control women at low risk for exposure. HIV-1-infected CSWs had significantly higher genital mucosal levels of TNF-α and IFN-γ compared with those in both the HIV-uninfected CSW and non-CSW groups. In contrast, the serum levels of all the cytokines tested were lower in HIV-1-infected CSWs compared with those in the other groups. The increased production of genital mucosal pro-inflammatory cytokines in HIV-1-infected CSWs possibly reflects susceptibility to HIV-1 infection and disease progression/perpetuation at the initial site of exposure.
INTRODUCTION
In 2007, there were 33.2 million people living with HIV/acquired immune deficiency syndrome worldwide, and the number of women infected with HIV was estimated at 15.4 million. Most HIV infections are acquired through heterosexual intercourse, and each year the rate of HIV-infected women increases dramatically. In Africa, 60 % of new HIV infections affect women who are considered the most vulnerable population. 1 Preventive vaccine and protective microbicide strategies are still under development, but until now clinical trials have failed to prove the efficacy and safety of commercially available compounds. Indeed, in a recent clinical trial, the most promising HIV vaccine has not only failed to protect but may actually have increased the risk of HIV infection in some study participants. 2 Furthermore, microbicides aiming to prevent HIV entry in the vaginal tract have often failed because some components, such as nonoxynol-9, can be toxic and may increase susceptibility to HIV infection. 3,4 The development of efficient preventive strategies against HIV requires a further understanding of the factors involved in HIV susceptibility, such as the mucosal immunity at the initial site of infection and its link to systemic immunity.
There are a number of factors that have been correlated with women’s susceptibility to HIV infection. Socio-demographic and sexual behavior factors, such as age, marital status, history of prostitution, the use of condoms, and the number of sexual partners, have been associated with HIV-1 infection. 5 –7 The practice of vaginal douching has also been shown to increase risk of HIV-1 infection. 8 Host genetic factors such as the CCR5 32-bp deletion, HIV-1 co-receptor mutation, and specific human leukocyte antigen class I alleles have also been associated with HIV-1 infection. 9,10 Biological factors such as the sexual partner’s high viral load and the presence of sexually transmitted infections may also have an impact on the risk of HIV-1 acquisition. For example, Herpes simplex-2 (HSV-2) seropositivity has been associated with increased risk of HIV acquisition. 11,12
The initial site of exposure to HIV-1 during heterosexual transmission occurs in the genital tract. However, little is known about the mechanisms of transmission and HIV-specific immune responses at this site. The female genital tract is related to the mucosal-associated lymphoid tissue, which contains the majority of immune cells within the body. 13 The particularity of the female genital tract immunity is that it is tightly regulated by a hormonal/inflammatory process throughout the menstrual cycle, having to deal with the pressure of procreation and with surveillance of the commensal microbial flora as well as with intrusion by pathogens. 14 The most obvious scenario for heterosexual HIV-1 transmission involves the passage of the virus across the mucosal epithelial barrier and its capture by Langerhans and/or intraepithelial dendritic cells, which may facilitate infection of target cells in the sub-mucosal layer and draining lymphoid organs. 15 Although the mechanism of HIV-1 transmission in the female genital tract has become an important question in recent years, the factors involved in HIV-1 susceptibility and its dissemination in the genital tract still remain unclear. Studies in Kenyan commercial sex workers (CSWs) have identified HIV-1-resistant women who present systemic and genital HIV-1-specific cytotoxic T cells 16–18 and neutralizing IgA. 19–21 Moreover, HIV-1-resistant CSWs showed increased cervical T-cell counts and RANTES (regulated on activation normal T cell expressed and secreted) expression compared with HIV-1-negative subjects. Importantly, these increases were not reflected in the systemic lymphocyte compartment. 22 Further characterization of the factors involved in mucosal immunity and their links to modulation of adaptive immune responses and systemic immunity are required for the development of efficient preventive strategies against HIV.
The purpose of this study was to characterize and compare the expression of immunoregulatory cytokines in the cervicovaginal lavage (CVL) samples and serum of HIV-1-infected CSWs, HIV-1-uninfected CSWs, and HIV-1-uninfected non-CSW control subjects at low risk for HIV exposure. We report that HIV-1-infected CSWs had significantly higher levels of TNF-α and interferon (IFN)-γ in their CVLs compared with those in both the HIV-uninfected CSW and non-CSW groups. In contrast, the serum levels of all the cytokines tested were lower in the HIV-1-infected CSWs compared with those in the other groups.
RESULTS
Demographic, sexual behavior, and genital infection characteristics of the study groups
These data were collected to address the issue of confounding variables for risk of HIV infection and mucosal immune responses. The three study groups were similar with respect to age, days from last menses, and the presence of genital tract infections as determined by gynecological exams, microscopic examination of vaginal specimens, and strand displacement assay for detection of Chlamydia trachomatis and Neisseria gonorrhoeae from endocervical swabs (Table 1). The HIV-1-uninfected non-CSW control subjects were more likely to have a regular partner (P = 0.005) and to be HSV-2-seronegative (P = 0.0001) than the HIV-1-infected and HIV-1-uninfected CSW women. The duration of sex work, average number of clients during the past week, vaginal douching, and condom use were equivalent between the HIV-1-infected and HIV-1-uninfected CSW groups.
