Abstract
We investigated the role of CD4+ T-cell–produced interferon (IFN)-γ on corneal epithelial apoptosis in a murine desiccating stress (DS) model that resembles Sjögren's syndrome. The DS model was generated in C57BL/6 (B6) and B6 IFN-γ–knockout (B6γKO) mice. Adoptive transfer of CD4+ T cells from DS-exposed donor to recombination activating gene (RAG)-1−/− recipient mice and topical neutralization of IFN-γ were performed to determine whether IFN-γ produced by pathogenic CD4+ T cells promotes corneal epithelial apoptosis. Apoptosis in corneal epithelia was assessed by evaluating the expression and activity of caspases 3, 8, and 9. The activation of caspase-8 mediated increased corneal epithelial apoptosis in B6 mice after DS, and this was exacerbated by subconjunctival IFN-γ injection. B6γKO mice were resistant to DS-induced apoptosis; however, B6γKO mice receiving IFN-γ developed apoptosis similar to that observed in B6 wild-type mice. Adoptive transfer of CD4+ T cells from donors subjected to DS increased corneal epithelial apoptosis via activation of caspase-8 in recipients, similar to that in the donor mice. Topical neutralization of IFN-γ in adoptive transfer recipients decreased corneal epithelial apoptosis. DS, IFN-γ administration, or CD4+ T-cell adoptive transfer had no effect on the expression and activation of the intrinsic apoptosis mediator, caspase-9. CD4+ T-cell–produced IFN-γ plays a pivotal role in DS-induced corneal epithelial apoptosis via activation of the extrinsic apoptotic pathway.
Sjögren's syndrome (SS) is a prevalent chronic autoimmune disorder characterized by infiltration of salivary and lacrimal glands by mononuclear cells, causing secondary destruction of the parenchymal tissue.1,2 Keratoconjunctivitis sicca (KCS) in SS is a severe and potentially sight-threatening ocular surface epithelial disease.3 The pathogenesis of KCS in SS is a multifactorial process, including activating stress pathways in the ocular surface epithelia by the hyperosmolar tear film and cytokines produced by resident intraepithelial lymphocytes and infiltrating CD4+ T cells producing IL-17 and interferon (IFN)-γ.4–7 In our murine model with features mimicking SS, we previously demonstrated that desiccating stress (DS) activated CD4+ T cells that, when adoptively transferred to T-cell–deficient nude mice, were sufficient to elicit autoimmune lacrimal keratoconjunctivitis (ALKC) with ocular surface and lacrimal gland inflammation, reduced tear production, and conjunctival goblet cell loss.8 This evidence suggests that CD4+ T-cell–mediated immunity makes a prominent contribution to the ocular surface epithelial disease in SS.
Although the exact mechanism by which CD4+ T cells promote damage of ocular surface epithelia in KCS is not completely understood, our previous report implicated the type 1 helper T-cell (Th-1) cytokine, IFN-γ, in this process. We found that DS promoted migration of CD4+ T cells and IFN-γ+ cells into goblet cell zones of the conjunctiva and increased the concentration of IFN-γ in tears.5 IFN-γ is exclusively secreted by T cells (cytotoxic and Th-1) and natural killer cells and is a pleiotropic cytokine with immunomodulatory effects on a variety of immune cells. In addition to its immunomodulatory role, IFN-γ has had anti-proliferative functions. Recent studies9–12 have demonstrated that IFN-γ can induce apoptosis or sensitize a variety of cell lines to apoptotic stimuli by activating the Fas-CD95 or tumor necrosis factor–related apoptosis-inducing ligand/Apo2L pathways.
Pathogenic apoptosis occurs in corneal epithelia in both SS and non–SS-like KCS13–16; however, the precise mechanisms triggering this process remain unclear. In the past two decades, it has been well documented that T lymphocytes have an important role in inducing apoptosis of salivary and lacrimal glands in SS by three different mechanisms: i) Fas-FasL interaction, ii) perforin and granzyme B release, and iii) production of cytokines, such as IFN-γ and tumor necrosis factor-α.9,17 However, the precise role of T-cell cytokines in inducing corneal epithelial apoptosis in SS has not been established.
The purpose of this study was to investigate the role of IFN-γ produced by CD4+ T cells in corneal epithelial apoptosis, using a murine DS model with features resembling SS.
Materials and Methods
Mouse Model of Dry Eye
This research protocol was approved by the Center for Comparative Medicine, Baylor College of Medicine (Houston, TX), and it conformed to the standards in the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research. DS was generated in female C57BL/6 (B6) mice, aged 6 to 8 weeks, by s.c. injection of 0.5 mg/0.2 mL scopolamine hydrobromide (Sigma-Aldrich, St Louis, MO) into alternating hindquarters, administered four times a day (8:30 AM, 11 AM, 1 PM, and 4:30 PM), and exposure to an air draft and <40% ambient humidity. Mice were euthanized after 5 days of DS (DS5). A group of age- and sex-matched mice that did not receive any treatment to induce dry eye served as nonstressed (NS) controls.
Exogenous Administration of IFN-γ
To evaluate the role of IFN-γ in corneal apoptosis, we subjected B6 and B6 IFN-γ- knockout (B6γKO) mice (B6.129s7-Ifn-gtm1Ts/J; Jackson Laboratories, Bar Harbor, ME) to DS, as described, for 5 days. Mice of each strain were divided into four groups: i) NS control mice; ii) DS5 mice that received no ocular injections; iii) DS5 vehicle control animals that received bilateral subconjunctival injections (20 μL/eye) of 0.1% bovine serum albumin (BSA) in PBS (DS5+BSA); and iv) DS5+IFN-γ mice that received bilateral subconjunctival injections of recombinant murine IFN-γ (1 × 104 U/eye per injection, dissolved in 20 μL of 0.1% BSA in PBS; Chemicon, Temecula, CA) at –4, –2, 0, 2, and 4 days of DS.
