Abstract
Background
Older patients often receive less guideline-concordant care for heart failure than younger patients.
Objective
To determine whether age differences in heart failure care are explained by patient, provider, and health system characteristics and/or by chart-documented reasons for non-adherence to guidelines.
Design and Patients
Retrospective cohort study of 2,772 ambulatory veterans with heart failure and left ventricular ejection fraction <40% from a 2004 nationwide medical record review program (the VA External Peer Review Program).
Main Measures
Ambulatory use of ACE inhibitors, angiotensin receptor blockers (ARBs), and beta blockers.
Results
Among 2,772 patients, mean age was 73 +/− 10 years, 87% received an ACE inhibitor or ARB, and 82% received a beta blocker. When patients with explicit chart-documented reasons for not receiving these drugs were excluded, 95% received an ACE inhibitor or ARB and 89% received a beta blocker. In multivariable analyses controlling for a variety of patient and health system characteristics, the adjusted odds ratio for ACE-inhibitor and ARB use was 0.43 (95% CI 0.24–0.78) for patients age 80 and over vs. those age 50–64 years, and the adjusted odds ratio for beta blocker use was 0.66 (95% CI 0.48–0.93) between the two age groups. The magnitude of these associations was similar but not statistically significant after excluding patients with chart-documented reasons for not prescribing ACE inhibitors or ARBs and beta blockers.
Conclusions
A high proportion of veterans receive guideline-recommended medications for heart failure. Older veterans are consistently less likely to receive these drugs, although these differences were no longer significant when accounting for patients with chart-documented reasons for not prescribing these drugs. Closely evaluating reasons for non-prescribing in older adults is essential to assessing whether non-treatment represents good clinical judgment or missed opportunities to improve care.
Electronic supplementary material
The online version of this article (doi:10.1007/s11606-011-1745-2) contains supplementary material, which is available to authorized users.
KEY WORDS: guideline adherence, heart failure, aging, health services research, quality of care
INTRODUCTION
Heart failure is the most common reason for hospitalization in older adults and accounts for approximately 300,000 deaths in the United States each year.1,2 Because angiotensin-converting enzyme inhibitors (ACE-inhibitors), angiotensin receptor blockers (ARBs), and beta blockers have each been shown to substantially reduce mortality in adults with heart failure and reduced ejection fraction, a number of intensive efforts have been launched to increase use of these drugs in eligible patients.3–8
The success of these efforts is tempered by the finding that older patients are less likely to receive guideline-concordant care for heart failure.7,9,10 In a number of studies in the United States and Europe, patients who were older were consistently less likely to receive beta blockers, and many (although not all) studies have found less frequent use of ACE inhibitors with advancing age.6,7,11–15
These age differences in use of guideline-recommended medications for heart failure have important implications for the care of older adults. The SENIORS trial and subgroup analyses of other trials suggest that the benefits and tolerability of angiotensin and beta blockade extend to older adults.16–21 From this perspective, lower rates of guideline-recommended treatment in older adults may represent a missed opportunity. On the other hand, very limited data exist about these treatments in patients of highly advanced age, and several studies suggest that the extent of risk reduction may diminish in the upper reaches of lifespan.16–23 Moreover, older patients with heart failure more often have comorbidities that limit life expectancy, add to medication burden, and confer relative or absolute contraindications to guideline-recommended medications.12,14,24,25
Understanding the reasons behind age differences in treatment can help differentiate the extent to which differences in treatment represent appropriate individualization of therapy or true quality gaps. Several previous studies that have evaluated age differences in heart failure care have been limited by incomplete access to potentially important confounders, particularly among studies conducted in ambulatory settings. In this study, we used clinically rich data from a nationwide cohort of veterans with heart failure to better understand differences in outpatient heart failure treatment across the age spectrum. First, we aimed to determine the association between age and outpatient use of guideline-recommended medications after controlling for a variety of clinical, demographic, health care utilization, and health system factors. Second, we aimed to evaluate whether the frequency of chart-documented reasons for not prescribing guideline-recommended drugs varied across age groups, and whether accounting for these reasons would close the age gap.
METHODS
Data Sources and Measures
We used data on veterans with heart failure from the VA’s External Peer Review Program (EPRP) for 2004. EPRP is a national program of structured chart review of patients at every VA medical center and community-based outpatient clinic. EPRP samples were overweighted to include a disproportionate share of women. Patients receiving hospice care, with a documented life expectancy of less than 6 months, or a diagnosis of esophageal, pancreatic, or hepatic cancer were not sampled by EPRP. Previous studies have demonstrated high inter-rater reliability for chart abstraction, with kappa of 0.9.26
To obtain information about heart failure care, abstractors extracted information from progress notes, diagnostic test reports, nurses’ notes, discharge summaries, and other areas of VA’s electronic medical record. These data included whether the patient had ever received an assessment of left ventricular ejection fraction (LVEF) and the result of the most recent test. Abstractors also reviewed whether patients were currently prescribed ACE inhibitors, angiotensin receptor blockers (ARBs), and beta blockers. Among patients not receiving these drugs, reviewers were instructed to identify reasons that were explicitly documented by the clinician as a reason for non-prescribing. Due to the way the coding scheme was developed, most reasons were classified into the “other” category, thus providing limited useful information on the specific types of reasons for non-prescribing.