Table 1.
Distribution of demographic, sexual behavior, and genital tract infection characteristics in HIV-1-uninfected and HIV-1-infected CSWs, and HIV-1-uninfected non-CSW control subjects
| HIV-1-uninfected CSWs | HIV-1-infected CSWs | HIV-1-uninfected non-CSW controls | P-value a | |
|---|---|---|---|---|
| N=57 | N=52 | N=73 | ||
| Age, mean (s.d.), years | 34.3 (12) | 33.5 (10.1) | 32.4 (9.4) | NS |
| Duration of sex work, mean (s.d.), years | 4.2 (3.1) | 3.8 (2.6) | NA | NS |
| Number of clients last week, mean (s.d.) | 17.1 (13.7) | 12.0 (11.0) | NA | NS |
| Days since last menses, mean (s.d.) | 14.5 (8.2) | 18.5 (15.5) | 20.7 (10.3) | NS |
| Regular partner | 32/55 (58.2%) | 31/52 (59.6%) | 59/72 (81.9%)b | 0.005 |
| Vaginal douching | 48/51 (94.1%) | 42/42 (100%) | 63/68 (92.6%) | NS |
| Condom always used with clients | 47/55 (85.5%) | 37/47 (78.7%) | NA | NS |
| Vaginosis | 21/49 (42.9%) | 24/46 (52.2%) | 22/73 (30.1%) | NS |
| Candidiasis | 5/49 (10.2%) | 6/46 (13.0%) | 14/73 (19.2%) | NS |
| NG and/or CT infection | 7/51 (13.7%) | 5/43 (11.6%) | 2/73 (2.7%) | NS |
| HSV-2-positive serology | 38/47 (80.9%) | 42/44 (95.5%) | 34/61 (55.7%) c | < 0.0001 |
CSW, commercial sex worker; HIV-1, human immunodeficiency virus type 1; HSV-2, Herpes simplex 2; N, number of participants; NA, non applicable; NS, nonsignificant; NG/CT, Neisseria gonorrhoeae/Chlamydia trachomatis.
All risk factor data were collected via a questionnaire administrated before samples were collected. Gynecological exams and biological sampling were performed by a physician without knowledge of HIV status of the women to avoid potential bias.
P-values for the comparison across all groups were calculated with one-way analysis of variance for the age, days since last menses; Mann–Whitney U-test for the duration of sex work and average number of clients; χ2 test for regular partner, vaginal douching, condom use, vaginosis, candidiasis, NG/CT, HSV-2.
P=0.005 for the comparison between HIV-1-uninfected non-CSWs and HIV-1-uninfected CSWs, and
P=0.008 for the comparison between HIV-1-uninfected non-CSWs and HIV-1-uninfected CSWs, and P=0.001 for the comparison between HIV-1-uninfected non-CSWs and HIV-1-infected CSWs.
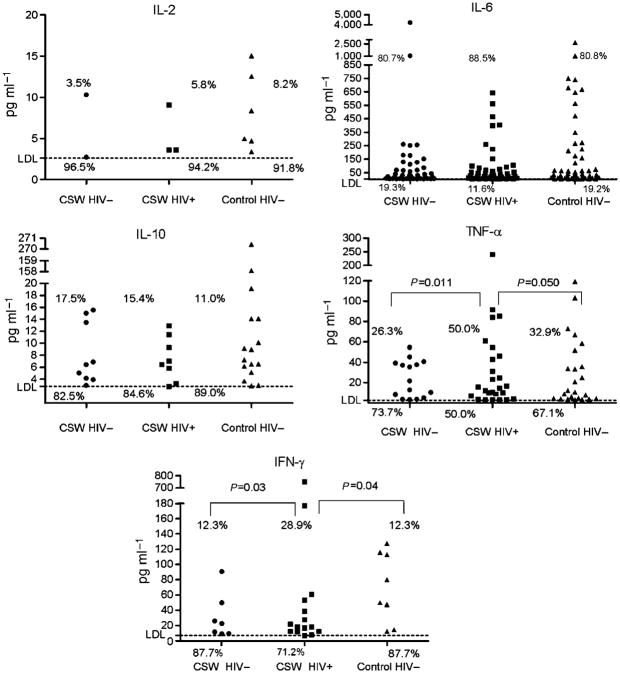
Cytokine expression patterns in cervicovaginal lavage samples
With the exception of interleukin (IL)-4, all investigated cytokines were detected in CVL samples of the three study groups (Table 2 ). The detection rate (Figure 1) and expression level (Table 2) of IL-2, IL-6, and IL-10 were similar in HIV-1-uninfected CSWs, HIV-1-infected CSWs, and HIV-uninfected non-CSW control subjects. However, TNF-α and IFN-γ detection rates and expression levels were significantly different between the HIV-1-infected and HIV-1-uninfected groups. HIV-1-infected CSWs had higher levels of TNF-α and IFN-γ than did the HIV-uninfected CSWs (P = 0.006, P = 0.031, respectively) and the HIV-uninfected non-CSW control women (P = 0.02, P = 0.006, respectively). Accordingly, the percentage of women producing significant amounts of TNF-α and IFN-γ (concentrations above the lower detection limit (LDL)) was significantly higher in the HIV-1-infected CSW group compared with that in both the HIV-uninfected CSW and non-CSW groups (Figure 1). There was no correlation between the HIV-1 viral load and the levels of cytokines in the CVL samples of the HIV-1-infected CSWs.
