Abstract
The treatment of patients with relapsed and refractory Hodgkin lymphoma (HL), especially those who relapse after autologous stem cell transplantation, remains challenging. Patients with HL whose disease relapses after stem cell transplantation are rarely cured with current treatment modalities, and have a median survival is less than 3 years. With no new drugs have been approved by the FDA for HL in more than three decades, there is a clear unmet medical need for drug development for this patients population. New treatment strategies that are based on targeting oncogenic signaling pathways are currently explored. This review will focus on emerging new treatment modalities that are currently under investigation for patients with relapsed classical HL.
Keywords: Reed Sternberg, Brentuximab vedotin, SGN-35, Panobinostat, HADC, Everolimus, mTOR, Rituximab
Hodgkin lymphoma (HL) is a rare human B cell lymphoid cancer representing 11.4% of all lymphomas in the United States.1 The treatment of HL has been evolved over the past three decades, and modern therapy is expected to successfully cure more than 80% of the patients. Despite this rather rare successful achievement in medical oncology, the current treatment continues to lack specificity and to induce unacceptable long-term toxicities that paradoxically shortens patients survival. Furthermore, patients who are not cured with front-line or second-line therapy, including stem cell transplantation, have an estimated median survival of less than 3 years.2 As the median age of this patient population is the mid-30s, the impact of early mortality on the number of years lost from productive life is more significant than many other cancers. However, because HL is a rare cancer that is highly curable, the development of new drugs for the treatment of HL has been very slow. Clearly, drug development in this area will address a significant unmet medical need.3 With recent advances in our understanding of HL pathology, biology, and immunology, several therapeutic targets have been identified and are currently under preclinical and clinical investigation.3,4 The aim of drug development in HL is not only to further improve the cure rate, but also to decrease toxic effects of therapy. This review will focus on the most promising new drugs that are currently in clinical trials for the treatment of patients with relapsed classical HL.
Brentuximab vedotin (SGN-35)
The dense expression of CD30 by HRS cells coupled with its highly restricted expression makes it an obvious target for therapeutic monoclonal antibody.5,6 Results from two clinical studies using first-generation naked anti-CD30 monoclonal antibodies in patients with relapsed HL have been disappointing, perhaps reflecting their poor antigen binding and/or effector cell activation properties (Table 1).7,8 In an alternate strategy, the anti-CD30 antibody cAC10 was conjugated to a synthetic anti-microtubule agent, monomethyl auristatin E (MMAE), resulting in a novel immunotoxin conjugate brentuximab vedotin (SGN-35).9 Brentuximab vedotin was recently evaluated in two phase I clinical trials in patients with relapsed HL and ALCL. In the first phase I study, brentuximab vedotin was administered on every three weeks schedule. Forty-five patients with relapsed HL and anaplastic large cell lymphoma (ALCL) were treated with escalating doses (0.1 to 3.6 mg/kg) by intravenous infusions every three weeks. The treatment was reasonably well tolerated, with neutropenia and hyperglycemia being dose limiting toxicities. Neuropathy was also observed in some patients, especially after repeated dosing. Remarkably, 88% of the patients demonstrated tumor reductions, of whom 17 (37%) achieved partial or complete remissions.10 In a second phase-I study, 37 patients (31 with HL) were treated with brentuximab vedotin that was administered on a weekly schedule for 3 consecutive weeks in four-week cycles. Dose-limiting toxicities included grade 3 gastrointestinal toxicity and grade 4 hyperglycemia. The overall response rate was 46% (29% CRs). 11 Based on these encouraging results, a pivotal phase II trial recently completed enrollment of 104 patients treated using 1.8 mg/kg given every three weeks.
Table 1.
HDACs indicates histone deacetylases; mTOR, mammalian target of rapamycin.
| Agent | Target | Route | Phase | N | PR | CR | PR + CR | 1st author |
|---|---|---|---|---|---|---|---|---|
| MDX060 [7] | CD30 | IV | II | 47 | 2 | 2 | 4 (8%) | Ansell |
| SGN30 [8] | CD30 | IV | II | 38 | 0 | 0 | 0 (0%) | Forrero-Torres |
| SGN35 [10] | CD30 | IV | I | 45 | 10 | 7 | 17 (37%) | Younes |
| SGN35 [11] | CD30 | IV | I | 31 | 6 | 10 | 16 (46%) | Fanale |
| Panobinostat [25] | HDACs | Oral | II | 13 | 7 | 0 | 7 (58%) | Dickinson |
| Panobinostat [26] | HDACs | Oral | II | 129 | 29 | 4 | 33 (26%) | Sureda |
| MGCD0103 [30] | HDACs | Oral | II | 21 | 6 | 2 | 8 (38%) | Bociek |
| Vorinostat [31] | HDACs | Oral | II | 25 | 1 | 0 | 1 (4%) | Kirshbaum |
| Everolimus [45] | mTOR | Oral | II | 19 | 8 | 1 | 9 (47%) | Johnston |
| Lenalidomide [47] | ? | Oral | II | 35 | 5 | 1 | 6 (17%) | Fehniger |
| Lenalidomide [48] | ? | Oral | II | 15 | 2 | 0 | 2 (13%) | Kuruvilla |
Panobinostat and other Histone Deacetylases (HDAC) Inhibitors
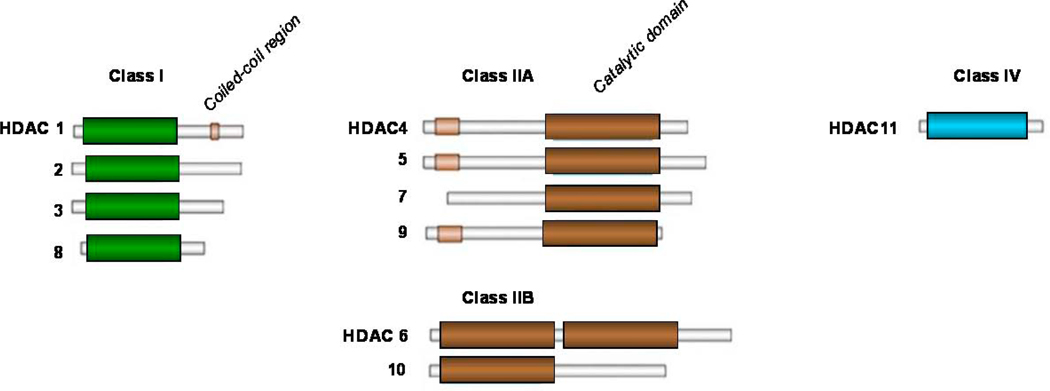