We merged with EPRP a variety of other VA databases. To identify comorbidities, we reviewed all outpatient encounter diagnoses and inpatient discharge diagnoses over the previous 2 years, defining a condition as present if it was encoded on two or more times during this period.27 We used analogous methods to apply the Deyo adaptation of the Charlson comorbidity score, a validated and widely-used measure of comorbid burden.28 Data from VA’s outpatient pharmacy were used to tabulate the number of unique medications used by each patient, and laboratory data were assessed to evaluate laboratory values over the prior 2 years. U.S. census file data on median income per census tract was merged at the level of the patient’s zip code. Finally, we used data from VA’s Office of Academic Affiliations to determine whether each facility had an academic affiliation.
Our principal method for determining reasons for not prescribing ACE inhibitors or ARBs and beta blockers was derived from the EPRP chart review. As EPRP requires that the documenting clinician make an explicit link between a reason and the decision not to prescribe, we assessed other data sources that might explain non-prescribing of these drugs even in the absence of a clear, direct explanation by a clinician (see online Appendix). For ACE inhibitors and ARBs, these included serum creatinine >2.5 mg/dL or serum potassium >5.5 mg/dL in the past year or a diagnosis of renal artery stenosis. For beta blockers, these included a diagnosis of asthma, sick sinus syndrome, second-degree or third-degree atrioventricular block, or hospitalization for chronic obstructive pulmonary disease (with hospitalization serving as a marker of disease severity).
Analytic Plan
Among 7,659 patients with heart failure sampled by EPRP, 6,220 had documented measurement of LVEF. We restricted our sample to the 2,772 patients with documented LVEF <40%. Because use of ARBs was not assessed during the first half of the study year, we restricted our analysis of ACE inhibitor and ARB use to patients assessed during the second half of the study year.
Because EPRP’s sampling strategy samples a similar number of patients in each facility regardless of facility size, patients from smaller facilities are overweighted relative to those from larger facilities. To account for this asymmetric weighting, we divided the sample into subjects who received their main care in hospital-based clinics and subjects from community-based outpatient clinics (i.e., larger vs. smaller facilities). This division resulted in relatively small numbers of patients in the community-based clinic sample. Thus, we conducted our main analyses on the hospital-based clinic group.
We constructed separate logistic regression models to identify predictors of each of our two outcomes (use of beta blockers and use of ACE inhibitors or ARBs). We conducted sensitivity analyses in which we narrowed the definition of beta blocker to include only those agents specifically recommended by clinical practice guidelines (bisoprolol, carvedilol, and metoprolol succinate).
We used a two-stage approach to preserve statistical power in our multivariable analyses. First, we organized our variables into five groups (age and comorbid burden, demographics, health services utilization, academic affiliation, and medication use and specific comorbidities). Within each group, we regressed all covariates against the medication outcome. Covariates with P value <0.20 on each of the group-based analyses were then entered into a final multivariable model without any further variable selection (in addition, age and Charlson comorbidity score were forced into all of the final multivariable models).
Analyses were conducted using SAS 9.2 (SAS Institute) and STATA 10.0 and 11.2 (StataCorp). This research was approved by the institutional review boards of the San Francisco VA Medical Center and the University of California, San Francisco.
RESULTS
Use of Guideline-Recommended Medications
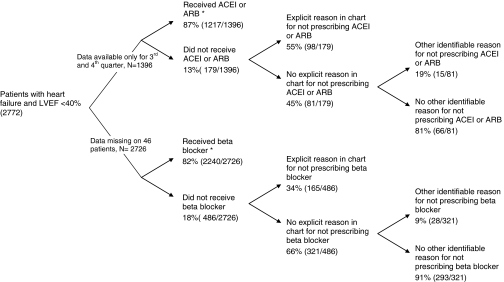
Of 2,772 patients with LVEF <40%, the mean (SD) age was 71 +/− 10 years, 92% (2,563) were men, and 58% (1,597) were alive after 5 years (Table 1). Use of recommended medications was high, with 87% of patients prescribed an ACE-inhibitor or ARB and 82% prescribed a beta blocker (Fig. 1). Use of beta blockers included 47% of patients prescribed beta blockers specifically recommended by guidelines, and 35% prescribed another type of beta blocker. Most patients not taking an ACE inhibitor or ARB were taking a beta blocker, and vice versa. Among 1,351 patients with complete data available for analysis, 12% (160) received an ACE-inhibitor or ARB but not a beta-blocker, 10% (131) received a beta-blocker but not an ACE inhibitor or ARB, and only 36 (3%) received neither type of drug.
Table 1.
Characteristics of Subjects
| Characteristic | n (%) |
|---|---|
| (N = 2,772) | |
| Age (years) | |
| 50 – 64 | 716 (26%) |
| 65 – 79 | 1,391 (50%) |
| 80 + | 665 (24%) |
| Male sex | 2,563 (92%) |
| Race | |
| White | 1,640 (59%) |
| Black | 370 (13%) |
| Asian / Pacific Islander or American Indian | 17 (1%) |
| Unknown / Missing | 745 (27%) |
| Median household income (median, IQR) * | $36,301 ($30,536, $46,344) |
| Charlson comorbidity score | |
| 1 | 597 (22%) |
| 2–3 | 1,245 (45%) |
| 4+ | 930 (34%) |
| Number of medications used (mean / SD) | 9.3 ± 4.3 |
| Common comorbidities | |
| Diabetes | 1,288 (46%) |
| Ischemic heart disease | 1,969 (71%) |
| Chronic obstructive pulmonary disease | 886 (32%) |
| Cancer | 342 (12%) |
| Alive 5 years after study | 1,597 (58%) |
| Number of visits in past two years (median / IQR) | |
| Primary care clinic visits | 4 (2, 7) |
| Cardiology clinic visits | 1 (0, 2) |
| Academic affiliation of primary site of care | 2,091 (75%) |
* Median income from zip-linked census tract, 1999. Income information missing for 75 subjects
Abbreviations: IQR, interquartile range
Figure 1.