Table 2.
Cytokine levels in cervicovaginal lavage samples from HIV-1-uninfected and HIV-1-infected CSWs, and HIV-1-uninfected non-CSW control subjects
| HIV-1-uninfected CSWs | HIV-1-infected CSWs | HIV-1-uninfected non-CSW controls | P-valuea | |
|---|---|---|---|---|
| N=57 | N=52 | N=73 | ||
| IL-2 | 0.2 (1.4) | 0.7 (2.6) | 0.3 (1.4) | NS |
| IL-4 | 0 | 0 | 0 | NS |
| IL-6 | 135.2 (571.0) | 83.8 (148.6) | 151.8 (371.4) | NS |
| IL-10 | 1.31 (3.6) | 3.0 (1.8) | 7.5 (36.4) | NS |
| TNF-α | 6.31 (14.0) | 17.7 (39.3)b | 9.6 (23.4) | 0.015 |
| IFN-γ | 3.95 (14.2) | 24.0 (106.3)c | 7.7 (26.2) | 0.008 |
CSW, commercial sex worker; HIV-1, human immunodeficiency virus type 1; IFN, interferon; IL, interleukin; N, number of participants; NS, nonsignificant; TNF, tumor necrosis factor.
Data are mean (s.d.) pg ml−1.
The lower detection limit for each assay was 2.6 pg ml−1 for IL-2 and IL-4, 3.0 pg ml−1 for IL-6, 2.8 pg ml−1 for IL-10 and TNF-α, and 7.1 pg ml−1 for IFN-γ.
P-values for the comparison across all groups were calculated with one-way analysis of variance test.
P=0.006 for the comparison between HIV-1-infected CSWs and HIV-1-uninfected CSWs, and P=0.02 for the comparison between HIV-1-infected CSWs and HIV-1-uninfected non-CSWs as determined by Mann–Whitney U-test.
P=0.031 for the comparison between HIV-1-infected CSWs and HIV-1-uninfected CSWs, and P=0.006 for the comparison between HIV-1-infected CSWs and HIV-1-uninfected non-CSWs as determined by Mann–Whitney U-test.
Figure 1.
Distribution of IL-2, IL-6, IL-10, TNF-α, and IFN-γ CVL levels according to the study groups. CVL cytokine levels were quantified by BD Cytometric Bead Array and normalized to a standard curve. The LDL for each assay was 2.6 pg ml− for IL-2, 3.0 pg ml−1 for IL-6, 2.8 pg ml−1 for IL-10 and TNF-α, and 7.1 pg ml−1 for IFN-γ. Sample measurements below the LDL were assigned a value of 0. Values are expressed in pg ml−1. Owing to the high number of samples below the assay LDL, cytokine levels were dichotomized as detectable and undetectable in all analyses. Comparisons of the cytokine detection rates (% of women producing cytokine levels above the LDL) between two study groups were examined with the χ2 test. Significant (or near significant) differences in the cytokine detection rates between the two groups are shown. Differences that were not statistically significant are not illustrated. CVL, cervicovaginal lavage; IFN, interferon; IL, interleukin; LDL, lower detection limit; TNF, tumor necrosis factor.
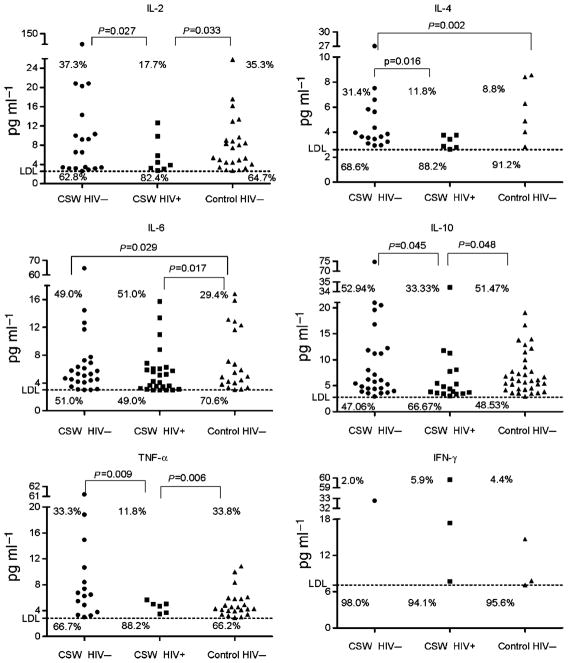
Cytokine expression patterns in serum
For the purpose of comparison between the mucosal and systemic compartments, we also examined the pattern of cytokine expression in the serum of all participants. In stark contrast to what is found in mucosal samples, we observed a decrease in the levels of cytokine expression in the serum of the HIV-1-infected CSWs compared with those in both the HIV-1-uninfected CSW and non-CSW groups (Table 3).
