Abstract
Purpose
To evaluate changes in clinical and pathological characteristics of prostate cancer in patients treated surgically at a large tertiary care center in the context of increased use of active surveillance (AS) and minimally invasive surgery (MIS).
Materials
We performed retrospective review of 6,624 patients with localized prostate cancer who underwent radical prostatectomy from 2000–2010 at Memorial Sloan-Kettering Cancer Center. Patients were stratified by surgical approach (open, laparoscopic or robotic) and risk categories (low, intermediate, or high). Patients with low-risk disease, without intervention and minimum followup of 6 months were considered to have elected AS.
Results
AS cases increased from <20 per year between 2000–2004 to ≥100 per year between 2007–2009. Over the same decade MIS cases (laparoscopic or robotic) increased from zero to 63% of all surgical cases. The percentage of patients in intermediate- and high-risk categories increased over time, while the percentage in the low-risk category decreased (OR per year 0.91, 95% CI 0.89, 0.92, p <0.0005). The proportion of surgery patients with Gleason 6 tumors decreased over time (OR per year 0.87, 95% CI 0.85, 0.88; p <0.0005) while pathologic stage and Gleason score increased (p <0.0005). The proportion of low-risk cases decreased across all types of surgery, with the largest decrease in robotic surgery (p <0.0005).
Conclusions
We observed a reverse stage shift in patients undergoing radical prostatectomy since 2000 despite the introduction and rapid proliferation of MIS. These findings may be due to increased use of AS and institutional focus on treatment of higher-risk disease.
Keywords: prostatic neoplasm, prostate cancer, risk, robotic, prostatectomy, active surveillance, minimally invasive surgery
Introduction
Prostate cancer is the most common cancer in men in the United States with 218,000 new diagnoses and 32,000 deaths each year.1 The great disparity between the high number of diagnoses and the smaller number of deaths strongly indicates that many men with prostate cancer are overtreated. While it is not clear specifically which patients need intervention or what type of intervention will be the most beneficial, it is broadly accepted that patients with higher-risk clinical features (high PSA level, more advanced clinical stage, or a Gleason score ≥ 7) are at greatest risk of metastases or death from their disease.2 Thus, it is these patients that preferentially need curative treatment for their disease.
It has been recognized that surgical intervention with curative intent for the treatment of localized prostate cancer has increased dramatically, perhaps by as much as 60% in recent years.3 This has been attributed, in large part, to increased use and marketing of robotic systems as a less invasive way of treating prostate cancer. Furthermore, it has been hypothesized that the increased use of surgery has been largely on patients with low-risk prostate cancer, who are less likely to benefit from intervention.3
Recognition that many men with prostate cancer are being overtreated has lead to increased use of AS as a method for carefully observing men with low-risk prostate cancer, with the intent to recognize progression and intervene if necessary.4 The increasing use of robotic systems and of AS are two competing factors that may have countervailing influences on the characteristics of men undergoing RP. In this paper, we aim to investigate how these trends might influence the clinical and pathologic characteristics of men undergoing RP at a large tertiary center.
Methods
We performed an institutional review board-approved retrospective review of 6,624 consecutive patients who underwent RP from 2000 – 2010 at MSKCC for localized prostate cancer. This time frame was chosen as it represents the contemporary era of prostate cancer care. Patients who had preoperative radiation therapy or androgen deprivation therapy were excluded from analysis. Patient data were collected and entered into a prospective prostate cancer database. The 6th edition of the AJCC TNM classification was used to define clinical stage, and histopathological grading was done according to the Gleason system.5 Biopsies performed at referring facilities were reviewed by MSKCC dedicated genitourinary pathologists.