Post-transcriptional histone modification plays an important role in regulating gene transcription and is mediated by a several of enzymes, including histone acetyltransferases (HATs) and histone deacetylation (HDACs).12 These enzymes mediate acetylation and deacetylation of specific lysine amino acid residues on histone and non-histone proteins that regulate variety of proteins that are involved in cell proliferation, survival, angiogenesis, and immunity.13–15 To date, 18 HDACs have been identified in humans, and are grouped in two major categories: zinc-dependent HDACs and NAD-dependent HDACs.16,17 Furthermore, HDACs are classified into four major classes: Class I (HDAC 1, 2, 3, 8, and 11); Class II (HDAC 4, 5, 6, 7, 9, and 10); Class III (SIRT 1–7), and Class IV (HDAC 11) (Figure 2). Class III is NAD-dependent, whereas classes I, II, and IV are zinc dependent. At the present time, several clinical grade pharmacologic inhibitors of the zinc-dependent HDACs are available for clinical trials, but only two inhibitors, vorinostat and romidepsin has been approved by the FDA for treating relapsed cutaneaus T-cell lymphoma. Vorinostat (SAHA) and panobinostat (LBH589) inhibit HDAC classes I and II (pan-HDAC inhibitors). MGCD0103 and entinostat (SNDX-275, formerly MS-275) preferentially inhibit class I HDACs (isotype selective HDAC inhibitors).
Figure 2.
There are several rationales for using HDAC inhibitors (HDACis) for the treatment of HL. For example, although HRS cells are of B-cell origin, they infrequently express B-cell antigens.18 This loss of B-cell phenotype has been reported to be epigenetically regulated and may be therapeutically reversible.19,20 Several HDAC inhibitors have antiproliferative activity in HL-derived cell lines in vitro. In a recent study, vorinostat was shown to induce cell cycle arrest and apoptosis in HL cell lines and to synergize with chemotherapy.21 Similarly, the isotype-selective MGCD0103 has a potent anti-lymphoma activity by modulating the expression of a variety of survival proteins and provides mechanistic rationale for combining class-I HDAC inhibitors with proteasome inhibitors and TRAIL.22 In vitro experiments with entinostat (SNDX-275) demonstrated that this HDACi has dual antiproliferative effect by downregulation of XIAP and induction of apoptosis, and possibly by modulation the immune response.23 Furthermore, vorinostat inhibited STAT6 phosphorylation and transcription in HL cell lines, an effect that was associated with a decrease in the expression and secretion of Th2-type cytokines and chemokines, including thymus and activation-regulated chemokine (TARC/CCL17) and IL-5, and an increase in Th1-type cytokines/chemokines, including a profound increase in IP-10 levels.21 HDACis, alone or in combination with hypomethylating agents, have been shown to induce cancer testis antigen (CTA) expression, including MAGE, SSX, and NY-ESO-1 family members in a variety of tumors, and therefore may induce favorable anti-tumor immune response in vivo.24
Clinically, the leading HDACi compound in HL is panobinostat (LBH589) (Table 1). Based on promising results from a phase I study that included 13 patients with relapsed HL,25 a large pivotal international phase II study was initiated to confirm these results. Oral panobinostat is administered at a dose of 40 mg three times per week, every week, in 21-day cycles. Dose delays and modifications for management of adverse events was permitted, but the lowest doses allowed on study was 20 mg. Efficacy was evaluated every 2 cycles by imaging studies. Remarkably, patients were enrolled in less than one year. The median age was 32 years (18–75), and the median number of prior chemotherapeutic regimens was 4 (1–7). Importantly, the median time to relapse after first ASCT was only 8 months, which represents a poor prognostic indicator. Moreover, 37% of the patients did not respond to their last prior therapy. Twelve patients also received prior allogeneic transplant. In preliminary efficacy analysis, 129 patients were evaluable for response or discontinued early. A total of 33 responses (4CR + 29PR) with an ORR of 26% and a disease-control rate (CR + PR + SD) of 86% were observed. As many patients continue to actively receive therapy, longer follow up will be required to determine PFS. Common drug-related Grade 1/2 adverse effects were diarrhea, nausea, fatigue, vomiting, and anorexia. Common drug-related Grade 3/4 adverse events were thrombocytopenia, anemia, and neutropenia. The thrombocytopenia was manageable and reversible with dose hold and modification.26
MGCD0103 is a novel oral nonhydroxymate benzamide-based HDAC inhibitor that selectively inhibits HDAC 1 and 2 (and to a lesser extent, 3 and 11) isoforms.27 Its IC50 for inhibiting recombinant HDAC1 activity is 0.082 mM compared with > 30 mM for HDAC6.28,29 The safety and efficacy of MGCD0103 given orally 3 times per week (85 mg to 110 mg starting doses) was recently evaluated in a phase II study in patients with relapsed and refractory HL. Patients were allowed to receive therapy for 1 year in the absence of disease progression or prohibitive toxicity. Of the 21 patients who were treated with 110 mg dose level, 8 (35%) patients achieved partial or complete remissions. However, this dose level was poorly tolerated resulting in dose interruptions and reductions, and discontinuation of therapy after a median of 4.5 months. Subsequently, the study was revised to allow a lower starting dose of 85 mg at the same schedule. Three of the 10 (30%) patients enrolled on the reduced dose achieved partial remissions. Furthermore, grade 3 and 4 toxicity (mainly fatigue, with no significant hematologic toxicity) was reduced to 20%. Overall, 80% of the 30 evaluable patients had some decrease in their tumor measurements. Although none of the patients developed significant EKG abnormalities, two patients developed significant pericardial effusions requiring discontinuation of therapy. Collectively, this data indicate that class I HDAC inhibitors have a potential therapeutic value in patients with HL.30 Finally, the Southwest Oncology Group (SWOG) conducted a phase II trial of vorinostat in patients with relapsed HL.31 Twenty-five patients were treated with 200 mg vorinostat given orally twice per day for 14 days every 21-day cycle. One patient (4%) achieved a partial remission.