Use of guideline-recommended interventions among veterans with heart failure. “Chart-documented reasons” for non-prescribing comprise reasons specifically cited by the treating clinician for not prescribing an ACE inhibitor or ARB and/or beta blocker. * Complete data on use of ACE inhibitors and ARBs were only available for the third and fourth quarters of the study year. In addition, six patients were participating in a clinical trial of heart failure therapy and were excluded from the analysis of ACE-inhibitor or ARB use. Data on beta blocker use were missing for 46 patients.
Association Between Age, Comorbidity, and Use of Guideline-Recommended Services
Guideline adherence was higher in hospital-based clinics than in community-based clinics, including use of ACE-inhibitors or ARBs (88% vs. 82%, P = 0.01) and use of beta blockers (83% vs. 76%, P = 0.001). Based on a priori decisions related to sampling methodology and sample size (as described in the methods), our main analyses of predictors of guideline adherence focused on hospital-based clinics.
Older patients were less likely to use ACE inhibitors or ARBs and beta blockers than their younger counterparts (P ≤ 0.01 for each; see Tables 2 and 3). In contrast, comorbid burden was not associated with receipt of ACE inhibitor or ARBs or of beta blockers (P > 0.20 for each drug type).
Table 2.
Age, Comorbidity, and Use of ACE Inhibitors or ARBs in Hospital-based Clinics
| N | Use of ACE inhibitors or ARBs | ||||
|---|---|---|---|---|---|
| Bivariate results | P-value | Multivariable results | P-value | ||
| n (%) | OR (95% CI) | ||||
| AGE AND COMORBID BURDEN | |||||
| Age group | |||||
| 50-64 | 311 | 290 (93) | 0.001 | -- | <0.001 |
| 65-79 | 573 | 502 (88) | 0.56 (0.32–0.96) | ||
| 80+ | 272 | 229 (84) | 0.43 (0.24–0.78) | ||
| Charlson comorbidity score | |||||
| 0–1 | 193 | 173 (90) | 0.21 | -- | 0.31 |
| 2–3 | 508 | 453 (89) | 0.88 (0.49–1.60) | ||
| 4–5 | 295 | 257 (87) | 0.74 (0.36–1.52) | ||
| 6+ | 160 | 138 (86) | 0.67 (0.29–1.57) | ||
| OTHER DEMOGRAPHIC CHARACTERISTICS | |||||
| Gender | |||||
| Female | 70 | 61 (87) | 0.75 | * | |
| Male | 1086 | 960 (88) | |||
| Race/ethnicity group | |||||
| White, non-Hispanic | 700 | 606 (87) | 0.05 | -- | 0.13 |
| Other | 170 | 156 (92) | 1.80 (0.93–3.49) | ||
| Missing/unknown | 286 | 259 (91) | 1.39 (0.85–2.27) | ||
| Income | |||||
| ≤ median income | 556 | 487 (87) | 0.22 | -- | 0.18 |
| > median income | 597 | 534 (89) | 1.31 (0.88–1.96) | ||
| HEALTH SERVICES UTILIZATION | |||||
| # primary care visits in past 2 years | |||||
| 0–1 | 63 | 56 (89) | 0.95 | * | |
| 2–4 | 420 | 361 (86) | |||
| 5–10 | 500 | 461 (92) | |||
| >10 | 173 | 143 (83) | |||
| # cardiology visits in past 2 years | |||||
| 0 | 423 | 376 (89) | 0.92 | * | |
| 1–2 | 344 | 300 (87) | |||
| >2 | 389 | 345 (89) | |||
| Hospitalization in past 2 years | |||||
| No hospital stay | 514 | 462 (90) | 0.04 | -- | 0.19 |
| ≥ 1 hospital stay where HF was primary diagnosis | 277 | 248 (90) | 1.17 (0.69–2.00) | ||
| ≥ 1 hospital stay, none of which where HF was primary diagnosis | 365 | 311 (85) | 0.75 (0.47–1.17) | ||
| ACADEMIC AFFILIATION OF TREATING FACILITY | |||||
| Affiliation of primary care site | |||||
| Not academically affiliated | 41 | 35 (85) | 0.75 | * | |
| Academically affiliated | 1037 | 919 (89) | |||
| Unknown/missing | 58 | 49 (84) | |||
| MEDICATION USE AND CONDITIONS OF INTEREST | |||||
| # medications used | |||||
| ≤ 5 | 201 | 171 (85) | 0.90 | * | |
| 6 – 11 | 599 | 542 (90) | |||
| ≥ 12 | 330 | 286 (87) | |||
| Comorbid conditions | |||||
| Atrial fibrillation | 370 | 317 (86) | 0.06 | 0.71 (0.47–1.07) | 0.10 |
| Ischemic heart disease | 853 | 750 (88) | 0.48 | * | |
| Diabetes | 576 | 519 (90) | 0.06 | 1.72 (1.09–2.71) | 0.02 |
| COPD | 576 | 346 (87) | 0.29 | * | |
| Hypertension | 621 | 545 (88) | 0.52 | * | |
| Chronic renal insufficiency | 176 | 141 (80) | <0.001 | 0.43 (0.25–0.73) | 0.002 |
| Dementia or cancer | 189 | 162 (86) | 0.22 | * | |
* Variables significant at P < 0.20 on an intermediate block-based analysis (not shown) were included in the final multivariable model, as indicated by those variables with non-missing values in the multivariable results
** All P values reflect a test of trend (where applicable)
Table 3.