Patients were stratified according to surgical approach: open radical prostatectomy (ORP), laparoscopic radical prostatectomy (LRP), robot-assisted laparoscopic prostatectomy (RALP). Patients were also stratified by National Comprehensive Cancer Network (NCCN) guidelines risk categories: low (PSA ≤10 ng/mL, ≤T2a, and Gleason score ≤6), intermediate (PSA 10-20 ng/mL, or T2b-T2c, or Gleason score 7) or high (PSA >20ng/mL, or ≥T3a, or Gleason score ≥8) risk.6 In a separate analysis, we identified patients who initiated AS at MSKCC from 2000 – 2010. For this study, AS patients were defined as those with low-risk prostate cancer who had been monitored at MSKCC for more than 6 months without any intervention.
Changes in clinical and pathologic features over time were analyzed using logistic or ordinal regression. Locally weighted scatterplot smoothing (lowess) methods were used to visualize the relationship between year of surgery and tumor characteristics. All analyses were conducted using Stata 11 (Stata Corp., College Station, Tx).
Results
RP for localized prostate cancer was performed on a total of 6,624 patients who met the inclusion criteria from 2000–2010 using the following surgical techniques, 4,145 (63%) by ORP, 1,759 (27%) by LRP, and 718 (11%) by RALP. Baseline characteristics are shown in Table 1. Treatment by ORP peaked in 2002 and has declined in recent years, while treatment by LRP (first performed in 2002) and RALP (first performed in 2005) have been increasing. Patients were stratified into low-, intermediate-, and high-risk categories with 2,836 (43%), 2,913 (44%), and 868 (13%) patients in each group, respectively. Seven patients (<1%) were missing clinical data and no risk category could be assigned. Over the course of study, the total number of RPs performed yearly by any approach doubled from fewer than 400 procedures in 2001 to more than 800 procedures in 2009.
PSA levels did not demonstrate any significant changes over time in either the total cohort (mean PSA 6.85 ng/mL) or in the individual risk groups. There was a reduction in clinical stage for the overall cohort with an increase in stage 1 tumors (55% in 2000 vs 71% in 2010) and a decrease in stage 2 tumors (45% in 2000 vs 29% in 2010) (p <0.0005 by ordinal logistic regression). The rates of clinical stage 3 tumors fluctuated between 2 and 5%.
For the entire group, the proportion of patients who fell into the intermediate- and high-risk categories increased, while the proportion of patients in the low-risk category decreased (OR per year for being at low risk 0.91, 95% CI 0.89, 0.92; p <0.0005) (Figure 1). This finding was consistent and independent of surgical technique indicating that the proportion of low-risk patients in the ORP, LRP, and RALP groups fell over time (Figure 2). The rate of change differed between groups (p <0.0005 by Cochran's Q test for heterogeneity), with RALP demonstrating the largest decrease in proportion of low-risk patients. Our principle result was unaffected after adjusting for BMI or race, which might have conceivably affected risk or treatment choice (OR 0.90; 95% C.I. 0.88, 0.92; p<0.0005).
Figure 1.
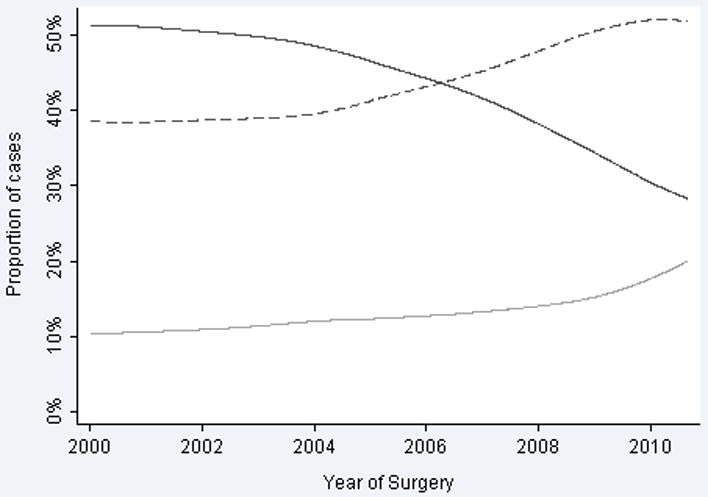
Proportion of radical prostatectomy cases classified by NCCN risk stratification. Solid line indicates low-risk. Dashed line indicates intermediate-risk. Grey line indicates high-risk.
