A 71-year old man was referred for recurrent bilateral, transudative pleural effusions of unknown etiology. Over the previous five months, he had had multiple admissions during which the effusions were drained a total of 15 times, with each procedure removing 1–2 L from both sides. When the effusions were present, he noted mild dyspnea and orthopnea, but these symptoms resolved after thoracentesis. He also noted anorexia and a 30-pound weight loss over the same time period. He denied paroxysmal nocturnal dyspnea, chest pain, lower extremity edema, lightheadedness, presyncope, palpitations, fever, chills, night sweats, nausea, vomiting or changes in his bowel and bladder habits. During his initial admission, an electrocardiogram (ECG) demonstrated a normal sinus rhythm with non-specific ST-TW changes but no other abnormalities. An echocardiogram demonstrated a normal ejection fraction, mild mitral and tricuspid regurgitation, normal right and left ventricle size with septal ventricular hypertrophy (14 mm, normal 7–11 mm), and mild right and left atrial enlargement. Left and right heart catheterizations were normal. After his initial admission, treatment with bumetanide 2 mg twice daily did not prevent reaccumulation of the effusions. He had undergone three thoracenteses in the last week, most recently on the day prior to his admission.
The presence of chronic, recurrent, bilateral transudative pleural effusions raises several possibilities. Heart failure is the most common cause of bilateral transudative pleural effusions. The presence of biatrial enlargement and normal ventricular systolic function in an elderly man with hypertension makes diastolic dysfunction an important consideration. The septal hypertrophy could imply an infiltrative process, but may also occur in hypertrophic or hypertensive cardiomyopathy. Nephrotic syndrome can present with pleural effusions but the weight loss and lack of peripheral edema make it less likely. Hepatic hydrothorax can uncommonly occur in patients without ascites, but bilateral effusions would be unusual. Considering the patient’s advanced age and significant weight loss, malignancy remains on the differential, although cancers typically cause exudative effusions. The weight loss makes hypothyroidism, in which the pleural effusions are usually small and asymptomatic, unlikely as well.
The problem representation is an abstract one-sentence summary of the key features of the case synthesized in the clinician’s mind and only sometimes explicitly spoken or written. At this point, the problem representation is refractory, recurrent, bilateral transudative pleural effusions and weight loss. In the traditional medical encounter the problem representation is usually developed after collecting clinical data. This case is more typical of referrals, transfers, and some consultations, where the problem may be clearly defined at the outset (the very first sentence of this case). In these instances, data collection focuses on verifying or further refining this representation and narrowing the list of possibilities.
He has a history of prostate and renal cell carcinomas that were resected in 1993. He was not treated with chemotherapy or radiation. The only other past medical history was hypertension and chronic kidney disease (baseline blood urea nitrogen 65 mg/dl and creatinine 2.4 mg/dl), which had been stable over the past year. He worked as a schoolteacher for most of his life, except for 7 years in the 1960s when he worked as a machinist building parts for airplanes (which exposed him to asbestos). He did not smoke, drink alcohol, or use illicit drugs. On admission his only medications were bumetanide, multivitamins, and fish oil. He previously took lisinopril, hydrochlorothiazide, montelukast, and albuterol inhaler, but discontinued these 2 months earlier based on his doctor’s recommendation. Over the last 5 months his blood pressure had gradually decreased from 140/90 to 70/50 mm Hg. Approximately one month prior to the current admission, he was started and then maintained on a stable dose of bumetanide 2 mg twice daily. His family history was significant for stomach cancer, colon cancer, diabetes, and coronary artery disease. He was born and raised in the United States and had not traveled recently.
His personal history of renal cell and prostate cancers, as well as his strong family history, increase concern for malignancy. It would be unusual for a solid tumor to cause a transudative effusion or to present only as bilateral effusions without evidence of metastases. Asbestos typically causes unilateral, exudative effusions and pleural plaques; his distant and limited exposure history makes asbestosis unlikely. The development of slowly progressive, asymptomatic hypotension raises the possibility of chronic conditions that impair cardiac output or lower systemic vascular resistance, such as end-stage liver disease or adrenal insufficiency, although he does not have risk factors for either condition. In an elderly patient with profound but slowly progressive hypotension, an early consideration is pump failure due to prior myocardial infarction or progressive cardiomyopathy, commonly caused by uncontrolled hypertension or valvular disease. The latter two possibilities are unlikely given the relatively normal echocardiogram several months earlier.