Age, Comorbidity, and Use of Beta Blockers in Hospital-based Clinics
| N | Use of beta blockers | ||||
|---|---|---|---|---|---|
| Bivariate results | P-value | Multivariable results | P-value | ||
| n (%) | OR (95% CI) | ||||
| AGE AND COMORBID BURDEN | |||||
| Age group | |||||
| 50–64 | 605 | 518 (86) | 0.001 | -- | 0.01 |
| 65–79 | 1110 | 937 (84) | 0.95 (0.70–1.28) | ||
| 80+ | 519 | 403 (78) | 0.66 (0.48–0.93) | ||
| Charlson comorbidity score | |||||
| 0–1 | 470 | 400 (85) | 0.43 | -- | 0.67 |
| 2–3 | 981 | 792 (81) | 0.77 (0.56–1.08) | ||
| 4–5 | 520 | 440 (85) | 1.00 (0.67–1.49) | ||
| 6+ | 263 | 226 (86) | 0.98 (0.59–1.62) | ||
| OTHER DEMOGRAPHIC CHARACTERISTICS | |||||
| Gender | |||||
| Female | 176 | 135 (77) | 0.03 | -- | 0.08 |
| Male | 2059 | 1723 (84) | 1.44 (0.95–2.17) | ||
| Race/ethnicity group | |||||
| White, non-Hispanic | 1343 | 1119 (83) | 0.70 | * | |
| Other | 343 | 287 (84) | |||
| Missing/unknown | 548 | 452 (83) | |||
| Income | |||||
| ≤ median income | 1099 | 897 (82) | 0.05 | -- | 0.25 |
| > median income | 1135 | 961 (85) | 1.15 (0.91–1.46) | ||
| HEALTH SERVICES UTILIZATION | |||||
| # primary care visits in past 2 years | |||||
| 0–1 | 271 | 210 (78) | <0.001 | -- | <0.001 |
| 2–4 | 919 | 750 (82) | 1.51 (1.04–2.19) | ||
| 5–10 | 784 | 671 (86) | 1.91 (1.28–2.84) | ||
| >10 | 260 | 227 (87) | 2.31 (1.38–3.88) | ||
| # cardiology visits in past 2 years | |||||
| 0 | 908 | 695 (77) | <0.001 | -- | <0.001 |
| 1–2 | 704 | 615 (87) | 1.96 (1.46–2.62) | ||
| >2 | 622 | 548 (88) | 1.99 (1.44–2.75) | ||
| Hospitalization in past 2 years | |||||
| No hospital stay | 1050 | 866 (82) | 0.65 | -- | 0.52 |
| ≥ 1 hospital stay where HF was primary diagnosis | 490 | 415 (85) | 0.99 (0.71–1.38) | ||
| ≥ 1 hospital stay, none of which where HF was primary diagnosis | 694 | 577 (83) | 0.86 (0.64–1.15) | ||
| ACADEMIC AFFILIATION OF TREATING FACILITY | |||||
| Affiliation of primary care site | |||||
| Not academically affiliated | 71 | 58 (82) | 0.05 | -- | 0.19 |
| Academically affiliated | 1987 | 1664 (84) | 0.91 (0.47–1.76) | ||
| Unknown/missing | 118 | 87 (74) | 0.60 (0.27–1.31) | ||
| MEDICATION USE AND CONDITIONS OF INTEREST | |||||
| # medications used | |||||
| ≤ 5 | 373 | 305 (82) | 0.53 | * | |
| 6 – 11 | 1170 | 979 (84) | |||
| ≥ 12 | 645 | 539 (84) | |||
| Comorbid conditions | |||||
| Atrial fibrillation | 679 | 550 (81) | 0.07 | * | |
| Ischemic heart disease | 1579 | 1344 (85) | <0.001 | 1.38 (1.07–1.77) | 0.01 |
| Diabetes | 1060 | 893 (84) | 0.20 | * | |
| COPD | 721 | 563 (78) | <0.001 | 0.56 (0.43–0.73) | <0.001 |
| Hypertension | 1112 | 905 (81) | 0.03 | * | |
| Chronic renal insufficiency | 289 | 243 (84) | 0.66 | * | |
| Dementia or cancer | 338 | 273 (81) | 0.20 | 0.76 (0.54–1.06) | 0.11 |
* Variables significant at P < 0.20 on an intermediate block-based analysis (not shown) were included in the final multivariable model, as indicated by those variables with non-missing values in the multivariable results
** All P values reflect a test of trend (where applicable)
Associations between age, comorbid burden, and receipt of guideline-recommended services were generally similar in community clinics. However, in part due to lower sample sizes no statistically significant difference in drug use across age groups was observed (P = 0.26 for ACE inhibitors or ARBs and P = 0.38 for beta blockers). There was no association between use of guideline-recommended drugs and comorbid burden (P = 0.96–0.99).
Associations between age and beta blocker use were almost identical when we restricted the analysis to include only beta blockers which are specifically recommended by guidelines (bisoprolol, carvedilol, and metoprolol succinate). Compared to patients age 50–64 years, the adjusted odds ratios of receiving a guideline-recommended beta blocker was 0.93 (95% CI, 0.75–1.15) for patients age 65–79 and 0.66 (95% CI, 0.51–0.85) for patients age 80 and older (P for trend = 0.002).