CD4+ T-Cell Isolation and Adoptive Transfer
To evaluate the role of pathogenic CD4+ T cells in corneal epithelial apoptosis induced by DS, adoptive transfer experiments were performed after CD4+ T-cell isolation. Spleens and cervical lymph nodes were collected from NS and DS5 B6 mice, and their cell suspensions were enriched for CD4+ T cells by negative selection using rat anti-mouse CD4-conjugated magnetic microbeads (MACS system; Miltenyi Biotec, Bergisch Gladbach, Germany). Cells were washed with PBS before being adoptively transferred. Untouched cells were used for flow cytometry, enzyme-linked immunosorbent spot (ELISPOT), or adoptive transfer experiments. CD4+ T cells, 5 × 106, were transferred (i.p.) to RAG-1–deficient mice (RAG-1−/−; B6.129S7/J; Jackson Laboratories) without mature B and T lymphocytes. RAG-1−/− mice were divided into two treatment groups: DS5 mice that received CD4+ T cells from DS5 B6 mice and NS mice that received CD4+ T cells from NS B6 mice. All RAG-1−/− mice were euthanized after 72 hours of adoptive transfer.
Mouse IFN-γ, IL-13, and IL-17A ELISPOT for Isolated CD4+ T Cells
Replicate 50-μL cell suspensions containing 1.0 × 106 freshly isolated CD4+ T cells (as previously described) from NS and DS5 B6 mice (five samples in each group) were added to 96- well polyvinylidene fluoride plates (Milipore, Billerica, MA), precoated with anti-mouse IFN-γ, IL-13, or IL-17A capture antibody (R & D Systems, Minneapolis, MN). Wells containing either cells or positive control (3 ng per well of recombinant mouse IFN-γ, IL-13, or IL-17A; R & D Systems) or media alone (negative control) were incubated at 37°C with 5% CO2 for 24 hours in RPMI 1640 medium (Invitrogen-Gibco, Carlsbad, CA). After washing, the plate was incubated overnight at 4°C with biotinylated anti-mouse IFN-γ, IL-13, or IL-17A detection antibody (R & D Systems), followed by a 2-hour incubation with streptavidin-horseradish peroxidase (R & D Systems) the next day. Red color development was achieved by incubating with NovaRed peroxidase substrate (Vector Laboratories, Burlingame, CA) for 15 minutes. The polyvinylidene fluoride membrane was dried, and the individual wells were punched out from the plate. The positive red spots on the membrane were counted under a dissecting microscope (model SMZ1500; Nikon, Melville, NY). Replicate wells were averaged from three individual experiments. The results are presented as number of spots.
Flow Cytometry Analysis of Splenocytes before or after CD4 Isolation
Single-cell suspensions of spleen, either before or after CD4 isolation, were stained with anti-CD16/32 (to block Fc receptors; BD Pharmingen, San Diego, CA), followed by cell surface staining with fluorescein isothiocyanate–anti-CD4 or CD11c, allophycocyanin–anti-B220 or CD11b, and phycoerythrin-anti-CD8 or DX5 antibodies (BD Pharmingen). Positive controls consisted of splenocytes processed at the same time under the same protocol. Negative controls consisted of cells stained with fluorescein isothiocyanate, phycoerythrin, or allophycocyanin-isotype antibodies (BD Pharmingen). Cells were resuspended in 0.2 μg/mL propidium iodide and then the analysis was performed. A BD LSRII Benchtop cytometer was used for flow cytometry, and data were analyzed using BD Diva Software (BD Pharmingen).
In Vivo Neutralization of IFN-γ
To evaluate the role of IFN-γ produced by pathogenic CD4+ T cells in corneal epithelial apoptosis in RAG-1−/− mice after adoptive transfer of CD4+ T cells, in vivo neutralization of IFN-γ was performed in DS5 RAG-1−/− mice by topical application of 10 μL of anti-IFN-γ hybridoma (1 mg/mL, R4-6A2; catalog no. HB-170; American Type Culture Collection, Rockville, MD; DS5+anti-IFN-γ) or vehicle control (rat IgG, 1 mg/mL; Vector Laboratories; DS5+RatIgG) four times daily from –2 to 3 days of CD4+ T-cell adoptive transfer.
Histological Features
The eyes and adnexa of mice were excised and embedded in optimal cutting temperature compound (VWR, Suwanee, GA; n = 3 eyes in each group/each strain) or paraffin (n = 3 eyes in each group/each strain). Optimal cutting temperature–embedded samples were cut into sagittal sections (8-μm thick) and placed onto glass slides that were stored at –80°C.
TUNEL Assay and Immunostaining
The TUNEL assay was performed using a commercially available kit (ApopTag; Intergen Co, Purchase, NY).
Cryosections stained for activated (AC) caspase-3 (5 μg/mL; BD Pharmingen), caspase-8 (neat serum, 1:100; Novus Biologicals, Littleton, CO), and caspase-9 (neat serum, 1:100; Novus Biologicals) were developed using goat anti-rabbit Alexa-Fluor 488-conjugated antibody. Negative controls performed at the same time consisted of sections incubated with PBS in place of primary antibody. Digital images (512 × 512 pixels) of representative areas of the central cornea were captured with a laser-scanning confocal microscope (Zeiss, Thornwood, NY). The intensity of the staining was measured using NIS Elements (Nikon).
Activation Fluorometric Assays for Caspases 3, 8, and 9
The activation of caspases 3, 8, and 9 was measured according to the protocol provided by the manufacturer of their respective fluorometric kits (Biovision K105-25, K112-25, and K189-100, respectively). Conjunctiva was surgically excited and placed in the lysis buffer provided with the kits. Protein concentration was measured using a Micro BSA protein assay kit (Thermo Fisher Scientific, Austin, TX), and 50 μg of corneal lysate was used for each assay. Lysis buffer without any protein was used as blank control. Three samples per group/strain were used, and one sample consisted of pooled corneal epithelial samples from four mice. Fluorescence was read using a Tecan Spectra Fluor instrument with a 400-nm excitation filter and a 505-nm emission filter.
