Abstract
Approximately 10% to 15% of human cancers lack detectable telomerase activity, and a subset of these maintain telomere lengths by the telomerase-independent telomere maintenance mechanism termed alternative lengthening of telomeres (ALT). The ALT phenotype, relatively common in subtypes of sarcomas and astrocytomas, has rarely been reported in epithelial malignancies. However, the prevalence of ALT has not been thoroughly assessed across all cancer types. We therefore comprehensively surveyed the ALT phenotype in a broad range of human cancers. In total, two independent sets comprising 6110 primary tumors from 94 different cancer subtypes, 541 benign neoplasms, and 264 normal tissue samples were assessed by combined telomere-specific fluorescence in situ hybridization and immunofluorescence labeling for PML protein. Overall, ALT was observed in 3.73% (228/6110) of all tumor specimens, but was not observed in benign neoplasms or normal tissues. This is the first report of ALT in carcinomas arising from the bladder, cervix, endometrium, esophagus, gallbladder, kidney, liver, and lung. Additionally, this is the first report of ALT in medulloblastomas, oligodendrogliomas, meningiomas, schwannomas, and pediatric glioblastoma multiformes. Previous studies have shown associations between ALT status and prognosis in some tumor types; thus, further studies are warranted to assess the potential prognostic significance and unique biology of ALT-positive tumors. These findings may have therapeutic consequences, because ALT-positive cancers are predicted to be resistant to anti-telomerase therapies.
Telomeres are the nucleoprotein complexes located at the extreme ends of eukaryotic chromosomes; they consist of 5 to 10 kb of the repeating hexanucleotide DNA sequence TTAGGG.1,2 The shelterin complex, a core set of six proteins integral for telomere function, associates with these repetitive DNA regions.3,4 Telomeres function primarily to mask double-strand break DNA damage signals at chromosomal termini, inhibit terminal exonucleolytic degradation, and prevent chromosomal fusions.5,6 In normal somatic cells, telomeres shorten with each cell division, and significant telomere shortening leads to p53-dependent senescence or apoptosis.7 As a result, there is a limited number of population doublings that a somatic cell lineage may undergo, at which point further proliferative expansion is blocked. During malignant transformation, these cell cycle checkpoints are abrogated (eg, through mutations in tumor suppressor proteins). If cellular proliferation continues unchecked, then genomic instability may ensue via chromosomal breakage-fusion-bridge cycles caused by eroded, dysfunctional telomeres.8 In 85% to 90% of human cancers, telomere dysfunction is attenuated and telomere length appears to be maintained, or increased, through up-regulation of the enzyme telomerase, a reverse transcriptase with the ability to synthesize new telomere DNA using an internal RNA template.9 However, telomere loss may also be compensated in some cancers by the telomerase-independent telomere maintenance mechanism termed alternative lengthening of telomeres (ALT), which is thought to be dependent on homologous recombination.10
The ALT phenotype is identified at the cellular level by the presence of ALT-associated promyelocytic leukemia (PML) protein nuclear bodies (APBs) that contain large amounts of telomeric DNA, in addition to PML protein and other proteins involved in telomere binding, DNA replication, and recombination.11,12 ALT-positive cells are characterized by striking telomere length heterogeneity, as well as increased chromosomal instability. APBs are cancer-specific and, in fixed tissues, can be visualized by combined telomere-specific fluorescence in situ hybridization (FISH) and immunofluorescence labeling for PML protein.13,14 This method has been extensively validated and allows for straightforward identification of ALT-positive cancers in fixed human tissue specimens.15
The ALT phenotype is common among certain sarcomas (eg, osteosarcomas and liposarcomas), as well as in subsets of central nervous system tumors, including astrocytomas16; however, the prevalence of ALT varies widely among these different tumor types. Our laboratory recently reported the presence of ALT in a small subset of breast carcinomas,17 but the ALT phenotype has rarely been reported in other epithelial malignancies.16
We have comprehensively surveyed the ALT phenotype in two independent sets of fixed specimens, comprising a total of 6110 primary tumors from a broad range of human cancer subtypes. Overall, the prevalence of the ALT phenotype is 3.73%; however, the prevalence varies drastically between different subtypes. Here, we describe the results of this extensive survey, including the novel finding of the ALT phenotype in carcinomas arising from the bladder, cervix, endometrium, esophagus, gallbladder, kidney, liver, and lung. In addition, this is the first report of the ALT phenotype in several tumor types of nonepithelial origin, including medulloblastomas, pediatric glioblastomas multiformes (GBMs), oligodendrogliomas, meningiomas, and schwannomas.
Materials and Methods
Sources of Tissue
Two independent sets of primary tumor tissues were used in the present study. Set 1 consisted of 4001 tumors from 68 different cancer subtypes. The vast majority of these cases were resected and processed at the Johns Hopkins Hospital and were arrayed in tissue microarray (TMA) format. This set consisted of 165 TMAs containing multiple cores of each tumor specimen and, in most instances, adjacent normal tissue. In addition to these TMAs from our institution, seven TMAs containing 195 cases (three cores per case from cancer and one core from matched normal intestinal mucosa) of primary small intestinal adenocarcinoma from 20 institutions of the Korean Small Intestinal Cancer Study Group were included. Moreover, 56 invasive breast carcinoma tissue sections from the Johns Hopkins Hospital and 29 neuroblastic tumor tissue sections from the University of Texas Southwestern Medical Center were also obtained. To validate and expand on the findings in this first set, a second set of multitumor arrays was obtained (set 2).18 TMAs in set 2 contained 2109 primary tumors from 61 cancer subtypes. In this set of tumors, each case was represented on the array by a single tissue core. In addition to the malignant tumors, 541 benign neoplasms (see Supplemental Table S1 at http://ajp.amjpathol.org) and 264 normal tissue samples (see Supplemental Table S2 at http://ajp.amjpathol.org) were also obtained. The study was approved by the Johns Hopkins University School of Medicine institutional review board.
