Abstract
Organ branching morphogenesis is a complex process that requires many coordinated cell functions, including cell migration, proliferation, and polarization. This process is regulated at numerous levels, including spatial and temporal expression of transcription factors and their regulators; growth factors and their receptors; as well as cell-cell and cell-extracellular matrix interactions. Integrins and dystroglycan are transmembrane receptors that control both the adhesion of cells to matrix components as well as transduction of signaling coming from and directed to the matrix. In this article we review current advances defining the roles of these receptors in branching morphogenesis focusing on the major epithelial cell derived structures in mammals, namely salivary gland, mammary gland, lung, pancreas, and kidney.
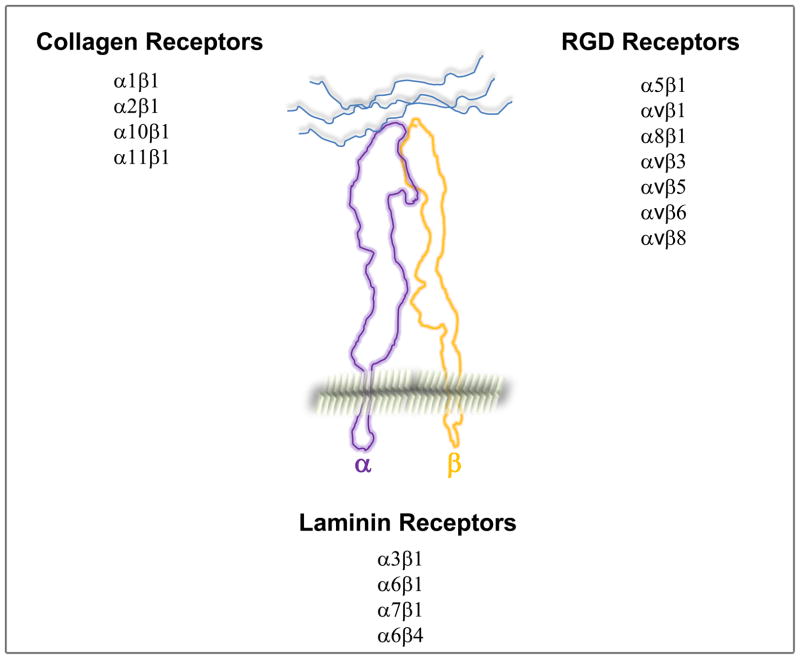
Organs with a branched structure are present in all mammals. Branching allows the organ to grow in multiple directions thus allowing it to fit into different body cavities as well as increasing its functional surface area. The diverse and complex mechanisms of branching morphogenesis are regulated at numerous levels, including spatial and temporal expression of transcription factors and their regulators; growth factors and their receptors (Reviewed in [1]); as well as cell-cell and cell-extracellular matrix (ECM) interactions. A key feature of epithelial derived branched structures is that the cells adhere to a basement membrane (BM); a specialized ECM structure composed primarily of collagen IV, different laminins, nidogen and proteoglycans. Cell adhesion to BMs is mediated by integrins and dystroglycan. There are 24αβ integrin heterodimers derived from 18α and 8β subunits which have distinct specificities for ECM and are classified into the collagen, laminin and arginine-glycine-aspartic acid (RGD) binding integrins (Fig. 1) [2]. In addition to anchoring cells to the ECM, integrins serve as signaling molecules that regulate functions such as migration, differentiation, proliferation, and survival by interacting with the cell cytoskeleton and intracellular signaling molecules. Thus, integrins act as a bridge for cells to bind to and transduce signals from ECM into cells as well as for cells to modify the extracellular environment to which they adhere [3]. Dystroglycan, which consist of an α and β subunuit, is an integral membrane component of the dystrophin-glycoprotein complex. The β subunit interacts with the intracellular cytoskeletal proteins, while the α subunit binds to several extracellular ligands such as laminin, agrin and perlecan. Dystroglycan has been shown to play a major role in in the assembly and maintenance of basement membranes [4]. In this article we review current advances defining the roles of integrins in branching morphogenesis focusing on the major epithelial cell derived structures in mammals, namely salivary gland, mammary gland, lung, pancreas, and kidney. There are many similarities as well as cell specific differences in how cell-ECM interactions regulate branching in these different organs.
Figure 1.
List of the major collagen, laminin, RGD binding integrins expressed in solid tissue organs. The platelet- and leukocyte-expressed integrins are not included.