Immunohistochemistry
Cryosections were immunostained for the expression of mouse CD4 (rat anti-mouse CD4; 10 μg/mL; rat IgG2a, k; clone H129.19; BD Pharmingen) and IFN-γ (rat anti-mouse IFN-γ; clone R4-6A2; 20 μg/mL; Biolegend, San Diego, CA) using Vectastain Elite ABC reagents (Vector Laboratories). The density of CD4+ T cells in conjunctiva was measured in digital images using NIS Elements and expressed as the number of positive cells per millimeter.
Measurement of Goblet Cell Density
Sections (6-μm thick) cut from paraffin-embedded globes were stained with PAS reagent. The goblet cell density was measured in the superior and inferior bulbar and tarsal conjunctiva using NIS Elements software and expressed as the number of positive cells per millimeter.
Total RNA Extraction, Reverse Transcription, and Quantitative Real-Time PCR
Total RNA from the corneal epithelia collected was extracted using a Qiagen RNeasy Micro Kit (Qiagen, Valencia, CA), according to the manufacturer's instructions, quantified by a NanoDrop ND-1000 Spectrophotometer (Thermo Fisher Scientific) and stored at –80°C. Six samples per group per stain were used. First-strand cDNA was synthesized with random hexamers by M-MuLV reverse transcription (Ready-To-Go You-Prime First-Strand Beads; GE Healthcare, Inc., Arlington Heights, NJ).
Real-time PCR was performed with specific MGB probes (Taqman; Applied Biosystems, Inc., Foster City, CA) and PCR master mix (Taqman Gene Expression Master Mix), in a commercial thermocycling system (Mx3005P QPCR System; Stratagene, La Jolla, CA), according to the manufacturer's recommendations. Murine MGB probes were glyceraldehyde-3-phosphate dehydrogenase (GAPDH; Mm99999915), caspase-3 (Mm00438045), caspase-8 (Mm00802247), and caspase-9 (Mm01348848). The GAPDH gene was used as an endogenous reference for each reaction. The results of the relative-quantitative real-time PCR were analyzed by the comparative CT method18 and normalized by GAPDH as an internal control.
Statistical Analysis
Two-way analysis of variance with Tukey's post hoc test was used for statistical comparison between groups. P ≤ 0.05 was considered statistically significant. These tests were performed using GraphPad Prism 5.0 software (GraphPad Software Inc, Interferon-γ and Apoptosis, San Diego, CA).
Results
DS Increased Corneal Epithelial Apoptosis via the Extrinsic Apoptotic Pathway
DS significantly increased AC caspase-3 and TUNEL immunoreactivity in corneal epithelia (Figures 1 and 2). The AC caspase-3 expression pattern in corneal epithelia was confirmed by real-time PCR and fluorometric assays at mRNA and activity levels (Figure 3, A and D). Because both AC caspase-3 and TUNEL are not specific for any of the apoptotic pathway, we then investigated the expression and activity of the extrinsic and intrinsic apoptotic pathway mediators (caspases 8 and 9, respectively), using real-time PCR, immunostaining, and fluorometric assays. DS significantly increased caspase-8 expression and activity, whereas it had no effect on casapse-9 expression and activity (Figures 1–3). Taken together, these results indicate that DS increases corneal epithelial apoptosis via the caspase-8–mediated extrinsic apoptotic pathway but not the caspase-9–mediated intrinsic apoptotic pathway.
Figure 1.
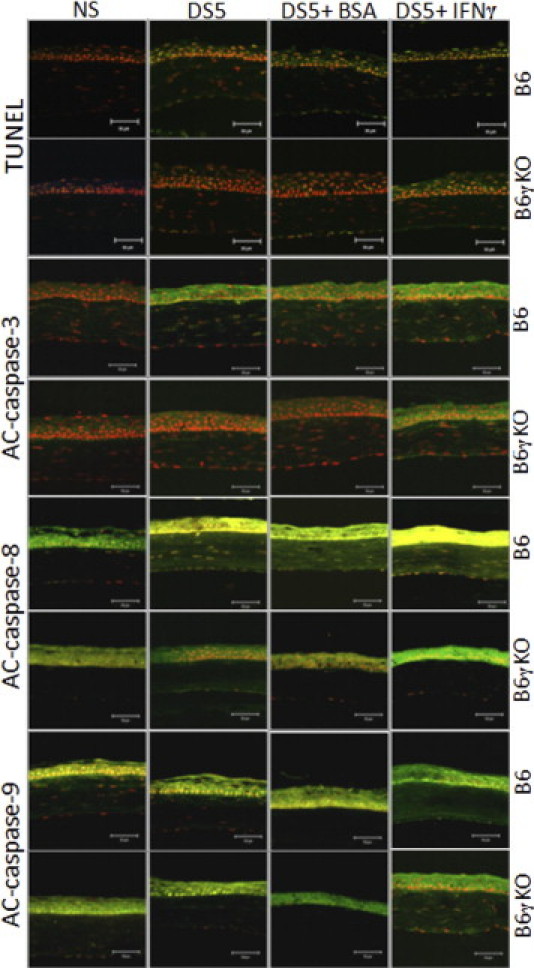
Laser-scanning confocal microscopy of immunofluorescent staining in tissue sections for TUNEL, AC caspases 3, 8, and 9 (green) with propidium iodide (red) nuclear counterstaining in NS control mice, DS5, and DS5 treated with BSA or IFN-γ subconjunctival injection in B6 or B6γKO mice. Increased TUNEL and caspase-3 and caspase-8 immunoreactivity was noted in corneal epithelia in B6 mice after DS, and subconjunctival IFN-γ administration further increased this process. B6γKO mice were resistant to DS-induced apoptosis; however, B6γKO mice receiving IFN-γ showed similar results to B6 wild-type mice. DS and IFN-γ administration had no effect on the immunoreactivity of caspase-9. Scale bars = 50 μm.