Telomere-Specific Immunostaining FISH
Combined telomere-specific FISH and immunofluorescence labeling of PML protein was performed as described previously.13,14 Briefly, deparaffinized slides were hydrated, steamed for 25 minutes in citrate buffer (Vector Laboratories, Burlingame, CA), dehydrated, and hybridized with a Cy3-labeled peptide nucleic acid (PNA) probe complementary to the mammalian telomere repeat sequence [(N-terminus to C-terminus) 5′-CCCTAACCCTAACCCTAA-3′]. As a positive control for hybridization efficiency, an Invitrogen (Carlsbad, CA) Alexa Fluor 610-labeled PNA probe having specificity for human centromeric DNA repeats (5′-ATTCGTTGGAAACGGGA-3′, CENP-B binding sequence) was included in the hybridization solution.19 After posthybridization washes, an anti-PML antibody (1:100 dilution; catalog no. PG-M3; Dako, Carpinteria, CA) was incubated for 45 minutes at room temperature, followed by incubation of anti-mouse Alexa Fluor 488 fluorescent secondary antibody (catalog no. A-11001; Molecular Probes, Eugene, OR) and counterstaining with DAPI. Slides were imaged with a Nikon 50i epifluorescence microscope equipped with X-Cite series 120 illuminator (EXFO Photonics Solutions, Mississauga, ON, Canada) and appropriate fluorescence excitation/emission filters. Grayscale images were captured using Nikon NIS-Elements software version 2.30 and an attached Photometrics (Tucson, AZ) CoolSNAP EZ digital camera, pseudo-colored, and merged.
ALT Assessment
All cases were assessed for the presence of the ALT phenotype. ALT-positive cases were identified by large, very bright intranuclear foci of telomere FISH signals marking ALT-associated telomeric foci throughout the tumor cells. Although telomere FISH signals from these individual bright foci often colocalized with PML protein, heterogeneity in this trait was observed, even within the same tumor. Given several instances in the literature of ALT-positive cell lines lacking telomere/PML colocalization,20–22 colocalization was not considered an absolute requirement for classifying a case as ALT-positive. Thus, cases were classified as ALT-positive if they met the following criteria: first, the presence of ultra-bright intranuclear foci of telomere FISH signals (ALT-associated telomeric foci), with integrated total signal intensities for individual foci being >10-fold that of the per cell mean integrated signal intensities for all telomeric signals in individual benign stromal cells within the same case; second, ≥1% of tumor cells displaying these ALT-associated telomeric foci. Cases lacking ALT-associated telomeric foci in which at least 500 cells were assessed were considered ALT-negative. Areas exhibiting necrosis were excluded from consideration.
Statistical Analysis
When appropriate, different tumor subtypes were compared with a two-sided Fisher's exact test using SAS version 9.2 statistical packages (SAS Institute, Cary, NC). P values of <0.05 were considered to be significant.
Results
Determination of the ALT Telomere Maintenance Mechanism in Human Cancer Subtypes
We identified the presence of the ALT phenotype by using telomere-specific FISH to visualize telomeric DNA in interphase nuclei of fixed tissue specimens. ALT-positive tumors are readily distinguishable by large ultra-bright telomere FISH signals, which are a nearly universal feature of ALT-positive cell populations.23 In Figure 1, we present for comparison an ALT-negative invasive urothelial carcinoma case (Figure 1A) and an ALT-positive invasive urothelial carcinoma case (Figure 1B). The ALT-negative case displays robust telomere signals in the tumor cells and adjacent stromal cells; in the ALT-positive case, distinctive large, very bright intranuclear foci of telomere FISH signals mark ALT-associated telomeric foci throughout the tumor cells. Other representative ALT-positive cases shown include a renal sarcomatoid carcinoma (Figure 1C) and an anaplastic medulloblastoma (Figure 1D), neither of which has been previously identified as using the ALT pathway. In ALT-positive tumors, the percentage of cells containing ALT-associated telomeric foci varied by tumor type, ranging from 1% to >95% of tumor cells. This trait also varied among different tumors from the same cancer subtype. Finally, in Figure 1 we present two additional ALT-positive cases, an oligodendroglioma (Figure 1E) and an angiosarcoma (Figure 1F). In both of these cases, PML protein colocalizes to most of the ALT-associated telomere foci. The inset for each case highlights a typical APB, displaying a targetoid appearance of telomere DNA signal with a peripheral rim of PML protein.
Figure 1.
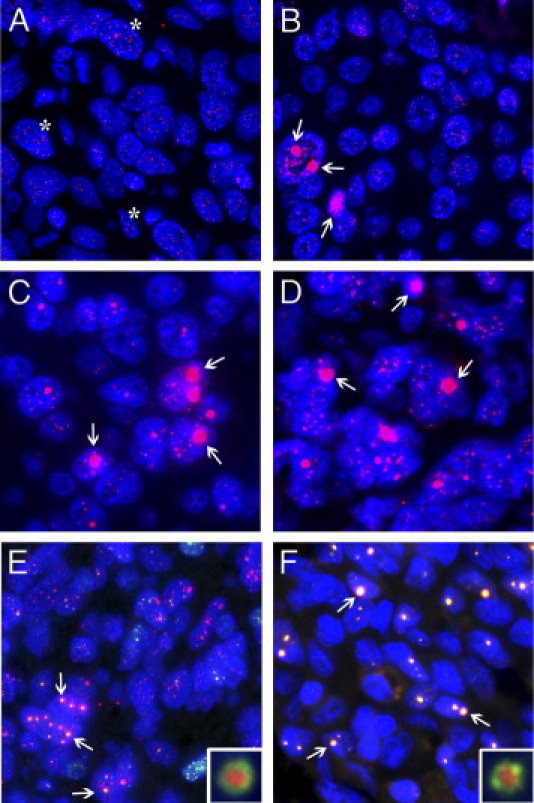
Representative examples of ALT-negative and ALT-positive tumors. A and B: Representative invasive urothelial carcinomas. In the ALT-negative case (A), robust telomere signals are present in tumor cells and adjacent stromal cells (asterisks). In the ALT-positive case (B), distinctive large, very bright intranuclear foci of telomere FISH signals mark ALT-associated telomeric foci throughout the tumor cells (arrows). Note the marked heterogeneity in the telomere signals, where visible, in the cancer cells. C–F: Representative ALT-positive cases of renal sarcomatoid carcinoma (C), anaplastic medulloblastoma (D), oligodendroglioma (E), and angiosarcoma (F). In all images (A–F), the DNA is stained with DAPI (blue) and telomere DNA is stained with the Cy3-labeled telomere-specific PNA probe (red). Two cases (E and F) are shown with costaining of PML protein (green), to demonstrate colocalization to most of the ALT-associated telomeric foci (arrows). The inset for each case highlights a typical APB that contains a targetoid appearance of telomere signal with a peripheral rim of PML protein. Original magnification: ×400 (A–F); ×1000 (inset).