Everolimus (RAD001)
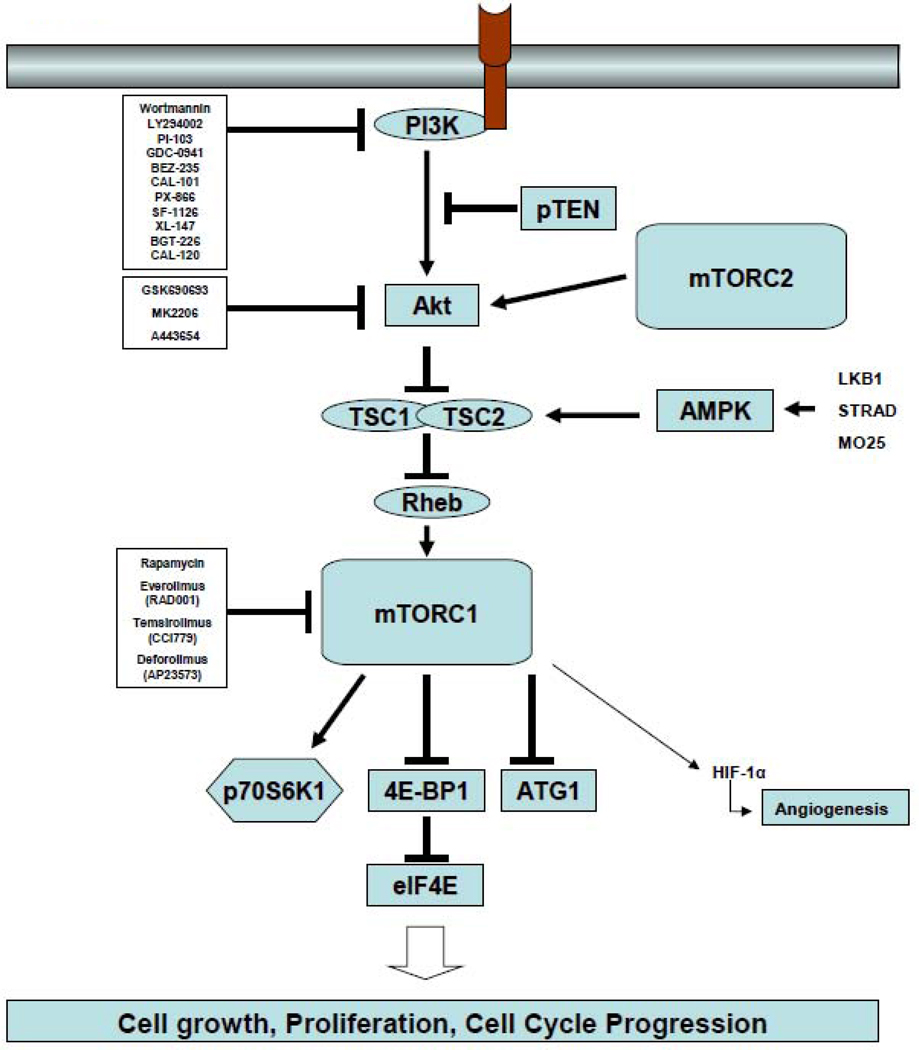
The phosphatidylinositol 3-kinase (PI3K)/Akt/mTOR signaling pathway (Figure 3) is one of the most aberrantly activated survival pathway in cancer, making it an important target for drug development.32,33 This pathway is negatively regulated by the tumor suppressor protein PTEN. Unlike most cancers, in which PI3K activation is frequently associated with PTEN deletion or mutation, other mechanisms have been reported to activate this pathway in HL, including activation of CD30, CD40, and RANK receptors, mutations in the p85a subunit of the PI3K, and inactivation of PTEN function by phosphorylation.34–39 In vitro experiments demonstrated that inhibition of PI3K, Akt, or mTOR by various small molecules can induce cell cycle arrest, autophagy, and apoptosis in HRS-derived cell lines in vitro.40–42 In addition to a direct antitumor effect, mTOR inhibitors may induce clinical responses by enhancing the immune response and inhibiting angiogenesis.43,44
Figure 3.