Table 3.
Cytokine levels in serum from HIV-1-uninfected and HIV-1-infected CSWs, and HIV-1-uninfected non-CSW control subjects
| HIV-1-uninfected CSWs | HIV-1-infected CSWs | HIV-1-uninfected non-CSW controls | P-valuea | |
|---|---|---|---|---|
| N=51 | N=51 | N=68 | ||
| IL-2 | 5.7 (19.3) | 0.9 (2.5)b | 2.9 (5.1) | 0.02 |
| IL-4 | 1.8 (4.2)c | 0.5 (1.1) | 0.5 (1.8) | 0.002 |
| IL-6 | 4.1 (9.4) | 2.9 (3.7) | 2.2 (4.2) | NS |
| IL-10 | 5.5 (11.4) | 2.4 (5.5)d | 3.7 (4.7) | 0.05 |
| TNF-α | 3.2 (9.2) | 0.5 (1.5)e | 1.7 (2.7) | 0.021 |
| IFN-γ | 0.6 (4.6) | 1.7 (8.7) | 0.5 (2.2) | NS |
CSW, commercial sex worker; HIV-1, human immunodeficiency virus type 1; IFN, interferon; IL, interleukin; N, number of participants; NS, nonsignificant; TNF, tumor necrosis factor.
Data are mean (s.d.) pg ml−1.
The lower detection limit for each assay was 2.6 pg ml−1 for IL-2 and IL-4, 3.0 pg ml−1 for IL-6, 2.8 pg ml−1 for IL-10 and TNF-α, and 7.1 pg ml−1 for IFN-γ.
P-values for the comparison across all groups were calculated with one-way analysis of variance test.
P=0.010 for the comparison between HIV-1-infected CSWs and HIV-1-uninfected CSWs, and P=0.012 for the comparison between HIV-1-infected CSWs and HIV-1-uninfected non-CSWs as determined by Mann–Whitney U-test.
P=0.016 for the comparison between HIV-1-uninfected CSWs and HIV-1-infected CSWs, and P=0.002 for the comparison between HIV-1-uninfected CSWs and HIV-1-uninfected non-CSWs as determined by Mann–Whitney U-test.
P=0.028 for the comparison between HIV-1-infected CSWs and HIV-1-uninfected CSWs, and P=0.028 for the comparison between HIV-1-infected CSWs and HIV-1-uninfected non-CSWs as determined by Mann–Whitney U-test.
P=0.019 for the comparison between HIV-1-infected CSWs and HIV-1-uninfected CSWs, and P=0.007 for the comparison between HIV-1-infected CSWs and HIV-1-uninfected non-CSWs as determined by Mann–Whitney U-test.
HIV-1-infected CSWs had lower levels of IL-2, IL-10, and TNF-α than did the HIV-uninfected CSWs (P = 0.010, P = 0.028, P = 0.019, respectively) and the HIV-uninfected non-CSW control women (P = 0.012, P = 0.028, P = 0.007, respectively). In addition, the percentage of women with detectable levels of IL-2, IL-10, and TNF-α was significantly lower in the HIV-1-infected CSW group compared with those in both the HIV-uninfected CSW and non-CSW groups (Figure 2 ). HIV-1-infected CSWs (P = 0.016) and HIV-1-uninfected non-CSW control subjects (P = 0.002) had lower levels of IL-4 than did the HIV-1-uninfected CSWs. The same finding was observed for IL-6, although the differences were not statistically significant. The IFN-γ levels were not significantly different in the three groups. There was no correlation between the HIV-1 viral load and the levels of cytokines in the serum of the HIV-1-infected CSWs.
Figure 2.
Distribution of IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ serum levels according to the study groups. Serum cytokine levels were quantified by BD Cytometric Bead Array and normalized to a standard curve. The LDL for each assay was 2.6 pg ml−1 for IL-2 and IL-4, 3.0 pg ml−1 for IL-6, 2.8 pg ml−1 for IL-10 and TNF-α, and 7.1 pg ml−1 for IFN-γ. Sample measurements below the LDL were assigned a value of 0. Values are expressed in pg ml−1. Owing to the high number of samples below the assay LDL, cytokine levels were dichotomized as detectable and undetectable in all analyses. Comparisons of the cytokine detection rates (% of women producing cytokine levels above the LDL) between two study groups were examined with the χ2 test. Significant (or near significant) differences in the cytokine detection rates between the two groups are shown. Differences that were not statistically significant are not illustrated. IFN, interferon; IL, interleukin; LDL, lower detection limit; TNF, tumor necrosis factor.