Figure 2.
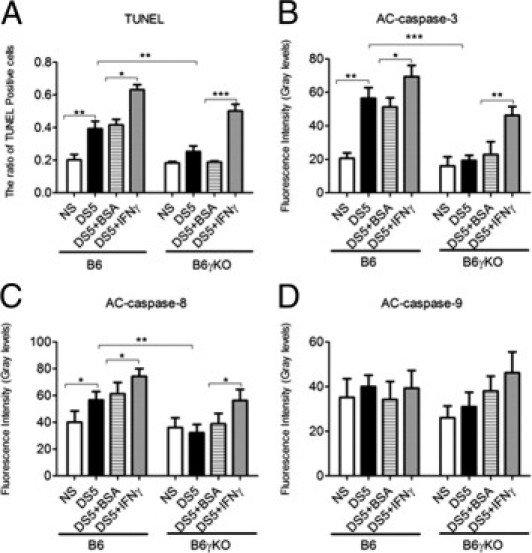
The ratio of TUNEL-positive cells (A) and AC caspase-3 (B), AC caspase-8 (C), and AC caspase-9 (D) immunofluorescent intensity in corneal epithelia. DS5+BSA, DS5 treated with BSA subconjunctival injection; DS5+IFN-γ, DS5 treated with an IFN-γ subconjunctival injection. Data are given as the mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001.
Figure 3.
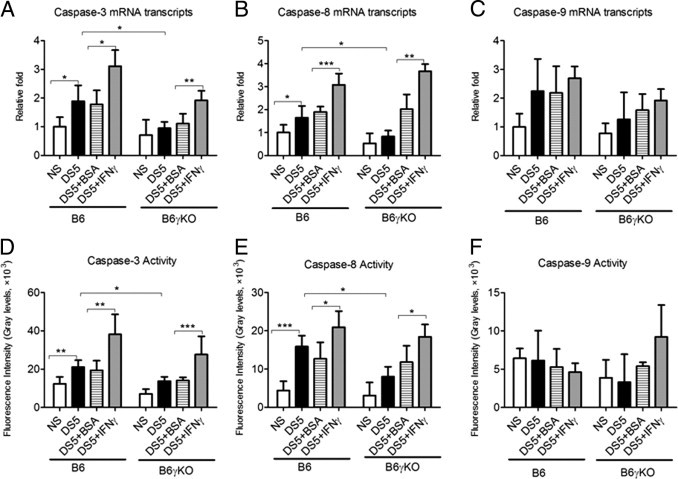
Relative mRNA levels of caspase-3 (A), caspase-8 (B), and caspase-9 (C), evaluated by real-time PCR; caspase-3 (D), caspase-8 (E), and caspase-9 (F) activities were evaluated by fluorometric assays. DS5+BSA, DS5 treated with BSA subconjunctival injections; DS5+ IFN-γ, DS5 treated with IFN-γ subconjunctival injections. Data are given as the mean ± SEM. *P < 0.05, **P < 0.01, and ***P < 0.001.
B6γKO Mice Show Resistance to DS-Induced Apoptosis in Corneal Epithelia
To investigate the role of IFN-γ in modulating apoptosis, we used B6γKO mice to evaluate if IFN-γ deficiency would prevent or alleviate DS-induced apoptosis in corneal epithelia. We found that DS had no effect on AC caspase-3 and caspase-8 and TUNEL immunoreactivity in this strain (Figures 1 and 2). Real-time PCR and fluorometric assays confirmed the results of AC caspase-3 and caspase-8 immunostaining in corneal epithelia at mRNA and activity levels (Figure 3).
Exogenous Administration of IFN-γ Increases DS-Induced Apoptosis in Corneal Epithelia in Both B6 and B6γKO Mice through the Extrinsic Apoptotic Pathway
To further investigate the role of IFN-γ in modulating corneal epithelial apoptosis, we performed subconjunctival injection of IFN-γ in B6 and B6γKO mice. Significantly increased TUNEL and AC caspase-3 and caspase-8 immunoreactivity in corneal epithelia was noted in the IFN-γ–injected group compared with the BSA-injected group in both strains of mice after 5 days of DS treatment (P < 0.05, Figures 1 and 2). Real-time PCR and fluorometric assays confirmed the results of immunostaining at caspase-3 and caspase-8 mRNA and activity levels (P < 0.05, Figure 3). Subconjunctival injection of IFN-γ had no effect on caspase-9 expression and activity in corneal epithelia in both strains of mice (P > 0.05, Figures 1–3).
Ocular Surface Inflammation Can Be Induced by Adoptive Transfer of CD4+ T Cells from Mice Subjected to DS
We have previously demonstrated that the SS-like ALKC mediated by DS-elicited pathogenic CD4+ T cells that, when adoptively transferred to T-cell–deficient nude mice, produced inflammation in the lacrimal gland and ocular surface.8 The purity of the isolated CD4+ T cell using MacBeads was determined by flow cytometry analysis of surface CD4 staining, and it was almost 90% (Figure 4A). Non-CD4+ T cells (ie, CD8+ T cells, B220+ B cells, DX5+ NK cells, CD11c+ dendritic cells, and CD11b+ macrophages) were almost completely depleted (each <1.5%, Figure 4A).
Figure 4.