Prevalence of the ALT Telomere Maintenance Mechanism in Human Cancer Subtypes
To determine the prevalence of the ALT phenotype in human cancers, we assessed two independent sets of malignant tissues comprising 6110 primary tumors from 94 different cancer subtypes. Set 1 consisted of 4001 specimens encompassing a broad range of malignant tumors, including tumors arising from the adrenal gland, biliary tract, breast, central nervous system, colon, esophagus, gallbladder, kidney, liver, lung, ovary, pancreas, prostate, salivary gland, skin, small intestine, soft tissue, stomach, urinary bladder, and uterus (Table 1). A total of 141 tumors were identified as ALT-positive in set 1, yielding a prevalence of 3.52%. To confirm and expand on these findings, we further assessed the ALT phenotype in a second set of multitumor TMAs (set 2), which had previously been used to validate other molecular markers.18 This set of tumors consisted of 2109 primary tumor specimens from 61 different cancer subtypes. Set 2 included types similar to the first set, but also included hematopoietic and neuroendocrine neoplasms, as well as tumors arising from the oral cavity, pleura, tendon sheath, testis, and thyroid (Table 1). A total of 87 tumors were identified as ALT-positive in set 2, representing a prevalence of 4.13%. With cases from both sets combined, a total of 228 ALT-positive tumors were identified, representing an overall prevalence of the ALT phenotype in human cancers of 3.73% (Table 1).
Table 1.
Prevalence of the ALT Phenotype in Human Cancer Subtypes
| Location/Tumor type | ALT+, set 1 |
ALT+, set 2 |
ALT+, overall |
|||
|---|---|---|---|---|---|---|
| n/N | % | n/N | % | n/N | % | |
| Adrenal gland/peripheral nervous system | ||||||
| Pheochromocytoma | 1/39 | 3 | 1/28 | 4 | 2/67 | 3 |
| Neuroblastoma | 2/22 | 9 | —⁎ | — | 2/22 | 9 |
| Ganglioneuroblastoma | 1/7 | 14 | — | — | 1/7 | 14 |
| Biliary | ||||||
| Cholangiocarcinoma, extrahepatic | 0/23 | 0 | — | — | 0/23 | 0 |
| Cholangiocarcinoma, intrahepatic | 0/10 | 0 | — | — | 0/10 | 0 |
| Breast | ||||||
| Ductal carcinoma† | 5/217 | 2 | 0/34 | 0 | 5/251 | 2 |
| Ductal carcinoma with lobular features | 0/20 | 0 | — | — | 0/20 | 0 |
| Lobular carcinoma | 1/14 | 7 | 0/13 | 0 | 1/27 | 4 |
| Mucinous carcinoma | — | — | 0/15 | 0 | 0/15 | 0 |
| Tubular carcinoma | — | — | 0/9 | 0 | 0/9 | 0 |
| Medullary carcinoma | 0/1 | 0 | 1/54 | 2 | 1/55 | 2 |
| Central nervous system | ||||||
| Pilocytic astrocytoma (grade 1) | 2/55 | 4 | 0/3 | 0 | 2/58 | 3 |
| Diffuse astrocytoma (grade 2) | 14/19 | 74 | 3/8 | 38 | 17/27 | 63 |
| Anaplastic astrocytoma (grade 3) | 17/19 | 89 | 2/11 | 18 | 19/30 | 63 |
| Glioblastoma multiforme (grade 4; adult) | 9/65 | 14 | 3/40 | 8 | 12/105 | 11 |
| Glioblastoma multiforme (grade 4; pediatric) | 14/32 | 44 | — | — | 14/32 | 44 |
| Oligodendroglioma | 6/20 | 30 | 2/20 | 10 | 8/40 | 20 |
| Medulloblastoma, anaplastic | 3/17 | 18 | — | — | 3/17 | 18 |
| Medulloblastoma, nonanaplastic | 1/38 | 3 | — | — | 1/38 | 3 |
| Other embryonal tumors | 1/10 | 10 | — | — | 1/10 | 10 |
| Meningioma | — | — | 1/46 | 2 | 1/46 | 2 |
| Schwannoma | — | — | 1/44 | 2 | 1/44 | 2 |
| Colon | ||||||
| Adenocarcinoma | 0/77 | 0 | 0/49 | 0 | 0/126 | 0 |
| Esophagus | ||||||
| Adenocarcinoma | 0/97 | 0 | 1/9 | 11 | 1/106 | 1 |
| Squamous cell carcinoma | — | — | 0/29 | 0 | 0/29 | 0 |
| Small cell carcinoma | — | — | 0/1 | 0 | 0/1 | 0 |
| Gallbladder | ||||||
| Adenocarcinoma | 1/27 | 4 | 0/33 | 0 | 1/60 | 2 |
| Hematopoietic neoplasms | ||||||
| non-Hodgkin's lymphoma, other subtypes | — | — | 0/54 | 0 | 0/54 | 0 |
| non-Hodgkin's lymphoma, diffuse large B-cell | — | — | 0/10 | 0 | 0/10 | 0 |
| Hodgkin's lymphoma, nodular sclerosis | — | — | 0/23 | 0 | 0/23 | 0 |
| Hodgkin's lymphoma, mixed cellularity | — | — | 0/17 | 0 | 0/17 | 0 |
| Thymoma | — | — | 0/37 | 0 | 0/37 | 0 |
| Kidney | ||||||
| Clear cell carcinoma | 1/69 | 1 | 0/48 | 0 | 1/117 | 1 |