Figure 2.
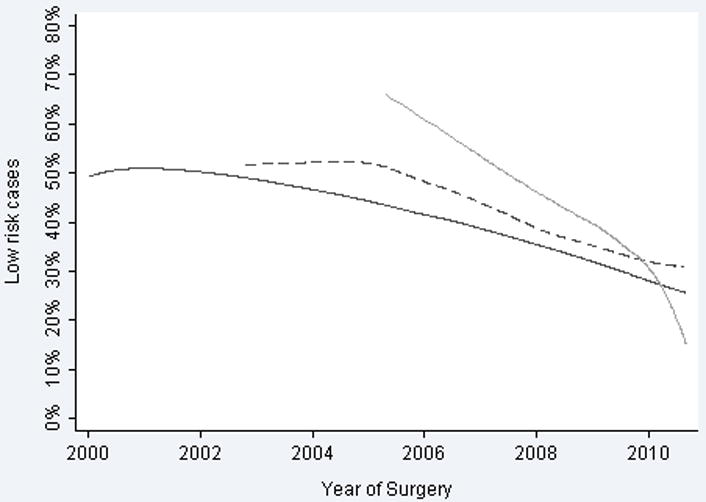
Proportion of radical prostatectomy cases classified as low-preoperative risk. Solid line indicates open radical prostatectomy. Dashed line indicates laparoscopic radical prostatectomy. Grey line indicates robotic-assisted laparoscopic prostatectomy.
Despite stable PSA and a decline in clinical stage, the proportion of patients in the higher NCCN risk categories rose due to higher Gleason scores over the study period (Figure 3). There was a dramatic decline in patients with Gleason 6 tumors receiving surgery (OR per year 0.87, 95% CI 0.85, 0.88; p <0.0005). In 2000, tumors with biopsy Gleason score of 6 or less accounted for 66% of patients undergoing RP, by 2010 this proportion had fallen to 32%. Gleason scores 3+4, 4+3, and 4+4 all increased in frequency over the period of study, although the most dramatic increase was in Gleason 3+4. Importantly, there were concurrent increases in pathologic grade (p <0.0005) and stage (p <0.0005); ≥pT3a increased from 26% of surgical cases in 2000 to 39% in 2010. Similarly, there was a decline in pT2 disease from 74% in 2000 to 60% in 2010 (Figure 4). AS cases increased dramatically over the course of the study, from fewer than 20 cases a year between 2000 and 2004 to 100 or more cases a year between 2007 and 2009 (Figure 5).
Figure 3.
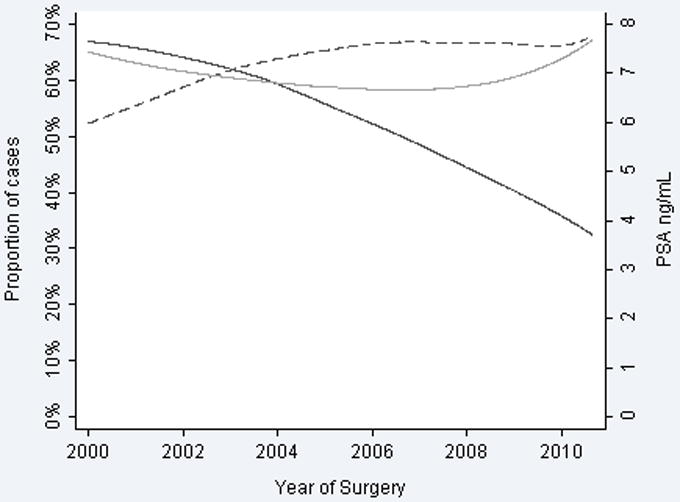
Biopsy features over time. Solid line indicates biopsy grade 6. Dashed line indicates T1c disease. Grey line indicates PSA.