The salivary gland
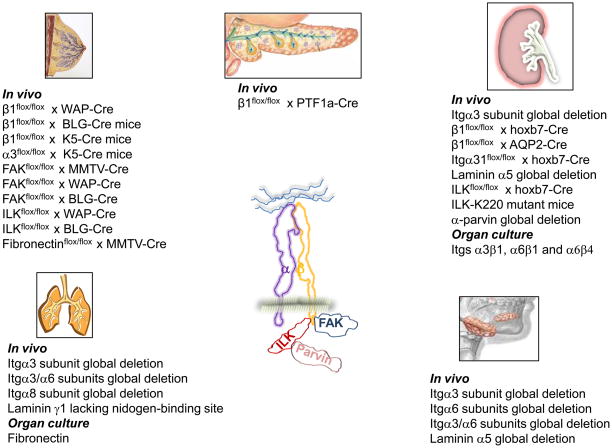
The salivary gland is an excellent model system to define branching morphogenesis mechanisms because it is amenable to in vitro organ culture. This system was used well to identify the requirement of the local expression and accumulation of fibronectin fibrils for branching of many epithelial organs, including the salivary gland [5**]. Fibronectin-mediated branching is likely mediated through integrin α5β1 and perhaps the αv containing integrins; however this has never been definitively demonstrated in vivo. The laminin receptors and specific laminins are required for salivary gland development. Early in vitro studies using antibodies against laminin 111 and integrin α6 demonstrated the importance of laminin integrin interactions in submandibular gland morphogenesis in vitro [6]. The results with inhibitory antibodies was confirmed by others [7], however genetic ablation of the integrin α6 subunit did not result in a branching morphogenesis defect in vivo [8]. By contrast, deletion of the integrin α3 subunit resulted in a branching defect in vivo [9] and loss of both integrin α3 and α6 subunits resulted in a worse phenotype [10] than only deleting the integrin α3 subunit, suggesting a synergistic role for laminin-binding integrins in submandibular gland development. Submandibular glands from mice lacking the laminin α5 chain, phenocopy those of the integrin α3/α6 double mutants [11**.] Because no laminin β2 chain is present in the submandibular BM, it is proposed that α3β1 and α6 integrin interactions with laminin 511 are required for submandibular gland development. Because both β1 and β4 integrin subunits are expressed in the submandibular gland [12], it is unclear as to the relative roles of α6β1 and α6β4 in this process. The only data suggesting a role for dystroglycan in salivary gland development is that antibodies that block its binding to laminin-1 perturbs epithelial branching morphogenesis in salivary gland organ cultures [13]. ECM and integrins analyzed in salivary gland development are summarized in Figure 2.
Figure 2.
List of mice or in vitro systems generated to analyze the selective role of integrins, ECM and integrin binding proteins in the development and branching of salivary gland, mammary gland, pancreas, lung and kidney.
The mammary gland
Mammary glands are comprised of epithelial cells that form collecting ducts and the milk-secreting alveoli (visible only during pregnancy and lactation). The mammary epithelium consists of luminal cells surrounded by myoepithelial cells that lie on BMs. A role for β1 containing integrins in alveolar development was established by deleting β1 integrin with the whey acidic protein(WAP)-Cre mice, where cre is expressed in the luminal cells after the initiation of mammary differentiation during pregnancy, or the β-lactoglobulin (BLG)-Cre mice, where the cre is expressed in luminal cells at 12 weeks of age [14*,15*]. No branching defect, occurred in either mouse, however the alveoli were disorganized with detached luminal epithelial cells, decreased focal adhesion kinase (FAK) activation and reduced cell proliferation. When β1 integrin was deleted from the basal cell layer of the mammary epithelium using the keratin5 (K5)-Cre mouse, a branching morphogenesis defect and altered lobulo-alveolar development was observed, suggesting β1 integrin-dependent basal mammary epithelial cell-ECM interactions is required for mammary gland morphogenesis and the maintenance of a functional stem cell population [16**]. Thus β1 integrin plays distinct roles in different cell populations in the developing mammary gland. The specific αβ1 integrins that regulate mammary gland morphogenesis is poorly understood because there is only one definitive study using floxed integrin α subunit mice in mammary gland development. In this study, when the α3 integrin was deleted using keratin 5 cre, there were no abnormalities with the integrity or functional differentiation of the mammary epithelium, however there were low rates of milk ejection due to a contractility defect of the myoepithelial cells, which was associated with decreased FAK phosphorylation and an alteration in Rho/Rac balance [17**]. Prior to this study, the role of specific integrin α subunits were performed by transplantation of rudiments of embryonic mammary tissue from the α3-,α6-, or β4-null mice into syngeneic mice [18]. As with the floxed α3 null mouse no developmental phenotypes were observed in any of the null mice. Furthermore due to the model limitations no functional abnormalities were seen either, thus highlighting the importance of deleting the specific integrin subunits in situ in in vivo models. Deleting the rest of the laminin receptors individually or in combination using inducible cre and tissue selective floxed mice will address their specific roles in mammary gland development.