| Papillary carcinoma | 0/54 | 0 | 1/32 | 3 | 1/86 | 1 |
| Chromophobe carcinoma | 3/37 | 8 | 1/10 | 10 | 4/47 | 9 |
| Sarcomatoid carcinoma | 2/27 | 7 | — | — | 2/27 | 7 |
| Larynx | ||||||
| Squamous cell carcinoma | — | — | 0/29 | 0 | 0/29 | 0 |
| Liver | ||||||
| Hepatocellular carcinoma | 7/91 | 8 | 1/30 | 3 | 8/121 | 7 |
| Lung | ||||||
| Adenocarcinoma | 0/64 | 0 | 0/89 | 0 | 0/153 | 0 |
| Squamous cell carcinoma | 0/55 | 0 | 0/45 | 0 | 0/100 | 0 |
| Papillary carcinoma | 0/45 | 0 | — | — | 0/45 | 0 |
| Bronchioloalveolar carcinoma | 0/40 | 0 | — | — | 0/40 | 0 |
| Small cell carcinoma | 0/16 | 0 | 1/47 | 2 | 1/63 | 2 |
| Large cell carcinoma | 0/10 | 0 | 1/25 | 4 | 1/35 | 3 |
| Carcinoma, other subtypes | 0/15 | 0 | — | — | 0/15 | 0 |
| Carcinoid tumor | 0/3 | 0 | — | — | 0/3 | 0 |
| Neuroendocrine neoplasms | ||||||
| Carcinoid tumor | — | — | 2/32 | 6 | 2/32 | 6 |
| Paraganglioma | — | — | 1/8 | 13 | 1/8 | 13 |
| Oral cavity | ||||||
| Squamous cell carcinoma | — | — | 0/41 | 0 | 0/41 | 0 |
| Ovary | ||||||
| Serous carcinoma | 0/163 | 0 | 0/42 | 0 | 0/205 | 0 |
| Clear cell carcinoma | 2/56 | 4 | — | — | 2/56 | 4 |
| Endometrioid carcinoma | 0/32 | 0 | 1/40 | 3 | 1/72 | 1 |
| Mucinous carcinoma | — | — | 0/21 | 0 | 0/21 | 0 |
| Pancreas | ||||||
| Adenocarcinoma | 0/420 | 0 | 0/28 | 0 | 0/448 | 0 |
| Pleura | ||||||
| Malignant mesothelioma | — | — | 1/28 | 4 | 1/28 | 4 |
| Prostate | ||||||
| Adenocarcinoma | 0/1071 | 0 | 0/81 | 0 | 0/1152 | 0 |
| Small cell carcinoma | 0/24 | 0 | — | — | 0/24 | 0 |
| Salivary gland | ||||||
| Carcinoma | 0/98 | 0 | — | — | 0/98 | 0 |
| Skin | ||||||
| Malignant melanoma | 2/47 | 4 | 5/59 | 8 | 7/106 | 7 |
| Basal cell carcinoma | — | — | 0/57 | 0 | 0/57 | 0 |
| Squamous cell carcinoma | — | — | 0/56 | 0 | 0/56 | 0 |
| Small intestine | ||||||
| Adenocarcinoma | 0/195 | 0 | 0/20 | 0 | 0/215 | 0 |
| Soft tissues | ||||||
| Gastrointestinal stromal tumor | 0/34 | 0 | — | — | 0/34 | 0 |
| Kaposi's sarcoma | 0/33 | 0 | 0/22 | 0 | 0/55 | 0 |
| Ewing's sarcoma/primitive neuroectodermal tumor | 0/23 | 0 | — | — | 0/23 | 0 |
| Undifferentiated pleomorphic sarcoma‡ | 15/22 | 68 | 18/30 | 60 | 33/52 | 63 |
| Fibrosarcoma and variants | 3/21 | 14 | — | — | 3/21 | 14 |
| Leiomyosarcoma | 11/13 | 85 | 20/46 | 43 | 31/59 | 53 |
| Liposarcoma | 3/10 | 30 | 6/28 | 21 | 9/38 | 24 |
| Angiosarcoma | 1/9 | 11 | — | — | 1/9 | 11 |
| Epithelioid sarcoma | 2/6 | 33 | — | — | 2/6 | 33 |
| Clear cell sarcoma | 0/5 | 0 | — | — | 0/5 | 0 |
| Malignant peripheral nerve sheath tumor | 0/4 | 0 | — | — | 0/4 | 0 |
| Rhabdomyosarcoma | 0/4 | 0 | — | — | 0/4 | 0 |
| Chondrosarcoma | 2/2 | 100 | — | — | 2/2 | 100 |
| Neurofibroma | — | — | 4/37 | 11 | 4/37 | 11 |
| Stomach | ||||||
| Adenocarcinoma | 0/80 | 0 | 0/75 | 0 | 0/155 | 0 |
| Tendon sheath | ||||||
| Giant cell tumor | — | — | 0/22 | 0 | 0/22 | 0 |
| Testis | ||||||
| Seminoma | — | — | 0/48 | 0 | 0/48 | 0 |
| Nonseminomatous germ cell tumor | — | — | 7/46 | 15 | 7/46 | 15 |
| Thyroid | ||||||
| Follicular carcinoma | — | — | 0/52 | 0 | 0/52 | 0 |
| Papillary carcinoma | — | — | 0/30 | 0 | 0/30 | 0 |
| Urinary bladder | ||||||
| Invasive urothelial carcinoma | 2/75 | 3 | 0/75 | 0 | 2/150 | 1 |
| Non-invasive urothelial carcinoma | — | — | 0/38 | 0 | 0/38 | 0 |
| Small cell carcinoma | 3/13 | 23 | — | — | 3/13 | 23 |
| Non-invasive papillary urothelial carcinoma | 0/5 | 0 | — | — | 0/5 | 0 |
| Squamous carcinoma | 0/2 | 0 | — | — | 0/2 | 0 |
| Sarcomatoid carcinoma | 0/1 | 0 | — | — | 0/1 | 0 |
| Uterus | ||||||
| Cervix, squamous carcinoma | 3/127 | 2 | 0/25 | 0 | 3/152 | 2 |
| Cervix, adenocarcinoma | 0/19 | 0 | — | — | 0/19 | 0 |
| Endometrium, endometrioid carcinoma | 0/16 | 0 | 0/48 | 0 | 0/64 | 0 |
| Endometrium, serous carcinoma | 1/9 | 11 | 2/32 | 6 | 3/41 | 7 |
| Endometrium, mixed mesodermal tumor | 0/4 | 0 | — | — | 0/4 | 0 |
| Endometrium, clear cell carcinoma | 0/3 | 0 | — | — | 0/3 | 0 |
Subtype not included in this set.