On admission he was a thin, well-appearing elderly gentleman with a temperature of 35.8°C, heart rate of 100 beats per minute sitting, blood pressure 60/46 mm Hg, respiratory rate of 20 breaths per minute, and oxygen saturation of 97% on room air. There was no significant change in blood pressure or heart rate from sitting to standing positions. The mucous membranes were moist. There was no lymphadenopathy. Cardiac exam revealed diminished heart sounds with no murmurs, rubs, or gallops. His jugular venous pressure was normal; no Kussmaul’s sign or pulsus paradoxus was detected. His chest was clear to auscultation; no accessory muscle use, decreased breath sounds, or dullness to percussion were noted. His abdominal, neurologic, skin and musculoskeletal exams were normal. He had no lower extremity edema.
The current exam reveals no signs of left or right heart failure, end-stage liver disease, or nephrotic syndrome. Having undergone thoracentesis the day previously, he no longer has evidence of pleural effusions. Overdiuresis might explain the low blood pressure and tachycardia, although in the past he had not apparently responded to diuretics. Diminished heart sounds are non-specific; but the combination of diminished heart sounds, tachycardia, and hypotension brings to mind cardiac tamponade, although this triad is seen in the minority of cases. While previous testing would have excluded a significant pericardial effusion, the possibility of this serious manifestation developing at this point in the illness warrants consideration. Constrictive pericarditis can present with refractory transudative effusions without pulmonary edema, normal left ventricular systolic function and characteristic diastolic abnormalities on echocardiogram. However pericardial imaging on echocardiogram has significant limitations and so should not be relied upon to exclude constrictive pericarditis. However, these patients typically have elevated jugular venous pressure with significant lower extremity edema, and severe hypotension is unusual. At this point, three leading problems have emerged: recurrent transudative pleural effusions, weight loss, and severe hypotension.
Problem representations evolve over time–spanning minutes in a patient encounter or days to months in complex undiagnosed cases. At this point the final solution must also explain severe hypotension. The low blood pressure in combination with decreased heart sounds triggers consideration of an emergent condition (pericardial tamponade), but does not yield a unifying diagnosis consistent with the physical examination (due to the absence of jugular venous distension).
His chemistries were notable for blood urea nitrogen of 65 mg/dl (normal range 10–20) and creatinine of 2.5 mg/dl (normal range 0.5–1.3). Bilirubin, alkaline phosphatase, and transaminases were normal. His albumin was 3gm/dl (normal range 3.4–4.8). His complete blood count and coagulation studies were normal. His creatine kinase (CK), and CK–MB fraction were normal, but the troponin I was 0.46 ng/ml (normal range <0.05). B-natriuretic peptide (BNP) was 2,466 pg/ml (normal range 0–100). An early morning cortisol was greater than 20ug/dl. Thyroid stimulating hormone, angiotensin converting enzyme, and antinuclear antibody levels were normal. Ferritin was 33 ng/ml (normal range 30–300), iron 20 μg/dl (normal: 49–181), total iron binding capacity 360 μg/dl (normal: 228–428), and iron saturation 6% (normal range 25–72). Urinalysis was normal. An electrocardiogram (ECG) showed sinus rhythm at 82 beats per minute, multiple premature ventricular complexes, left atrial enlargement, and decreased voltage in the limb leads (Fig. 1). A chest x-ray showed a normal heart size, bilateral hilar fullness, and mild bilateral costophrenic angle blunting.
Figure 1.
Admission ECG for current hospitalization.