Reasons for not Prescribing Guideline-Recommended Drugs
Among 179 patients not receiving an ACE inhibitor or ARB, 55% (98) had a reason explicitly documented in the chart for not prescribing these medications (Fig. 1). Available data do not permit an accurate accounting of the specific reasons. An additional 15 patients without an explicit chart-documented reason had a clinical condition recorded in the electronic medical record which commonly contraindicates use of these drugs. Thus, 95% of patients (1,217/1,283) who did not have an identifiable reason for avoiding ACE inhibitors or ARBs were prescribed these drugs. The presence of chart-documented reasons for not prescribing guideline-recommended drugs did not vary by age or comorbid burden (P ≥ 0.15 for each; Table 4).
Table 4.
Prevalence of Reasons Documented in the Clinical Chart for not Prescribing ACE-inhibitors or ARBs and Beta Blockers: Variation by Age and Comorbid Burden
| Patients with chart-documented reasons for not prescribing… | ||||||||
|---|---|---|---|---|---|---|---|---|
| ACE inhibitors or ARBs | Beta Blockers | |||||||
| Characteristic | Bivariate results | P value | Multivariable results * | P value | Bivariate results | P value | Multivariable results * | P value |
| Age (years) | ||||||||
| 50-64 | 43% (12/28) | 0.73 | -- | 0.68 | 32% (33/103) | 0.90 | -- | 0.91 |
| 65-79 | 60% (56/93) | 2.04 (0.86-4.85) | 36% (84/233) | 1.20 (0.73-1.97) | ||||
| 80 + | 52% (30/58) | 1.48 (0.59-3.72) | 32% (48/150) | 1.01 (0.59-1.74) | ||||
| Charlson comorbidity score | ||||||||
| 1 | 47% (15/32) | 0.15 | -- | 0.15 | 28% (27/95) | 0.49 | -- | 0.49 |
| 2 to 3 | 52% (38/73) | 1.18 (0.51-2.74) | 36% (89/246) | 1.41 (0.84-2.37) | ||||
| 4 or more | 61% (45/74) | 1.73 (0.74-4.02) | 34% (49/145) | 1.25 (0.71-2.21) | ||||
* Analysis included only patients not receiving the medications of interest. Multivariable results adjusted for age and Charlson comorbidity score. All P values calculated using tests for trend.
Fewer patients not receiving beta blockers had clearly documented reasons for not receiving these medications (Fig. 1). Among 486 patients not receiving beta blockers, 34% (165) had a reason explicitly documented in the chart for not prescribing these medications. An additional 28 patients had a clinical condition which commonly contraindicates use of these drugs. Thus, 88% of patients (2,240/2,533) who did not have an identifiable reason for avoiding beta blockers were prescribed this type of drug. Patients with advanced age or high comorbid burden were no more or less likely to have a chart-documented reason for not prescribing beta blockers (P ≥ 0.49 for each; Table 4).
Finally, we repeated our analyses of the association between age, comorbid burden, and drug prescribing after excluding patients with explicit chart-documented reasons for not using our drugs of interest. Point estimates identified a similar degree of reduced use of ACE inhibitor or ARBs (OR 0.41, 95% CI 0.16–1.07), beta blockers overall (OR 0.73, 95% CI 0.48–1.10), and guideline-recommended beta blockers (OR 0.67, 95% CI 0.51–0.87) in patients age 80 years and older compared to those age 50–64 years. These associations no longer achieved statistical significance for ACE inhibitors or ARBs (P for trend = 0.07) or for beta blockers overall (P = 0.12), reflecting the smaller number of outcomes available for analysis, although they were significant for guideline-recommended beta blockers (P = 0.004). For comorbid burden, results were similar to our main analysis insofar as there was no significant or near-significant association between comorbid burden and use of either type of drug.
DISCUSSION
In this national study of ambulatory veterans with heart failure and reduced systolic function, 87% of subjects were prescribed an ACE inhibitor or ARB, and 82% were prescribed a beta blocker. Moreover, an additional one-third to one-half of patients not receiving these drugs had explicit documentation in the chart of reasons why they were not prescribed. On multivariable analyses, older patients were significantly less likely to receive ACE inhibitors or ARBs and beta blockers, with a substantial drop in use starting in patients age 65–79 years for ACE inhibitors/ARBs and in patients age 80 years and older for beta blockers. These age differences in drug treatment were no longer statistically significant after accounting for reasons explicitly cited by clinicians for not prescribing these drugs. However, we are unable to determine the specific reasons cited for non-prescribing and the extent to which they had clinical merit.
Our most straightforward—and perhaps most important—finding is that across a wide range of ages the strong majority of veterans with heart failure received guideline-recommended medications. This finding is consistent with previous studies in the United States. In the inpatient setting, rates of guideline-recommended medication use are high, with three recent national reports finding that at hospital discharge 78-85% of patients received an ACE inhibitor or ARB and 83-89% a beta blocker.6–8 Data on heart failure care in outpatients come from a variety of settings and time frames, and show greater heterogeneity in rates of guideline adherence. Several studies have found ambulatory treatment rates of approximately 58-82% for ACE inhibitors and ARBs and approximately 43-86% for beta blockers.29–32 The relatively high rates of guideline-concordant prescribing that we observed are consistent with reports for other conditions from VA, which have in part been attributed to VA’s coordinated efforts to improve care quality, including extensive use of clinical reminders (although the type and nature of reminders, including for heart failure, varies between facilities).26,33
Our other main finding—that use of guideline-recommended medications for heart failure is lower in older patients—is consistent with previous research as well.6,11–15 In recent studies of prescribing at hospital discharge, older patients were approximately 6-10% less likely to receive ACE inhibitors or ARBs and 5-8% less likely to receive beta blockers.6–8 These differences were attenuated—but for the most part did not entirely disappear– after controlling for a extensive set of potential confounders. Fewer studies have focused on age differences in care in outpatient settings in the United States. However, a number of studies from Europe have found consistently lower use of ACE-inhibitors and beta blockers in older outpatients, although many of these studies have methodologic limitations and had limited ability to account for potential confounders.13,34–36
Our results suggest that interpreting age differences in prescribing requires nuance. In our study, older patients had meaningfully lower rates of ACE inhibitor/ARB and beta blocker use in both absolute and relative terms, and the frequency of chart-documented reasons for not prescribing these drugs did not vary between age groups. In addition, differences in the inflection point between the drug types—with usage rates for ACE inhibitors and ARBs substantially dropping starting at age 65–79 years, and for beta blockers starting at age 80 and older—suggest a complex relation between age and treatment decisions for different drugs, even for the same condition. After removing patients with reasons for non-treatment from the denominator, point estimates of effect size for different age groups were similar but the associations between age and use of these treatments were no longer significant. It is difficult to know whether these reasons truly account for the observed treatment differences by age, or whether the associations persisted but were undetectable due to limited sample size. The presence of a persistently significant age effect when considering only the 3 beta blockers types specifically recommended by guidelines argues for (but does not prove) the latter explanation.
