Abstract
The thalamus serves as the obligatory gateway to the neocortex for sensory processing, and also serves as a pathway for corticocortical communication. In addition, the reciprocal synaptic connectivity between the thalamic reticular nucleus (TRN) and adjacent thalamic relay nuclei generates rhythmic activities similar to that observed during different arousal states and certain neurological conditions such as absence epilepsy. Epileptiform activity can arise from a variety of neural mechanisms, but in addition glia are thought to have an important role in such activities as well. Glia serve a central role in glutamine synthesis, a precursor for glutamate or GABA in nerve terminals. While alterations in glutamine shuttling from glia to neurons can influence GABA and glutamate neurotransmission; the consequences of such action on synaptic transmission and subsequent network activities within thalamic circuits is less understood. We investigated the consequences of altering glutamine transport on inhibitory transmission and intrathalamic activities using the in vitro thalamic slice preparation. Disruption of the glutamine shuttling by the neuronal glutamine transporter (system A transporter) antagonist, α-(methylamino)isobutyric acid (MeAIB) or the selective gliotoxic drug, fluorocitric acid (Fc) dramatically decreased intrathalamic rhythmic activities. At the single cell level, MeAIB and Fc significantly attenuated electrically evoked inhibitory postsynaptic currents (eIPSCs) in thalamic relay neurons; however, miniature IPSCs were unaffected. These data indicate that glutamate-glutamine shuttle is critical for sustaining thalamic synaptic transmission, and thereby alterations in this shuttle can influence intrathalamic rhythmic activities associated with absence epilepsy.
Keywords: glutamine, thalamus, inhibition, thalamic reticular nucleus
INTRODUCTION
Information gating via thalamocortical circuits is a dynamic process arising from the intrinsic properties of thalamic neurons in coordination with synaptic afferents in internal and external sources (Jones 1985, Sherman & Guillery, 2001, Steriade, et. al., 1997). Inhibitory afferents arise predominantly from the thalamic reticular nucleus (TRN) as well as local interneurons (Jones, 1985). In thalamocortical circuits, inhibition regulates sensory information transfer from the dorsal thalamus to the neocortex as exhibited by receptive field changes in thalamocortical neurons following alteration of TRN activity (Hale et al. 1982; Ahlsen et al. 1985; Shosaku et al. 1989; Lee et al. 1994). Furthermore, the reciprocal synaptic relationship between TRN and thalamic relay nuclei can give rise to rhythmic activities associated with different levels of arousal as well as the slow 3 Hz rhythms similar to that observed in absence epileptic seizures (Steriade & Llinas, 1988; Steriade et al., 1993; von Krosigk et al., 1993).
The glutamate-glutamine shuttle clearly plays an important role in “normal” glutamatergic and GABAergic synaptic transmission, and not only during periods of abnormal activities such as epileptiform discharge (Armano et al., 2002; Bacci et al., 2002; Liang et al., 2006; Fricke et al., 2007). Extracellular glutamate is taken up into glial cells and is metabolized to glutamine via activation of glutamine synthetase. Glutamine then exits glia via system N transporter, and is subsequently taken up by neuronal presynaptic terminals through a system A transporter where it is then converted to glutamate and repackaged into synaptic vesicles for release (Chaudhry et al., 1999; Chaudhry et al., 2002). In inhibitory neurons, glutamate is decarboxylated to synthesize GABA and repackaged into vesicles for synaptic release. Pharmacological interruption of glutamine supply ultimately reduces GABA release (Liang et al., 2006; Fricke et al., 2007) and glutamate release (Armano et al., 2002; Bacci et al., 2002) at individual synapses. Neuronal glutamate uptake via excitatory amino acid carrier-1 (EAAC1) is also likely a predominant contributor in maintaining the synaptic pool of neurotransmitter in inhibitory neurons (Sepkuty et al., 2002; Mathews & Diamond, 2003).
Alterations in the metabolism of major neurotransmitters such as glutamate or GABA can produce an imbalance in neuronal excitability that may contribute to epileptiform activities. Knockdown of the neuronal glutamate transporter, excitatory amino acid transporter 3 (EAAT3), reduces GABA synthesis and subsequently results in epileptiform activity (Sepkuty et al., 2002). The glutamate-glutamine shuttle between glia and neurons serves to recycle glutamate or GABA for subsequent release. Impairment of glutamate-glutamine cycling leads to elevated levels of extracellular glutamate in the hippocampus of temporal lobe epilepsy patients (Petroff et al., 2002; Eid et al., 2004). Elevated glutamine concentrations in thalamus have been observed in patients with idiopathic generalized epilepsy, supporting the possible involvement of the glutamate-glutamine shuttle on epilepsy-related excitability (Helms et al., 2006). In animal studies, attenuation of this shuttle reduces epileptiform discharges in the hippocampus in vitro (Bacci et al., 2002). Epileptiform activity produced in a cortical injury model of epilepsy increased glutamine uptake via up-regulation of neuronal glutamine transporters and exogenous glutamine enhances the abnormal epileptic activities (Tani et al., 2007). Alterations in shuttle activity may contribute to epileptiform activity by altering the balance of excitatory and inhibitory synaptic transmission. Glia-mediated glutamate glutamine cycle has been demonstrated as critical role on epileptic activities in hippocampus and cortex. Glutamine transport has been demonstrated as an important contributor to persistent thalamic oscillation (Bryant et al., 2009); however, the influence and underlying mechanisms of glutamine transport manipulations on synaptic transmission and subsequent intrathalamic rhythmic activity is unknown. Our results indicate that pharmacological blockage of system A transporter and malfunction of glia dramatically attenuate GABA release thereby suppressing inhibitory synaptic transmission, and such actions can contribute to the observed dampened intrathalamic oscillations.
MATERIALS AND METHODS
Brain slice preparation
All experimental procedures carried out in accordance with University of Illinois guidelines, and approved by the Institutional Animal Care and Use Committee. Sprague-Dawley rats (postnatal age: 10–16 days) were deeply anesthetized with sodium pentobarbital (55 mg/kg). The brains were quickly removed and placed into chilled (4°C), oxygenated (5% CO2 and 95% O2) slicing medium containing (in mM): 2.5 KCl, 1.25 NaH2PO4, 10.0 MgSO4, 0.5 CaCl2, 26.0 NaHCO3, 11.0 glucose, and 234.0 sucrose. Horizontal slices (300 µm thickness) were cut using a vibrating tissue slicer and transferred to a holding chamber containing oxygenated physiological saline that contained (in mM): 126.0 NaCl, 2.5 KCl, 1.25 NaH2PO4, 2.0 MgCl2, 2.0 CaCl2, 26.0 NaHCO3, and 10.0 glucose. Individual slices were then transferred to a recording chamber maintained at 32°C, and oxygenated physiological saline was continuously superfused at a rate of 2.0 ml/min.