The role of integrin associated proteins in mammary gland branching morphogenesis is also poorly understood. Recently FAK was deleted from the mammary epithelial cells with the MMTV-Cre mouse and consistent with deletion of the integrin β1 subunit using the K5-Cre mice, a mild branching defect and severe lobulo-alveolar hypoplasia during pregnancy and lactation were detected [19**]. This phenotype was more severe than the one observed when the β1 integrin was deleted using the WAP- or BLG-Cre. However, when FAK was deleted using the WAP- or BLG-Cre mice, no mammary phenotype was observed [20]**, suggesting that FAK is not involved in mediating integrin β1-mediated signaling after the initiation of mammary differentiation during pregnancy or in adulthood. Interestingly, deletion of integrin linked kinase (ILK), another integrin binding protein, with the WAP- or BLG-Cre mice resulted in morphologically imperfect alveoli in late pregnancy [20] with smaller alveolar lumina and epithelial cells that protruded into the lumen resulting in a lactation defect. This phenotype is similar to that seen when the integrin β1 subunit was deleted using the same Cre mice. These data suggest that signaling molecules such as FAK and ILK that bind to the integrin β1 tails transduce specific integrin-dependent signals that are important at different developmental stages of mammary gland morphogenesis.
Although the mammary gland has a well-developed BM, there is no in vivo data demonstrating the specific roles of different BM components in mammary gland morphogenesis. The only matrix investigated in this context is fibronectin which was deleted using the MMTV-Cre mouse. These mice developed moderate retardation in outgrowth and branching of the ductal tree and the epithelial cells failed to undergo normal lobulo-alveolar differentiation during pregnancy [21*]. This defect was associated with decreased integrin β1 expression and FAK autophosphorylation, suggesting disruption of the fibronectin/integrin β1/FAK signaling axis. This study is also in vivo confirmation of the requirement for fibronectin in organ branching morphogenesis. There are currently no studies showing a role for dystroglycan in mammary gland development. Studies of ECM, integrins and their binding partners in mammary gland development are summarized in Figure 2.
The lung
Despite extensive studies on lung development, almost nothing is known about the role of integrins in this process (Fig. 2). The laminin receptors are important as lungs from mice lacking the integrin α3 subunit fail to branch into smaller bronchioles [22] and deleting both integrin α3 and α6 subunits resulted in marked lung hypoplasia [10]. Integrin α8β1 is the only other integrin shown to play a role in lung development as the integrin α8-null mice develop fusion of the medial and caudal lobes of the lung as well as subtle abnormalities in airway division [23*]. The mechanism proposed for this phenotype is that integrin α8β1 regulates mesenchymal cell adhesion and migration. There is almost no published literature defining the in vivo roles for the specific matrix components in the lung. In fact, the only published lung phenotype in mice is that specific ablation of the nidogen-binding site in the laminin γ1 chain causes abnormal lung development [24]. In vitro organ culture systems suggest a role for fibronectin in mediating lung branching morphogenesis [5]. There is no in vivo evidence for a role for dystroglycan in lung development, although antibodies that blocked interactions between dystroglycan and laminin-111 inhibited lung branching morphogenesis in an in vitro model [13]. Thus although it is clear that there is a significant role for integrins, integrin binding proteins and ECM in lung branching morphogenesis, this is an understudied area that requires extensive research. Likewise the role of dystrgoglycans in lung morphogenesis has not been defined.
The Pancreas
The exocrine pancreas, which develops by branching morphogenesis, consists of a multi-branched ductal system with enzyme secreting acinar cells at the tips of the branches. Pancreatic development starts at E9.5 in the mouse and is a highly regulated and complex process. At present, only the role of the integrin β1 subunit has been investigated in pancreas development (Fig. 2). Surprisingly, deleting this subunit in most cells of the nascent pancreatic bud at E9.5 using the Ptf1a-Cre mouse did not result in developmental or early functional abnormalities [25**]. However there was pancreas degeneration over time due to loss of contact of acinar cells with the BM. This study suggests that either β1-containing integrins play little or no role in pancreas development or there is compensation by non β1 integrins such as the αv containing and/or the α6β4 integrins. Dystroglycan has not been studied with respect to exocrine pancreas development. This area of development needs further study.