The normal urinalysis and coagulation studies rule out nephrotic syndrome and end-stage liver disease, respectively, given the low pre-test probabilities for these conditions. The elevated BNP suggests an underlying cardiac process, although some BNP elevation might be expected from his reduced glomerular filtration rate. The low voltage on ECG observed in the limb leads suggests pericardial disease (particularly in a thin patient), but typically such diseases lead to a diffuse reduction in voltage. The extremely elevated BNP favors a myocardial process such as restrictive cardiomyopathy over constrictive pericarditis. A small proportion of patients with restrictive cardiomyopathies present without elevated jugular venous pressure or lower extremity edema. Infiltrative restrictive cardiomyopathies, including sarcoidosis and amyloidosis, could also explain the mildly elevated troponin due to direct myocardial injury. Hemochromatosis would be an important consideration, but his age, absence of diabetes or liver function abnormalities, and low iron saturation exclude this diagnosis. His unchanged chronic renal dysfunction and bland urine sediment lower the concern for superimposed renal pathology. The cortisol level excludes adrenal insufficiency as a cause of his hypotension.
The priority given to certain findings by the discussant drives the revised problem representation and ensuing diagnostic considerations. The decreased limb voltage on ECG and elevated biomarkers are emphasized (although a number of negative findings are integrated as well). What follows is a comparison of two competing illness scripts (mental representations of diseases in memory)—constrictive pericarditis (discussed previously) and restrictive cardiomyopathy (new hypothesis). Some laboratory values change disease probabilities significantly, such as the normal urinalysis and serum cortisol, while others are equivocal but suggestive. The significantly elevated BNP and mildly elevated troponin suggest a myocardial rather than pericardial disorder. The normal urinalysis dissuades the discussant from linking the chronic kidney disease to the final solution.
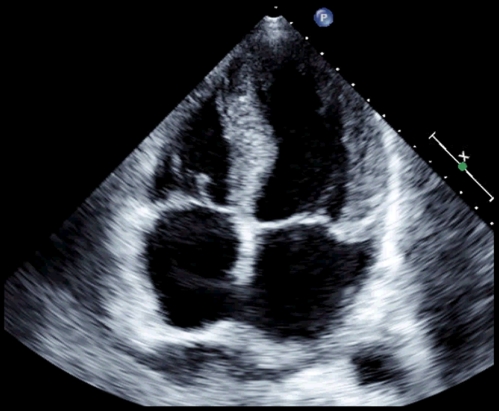
A transthoracic echocardiogram (Fig. 2) showed a left ventricular ejection fraction of 40%, increased left ventricular wall thickness, mildly reduced right ventricular systolic function, normal sized right and left ventricles, severe biatrial enlargement, increased right ventricular systolic pressure of 40–50 mmHg (normal 15–25), and no pericardial effusion. A computed tomography of the chest demonstrated reaccumulation of bilateral pleural effusions and no parenchymal infiltrates or lymphadenopathy.
Figure 2.
Transthoracic echocardiogram, apical four chamber view, systolic phase of cardiac cycle.
The normal sized ventricles with mild systolic dysfunction and severe biatrial enlargement suggest a restrictive cardiomyopathy. The increased left ventricular wall thickness may be consistent with his previous history of hypertension (particularly if uncontrolled) but decreased voltage in the limb leads contradict that interpretation, once again raising suspicion for an infiltrative cardiomyopathy. The absence of thoracic lymphadenopathy or pulmonary infiltrates on CT make sarcoidosis unlikely; although a rare presentation of isolated cardiac sarcoidosis can only be excluded with an endomyocardial biopsy. As hemochromatosis and sarcoidosis appear unlikely, amyloidosis emerges as a leading diagnosis. The hypotension could be explained by amyloidosis-associated autonomic neuropathy. Although normal cardiac hemodynamic measurements by catheterization during his initial hospitalization would not be expected, he may have been hypovolemic at the time, leading to a falsely negative result.