Recording procedures
Extracellular multiple unit recordings were obtained using sharpened tungsten microelectrodes (1–3 MΩ, Frederick Haer, Inc., Bowdoinham, ME). All data were digitized (1–2 kHz) and stored using pClamp software (Molecular Devices, Sunnyvale, CA, USA). Monopolar electrical stimulation was applied to either TRN or internal capsule with sharpened tungsten electrodes (200–600 kΩ, Figure 1C). Analyses of intrathalamic rhythmic activities were similar to those described previously (Lee & Cox, 2006). In brief, to quantify the oscillatory activity, Mini Analysis software (Synaptosoft, Leonia, NJ, USA) was used to detect unit discharge and generate autocorrelograms from which we calculated peak number (# peaks; number of cycles per rhythmic event), oscillation amplitude (Amposc; number of unit discharges per stimulus), and period (interburst interval).
Figure 1.
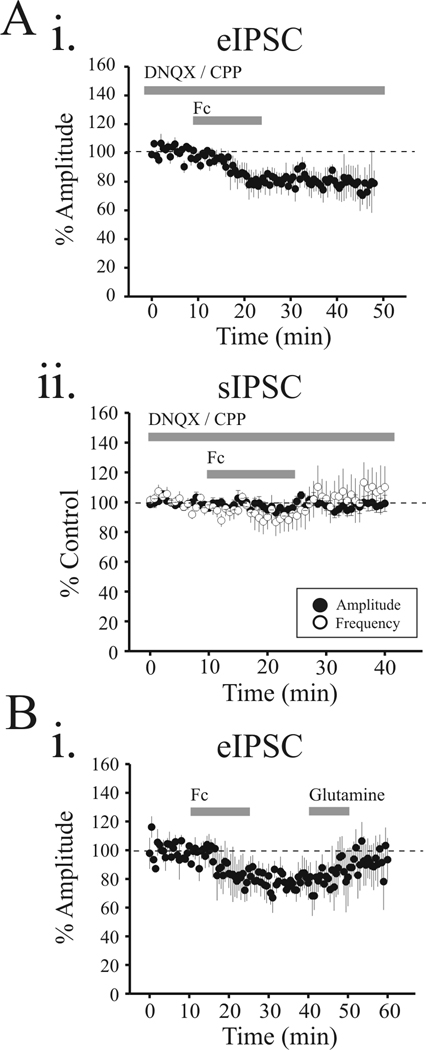
MeAIB and TBOA attenuate eIPSCs in thalamic relay neurons. Ai. In voltage clamp recording from VB relay neurons, stimulation of TRN evokes multi-peaked IPSC. Time course of MeAIB-mediated action with representative traces of eIPSC in control (a), MeAIB (10 mM) (b), wash (c) and SR95531 (10 µM) (d). MeAIB reduces the eIPSC and following recovery of the MeAIB-mediated suppression. The GABAA receptor antagonist SR95531 (10 µM) completely attenuates all IPSCs. Aii. Population data illustrate attenuation of eIPSC amplitude (closed circle) and eIPSC area (open circle). Bi. Time course of TBOA-mediated suppression of eIPSC with representative responses in control (a), TBOA (30 µM) (b), and wash (c). Bii. Population data illustrate the robust TBOA-mediated reduction of eIPSC amplitude (closed circles) and eIPSC area (open circles).
Whole cell recordings were obtained as previously described (Yang & Cox, 2007). Briefly, recording pipettes had tip resistances of 3–7 MΩ when filled with a solution containing (in mM): 117.0 Cs-gluconate, 13.0 CsCl, 1.0 MgCl2, 0.07 CaCl2, 0.1 EGTA, 10.0 HEPES, 2.0 Na2-ATP, 0.4 Na-GTP, and 0.3% biocytin. The pH and osmolarity of intracellular solution were adjusted to 7.3 and 290 mOsm, respectively. The internal solution resulted in ~10 mV junction potential that was corrected in the voltage measures. A fixed-stage microscope (Axioskop2, Carl Zeiss, Inc.) equipped with differential interference contrast optics and water-immersion objective was used to view individual neurons within the slice. Recordings were obtained using a Multiclamp 700B amplifier (Molecular Devices). Signals were low-pass filtered at 2.5 kHz, digitized at 10 kHz, and stored on computer for subsequent analyses using pCLAMP software (Molecular Devices). Synaptic responses were evoked by a bipolar stimulating electrode placed on TRN or internal capsule. Inhibitory postsynaptic currents (IPSCs) were recorded using a holding potential of 0 mV. In a subpopulation of recordings the TRN, which lies lateral to VB, was dissected and removed from the horizontal brain slice. The two brain regions are clearly identifiable using a dissecting microscope, and dissection using a scalpel. The remaining slice is referred to in the results as the isolated VB slice.
Spontaneous IPSC acquisition and analyses
Spontaneous synaptic events were analyzed off-line using Mini Analysis software. Cumulative probability plots were calculated from 30-second windows just prior to drug application (control), and during the peak drug response, which normally reached a maximum effect approximately two minutes after drug application and typically sustained for at least three minutes. A Kolmogorov-Smirnov test was used to test statistical significance between different experimental conditions. Time series illustrating the population data regarding spontaneous IPSC (sIPSC) frequency and amplitude were constructed using 30-second bins and normalized to pre-drug baseline level that was calculated from five minutes prior to drug application Data are presented as mean ± SEM and most statistical analyses consisted of a student’s t test unless noted otherwise.
Pharmacological agents
Concentrated stock solutions of various pharmacological agents were initially prepared and diluted in physiological saline to a final concentration before use. α-(methyamino) isobutyric acid (MeAIB) was prepared fresh each day at final concentration in physiological solution. Fluorocitric acid (Fc) was obtained after precipitation of barium from DL-Fluorocitric acid barium salt as described previously (Paulsen et al., 1987). Agonists were applied via a short bolus into the input line of the recording chamber using a syringe pump, and antagonists were bath applied. All compounds were purchased from Sigma (St. Louis, MO) or Tocris (Ellisville, MO).
RESULTS
Voltage clamp recordings of inhibitory postsynaptic currents (IPSCs) were obtained using a Cs+-containing intracellular solution and holding potential of 0 mV to optimize recordings. Under these conditions, electrical stimulation of TRN evoked an inhibitory postsynaptic current (eIPSC) in thalamic relay neurons which contained multiple peaks presumably resulting from burst discharge of the presynaptic TRN neurons (Figure 1Ai; (Cox et al., 1997)). In the presence of the neuronal glutamine transporter (system A transporter) antagonist, MeAIB (10 mM), the peak amplitude and area of the eIPSC were strongly attenuated without changing the multi-peaked shape of the eIPSC (Figure 1Ai). The MeAIB-mediated suppression completely recovered following washout (5 min) and subsequent application of the GABAA receptor antagonist, SR95531 (10 µM) completely blocked the eIPSC indicating the response is mediated by GABAA receptors (Figure 1Ai). Within the population data, MeAIB significantly decreased the initial eIPSC peak amplitude by 23.5 ± 6.9% and eIPSC area by 34.0 ± 3.9% (Figure 1Aii, n=6, p<0.01, paired t-test).
