Abstract
Background
Exercise stimulates coordinated release of the sympathoadrenal hormones, ACTH, cortisol, norepinephrine (NE) and epinephrine (Epi).
Hypothesis
Chronic obstructive pulmonary disease (COPD) is marked by heightened sympathoadrenal outflow at comparable relative workloads.
Location
Clinical research unit.
Subjects
Eight healthy men and 9 men with stable COPD (forced expiratory volume at one second < 75% predicted).
Interventions
Volunteers rested (baseline) or exercised at individual submaximal (35 ± 5%) or maximal oxygen consumption (VO2 max). Blood was sampled every 2 min for 40 min concurrently.
Methods
Two-way ANCOVA was applied to examine group (normal/COPD) and exercise (3 levels) effects on ACTH, cortisol, NE and Epi release and regularity (estimable by approximate entropy, ApEn).
Results
The timing of peak hormone concentrations was Epi (14 min), NE (16 min), ACTH (22 min) and cortisol (34 min) in both cohorts. Type of exercise regimen influenced all 4 hormones (each P < 0.001), and subject group [control vs COPD] affected cortisol (P < 0.001) and Epi (P = 0.048) responses. Exercise regimen and group together controlled ACTH, cortisol and Epi (each P < 0.001), but not NE, responses. In particular, endocrine responses were attenuated in COPD compared with control subjects. ApEn analysis also identified loss of maximal exercise-induced ACTH-secretory regularity in COPD patients (P = 0.042).
Conclusion
These outcomes demonstrate impaired rather than augmented exercise-associated sympathocorticotropic-axis outflow in patients with COPD even when outcomes are normalized to VO2 max, suggesting that factors other than fitness are at work.
Keywords: dyspnea, ACTH, cortisol, epinephrine, norepinephrine
Introduction
Exercise stimulates sympathoadrenal outflow, viz., secretion of stress-adaptive noradrenergic (NE), adrenal medullary (Epi), corticotropic (ACTH) and adrenal (cortisol) signals [1, 2]. The majority of exercise-related endocrine studies have been performed in normal volunteers rather than in patients with organ-system pathophysiology. In fact, the impact of catabolic illnesses like chronic obstructive pulmonary disease (COPD) on corticotropin secretion is not well studied. Higher basal sympathetic neural activity, reduced glycolytic muscle fibers, (severe) hypoxia-induced ACTH but not cortisol elevation, cachexia, and relative tissue resistance to insulin and growth hormone have been suggested in COPD [3–5]. A major clinical implication of these findings is that stress-evoked sympathoadrenal outflow, if excessive, could exacerbate morbidity due to accentuated vasoreactivity (NE), glucose intolerance (Epi and cortisol), muscle wasting (cortisol) and hypertension (NE, Epi, cortisol).
Chronic catabolic processes, such as COPD, are often associated with elevated systemic concentrations of certain inflammatory cytokines such as IL-1, IL-6 and TNF-α [6]. Cytokines constitute major stimuli of corticotrope-adrenal secretion [7]. Low physical fitness, a characteristic feature of COPD, likewise predicts greater responsiveness of beta-endorphin, ACTH, cortisol and Epi to an exercise stimulus [8]. Greater hypoxia and/or dyspnea (perceived respiratory distress) could be expected to potentiate ACTH-cortisol secretion at equal power output [9, 10]. Conversely, reduced renin-angiotensin activity, also reported in COPD [11], might attenuate stress-induced ACTH and cortisol secretion [12]. These predictions follow from studies in healthy volunteers with normal baseline pulmonary function. To our knowledge, no comparable data exist in patients with stable COPD not receiving glucocorticoids. We hypothesized that COPD is marked by excessive exercise-associated ACTH, cortisol, NE and Epi outflow during maximal voluntary exertion.