Figure 4.
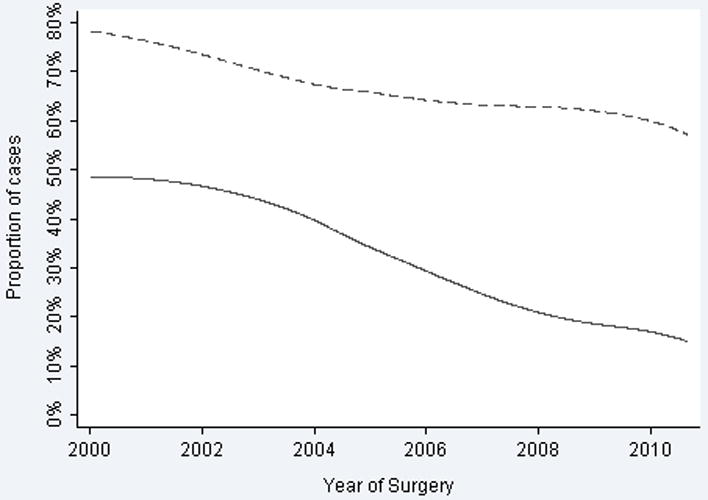
Pathologic features over time. Solid line indicates pathologic Gleason 6. Dashed line indicates organ-confined disease.
Figure 5.
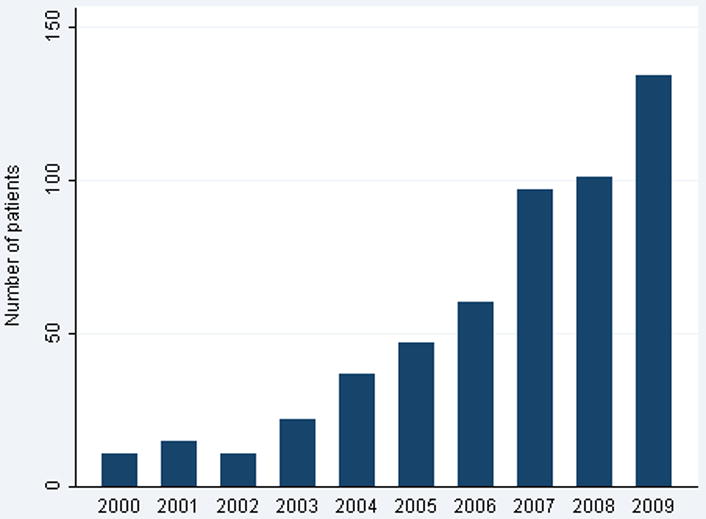
Number of patients enrolled into active surveillance by year.
Discussion
The widespread implementation of PSA screening during the 1990's led to earlier presentation of prostate cancer.7 The decrease in risk consequently observed in surgical cohorts is widely described as a stage shift.8 Since 2000 we have observed a reverse stage shift at MSKCC, with stage and grade increasing in a RP cohort over the years of study (Table 1). While overall surgical volume increased, a declining percentage of operations were performed in men with low-risk disease (Figure 1). This was observed across all three surgical approaches (Figure 2). While PSA remained relatively stable and clinical stage decreased slightly, biopsy Gleason tumors ≤6 declined dramatically, indicating that the increase in NCCN risk category was largely a result of an increase in Gleason score (Figure 3). This resulted in increased aggressive pathological features on RP specimens with a decline in organ-confined disease and a decrease in Gleason tumors ≤6 (Figure 4). While the reasons for these findings are multifactorial, a significant increase in the number of men electing AS over this time period suggests that AS is likely a factor in influencing the relative decrease of men with low-risk disease undergoing RP (Figure 5).