The therapeutic value of inhibiting the PI3K/Akt/mTOR axis has been recently studied using the oral mTOR inhibitor everolimus (Figure 3).45 Nineteen evaluable patients with relapsed HL were treated with daily doses of 10 mg everolimus, ORR rate was 47%, 8 patients achieved partial remission and 1 to complete remission (Table 1). Grade 3 adverse events included thrombocytopenia and anemia. If confirmed in a larger number of patients, everolimus may become one of the most active agents in relapsed HL. Because HRS cells frequently demonstrate aberrant and simultaneous activation of several survival pathways, including NF-κB, ERK, PI3K/Akt (Figure 3), rationally designed combination strategies will be required to improve the response rate and to prolong the response duration of mTOR inhibitors. In vitro experiments suggested that mTOR inhibitors may synergize with chemotherapy, PI3K inhibitors, and HDAC inhibitors in a variety of tumor models, including HL.40,46 A phase I clinical trial combining the HDAC inhibitor panobinostat with the mTOR inhibitor everolimus is currently enrolling patients with NHL and HL.
Lenalidomide
Two independent groups evaluated the safety and efficacy of lenalidomide in patients with relapsed HL. In the first study, Fehniger et al reported their experience with 25 mg/ day of lenalidomide on days 1–21 of a 28-day cycle.47 Treatment continued until progressive disease or an unacceptable adverse event. Despite the liberal dose reductions that were allowed for hematologic and non-hematologic toxicity, 6 of the 35 evaluable patients responded (1 CR and 5 PRs). Grade 3 and 4 neutropenia was observed in 40%, anemia (24%), leukopenia (21%), and thrombocytopenia was seen in 16% of the patients. In a second study, Kuruvilla and colleagues treated 15 patients with relapsed HL using the same dose and schedule of lenalidomide in the previous study.48 Two patients achieved PRs and 7 achieved stable disease, with a median time to progression of 3.2 months. Six patients discontinued therapy because of disease progression and 5 due to toxicity. Four patients developed grade 3 or 4 neutropenia and thrombocytopenia and 5 patients developed skin grade 1 or 2 skin rash. Collectively, these data suggest that lenalidomide has promising single-agent activity in relapsed HL. A French group conducted a small study of 8 patients with refractory or relapsed HL receiving ESAP salvage regimen in combination with lenalidomide. Response was evaluated by PET/CT. 7 CRs and 1 PRs were seen. The toxicity was essentially hematological, neutropenia and trombocytopenia.49
Rituximab
The anti-CD20 monoclonal antibody rituximab was evaluated in the treatment of patients with classical HL. Although CD20 antigen is infrequently expressed by HRS cells, it is highly expressed by the reactive B cells in the microenvironment. Thus, it was hypothesized that rituximab may induce clinical remissions in classical HL by depleting B cells from the microenvironment, by directly killing the few cases of CD20-expressing HRS cells, and perhaps by killing the putative HRS stem cells.50 In a pilot study, investigators from the M.D. Anderson Cancer Center treated 22 patients with relapsed classical HL with six weekly doses of rituximab, of whom 6 demonstrated CD20 expression by HRS cells.51 Five (22%) patients achieved partial or complete remissions, and 8 additional patients had stable disease. Clinical remissions were observed in patients regardless of CD20 expression by HRS cells, and were limited in patients whose disease was confined to the lymph nodes. In a follow-up study, the same investigators combined rituximab with ABVD (adriamycin, bleomycin, vinblasine, dacarbazine) chemotherapy to treat patients with newly diagnosed classical HL.52 Fifty-two patients with newly diagnosed classical HL were treated on a phase II study. With a median follow-up of 32 months, the estimated event-free survival (EFS) was 82% and overall survival 100%. Importantly, the EFS was improved for all risk categories: for patients with a prognostic score of 0 to 1 the EFS was 92%, for scores 0 to 2, 86%, and for scores 3 to 5, 73%. These data are currently being confirmed in a multicenter randomized study comparing ABVD with rituximab plus ABVD. In the final report of this study 104 patients with a median age of 35 years were enrolled in. With a median of 5 year follow up (6–94 months) the projected EFS for RABVD is 87% which is significantly better than institutional results with ABVD (p=0.0036). Improvement in EFS was observed with RABVD in patients with IPS 0–2 (EFS 89% vs. 71%, p=0.0248); and those with IPS >2 (80% vs. 55%; p=0.0532). This data serves as the rationale for a multicenter randomized trial comparing ABVD with RABVD in newly diagnosed patients with classical HL with stage III and IV and IPS >2. The study is currently enrolling patients.53
Proteasome inhibitors
