Abstract
Anterior cruciate ligament (ACL) tears are known to be a risk factor for incident knee osteoarthritis (OA). At the present time, it is unknown whether an incidental ACL tear in those with established knee OA alters the pattern of synovial joint damage. Therefore, our aim was to assess whether ACL tears in persons with knee OA are associated with specific patterns of cartilage loss, meniscal degeneration, and bone marrow lesion (BML) location. We included 160 participants from the progression subcohort of the Osteoarthritis Initiative (OAI) Study, an ongoing 4-year, multicenter study, focusing on knee OA. Regional cartilage morphometry measures including cartilage volume (mm3), denuded area, normalized cartilage volume, bone surface area, as well as location of meniscal pathology and BMLs in index knees on the same side were compared between those with and without ACL tears. Of the 160 subjects (51% women, age 62.1 (±9.9), BMI 30.3 (±4.7) kg/m2), 14.4% had an ACL tear. After adjusting for age, BMI and gender participants with ACL tears had significantly greater cartilage volume in the posterior lateral femur (P = 0.04) and the central medial tibia (0.001) compared to those without ACL tears. Normalized cartilage volume was not different between those with and without ACL tears. In addition, individuals with ACL tears had significantly larger bone surface areas in the medial tibia (P = 0,006), the central medial tibia (P = 0.008), the posterior lateral femur (P = 0.004), and the posterior medial femur (P = 0.04). Furthermore, participants with ACL tears showed significantly more meniscal derangement in the lateral posterior horn (P = 0.019) and significantly more BMLs in the lateral femur (P = 0.0025). We found clear evidence of predominant lateral tibiofemoral involvement, with OA-associated findings on MRI, including increased denuded area and bone surface area, BMLs, and meniscal derangement in knees of individuals with ACL tears compared to those without.
Keywords: Knee osteoarthritis, Anterior cruciate ligament tear, Cartilage loss, Bone marrow lesions, Meniscal pathology
Introduction
Osteoarthritis (OA) is the leading cause of disability in older adults [1]. Although radiographic features such as joint space narrowing (JSN) and the presence of osteophytes define the presence of OA [2], new technologies such as magnetic resonance imaging (MRI) may improve the assessment of early disease development and progression. MRI has shown a higher sensitivity and specificity for the diagnosis of acute traumatic lesions within the knee and its early-stage post-traumatic degenerative changes [3–5] and the assessment of joint morphology [6, 7].
Knee injuries are an important risk factor for the development of knee OA [8–11]. Rupture of the anterior cruciate ligament (ACL) occurs more often at a younger age [12, 13] with an incidence for different sports between 18/1,000 players in soccer [14] and 1/1,000 game hours in handball [15]. Persons with a rupture of the ACL are at an elevated risk for knee OA [16–20], shown severalfold in young, athletic persons [14, 17, 20–22], due to subsequent anteroposterior instability of the knee.
There are some data suggesting that persons with ACL tears are at increased risk for cartilage loss [23, 24]; however, there are little data on the association of ACL tears with meniscal degeneration or bone marrow lesions (BMLs) in persons with OA. The presence of both of these structural features could facilitate further structural deterioration if ACL tears increased their frequency and severity.
In knees with acute ACL tears, it has been shown that chondral lesions seem to appear more frequently in the lateral tibiofemoral compartment [25]. Furthermore, the contact pattern of ACL-injured knees compared to healthy controls was shown to be more posterior in acutely injured knees, and this seemed to be associated with severity of symptoms [26, 27].
Therefore, the aim of this study was to explore the pattern of articular damage and the relationship to bone interface in persons with OA and ACL tears by testing the following hypothesis.
ACL tears in persons with knee OA will be associated with increased cartilage loss, meniscal degeneration, specific patterns of BMLs location, and other bony changes. This pattern of damage would be consistent with the predominant initial location of the osteochondral injury in those with ACL tears in the lateral tibiofemoral compartment.
Materials and methods
Study sample
The Osteoarthritis Initiative (OAI) is an ongoing 4-year, multicenter, longitudinal, prospective observational cohort study designed to identify biomarkers of the development and/or progression of knee OA. On an annual basis, 4,796 participants undergo detailed evaluation, including knee MRIs, fixed-flexion knee radiographs, detailed questionnaires on pain, demographic information, and physical examination (e.g., BMI). The local institutional review boards approved and reviewed the study protocol, amendments, and informed consent documentation. The data for this research were drawn from the OAI and are available for public access at http://www.oai.ucsf.edu/. The specific datasets used are clinical dataset 0.1.1 and Image Release 0.B.1.
The OAI consists of a progression subcohort, from which our sample is drawn, and an incidence subcohort. A total of 1,389 participants with radiographic signs (radio-graphic evidence of OA (ROA) defined as definite tibiofemoral osteophytes (OARSI atlas grade ≥ 1) on X-ray) and symptoms of knee OA, such as pain, stiffness and aching on most days of a month during the past year at baseline, were recruited for the progression subcohort. Subjects with severe narrowing (OARSI grade 3 narrowing or bone on bone) in both knees were excluded. The X-ray readings of osteophytes and joint space narrowing were taken at each OAI Clinical Center.
For the purposes of this study, a group of 160 participants was selected from the progression subcohort of the OAI, due to previously available data including high-quality MRI readings for ACL tears and data on chondral morphometry. For this convenience, sample of 160 participants, imaging data from baseline with the abovementioned data from a previous investigation and imaging data from one-year follow-up, was available. The detailed selection criteria for these participants and the index knee have been described previously [28].
Assessment of joint injury
History of joint surgery was evaluated at the enrollment visit by asking the participants whether they ever had any kind of knee surgery including arthroscopy, ligament repair surgery or meniscectomy, or they ever had a hip replacement surgery. In addition, participants were asked whether they ever injured their knee(s) badly enough to limit their ability to walk for at least 2 days. Results from this survey for our study population can be found in Table 2.
Table 2.
Descriptive characteristics of study sample (n = 160)
| All (n = 160) | No ACL tear (n = 137) | ACL tear (n = 23) | |
|---|---|---|---|
| Gender (female n (%)) | 81 (51) | 76 (56) | 5 (22) |
| Age (mean, SD) years | 62.1 (9.9) | 62.9 (9.5) | 57.5 (10.8) |
| Age group (≤65 years n (%)) | 95 (59) | 77 (56) | 18 (78) |
| BMI (mean, SD) kg/m2 | 30.3 (4.7) | 30.3 (4.7) | 30.5 (4.8) |
| Index knee (left n (%)) | 78 (49) | 69 (50) | 9 (39) |
| Kellgren and Lawrence grade of index knee, no. (%) | |||
| 0 | 6 (4) | 6 (4) | 0 (0) |
| 1 | 18 (11) | 17 (12) | 1 (4) |
| 2 | 58 (36) | 51 (37) | 7 (30) |
| 3 | 64 (40) | 54 (39) | 10 (44) |
| 4 | 14 (9) | 9 (7) | 5 (22) |
| History of knee and hip surgery including arthroscopy (yes for study knee n (%)) | 45 (28.9) | 31 (22.6) | 14 (60.9) |
| History of knee injury (yes for study knee n (%)) ever injured badly enough to limit ability to walk for at least 2 days | 75 (47.2) | 53 (38.7) | 22 (95.7) |
Radiographic assessment
A SynaFlexer™ frame (Synarc, Inc., San Francisco, CA) was used to obtain bilateral posteroanterior (PA) views of the knee to position the subject's feet reproducibly. The acquisition protocol required that the participant's body weight was distributed equally between their two legs and knees, and their thighs were pressed directly against the wall of the frame. The anterior wall of the frame was in contact with the Bucky or reclining tabletop of the radio-graphic unit. The goal of this positioning was to achieve a fixed angle of knee flexion of about 20°. Additionally, a V-shaped angulation support on the base of the frame was used to fix the foot below the index knee in 10° external rotation. The X-ray beam is angled 10° caudal [29].