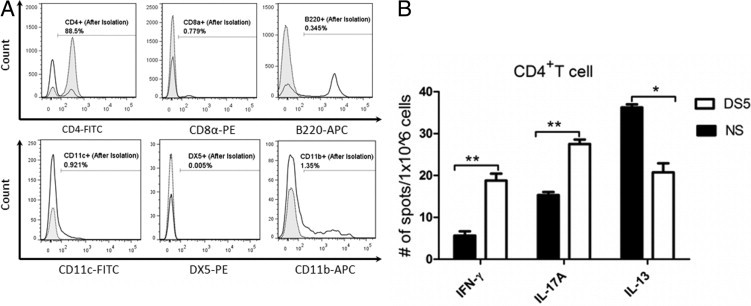
A: The purity of the isolated CD4+ T cell was almost 90%, whereas the non-CD4+ T cell (ie, CD8+ T cell, B220+ B cell, DX5+ NK cell, CD11c+ dendritic cell, and CD11b+ macrophage) was almost depleted. Clear histograms indicate flow cytometry analysis before isolation, whereas tinted histograms are after CD4+ isolation. The percentage of isolated cells in the CD4+ T-cell fraction is depicted in the graph. APC, allophycocyanin; FITC, fluorescein isothiocyanate; PE, phosphatidylethanolamine. B: DS increased the production of IFN-γ and IL-17A, whereas it decreased the production of IL-13 by CD4+ T cells isolated from the B6 donor mice, as determined by ELISPOT. Data are given as the mean ± SEM. *P < 0.05, and **P < 0.001.
Our DS model elicits a Th-17 and Th-1 response in the mouse conjunctiva.5,7 We sought to determine by ELISPOT the phenotype of primed CD4+ T cells in our DS model (Figure 4B). We observed that DS significantly increased the production of IFN-γ and IL-17A (P < 0.01), whereas it decreased the production of IL-13 by CD4+ T cells isolated from the B6 donor mice (P < 0.05).
In this study, we also found adoptive transfer of CD4+ T cells from donor mice subjected to DS to RAG-1−/− mice promoted ocular surface inflammation with increased CD4+ T-cell infiltration and increased IFN-γ expression and goblet cell loss in the recipient ocular surface (Figure 5). These findings indicate that SS-like KCS can be induced by adoptive transfer of CD4+ T cells from donor mice subjected to DS to RAG-1−/− recipients housed in the NS condition.
Figure 5.
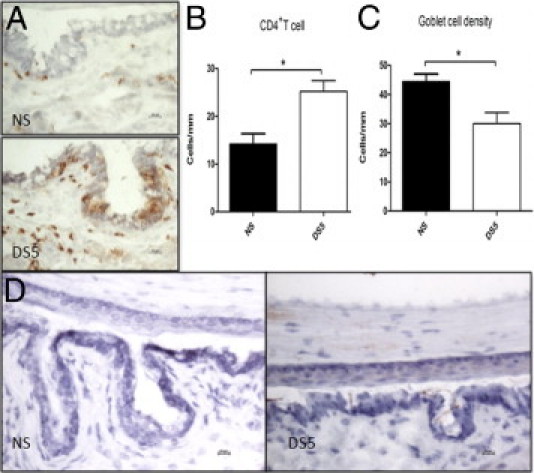
Adoptive transfer of CD4+ T cells elicited by DS to RAG-1−/− mice promoted ocular surface inflammation with increased CD4+ T-cell infiltration (A and B), goblet cell loss (C), and increased IFN-γ expression (D) in the ocular surface. NS, RAG-1−/− mice received CD4+ T cells from NS donor mice; DS5, RAG-1−/− mice received CD4+ T cells from DS5 donor mice. *P < 0.05.
Corneal Epithelial Apoptosis Can Be Induced by Adoptive Transfer of CD4+ T Cells from Mice Subjected to DS
We performed adoptive transfer experiments to determine the role of pathogenic CD4+ T cells in the corneal epithelial apoptosis that is induced by DS. We found that adoptive transfer of CD4+ T cells from donor mice subjected to DS increased caspase-3 and caspase-8 immunoreactivity and mRNA levels in corneal epithelia, whereas it had no effect on caspase-9 expression and activity (Figure 6). This evidence indicates that CD4+ T cells elicited by DS can induce corneal epithelial apoptosis via the caspase-8–mediated extrinsic apoptotic pathway but not the caspase-9–mediated intrinsic apoptotic pathway.
Figure 6.
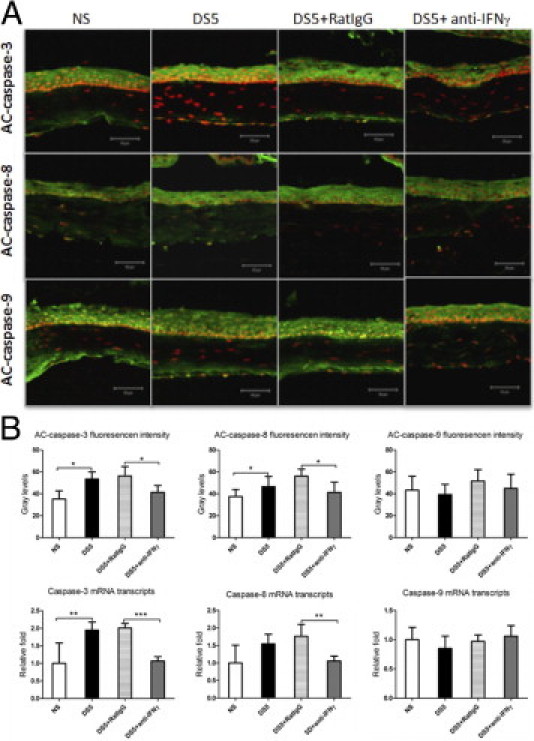
A: Laser-scanning confocal microscopy of immunofluorescent staining in tissue sections stained for AC caspases 3, 8, and 9 (green) with propidium iodide (red) nuclear counterstaining in RAG-1−/− adoptive transfer recipient mice. B: AC caspases 3, 8, and 9 immunofluorescent intensity and relative mRNA levels of caspases 3, 8, and 9 in corneal epithelia in the recipient mice. Data are given as the mean ± SEM. NS, RAG-1−/− mice received CD4+ T cells from NS donor mice; DS5, RAG-1−/− mice received CD4+ T cells from DS5 donor mice; DS5+RatIgG, DS5 RAG-1−/− adoptive transfer recipients treated with topical application of vehicle control (RatIgG); DS5+anti-IFN-γ, DS5 RAG-1−/− adoptive transfer recipients treated with topical application of anti-IFN-γ antibody. *P < 0.05, **P < 0.01, and ***P < 0.001. Scale bars = 50 μm.