The Kidney
The kidney is a complex organ that consists of multiple nephrons that drain into the collecting system. The nephrons develop from the metanephric mesenchyme (MM) while the collecting system develops from the ureteric bud (UB), which undergoes branching morphogenesis. The laminin receptors have been shown to be the principal integrins involved in UB development (Fig. 2). The first evidence for their role came from in vivo studies demonstrating that antibodies directed against integrin α6β1 inhibited kidney branching in in vitro culture models [26,27]. A major branching morphogenesis defect was then found in the global integrin α3-null mouse [22]. This finding was substantiated by others in organ culture assays utilizing blocking antibodies and the same group confirmed that blocking the integrin α6 subunit resulted in branching defects, however they proposed this effect was predominantly mediated by integrin α6β4 [28]. β1 integrin was recently deleted in the UB using a hoxb7-Cre mouse, which is expressed in the UB at day E10.5 [29**,30]. There was a severe branching morphogenesis defect with decreased nephron formation, a major proliferation defect and decreased activation of many of integrin-activated signaling pathways. In contrast, deleting the integrin β1 subunit in the collecting ducts at E18.5 using the AQP2-Cre mouse, did not result in a developmental phenotype; however the mice were rendered susceptible to renal tubular injury [29]. These data suggest that integrin β1 primarily regulates the early stages of UB development and is required to maintain structural integrity of the tubules when subjected to injury. α3 is the only α subunit that has been specifically deleted in the UB using the hoxb7-cre mouse. Whereas no abnormalities in collecting duct branching morphogenesis were observed, there was a papillary outgrowth defect that was attributed to abnormal Wnt7b signaling that modulate cell survival. Like the developing submandibular gland, a similar phenotype was seen in laminin α5 chain-null mutant embryos [31*], suggesting that an interaction between integrin α3β1 and α5 chain containing laminins is required for normal papillary development.
The only integrin binding protein studied in renal UB development so far is ILK. ILK deletion in the UB, resulted in a moderate decrease in branching, but the mice died due to intraluminal collecting duct cellular proliferation. ILK-null collecting duct cells were unable to stimulate p38 MAPK a key step required to induce cell growth contact inhibition [32**]. Unlike the mammary gland, deleting β1 and ILK in the UB results in markedly different phenotypes, suggesting ILK only transduces a distinct subset of signaling pathways activated by β1 integrin. Thus, ILK has very different functional roles in the kidney and mammary gland. Another study demonstrating a key role for ILK in renal develop was the observation that transgenic mice carrying mutations in the highly conserved lysine 220 in the ATP binding domain of ILK had severe renal agenesis/dysgenesis due to abnormalities in the UB and the MM, and the inductive tissue interactions between them [33**]. The phenotype of these transgenic mice was shown to be similar to mice lacking α-parvin, an ILK binding partner, suggesting that lysine 220 of ILK mediates α-parvin binding and loss of this ILK/α-parvin interaction is responsible for the renal defect observed. This study also showed that no kinase activity by ILK was required for its role in renal development. This observation was corroborated by recent structural studies demonstrating that ILK is a pseudo-kinase [34**].