DISCUSSION
It is of primary importance to design strategies that will protect human mucosal ports of entry, such as the female genital tract, from HIV infection. This will be better achieved by furthering our understanding of mucosal immunology and its link to the systemic immune system. The present study shows that during HIV infection, the production of immunoregulatory cytokines in the genital mucosa displays characteristic features that are distinct from those of the systemic immune compartment.
The relatively lower levels of cytokines detected in the serum of HIV-1-infected CSWs compared with those in HIV-1-uninfected women is in agreement with a recent report demonstrating that the capacity of blood-derived lymphocytes to produce in vitro pro-inflammatory cytokines such as IL-2, TNF-α, and IFN-γ correlated with a better prognosis and slow HIV-1 disease progression. 23 Moreover, the significant increase in the IL-4 levels observed in the serum of HIV-1-unifected CSWs may suggest that in these women the blood CD4 + T-cell compartment could have the capacity to produce a more vigorous response by increasing the avidity and affinity of both the humoral and cellular immune responses. In contrast to what is found in blood samples, HIV-1-infected CSWs had relatively higher levels of pro-inflammatory cytokines TNF-α and IFN-γ in the genital mucosal tract compared with those in HIV-1-uninfected women. The local inflammatory reaction observed in the genital tract of HIV-1-infected CSWs is probably a consequence of the combinatory direct and indirect effects caused by HIV. 13 This has been demonstrated in the gastrointestinal tract where the levels of HIV-1 replication and CD4 + T-cell depletion coincided with a marked increase in transcription of human activation- and inflammation-associated genes. 24 Studies of female rhesus macaques infected genitally with simian immunodeficiency virus have shown that pro-inflammatory cytokine responses are earliest and strongest in the genital mucosa and draining lymphoid tissues and that they are positively correlated with virus replication within these tissues. 25 CD8 + T cells from the genital mucosa of HIV-1-infected CSWs have been shown to produce IFN-γ in response to HIV in vitro.18,26 The significant increase in the levels of the pro-inflammatory cytokines TNF-α and IFN-γ in the genital mucosal tract of HIV-1-infected CSWs may favor disease progression/perpetuation by the recruitment of a continuum of target cells in view of augmenting viral replication and dissemination beyond the initial site of infection. 14,15
As the lumen of the female genital tract is a “non-sterile” environment, the microbial flora as well as the presence of genital infections and lesions can enhance susceptibility to HIV-1 by breaching the epithelial barrier, recruiting target cells, or generating a pro-inflammatory milieu. 27 This has been shown at the level of the gastrointestinal mucosa barrier, where microbial translocation is believed to be the major cause of HIV-1-related chronic inflammation. 28,29 We have investigated if there was an imbalance in the female genital tract microbial flora by verifying the presence of bacterial vaginosis (BV), as any imbalance and loss at the level of the natural flora is likely to affect immune responses and their control. Indeed, BV has been associated with increased levels of cytokines such as IL-1, IL-6, IL-10, and TNF-α and of chemokines such as RANTES, macrophage inflammatory protein-1α, and macrophage inflammatory protein-1βin CVLs and secretions. 30,31 The assessment of BV was done by measuring the lactobacillus/Gardnerella + Mobiluncus ratio the vaginal secretions of all the study participants. However, we found no correlation between the presence of BV and the risk of HIV-1 infection or the cytokine expression patterns. Sexually transmitted infections could also have an impact on the genital mucosal immunity and have been shown to increase the risk of HIV-1 acquisition and modulate the immune factors associated with HIV-1 infection. N. gonorrhoeae infection was shown to impair HIV-1-specific cellular immune responses and increase the levels of IL-6 and IL-8 produced by cervical and vaginal epithelial cells. 32,33 HSV-2 seropositivity has been associated with increased risk of HIV-1 acquisition. 11,12 In the present study, we carefully looked for genital infections by clinical examination and by testing specifically for the presence of syphilis, Trichomonas vaginalis, candidiasis, C. trachomatis, N. gonorrhoeae, and HSV-2. All participants tested negative for syphilis, and T. vaginalis infection was detected only in two women (data not shown). Candidiasis, C. trachomatis, and N. gonorrhoeae were more prevalent in our study population, but we found no correlation between the presence of these genital infections and the risk of HIV-1 infection or the cytokine expression patterns (Table 1). Although we found a positive association between HSV-2 seropositivity and the practice of prostitution (Table 1), none of these women had active genital infection upon clinical examination. HSV IgG serology does not correlate with active infection of HSV but rather reflects a past exposure to the virus. We cannot exclude the possibility that other genital infections such as chancroid, donovanosis, and human papillomavirus could have influenced the production of cytokines. Although we did not test specifically for these infections, none of the participants had evidence of characteristic genital chancres, ulcers, and/or condylomas associated with these illnesses (data not shown). Thus, these observations suggest that HIV-1 is probably the main pathogenic cause of the increased pro-inflammatory cytokine levels found in the female genital tract of HIV-1-infected CSWs.