GABA derived from decarboxylation of glutamate by glutamic acid decarboxylase (GAD) may not only originate from glutamine uptake but also from glutamate uptake via glutamate transporters in presynaptic terminals. We next tested if inhibiting glutamate transporters using the nonspecific glutamate transporter inhibitor, threo-beta-benzyloxyaspartic acid (TBOA), affects GABAergic synaptic events. Bath application of TBOA (30 µM) strongly attenuated the eIPSC in relay neurons (Figure 1B). Unlike MeAIB, not only was the initial eIPSC significantly attenuated, but the multiple peaks were also blocked (Figure 1Bi). Overall, TBOA significantly attenuated the eIPSC amplitude by 67.6 ± 7.8% and eIPSC area by 76.6 ± 4.0% (Figure 1Bii, n=7, p<0.01, paired t-test).
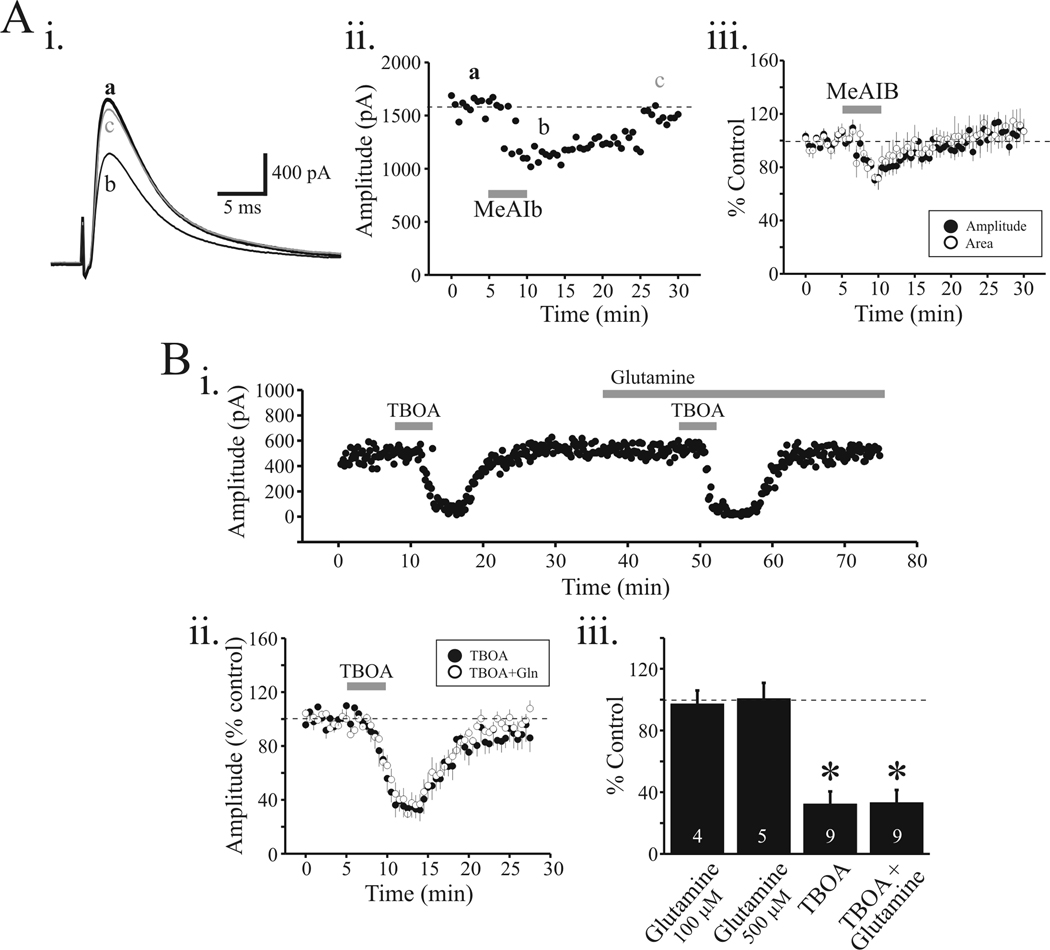
Considering the horizontal thalamic slice preparation maintains intact connections between TRN and VB thalamocortical neurons, the reduction of the eIPSC by MeAIB could arise from a direct action on TRN neurons or perhaps alteration of afferent excitatory synaptic transmission onto TRN neurons. In our experiments, MeAIB (2, 10 mM) produced minimal depolarization in TRN neurons that averaged 0.4 ± 0.1 mV (n=5) and 1.1 ± 0.8 mV (n=5), respectively. This small depolarization is probably not sufficient to change the firing mode of these neurons to attenuate the rhythmic activities; however, to avoid these upstream influences of somatic TRN effects or afferents onto TRN neurons, the TRN was dissected from the thalamic slice. Electrical stimulation of the lateral portion of VB activated TRN afferents resulting in a single peaked eIPSC (Figure 2Ai). Under these conditions, MeAIB (10 mM) significantly reduced eIPSC amplitude by 21.5 ± 5.9% (n=5) and eIPSC area by 21.3 ± 6.1% (n=5; Figure 2Aii, iii, p<0.01, paired t-test). In the isolated VB slice, the effect of MeAIB on eIPSC area was significantly less than that in the intact VB-TRN slice (p<0.01, t-test).
Figure 2.
MeAIB and TBOA reduced eIPSC in isolated VB slice. Ai. In this preparation, the TRN was dissected from the slice. Representative recording from VB neuron and the eIPSC evoked by local electrical stimulation in control (a), following MeAIB application (10 mM) (b) and 15 min wash (c). Note the decrease in amplitude of eIPSC following MeAIB application. Aii. Time course of MeAIB-mediated action on eIPSC. Aiii. Population data indicating the reduction of eIPSC amplitude (closed circles) and eIPSC area (open circles) by MeAIB. Bi. In a different relay neuron, TBOA strongly attenuates the eIPSC that recovers to baseline levels within 10 minutes. In the presence of glutamine (100 µM, 500 µM), the reduction in eIPSC by TBOA is unaltered. Bii. Population data indicate similar decreases in eIPSC amplitude by TBOA before (filled circles) and in the presence of glutamine (open circles). Biii. The histogram plot summarized the average change in eIPSC amplitude by glutamine alone (100 µM, 500 µM), TBOA alone, and TBOA + glutamine. Data are presented as percent of control levels. * p<0.01.
In the isolated VB slice, TBOA (30 µM) significantly attenuated the eIPSC by 67.7 ± 8.2% (Figure 2Bi, ii; n=9; p<0.01, paired t-test). The magnitude of the TBOA attenuation did not significantly differ from that observed in the VB-TRN slice (c.f., Figure 2Bii, 2Bii; p>0.1, t-test). These data clearly indicate a robust action of TBOA on inhibitory synaptic transmission but the site of action is unclear considering TBOA can block not only glutamate transporters located in presynaptic terminals, but also glutamate transporters in glia which can disrupt glia-mediated glutamate-glutamine cycle. In order to dissect these two potential sites of action, we next applied glutamine to see if it would override the TBOA-mediated attenuation. A positive action of glutamine would be consistent with the glial site of TBOA action. As illustrated in Figure 2Bi, the initial TBOA application (30 µM) produced a strong reduction in the eIPSC that recovered within 10 minutes. In the presence of glutamine (100 µM), the subsequent TBOA application produced a similar degree of attenuation of the eIPSC. It is important to note that glutamine alone did not significantly alter the eIPSC amplitude (100 µM, n=4; 500 µM, n=5; Figure 2Biii; p>0.1, paired t-test). In the presence of glutamine, TBOA reduced eIPSC amplitude by 66.7 ± 7.1% which did not significantly differ from the action of TBOA alone (Figure 2Bii, iii; p>0.1, paired t-test). These data suggest that the neuronal glutamate transporter is crucial for GABAergic synaptic transmission within the thalamocortical circuit.