The manner in which data on reasons for non-prescribing were collected makes it impossible to closely examine the distribution of these reasons and the extent to which they were clinically justifiable. However, results from other studies in the outpatient setting present a mixed picture of the clinical appropriateness of not prescribing guideline-recommended medications to older patients. In a VA-based study of an intervention to improve guideline-concordant care in heart failure, physicians commonly cited patients’ inability to tolerate therapy as the reason for not following guideline recommendations.37 In other studies, half of patients not prescribed beta blockers and two-thirds to nearly all patients not prescribed ACE inhibitors had an identifiable clinical contraindication to drug therapy.38,39 However, many of these clinical contraindications are relative, not absolute. Previous work has shown that most older adults, even those with relative contraindications, can tolerate these medications, yet that many clinicians cite concern over side effects to ACE inhibitors and beta blockers as major barriers to treatment.7,23,40–42 Moreover, other work suggests that clinical inertia, lack of understanding of guideline recommendations and doubts about their applicability, and difficulty accessing care are substantial barriers to more complete use of these drugs.23,41–43
Our study has several important limitations. The chart review process used only the most recent test result to assess LVEF, although this measure varies over time and often improves with treatment. Nonetheless, this method is consistent with other major studies of adherence to heart failure guidelines.6 In addition, it is unclear to what extent our findings generalize to other health care systems. However, our overall findings are consistent with those observed in other, non-VA studies. Moreover, the VA has served as a bellwether and model system for evaluating many quality issues with wide applicability.26,44
In summary, we found that there was a clinically important drop in prescribing rates in older age groups that was not explained by a wide variety of factors, but that these differences were more difficult to detect after accounting for reasons cited in the clinical chart for non-prescribing. Patients in the upper reaches of age are different from their younger counterparts, and unique considerations need to be evaluated in making treatment decisions for this vulnerable group. Nonetheless, given the major clinical benefits of ACE inhibitors and beta blockers for the treatment of systolic heart failure, reasons for not prescribing these drugs should be compelling and clearly documented, and further work is needed to determine whether the reasons clinicians cite for non-prescribing to older patients represent good clinical judgment or suboptimal care. This approach can account for the special circumstances of older patients while avoiding the clinical inertia, unjustified fears, and subtle ageism that can result in underuse of these valuable agents.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Online Appendix: Classification of reasons for not prescribing guideline-recommended drugs (DOC 40 kb).
Acknowledgements
Contributors The authors thank Sharon Goodman for her help procuring and interpreting data from VA’s EPRP system.
Funding Sources This work was funded by the VA Health Services Research and Development Service (IIR 06-080-2, Dr. Steinman) and by the National Institute on Aging and American Federation for Aging Research (K23-AG030999, Dr. Steinman). The funding sources had no control over the analysis, writing, or decision to publish this manuscript.
Prior Presentation This work was presented at the annual meeting of the Society for General Internal Medicine, Minneapolis, MN, May 2010.
Disclaimer Any opinions expressed in this manuscript are those of the authors and do not reflect the official position of the Department of Veterans Affairs.
Conflicts of Interest None disclosed.