Our study shows that during HIV infection, the production of immunoregulatory cytokines in the genital mucosa displays characteristic features that are distinct from those of the systemic immune compartment. The increased production of genital mucosal pro-inflammatory cytokines in the HIV-1-infected CSWs possibly reflects susceptibility to HIV-1 infection and modulates disease progression/perpetuation at the initial site of exposure. This clearly reinforces the importance of well understanding mucosal immunity and the factors involved in linking innate to adaptive responses while preserving the integrity of the mucosal/systemic balance to develop preventive strategies such as microbicides and vaccines.
METHODS
Study populations
Female CSWs were enrolled through a dedicated sex worker clinic in Cotonou, Benin and were divided into two groups: HIV-1-uninfected CSWs (n = 57) and HIV-1-infected CSWs (n=52). The HIV-1-uninfected non-CSW control subjects at low risk for exposure (n = 73) were enrolled from a general health clinic in Cotonou. This study was approved by the Ministère de la Santé du Bénin and by the CHUM, Hôpital Maisonneuve-Rosemont, and Université Laval human research ethics boards. Women were allowed to participate in the study as they attended clinics. All subjects provided written informed consent. At enrollment, participants were asked to answer a questionnaire about demographic information, sexual behavior, duration of prostitution, number of sex partners, condom use, vaginal douching practices, and reproductive history. Women were excluded from the study if < 18 years old, menstruating, or pregnant. Each participant underwent a genital examination by a physician. Vaginal specimens were obtained for diagnosis of candidiasis, T. vaginalis infection, and BV by microscopic examination. Endocervical swabs were obtained to test for N. gonorrhoeae and C. trachomatis infection using BD ProbeTec ET system (Strand Displacement Assay, Becton Dickinson, Heidelberg, Germany). Peripheral blood was taken for HIV, syphilis, and HSV-2 serologies and for HIV-1 viral load, CCR5 genotype, and cytokine determination. Plasma and serum were kept frozen at −80°C until use. HIV-1 positivity was defined by the presence of HIV-1 antibodies tested with Vironostika HIV Uni-Form II Ag/Ab (Organon Teknika, Boxtel, The Netherlands). Non-reactive samples were considered HIV-seronegative, whereas reactive samples were tested with Genie II HIV-1/HIV-2 (Bio-Rad, Hercules, CA, USA). Genie II dually reactive samples (to HIV-1 and HIV-2) and discordant samples (Vironostika reactive/Genie II non-reactive) were further tested by INNO-LIA HIV I/II Score (Innogenetics NV, Technologiepark 6, Gent, Belgium) to resolve the ambiguities. Plasmatic HSV-2 IgG detection was determined with the Captia anti-HSV-2 IgG specific test (Trinity Biotech, Bray, Ireland). HIV-1 viral loads were determined in the plasma of all HIV-1-infected CSWs using VERSANT HIV-1 RNA 3.0 Assay (bDNA) (Siemens Medical Solutions Diagnostics, Tarrytown, NY). DNA samples were genotyped for the CCR5 32-bp deletion allele, and all women were found to be homozygous for the wild-type allele.
Mucosal sample collection and preparation
Cervicovaginal lavage samples were obtained from all study participants by a physician using a 10-ml syringe filled with sterile phosphate-buffered solution and aimed directly into the cervical os. CVL fluids were then collected, transferred immediately into 20 ml of RPMI-1640, kept on ice, and processed within 1 h. CVL samples were centrifuged at 1500 r.p.m. for 10 min to remove cells and debris, and supernatants were stored at −80°C until shipped on dry ice to Montreal, Canada. CVL samples were concentrated with Amicon Ultra-15 5 kDa (Millipore, Billerica, MA) prior to cytokine measurement.
Cytokine measurement
Cytokine levels were determined in serum and CVL samples using the human TH1/TH2 kit II of the BD Cytometric Bead Array technology (Becton Dickinson, Franklin Lakes, NJ), which allows simultaneous detection of IL-2, IL-4, IL-6, IL-10, TNF-α, and IFN-γ. Analysis was performed on a BD FACSAria apparatus. The final concentration for a given cytokine in the CVL sample was determined as follows: concentration obtained with BD Cytometric Bead Array (pg ml−1)/(CVL concentration factor) × total CVL volume prior to concentration. The LDL for each assay was 2.6 pg ml−1 for IL-2 and IL-4, 3.0 pg ml−1 for IL-6, 2.8 pg ml−1 for IL-10 and TNF-α, and 7.1 pg ml−1 for IFN-γ. Sample measurements below the LDL were assigned a value of 0 pg ml−1.