The role of dystroglycan in branched organ morphogenesis has been best studied in the kidney. Early experiments demonstrated that monoclonal antibodies known to block binding of dystroglycan to laminin-111 inhibited development of the kidney in organ culture systems [35]. More recently a role for dystroglycan was shown for development of the pronephros in xenopus, because when it was targeted with morpholinos there was BM disorganization and a drastic decrease in pronephric tubules [36*]. However when dystroglycan was deleted from either podocytes, the ureteric bud, metanephric mesenchyme or from the whole kidney, no significant morphological or functional abnormalities were seen [37**]. These data suggest that in mammals integrins are the primary extracellular matrix receptors in renal epithelia. Consistent with the conclusions drawn from this study, when both the β1 integrin subunit and dystroglycan was deleted from the ureteric bud, the phenotype was no worse than only deleting β1 (Peter Yurchenco personal communication)
Conclusions
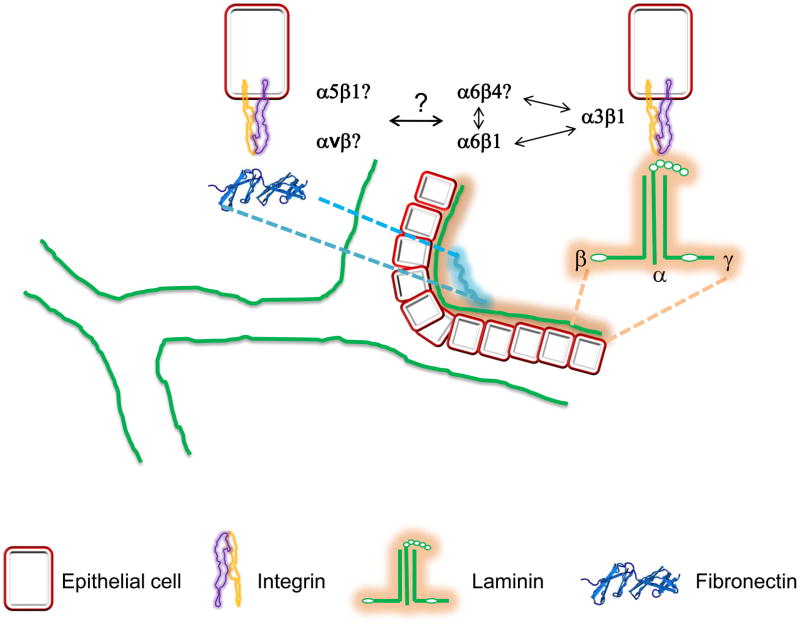
The role of integrins and the dystroglycans, their ligands and their intracellular binding partners in the morphogenesis of branched organs has been poorly investigated over the years. While the data so far suggests either no or a minor role for dystroglycans in morphogenesis of these organs, it is clear that the laminin binding integrins are required for branching and development of many organs, however the contribution of the specific laminin receptors is vague. The laminin receptors appear to function synergistically in branched organ development as phenotypes in double-null integrin mutants (i.e. α3/α6 null mice) are invariably worse than the single mutants. How these integrins co-operate and whether they synergize with dystroglycan in morphogenesis is unknown (Figure 3). This is made even more difficult to study and understand because α6 heterodimerizes with both β1 and β4 subunits and the relative contributions of α6β1 and α6β4 in branching morphogenesis is completely unexplored.
Figure 3.
Schematic representation of a tubule undergoing branching. Laminin binding integrins and fibronectin have been shown to control different steps in branching morphogenesis. However, which specific laminin or fibronectin receptors control branching, whether laminin and fibronectin receptors cross talk with each other during branching and how laminin receptors work synergistically with each other in governing branch formation are still unanswered questions that need to be addressed.
Although some studies suggest a role for fibronectin in regulating branching morphogenesis, the cellular receptors interacting with fibronectin and modulating cellular responses necessary for branching morphogenesis are currently unknown (Figure 3). Indeed, it is not known whether the RGD binding integrins are required at all for branching to occur in vivo and how their deletion might affect branching and epithelial cell functions.
Recent work has begun to define how certain integrin binding proteins play a role in transducing specific signals in branching morphogenesis. Defining how these proteins bind to the integrin cytoplasmic domains and what specific signals they transduce in different organs needs to be studied in detail. Finally, and perhaps most importantly, it is unclear how the signaling specificity of the cytoplasmic tails of the different collagen, laminin and RGD binding integrins is governed. Defining the mechanisms whereby specific integrin α subunit signaling occurs is probably the biggest unanswered question in integrin biology, especially when multiple integrins can bind to the same ECM ligands. Answering this question will certainly improve our understanding of how cell-ECM interactions regulate organ branching morphogenesis.
Highlights.
Branching morphogenesis requires cell-extracellular matrix (ECM) interactions.
ECM and ECM receptors are required for branched organ development.
Laminins and fibronectin are the principal ECM required for branching.
Laminin receptors are the major integrins.
Dystroglycan plays a minor role in branched organ development.