For a prior study [28], the baseline radiographs of these 160 subjects had been read independently by one bone and joint radiologist and a rheumatologist (DH). The readers were blinded to sequence and performed paired readings of the knee X-rays using the Kellgren and Lawrence (KL) grade on a 0–4 scale [30]. If disagreements occurred, they used adjudicated readings that were arrived at by a consensus of both readers, for KL grade and JSN.
Selection of knee for analysis
Both the presence of symptoms (frequent knee pain) and ROA were required to be present in the same knee, to meet the inclusion criteria. There were 100 participants with unilateral OA that met these criteria. For those participants with bilateral symptomatic knee OA, we chose the knee with moderate disease and with a higher chance of disease progression. In individuals with KL grade 2 or 3 in both knees, we employed the following features to rank the risk of progression for both knees in order to select the knee at greatest risk for medial tibiofemoral progression. If knees still ranked equal after applying the first feature, we moved to the next feature listed below:
greater anatomic axis varus angulation,
≥2.0 mm medial minimum joint space width (JSW),
greater grade of medial JSN (grade 1–3),
the presence of any medial tibial or femoral osteophyte grade ≥ 2 with greater grade than lateral osteophytes,
the presence of any medial tibial or femoral osteophyte, and finally
the right knee
If neither of the knees was graded KL grade 2 or 3, the knee with the higher KL grade was chosen. If the participant was graded KL 0, 1, or 4 on both knees, then the same process described earlier was followed.
MRI sequence parameters
Images were acquired on identical 3 T MRI scanners (Siemens Magnetom Trio, Erlangen, Germany) using a quadrature transmit-receive knee coil (USA Instruments, Aurora, OH). For the purposes of reading BMLs, we used the sagittal intermediate-weighted (IW) turbo spin echo (TSE) fat-suppressed (FS) images. The Dual Echo in the Steady State (DESS) sequences was used to assist with the localization of some lesions. The menisci were scored with the same sequences and in addition on the 2D coronal IW TSE sequences. For the purpose of the cartilage segmentation, an unsupervised segmentation was done using the DESS sequences. Sequence parameters can be found in detail in Table 1 and also on the OAI homepage http://www.oai.ucsf.edu/.
Table 1.
MRI sequence parameters: also see OAI homepage http://www.oai.ucsf.edu
| MRI parameters | |||
|---|---|---|---|
| 2D TSE | 2D TSE | DESS | |
| Weighting | Int | Int | T2 |
| Plane | Coronal | Sagittal | Sagittal |
| Fat sat | No | Yes | WE |
| Matrix (phase) | 307 | 313 | 307 |
| Matrix (freq) | 384 | 448 | 384 |
| No. of slices | 41 | 37 | 160 |
| FOV (mm) | 140 | 160 | 140 |
| Slice thickness (mm) | 3 | 3 | 0.7 |
| Skip (mm) | 0 | 0 | 0 |
| Flip angle (°) | 180 | 180 | 25 |
| TE/TI (ms) | 29 | 30 | 4.7 |
| TR (ms) | 3,850 | 3,200 | 16.3 |
| BW (Hz/pixel) | 352 | 248 | 185 |
| Chemical shift (pixel) | 1.3 | 0 | 0 |
| NAV (NEX) | 1 | 1 | 1 |
| Echo train length | 7 | 5 | 1 |
| Phase encode axis | R/L | S/I | A/P |
| Phase partial fourier (8/8 = 1) | 1 | 1 | 0.875 |
| Readout partial fourier (8/8 = 1) | 1 | 1 | 1 |
| Slice partial fourier (8/8 = 1) | 1 | 1 | 0.875 |
| Options | Elliptical k-space filter and large FOV filter | Elliptical k-space filter and large FOV filter | Elliptical k-space filter, elliptical sampling, and lager FOV filter |
| Distance factor (%) | 0 | 0 | 0 |
| Phase oversampling | 20 | 4 | 0 |
| Slice oversampling | 0 | 0 | 10 |
| Phase resolution | 80 | 70 | 80 |
| Averaging technique | Short term | Short term | Short term |
| Gradient rise time | Fast | Fast | Fast |
| RF amplitude | Normal | Normal | Fat |
| X-resolution (mm) | 0.365 | 0.357 | 0.365 |
| Y-resolution (mm) | 0.456 | 0.511 | 0.456 |
| Calc time (min) | 3.4 | 4.7 | 11.2 |
| Scan time (min) | 3.4 | 4.7 | 10.2 |
MRI readings
The bilateral MRIs from the enrollment visit for 160 participants included in this study were obtained from the OAI Coordinating Center.
Bone marrow lesions (BMLs)
BMLs and meniscal derangement were evaluated in two different reading sessions by a rheumatologist (GHL) with expertise in musculoskeletal MRI readings and centrally involved in the development of the Boston Leeds Osteo-arthritis Knee Score (BLOKS) [31]. This reader was blinded to subject data and scored each knee MRI for BMLs and MRI meniscal derangement using the BLOKS grading system. The intra-rater reliability was kappa = 0.88 and was assessed by reading a sample of 10 knee MRIs twice by the same reader, at least 2 days separated from each other.
On the sagittal IW TSE FS images (Table 1), a BML was described when seeing an ill-defined hyperintense signal in the subchondral bone, proximal to the epiphyseal line (Fig. 1). Additionally, the DESS sequences (Table 1) were consulted to assist with the localization of lesions. The BMLs were evaluated for size on the IW TSE FS images from 0 to 3 at each of the following locations using BLOKS [31]: medial and lateral patella, medial and lateral trochlea, medial and lateral weight-bearing femur, medial and lateral tibia, and subspinous tibia with 0 = none, 1 = <10% of the whole bone volume, 2 = 10–25% of the whole bone volume, and 3 = >25% of the whole bone volume.
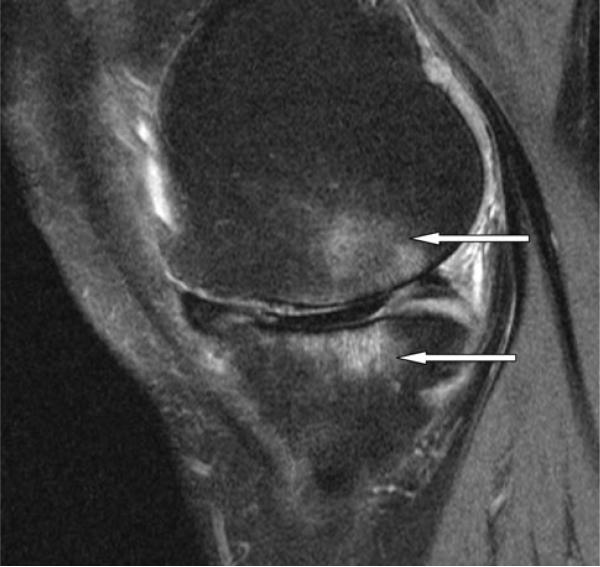
Fig. 1.
Sagittal view of the medial tibiofemoral compartment with large ill-defined subchondral bone marrow lesions in the tibia and femur (white arrows). There is extensive cartilage loss in the weight-bearing tibia and femur and partial maceration of the posterior horn of medial meniscus
Only those BMLs with greater than 25% of the surface area adjacent to the subchondral plate were included. We classified large BMLs as those with BLOKS score ≥ 2.