It has been suggested that RALP should be reserved for men with low-risk prostate cancer and that tactile sensation is required for higher-risk tumors.9, 10 Additionally, because robotic surgery is a rather novel field, a selection of patients with lower-risk disease might have been anticipated in this cohort. Interestingly this was not the case; there was a declining proportion of patients with low-risk prostate cancer who underwent RALP, similar to the decline seen with ORP (fig. 2). Thus, robotic surgeons selected patients with increasing risk based on institutional selection criteria and not technological bias, similar to surgeons using other approaches.
Studies suggest that upgrading of disease is largely the result of changes in pathologic criteria rather than changes in actual tumor biology.11 A consensus statement created by 80 urologic pathologists was released in late 2005 and may have influenced subsequent pathologic analyses of biopsy Gleason scores.12 Most notably this panel determined that in cases where there was a predominant low-grade pattern with an element of a higher-grade pattern, the pathologic grade should reflect the high-grade pattern. For example, if a needle biopsy entirely involved by cancer has a 98% Gleason pattern of 3 and 2% Gleason pattern of 4, the Gleason score would be 3+4=7.13 The panel's decision to include any high-grade pattern in the final score may not have been used by all pathologists prior to 2006, and it may be hypothesized to be responsible for Gleason score upgrading since 2006. Indeed in our study, Gleason scores >6 did increase after 2006; and the average proportion of Gleason scores ≥7 was 39% from 2000 to 2005 compared with 57% from 2006 to 2010. However, we find it unlikely that changes in grading are solely responsible for the changes in risk categorization we observed. Firstly, pathologic stage increased over time along with Gleason grade (Figure 4). Secondly, as the panel's recommendations reflected the evolution in Gleason grading over a number of years, many of these modifications were already employed at MSKCC prior to the 2005 report. Thirdly, we saw increases in Gleason pattern 4+3, 4+4, and 4+5 concomitant to increases in pattern 3+4. Fourthly, the number of patients with low-risk disease placed into AS cohorts dramatically increased over the study period, with the corresponding decrease in RP performed in patients with Gleason pattern 6. The totality of evidence suggests that the increase in risk seen in patients undergoing RP at MSKCC is not a result of isolated biopsy upgrading phenomenon but a specific and coordinated effort to identify men who are less likely to benefit from surgical intervention and minimize their exposure to that risk.
Within the study design we cannot definitively demonstrate why there has been a decline in the use of RP for men with low-risk prostate cancer. We have suggested that these declines may be due in large part to an increased use of AS for patients with low-risk disease over this same time period. However a weakness of this study is our inability to reject alternative possibilities such as increased use of radiotherapy or changing referral patterns. RP is the most common form of treatment for prostate cancer, more than double external beam radiotherapy and brachytherapy combined.14 Given the overall increase in the number of patients undergoing RP at our institution, we find it implausible that changes in referral patterns or radiation therapy are the sole reasons for the shift seen in this study; however the possibility cannot be excluded. This study is also limited because of its retrospective single institution nature and thus subject to all the inherent biases associated with such a design.