NF-κB plays a central role in regulating the expression of various genes involved in cell survival, apoptosis, carcinogenesis, and inflammation, making it a potential therapeutic target.54 The NF-κB family is composed of five proteins: NF-κB1 (p50/p105), NF-B2 (p52/p100), RelA (p65), RelB, and c-Rel. These members exist as homodimers and heterodimers that are organized into two distinctive pathways: the classical (or cononical) and the alternative (non-cononical) pathways. Both pathways have shown to be activated in primary and cultured HRS cells of HL and to be involved in promoting HRS cell survival.4,55–58 In addition to autocrine and paracrine cytokine loops that can activate NF-κB in HRS cells, mutations in the IκB and A20 genes were also reported to be involved in the aberrant activation of NF-κB in HRS cells.4,59,60 The first attempt to therapeutically inhibit NF-κB activation in HL used the proteasome inhibitor bortezomib. By inhibiting the degradation of cytoplasmic IκBα, bortezomib inhibits the activation of NF-κB. Furthermore, bortezomib has been reported to alter the levels of p21, p27, Bcl-2, Bax, XIAP, survivin, and p53, leading to cell cycle arrest and apoptosis in several tumor types.61 In preclinical studies, bortezomib inhibited HL cell line proliferation and induced cell cycle arrest and apoptosis in a time-dependent and dose-dependent manner, and was effective even in HL cell lines that harbored mutations in the IκBα gene.62 Despite these favorable preclinical results, bortezomib demonstrated no significant clinical activity in patients with relapsed HL.63,64
Based on preclinical experiments that demonstrated synergy between bortezomib and chemotherapy, two independent groups evaluated bortezomib-based combinations in patients with relapsed classical HL. In the first study, a phase I trial was conducted to evaluate the combination of bortezomib with the ICE regimen.65 Escalating doses of bortezomib were given on days 1 and 4 of each ICE cycle. Twelve patients were enrolled, of whom 6 achieved PRs and 3 achieved CRs, for an overall response rate of 75%. Treatment was well tolerated and was associated with reversible grade 4 neutropenia and thrombocytopenia in 33% and 50% of the patients, respectively. Based on these encouraging data, a randomized phase II study comparing ICE with bortezomib plus ICE is currently enrolling patients to determine the contribution of bortezomib to the ICE regimen. In a second study, bortezomib was combined with gemcitabine for the treatment of patients with relapsed HL.66 Bortezomib 1 mg/ m2 was given on days 1, 4, 8, and 11, and gemcitabine 800 mg/m2 was given on days 1 and 8. Treatments were repeated every 21 days. The overall response rate in 18 patients was 22%. However, because of the relatively low response rate, coupled with treatment-related liver toxicity, the authors concluded that this regimen should not be further developed for the treatment of HL.
Summary and Future Directions
Several compounds have been identified as promising agents for the treatment of patients with relapsed classical HL. Brentuximab vedotin and panobinostat, are currently being examined in pivotal clinical trials seeking potential approval by the FDA. If approved, these compounds will be incorporated with conventional chemotherapy regimens that will likely change the standard of care for this disease. As more novel drugs are identified, future investigations should focus on identifying predictive markers that will lead to more personalized therapeutic approach.
Practice Points.
HL is a highly curable lymphoma, although treatment of patients with relapsed and refractory disease remains challenging
Cured patients frequently develop late toxic complications that may impact on their expected life span
No new drugs approved by FDA for HL in the past three decades
Brentuximab vedotin and panobinostat are the leading new compounds, and both completed pivotal trials in patients with relapsed HL
Figure 1.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
A. Younes declares the following conflicts of interest:
Research funding and honoraria from Genetech.
Research funding and honoraria from SBIO.
Research funding and honoraria from Novartis.
Research funding and honoraria from Seattle Genetics.
A. Jona has no conflicts of interest to declare.
References
- 1.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer Statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 2.Horning S, Fanale MSd. Defining a population of Hodgkin lymphoma patients for novel therapeutics: an international effort [abstract] Ann Oncol. 2008;20:118. [Google Scholar]
- 3.Buglio D, Georgakis G, A Y. Novel small-molecule therapy of Hodgkin lymphoma. Expert Rev Anticancer Ther. 2007;7:735–740. doi: 10.1586/14737140.7.5.735. [DOI] [PubMed] [Google Scholar]
- 4.Kuppers R. The biology of Hodgkin’s lymphoma. Nat Rev Cancer. 2009;9:15–17. doi: 10.1038/nrc2542. [DOI] [PubMed] [Google Scholar]
- 5.Younes A, Carbone A. CD30/CD30 ligand and CD40/ CD40 ligand in malignant lymphoid disorders. Int J Biol Markers. 1999;14:135–143. doi: 10.1177/172460089901400303. [DOI] [PubMed] [Google Scholar]