Menisci
The same sagittal IW TSE FS (Table 1) images as well as the coronal 2D IW TSE images (Table 1) were used to score the meniscal integrity. Those menisci with disruption of the overall morphology of the meniscus and diffuse hyperintense signal in the body of the meniscus were defined as MRI meniscal derangement. The meniscal derangement was graded in each of the following locations: the anterior horn, body, and posterior horn of the medial and lateral menisci. To assess intra-rater reliability, a sample of 10 knee MRIs were read for MRI meniscal derangement twice by the same reader separated by at least 2 days for a simple kappa of 0.87.
ACL tears
The presence of an ACL tear at baseline was read using sagittal and coronal views and scored on a 0–2 scale, with 0 = normal, 1 = partial tear, and 2 = complete tear. A complete tear was defined as complete disruption of ACL fibers with ligament discontinuity, while a partial tear was defined as residual straight and tight ACL fibers in at least one-pulse sequence, while the anteromedial and posterolateral bundle of the ACL were not evaluated separately in the coronal slices. All ACL tears were read by one board-certified musculoskeletal radiologist (AG) (intra-reader weighted kappa = 0.75), separate from the scoring of other articular features and unaware of the hypothesis being tested. Due to the fact that even partial tears may change the bio-mechanics, and therefore the pattern of joint damage and the small sample size, we combined partial and complete tears.
Cartilage morphometry
For the analysis of the cartilage parameters, we used the DESS sequences. The segmentation was semiautomated and has been described in detail elsewhere [28].
The following measures were evaluated after the segmentation of the images in different areas of the knee joint:
-
1
Cartilage volume.
-
2
Normalized cartilage volume (volume normalized to bone surface interface area).
The bone surface interface area is the area of the cartilage in contact with bone. The normalization was done by dividing the measured cartilage volume by the area of measured cartilage in contact with bone plus the area of full thickness defects (denuded area of bone).
-
3
Denuded area (total cartilage bone interface area denuded of cartilage). The denuded area is the area of bone where a full-thickness cartilage defect is present.
-
4
Bone interface area (total bone surface area, regardless of cartilage denudation)
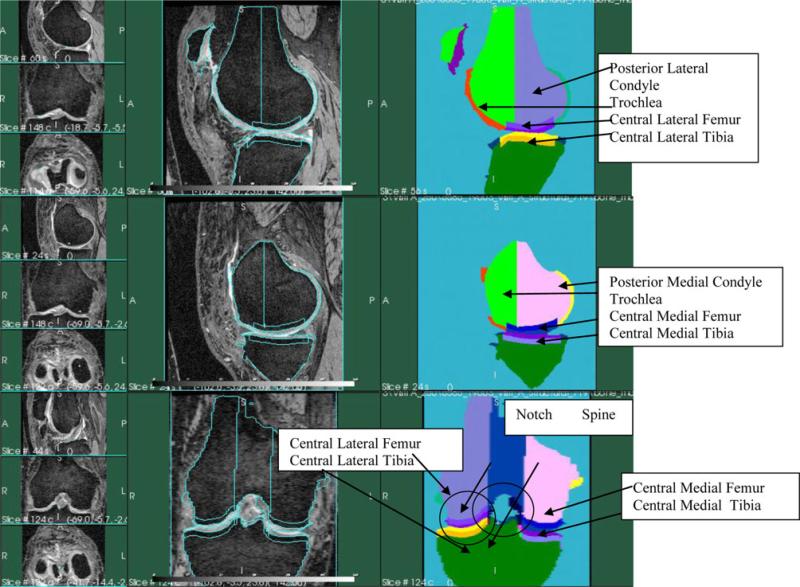
Depiction of the different areas is shown in Fig. 2.
Fig. 2.
MRI slices and schematics of depicting the cartilage areas. Top panel: lateral sagittal slice of the human knee. Middle panel: medial sagittal slice of the human knee. Bottom panel: coronal slice of the human knee
Statistical analysis
Statistical analyses were performed using SAS software (SAS Institute Inc., Cary, NC, release 8.2). Descriptive statistics were performed using a nonparametric Wilcoxon test for continuous variables and Chi-square tests for categorical variables. The sample was divided into those with ACL tears and those without. We examined whether an ACL tear was associated with increased meniscal degeneration, specific patterns of BMLs location, bone morphology, and cartilage morphometry in the index knee compared with the index knee of those without an ACL tear. We adjusted the analysis of cartilage volume, normalized cartilage volume, and bone cartilage interface surface areas for confounders including age, BMI, and gender. Since denuded area was not normally distributed, we used a quantile regression model—a nonparametric analysis method while adjusting for age, BMI, and gender. The analysis of BMLs and meniscal degeneration were not adjusted for any of the above-mentioned parameters or multiple testing due to a cell frequency of less than five in multiple cells and the nonsignificant result in the simple relationship test at most locations.
Results
The demographic characteristics of the overall study sample and those with and without ACL tears are shown in Table 2. Fifty-one percent of the study population were women, the average age of the subjects was 62.1 years with a standard deviation (SD) of 9.9 years, and 59% of the participants were 65 years of age or older. The mean BMI was 30.3 kg/m2 with a SD of 4.7. The left knee was picked as the index knee in 78 participants (49%). Fifteen percent of the participants in this study sample did not meet the criteria of ROA defined by a centralized reading of a KL grade ≥ 2. The eligibility criteria used for the OAI progression subcohort were based on the identification of a definite tibiofemoral osteophyte at each OAI Clinical Center, and some disagreement in radiographic assessment with the adjudicated central reading of KL grades was expected.
A partial or complete ACL tear was present in 23 study participants, with 10 ACLs being graded as partially torn and 13 as completely torn, and 22 of these participants reporting a history of substantive knee injury and 2 participants showed an ACL repair that was graded as an intact ACL. For reasons of statistical power, we decided to combine those with complete and partial tears of the ACL.
Results of the cartilage volume, normalized cartilage volume, denuded area, and bone interface area in participants with and without ACL tears are shown in Table 3. Individuals with ACL tears had significantly greater cartilage volume in the following locations: femur plate (P = 0.008), posterior lateral femur (P = 0.004), posterior medial femur (P = 0.006), central lateral femur (P = 0.012), and central lateral tibia (P = 0.024). After adjusting for age, gender, and BMI, only the central medial tibial (P = 0.04) and the posterior lateral femur (P = 0.001) remained significant.
Table 3.