METHODS
Subjects
The study was reviewed and approved by the Human Investigation Committee of the Veterans Affairs Medical Center, Salem, Virginia. Written informed consent was given by all subjects. The study sample comprised 8 healthy men (FEV2 > 75%) and 9 ambulatory male patients with documented stable (2–8 yr) COPD (FEV1 < 75%), unchanged weight over 3 mo., the absence of cor pulmonale, and no exposure to glucocorticoids within 3 mo. Volunteers with angina pectoris, congestive heart failure, arrhythmia, blood pressure greater than 200/120 or lower than 90/60 mm Hg, any acute illness, acute or chronic pain, narcotic or progestin exposure, impaired mentation, balance disturbance or orthopedic compromise were excluded. Anemia, recent transfusion, anabolic steroid use, pituitary disease, FEV1 < 15%, history of syncope or hypotension were exclusion criteria also. Other subject characteristics are summarized in Table 1.
TABLE 1.
Baseline Subject Characteristics
| Variable | Normal (N = 8) | COPD (N = 9) | P Value |
|---|---|---|---|
| Age (years) | 53 ± 2.5 | 65 ± 4.3 | NS |
| BMI (kg/m2) | 28 ± 0.63 | 24 ± 1.1 | < 0.010 |
| Peak Flow (L/min) | 4.7 ± 0.14 | 1.3 ± 0.20 | < 0.001 |
| FEV1 (% predicted) | 93 ± 4.4 | 43 ± 6.4 | < 0.001 |
| NE baseline (nmol/L) | 2.8 ± 0.22 | 3.2 ± 0.36 | NS |
| Epi baseline (nmol/L) | 0.33 ± 0.052 | 0.44 ± 0.098 | NS |
| ACTH baseline (pmol/L) | 6.6 ± 1.4 | 6.2 ± 2.9 | NS |
| Cortisol baseline (nmol/L) | 364 ± 48 | 280 ± 53 | NS |
|
|
|||
Data are the mean ± SEM. NS denotes P ≥ 0.01.
FEV1, forced expiratory volume in 1 sec; baseline, mean of 40 resting samples in each subject.
Protocol
Subjects were studied on 4 separate days always at least 72 hr apart. COPD and controls exercised and rested in the same order of sessions. The first day was always a practice session without blood sampling to allow estimation of individual maximal exercise-associated heart rate (HR), oxygen consumption (VO2) and dyspnea score. The second entailed maximal voluntary exercise with 2-min sampling for 40 min. On the third day, participants remained seated and resting while sampled. The fourth day comprised submaximal exercise defined by 35 ± 5% of VO2 max with venous sampling every 2 min for 40 min. Thus, the second and fourth days of study were at least 144 hr apart.
Volunteers performed bicycle ergometry (SensorMedics® Exercise Test Analyzer) incremented in a continuous ramp fashion by 10–25 watts per min. Participants initially rested on the bicycle for 5 min. At time zero, subjects began to pedal with no load for 4 min, and then against increasing load for up to 15 min or until symptoms of limiting breathlessness or fatigue intervened. Dyspnea was quantified using the modified Borg score [13], in which exercising subjects pointed every 2 min to a card marked with numbers ranging from 0 for no dyspnea to 10 for extreme dyspnea. HR, minute ventilation (VE) and VO2 were monitored every 20 sec, and BP and arterial oxyhemoglobin saturation (Sa02, by pulse oximetry) every 2 min.
No subject experienced any untoward event during the exercise phases of the protocol. Two normal men had transient hypotension and dizziness without syncope in the recovery phase of the protocol.
Borg dyspnea-score (perceived exertion) and catecholamine thresholds
Baseline dyspnea scores were obtained during unloaded pedaling. The time-dyspnea threshold was defined as the time at which the Borg estimate departed exponentially from mean baseline. The same concept was applied for NE and Epi thresholds. Borg scores were also regressed against VO2, expressed as percentage of observed individual maximal VO2. The VO2-dyspnea threshold was defined as VO2 when the Borg estimate deviated from baseline.