The discussant notes the ventricular wall thickness and paradoxically decreased limb voltage. This discrepancy is both at odds with the illness script for hypertension-associated ventricular hypertrophy and may have triggered a rule-of-thumb acquired from experience (“LVH on echo with low voltage on EKG—think infiltrative cardiomyopathy”). Although the case for amyloidosis cannot be made stronger with the information at hand, its probability is elevated by excluding the competing diagnoses of sarcoidosis and hemochromatosis. Possible explanations for the discrepancy between the original echocardiogram and the more recent study include: incomplete original information (e.g. summary instead of complete report, or even a synopsis such as “normal”); variations in the patient’s physiology or image acquisition; or evolution of the disease over time.
On hospital day 3 the patient reported dyspnea and orthopnea. A chest x-ray showed worsening large bilateral pleural effusions. A repeat computed tomography of the chest demonstrated bilateral pleural effusions. Pleural fluid was transudative with 450 nucleated cells; the differential count was 90% lymphocytes, 7% monocytes, 1% neutrophils. Adenosine deaminase, cytology, gram stain, and acid-fast bacilli culture of the fluid were all normal.
The rapidity of the accumulation could reflect intravenous fluid administration for the patient’s hypotension. However, in decompensated heart failure, it is unusual for such marked pleural transudation to occur without any pulmonary edema, suggesting the possibility of pleural disease as an additional contributor to the effusions. Although the effusion is transudative, the preponderance of lymphocytic cells suggests chronic pleural inflammation or invasion, as can be seen in amyloidosis. The clinical picture and echocardiogram support a diagnosis of restrictive cardiomyopathy secondary to amyloidosis. An endomyocardial biopsy could provide pathological confirmation.
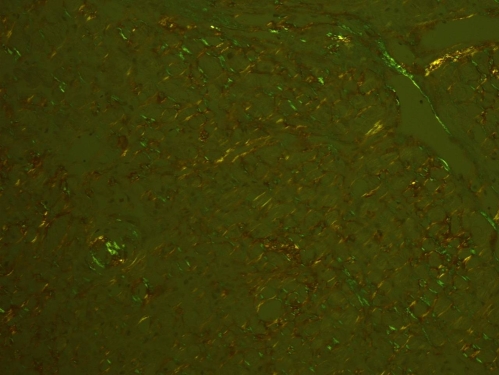
On hospital day 4, a right heart catheterization showed a mildly decreased cardiac output, and increased right atrial, right ventricular, pulmonary arterial, and pulmonary wedge pressures. Endomyocardial biopsy showed classic apple green birefringence on Congo red staining in a pattern consistent with amyloid, myocyte hypertrophy and degenerative changes (Fig. 3). A SPEP showed moderate hypoproteinemia, moderate hypoalbuminemia, and moderate hypogammaglobulinemia. A serum immunofixation electrophoresis showed a small band in the IgM and corresponding kappa lane within a polyclonal background, and two additional small bands in the lambda lane. A UPEP and urine immunoelectrophoresis were both normal. Serum free light chain assay showed normal kappa light chain levels and an elevated lambda light chain level of 88.40 mg/L (normal range 5.70–26.30).
Figure 3.
Right ventricular endomyocardial biopsy demonstrating apple green birefringence.
The patient deteriorated rapidly on hospital day 6, requiring hemodynamic support with multiple vasopressors. A repeat transthoracic echocardiogram showed an ejection fraction of 20%, further biatrial enlargement, and increased biventricular dysfunction and wall thickness. The patient died on hospital day 9 prior to receiving treatment for amyloidosis. The myocardial tissue was characterized post-mortem by the Mayo Clinic laboratory as containing AL amyloid.
DISCUSSION
The development and refinement of a problem representation, as highlighted in earlier articles in this series, is the critical step that allows the clinician to match the patient’s words and data with illness scripts in the physician’s memory.1,2 The traditional patient encounter requires sequential data collection to develop a problem representation. There are other situations (e.g., consultation, referral, or transfer) in which an explicit problem representation is presented at the outset, as in this case. This early framing does not free the consulting clinician from doing repeated or more in depth data collection to verify the primary data; but subtly changes the major cognitive duty from problem representation development to problem representation verification/rejection and further characterization. In return for having a large amount of data acquired and filtered, the accepting physician must be mindful of premature closure, confirmation bias, and framing effects that may influence their thinking.