Statistical analysis
Statistical analysis was performed using the GraphPad PRISM 5.0. for Windows (GraphPad Software, San Diego, CA). One-way analysis of variance and χ2 tests were used to assess the significance of the associations between continuous and categorical variables across all study groups. Comparisons of continuous and categorical variables between two groups were assessed by the Mann–Whitney U and χ2 tests, respectively.
Acknowledgments
This work was supported by Grant HOP-79213 from the Canadian Institutes of Health Research (CIHR) and by the Réseau SIDA from the Fonds de la Recherche en Santé du Québec (FRSQ). Julie Lajoie and Marguerite Massinga Loembe hold Student Research awards from the CIHR. Michel Alary and Michel Roger are recipients of Research Scholar awards from the FRSQ. We are indebted to Nassirou Geraldo, Aina Gabin, and Carmelle Assogba for their clinical expertise, to Gérard Ahotin, Laurette Djossou, and Ella Goma for their technical assistance, and to Georges Batona and other field workers who helped with recruitment of commercial sex workers. We are grateful to Sylvain Gimmig for invaluable assistance in flow cytometry analysis and helpful discussion.
Footnotes
This work was presented in part at the 13thInternational Congress of Immunology, Rio de Janeiro, Brazil, August 21–25, 2007
DISCLOSURE
The authors declare no conflict of interest.
References
- 1.http://www.unaids.org/en/KnowledgeCentre/HIVData/EpiUpdate/EpiUpdArchive/2007default.asp.
- 2.Cohen J. Did Merk’s failed HIV vaccine cause harm? Science. 2007;318:1048–1049. doi: 10.1126/science.318.5853.1048. [DOI] [PubMed] [Google Scholar]
- 3.Cone RA, Hoen T, Wong X, Abusuwwa R, Anderson DJ, Moench TR. Vaginal microbicides: detecting toxicities in vivo that paradoxically increase pathogen transmission. BMC Infect Dis. 2006;6:90–106. doi: 10.1186/1471-2334-6-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lederman MM, Offord RE, Hartley O. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat Rev Immunol. 2006;6:371–382. doi: 10.1038/nri1848. [DOI] [PubMed] [Google Scholar]
- 5.Cohen B, Trussell J. Preventing and Mitigating AIDS in Sub-Saharan Africa: Research and Data Priorities for the Social and Behavioural Sciences. National Academy Press; NW, Washington DC: 1996. pp. 105–154. [PubMed] [Google Scholar]
- 6.Prohaska TR, Albrecht G, Levy JA, Sugrue N, Kim JH. Determinants of self-perceived risk for AIDS. J Health Soc Behav. 1990;31:384–394. [PubMed] [Google Scholar]
- 7.Ahlburg DA, Jensen ER, Perez AE. Determinants of extramarital sex in the Philippines. Health Transit Rev. 1997;7 (supplement):467–479. [PubMed] [Google Scholar]
- 8.McClelland RS, Lavreys L, Hassan WM, Mandaliya K, Ndinya-Achola JO, Baeten JM. Vaginal washing and increased risk of HIV-1 acquisition among African women: a 10 years prospective study. AIDS. 2006;20:269–273. doi: 10.1097/01.aids.0000196165.48518.7b. [DOI] [PubMed] [Google Scholar]
- 9.Lajoie J, Hargrove J, Zijenah LS, Humphrey J, Ward BJ, Roger M. Genetic variants in nonclassical major histocompatibility complex class I human leukocyte antigen (HLA)-E and HLA-G molecules are associated with susceptibility to heterosexual Acquisition of HIV-1. J Infect Dis. 2006;193:298–301. doi: 10.1086/498877. [DOI] [PubMed] [Google Scholar]
- 10.Kaslow RA, Dorak T, Tang JJ. Influence of Host genetic variation on susceptibility to HIV type 1 infection. J Infect Dis. 2005;191:S68–S77. doi: 10.1086/425269. [DOI] [PubMed] [Google Scholar]
- 11.Freeman EE, Weiss HA, Glynn JR, Cross PL, Whitworth JA, Hayes RJ. Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. AIDS. 2006;20:73–83. doi: 10.1097/01.aids.0000198081.09337.a7. [DOI] [PubMed] [Google Scholar]
- 12.Rebbapragada A, et al. Negative mucosal synergy between Herpes simplex type 2 and HIV in the female genital tract. AIDS. 2007;21:589–898. doi: 10.1097/QAD.0b013e328012b896. [DOI] [PubMed] [Google Scholar]
- 13.Haynes BF. Gut microbes out of control in HIV infection. Nat Med. 2006;33:915–933. doi: 10.1038/nm1206-1351. [DOI] [PubMed] [Google Scholar]
- 14.Wira CR, Fahey JV, Sentman CL, Pioli PA, Shen L. Innate and adaptive immunity in female genital tract: cellular responses and interactions. Immunol Rev. 2005;206:306–335. doi: 10.1111/j.0105-2896.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 15.Sugaya M, Loré K, Koup RA, Douek DC, Blauvelt A. HIV-infected Langerhans cells preferentially transmit virus to proliferating autologous CD4+ memory T cells located within Langerhans cell-T cell clusters. J Immunol. 2004;172:2219–2224. doi: 10.4049/jimmunol.172.4.2219. [DOI] [PubMed] [Google Scholar]