Because of the slow growth and indolent nature of prostate cancer, the majority of men who undergo RP do not benefit from this intervention.15 Increased awareness of the burden of overtreatment and greater understanding of the disease has lead to rising interest in AS as a reasonable alternative to RP for a subgroup of patients with low-risk disease.16 While AS is generally reserved for men with low risk prostate cancer, there is little uniformity amongst practitioners regarding the ideal characteristics for recommendation of AS, the definition of progression on AS, or the timing and intensity of follow up physical exams, PSA measurements and repeat biopsies for patients electing AS. Over the past decade at MSKCC we have undergone an evolution in our strategy for enrollment criteria in to AS. In recent years we have begun to recommend a repeat biopsy within 3 months of diagnosis for all patients with low risk, low volume prostate cancer electing AS. We have previously reported that 17% to 25% of these patients will be upgraded on repeat biopsy and are generally excluded from our AS cohort.17, 18 Once placed on an AS program about 40% of patients have progression of their disease within 5 years.18
Perhaps not surprisingly, by reducing the number of patients with low-risk disease in the cohort undergoing RP, the overall risk of the group that does undergo intervention is increased, as demonstrated in this study. Additionally, repeat biopsies in patients electing AS will result in upstaging in a minority of these patients and may further increase the overall risk of the group electing intervention. This will be a result of more accurate staging rather than any true change in risk. Finally as AS cohorts mature more patients will experience progression and then elect treatment, this may serve to increase the number of intermediate and high risk patients in treatment groups. Due to the increasing use of AS, physicians should be aware of these phenomena and recognize that patients remaining in operative cohorts will be at higher-risk than they had been previously.
In this study we the used the NCCN risk stratification schemas to broadly separate patients into low, intermediate and high risk categories. This schema which is largely similar to those offered by the American Urologic Association and the European Association of Urologists, broadly groups patients with divergent features into the same risk category.6, 19, 20 Also, all of these tools fail to incorporate important clinical factors such as volume of disease, patient's age, or comorbidity status. Thus while risk stratification schema maybe useful in determining treatment trends as in this study, clinical recommendations should be tailored for individual patients based on all available data.
We have demonstrated that at a high volume tertiary care center with significant increases in surgical volume, the proportion of men with low-risk prostate cancer undergoing RP has decreased, what we have termed as a reverse stage shift. This is true despite an evolution in surgical approach from open procedures to laparoscopic and robotic procedures. This finding is contrary to the suggestion of Barbash and Giled, that the introduction of MIS has resulted not only in an increase in surgical procedures for patients with prostate cancer but also “that robotic technology may have contributed to the substitution of surgical for nonsurgical treatments for this disease”.3 While our findings are limited to a single institution and may not be broadly reflective of US practice patterns, they would suggest that, at least at MSKCC, surgical approach is not a determining factor in temporal treatment trends.
Furthermore, the finding of a reverse stage shift appears largely independent of changes in pathological grading over the study period and demonstrates increasingly aggressive tumor biology in the patients selected to have surgery. As such, we believe our data are largely reflective of a selection preference for performing surgery in men with higher-risk prostate cancer and encouraging men with low-risk disease to consider AS. Further studies should be aimed at understanding how these selection practices impact long-term oncologic outcomes.
Acknowledgments
Attributions: Supported by the Sidney Kimmel Center for Prostate and Urologic Cancers and by funds provided by David H. Koch through the Prostate Cancer Foundation and by the National Cancer institute (NCI) (U54CA137788 and U54CA132378)
References