Cartilage morphology parameters and bone surface area between patients with and without ACL tears
| Location | Cartilage volume at baseline (mm3) |
Denuded area at baseline (mm2) |
Normalized cartilage volume at baseline |
Bone cartilage interface surface Areas (mm2) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| W/o ACL Mean (Std) | W/ACL Mean (Std) | Unad p | Adj p | W/o ACL Median (IQR) | W/ACL Median (IQR) | Unadj p | Adj p | W/o ACL Mean (Std) | W/ACL Mean (Std) | Unadj p | Adj p | W/o ACL Mean (Std) | W/ACL Mean (Std) | Unadj p | Adj p | |
| Femur | 11722.5 (3001.6) | 14043.3 (3927.8) | 0.008 | 0.26 | 58.1 (1.8, 376.4) | 74.5 (36.3, 353.7) | 0.25 | 0.20 | 2.3 (0.3) | 2.5 (0.5) | 0.18 | 0.95 | 4869.2 (830.6) | 5423.8 (840.1) | 0.01 | 0.35 |
| Lateral tibia | 2228.5 (719.0) | 2555.6 (778.0) | 0.06 | 0.53 | 1.1 (0.0, 1.1) | 2.9 (1.1, 30.0) | 0.0004 | 0.69 | 2.3 (0.4) | 2.3 (0.4) | 0.60 | 0.15 | 967.3 (187.8) | 1068.0 (216.2) | 0.04 | 0.99 |
| Medial tibia | 1973.7 (637.4) | 2145.3 (673.1) | 0.11 | 0.15 | 3.4 (0.0, 8.5) | 3.4 (0.0, 131.5) | 0.56 | 1.00 | 1.8 (0.3) | 1.9 (0.5) | 0.21 | 0.54 | 1039.1 (208.7) | 1035.2 (242.2) | 0.51 | 0.006 |
| Patella | 2656.4 (1214.3) | 3180.0 (1127.0) | 0.06 | 0.71 | 1.9 (0.0, 61.1) | 0.06 (0.0, 103.3) | 0.68 | 0.92 | 2.2 (0.8) | 2.5 (0.8) | 0.20 | 0.89 | 1050.0 (304.7) | 1175.5 (263.3) | 0.07 | 0.89 |
| Trochlea | 4510.5 (1440.1) | 4969.1 (1662.0) | 0.30 | 0.28 | 5.4 (0.0, 58.0) | 12.4 (0.1, 43.6) | 0.55 | 0.32 | 2.3 (0.5) | 2.5 (0.7) | 0.35 | 0.73 | 1841.8 (387.2) | 1918.5 (304.4) | 0.47 | 0.18 |
| Cent lat femur | 1703.2 (524.6) | 2066.9 (660.3) | 0.01 | 0.16 | 0.6 (0.0, 0.6) | 0.6 (0.3, 5.0) | 0.03 | 1.00 | 2.2 (0.4) | 2.3 (0.5) | 0.16 | 0.55 | 765.0 (138.2) | 862.5 (142.4) | 0.01 | 0.10 |
| Cent lat tibia | 1838.4 (571.9) | 2240.3 (732.6) | 0.02 | 0.46 | 1.1 (0.0, 1.1) | 1.1 (1.1, 19.8) | 0.003 | 0.93 | 2.3 (0.5) | 2.4 (0.4) | 0.41 | 0.21 | 779.6 (152.6) | 905.7 (210.8) | 0.01 | 0.06 |
| Cent med femur | 1471.6 (690.8) | 1755.3 (808.6) | 0.12 | 0.76 | 7.1 (0.0, 66.5) | 7.1 (0.0, 101.1) | 0.69 | 1.00 | 1.8 (0.7) | 2.0 (0.8) | 0.21 | 0.99 | 730.7 (212.0) | 767.9 (193.3) | 0.29 | 0.21 |
| Cent med tibia | 1313.3 (503.9) | 1370.1 (527.8) | 0.41 | 0.04 | 3.3 (0.0, 8.3) | 3.3 (0.0, 131.3) | 0.51 | 1.00 | 1.7 (0.4) | 1.8 (0.6) | 0.29 | 0.33 | 713.1 (193.3) | 682.0 (223.6) | 0.95 | 0.008 |
| Post lat femur | 1585.6 (441.7) | 2139.9 (899.9) | 0.004 | 0.001 | 0.0 (0.0, 0.0) | 0.0 (0.0, 0.0) | 0.17 | 1.00 | 2.5 (0.4) | 2.7 (0.5) | 0.22 | 0.62 | 622.5 (142.4) | 777.0 (233.8) | 0.01 | 0.004 |
| Post med femur | 1694.8 (464.0) | 2047.5 (607.9) | 0.006 | 0.10 | 0.3 (0.0, 0.3) | 0.3 (0.0, 2.7) | 0.27 | 1.00 | 2.3 (0.4) | 2.4 (0.5) | 0.74 | 0.68 | 716.7 (143.3) | 830.2 (198.4) | 0.01 | 0.04 |
| c + p med femur | 3166.5 (1062.2) | 3802.8 (1235.1) | 0.04 | 0.56 | 7.4 (0.0, 66.5) | 7.4 (0.0, 110.0) | 0.56 | 0.42 | 4.1 (1.0) | 4.4 (1.1) | 0.38 | 0.85 | ||||
| Cent med F + T | 2785.0 (1160.3) | 3125.3 (1314.5) | 0.191 | 0.30 | 10.4 (0.0, 87.9) | 10.4 (0.0, 235.5) | 0.75 | 0.42 | 3.5 (1.1) | 3.8 (1.4) | 0.23 | 0.70 | ||||
Cartilage volume and normalized cartilage volume adjusted for age, BMI, and gender
Bold values are significant at P < 0.05 level
Analysis of the denuded area (total cartilage bone interface area denuded of cartilage) also showed significantly greater denuded area in persons with ACL tears in the lateral tibia plate (0.0004), central lateral tibia (P = 0.003), and central lateral femur (P = 0.03). After adjusting for age, gender, and BMI, none of these regions remained significant.
There were no significant differences in any region, however, when the cartilage volume was normalized to the bone surface area. Therefore, we also examined the bone interface area between participants with and without ACL tears and found a significantly larger bone interface area in participants when compared to those without ACL tears. The regions with significantly greater bone cartilage interface areas were the femur (P = 0.01), lateral tibia (P = 0.04), central lateral femur (P = 0.01), central lateral tibia (P = 0.01), posterior lateral femur (P = 0.01), and posterior medial femur (P = 0.01). After adjusting for age, gender, and BMI, the medial tibia (P = 0.006), central medial tibia (P = 0.008), posterior lateral femur (P = 0.004), and the posterior medial femur (P = 0.04) remained significant.
Analysis of the meniscal pathology showed significantly (P = 0.019) more meniscal derangement in the lateral posterior horn when comparing participants with to those without ACL tears (Table 4).
Table 4.
Meniscal pathology at baseline between participants with and without ACL tears
| ACL tear |
Fisher's exact test P values | ||||
|---|---|---|---|---|---|
| No |
Yes |
||||
| n | Percent | n | Percent | ||
| Medial anterior derangement | |||||
| No | 80 | 61.5 | 10 | 50.0 | 0.33 |
| Yes | 50 | 38.5 | 10 | 50.0 | |
| Medial body derangement | |||||
| No | 42 | 32.3 | 4 | 20.0 | 0.31 |
| Yes | 88 | 67.7 | 16 | 80.0 | |
| Medial posterior derangement | |||||
| No | 39 | 30.0 | 2 | 10.0 | 0.10 |
| Yes | 91 | 70.0 | 18 | 90.0 | |
| Lateral anterior derangement | |||||
| No | 110 | 84.6 | 19 | 95.0 | 0.31 |
| Yes | 20 | 15.4 | 1 | 5.0 | |
| Lateral body derangement | |||||
| No | 103 | 79.2 | 16 | 80.0 | 1.00 |
| Yes | 27 | 20.8 | 4 | 20.0 | |
| Lateral posterior derangement | |||||
| No | 105 | 80.8 | 11 | 55.0 | 0.02 |
| Yes | 25 | 19.2 | 9 | 45.0 | |
Bold values are significant at P < 0.05 level
As shown in Table 5, participants with ACL tears had larger BMLs in the lateral femur (P = 0.0025) compared to those without.
Table 5.