Includes data from samples previously published.17
Includes cases classified as malignant fibrous histiocytoma.
First Description of the ALT Telomere Maintenance Mechanism in Numerous Cancer Subtypes
Although we recently described the presence of the ALT phenotype in a small subset of breast carcinomas,17 the ALT phenotype has rarely been reported in other epithelial malignancies.16 Here, we report the presence of the ALT phenotype in numerous epithelial malignancies. The ALT phenotype was present in 8/121 (7%) cases of hepatocellular carcinoma, 3/41 (7%) cases of serous endometrial carcinoma, 3/152 (2%) cases of squamous cervical carcinoma, 1/60 (2%) case of gallbladder adenocarcinoma, and 1/106 (1%) case of esophageal adenocarcinoma. In renal carcinoma, the ALT phenotype was observed in 4/47 (9%) cases of chromophobe carcinoma, 2/27 (7%) cases of sarcomatoid carcinoma, 1/117 (1%) case of clear cell carcinoma, and 1/86 (1%) case of papillary carcinoma. In urinary bladder carcinomas, we observed the presence of ALT in 3/13 (7%) cases of small cell bladder carcinoma and 2/150 (1%) cases of invasive urothelial carcinoma. Although ALT was not observed in most lung carcinoma subtypes, we did observe a single case each of large cell [1/35 (3%)] and small cell [1/63 (2%)] carcinomas that exhibited the ALT phenotype.
In addition to the novel findings in epithelial malignancies, we present here the first report of ALT in several tumor types of nonepithelial origin, including medulloblastomas, pediatric GBMs, oligodendrogliomas, meningiomas, and schwannomas. In medulloblastomas, the prevalence of ALT-positive tumors varied across subtypes: 18% in anaplastic medulloblastomas and 3% in nonanaplastic medulloblastomas. The prevalence of the ALT phenotype in adult GBM cases was 11%. We also assessed 32 cases of pediatric GBM and observed a statistically significant increase in the prevalence of the ALT phenotype in the pediatric cases (44%), compared with the adult cases (P = 0.0002). In other central nervous system tumors, the prevalence of ALT was 20% in oligodendrogliomas, 2% in meningiomas, and 2% in schwannomas.
The ALT Telomere Maintenance Mechanism Is Not Observed in Numerous Cancer Subtypes
There appear to be several cancer subtypes that rarely, if ever, use the ALT telomere maintenance mechanism. In particular, we did not observe the ALT phenotype in adenocarcinomas arising from the prostate (N = 1152), pancreas (N = 448), small intestine (N = 215), stomach (N = 155), or colon (N = 126). Although the numbers of cases were smaller, we also did not observe the ALT phenotype in cholangiocarcinomas, laryngeal squamous cell carcinomas, oral squamous cell carcinomas, salivary gland carcinomas, follicular and papillary thyroid carcinomas, giant cell tumors of the tendon sheath, or hematopoietic neoplasms. Although malignancies arising from certain organs (eg, prostate cancer) rarely, if ever, develop ALT, there are malignancies from other organ sites that are capable of developing the ALT phenotype, but apparently only in particular cancer subtypes. Notably, in lung carcinoma, the ALT phenotype was observed only in a small subset of carcinomas originating from neuroendocrine cells; it was not observed in any other subtype. Other specific subtypes in which we did not observe the ALT phenotype include ovarian serous carcinoma, endometrioid carcinoma of the endometrium, seminoma, and basal cell and squamous cell carcinoma of the skin. Although the ALT phenotype is highly prevalent in certain types of sarcomas, we did not find evidence of the ALT phenotype in gastrointestinal stromal tumors, Kaposi's sarcomas, or Ewing's sarcomas/primitive neuroectodermal tumors.
The ALT Telomere Maintenance Mechanism Is Not Observed in Normal Tissue Samples or Benign Neoplasms
Next, we assessed a set of 264 normal tissues encompassing a wide range of tissue types. The ALT phenotype was not observed in these non-neoplastic tissue samples (see Supplemental Table S1 at http://ajp.amjpathol.org). In accord with this observation, we did not observe the ALT phenotype in non-neoplastic tissue entrapped in or adjacent to any of the tumors assessed. We also assessed a set of 541 benign neoplasms arising from a range of different tissues. The ALT phenotype was not observed in these benign neoplasms (see Supplemental Table S1 at http://ajp.amjpathol.org). Although we did not specifically assess intraepithelial neoplasms, we did observe the ALT phenotype in two individual cases: a melanoma in situ and a case of cervical intraepithelial neoplasia (grade 3). For representative images demonstrating the presence of ALT-associated telomeric foci in these cases (see Supplemental Figure S1 at http://ajp.amjpathol.org).
Discussion
In the present study, we comprehensively surveyed the ALT telomere maintenance mechanism in a broad range of human cancer subtypes. We used telomere-specific FISH and immunofluorescence labeling for PML protein to assess the ALT status in fixed tissue specimens of 6110 primary tumors from 94 different cancer subtypes. Across all cancer subtypes, the prevalence of the ALT phenotype was 3.73%; however, the prevalence varied widely between different cancer subtypes. The results obtained in sets 1 and 2 are similar, except in leiomyosarcomas, for which there was a statistically significant difference in the prevalence between the two independent sets (85% versus 43%; P = 0.012).