The textbook approach for reaching a diagnosis is finding supporting evidence from the history, physical examination, laboratory, and imaging studies that matches a particular illness script. A complementary strategy highlighted less often but used regularly in practice is the elimination of competing hypotheses. Theoretically, the sum of all possible diagnoses must equal 100%. In this case, when the discussant discovered features going against constrictive pericarditis (e.g. lack of edema), restrictive cardiomyopathy increased in probability. When no further information supporting amyloidosis as the explanation for the restrictive cardiomyopathy could be gleaned (e.g., nephrotic syndrome or sensorimotor peripheral neuropathy), exclusion of competing infiltrative cardiomyopathies (e.g., sarcoidosis and hemochromatosis) bolstered the likelihood of amyloidosis. This justified an invasive test, endomycardial biopsy, to confirm the diagnosis. Both the “rule in” (textbook) and “rule out” (utilized in this case) strategy require robust illness scripts buttressed by literature searches when rare disorders are being considered.
The framework assumes that the ultimate unifying diagnoses are mutually exclusive. However the clinician is frequently left trying to bolster the case for Dx1 only by downgrading the probability of Dx2 with the nagging feeling that an unsuspected or unknown (either to the clinician or to medical science) diagnosis (Dx3) explains the patient’s illness. There is no fail-proof way to safeguard against this possibility, but the establishment of a clear problem representation is the critical step in leveraging other human and electronic resources to ponder the same question before crossing a diagnostic or treatment threshold.
CLINICAL TEACHING POINTS
Unexplained recurrent transudative bilateral pleural effusions should prompt an evaluation for constrictive pericarditis and restrictive cardiomyopathy after more common causes, such as other cardiomyopathies, cirrhosis, nephrotic syndrome, and pulmonary embolism have been excluded.3 Unlike other cardiac causes, constrictive pericarditis and restrictive cardiomyopathy sometimes present without pulmonary edema.4,5
Clinicians can use basic bedside, laboratory, and echocardiographic information to distinguish restrictive cardiomyopathy from constrictive pericarditis. Though elevated jugular venous pressure and peripheral edema are nearly always associated with constrictive pericarditis, these findings may be absent in cardiac amyloidosis.4 In addition, a significantly elevated B-natriuretic peptide may help distinguish intrinsic myocardial disorders such as restrictive cardiomyopathy from constrictive pericarditis; although the values may overlap in these two conditions and lose specificity in patients with a reduced glomerular filtration rate.6–8 Ultimately, advanced echocardiographic findings (particularly Doppler imaging) and hemodynamic measurements by cardiac catheterization are required for definitive diagnosis.
The pattern of biatrial enlargement with increased ventricular wall thickness and normal ventricular size on echocardiography suggests a restrictive cardiomyopathy, often due to amyloidosis.9 Common ECG abnormalities include low limb lead voltage and a pseudoinfarct pattern.10
The combination of restrictive cardiomyopathy and neuropathy is suggestive of amyloidosis. In this case, the progressive hypotension raised suspicion for an autonomic neuropathy. Although hypotension may be seen in 11–44% of patients with amyloidosis, orthostatic hypotension is unusual.4,11
Primary Amyloidosis (AL) is a rare plasma cell dyscrasia in which the lambda or kappa light chains of antibodies are overproduced and deposited in various organs (e.g., heart, kidney, peripheral nerves), leading to dysfunction. AL amyloidosis can only be diagnosed by tissue biopsy from either the involved organs or abdominal fat pad.12,13
The median survival for AL amyloidosis is six to nine months in patients with heart failure, but may be as low as 2 months in patients with persistent pulmonary pleural effusions.4,14,15
Acknowledgments
Conflict of Interest Statement None disclosed.
Funding Source None.