Acknowledgments
These studies were in part supported by a Merit Review from the Department of Veterans Affairs (AP and RZ) and the NIH grants: 2P01DK065123 (AP and RZ); DK075594, DK65123 and DK083187 (RZ); AHA established investigator award (RZ); the O’Brien P30DK79341-01 (AP, RZ).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Affolter M, Zeller R, Caussinus E. Tissue remodelling through branching morphogenesis. Nat Rev Mol Cell Biol. 2009;10:831–842. doi: 10.1038/nrm2797. [DOI] [PubMed] [Google Scholar]
- 2.Hynes R. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 3.Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol. 2002;4:E65–68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- 4.Barresi R, Campbell KP. Dystroglycan: from biosynthesis to pathogenesis of human disease. J Cell Sci. 2006;119:199–207. doi: 10.1242/jcs.02814. [DOI] [PubMed] [Google Scholar]
- **5.Sakai T, Larsen M, Yamada KM. Fibronectin requirement in branching morphogenesis. Nature. 2003;423:876–881. doi: 10.1038/nature01712. This is the seminal paper which shows that fibronectin is required for for cleft formation in salivary gland branching morphogenesis. [DOI] [PubMed] [Google Scholar]
- 6.Kadoya Y, Kadoya K, Durbeej M, Holmvall K, Sorokin L, Ekblom P. Antibodies against domain E3 of laminin-1 and integrin alpha 6 subunit perturb branching epithelial morphogenesis of submandibular gland, but by different modes. J Cell Biol. 1995;129:521–534. doi: 10.1083/jcb.129.2.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koyama N, Hayashi T, Gresik EW, Kashimata M. Role of alpha 6 integrin subunit in branching morphogenesis of fetal mouse submandibular gland: investigation by mesenchyme-free epithelial culture system. J Med Invest. 2009;56 (Suppl):247–249. doi: 10.2152/jmi.56.247. [DOI] [PubMed] [Google Scholar]
- 8.Georges-Labouesse E, Messaddeq N, Yehia G, Cadalbert L, Dierich A, Le Meur M. Absence of integrin alpha 6 leads to epidermolysis bullosa and neonatal death in mice. Nat Genet. 1996;13:370–373. doi: 10.1038/ng0796-370. [DOI] [PubMed] [Google Scholar]
- 9.Menko AS, Kreidberg JA, Ryan TT, Van Bockstaele E, Kukuruzinska MA. Loss of alpha3beta1 integrin function results in an altered differentiation program in the mouse submandibular gland. Dev Dyn. 2001;220:337–349. doi: 10.1002/dvdy.1114. [DOI] [PubMed] [Google Scholar]
- 10.De Arcangelis A, Mark M, Kreidberg J, Sorokin L, Georges-Labouesse E. Synergistic activities of alpha3 and alpha6 integrins are required during apical ectodermal ridge formation and organogenesis in the mouse. Development. 1999;126:3957–3968. doi: 10.1242/dev.126.17.3957. [DOI] [PubMed] [Google Scholar]
- **11.Rebustini IT, Patel VN, Stewart JS, Layvey A, Georges-Labouesse E, Miner JH, Hoffman MP. Laminin alpha5 is necessary for submandibular gland epithelial morphogenesis and influences FGFR expression through beta1 integrin signaling. Dev Biol. 2007;308:15–29. doi: 10.1016/j.ydbio.2007.04.031. This is the most up to date major study defining the role of integrins and the laminin α5 chain in the development of the salivary gland. In additon, It also integrates other work performed in this area of research. [DOI] [PMC free article] [PubMed]
- 12.Kashimata M, Gresik EW. Epidermal growth factor system is a physiological regulator of development of the mouse fetal submandibular gland and regulates expression of the alpha6-integrin subunit. Dev Dyn. 1997;208:149–161. doi: 10.1002/(SICI)1097-0177(199702)208:2<149::AID-AJA2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 13.Durbeej M, Talts JF, Henry MD, Yurchenco PD, Campbell KP, Ekblom P. Dystroglycan binding to laminin alpha1LG4 module influences epithelial morphogenesis of salivary gland and lung in vitro. Differentiation. 2001;69:121–134. doi: 10.1046/j.1432-0436.2001.690206.x. [DOI] [PubMed] [Google Scholar]
- *14.Li N, Zhang Y, Naylor MJ, Schatzmann F, Maurer F, Wintermantel T, Schuetz G, Mueller U, Streuli CH, Hynes NE. Beta1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. Embo J. 2005;24:1942–1953. doi: 10.1038/sj.emboj.7600674. This manuscript described the phenotype of the mammary gland in which the beta1 integrins subunit was deleted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *15.Naylor MJ, Li N, Cheung J, Lowe ET, Lambert E, Marlow R, Wang P, Schatzmann F, Wintermantel T, Schuetz G, et al. Ablation of beta1 integrin in mammary epithelium reveals a key role for integrin in glandular morphogenesis and differentiation. J Cell Biol. 2005;171:717–728. doi: 10.1083/jcb.200503144. This manuscript described the phenotype of the mammary gland in which the beta1 integrins subunit was