- 6.Younes A, Aggarwall BB. Clinical implications of the tumor necrosis factor family in benign and malignant hematologic disorders. Cancer. 2003;98:458–467. doi: 10.1002/cncr.11524. [DOI] [PubMed] [Google Scholar]
- 7.Ansell SM, Horwitz SM, Engert A. Phase I/II study of an anti-CD30 monoclonal antibody (MDX-60) in Hodgkin’s lymphoma and anaplastic large-cell lymphoma. J Clin Oncol. 2007;25:2764–2769. doi: 10.1200/JCO.2006.07.8972. [DOI] [PubMed] [Google Scholar]
- 8.Forero-Torres A, Leonard JP, Younes A, et al. A Phase II study of SGN-30 (anti-CD30 mAb) in Hodgkin lymphoma or systemic anaplastic large cell lymphoma. Br J Haematol. 2009;146:171–179. doi: 10.1111/j.1365-2141.2009.07740.x. [DOI] [PubMed] [Google Scholar]
- 9.Oflazoglu E, Kissler KM, Sievers EL, Grewal IS, Gerber HP. Combination of the anti-CD30-auristatin-E antibody-drug conjugate (SGN-35) with chemo-therapy improves antitumour activity in Hodgkin lymphoma. Br J Haematol. 2008;142:69–73. doi: 10.1111/j.1365-2141.2008.07146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Younes A, Forero-Torres A, Bartlett NL, et al. Multiple Complete Responses in a Phase 1 Dose-Escalation Study of the Antibody-Drug Conjugate SGN-35 in Patients with Relapsed or Refractory CD30-Positive Lymphomas. Blood (ASH Annual Meeting Abstracts) 2008;112:1006. [Google Scholar]
- 11.Fanale M, Bartlett NL, Forero-Torres A, et al. The Antibody-Drug Conjugate Brentuximab Vedotin (SGN-35) Induced Multiple Objective Responses in Patients with Relapsed or Refractory CD30-Positive Lymphomas in a Phase 1 Weekly Dosing Study. Blood (ASH Annual Meeting Abstracts) 2009;114:2731. [Google Scholar]
- 12.Glozak MA, Seto E. Histone deacetylases and cancer. Oncogene. 2007;26:5420–5432. doi: 10.1038/sj.onc.1210610. [DOI] [PubMed] [Google Scholar]
- 13.Heider U, Kaiser M, Sterz J. Histone deacetylase inhibitors reduce VEGF production and induce growth suppression and apoptosis in human mantle cell lymphoma. Eur J Haematol. 2006;76:42–50. doi: 10.1111/j.1600-0609.2005.00546.x. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Yan-Neale Y, Cai R, Alimov I, D C. Activation of mitochondrial pathway is crucial for tumor selective induction of apoptosis by LAQ824. Cell Cycle. 2006;5:1662–1668. doi: 10.4161/cc.5.15.3099. [DOI] [PubMed] [Google Scholar]
- 15.Brogdon JL, Xu Y, Szabo SJ. Histone deacetylase activities are required for innate immune cell control of Th1 but not Th2 effector cell function. Blood. 2007;109:1123–1130. doi: 10.1182/blood-2006-04-019711. [DOI] [PubMed] [Google Scholar]
- 16.Bolden JE, Peart MJ, Johnstone RW. Anticancer activities of histone deacetylase inhibitors. Nat Rev Drug Discov. 2006;5:769–784. doi: 10.1038/nrd2133. [DOI] [PubMed] [Google Scholar]
- 17.Minucci S, Pelicci PG. Histone deacetylase inhibitors and the promise of epigenetic (and more) treatments for cancer. Nat Rev Cancer. 2006;6:38–51. doi: 10.1038/nrc1779. [DOI] [PubMed] [Google Scholar]
- 18.Schwering I, Brauninger A, Klein U. Loss of the B-lineage-specific gene expression program in Hodgkin and Reed-Sternberg cells of Hodgkin lymphoma. Blood. 2003;101:1505–1512. doi: 10.1182/blood-2002-03-0839. [DOI] [PubMed] [Google Scholar]
- 19.Ushmorov A, Ritz O, Hummel M. Epigenetic silencing of the immunoglobulin heavy-chain gene in classical Hodgkin lymphoma-derived cell lines contributes to the loss of immunoglobulin expression. Blood. 2004;104:3326–3334. doi: 10.1182/blood-2003-04-1197. [DOI] [PubMed] [Google Scholar]
- 20.Ushmorov A, Leithauser F, Sakk O. Epigenetic processes play a major role in B-cell-specific gene silencing in classical Hodgkin lymphoma. Blood. 2006;107:2493–2500. doi: 10.1182/blood-2005-09-3765. [DOI] [PubMed] [Google Scholar]
- 21.Buglio D, Georgiakis GV, Hanabuchi S. Vorinostat inhibits STAT6-mediated TH2 cytokine and TARC production and induces cell death in Hodgkin lymphoma cell lines. Blood. 2008;112:1424–1433. doi: 10.1182/blood-2008-01-133769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buglio D, Mamidipudi V, Khaskhely NM, et al. The Histone Deacetylase Inhibitor MGCD0103 Down Regulates CD30, Activates NF-Kb, and Synergizes with Proteasome Inhibitors by HDAC6 Independent Mechanism in Hodgkin Lymphoma. Blood (ASH Annual Meeting Abstracts) 2009;114:3735. [Google Scholar]
- 23.Khaskhely NM, Buglio D, Shafer J, Bollard CM, Younes A. The Histone Deacetylase (HDAC) Inhibitor Entinostat (SNDX-275) Targets Hodgkin Lymphoma through a Dual Mechanism of Immune Modulation and Apoptosis Induction. Blood (ASH Annual Meeting Abstracts) 2009;114:1562. [Google Scholar]
- 24.Shichijo S, Yamada A, Sagawa K. Induction of MAGE genes in lymphoid cells by the demethylating agent 5-aza-2'-deoxycytidine. Jpn J Cancer Res. 1996;87:751–756. doi: 10.1111/j.1349-7006.1996.tb00288.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dickinson M, Ritchie D, DeAngelo DJ, et al. Preliminary evidence of disease response to the pan deacetylase inhibitor panobinostat (LBH589) in refractory Hodgkin Lymphoma. Br J Haematol. 2009;147:97–101. doi: 10.1111/j.1365-2141.2009.07837.x. [DOI] [PubMed] [Google Scholar]