In order to test the potential role of glia in GABAergic synaptic transmission, the selective gliotoxic drug, fluorocitric acid (Fc) was bath applied in a similar manner as experiments described in Figure 2. Fc (50 µM, 15 min duration) suppressed the eIPSC by 20.8 ± 7.7% in an irreversible manner in all 6 cells tested (Figure 3Ai); however the amplitude or frequency of the spontaneous IPSCs (sIPSCs) were unaltered by Fc (amplitude: 95.0 ± 2.9% control; frequency: 90.0 ± 9.3% control, Figure 3Aii, p>0.05, paired t-test). We next tested whether the Fc-mediated reduction could be rescued by glutamine. In 10 cells tested, Fc reduced the eIPSC amplitude by 16.0 ± 6.0%. In a subpopulation of neurons, glutamine application (100 µM, 10 min) restored the eIPSC to 95.7 ± 12.6 % of control levels (Figure 3Bi, n=4).
Figure 3.
The gliotoxin Fc suppresses eIPSCs in VB neurons. Ai. Population data indicating the reduction of eIPSC amplitude by Fc (50 µM) in presence of glutamate antagonists, DNQX (20 µM) and CPP (10 µM). Aii. Population data illustrate the lack of action of Fc on sIPSC amplitude (closed circle) and sIPSC frequency (open circle). B. In a subset of neurons (n=4), the suppressive action of Fc was reversed following glutamine application (10 min, 100 µM).
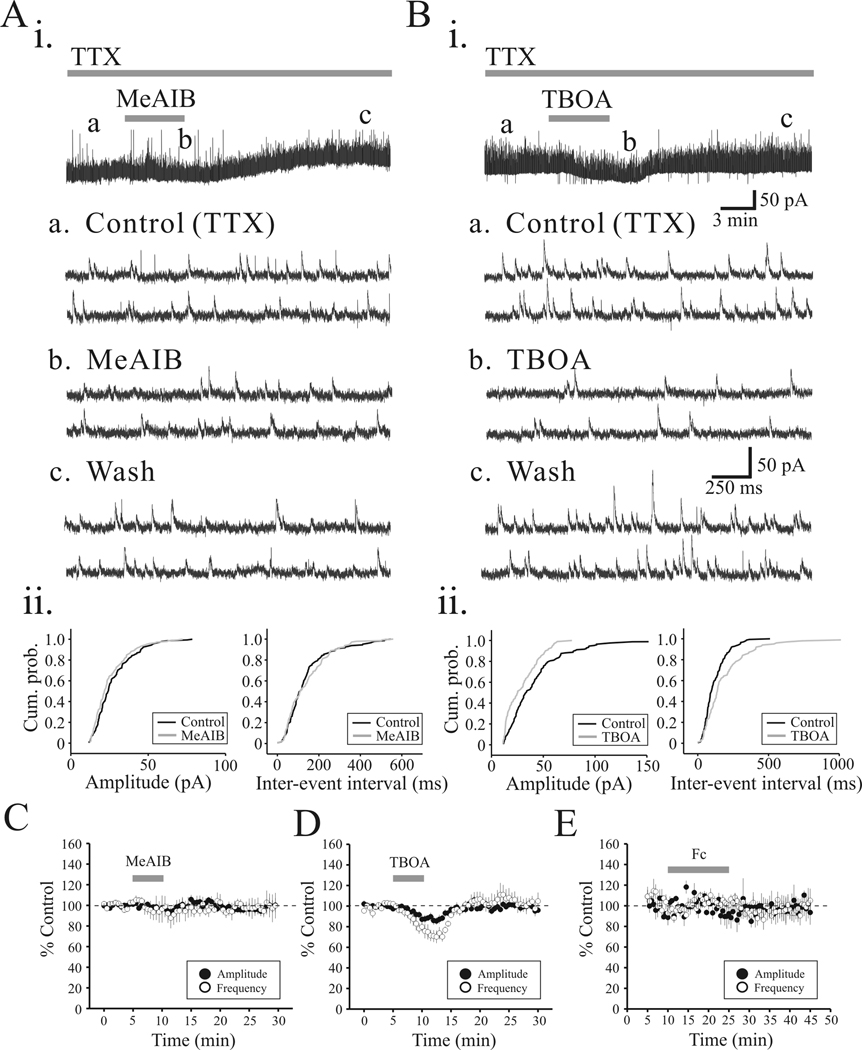
To tease out the possible pre- and/or post-synaptic site(s) of action, we next recorded miniature IPSCs (mIPSCs) in the presence of TTX (1 µM). MeAIB (10 mM) did not significantly alter mIPSC amplitude or mIPSC frequency (Figure 4Ai, ii, p>0.1, Kolmogorov-Smirnov test). The lack of alteration of mIPSCs by 10mM MeAIB suggests that the attenuation of the eIPSC is not attributed to postsynaptic changes in GABA receptor sensitivity. In the population data, MeAIB did not significantly alter mIPSC amplitude (95.0 ± 3.1% of control) or frequency (93.3 ± 9.9% of control; Figure 4C, n = 5, p>0.05, paired t-test). In contrast, TBOA (30 µM) significantly decreased mIPSC amplitude by 13.2 ± 3.8% and mIPSC frequency by 28.4 ± 5.9%, (Figure 4B, D, n = 5, p<0.01, paired t-test). Similar to MeAIB, Fc (50 µM) did not significantly alter mIPSC amplitude (6.4 ± 8.1%, n=5) or mIPSC frequency (6.2 ± 6.5%, n=5; Figure 4E; p>0.1, paired t-tests). These data suggest that under conditions in which action potential generation is blocked, inhibiting glutamine transporter with MeAIB and Fc does not alter inhibitory synaptic transmission, whereas inhibiting neuronal glutamate transporter with TBOA is attributable to decreased inhibitory action.
Figure 4.
TBOA, but not MeAIB or Fc, alter mIPSC activity. Ai. mIPSCs were recorded from VB relay neuron in presence of TTX (1 µM). Representative traces illustrate mIPSC activity in control (a), MeAIB (b) and 15 minute wash of MeAIB (c). Aii. Cumulative probability plots of mIPSC amplitude and inter-event intervals from neuron illustrated in Ai. Bi. In a different VB relay neuron, representative traces illustrating mIPSC activity in control (a), TBOA (30 µM)(b) and 15 minute wash of TBOA (c). Bii. Cumulative probability plots of amplitude and inter-event intervals from neuron in Bi indicate significant change in mIPSC amplitude and inter-event intervals. C.D.E. Population data illustrating the action of MeAIB (C), TBOA (D), and Fc (E) on mIPSC amplitude (closed circles) and mIPSC frequency (open circles).