In Vivo Neutralization of IFN-γ Alleviates Corneal Epithelial Apoptosis Induced by Adoptively Transferred CD4+ T Cells
In vivo topical neutralization of IFN-γ was performed to determine whether CD4+ T cells induced corneal epithelial apoptosis by production of IFN-γ in the RAG-1−/− adoptive transfer recipients. We found that topical neutralization of IFN-γ decreased corneal epithelial caspase-3 and caspase-8 immunoreactivity and mRNA levels in the recipient mice (Figure 6), indicating that IFN-γ plays an important role in corneal epithelial apoptosis induced by pathogenic CD4+ T cells.
Discussion
SS is a common autoimmune exocrinopathy typically characterized by chronic dysfunction of salivary and lacrimal glands, leading to persistent dryness and oral and ocular mucosal damage.19,20 We have developed a murine model mimicking the ALKC in human SS. Mice subjected to DS develop profound dysfunction of the lacrimal functional unit with decreased secretion of tear fluid, loss of conjunctival goblet cells, and transformation of the lubricated ocular surface epithelia to a keratinized poorly wettable surface.5,21,22 Immunopathological changes, including immune/inflammatory cell infiltration and increased production of inflammatory chemokines and cytokines, have been detected in the ALKC of this model.5–7,23,24 After adoptive transfer of CD4+ T cells isolated from donor mice exposed to DS, naïve T-cell deficient (nude) mice developed SS-like ALKC,8 suggesting that CD4+ T-cell–mediated immunity makes an important contribution to the tissue destruction in this model. However, the specific role of CD4+ T cells in the tissue damage in this model remains largely unknown.
In this study, we found that DS increased apoptosis in the corneal epithelia in B6 wild-type mice, whereas B6γKO mice showed resistance to DS-induced apoptosis in their corneal epithelia; exogenous administration of IFN-γ increased corneal epithelial apoptosis in both B6 and B6γKO mice under DS. This evidence implicates the Th-1 cytokine IFN-γ in the induction of corneal epithelial apoptosis in SS. A growing body of clinical and experimental studies13–16 has shown that pathological apoptosis can play a key component in the pathogenesis of KCS and is a therapeutic target for dry eye. The mechanism responsible for this pathogenesis remains to be determined. We previously reported that, in addition to suppressing the CD4+ T-cell infiltration and IL-17A and IFN-γ expression in the conjunctiva, ophthalmic emulsion of cyclosporine A suppressed ocular surface apoptosis in our SS-like ALKC model,25,26 suggesting that CD4+ T-cell–mediated immune inflammation may have an important role in ocular surface apoptosis that develops in SS-like ALKC. The findings of this study suggest that cyclosporine A may alleviate ocular surface apoptosis by inhibiting production of IFN-γ.
Apoptosis can be mediated by two predominant pathways.12 One apoptotic pathway is the extrinsic or death receptor pathway mediated by ligation of cell surface death receptors, such as Fas and tumor necrosis factor–related apoptosis-inducing ligand, and subsequent activation of caspase-8. The other is called an intrinsic or mitochondrial pathway mediated by activation of caspase-9. In this study, DS and exogenous administration of IFN-γ with DS exposure increased corneal epithelial apoptosis via activation of caspase-8 but not caspase-9, indicating that IFN-γ promotes corneal epithelial apoptosis through the extrinsic apoptosis pathway in SS.
IFN-γ is secreted mainly by (cytotoxic and Th-1) T cells and NK cells. We previously reported that increased IFN-γ was noted in B6 mice after 5 days of DS.5 More recently, we observed that conjunctival intraepithelial NK+ cells could be a resource of IFN-γ, in eyes subjected to DS,26 and that the IFN-γ mRNA level increased in NK+ cells isolated from the conjunctiva of B6 mice after 1 day of DS compared with NS (unpublished data). These results indicate that activated NK+ cells may be contributed to the IFN-γ–induced apoptosis in mice subjected to DS. To establish a definite role for pathogenic CD4+ T cells in the corneal epithelial apoptosis induced by DS, adoptive transfer of isolated CD4+ T cells was performed. In this study, the purity of the isolated CD4+ T cell was almost 90%, whereas the other potential IFN-γ–secreting cells, including DX5+ NK cells and CD8+ T cells, were almost depleted. Adoptive transfer of CD4+ T cells from DS-exposed donor mice, RAG-1−/− recipients, promoted ocular surface inflammation with increased CD4+ T-cell infiltration and increased IFN-γ expression. Consistent with the finding in the donor mice, the recipient mice showed increased corneal epithelial apoptosis via activation of the caspase-8–mediated extrinsic apoptotic pathway but not the caspase-9–mediated intrinsic apoptotic pathway. In vivo topical neutralization of IFN-γ in the adoptive transfer recipients decreased the corneal epithelial apoptosis induced by pathogenic CD4+ T cells. This evidence indicates that IFN-γ derived from pathogenic CD4+ T cells plays a pivotal role in promoting corneal epithelial apoptosis via activation of the extrinsic apoptosis pathway in our SS-like ALKC model.
Th-1 and Th-17 cells were initially viewed as distinct and possibly antagonistic differentiation pathways27 However, recently, a wave of published reports28–30 shows that human Th cells can coexpress IL-17A with a variety of other cytokines, including IFN-γ, IL-4, or IL-10, with different functional consequences. Th-17 cells coexpressing IFN-γ (known as Th-17-1 cells) have also been described in murine models of graft-versus-host disease.31 In our study, the pure population of adoptively transferred cells contained both IFN-γ and IL-17A producers. Whether the cells producing IFN-γ were Th-1 cells alone or combined with Th-17-1 cells, the results of this study clearly implicate a dominant role for IFN-γ in inducing corneal epithelial apoptosis in this SS-like ALKC model.