- 26.Sureda A, Engert A, Browett PJ, et al. Interim results for the phase II study of panobinostat (LBH589) in patients (Pts) with relapsed/refractory Hodgkin's lymphoma (HL) after autologous hematopoietic stem cell transplant (AHSCT) ASCO Meeting Abstracts. 2010;28:8007. [Google Scholar]
- 27.Khan N, Jeffers M, Kumar S. Determination of the class and isoform selectivity of small-molecule histone deacetylase inhibitors. Biochem J. 2008;409:581–589. doi: 10.1042/BJ20070779. [DOI] [PubMed] [Google Scholar]
- 28.Beckers T, Burkhardt C, Wieland H. Distinct pharmacological properties of second generation HDAC inhibitors with the benzamide or hydroxamate head group. Int J Cancer. 2007;121:1138–1148. doi: 10.1002/ijc.22751. [DOI] [PubMed] [Google Scholar]
- 29.Riester D, Hildmann C, Grunewald S, Beckers T, Schwienhorst A. Factors affecting the substrate specificity of histone deacetylases. Biochem Biophys Res Commun. 2007;357:439–445. doi: 10.1016/j.bbrc.2007.03.158. [DOI] [PubMed] [Google Scholar]
- 30.Bociek RG, Kuruvilla J, Pro B, et al. Isotype-selective histone deacetylase (HDAC) inhibitor MGCD0103 demonstrates clinical activity and safety in patients with relapsed/refractory classical Hodgkin Lymphoma (HL) ASCO Meeting Abstracts. 2008;26:8507. [Google Scholar]
- 31.Kirschbaum MH, Goldman BH, Zain JM. Vorinostat (suberoylanilide hydroxamic acid) in relapsed or refractory Hodgkin lymphoma. SWOG 0517 [abstract]. Blood. 2007;110:2574. [Google Scholar]
- 32.Ihle NT, Powis G. Take your PIK: phosphatidylinositol 3-kinase inhibitors race through the clinic and toward cancer therapy. Mol Cancer Ther. 2009;8:1–9. doi: 10.1158/1535-7163.MCT-08-0801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Franke TF. PI3K/Akt: getting it right matters. Oncogene. 2008;27:6473–6488. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- 34.Georgakis GV, Yazbeck VY, Li Y, Younes A. Preclinical rationale for therapeutic targeting of mTOR by CC-I779 and rapamycin in Hodgkin lymphoma [abstract] Proc ASCO. 2006;24:10070. [Google Scholar]
- 35.Jucker M, Sudel K, Horn S. Expression of a mutated form of the p85alpha regulatory subunit of phosphatidylinositol 3-kinase in a Hodgkin’s lymphoma-derived cell line (CO) Leukemia. 2002;16:894–901. doi: 10.1038/sj.leu.2402484. [DOI] [PubMed] [Google Scholar]
- 36.Morrison JA, Gulley ML, Pathmanathan R, Raab-Traub N. Differential signaling pathways are activated in the Epstein-Barr virus-associated malignancies nasopharyngeal carcinoma and Hodgkin lymphoma. Cancer Res. 2004;64:5251–5260. doi: 10.1158/0008-5472.CAN-04-0538. [DOI] [PubMed] [Google Scholar]
- 37.Nagel S, Scherr M, Quentmeier H. HLXB9 activates IL6 in Hodgkin lymphoma cell lines and is regulated by PI3K signalling involving E2F3. Leukemia. 2005;19:841–846. doi: 10.1038/sj.leu.2403716. [DOI] [PubMed] [Google Scholar]
- 38.Renne C, Willenbrock K, Martin-Subero JI. High expression of several tyrosine kinases and activation of the PI3K/AKT pathway in mediastinal large B cell lymphoma reveals further similarities to Hodgkin lymphoma. Leukemia. 2007;21:780–787. doi: 10.1038/sj.leu.2404594. [DOI] [PubMed] [Google Scholar]
- 39.Dutton A, Reynolds GM, Dawson CW, Young LS, PG M. Constitutive activation of phosphatidyl-inositide 3 kinase contributes to the survival of Hodgkin’s lymphoma cells through a mechanism involving Akt kinase and mTOR. J Pathol. 2005;205:498–506. doi: 10.1002/path.1725. [DOI] [PubMed] [Google Scholar]
- 40.Georgakis GV, Yazbeck VY, Li Y, Younes A. The mTOR inhibitor temsirolimus (CCI-779) induces cell cycle arrest and autophagy in Hodgkin lymphoma (HL) cell lines and enhances the effect of the PI3-kinase inhibitor LY294002 [abstract] Blood. 2006;108:2259. [Google Scholar]
- 41.Georgakis GV, Li Y, Rassidakis GZ, Medeiros LJ, Mills GB, Younes A. Inhibition of the phosphatidylinositol-3 kinase/Akt promotes G1 cell cycle arrest and apoptosis in Hodgkin lymphoma. Br J Haematol. 2006;132:503–511. doi: 10.1111/j.1365-2141.2005.05881.x. [DOI] [PubMed] [Google Scholar]
- 42.Jundt F, Raetzel N, Muller C. A rapamycin derivative (everolimus) controls proliferation through down-regulation of truncated CCAAT enhancer binding protein {beta} and NF-{kappa}B activity in Hodgkin and anaplastic large cell lymphomas. Blood. 2005;106:1801–1807. doi: 10.1182/blood-2004-11-4513. [DOI] [PubMed] [Google Scholar]
- 43.Zheng Y, Collins SL, Lutz MA. A role for mammalian target of rapamycin in regulating T cell activation versus anergy. J Immunol. 2007;178:2163–2170. doi: 10.4049/jimmunol.178.4.2163. [DOI] [PubMed] [Google Scholar]
- 44.Del Bufalo DCL, Trisciuoglio D. Antiangiogenic potential of the mammalian target of rapamycin inhibitor temsirolimus. Cancer Res. 2006;66:5549–5554. doi: 10.1158/0008-5472.CAN-05-2825. [DOI] [PubMed] [Google Scholar]