References
- 1.Fonarow GC. Quality indicators for the management of heart failure in vulnerable elders. Ann Intern Med. 2001;135(8 Pt 2):694–702. doi: 10.7326/0003-4819-135-8_part_2-200110161-00008. [DOI] [PubMed] [Google Scholar]
- 2.Hunt SA, Abraham WT, Chin MH, et al. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112(12):e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 3.Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med. 1991;325(5):293–302. [DOI] [PubMed]
- 4.Foody JM, Farrell MH, Krumholz HM. beta-Blocker therapy in heart failure: scientific review. JAMA. 2002;287(7):883–889. doi: 10.1001/jama.287.7.883. [DOI] [PubMed] [Google Scholar]
- 5.Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med. 1999;341(10):709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 6.Masoudi FA, Rathore SS, Wang Y, et al. National patterns of use and effectiveness of angiotensin-converting enzyme inhibitors in older patients with heart failure and left ventricular systolic dysfunction. Circulation. 2004;110(6):724–731. doi: 10.1161/01.CIR.0000138934.28340.ED. [DOI] [PubMed] [Google Scholar]
- 7.Fonarow GC, Abraham WT, Albert NM, et al. Age- and gender-related differences in quality of care and outcomes of patients hospitalized with heart failure (from OPTIMIZE-HF) Am J Cardiol. 2009;104(1):107–115. doi: 10.1016/j.amjcard.2009.02.057. [DOI] [PubMed] [Google Scholar]
- 8.Forman DE, Cannon CP, Hernandez AF, Liang L, Yancy C, Fonarow GC. Influence of age on the management of heart failure: findings from Get With the Guidelines-Heart Failure (GWTG-HF) Am Heart J. 2009;157(6):1010–1017. doi: 10.1016/j.ahj.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhry SI, Berlowitz DR, Concato J. Do age and comorbidity affect intensity of pharmacological therapy for poorly controlled diabetes mellitus? J Am Geriatr Soc. 2005;53(7):1214–1216. doi: 10.1111/j.1532-5415.2005.53370.x. [DOI] [PubMed] [Google Scholar]
- 10.Glynn RJ, Monane M, Gurwitz JH, Choodnovskiy I, Avorn J. Aging, comorbidity, and reduced rates of drug treatment for diabetes mellitus. J Clin Epidemiol. 1999;52(8):781–790. doi: 10.1016/S0895-4356(99)00055-4. [DOI] [PubMed] [Google Scholar]
- 11.Hood S, Taylor S, Roeves A, et al. Are there age and sex differences in the investigation and treatment of heart failure? A population-based study. Br J Gen Pract. 2000;50(456):559–563. [PMC free article] [PubMed] [Google Scholar]
- 12.Hulsmann M, Berger R, Mortl D, Pacher R. Influence of age and in-patient care on prescription rate and long-term outcome in chronic heart failure: a data-based substudy of the EuroHeart Failure Survey. Eur J Heart Fail. 2005;7(4):657–661. doi: 10.1016/j.ejheart.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Muntwyler J, Cohen-Solal A, Freemantle N, Eastaugh J, Cleland JG, Follath F. Relation of sex, age and concomitant diseases to drug prescription for heart failure in primary care in Europe. Eur J Heart Fail. 2004;6(5):663–668. doi: 10.1016/j.ejheart.2004.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Lee DS, Tu JV, Juurlink DN, et al. Risk-treatment mismatch in the pharmacotherapy of heart failure. JAMA. 2005;294(10):1240–1247. doi: 10.1001/jama.294.10.1240. [DOI] [PubMed] [Google Scholar]
- 15.Bertoni AG, Duren-Winfield V, Ambrosius WT, et al. Quality of heart failure care in managed Medicare and Medicaid patients in North Carolina. Am J Cardiol. 2004;93(6):714–718. doi: 10.1016/j.amjcard.2003.11.053. [DOI] [PubMed] [Google Scholar]
- 16.Scott IA, Guyatt GH. Cautionary tales in the interpretation of clinical studies involving older persons. Arch Intern Med. 2010;170(7):587–595. doi: 10.1001/archinternmed.2010.18. [DOI] [PubMed] [Google Scholar]
- 17.Dulin BR, Krum H. Drug therapy of chronic heart failure in the elderly: the current state of clinical-trial evidence. Curr Opin Cardiol. 2006;21(4):393–399. doi: 10.1097/01.hco.0000231411.15049.20. [DOI] [PubMed] [Google Scholar]
- 18.Flather MD, Shibata MC, Coats AJ, et al. Randomized trial to determine the effect of nebivolol on mortality and cardiovascular hospital admission in elderly patients with heart failure (SENIORS) Eur Heart J. 2005;26(3):215–225. doi: 10.1093/eurheartj/ehi115. [DOI] [PubMed] [Google Scholar]
- 19.Erdmann E, Lechat P, Verkenne P, Wiemann H. Results from post-hoc analyses of the CIBIS II trial: effect of bisoprolol in high-risk patient groups with chronic heart failure. Eur J Heart Fail. 2001;3(4):469–479. doi: 10.1016/S1388-9842(01)00174-X. [DOI] [PubMed] [Google Scholar]
- 20.Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344(22):1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 21.Deedwania PC, Gottlieb S, Ghali JK, Waagstein F, Wikstrand JC. Efficacy, safety and tolerability of beta-adrenergic blockade with metoprolol CR/XL in elderly patients with heart failure. Eur Heart J. 2004;25(15):1300–1309. doi: 10.1016/j.ehj.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 22.Boyd CM, Darer J, Boult C, Fried LP, Boult L, Wu AW. Clinical practice guidelines and quality of care for older patients with multiple comorbid diseases: implications for pay for performance. JAMA. 2005;294(6):716–724. doi: 10.1001/jama.294.6.716. [DOI] [PubMed] [Google Scholar]
- 23.Fuat A, Hungin AP, Murphy JJ. Barriers to accurate diagnosis and effective management of heart failure in primary care: qualitative study. BMJ. 2003;326(7382):196. doi: 10.1136/bmj.326.7382.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masoudi FA, Havranek EP, Smith G, et al. Gender, age, and heart failure with preserved left ventricular systolic function. J Am Coll Cardiol. 2003;41(2):217–223. doi: 10.1016/S0735-1097(02)02696-7. [DOI] [PubMed] [Google Scholar]