- 16.Fowke KR, et al. Resistance to HIV-1 infection among persistently seronegative prostitutes in Nairobi, Kenya. Lancet. 1996;348:1347–1351. doi: 10.1016/S0140-6736(95)12269-2. [DOI] [PubMed] [Google Scholar]
- 17.Fowke KR, et al. HIV-1 specific cellular immune responses among HIV-1 resistant sex workers. Immunol Cell Biol. 2000;78:586–895. doi: 10.1046/j.1440-1711.2000.00944.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaul R, et al. HIV-1 specific mucosal CD8+ lymphocyte responses in the cervix of HIV-1 resistant prostitutes in Nairobi. J Immunol. 2000;164:1602–1611. doi: 10.4049/jimmunol.164.3.1602. [DOI] [PubMed] [Google Scholar]
- 19.Kaul R, et al. HIV-1 specific mucosal IgA in a cohort of HIV-1 resistant Kenyan sex worker. AIDS. 1999;13:23–29. doi: 10.1097/00002030-199901140-00004. [DOI] [PubMed] [Google Scholar]
- 20.Devito C, et al. Cross-Clade HIV-1 specific neutralizing IgA in mucosal and systemic compartments of HIV-1 exposed persistently seronegative subjects. J Acquir Immune Defic Syndr. 2002;30:413–420. doi: 10.1097/00042560-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 21.Broliden K, et al. Functional HIV-1 specific IgA antibodies in HIV-1 exposed persistently IgG seronegative female sex workers. Immunol Lett. 2001;79:29–36. doi: 10.1016/s0165-2478(01)00263-2. [DOI] [PubMed] [Google Scholar]
- 22.Iqbal SM, et al. Elevated T cell counts and Rantes expression in the genital mucosa of HIV-1-resistant Kenyan commercial sex workers. J Infec Dis. 2005;192:728–738. doi: 10.1086/432482. [DOI] [PubMed] [Google Scholar]
- 23.Kannanganat S, et al. Human immunodeficiency virus type 1 controllers but not noncontrollers maintain CD4T cells coexpressing three cytokines. J Virol. 2007;81:12071–12075. doi: 10.1128/JVI.01261-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sankaran S, et al. Rapid onset of intestinal epithelial barrier dysfunction in primary HIV infection is driven by imbalance between immune response and mucosal repair and regeneration. J Virol. 2008;82:538–545. doi: 10.1128/JVI.01449-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abel K, Rocke DM, Chohan B, Fritts L, Miller CJ. Temporal and anatomic relationship between virus replication and cytokine gene expression after vaginal simian immunodeficiency virus infection. J Virol. 2005;79:12164–12172. doi: 10.1128/JVI.79.19.12164-12172.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Musey L, Hu Y, Eckert L, Christensen M, Karchmer T, McElrath MJ. HIV-1 induces cytotoxic T lymphocytes in the cervix of infected women. J Exp Med. 1997;185:293–303. doi: 10.1084/jem.185.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kaul R, et al. The genital tract immune milieu: An important determinant of HIV-1 susceptibility and secondary transmission. J Reprod Immunol. 2008;77:32–40. doi: 10.1016/j.jri.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Brenchley JM, Price DA, Douek DC. HIV disease: fallout from a mucosal catastrophe? Nat immunol. 2006;7:235–239. doi: 10.1038/ni1316. [DOI] [PubMed] [Google Scholar]
- 29.Brenchley JM, Douek DC. HIV infection and gastrointestinal immune system. Mucosal Immunol. 2008;1:23–30. doi: 10.1038/mi.2007.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen CR, et al. Increased interleukin-10 in the endocervical secretions of women with non-ulcerative sexually transmitted diseases: a mechanism for enhanced HIV-1 transmission? AIDS. 1999;13:327–332. doi: 10.1097/00002030-199902250-00004. [DOI] [PubMed] [Google Scholar]
- 31.Yasodhara P, Raghunath M, Sreeramulu D, Venu L, Hemalatha R, Krishna TP. Local immunity in Indian women with bacterial vaginosis. J Reprod Immunol. 2006;70:133–141. doi: 10.1016/j.jri.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 32.Boulton IC, Gray-Owen SD. Neisserial binding to CEACAM-1 arrests the activation and proliferation of CD4+ T lymphocytes. Nat Immunol. 2002;3:229–236. doi: 10.1038/ni769. [DOI] [PubMed] [Google Scholar]
- 33.Fichorova RN, Desai PJ, Gibson FC, III, Genco CA. Distinct proinflammatory host responses to Neisseria gonorrhoeae infection in immortalized human cervical and vaginal epithelial cells. Infect Immun. 2001;69:5840–5848. doi: 10.1128/IAI.69.9.5840-5848.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]