In conclusion, this study provides evidence that the IFN-γ–secreting CD4+ T cell plays a significant role in the pathological corneal epithelial apoptosis in a mouse model mimicking SS. This study provides new insight into the pathogenesis of mucosal autoimmune diseases characterized by CD4+ T-cell infiltration.
Footnotes
Supported by the NIH (grant EY11915 to S.C.P.), Research to Prevent Blindness, the Oshman Foundation, and the William Stamps Farish Fund.
X.Z. and W.C. contributed equally to this work.
References
- 1.Mitsias D.I., Tzioufas A.G., Veiopoulou C., Zintzaras E., Tassios I.K., Kogopoulou O., Moutsopoulos H.M., Thyphronitis G. The Th1/Th2 cytokine balance changes with the progress of the immunopathological lesion of Sjogren's syndrome. Clin Exp Immunol. 2002;128:562–568. doi: 10.1046/j.1365-2249.2002.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nguyen C.Q., Hu M.H., Li Y., Stewart C., Peck A.B. Salivary gland tissue expression of interleukin-23 and interleukin-17 in Sjogren's syndrome: findings in humans and mice. Arthritis Rheum. 2008;58:734–743. doi: 10.1002/art.23214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pflugfelder S.C., Tseng S.C.G., Sanabria O., Kell H., Garcia C.G., Felix C., Feuer W., Reis B.L. Evaluation of subjective assessments and objective diagnostic tests for diagnosing tear-film disorders known to cause ocular irritation. Cornea. 1998;17:38–56. doi: 10.1097/00003226-199801000-00007. [DOI] [PubMed] [Google Scholar]
- 4.de Paiva C.S., Hwang C.S., Pitcher J.D., III, Pangelinan S.B., Rahimy E., Chen W., Yoon K.C., Farley W.J., Niederkorn J.Y., Stern M.E., Li D.Q., Pflugfelder S.C. Age-related T-cell cytokine profile parallels corneal disease severity in Sjogren's syndrome-like keratoconjunctivitis sicca in CD25KO mice. Rheumatology (Oxford) 2010;49:246–258. doi: 10.1093/rheumatology/kep357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Paiva C.S., Villarreal A.L., Corrales R.M., Rahman H.T., Chang V.Y., Farley W.J., Stern M.E., Niederkorn J.Y., Li D.Q., Pflugfelder S.C. Dry eye-induced conjunctival epithelial squamous metaplasia is modulated by interferon-gamma. Invest Ophthalmol Vis Sci. 2007;48:2553–2560. doi: 10.1167/iovs.07-0069. [DOI] [PubMed] [Google Scholar]
- 6.Luo L., Li D.Q., Doshi A., Farley W., Corrales R.M., Pflugfelder S.C. Experimental dry eye stimulates production of inflammatory cytokines and MMP-9 and activates MAPK signaling pathways on the ocular surface. Invest Ophthalmol Vis Sci. 2004;45:4293–4301. doi: 10.1167/iovs.03-1145. [DOI] [PubMed] [Google Scholar]
- 7.de Paiva C.S., Chotikavanich S., Pangelinan S.B., Pitcher J.D., III, Fang B., Zheng X., Ma P., Farley W.J., Siemasko K.F., Niederkorn J.Y., Stern M.E., Li D.Q., Pflugfelder S.C. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2:243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Niederkorn J.Y., Stern M.E., Pflugfelder S.C., de Paiva C.S., Corrales R.M., Gao J., Siemasko K. Desiccating stress induces T cell-mediated Sjogren's syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006;176:3950–3957. doi: 10.4049/jimmunol.176.7.3950. [DOI] [PubMed] [Google Scholar]
- 9.Abu-Helu R.F., Dimitriou I.D., Kapsogeorgou E.K., Moutsopoulos H.M., Manoussakis M.N. Induction of salivary gland epithelial cell injury in Sjogren's syndrome: in vitro assessment of T cell-derived cytokines and Fas protein expression. J Autoimmun. 2001;17:141–153. doi: 10.1006/jaut.2001.0524. [DOI] [PubMed] [Google Scholar]
- 10.Wen L.P., Madani K., Fahrni J.A., Duncan S.R., Rosen G.D. Dexamethasone inhibits lung epithelial cell apoptosis induced by IFN-gamma and Fas. Am J Physiol. 1997;273:L921–L929. doi: 10.1152/ajplung.1997.273.5.L921. [DOI] [PubMed] [Google Scholar]
- 11.Trautmann A., Kruger K., Akdis M., Muller-Wening D., Akkaya A., Brocker E.B., Blaser K., Akdis C.A. Apoptosis and loss of adhesion of bronchial epithelial cells in asthma. Int Arch Allergy Immunol. 2005;138:142–150. doi: 10.1159/000088436. [DOI] [PubMed] [Google Scholar]
- 12.Chawla-Sarkar M., Lindner D.J., Liu Y.F., Williams B.R., Sen G.C., Silverman R.H., Borden E.C. Apoptosis and interferons: role of interferon-stimulated genes as mediators of apoptosis. Apoptosis. 2003;8:237–249. doi: 10.1023/a:1023668705040. [DOI] [PubMed] [Google Scholar]
- 13.Chen W., Zhang X., Zhang J., Chen J., Wang S., Wang Q., Qu J. A murine model of dry eye induced by an intelligently controlled environmental system. Invest Ophthalmol Vis Sci. 2008;49:1386–1391. doi: 10.1167/iovs.07-0744. [DOI] [PubMed] [Google Scholar]