- 25.Masoudi FA, Baillie CA, Wang Y, et al. The complexity and cost of drug regimens of older patients hospitalized with heart failure in the United States, 1998–2001. Arch Intern Med. 2005;165(18):2069–2076. doi: 10.1001/archinte.165.18.2069. [DOI] [PubMed] [Google Scholar]
- 26.Jha AK, Perlin JB, Kizer KW, Dudley RA. Effect of the transformation of the Veterans Affairs Health Care System on the quality of care. N Engl J Med. 2003;348(22):2218–2227. doi: 10.1056/NEJMsa021899. [DOI] [PubMed] [Google Scholar]
- 27.Borzecki AM, Wong AT, Hickey EC, Ash AS, Berlowitz DR. Identifying hypertension-related comorbidities from administrative data: what's the optimal approach? Am J Med Qual. 2004;19(5):201–206. doi: 10.1177/106286060401900504. [DOI] [PubMed] [Google Scholar]
- 28.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi: 10.1016/0895-4356(92)90133-8. [DOI] [PubMed] [Google Scholar]
- 29.Fonarow GC, Yancy CW, Albert NM, et al. Heart failure care in the outpatient cardiology practice setting: findings from IMPROVE HF. Circ Heart Fail. 2008;1(2):98–106. doi: 10.1161/CIRCHEARTFAILURE.108.772228. [DOI] [PubMed] [Google Scholar]
- 30.Matthews JC, Johnson ML, Koelling TM. The impact of patient-specific quality-of-care report cards on guideline adherence in heart failure. Am Heart J. 2007;154(6):1174–1183. doi: 10.1016/j.ahj.2007.08.007. [DOI] [PubMed] [Google Scholar]
- 31.Goff DC, Jr, Massing MW, Bertoni AG, et al. Enhancing quality of heart failure care in managed Medicare and Medicaid in North Carolina: results of the North Carolina Achieving Cardiac Excellence (NC ACE) Project. Am Heart J. 2005;150(4):717–724. doi: 10.1016/j.ahj.2004.12.025. [DOI] [PubMed] [Google Scholar]
- 32.Smith NL, Chan JD, Rea TD, et al. Time trends in the use of beta-blockers and other pharmacotherapies in older adults with congestive heart failure. Am Heart J. 2004;148(4):710–717. doi: 10.1016/j.ahj.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Sinha S, Goldstein M, Penrod J, et al. Brief report: beta-blocker use among veterans with systolic heart failure. J Gen Intern Med. 2006;21(12):1306–1309. doi: 10.1111/j.1525-1497.2006.00601.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Groote P, Isnard R, Assyag P, et al. Is the gap between guidelines and clinical practice in heart failure treatment being filled? Insights from the IMPACT RECO survey. Eur J Heart Fail. 2007;9(12):1205–1211. doi: 10.1016/j.ejheart.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 35.Shah SM, Carey IM, DeWilde S, Richards N, Cook DG. Trends and inequities in beta-blocker prescribing for heart failure. Br J Gen Pract. 2008;58(557):862–869. doi: 10.3399/bjgp08X376195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koschack J, Jung HH, Scherer M, Kochen MM. Prescriptions of recommended heart failure medications can be correlated with patient and physician characteristics. Int J Clin Pract. 2009;63(2):226–232. doi: 10.1111/j.1742-1241.2008.01937.x. [DOI] [PubMed] [Google Scholar]
- 37.Keeffe B, Subramanian U, Tierney WM, et al. Provider response to computer-based care suggestions for chronic heart failure. Med Care. 2005;43(5):461–465. doi: 10.1097/01.mlr.0000160378.53326.f3. [DOI] [PubMed] [Google Scholar]
- 38.Baker DW, Persell SD, Thompson JA, et al. Automated review of electronic health records to assess quality of care for outpatients with heart failure. Ann Intern Med. 2007;146(4):270–277. doi: 10.7326/0003-4819-146-4-200702200-00006. [DOI] [PubMed] [Google Scholar]
- 39.Bart BA, Gattis WA, Diem SJ, O'Connor CM. Reasons for underuse of angiotensin-converting enzyme inhibitors in patients with heart failure and left ventricular dysfunction. Am J Cardiol. 1997;79(8):1118–1120. doi: 10.1016/S0002-9149(97)00060-X. [DOI] [PubMed] [Google Scholar]
- 40.Witham MD, Gillespie ND, Struthers AD. Age is not a significant risk factor for failed trial of beta-blocker therapy in older patients with chronic heart failure. Age Ageing. 2004;33(5):467–472. doi: 10.1093/ageing/afh150. [DOI] [PubMed] [Google Scholar]
- 41.Phillips SM, Marton RL, Tofler GH. Barriers to diagnosing and managing heart failure in primary care. Med J Aust. 2004;181(2):78–81. doi: 10.5694/j.1326-5377.2004.tb06178.x. [DOI] [PubMed] [Google Scholar]
- 42.Steinman MA, Patil S, Kamat P, Peterson C, Knight SJ. A taxonomy of reasons for not prescribing guideline-recommended medications for patients with heart failure. Am J Geriatr Pharmacother. 2010;8(6):583–594. doi: 10.1016/S1543-5946(10)80007-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Parameswaran AC, Tang WH, Francis GS, Gupta R, Young JB. Why do patients fail to receive beta-blockers for chronic heart failure over time? A "real-world" single-center, 2-year follow-up experience of beta-blocker therapy in patients with chronic heart failure. Am Heart J. 2005;149(5):921–926. doi: 10.1016/j.ahj.2004.07.026. [DOI] [PubMed] [Google Scholar]
- 44.Kerr EA, Zikmund-Fisher BJ, Klamerus ML, Subramanian U, Hogan MM, Hofer TP. The role of clinical uncertainty in treatment decisions for diabetic patients with uncontrolled blood pressure. Ann Intern Med. 2008;148(10):717–727. doi: 10.7326/0003-4819-148-10-200805200-00004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Below is the link to the electronic supplementary material.
Online Appendix: Classification of reasons for not prescribing guideline-recommended drugs (DOC 40 kb).