deleted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **16.Taddei I, Deugnier MA, Faraldo MM, Petit V, Bouvard D, Medina D, Fassler R, Thiery JP, Glukhova MA. Beta1 integrin deletion from the basal compartment of the mammary epithelium affects stem cells. Nat Cell Biol. 2008;10:716–722. doi: 10.1038/ncb1734. The most recent paper on the role of integrins in mammary gland morphogenesis that, in conjunction with references 14 and 15, shows that deleting integrin β1 with different cre mice results in distinct phenotypes, including a branching mophogenesis phentoype. It also demonstrates that interactions of basal cells with the ECM via β1 integrins are important in the maintenance of the mammary stem-cell population. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **17.Raymond K, Cagnet S, Kreft M, Janssen H, Sonnenberg A, Glukhova MA. Control of mammary myoepithelial cell contractile function by alpha3beta1 integrin signalling. Embo J. doi: 10.1038/emboj.2011.113. In press. This is the only manuscript so far to describe the role of a specific integrin in mammmary gland development in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Klinowska TC, Alexander CM, Georges-Labouesse E, Van der Neut R, Kreidberg JA, Jones CJ, Sonnenberg A, Streuli CH. Epithelial development and differentiation in the mammary gland is not dependent on alpha 3 or alpha 6 integrin subunits. Dev Biol. 2001;233:449–467. doi: 10.1006/dbio.2001.0204. [DOI] [PubMed] [Google Scholar]
- **19.Nagy T, Wei H, Shen TL, Peng X, Liang CC, Gan B, Guan JL. Mammary epithelial-specific deletion of the focal adhesion kinase gene leads to severe lobulo-alveolar hypoplasia and secretory immaturity of the murine mammary gland. J Biol Chem. 2007;282:31766–31776. doi: 10.1074/jbc.M705403200. This manuscript shows a role for FAK in mammary gland branching morphogenesis. It contrasts with the studies in reference 16 demonstrating the importance of interpreting results in the context of the cre mice used. [DOI] [PubMed] [Google Scholar]
- **20.Akhtar N, Marlow R, Lambert E, Schatzmann F, Lowe ET, Cheung J, Katz E, Li W, Wu C, Dedhar S, et al. Molecular dissection of integrin signalling proteins in the control of mammary epithelial development and differentiation. Development. 2009;136:1019–1027. doi: 10.1242/dev.028423. This manuscript, in contrast to reference 15, shows that FAK is not important for certain aspects of mammary gland development. It does however show a critical role for ILK as deleting this integrin binding partner phenocopies the β1 integrin null phenotype. These data are different to studies performed in kidney emphasizing the different functions of these proteins in specific organs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *21.Liu K, Cheng L, Flesken-Nikitin A, Huang L, Nikitin AY, Pauli BU. Conditional knockout of fibronectin abrogates mouse mammary gland lobuloalveolar differentiation. Dev Biol. 2010;346:11–24. doi: 10.1016/j.ydbio.2010.07.001. This manuscript describes the branching morphogenesis defects in the maamry gland associated with deleting fibronectin in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kreidberg JA, Donovan MJ, Goldstein SL, Rennke H, Shepherd K, Jones RC, Jaenisch R. Alpha 3 beta 1 integrin has a crucial role in kidney and lung organogenesis. Development. 1996;122:3537–3547. doi: 10.1242/dev.122.11.3537. [DOI] [PubMed] [Google Scholar]
- *23.Benjamin JT, Gaston DC, Halloran BA, Schnapp LM, Zent R, Prince LS. The role of integrin alpha8beta1 in fetal lung morphogenesis and injury. Dev Biol. 2009;335:407–417. doi: 10.1016/j.ydbio.2009.09.021. This manuscript defines the role of integrin alpha8beta1 in lunge development in vitro and in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willem M, Miosge N, Halfter W, Smyth N, Jannetti I, Burghart E, Timpl R, Mayer U. Specific ablation of the nidogen-binding site in the laminin gamma1 chain interferes with kidney and lung development. Development. 2002;129:2711–2722. doi: 10.1242/dev.129.11.2711. [DOI] [PubMed] [Google Scholar]
- **25.Bombardelli L, Carpenter ES, Wu AP, Alston N, DelGiorno KE, Crawford HC. Pancreas-specific ablation of beta1 integrin induces tissue degeneration by disrupting acinar cell polarity. Gastroenterology. 2010;138:2531–2540. e2531–2534. doi: 10.1053/j.gastro.2010.02.043. This is currently the only paper that investigates the role of integrins in pancreas development. Surprisingly, in this instance integrins do not appear to play a role in pancreas development. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Falk M, Salmivirta K, Durbeej M, Larsson E, Ekblom M, Vestweber D, Ekblom P. Integrin alpha 6B beta 1 is involved in kidney tubulogenesis in vitro. J Cell Sci. 1996;109 (Pt 12):2801–2810. doi: 10.1242/jcs.109.12.2801. [DOI] [PubMed] [Google Scholar]