- 14.Chen W., Zhang X., Liu M., Zhang J., Ye Y., Lin Y., Luyckx J., Qu J. Trehalose protects against ocular surface disorders in experimental murine dry eye through suppression of apoptosis. Exp Eye Res. 2009;89:311–318. doi: 10.1016/j.exer.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 15.Yeh S., Song X.J., Farley W., Li D.Q., Stern M.E., Pflugfelder S.C. Apoptosis of ocular surface cells in experimentally induced dry eye. Invest Ophthalmol Vis Sci. 2003;44:124–129. doi: 10.1167/iovs.02-0581. [DOI] [PubMed] [Google Scholar]
- 16.Strong B., Farley W., Stern M.E., Pflugfelder S.C. Topical cyclosporine inhibits conjunctival epithelial apoptosis in experimental murine keratoconjunctivitis sicca. Cornea. 2005;24:80–85. doi: 10.1097/01.ico.0000133994.22392.47. [DOI] [PubMed] [Google Scholar]
- 17.Manganelli P., Fietta P. Apoptosis and Sjogren syndrome. Semin Arthritis Rheum. 2003;33:49–65. doi: 10.1053/sarh.2003.50019. [DOI] [PubMed] [Google Scholar]
- 18.Lowe B., Avila H.A., Bloom F.R., Gleeson M., Kusser W. Quantitation of gene expression in neural precursors by reverse-transcription polymerase chain reaction using self-quenched, fluorogenic primers. Anal Biochem. 2003;315:95–105. doi: 10.1016/s0003-2697(02)00695-4. [DOI] [PubMed] [Google Scholar]
- 19.Manoussakis M.N., Moutsopoulos H.M. Sjogren's syndrome: autoimmune epithelitis. Baillieres Best Pract Res Clin Rheumatol. 2000;14:73–95. doi: 10.1053/berh.1999.0078. [DOI] [PubMed] [Google Scholar]
- 20.Manoussakis M.N., Moutsopoulos H.M. Sjogren's syndrome: current concepts. Adv Intern Med. 2001;47:191–217. [PubMed] [Google Scholar]
- 21.Chen Z., Tong L., Li Z., Yoon K.C., Qi H., Farley W., Li D.Q., Pflugfelder S.C. Hyperosmolarity-induced cornification of human corneal epithelial cells is regulated by JNK MAPK. Invest Ophthalmol Vis Sci. 2008;49:539–549. doi: 10.1167/iovs.07-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dursun D., Wang M., Monroy D., Li D.Q., Lokeshwar B.L., Stern M., Pflugfelder S.C. Experimentally induced dry eye produces ocular surface inflammation and epithelial disease. Adv Exp Med Biol. 2002;506:647–655. doi: 10.1007/978-1-4615-0717-8_91. [DOI] [PubMed] [Google Scholar]
- 23.Corrales R.M., Villarreal A., Farley W., Stern M.E., Li D.Q., Pflugfelder S.C. Strain-related cytokine profiles on the murine ocular surface in response to desiccating stress. Cornea. 2007;26:579–584. doi: 10.1097/ICO.0b013e318033a729. [DOI] [PubMed] [Google Scholar]
- 24.de Paiva C.S., Corrales R.M., Villarreal A.L., Farley W.J., Li D.Q., Stern M.E., Pflugfelder S.C. Corticosteroid and doxycycline suppress MMP-9 and inflammatory cytokine expression: MAPK activation in the corneal epithelium in experimental dry eye. Exp Eye Res. 2006;83:526–535. doi: 10.1016/j.exer.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Gao J., Schwalb T.A., Addeo J.V., Ghosn C.R., Stern M.E. The role of apoptosis in the pathogenesis of canine keratoconjunctivitis sicca: the effect of topical Cyclosporin A therapy. Cornea. 1998;17:654–663. doi: 10.1097/00003226-199811000-00014. [DOI] [PubMed] [Google Scholar]
- 26.De Paiva C.S., Raince J.K., McClellan A.J., Shanmugam K.P., Pangelinan S.B., Volpe E.A., Corrales R.M., Farley W.J., Corry D.B., Li D.Q., Pflugfelder S.C. Homeostatic control of conjunctival mucosal goblet cells by NKT-derived IL-13. Mucosal Immunol. 2011;4:397–408. doi: 10.1038/mi.2010.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weaver C.T., Hatton R.D., Mangan P.R., Harrington L.E. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. doi: 10.1146/annurev.immunol.25.022106.141557. [DOI] [PubMed] [Google Scholar]
- 28.Annunziato F., Cosmi L., Santarlasci V., Maggi L., Liotta F., Mazzinghi B., Parente E., Filì L., Ferri S., Frosali F., Giudici F., Romagnani P., Parronchi P., Tonelli F., Maggi E., Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Amadi-Obi A., Yu C.R., Liu X., Mahdi R.M., Clarke G.L., Nussenblatt R.B., Gery I., Lee Y.S., Egwuagu C.E. TH17 cells contribute to uveitis and scleritis and are expanded by IL-2 and inhibited by IL-27/STAT1. Nat Med. 2007;13:711–718. doi: 10.1038/nm1585. [DOI] [PubMed] [Google Scholar]
- 30.van Beelen A.J., Zelinkova Z., Taanman-Kueter E.W., Muller F.J., Hommes D.W., Zaat S.A., Kapsenberg M.L., de Jong E.C. Stimulation of the intracellular bacterial sensor NOD2 programs dendritic cells to promote interleukin-17 production in human memory T cells. Immunity. 2007;27:660–669. doi: 10.1016/j.immuni.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 31.Chen X., Vodanovic-Jankovic S., Johnson B., Keller M., Komorowski R., Drobyski W.R. Absence of regulatory T-cell control of TH1 and TH17 cells is responsible for the autoimmune-mediated pathology in chronic graft-versus-host disease. Blood. 2007;110:3804–3813. doi: 10.1182/blood-2007-05-091074. [DOI] [PMC free article] [PubMed] [Google Scholar]