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60(5):277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.D'Amico AV, Desjardin A, Chung A, et al. Assessment of outcome prediction models for patients with localized prostate carcinoma managed with radical prostatectomy or external beam radiation therapy. Cancer. 1998;82(10):1887–96. [PubMed] [Google Scholar]
- 3.Barbash GI, Glied SA. New technology and health care costs--the case of robot-assisted surgery. N Engl J Med. 2010;363(8):701–4. doi: 10.1056/NEJMp1006602. [DOI] [PubMed] [Google Scholar]
- 4.Klotz L. Active surveillance with selective delayed intervention: using natural history to guide treatment in good risk prostate cancer. J Urol. 2004;172(5 Pt 2):S48–50. doi: 10.1097/01.ju.0000141712.79986.77. discussion S50-1. [DOI] [PubMed] [Google Scholar]
- 5.Greene FL American Joint Committee on Cancer., American Cancer Society. AJCC cancer staging manual. 6th. New York: Springer-Verlag; 2002. [Google Scholar]
- 6.Mohler J, Bahnson RR, Boston B, et al. NCCN clinical practice guidelines in oncology: prostate cancer. J Natl Compr Canc Netw. 2010;8(2):162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 7.Derweesh IH, Kupelian PA, Zippe C, et al. Continuing trends in pathological stage migration in radical prostatectomy specimens. Urol Oncol. 2004;22(4):300–6. doi: 10.1016/j.urolonc.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Brawley OW. Prostate carcinoma incidence and patient mortality: the effects of screening and early detection. Cancer. 1997;80(9):1857–63. doi: 10.1002/(sici)1097-0142(19971101)80:9<1857::aid-cncr26>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 9.Ellis WJ, Lange PH. Point: open radical prostatectomy should not be abandoned. J Natl Compr Canc Netw. 2007;5(7):685–8. doi: 10.6004/jnccn.2007.0058. [DOI] [PubMed] [Google Scholar]
- 10.Lee EK, Baack J, Duchene DA. Survey of practicing urologists: robotic versus open radical prostatectomy. Can J Urol. 2010;17(2):5094–8. [PubMed] [Google Scholar]
- 11.Kuroiwa K, Uchino H, Yokomizo A, Naito S. Impact of reporting rules of biopsy Gleason score for prostate cancer. J Clin Pathol. 2009;62(3):260–3. doi: 10.1136/jcp.2008.060632. [DOI] [PubMed] [Google Scholar]
- 12.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL. The 2005 International Society of Urological Pathology (ISUP) Consensus Conference on Gleason Grading of Prostatic Carcinoma. Am J Surg Pathol. 2005;29(9):1228–42. doi: 10.1097/01.pas.0000173646.99337.b1. [DOI] [PubMed] [Google Scholar]
- 13.Epstein JI, Allsbrook WC, Jr, Amin MB, Egevad LL. Update on the Gleason grading system for prostate cancer: results of an international consensus conference of urologic pathologists. Adv Anat Pathol. 2006;13(1):57–9. doi: 10.1097/01.pap.0000202017.78917.18. [DOI] [PubMed] [Google Scholar]
- 14.Cooperberg MR, Broering JM, Carroll PR. Time trends and local variation in primary treatment of localized prostate cancer. J Clin Oncol. 28(7):1117–23. doi: 10.1200/JCO.2009.26.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schroder FH, Hugosson J, Roobol MJ, et al. Screening and prostate-cancer mortality in a randomized European study. N Engl J Med. 2009;360(13):1320–8. doi: 10.1056/NEJMoa0810084. [DOI] [PubMed] [Google Scholar]
- 16.Cooperberg MR, Broering JM, Kantoff PW, Carroll PR. Contemporary trends in low risk prostate cancer: risk assessment and treatment. J Urol. 2007;178(3 Pt 2):S14–9. doi: 10.1016/j.juro.2007.03.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berglund RK, Masterson TA, Vora KC, Eggener SE, Eastham JA, Guillonneau BD. Pathological upgrading and up staging with immediate repeat biopsy in patients eligible for active surveillance. J Urol. 2008;180(5):1964–7. doi: 10.1016/j.juro.2008.07.051. discussion 67-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adamy A, Yee DS, Matsushita K, et al. Role of prostate specific antigen and immediate confirmatory biopsy in predicting progression during active surveillance for low risk prostate cancer. J Urol. 185(2):477–82. doi: 10.1016/j.juro.2010.09.095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heidenreich A, Bellmunt J, Bolla M, et al. EAU Guidelines on Prostate Cancer. Part 1: Screening, Diagnosis, and Treatment of Clinically Localised Disease. Eur Urol. 2010 doi: 10.1016/j.eururo.2010.10.039. [DOI] [PubMed] [Google Scholar]
- 20.Thompson I, Thrasher JB, Aus G, et al. Guideline for the management of clinically localized prostate cancer: 2007 update. J Urol. 2007;177(6):2106–31. doi: 10.1016/j.juro.2007.03.003. [DOI] [PubMed] [Google Scholar]