Size and location of BMLs in participants with and without ACL tears
| Location | BML size | ACL tear |
Fisher's exact test P values | |||
|---|---|---|---|---|---|---|
| No |
Yes |
|||||
| n | Percent | n | Percent | |||
| Lateral trochlea | 0 | 80 | 61.5 | 13 | 65 | 0.09 |
| 1 | 16 | 12.3 | 2 | 10 | ||
| 2 | 8 | 6.2 | 4 | 20 | ||
| 3 | 26 | 20 | 1 | 5 | ||
| Lateral femur | 0 | 103 | 79.2 | 9 | 45 | 0.003 |
| 1 | 21 | 16.2 | 7 | 35 | ||
| 2 | 5 | 3.8 | 2 | 10 | ||
| 3 | 1 | 0.8 | 2 | 10 | ||
| Medial trochlea | 0 | 111 | 85.4 | 18 | 90 | 0.92 |
| 1 | 10 | 7.7 | 1 | 5 | ||
| 2 | 4 | 3.1 | 1 | 5 | ||
| 3 | 5 | 3.8 | – | – | ||
| Medial femur | 0 | 61 | 46.9 | 7 | 35 | 0.13 |
| 1 | 44 | 33.8 | 7 | 35 | ||
| 2 | 15 | 11.5 | 6 | 30 | ||
| 3 | 10 | 7.7 | – | – | ||
| Lateral patella | 0 | 74 | 56.9 | 13 | 65 | 0.45 |
| 1 | 22 | 16.9 | 3 | 15 | ||
| 2 | 11 | 8.5 | 3 | 15 | ||
| 3 | 23 | 17.7 | 1 | 5 | ||
| Medial patella | 0 | 100 | 76.9 | 18 | 90 | 0.63 |
| 1 | 15 | 11.5 | 2 | 10 | ||
| 2 | 3 | 2.3 | – | – | ||
| 3 | 12 | 9.2 | – | – | ||
| Lateral tibia | 0 | 87 | 66.9 | 10 | 50 | 0.28 |
| 1 | 31 | 23.8 | 7 | 35 | ||
| 2 | 6 | 4.6 | 2 | 10 | ||
| 3 | 6 | 4.6 | 1 | 5 | ||
| Medial tibia | 0 | 41 | 31.5 | 3 | 15 | 0.14 |
| 1 | 47 | 36.2 | 7 | 35 | ||
| 2 | 27 | 20.8 | 4 | 20 | ||
| 3 | 15 | 11.5 | 6 | 30 | ||
| Subspinous tibia | 0 | 99 | 76.2 | 15 | 75 | 0.69 |
| 1 | 23 | 17.7 | 4 | 20 | ||
| 2 | 5 | 3.8 | – | – | ||
| 3 | 3 | 2.3 | 1 | 5 | ||
Bold values are significant at P < 0.05 level
Discussion
In this study of 160 participants with knee OA, we demonstrated an altered pattern of cartilage loss, increased bone area, meniscal degeneration, and location of BMLs between participants with and without ACL tears. This study demonstrated a clear predisposition to lateral tibiofemoral articular damage in persons with concomitant OA and ACL tear.
With acute tears of the ACL being one of the most common severe sports injuries [32], the fact that patients with ACL injuries may also suffer from associated chondral and subchondral damages, as well as meniscal injuries, has been extensively investigated [33–37].
Several recent studies [27, 38–41] suggested a relation between ACL injuries and cartilage damage predominant in the lateral compartment. In a radiographic case–control study, Swaerd et al. [38] compared the location of structural changes of 176 post-traumatic patients with 155 controls who suffered from knee pain without prior injury. JSN and osteophytes were graded accordingly to the OARSI atlas, and the results showed a lateral shift of structural changes in the traumatic group.
Nishimori et al. [41] reported a high proportion of cartilage injuries in the lateral femur and lateral tibial plateau in addition to the posterior horn of the lateral meniscus in 39 patients with acute ACL tears. Even though this study design did not provide a noninjured control group, the arthroscopically described pattern of cartilage and meniscal injury after acute tears of the ACL is similar to that found in our study.
Tibiofemoral contact pattern between 31 participants with and without ACL tears was investigated in an MRI study by Scarvell et al. [27]. The lateral compartment loading pattern in ACL-injured knees was more posterior on the tibial plateau than in the healthy knees, and the different loading pattern was accompanied by more severe knee symptoms. These findings relate to the structural changes in our study, which may be determined by changed kinematics after unrepaired ACL injury.
Meniscal injuries are common findings in ACL-injured patients. Both lateral and medial meniscal tears in ACL-injured knees are described [42–44]. Two studies reported a relationship between lateral meniscal damage and acute injury, and between medial injuries in chronic ACL tears. Frequency differences in meniscal tears may be explained by various reasons, including different injury mechanisms, alignment, and timing of investigation with regards to the time point of the injury. Several investigators have demonstrated an association between ACL tears and the presence of meniscal tears, especially medially [24, 39, 45]. While any injury to the knee could result in concurrent meniscal damage and ACL tear, some have demonstrated that ACL tears themselves may cause secondary meniscal damage [39, 44, 45], which, in turn, could contribute to cartilage loss, BML formation, and potentially increasing malalignment. The extent of this risk needs to be clarified in order to ascertain whether this should be the target of future therapeutic interventions.
Bone bruises in association with acute ACL tears are very common [46–50] and have been investigated in several MRI studies [51–53], with the common finding of increased bruises in the lateral compartment. Additional histological research [51] showed chondrocyte degeneration, proteoglycan loss, and osteochondral necrosis in the bruise overlying cartilage. These findings correspond with our results on subchondral BMLs, which were significantly higher in the lateral femur for participants with ACL tears.
In addition to the changes in the acutely injured knee, the presence of osteoarthritic findings in the knee joint after ACL injury have been investigated in several long-term studies [14, 18, 54–56]. Jones et al. [57] summarized how acute and chronic ACL injuries associated with chondral and meniscal loss may lead to OA of the knee.
In a recent study by Amin et al. [58], cartilage loss in 49 participants with ACL tears over 15 or 30 months was compared to that in 216 without an ACL tear over the same period. Unadjusted odds ratios showed a significantly higher risk for cartilage loss in the medial compartment for those with ACL tears. However, after adjusting for age, BMI, gender, and meniscal damage, no increase in risk for cartilage loss could be detected. Our study results suggest that in participants with a predominant ACL tear, the pattern of cartilage loss changes toward the pattern found in acute ACL-injured knees. Therefore, ACL tears may act as an influencing factor regarding to the location and pattern of osteoarthritic changes. In our study, we found some differences in cartilage volume (with increase volume in certain regions), but after normalizing for area (analogous to cartilage thickness), these differences were attenuated and no longer reached statistical significance. These findings, in addition to the differences we found in bone surface area, seem to indicate that much of the morphologic adaptation to ACL injury occurs in bone rather than cartilage morphometry.
Our findings of significantly higher meniscal maceration in the lateral posterior horn in participants with ACL tears suggest a higher risk for lateral disease progression in patients with symptomatic knee OA and a prevalent ACL tear.
The location of BMLs may also determine progression pattern of knee OA in patients with ACL tears. In our study, the lateral predominance of BML in participants with ACL tears underlines the lateral propensity in post-traumatic knee OA.