We next tested whether the eIPSC reduction resulted from altering release probability from afferent fibers or a different possibility such as reducing GABA contents in presynaptic terminal of TRN. In the isolated VB slice, paired pulse stimulation produced a paired pulse depression when tested with inter-pulse intervals ranging from 10 to 640 ms. Paired pulse depression ratio (PPD) averaged 0.81 ± 0.02 with an inter-pulse interval of 320 ms. In this condition, MeAIB reduced both eIPSC in response to both the 1st and 2nd stimuli; however, the PPD was unaltered (Figure 5). Since PPD is interpreted to indicate changes in probability of release, this observation is consistent with a decrease of GABA content in previous observation (Fricke et al., 2007).
Figure 5.
MeAIB suppresses eIPSCs, but does not alter paired pulse depression. Representative traces illustrating paired pulse stimulation at 320ms interstimulus interval produces paired pulse depression. As illustrated in the population data, bath application of MeAIB reduces the amplitude of both the 1st eIPSC and 2nd eIPSC to similar level; however, the paired pulse ratio (eIPSC2/eIPSC1) was unaltered.
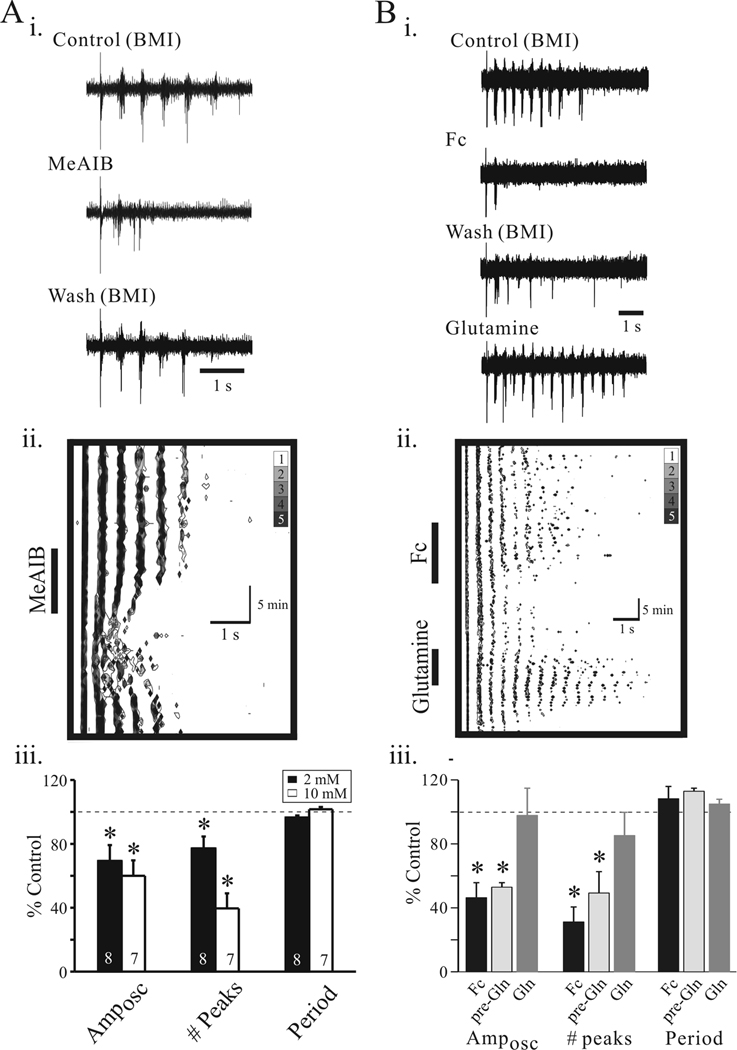
Considering intrathalamic rhythmic activities arise from reciprocal synaptic connections between glutamatergic thalamic relay neurons and GABAergic TRN neurons, we next tested if the suppression of inhibition described above may alter the intrathalamic circuit activity. In the presence of the GABAA receptor antagonist, bicuculline methiodide (BMI, 10 µM), electrical stimulation of internal capsule (IC) or TRN produced a slow rhythmic oscillation ranging from 1.5 Hz to 4.0 Hz (n=22 slices, Figure 6Ai). Under these conditions, we found that the slow oscillations evoked in the presence of BMI were stable. Bath application of MeAIB (5 mM), produced a strong attenuation of the rhythmic activity (Figure 6Ai) that returned to control levels after 10 minutes washout of MeAIB (Figure 6Aii). In the overall population, the number of peaks (# peaks) within each oscillatory episode was significantly reduced by MeAIB (Figure 6Aiii; 2 mM: 77.1 ± 7.5% of control, n=8 slices; 10 mM: 39.6 ± 9.4% of control, n=7 slices, p<0.01, paired t-test). Furthermore, the oscillation amplitude (Amposc) was significantly reduced at both MeAIB concentrations tested (Figure 6B; 2 mM: 69.3 ± 9.9% of control, n=8 slices; 10 mM: 60.0 ± 9.6% of control, n=7 slices, p<0.01, paired t-test). The interburst interval (period) was unaltered by MeAIB (2 mM: 96.4 ± 1.3% of control, 10 mM: 101.6 ± 1.5 of control, p>0.1, paired t-test).
Figure 6.
Disrupting glial function attenuates intrathalamic oscillations. Ai. Representative traces of extracellular recording from VB illustrating slow intrathalamic oscillation in Control (10µM BMI, a), following MeAIB (10 mM, 5 min) application (b), and 15 min wash (c). Aii. Contour plot of experiment in Ai. The abscissa corresponds to a single oscillatory in response to TRN stimulation. The time course of the overall experiment is represented by the ordinate starting with time equal to zero at the top. Aiii. Population data indicate significant suppression of Amposc and # peak, but no change in period at two different concentrations of MeAIB (2 and 10 mM). B: Fc attenuates intrathalamic oscillations. Bi. Representative traces of extracellular recordings in Control (10µM BMI, a), following Fc (50 µM, 15 min) application (b), 15 min wash (c), and glutamine application (Gln; d). Bii. Contour plot of experiments in Bi illustrate the anti-oscillatory actions of Fc and subsequent reversal by glutamine. Biii. Population data calculated from the autocorrelograms indicate anti-oscillatory actions of Fc on Amposc and # peak. Error bars represent SD. *, p<0.05.
Considering a primary source of glutamine is from glia-mediated glutamate-glutamine cycle, we next tested if the gliotoxic drug, Fc would alter intrathalamic rhythmic activities. Bath application of Fc (50 µM) strongly suppressed the rhythmic activities in an irreversible manner (Figure 6Bi, ii). Following Fc washout (10 minutes), the rhythmic activity remained suppressed; however, subsequent application of glutamine (Gln; 100 µM) reversed the suppression and returned the rhythmic activity near control levels (Figure 6Bi, ii). Overall, Fc significantly suppressed both Amposc (Figure 6Biii; 46.3 ± 9.4 % of control, n=7 slices, p<0.01, paired t-test) and the number of peaks (31.2 ± 9.3 % of control, p<0.01, paired t-test). As with MeAIB, the oscillation period was not significantly altered (n=7, p>0.1, paired t-test). In five slices tested, glutamine produced significant recovery of both number of peaks (85.2 ± 14.5 % of control) and Amposc (97.8 ± 17.0 % of control).