Footnotes
An erratum to this article can be found at http://dx.doi.org/10.1007/s11606-011-1846-y
References
- 1.Keenan CR, Dhaliwal G, Henderson MC, Bowen JL. Exercises in clinical reasoning: a 43-year-old woman with abdominal pain and fever. J Gen Intern Med. 2010;25(8):874–877. doi: 10.1007/s11606-010-1372-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Henderson MC, Dhaliwal G, Jones SR, Culbertson C, Bowen JL. Exercises in clinical reasoning: doing what comes naturally. J Gen Intern Med. 2010;25:84–87. doi: 10.1007/s11606-009-1187-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bope ET, Rakel RE, Kellerman R, editors. Conn's current therapy. Philadelphia: Saunders; 2011. [Google Scholar]
- 4.Dubrey SW, Cha K, Anderson J, Chamarthi B, Eisinger JR, Skinner M, Falk RH. The clinical features of immunoglobulin light-chain (al) amyloidosis with heart involvement. Q J Med. 1998;91:141–157. doi: 10.1093/qjmed/91.2.141. [DOI] [PubMed] [Google Scholar]
- 5.Goldstein JA. Cardiac tamponade, constrictive pericarditis, and restrictive cardiomyopathy. CurrProblCardiol. 2004;29:503–567. doi: 10.1016/j.cpcardiol.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 6.Leya FS, Arab D, Joyal D, Shioura KM, Lewis BE, Steen LH, Cho L. The efficacy of brain natriuretic peptide levels in differentiating constrictive pericarditis from restrictive cardiomyopathy. J Am CollCardiol. 2005;45(11):1900–1902. doi: 10.1016/j.jacc.2005.03.050. [DOI] [PubMed] [Google Scholar]
- 7.Miller WL, Wright RS, McGregor CG, et al. Troponin levels in patients with amyloid cardiomyopathy undergoing cardiac transplantation. Am J Cardiol. 2001;88:813–815. doi: 10.1016/S0002-9149(01)01877-X. [DOI] [PubMed] [Google Scholar]
- 8.Reddy PR, Dieter RS, Das P, Steen LH, Lewis BE, Leya FS. Utility of BNP in differentiating constrictive pericarditis from restrictive cardiomyopathy in patients with renal insufficiency. J Card Fail. 2007;13(8):668–671. doi: 10.1016/j.cardfail.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 9.Siqueira-Filho AG, Cunha CL, Tajik AJ, Seward JB, Schattenberg TT, Giuliani ER. M-mode and two-dimensional echocardiographic features in cardiac amyloidosis. Circulation. 1981;63(1):188–196. doi: 10.1161/01.CIR.63.1.188. [DOI] [PubMed] [Google Scholar]
- 10.Murtagh B, Hammill SC, Gertz MA, Kyle RA, Tajik AJ, Grogan M. Electrocardiographic findings in primary systemic amyloidosis and biopsy-proven cardiac involvement. Am J Cardiol. 2005;95(4):535–537. doi: 10.1016/j.amjcard.2004.10.028. [DOI] [PubMed] [Google Scholar]
- 11.Kyle RA, Kattke BA, Schirger A. Orthostatic hypotension as a clue to primary systemic amyloidosis. Circulation. 1966;34:883–888. doi: 10.1161/01.cir.34.5.883. [DOI] [PubMed] [Google Scholar]
- 12.Duston MA, Skinner M, Shirahama T, Cohen AS. Diagnosis of amyloidosis by abdominal fat aspiration. Analysis of four years' experience. Am J Med. 1987;82(3):412–414. doi: 10.1016/0002-9343(87)90439-6. [DOI] [PubMed] [Google Scholar]
- 13.Gertz MA, Rajkumar SV, editors. Amyloidosis: Diagnosis and Treatment. New York: Humana Press; 2010. [Google Scholar]
- 14.Falk RH. Diagnosis and management of the cardiac amyloidoses. Circulation. 2005;112:2047–2060. doi: 10.1161/CIRCULATIONAHA.104.489187. [DOI] [PubMed] [Google Scholar]
- 15.Berk J, Keane J, Seldin D, Sanchorawala V, Koyama J, Dember L, Falk R. Persistent pleural effusions in primary systemic amyloidosis: etiology and prognosis. Chest. 2003;124:969–977. doi: 10.1378/chest.124.3.969. [DOI] [PubMed] [Google Scholar]