- 45.Johnston PB, Inwards DJ, Colgan JP, et al. A Phase II trial of the oral mTOR inhibitor everolimus in relapsed Hodgkin lymphoma. Am J Hematol. 2010 doi: 10.1002/ajh.21664. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yazbeck VY, Buglio D, Georgakis GV. Temsirolimus downregulates p21 without altering cyclin D1 expression and induces autophagy and synergizes with vorinostat in mantle cell lymphoma. Exp Hematol. 2008;36:443–450. doi: 10.1016/j.exphem.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 47.Fehniger TA, Larson S, Trinkaus K, et al. A Phase II Multicenter Study of Lenalidomide in Relapsed or Refractory Classical Hodgkin Lymphoma. Blood (ASH Annual Meeting Abstracts) 2009;114:3693. doi: 10.1182/blood-2011-07-362475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuruvilla J, Taylor D, Wang L, Blattler C, Keating A, Crump M. Phase II trial of lenalidomide in patients with relapsed or refractory Hodgkin lymphoma [abstract] Blood. 2008;112:3052. [Google Scholar]
- 49.Tempescul A, Ianotto JC, Guillerm G, Berthou C. ESAP-Lenalidomide - a Highly Active Regimen in Refractory or Relapsed Hodgkin's Lymphoma. Blood (ASH Annual Meeting Abstracts) 2009;114:4797. [Google Scholar]
- 50.Jones RJ, Gocke CD, Kasamon YL. Circulating clonotypic B cells in classic Hodgkin lymphoma. Blood. 2009;113:5920–5926. doi: 10.1182/blood-2008-11-189688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Younes A, Romaguera J, Hagemeister F. A pilot study of rituximab in patients with recurrent, classic Hodgkin disease. Cancer. 2003;98:310–314. doi: 10.1002/cncr.11511. [DOI] [PubMed] [Google Scholar]
- 52.Wedgwood AR, Fanale MA, Fayad LE. Rituximab + ABVD improves event-free survival (EFS) in patients with classical Hodgkin lymphoma in all International Prognostic Score (IPS) groups and in patients who have PET positive disease after 2–3 cycles of therapy [abstract] Blood. 2007;110:215. [Google Scholar]
- 53.Copeland AR, Cao Y, Fanale M, et al. Final Report of a Phase-II Study of Rituximab Plus ABVD for Patients with Newly Diagnosed Advanced Stage Classical Hodgkin Lymphoma.: Results of Long Follow up and Comparison to Institutional Historical Data. Blood (ASH Annual Meeting Abstracts) 2009;114:1680. [Google Scholar]
- 54.Baud V, Karin M. Is NF-kappaB a good target for cancer therapy? Hopes and pitfalls. Nat Rev Drug Discov. 2009;8:33–40. doi: 10.1038/nrd2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Younes A, Garg A, BB A. Nuclear transcription factor-kappa B in Hodgkin’s disease. Leuk Lymphoma. 2003;44:929–935. doi: 10.1080/1042819031000067558. [DOI] [PubMed] [Google Scholar]
- 56.Staudt LM. The molecular and cellular origins of Hodgkin’s disease. J Exp Med. 2000;191:207–212. doi: 10.1084/jem.191.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bargou RC, Leng C, Krappmann D. High-level nuclear NF-kappa B and Oct-2 is a common feature of cultured Hodgkin/Reed-Sternberg cells. Blood. 1996;87:4340–4347. [PubMed] [Google Scholar]
- 58.Bargou RC, Emmerich F, Krappmann D. Constitutive nuclear factor-kappaB-RelA activation is required for proliferation and survival of Hodgkin’s disease tumor cells. J Clin Invest. 1997;100:2961–2969. doi: 10.1172/JCI119849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kato M, Sanada M, Kato I. Frequent inactivation of A20 in B-cell lymphomas. Nature. 2009;459:712–716. doi: 10.1038/nature07969. [DOI] [PubMed] [Google Scholar]
- 60.Schmitz R, Hansmann ML, Bohle V. TNFAIP3 (A20) is a tumor suppressor gene in Hodgkin lym-phoma and primary mediastinal B cell lymphoma. J Exp Med. 2009;206:981–989. doi: 10.1084/jem.20090528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Adams J. Potential for proteasome inhibition in the treatment of cancer. Drug Discov Today. 2003;8:307–315. doi: 10.1016/s1359-6446(03)02647-3. [DOI] [PubMed] [Google Scholar]
- 62.Zheng B, Georgakis GV, Li Y. Induction of cell cycle arrest and apoptosis by the proteasome inhibitor PS-341 in Hodgkin disease cell lines is independent of inhibitor of nuclear factor-kappaB mutations or activation of the CD30, CD40, and RANK receptors. Clin Cancer Res. 2004;10:3207–3215. doi: 10.1158/1078-0432.ccr-03-0494. [DOI] [PubMed] [Google Scholar]
- 63.Younes A, Pro B, Fayad L. Experience with bortezomib for the treatment of patients with relapsed classical Hodgkin lymphoma. Blood. 2006;107:1731–1732. doi: 10.1182/blood-2005-09-3731. [DOI] [PubMed] [Google Scholar]
- 64.Blum KA, Johnson JL, Niedzwiecki D, Canellos GP, Cheson BD, Bartlett NL. Single agent bortezomib in the treatment of relapsed and refractory Hodgkin lymphoma: cancer and leukemia Group B protocol 50206. Leuk Lymphoma. 2007;48:1313–1319. doi: 10.1080/10428190701411458. [DOI] [PubMed] [Google Scholar]
- 65.Fanale MA, Fayad LE, Pro B. A phase I study of bortezomib in combination with ICE (BICE) in patients with relapsed/refractory classical Hodgkin lymphoma [abstract] Blood. 2008;112:3048. [Google Scholar]
- 66.Mendler JH, Kelly J, Voci S. Bortezomib and gemcitabine in relapsed or refractory Hodgkin’s lymphoma. Ann Oncol. 2008;19:1759–1764. doi: 10.1093/annonc/mdn365. [DOI] [PMC free article] [PubMed] [Google Scholar]