DISCUSSION
In this study, we have focused on the potential contribution of the glutamate-glutamine cycle between neurons and glia for maintaining inhibitory synaptic transmission and the functional influence on circuit related activities. Disruption of glutamine transport by either MeAIB or Fc significantly altered the evoked inhibitory responses in thalamic relay neurons arising from TRN neurons. The MeAIB-mediated action on inhibitory activity appeared action-potential (AP)-dependent, which is consistent with previous report in hippocampal neurons (Liang et al., 2006). The AP-independent IPSC activities were dramatically attenuated by the blockade of neuronal glutamate transporter, but not by glutamine transporter block indicating the major contribution of glutamate transporter to spontaneously releasable GABA. This is the first direct demonstration that glutamine transporter has an important role on regulating inhibitory synaptic transmission in the thalamus, and thus can attenuate intrathalamic oscillations.
There are multiple mechanisms that could account for MeAIB/Fc-mediated reduction in GABAergic transmission on thalamocortical neurons in our study: 1) direct depolarization of TRN neurons, 2) alteration of GABA receptor sensitivity on thalamocortical neurons, 3) alteration of afferent glutamatergic synapses on TRN neurons, and 4) alteration of inhibitory synapses on thalamocortical neurons. MeAIB/Fc-mediated actions persisted in the isolated VB slice preparation (lacking TRN), suggesting that the effect is not due to a direct action on TRN neurons and/or an alteration of afferent excitatory synapses onto TRN cells. We also found that inhibition of the glutamine transporter did not alter mIPSC amplitude, suggesting the lack of change in GABA receptor sensitivity. Although an alteration in TRN-relay inhibitory synaptic transmission is a possible site of action, it is still unclear whether this reduction arises from a reduction in GABA release probability or GABA content. However, the lack of alteration in paired pulse depression would be consistent with a change in content rather than release probability. Currently, we cannot rule out the possibility that blockade of glutamate-glutamine cycle by MeAIB and Fc could also reduce glutamate synthesis leading to a decreased glutamate release from VB-TRN synapses, and thus also contribute to the reduction of intrathalamic oscillations observed in this study.
The influence of glutamine transport on inhibitory synaptic transmission has been studied in the hippocampus (Liang et al., 2006; Fricke et al., 2007). Our studies demonstrate that blocking the glutamine transporter reduced inhibitory synaptic transmission in the thalamic circuit, ultimately dampening intrathalamic oscillations. Blocking the glutamine transporter leads to reduced GABA release from presynaptic terminals of TRN neurons. In the case of the slow oscillations in which GABAB-receptor mediated inhibition is important for the periodicity of the oscillation, decreased GABA release would subsequently decrease postsynaptic GABAB receptor activation and ultimately dampen the slow intrathalamic oscillation. It is even more interesting when such an action has been shown to be dependent on the activity of inhibitory synapse. MeAIB produced a significantly greater suppression of the multi-peaked IPSC compared to the single peak IPSC. Considering the multi-peaked eIPSC results from multiple presynaptic action potentials (Cox et al., 1997), and mIPSC activity was unaltered by MeAIB, these results indicate that alterations in glutamine uptake produces greater effects on activity dependent processes such as the response to burst discharge from presynaptic TRN neurons.
Fc and fluoroacetate have been frequently used as a selective metabolic poison on glia, lacking direct actions on neuronal metabolism-linked excitability and energy metabolism (Waniewski & Martin, 1998; Stringer & Aribi, 2003; Larrosa et al., 2006). In current clamp recordings, Fc did not alter the membrane potential of TRN and thalamic relay neurons, but did produce a reversible depolarization of glial cells, indicating Fc specificity on glia (unpublished results). Previous studies indicate that Fc reduces excitatory synaptic transmission in vitro and these actions were reversed following glutamine addition (Bacci et al., 2002; Lee et al., 2005). Our results indicate a similar attenuation and recovery; however, in thalamus inhibitory synaptic transmission was affected. Considering that glutamine uptake normally serves as a buffered reservoir of a precursor for glutamate and GABA synthesis, alteration in glutamate transport activity is likely to influence the both excitatory and inhibitory neurotransmitters through glutamine transporter on demand. In the thalamocortical circuit, we have found a significant influence on inhibitory activity. Based on this, glia likely plays a critical role in extracellular glutamine levels considering our finding that glia malfunction via Fc decreased evoked IPSCs and intrathalamic activities that were then reversed by glutamine application.
Our results also indicate that blockade of glutamate transporter dramatically reduces inhibitory synaptic transmission in thalamus (e.g. eIPSC, mIPSC). Among three suggested sources for GABA in presynaptic terminals: 1) glutamine uptake, 2) GABA recycling (reuptake), and 3) glutamate uptake, the mechanism through neuronal glutamate transporter appears to be a predominant contributor in maintaining synaptic pool of GABA. First, inhibiting neuronal glutamate transporter significantly reduced mIPSC activity unlike that observed following inhibition of the glutamine transporter. Second, no change in mIPSC characteristics was observed in the mice lacking the GABA transporter subtype 1 (GAT-1) which is only expressed in presynaptic terminals (Jensen et al., 2003; Chiu et al., 2005). Furthermore, the functional and structural coupling between GAD-mediated synthesis and GABA packaging into synaptic vesicle has been reported as a main contributor of GABA filling (Jin et al., 2003).
Functional role of glutamine transporter on intrathalamic oscillation
The majority of previous studies regarding the modulation of intrathalamic oscillations have primarily focused on the regulation of neuronal excitability and reciprocal synaptic connectivity. For example, altering the firing mode of thalamic neurons (e.g., burst to tonic mode) is a potent mechanism to terminate intrathalamic oscillations. Numerous neuromodulators arising primarily from brainstem sources can alter firing mode of either TRN and relay neurons or both from burst to tonic discharge mode, and thereby attenuate intrathalamic activity (Lee & McCormick, 1996; Lee & McCormick, 1997; Cox et al., 1997; Sun et al., 2002; Lee & Cox, 2003; Brogerger & McCormick, 2005; Yang & Cox, 2008). However, it is worthwhile to re-focus on the mechanism of intrathalamic rhythmic activity in the aspect of interactions between neuron and glia.