Limitations
The frequency of ACL tears in this study sample was smaller than previously described in the literature with 13 (8.1%) complete ACL tears and 10 (6.25%) partial ACL tears and a tear rate of 14.4% in the whole study population. Three previous studies investigating ACL tears with other findings in knee OA presented rates from 22% [23], over 28% [59] to 35% [60], for full-thickness tears. This decreased prevalence of ACL tears may be related to the selection factors used to define the 160 participants chosen from OAI study to part of this substudy. Also 85% of the participants in our study had KL grades ≥ 2, which may influence this discrepancy. Another concern may be misclassification bias in the defining an ACL tear. The fidelity of MRI in ACL diagnosis has an accuracy between 90 and 100% [61–64] compared to the gold standard knee arthroscopy but is yet to be demonstrated in patients with knee OA. Such misclassification would bias toward the null, so our findings may underestimate the true associations. Regarding the statistical power of our analysis, the sample size of 160 participants with 23 suffering from a complete or a partial tear is a rather small sample size; hence, the results need to be confirmed in a larger cohort. Previous studies [27, 41, 58] investigating joint changes in participants with ACL tears did have a similar sample size to our study. As a result of the cross-sectional design of the study, we are unable to make any cause and effect conclusions based on our findings and more longitudinal research is needed.
Conclusion
In summary, we found a significant lateral tibiofemoral tendency of OA-associated findings including BMLs, increased bone surface area, and meniscal derangement in participants when compared to those without ACL tears. These findings suggest prevalent ACL tears in symptomatic OA patients may be an indicator for a specific pattern and location of disease progression. Further longitudinal research is necessary to corroborate our findings of the pattern of articular damage in persons with OA and ACL tears.
Acknowledgments
We would like to thank the participants and staff of the OAI. We would like to thank the Principal Investigators (Michael Nevitt, Kent Kwoh, Charles B. Eaton, Rebecca Jackson, Marc Hochberg, Joan Bathon), Co-Investigators, and staff of the Osteoarthritis Initiative. The OAI is a public–private partnership comprised of five contracts (N01-AR-2-2258; N01-AR-2-2259; N01-AR-2-2260; N01-AR-2-2261; N01-AR-2-2262) funded by the National Institutes of Health, a branch of the Department of Health and Human Services, and conducted by the OAI Study Investigators. Private funding partners include Merck Research Laboratories; Novartis Pharmaceuticals Corporation, GlaxoSmithKline; and Pfizer, Inc. Private sector funding for the OAI is managed by the Foundation for the National Institutes of Health. This manuscript has received the approval of the OAI Publications Committee based on a review of its scientific content and data interpretation. We would also like to acknowledge the following persons who contributed to this work: Piran Aliabadi (read the knee—X-ray films) and David Felson (chaired the X-ray adjudication sessions).
The Osteoarthritis Initiative and this pilot study are conducted and supported by the National Institute of Arthritis and Musculoskeletal and Skin Diseases (N01-AR-2-2262, N01-AR-2-2262, and N01-AR-2-2258) in collaboration with the OAI Investigators and Consultants. This manuscript has been reviewed by the OAI Publication committee for scientific content and data interpretation.
Footnotes
Conflict of interest Nothing to declare. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Contributor Information
Verena Stein, Division of Research, New England Baptist Hospital, Boston, MA, USA.
Ling Li, Division of Research, New England Baptist Hospital, Boston, MA, USA.
Grace Lo, Division of Rheumatology, Tufts University School of Medicine, Boston, MA, USA.
Ali Guermazi, Boston University School of Medicine at Boston Medical Center, Boston, MA, USA.
Yuqing Zhang, Boston University School of Medicine at Boston Medical Center, Boston, MA, USA.
C. Kent Kwoh, University of Pittsburgh and Pittsburgh VA Healthcare System, Pittsburgh, PA, USA.
Charles B. Eaton, Warren Alpert Medical School of Brown University, Providence, RI, USA
David J. Hunter, Division of Research, New England Baptist Hospital, Boston, MA, USA Northern Clinical School, University of Sydney, Sydney, NSW, Australia.
References
- 1.Prevalence of disabilities and associated health conditions among adults–United States (1999) MMWR Morb Mortal Wkly Rep. 2001;50(7):120–125. [PubMed] [Google Scholar]
- 2.Felson DT, McAlindon TE, Anderson JJ, Naimark A, Weissman BW, Aliabadi P, et al. Defining radiographic osteoarthritis for the whole knee. Osteoarthr Cartil. 1997;5(4):241–250. doi: 10.1016/s1063-4584(97)80020-9. [DOI] [PubMed] [Google Scholar]
- 3.Mackenzie R, Palmer CR, Lomas DJ, Dixon AK. Magnetic resonance imaging of the knee: diagnostic performance studies. Clin Radiol. 1996;51(4):251–257. doi: 10.1016/s0009-9260(96)80341-2. [DOI] [PubMed] [Google Scholar]
- 4.Cotten A, Delfaut E, Demondion X, Lapegue F, Boukhelifa M, Boutry N, et al. MR imaging of the knee at 0.2 and 1.5 T: correlation with surgery. AJR Am J Roentgenol. 2000;174(4):1093–1097. doi: 10.2214/ajr.174.4.1741093. [DOI] [PubMed] [Google Scholar]
- 5.Vincken PW, ter Braak BP, van Erkell AR, de Rooy TP, Mallens WM, Post W, et al. Effectiveness of MR imaging in selection of patients for arthroscopy of the knee. Radiology. 2002;223(3):739–746. doi: 10.1148/radiol.2233010849. [DOI] [PubMed] [Google Scholar]
- 6.Eckstein F, Glaser C. Measuring cartilage morphology with quantitative magnetic resonance imaging. Semin Musculoskelet Radiol. 2004;8(4):329–353. doi: 10.1055/s-2004-861579. [DOI] [PubMed] [Google Scholar]
- 7.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-organ magnetic resonance imaging score (WORMS) of the knee in osteoarthritis. Osteoarthr Cartil. 2004;12(3):177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 8.Felson DT, Anderson JJ, Naimark A, Kannel W, Meenan RF. The prevalence of chondrocalcinosis in the elderly and its association with knee osteoarthritis: the Framingham study. J Rheumatol. 1989;16(9):1241–1245. [PubMed] [Google Scholar]
- 9.Davis MA, Ettinger WH, Neuhaus JM, Cho SA, Hauck WW. The association of knee injury and obesity with unilateral and bilateral osteoarthritis of the knee. Am J Epidemiol. 1989;130(2):278–288. doi: 10.1093/oxfordjournals.aje.a115334. [DOI] [PubMed] [Google Scholar]
- 10.Wilder FV, Hall BJ, Barrett JP, Jr, Lemrow NB. History of acute knee injury and osteoarthritis of the knee: a prospective epidemiological assessment. The clearwater osteoarthritis study. Osteoarthr Cartil. 2002;10(8):611–616. doi: 10.1053/joca.2002.0795. [DOI] [PubMed] [Google Scholar]