Through this intensive characterization of the ALT phenotype in human cancer, we describe for the first time ALT-positive carcinomas arising from the bladder, cervix, endometrium, esophagus, gallbladder, kidney, liver, and lung. Although in some of these epithelial malignancies we observed only single ALT-positive cases, other carcinoma subtypes displayed considerable frequencies of ALT-positivity. For example, 7% of hepatocellular carcinomas were ALT-positive; within carcinomas of the kidney, 9% of chromophobe carcinomas and 7% of sarcomatoid carcinomas were ALT-positive. Of note, in some tissues, it appears that the ALT phenotype is more prevalent in tumors arising from neuroendocrine cells. For example, the prevalence of ALT in small cell bladder cases was 23%, compared with only 1% in invasive urothelial carcinoma. Similarly, individual cases of small cell and large cell lung carcinomas were observed; ALT was not present in any other lung carcinoma subtype.
In contrast to a previous study by Au et al,24 wherein ALT was not observed in malignant pleural mesotheliomas (N = 43), in the present study we observed a single ALT-positive case of this cancer (N = 28). Two additional cancer types that had previously been determined to contain a small subset of ALT-positive tumors were confirmed and extended. Previously, Bryan et al10 observed abnormally long telomeres (by Southern blotting) in 1/9 malignant melanomas and 2/15 ovarian carcinomas. In the present study, we found a prevalence of 7% in malignant melanomas, 4% in ovarian clear cell carcinomas, and 1% in endometrioid carcinomas of the ovary.
This is the first report of the ALT telomere maintenance mechanism in pediatric GBMs, medulloblastomas, oligodendrogliomas, meningiomas, and schwannomas. The presence of the ALT phenotype in GBM has been described previously,15,25–27 but all cases assessed were in adults. The prevalence of the ALT phenotype in adult GBM cases in the present study (11%) is similar to that in a recently published large retrospective series using the same assay (15%).27 Previous studies on smaller sets of adult GBM reported prevalence at 22% (7/32)15 and 25% (19/77).26 Notably, Henson et al15 observed an inverse relationship between ALT-positivity and patient age, and this observation was confirmed by McDonald et al27 in a retrospective cohort. Here, we report a significantly increased prevalence in pediatric GBM (44%), compared with adult GBM (11%). In adult GBM, recent results have shown a significantly longer overall survival in patients with mutations of the isocitrate dehydrogenase 1 gene (IDH1) in the presence of ALT.27 It would be worthwhile to examine the prognostic associations of ALT in pediatric GBM, although these tumors almost never show mutations in IDH1.
We identified many tumor types that apparently may not use the ALT pathway for telomere maintenance. In particular, we assessed hundreds of cases of adenocarcinomas arising from the prostate, colon, pancreas, and small intestine and did not observe a single ALT-positive tumor. These results suggest that particular tumor types preferentially use telomerase activation for stabilization of telomeres and emphasize the previous findings that the ALT phenotype is more common in tumors with mesenchymal and neuroepithelial cell origins.12,16 The ability of epithelial cells to up-regulate telomerase more easily than mesenchymal cells may account for these differences.
In sarcomas, the ALT phenotype has been described previously in specific subtypes.16 In agreement with these reports,16,28 we found the ALT phenotype in 24% of liposarcomas, 53% of leiomyosarcomas, and 63% of undifferentiated pleomorphic sarcomas (which includes malignant fibrous histiocytomas). In contrast to GBM, in liposarcomas the presence of the ALT phenotype confers a poor prognosis.28,29 Other genetic changes associated with the ALT phenotype presumably play important roles in determining the prognostic significance within a given tumor type. Although the ALT phenotype is extremely prevalent in certain sarcomas, we did not observe ALT in Ewing's sarcomas/primitive neuroectodermal tumors, gastrointestinal stromal tumors, or Kaposi's sarcomas. These findings are consistent with previous investigations showing that sarcomas characterized by specific chromosomal translocations tend to maintain telomeres via telomerase activation, whereas sarcomas with complex karyotypes are capable of using the ALT pathway.14,30
As outlined above, the results presented in the current study are in broad agreement with previous reports on ALT in human cancers.16 However, there is a major difference in one previously assessed cancer subtype: we did not observe the ALT phenotype in gastric carcinomas (n = 155). This is in contrast to findings of Omori et al,31 who recently reported a 38% overall ALT prevalence in gastric carcinomas, with an even higher prevalence reported in gastric carcinomas with microsatellite instability. One potential explanation for these discrepant results is that the previous study included an amplification protocol to intensify the telomere FISH signals, whereas in the current study we assessed telomere signals directly, without the use of amplification. Thus, it is possible that signal amplification in the prior study may have resulted in overly bright telomere FISH intensities, causing them to be mistaken for ALT-associated telomeric foci.
Despite the large number of cases and cancer subtypes assessed, the present study has limitations. Because of the TMA sampling methodology, prevalence estimates may underestimate the true prevalence of the ALT phenotype in some cancer subtypes. In addition, estimates of the prevalence of the ALT phenotype in certain subtypes may be too high or too low, particularly if the absolute numbers of cases examined are small. However, the present findings may help guide future studies to determine the actual prevalence of ALT within a given cancer subtype. All of the cases assessed in this survey were primary tumors; therefore, future studies are needed to assess the prevalence of ALT in metastatic lesions. Similarly, we assessed only a small number of premalignant lesions, and the role of the ALT phenotype in cancer progression is not yet elucidated. Although the present study comprehensively covered many cancer subtypes, some subtypes were not assessed; in particular, no leukemia cases were assessed. Finally, although several telomerase antibodies are available, none have been adequately validated for use in formalin-fixed, paraffin-embedded tissues. Thus, the use of such fixed specimens in the present study precluded additional analyses that would have required either fresh or frozen tissue samples, such as assessment of telomerase enzymatic activity.