Glia has been demonstrated as a primary contributor to generate intrinsic thalamic oscillations. Spontaneously synchronized Ca2+ wave propagation triggers NMDA-mediated neuronal activity, (Crunelli et al., 2002; Parri et al., 2001). In addition to its role as glutamate source, glial also function to regulate extracellular glutamine levels has been shown in thalamic inhibitory synapses in our studies. Disruption of those glia-mediated actions has been suggested to be tightly correlated to the onset and maintaining of epileptic discharges (Wetherington et al., 2008). Indeed, elevated glutamine concentrations have been observed in the thalamus of patients with idiopathic generalized epilepsy (Helms et al., 2006) and the impairment of glutamate-glutamine cycling has been critical in maintaining epileptic discharge in vitro as well (Bacci et al., 2002; Tani et al., 2007; Bryant et al., 2009). In our studies, disruption of this cycle by gliotoxin or inhibiting glutamine uptake attenuated intrathalamic oscillation. The MeAIB/Fc-mediated anti-oscillatory actions is an alternative means of regulating intrathalamic rhythmic activities, considering MeAIB modulates inhibitory synaptic efficacy rather than altering the firing mode of thalamic neurons. A similar interpretation holds true for the gliotoxic agent Fc. The glutamine transporter may be crucial in maintaining burst-driven action such as intrathalamic oscillation rather than tonic-mediated action and much less active conditions (e.g. mIPSC), whereas the glutamate transporter plays a more powerful role on overall inhibitory activity within the thalamus. Therefore, disruption of glutamine-glutamate shuttle may serve as a potential site for future anti-epileptic drug development.
Acknowledgements
This research was supported by the National Institutes of Health (EY014024). We thank Dr. S-H Lee and A. Cruz-Torres for excellent technical assistance.
REFERENCES
- Ahlsen G, Lindstrom S, Lo FS. Interaction between inhibitory pathways to principal cells in the lateral geniculate nucleus of the cat. Exp Brain Research. 1985;58:134–143. doi: 10.1007/BF00238961. [DOI] [PubMed] [Google Scholar]
- Armano S, Coco S, Bacci A, Pravettoni E, Schenk U, Verderio C, Varoqui H, Erickson JD, Matteoli M. Localization and functional relevance of system a neutral amino acid transporters in cultured hippocampal neurons. J Biol Chem. 2002;277:10467–10473. doi: 10.1074/jbc.M110942200. [DOI] [PubMed] [Google Scholar]
- Bacci A, Sancini G, Verderio C, Armano S, Pravettoni E, Fesce R, Franceschetti S, Matteoli M. Block of glutamate-glutamine cycle between astrocytes and neurons inhibits epileptiform activity in hippocampus. J Neurophysiol. 2002;88:2302–2310. doi: 10.1152/jn.00665.2001. [DOI] [PubMed] [Google Scholar]
- Broberger C, McCormick DA. Excitatory effects of thyrotropin-releasing hormone in the thalamus. J Neurosci. 2005;25:1664–1673. doi: 10.1523/JNEUROSCI.3198-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant AS, Li B, Beenhakker MP, Huguenard JR. Maintenance of thalamic epileptiform activity depends on the astrocytic glutamate-glutamine cycle. J Neurophysiol. 2009;102:2880–2888. doi: 10.1152/jn.00476.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhry FA, Reimer RJ, Krizaj D, Barber D, Storm-Mathisen J, Copenhagen DR, Edwards RH. Molecular analysis of system N suggests novel physiological roles in nitrogen metabolism and synaptic transmission. Cell. 1999;99:769–780. doi: 10.1016/s0092-8674(00)81674-8. [DOI] [PubMed] [Google Scholar]
- Chaudhry FA, Schmitz D, Reimer RJ, Larsson P, Gray AT, Nicoll R, Kavanaugh M, Edwards RH. Glutamine uptake by neurons: interaction of protons with system a transporters. J Neurosci. 2002;22:62–72. doi: 10.1523/JNEUROSCI.22-01-00062.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu CS, Brickley S, Jensen K, Southwell A, McKinney S, Cull-Candy S, Mody I, Lester HA. GABA transporter deficiency causes tremor, ataxia, nervousness, and increased GABA-induced tonic conductance in cerebellum. J Neurosci. 2005;25:3234–3245. doi: 10.1523/JNEUROSCI.3364-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox CL, Huguenard JR, Prince DA. Nucleus reticularis neurons mediate diverse inhibitory effects in thalamus. Proc Nat Acad Sci. 1997;94:8854–8859. doi: 10.1073/pnas.94.16.8854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crunelli V, Blethyn KL, Cope DW, Hughes SW, Parri HR, Turner JP, Toth TI, Williams SR. Novel neuronal and astrocytic mechanisms in thalamocortical loop dynamics. Philos Trans R Soc Lond BBiol Sci. 2002;357:1675–1693. doi: 10.1098/rstb.2002.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eid T, Thomas MJ, Spencer DD, Runden-Pran E, Lai JC, Malthankar GV, Kim JH, Danbolt NC, Ottersen OP, de Lanerolle NC. Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet. 2004;363:28–37. doi: 10.1016/s0140-6736(03)15166-5. [DOI] [PubMed] [Google Scholar]
- Fricke MN, Jones-Davis DM, Mathews GC. Glutamine uptake by System A transporters maintains neurotransmitter GABA synthesis and inhibitory synaptic transmission. J Neurochem. 2007;102:1895–1904. doi: 10.1111/j.1471-4159.2007.04649.x. [DOI] [PubMed] [Google Scholar]
- Hale PT, Sefton AJ, Baur LA, Cottee LJ. Interrelations of the rat's thalamic reticular and dorsal lateral geniculate nuclei. Exp Brain Research. 1982;45:217–229. doi: 10.1007/BF00235781. [DOI] [PubMed] [Google Scholar]
- Helms G, Ciumas C, Kyaga S, Savic I. Increased thalamus levels of glutamate and glutamine (Glx) in patients with idiopathic generalised epilepsy. J Neurol Neurosurg Psychiatry. 2006;77:489–494. doi: 10.1136/jnnp.2005.074682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Jahr CE. Extracellular glutamate concentration in hippocampal slice. J Neurosci. 2007;27:9736–9741. doi: 10.1523/JNEUROSCI.3009-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. Clonazepam suppresses GABA B -mediated inhibition in thalamic relay neurons through effects in nucleus reticularis. J Neurophysiol. 1994a;71:2576–2581. doi: 10.1152/jn.1994.71.6.2576. [DOI] [PubMed] [Google Scholar]
- Huguenard JR, Prince DA. Intrathalamic rhythmicity studies in vitro : Nominal t-current modulation causes robust antioscillatory effects. J Neurosci. 1994b;14:5485–5502. doi: 10.1523/JNEUROSCI.14-09-05485.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen K, Chiu CS, Sokolova I, Lester HA, Mody I. GABA transporter-1 (GAT1)-deficient mice: differential tonic activation of GABAA versus GABAB receptors in the hippocampus. J Neurophysiol. 2003;90:2690–2701. doi: 10.1152/jn.00240.2003. [DOI] [PubMed] [Google Scholar]