- 11.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 12.Arendt EA, Agel J, Dick R. Anterior cruciate ligament injury patterns among collegiate men and women. J Athl Train. 1999;34(2):86–92. [PMC free article] [PubMed] [Google Scholar]
- 13.Boden BP, Dean GS, Feagin JA, Jr, Garrett WE., Jr Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- 14.Roos H, Adalberth T, Dahlberg L, Lohmander LS. Osteoarthritis of the knee after injury to the anterior cruciate ligament or meniscus: the influence of time and age. Osteoarthr Cartil. 1995;3(4):261–267. doi: 10.1016/s1063-4584(05)80017-2. [DOI] [PubMed] [Google Scholar]
- 15.Myklebust R, Mayhew TM. Further evidence of species variation in mechanisms of epithelial cell loss in mammalian small intestine: ultrastructural studies on the reindeer (Rangifer tarandus) and seal (Phoca groenlandica). Cell Tissue Res. 1998;291(3):513–523. doi: 10.1007/s004410051021. [DOI] [PubMed] [Google Scholar]
- 16.Daniel DM, Stone ML, Dobson BE, Fithian DC, Rossman DJ, Kaufman KR. Fate of the ACL-injured patient. A prospective outcome study. Am J Sports Med. 1994;22(5):632–644. doi: 10.1177/036354659402200511. [DOI] [PubMed] [Google Scholar]
- 17.Kannus P, Jarvinen M. Posttraumatic anterior cruciate ligament insufficiency as a cause of osteoarthritis in a knee joint. Clin Rheumatol. 1989;8(2):251–260. doi: 10.1007/BF02030082. [DOI] [PubMed] [Google Scholar]
- 18.Lohmander LS, Ostenberg A, Englund M, Roos H. High prevalence of knee osteoarthritis, pain, and functional limitations in female soccer players twelve years after anterior cruciate ligament injury. Arthritis Rheum. 2004;50(10):3145–3152. doi: 10.1002/art.20589. [DOI] [PubMed] [Google Scholar]
- 19.Maletius W, Messner K. Eighteen- to twenty-four-year follow-up after complete rupture of the anterior cruciate ligament. Am J Sports Med. 1999;27(6):711–717. doi: 10.1177/03635465990270060501. [DOI] [PubMed] [Google Scholar]
- 20.von Porat A, Roos EM, Roos H. High prevalence of osteoarthritis 14 years after an anterior cruciate ligament tear in male soccer players: a study of radiographic and patient relevant outcomes. Ann Rheum Dis. 2004;63(3):269–273. doi: 10.1136/ard.2003.008136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McDaniel WJ, Jr, Dameron TB., Jr The untreated anterior cruciate ligament rupture. Clin Orthop Relat Res. 1983;172:158–163. [PubMed] [Google Scholar]
- 22.Clatworthy M, Amendola A. The anterior cruciate ligament and arthritis. Clin Sports Med. 1999;18(1):173–198. vii. doi: 10.1016/s0278-5919(05)70134-4. [DOI] [PubMed] [Google Scholar]
- 23.Hill CL, Seo GS, Gale D, Totterman S, Gale ME, Felson DT. Cruciate ligament integrity in osteoarthritis of the knee. Arthritis Rheum. 2005;52(3):794–799. doi: 10.1002/art.20943. [DOI] [PubMed] [Google Scholar]
- 24.Maffulli N, Binfield PM, King JB. Articular cartilage lesions in the symptomatic anterior cruciate ligament-deficient knee. Arthroscopy. 2003;19(7):685–690. doi: 10.1016/s0749-8063(03)00403-1. [DOI] [PubMed] [Google Scholar]
- 25.Costa-Paz M, Muscolo DL, Ayerza M, Makino A, Aponte-Tinao L. Magnetic resonance imaging follow-up study of bone bruises associated with anterior cruciate ligament ruptures. Arthroscopy. 2001;17(5):445–449. doi: 10.1053/jars.2001.23581. [DOI] [PubMed] [Google Scholar]
- 26.Scarvell JM, Smith PN, Refshauge KM, Galloway HR, Woods KR. Association between abnormal kinematics and degenerative change in knees of people with chronic anterior cruciate ligament deficiency: a magnetic resonance imaging study. Aust J Physiother. 2005;51(4):233–240. doi: 10.1016/s0004-9514(05)70004-0. [DOI] [PubMed] [Google Scholar]
- 27.Scarvell JM, Smith PN, Refshauge KM, Galloway HR, Woods KR. Comparison of kinematic analysis by mapping tibiofemoral contact with movement of the femoral condylar centres in healthy and anterior cruciate ligament injured knees. J Orthop Res. 2004;22(5):955–962. doi: 10.1016/j.orthres.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 28.Hunter DJ, Niu J, Zhang Y, Totterman S, Tamez J, Dabrowski C, et al. Change in cartilage morphometry: a sample of the progression cohort of the osteoarthritis initiative. Ann Rheum Dis. 2009;68(3):349–356. doi: 10.1136/ard.2007.082107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peterfy C, Li J, Zaim S, Duryea J, Lynch J, Miaux Y, et al. Comparison of fixed-flexion positioning with fluoroscopic semi-flexed positioning for quantifying radiographic joint-space width in the knee: test-retest reproducibility. Skeletal Radiol. 2003;32(3):128–132. doi: 10.1007/s00256-002-0603-z. [DOI] [PubMed] [Google Scholar]
- 30.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16(4):494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hunter DJ, Lo GH, Gale D, Grainger AJ, Guermazi A, Conaghan PG. The reliability of a new scoring system for knee osteoarthritis MRI and the validity of bone marrow lesion assessment: BLOKS (Boston leeds osteoarthritis knee score). Ann Rheum Dis. 2008;67(2):206–211. doi: 10.1136/ard.2006.066183. [DOI] [PubMed] [Google Scholar]
- 32.Frobell RB, Lohmander LS, Roos HP. Acute rotational trauma to the knee: poor agreement between clinical assessment and magnetic resonance imaging findings. Scand J Med Sci Sports. 2007;17(2):109–114. doi: 10.1111/j.1600-0838.2006.00559.x. [DOI] [PubMed] [Google Scholar]
- 33.Noyes FR, Bassett RW, Grood ES, Butler DL. Arthroscopy in acute traumatic hemarthrosis of the knee. Incidence of anterior cruciate tears and other injuries. J Bone Joint Surg Am. 1980;62(5):687–695. 757. [PubMed] [Google Scholar]
- 34.Indelicato PA, Bittar ES. A perspective of lesions associated with ACL insufficiency of the knee. A review of 100 cases. Clin Orthop Relat Res. 1985;198:77–80. [PubMed] [Google Scholar]
- 35.Escalas F, Curell R. Occult posttraumatic bone injury. Knee Surg Sports Traumatol Arthrosc. 1994;2(3):147–149. doi: 10.1007/BF01467916. [DOI] [PubMed] [Google Scholar]
- 36.Even-Sapir E, Arbel R, Lerman H, Flusser G, Livshitz G, Halperin N. Bone injury associated with anterior cruciate ligament and meniscal tears: assessment with bone single photon emission computed tomography. Invest Radiol. 2002;37(9):521–527. doi: 10.1097/00004424-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 37.Lahm A, Erggelet C, Steinwachs M, Reichelt A. Articular and osseous lesions in recent ligament tears: arthroscopic changes compared with magnetic resonance imaging findings. Arthroscopy. 1998;14(6):597–604. doi: 10.1016/s0749-8063(98)70056-8. [DOI] [PubMed] [Google Scholar]
- 38.Swaerd P, Kostogiannis I, Neuman P, Boegard T, Roos H. Differences in radiological characteristics between post-traumatic and non-traumatic knee osteoarthritis. Scand J Med Sci Sports. 2010;20(5):731–739. doi: 10.1111/j.1600-0838.2009.01000.x. [DOI] [PubMed] [Google Scholar]