The ALT telomere maintenance mechanism provides prognostic information in some cancers.16 Further studies are needed to assess the prognostic significance and unique biology of tumors that express ALT. The present study offers a springboard to guide future investigations in large cohorts that specifically focus on the tumor types exhibiting ALT to determine the true prevalence and potential prognostic value of this phenotype. Finally, these results may have therapeutic consequences, given that cancers using the ALT pathway are predicted to be resistant to anti-telomerase therapies, some of which have entered phase I/II clinical trials. Further understanding of the molecular mechanisms of ALT will be paramount in designing novel anti-cancer therapeutics targeting the ALT pathway.
Acknowledgment
We thank staff members Helen Fedor, Marcella Southerland, and Bonnie Gambichler of the Tissue Microarray Facility at Johns Hopkins.
Footnotes
Supported by an NIH postdoctoral training fellowship (T32 CA067751) to C.M.H. and a Department of Defense Breast Cancer Research Program postdoctoral fellowship (W81XWH-09-1-0650) to C.M.H. and by SPORE programs of the National Cancer Institute (P50 CA88843, P50 CA58236, P50 CA62924, P50 CA058184, P50 CA098252) to Johns Hopkins University School of Medicine.
Supplemental material for this article can be found at http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.06.018.
Supplementary data
Two ALT-positive intraepithelial neoplasms. The ALT phenotype was observed in two individual cases; (A) a melanoma in situ and (B) a cervical intraepithelial neoplasm (CIN grade 3). The arrows indicate the distinctive large, very bright, intra-nuclear foci of telomere FISH signals marking ALT-associated telomeric foci throughout the tumor cells. In both images, the DNA is stained with DAPI (blue) and telomere DNA is stained with the Cy3-labeled telomere-specific PNA probe (red). Original magnification × 400.
References
- 1.Blackburn E.H. Structure and function of telomeres. Nature. 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 2.Moyzis R.K., Buckingham J.M., Cram L.S., Dani M., Deaven L.L., Jones M.D., Meyne J., Ratliff R.L., Wu J.R. A highly conserved repetitive DNA sequence, (TTAGGG)n, present at the telomeres of human chromosomes. Proc Natl Acad Sci USA. 1988;85:6622–6626. doi: 10.1073/pnas.85.18.6622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Palm W., de Lange T. How shelterin protects mammalian telomeres. Annu Rev Genet. 2008;42:301–334. doi: 10.1146/annurev.genet.41.110306.130350. [DOI] [PubMed] [Google Scholar]
- 4.de Lange T. Shelterin: the protein complex that shapes and safeguards human telomeres. Genes Dev. 2005;19:2100–2110. doi: 10.1101/gad.1346005. [DOI] [PubMed] [Google Scholar]
- 5.de Lange T. How telomeres solve the end-protection problem. Science. 2009;326:948–952. doi: 10.1126/science.1170633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O'Sullivan R.J., Karlseder J. Telomeres: protecting chromosomes against genome instability. Nat Rev Mol Cell Biol. 2010;11:171–181. doi: 10.1038/nrm2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vaziri H. Critical telomere shortening regulated by the ataxia-telangiectasia gene acts as a DNA damage signal leading to activation of p53 protein and limited life-span of human diploid fibroblasts: A review. Biochemistry (Mosc) 1997;62:1306–1310. [PubMed] [Google Scholar]
- 8.Maser R.S., DePinho R.A. Connecting chromosomes, crisis, and cancer. Science. 2002;297:565–569. doi: 10.1126/science.297.5581.565. [DOI] [PubMed] [Google Scholar]
- 9.Shay J.W., Bacchetti S. A survey of telomerase activity in human cancer. Eur J Cancer. 1997;33:787–791. doi: 10.1016/S0959-8049(97)00062-2. [DOI] [PubMed] [Google Scholar]
- 10.Bryan T.M., Englezou A., Dalla-Pozza L., Dunham M.A., Reddel R.R. Evidence for an alternative mechanism for maintaining telomere length in human tumors and tumor-derived cell lines. Nat Med. 1997;3:1271–1274. doi: 10.1038/nm1197-1271. [DOI] [PubMed] [Google Scholar]
- 11.Royle N.J., Foxon J., Jeyapalan J.N., Mendez-Bermudez A., Novo C.L., Williams J., Cotton V.E. Telomere length maintenance–an ALTernative mechanism. Cytogenet Genome Res. 2008;122:281–291. doi: 10.1159/000167814. [DOI] [PubMed] [Google Scholar]
- 12.Cesare A.J., Reddel R.R. Alternative lengthening of telomeres: models, mechanisms and implications. Nat Rev Genet. 2010;11:319–330. doi: 10.1038/nrg2763. [DOI] [PubMed] [Google Scholar]
- 13.Meeker A.K., Gage W.R., Hicks J.L., Simon I., Coffman J.R., Platz E.A., March G.E., De Marzo A.M. Telomere length assessment in human archival tissues: combined telomere fluorescence in situ hybridization and immunostaining. Am J Pathol. 2002;160:1259–1268. doi: 10.1016/S0002-9440(10)62553-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Montgomery E., Argani P., Hicks J.L., DeMarzo A.M., Meeker A.K. Telomere lengths of translocation-associated and nontranslocation-associated sarcomas differ dramatically. Am J Pathol. 2004;164:1523–1529. doi: 10.1016/S0002-9440(10)63710-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Henson J.D., Hannay J.A., McCarthy S.W., Royds J.A., Yeager T.R., Robinson R.A., Wharton S.B., Jellinek D.A., Arbuckle S.M., Yoo J., Robinson B.G., Learoyd D.L., Stalley P.D., Bonar S.F., Yu D., Pollock R.E., Reddel R.R. A robust assay for alternative lengthening of telomeres in tumors shows the significance of alternative lengthening of telomeres in sarcomas and astrocytomas. Clin Cancer Res. 2005;11:217–225. [PubMed] [Google Scholar]