- 27.Sorokin L, Sonnenberg A, Aumailley M, Timpl R, Ekblom P. Recognition of the laminin E8 cell-binding site by an integrin possessing the alpha 6 subunit is essential for epithelial polarization in developing kidney tubules. J Cell Biol. 1990;111:1265–1273. doi: 10.1083/jcb.111.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zent R, Bush KT, Pohl ML, Quaranta V, Koshikawa N, Wang Z, Kreidberg JA, Sakurai H, Stuart RO, Nigam SK. Involvement of Laminin Binding Integrins and Laminin-5 in Branching Morphogenesis of the Ureteric Bud during Kidney Development. Dev Biol. 2001;238:289–302. doi: 10.1006/dbio.2001.0391. [DOI] [PubMed] [Google Scholar]
- **29.Zhang X, Mernaugh G, Yang DH, Gewin L, Srichai MB, Harris RC, Iturregui JM, Nelson RD, Kohan DE, Abrahamson D, et al. beta1 integrin is necessary for ureteric bud branching morphogenesis and maintenance of collecting duct structural integrity. Development. 2009;136:3357–3366. doi: 10.1242/dev.036269. This manuscript describes the role of β1 integrins in UB development. It shows that only early, but not late deletion causes branching defects. It also shows that integrins are required to protect tubules from injury and it demonstrates how integrins and growth factors interact with each other in renal tubule cell branching morphogenesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu W, Kitamura S, Truong DM, Rieg T, Vallon V, Sakurai H, Bush KT, Vera DR, Ross RS, Nigam SK. {beta}1-Integrin is required for kidney collecting duct morphogenesis and maintenance of renal function. Am J Physiol Renal Physiol. 2009;297:F210–217. doi: 10.1152/ajprenal.90260.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *31.Liu Y, Chattopadhyay N, Qin S, Szekeres C, Vasylyeva T, Mahoney ZX, Taglienti M, Bates CM, Chapman HA, Miner JH, et al. Coordinate integrin and c-Met signaling regulate Wnt gene expression during epithelial morphogenesis. Development. 2009;136:843–853. doi: 10.1242/dev.027805. This manuscript describes the phenotype of the kidney when the integrin alpha3 subunit is deleted in vivo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **32.Smeeton J, Zhang X, Bulus N, Mernaugh G, Lange A, Karner CM, Carroll TJ, Fassler R, Pozzi A, Rosenblum ND, et al. Integrin-linked kinase regulates p38 MAPK-dependent cell cycle arrest in ureteric bud development. Development. 2010;137:3233–3243. doi: 10.1242/dev.052845. This manuscript describes the phenotype of the developing UB when ILK is deleted. Unlike the mammary gland, there is a major difference between the β1 integrin and ILK null mice. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **33.Lange A, Wickstrom SA, Jakobson M, Zent R, Sainio K, Fassler R. Integrin-linked kinase is an adaptor with essential functions during mouse development. Nature. 2009;461:1002–1006. doi: 10.1038/nature08468. This paper demonstrates that mutating the parvin binding site of ILK results in a severe renal phenotype. This manuscript also demonstrates that ILK is not a kinase in vivo. [DOI] [PubMed] [Google Scholar]
- **34.Fukuda K, Gupta S, Chen K, Wu C, Qin J. The pseudoactive site of ILK is essential for its binding to alpha-Parvin and localization to focal adhesions. Mol Cell. 2009;36:819–830. doi: 10.1016/j.molcel.2009.11.028. This manuscript shows elegant structural studies demonstrating that ILK is a pseudokinase. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Durbeej M, Larsson E, Ibraghimov-Beskrovnaya O, Roberds SL, Campbell KP, Ekblom P. Non-muscle alpha-dystroglycan is involved in epithelial development. J Cell Biol. 1995;130:79–91. doi: 10.1083/jcb.130.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *36.Bello V, Sirour C, Moreau N, Denker E, Darribere T. A function for dystroglycan in pronephros development in Xenopus laevis. Dev Biol. 2008;317:106–120. doi: 10.1016/j.ydbio.2008.02.024. This manuscript demonstrates the requiremnt of dystroglycan in renal development of Xenopus laevis. [DOI] [PubMed] [Google Scholar]
- **37.Jarad G, Pippin JW, Shankland SJ, Kreidberg JA, Miner JH. Dystroglycan does not contribute significantly to kidney development or function, in health or after injury. Am J Physiol Renal Physiol. 2011;300:F811–820. doi: 10.1152/ajprenal.00725.2010. This manuscript shows that despite all the in vitro evidence and in vivo evidence in frogs, dystroglycan does not play a role in renal development as deleting it does not cause any developmental phenotypes or alterations in response to injury. [DOI] [PMC free article] [PubMed] [Google Scholar]