- Jin H, Wu H, Osterhaus G, Wei J, Davis K, Sha D, Floor E, Hsu CC, Kopke RD, Wu JY. Demonstration of functional coupling between gamma -aminobutyric acid (GABA) synthesis and vesicular GABA transport into synaptic vesicles. Proc Natl Acad Sci USA. 2003;100:4293–4298. doi: 10.1073/pnas.0730698100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EG. The Thalamus. New York: Plenum Press; 1985. [Google Scholar]
- Larrosa B, Pastor J, Lopez-Aguado L, Herreras O. A role for glutamate and glia in the fast network oscillations preceding spreading depression. Neuroscience. 2006;141:1057–1068. doi: 10.1016/j.neuroscience.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Lee J, Tommerdahl M, Favorov OV, Whitsel BL. Optically recorded response of the superficial dorsal horn: dissociation from neuronal activity, sensitivity to formalin-evoked skin nociceptor activation. J Neurophysiol. 2005;94:852–864. doi: 10.1152/jn.00976.2004. [DOI] [PubMed] [Google Scholar]
- Lee KH, McCormick DA. Abolition of spindle oscillations by serotonin and norepinephrine in the ferret lateral geniculate and perigeniculate nuclei in vitro. Neuron. 1996;17:309–321. doi: 10.1016/s0896-6273(00)80162-2. [DOI] [PubMed] [Google Scholar]
- Lee KH, McCormick DA. Modulation of spindle oscillations by acetylcholine, cholecystokinin and 1S,3R-ACPD in the ferret lateral geniculat and perigeniculate nuclei in vitro. Neuroscience. 1997;77:335–350. doi: 10.1016/s0306-4522(96)00481-2. [DOI] [PubMed] [Google Scholar]
- Lee SH, Cox CL. Vasoactive intestinal peptide selectively depolarizes thalamic relay neurons and attenuates intrathalamic rhythmic activity. J Neurophysiol. 2003;90:1224–1234. doi: 10.1152/jn.00280.2003. [DOI] [PubMed] [Google Scholar]
- Lee SH, Cox CL. Excitatory actions of vasoactive intestinal peptide on mouse thalamocortical neurons are mediated by VPAC2 receptors. J Neurophysiol. 2006;96:858–871. doi: 10.1152/jn.01115.2005. [DOI] [PubMed] [Google Scholar]
- Lee SM, Friedberg MH, Ebner FF. The role of GABA-mediated inhibition in the rat ventral posterior medial thalamus. I.Assessment of receptive field changes following thalamuc reticular nucleus lesions. J Neurophysiol. 1994;71:1702–1715. doi: 10.1152/jn.1994.71.5.1702. [DOI] [PubMed] [Google Scholar]
- Lerma J, Herranz AS, Herreras O, Abraira V, Martin del Rio R. In vivo determination of extracellular concentration of amino acids in the rat hippocampus. A method based on brain dialysis and computerized analysis. Brain Research. 1986;384:145–155. doi: 10.1016/0006-8993(86)91230-8. [DOI] [PubMed] [Google Scholar]
- Liang SL, Carlson GC, Coulter DA. Dynamic regulation of synaptic GABA release by the glutamate-glutamine cycle in hippocampal area CA1. J Neurosci. 2006;26:8537–8548. doi: 10.1523/JNEUROSCI.0329-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathews GC, Diamond JS. Neuronal glutamate uptake Contributes to GABA synthesis and inhibitory synaptic strength. J Neurosci. 2003;23:2040–2048. doi: 10.1523/JNEUROSCI.23-06-02040.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Contreras D. On the cellular and network bases of epileptic seizures. Annu Rev Physiol. 2001;63:815–846. doi: 10.1146/annurev.physiol.63.1.815. [DOI] [PubMed] [Google Scholar]
- Parri HR, Gould TM, Crunelli V. Spontaneous astrocytic Ca2+ oscillations in situ drive NMDAR-mediated neuronal excitation. Nat Neurosci. 2001;4:803–812. doi: 10.1038/90507. [DOI] [PubMed] [Google Scholar]
- Paulsen RE, Contestabile A, Villani L, Fonnum F. An in vivo model for studying function of brain tissue temporarily devoid of glial cell metabolism: the use of fluorocitrate. J Neurochem. 1987;48:1377–1385. doi: 10.1111/j.1471-4159.1987.tb05674.x. [DOI] [PubMed] [Google Scholar]
- Petroff OA, Errante LD, Rothman DL, Kim JH, Spencer DD. Glutamate-glutamine cycling in the epileptic human hippocampus. Epilepsia. 2002;43:703–710. doi: 10.1046/j.1528-1157.2002.38901.x. [DOI] [PubMed] [Google Scholar]
- Sepkuty JP, Cohen AS, Eccles C, Rafiq A, Behar K, Ganel R, Coulter DA, Rothstein JD. A neuronal glutamate transporter contributes to neurotransmitter GABA synthesis and epilepsy. J Neurosci. 2002;22:6372–6379. doi: 10.1523/JNEUROSCI.22-15-06372.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM, Guillery RW. Exploring the thalamus. San Diego: Academic Press; 2001. [Google Scholar]
- Shosaku A, Kayama Y, Sumitomo I, Sugitani M, Iwama K. Analysis of recurrent inhibitory circuit in rat thalamus: neurophysiology of the thalamic reticular nucleus. Prog Neurobiology. 1989;32:77–102. doi: 10.1016/0301-0082(89)90011-7. [DOI] [PubMed] [Google Scholar]
- Steriade M, Llinas R. The functional states of the thalamus and the associated neuronal interplay. Physiological Reviews. 1988;68:649–742. doi: 10.1152/physrev.1988.68.3.649. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCormick DA, Sejnowski TJ. Thalamocortical oscillations in the sleeping and aroused brain. Science. 1993;262:679–685. doi: 10.1126/science.8235588. [DOI] [PubMed] [Google Scholar]
- Steriade M, Jones EG, McCormick DA. Thalamus: volume 1, Organization and Function. New York: Elsevier; 1997. [Google Scholar]
- Stringer JL, Aribi AM. Effects of glial toxins on extracellular acidification in the hippocampal CA1 region in vivo. Epilepsy Res. 2003;54:163–170. doi: 10.1016/s0920-1211(03)00064-0. [DOI] [PubMed] [Google Scholar]
- Sun QQ, Huguenard JR, Prince DA. Somatostatin inhibits thalamic network oscillations in vitro: actions on the GABAergic neurons of the reticular nucleus. J Neurosci. 2002;22:5374–5386. doi: 10.1523/JNEUROSCI.22-13-05374.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tani H, Bandrowski AE, Parada I, Wynn M, Huguenard JR, Prince DA, Reimer RJ. Modulation of epileptiform activity by glutamine and system A transport in a model of post-traumatic epilepsy. Neurobiol Dis. 2007;25:230–238. doi: 10.1016/j.nbd.2006.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261:361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- Waniewski RA, Martin DL. Preferential utilization of acetate by astrocytes is attributable to transport. J Neurosci. 1998;18:5225–5233. doi: 10.1523/JNEUROSCI.18-14-05225.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherington J, Serrano G, Dingledine R. Astrocytes in the epileptic brain. Neuron. 2008;58:168–178. doi: 10.1016/j.neuron.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Cox CL. Modulation of inhibitory activity by nitric oxide in the thalamus. J Neurophysiol. 2007;97:3386–3395. doi: 10.1152/jn.01270.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S, Cox CL. Excitatory and anti-oscillatory actions of nitric oxide in thalamus. J Physiol. 2008;586:3617–3628. doi: 10.1113/jphysiol.2008.153312. [DOI] [PMC free article] [PubMed] [Google Scholar]