- 16.Henson J.D., Reddel R.R. Assaying and investigating Alternative Lengthening of Telomeres activity in human cells and cancers. FEBS Lett. 2010;584:3800–3811. doi: 10.1016/j.febslet.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 17.Subhawong A.P., Heaphy C.M., Argani P., Konishi Y., Kouprina N., Nassar H., Vang R., Meeker A.K. The alternative lengthening of telomeres phenotype in breast carcinoma is associated with HER-2 overexpression. Mod Pathol. 2009;22:1423–1431. doi: 10.1038/modpathol.2009.125. [DOI] [PubMed] [Google Scholar]
- 18.Baumhoer D., Tornillo L., Stadlmann S., Roncalli M., Diamantis E.K., Terracciano L.M. Glypican 3 expression in human nonneoplastic, preneoplastic, and neoplastic tissues: a tissue microarray analysis of 4,387 tissue samples. Am J Clin Pathol. 2008;129:899–906. doi: 10.1309/HCQWPWD50XHD2DW6. [DOI] [PubMed] [Google Scholar]
- 19.Chen C., Hong Y.K., Ontiveros S.D., Egholm M., Strauss W.M. Single base discrimination of CENP-B repeats on mouse and human chromosomes with PNA-FISH. Mamm Genome. 1999;10:13–18. doi: 10.1007/s003359900934. [DOI] [PubMed] [Google Scholar]
- 20.Cerone M.A., Autexier C., Londoño-Vallejo J.A., Bacchetti S. A human cell line that maintains telomeres in the absence of telomerase and of key markers of ALT. Oncogene. 2005;24:7893–7901. doi: 10.1038/sj.onc.1208934. [DOI] [PubMed] [Google Scholar]
- 21.Fasching C.L., Bower K., Reddel R.R. Telomerase-independent telomere length maintenance in the absence of alternative lengthening of telomeres-associated promyelocytic leukemia bodies. Cancer Res. 2005;65:2722–2729. doi: 10.1158/0008-5472.CAN-04-2881. [DOI] [PubMed] [Google Scholar]
- 22.Marciniak R.A., Cavazos D., Montellano R., Chen Q., Guarente L., Johnson F.B. A novel telomere structure in a human alternative lengthening of telomeres cell line. Cancer Res. 2005;65:2730–2737. doi: 10.1158/0008-5472.CAN-04-2888. [DOI] [PubMed] [Google Scholar]
- 23.Yeager T.R., Neumann A.A., Englezou A., Huschtscha L.I., Noble J.R., Reddel R.R. Telomerase-negative immortalized human cells contain a novel type of promyelocytic leukemia (PML) body. Cancer Res. 1999;59:4175–4179. [PubMed] [Google Scholar]
- 24.Au A.Y., Hackl T., Yeager T.R., Cohen S.B., Pass H.I., Harris C.C., Reddel R.R. Telomerase activity in pleural malignant mesotheliomas. Lung Cancer. 2011;73:283–288. doi: 10.1016/j.lungcan.2010.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Slatter T., Gifford-Garner J., Wiles A., Tan X., Chen Y.J., MacFarlane M., Sullivan M., Royds J., Hung N. Pilocytic astrocytomas have telomere-associated promyelocytic leukemia bodies without alternatively lengthened telomeres. Am J Pathol. 2010;177:2694–2700. doi: 10.2353/ajpath.2010.100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hakin-Smith V., Jellinek D.A., Levy D., Carroll T., Teo M., Timperley W.R., McKay M.J., Reddel R.R., Royds J.A. Alternative lengthening of telomeres and survival in patients with glioblastoma multiforme. Lancet. 2003;361:836–838. doi: 10.1016/s0140-6736(03)12681-5. [DOI] [PubMed] [Google Scholar]
- 27.McDonald K.L., McDonnell J., Muntoni A., Henson J.D., Hegi M.E., von Deimling A., Wheeler H.R., Cook R.J., Biggs M.T., Little N.S., Robinson B.G., Reddel R.R., Royds J.A. Presence of alternative lengthening of telomeres mechanism in patients with glioblastoma identifies a less aggressive tumor type with longer survival. J Neuropathol Exp Neurol. 2010;69:729–736. doi: 10.1097/NEN.0b013e3181e576cf. [DOI] [PubMed] [Google Scholar]
- 28.Venturini L., Motta R., Gronchi A., Daidone M., Zaffaroni N. Prognostic relevance of ALT-associated markers in liposarcoma: a comparative analysis. BMC Cancer. 2010;10:254. doi: 10.1186/1471-2407-10-254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Costa A., Daidone M.G., Daprai L., Villa R., Cantù S., Pilotti S., Mariani L., Gronchi A., Henson J.D., Reddel R.R., Zaffaroni N. Telomere maintenance mechanisms in liposarcomas: association with histologic subtypes and disease progression. Cancer Res. 2006;66:8918–8924. doi: 10.1158/0008-5472.CAN-06-0273. [DOI] [PubMed] [Google Scholar]
- 30.Ulaner G.A., Hoffman A.R., Otero J., Huang H.Y., Zhao Z., Mazumdar M., Gorlick R., Meyers P., Healey J.H., Ladanyi M. Divergent patterns of telomere maintenance mechanisms among human sarcomas: sharply contrasting prevalence of the alternative lengthening of telomeres mechanism in Ewing's sarcomas and osteosarcomas. Genes Chromosomes Cancer. 2004;41:155–162. doi: 10.1002/gcc.20074. [DOI] [PubMed] [Google Scholar]
- 31.Omori Y., Nakayama F., Li D., Kanemitsu K., Semba S., Ito A., Yokozaki H. Alternative lengthening of telomeres frequently occurs in mismatch repair system-deficient gastric carcinoma. Cancer Sci. 2009;100:413–418. doi: 10.1111/j.1349-7006.2008.01063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Two ALT-positive intraepithelial neoplasms. The ALT phenotype was observed in two individual cases; (A) a melanoma in situ and (B) a cervical intraepithelial neoplasm (CIN grade 3). The arrows indicate the distinctive large, very bright, intra-nuclear foci of telomere FISH signals marking ALT-associated telomeric foci throughout the tumor cells. In both images, the DNA is stained with DAPI (blue) and telomere DNA is stained with the Cy3-labeled telomere-specific PNA probe (red). Original magnification × 400.