- 39.Tandogan RN, Taser O, Kayaalp A, Taskiran E, Pinar H, Alparslan B, et al. Analysis of meniscal and chondral lesions accompanying anterior cruciate ligament tears: relationship with age, time from injury, and level of sport. Knee Surg Sports Traumatol Arthrosc. 2004;12(4):262–270. doi: 10.1007/s00167-003-0398-z. [DOI] [PubMed] [Google Scholar]
- 40.Scarvell JM, Smith PN, Refshauge KM, Galloway H, Woods K. Comparison of kinematics in the healthy and ACL injured knee using MRI. J Biomech. 2005;38(2):255–262. doi: 10.1016/j.jbiomech.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Nishimori M, Deie M, Adachi N, Kanaya A, Nakamae A, Motoyama M, et al. Articular cartilage injury of the posterior lateral tibial plateau associated with acute anterior cruciate ligament injury. Knee Surg Sports Traumatol Arthrosc. 2008;16(3):270–274. doi: 10.1007/s00167-007-0458-x. [DOI] [PubMed] [Google Scholar]
- 42.Cipolla M, Scala A, Gianni E, Puddu G. Different patterns of meniscal tears in acute anterior cruciate ligament (ACL) ruptures and in chronic ACL-deficient knees. Classification, staging and timing of treatment. Knee Surg Sports Traumatol Arthrosc. 1995;3(3):130–134. doi: 10.1007/BF01565470. [DOI] [PubMed] [Google Scholar]
- 43.Nikolic DK. Lateral meniscal tears and their evolution in acute injuries of the anterior cruciate ligament of the knee. Arthroscopic analysis. Knee Surg Sports Traumatol Arthrosc. 1998;6(1):26–30. doi: 10.1007/s001670050068. [DOI] [PubMed] [Google Scholar]
- 44.Smith JP, III, Barrett GR. Medial and lateral meniscal tear patterns in anterior cruciate ligament-deficient knees. A prospective analysis of 575 tears. Am J Sports Med. 2001;29(4):415–419. doi: 10.1177/03635465010290040501. [DOI] [PubMed] [Google Scholar]
- 45.Smith GN, Mickler EA, Albrecht ME, Myers SL, Brandt KD. Severity of medial meniscus damage in the canine knee after anterior cruciate ligament transection. Osteoarthr Cartil. 2002;10(4):321–326. doi: 10.1053/joca.2002.0520. [DOI] [PubMed] [Google Scholar]
- 46.Fowler PJ. Bone injuries associated with anterior cruciate ligament disruption. Arthroscopy. 1994;10(4):453–460. doi: 10.1016/s0749-8063(05)80198-7. [DOI] [PubMed] [Google Scholar]
- 47.Rosen MA, Jackson DW, Berger PE. Occult osseous lesions documented by magnetic resonance imaging associated with anterior cruciate ligament ruptures. Arthroscopy. 1991;7(1):45–51. doi: 10.1016/0749-8063(91)90077-b. [DOI] [PubMed] [Google Scholar]
- 48.Speer KP, Spritzer CE, Bassett FH, III, Feagin JA, Jr, Garrett WE., Jr Osseous injury associated with acute tears of the anterior cruciate ligament. Am J Sports Med. 1992;20(4):382–389. doi: 10.1177/036354659202000403. [DOI] [PubMed] [Google Scholar]
- 49.Spindler KP, Schils JP, Bergfeld JA, Andrish JT, Weiker GG, Anderson TE, et al. Prospective study of osseous, articular, and meniscal lesions in recent anterior cruciate ligament tears by magnetic resonance imaging and arthroscopy. Am J Sports Med. 1993;21(4):551–557. doi: 10.1177/036354659302100412. [DOI] [PubMed] [Google Scholar]
- 50.Stein LN, Fischer DA, Fritts HM, Quick DC. Occult osseous lesions associated with anterior cruciate ligament tears. Clin Orthop Relat Res. 1995;313:187–193. [PubMed] [Google Scholar]
- 51.Johnson DL, Urban WP, Jr, Caborn DN, Vanarthos WJ, Carlson CS. Articular cartilage changes seen with magnetic resonance imaging-detected bone bruises associated with acute anterior cruciate ligament rupture. Am J Sports Med. 1998;26(3):409–414. doi: 10.1177/03635465980260031101. [DOI] [PubMed] [Google Scholar]
- 52.Murphy BJ, Smith RL, Uribe JW, Janecki CJ, Hechtman KS, Mangasarian RA. Bone signal abnormalities in the posterolateral tibia and lateral femoral condyle in complete tears of the anterior cruciate ligament: a specific sign? Radiology. 1992;182(1):221–224. doi: 10.1148/radiology.182.1.1727286. [DOI] [PubMed] [Google Scholar]
- 53.Viskontas DG, Giuffre BM, Duggal N, Graham D, Parker D, Coolican M. Bone bruises associated with ACL rupture: correlation with injury mechanism. Am J Sports Med. 2008;36(5):927–933. doi: 10.1177/0363546508314791. [DOI] [PubMed] [Google Scholar]
- 54.Segawa H, Omori G, Koga Y. Long-term results of non-operative treatment of anterior cruciate ligament injury. Knee. 2001;8(1):5–11. doi: 10.1016/s0968-0160(00)00062-4. [DOI] [PubMed] [Google Scholar]
- 55.Gillquist J, Messner K. Anterior cruciate ligament reconstruction and the long-term incidence of gonarthrosis. Sports Med. 1999;27(3):143–156. doi: 10.2165/00007256-199927030-00001. [DOI] [PubMed] [Google Scholar]
- 56.Myklebust G, Holm I, Maehlum S, Engebretsen L, Bahr R. Clinical, functional, and radiologic outcome in team handball players 6 to 11 years after anterior cruciate ligament injury: a follow-up study. Am J Sports Med. 2003;31(6):981–989. doi: 10.1177/03635465030310063901. [DOI] [PubMed] [Google Scholar]
- 57.Jones HP, Appleyard RC, Mahajan S, Murrell GA. Meniscal and chondral loss in the anterior cruciate ligament injured knee. Sports Med. 2003;33(14):1075–1089. doi: 10.2165/00007256-200333140-00004. [DOI] [PubMed] [Google Scholar]
- 58.Amin S, Guermazi A, Lavalley MP, Niu J, Clancy M, Hunter DJ, et al. Complete anterior cruciate ligament tear and the risk for cartilage loss and progression of symptoms in men and women with knee osteoarthritis. Osteoarthr Cartil. 2008;16(8):897–902. doi: 10.1016/j.joca.2007.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Link TM, Steinbach LS, Ghosh S, Ries M, Lu Y, Lane N, et al. Osteoarthritis: MR imaging findings in different stages of disease and correlation with clinical findings. Radiology. 2003;226(2):373–381. doi: 10.1148/radiol.2262012190. [DOI] [PubMed] [Google Scholar]
- 60.Chan WP, Lang P, Stevens MP, Sack K, Majumdar S, Stoller DW, et al. Osteoarthritis of the knee: comparison of radiography, CT, and MR imaging to assess extent and severity. AJR Am J Roentgenol. 1991;157(4):799–806. doi: 10.2214/ajr.157.4.1892040. [DOI] [PubMed] [Google Scholar]
- 61.Lee JK, Yao L, Phelps CT, Wirth CR, Czajka J, Lozman J. Anterior cruciate ligament tears: MR imaging compared with arthroscopy and clinical tests. Radiology. 1988;166(3):861–864. doi: 10.1148/radiology.166.3.3340785. [DOI] [PubMed] [Google Scholar]
- 62.Rose NE, Gold SM. A comparison of accuracy between clinical examination and magnetic resonance imaging in the diagnosis of meniscal and anterior cruciate ligament tears. Arthroscopy. 1996;12(4):398–405. doi: 10.1016/s0749-8063(96)90032-8. [DOI] [PubMed] [Google Scholar]
- 63.Glashow JL, Katz R, Schneider M, Scott WN. Double-blind assessment of the value of magnetic resonance imaging in the diagnosis of anterior cruciate and meniscal lesions. J Bone Joint Surg Am. 1989;71(1):113–119. [PubMed] [Google Scholar]
- 64.Polly DW, Jr, Callaghan JJ, Sikes RA, McCabe JM, McMahon K, Savory CG. The accuracy of selective magnetic resonance imaging compared with the findings of arthroscopy of the knee. J Bone Joint Surg Am. 1988;70(2):192–198. [PubMed] [Google Scholar]