Sampling
After the subject arrived in the laboratory on the exercise test day, an i.v. catheter was inserted into each forearm. Blood samples (3 mL) were withdrawn beginning with the onset of unloaded pedaling (time zero) every 2 min as plasma in chilled plastic tubes with EDTA, yielding a total of 20 samples per session.
Hormone Assays
Plasma NE and Epi concentrations were measured by radioimmunoassay (RIA) using the KatCombi RIA kit (ALPCO, Windham, NH), which includes an affinity-extraction step performed on a macrotiter plate. Intra- and interassay CV’s were 8.5% and 11.2%, respectively. Plasma ACTH and cortisol concentrations were measured in duplicate in each 2-min sample using a robotics-automated two-site immunoradiometric assay and solid-phase RIA, respectively [14, 15]. Median within-assay coefficients of variation were 8% and 5%, and sensitivities 1.1 pmol/L and 38.8 nmol/L, respectively. No sample had undetectable measurements of any hormone.
Analyses by deconvolution and approximate entropy (ApEn)
NE, Epi, ACTH and cortisol time series were subjected to deconvolution and approximate entropy, ApEn, analyses. The deconvolution method was described in [16], except that only a single secretory-burst waveform was allowed. The framework is a maximum-likelihood model [17] with the additional threshold requirement that valid hormone-concentration peaks reduce the Akaike information criterion by P<0.05. Outcomes for ACTH were ACTH secretory-burst mass (pmol/L) and number (per 40 min), pulsatile and nonpulsatile (basal) ACTH secretion (pmol/L/40 min), ACTH half-life (min) and secretory-burst shape (mode or time delay to maximal secretion in min). Catecholamine units were nmol/L, and cortisol units nmol/L. Sensitivity and specificity of pulse detection both exceed 92.5% with this methodology [18].
The ApEn statistic was used to discriminate changes in secretory regularity, as a barometer of altered feedback state during exercise [19]. ApEn quantifies the relative orderliness or subpattern consistency in a time series, with higher ApEn corresponding to less regularity [greater randomness]. Cross-ApEn by analogy quantifies the relative synchrony (joint regularity) of subpatterns in paired time series. Higher cross-ApEn denotes less synchrony [greater asynchrony]. The terms feedforward and feedback cross-ApEn refer to ACTH-cortisol and cortisol-ACTH linkages, respectively, as defined earlier [20], and analogously for NE-Epi and Epi-NE pairs.
Statistical Analysis
Because each subject had a baseline resting session of identical 2-min sampling, ANCOVA was used to adjust for intraindividual correlations among baseline, low- and maximal-exercise responses. The covariate was the subject’s parameter value on the baseline day. The model structure comprised hierarchical mixed-effects two-way ANCOVA with 3 exercise levels (resting, low and maximal exercise) and 2 specification parameters (diagnostic subject groups: healthy control and COPD patients) [21]. Logarithmic transformation was utilized to limit heterogeneity of variance. The equal-slopes assumption of the ANCOVA structure was verified by a generalized F-ratio test, followed by restricted maximum-likelihood estimation of parameters. Rejection of pre-specified hypotheses was based on a multiple-comparison experiment-wise Type I error rate of < 0.05 using Tukey’s honestly significantly different (HSD) post hoc test.
Data Presentation
Values are given as the mean ± standard error (SEM). Differences in baseline characteristics (e.g. age, BMI) values between normal subjects and COPD patients were evaluated using an unpaired two-tailed Student’s t-test at a protected critical value of P ≤ 0.01, given that eight comparisons were required [22].
RESULTS
Characteristics of subjects
COPD patients were nonsignificantly older and had significantly lower BMI than control subjects (Table 1). FEV1 ranged from 79 to 114 % predicted in normal men and from 17 to 73% in COPD patients (P < 0.001). Mean baseline (resting) plasma ACTH, cortisol, NE and Epi concentrations did not differ by group.
Threshold values
The times (min) at which Borg-dyspnea (perceived exertion) scores departed from baseline (thresholds) overlapped in normal subjects (6.6 ± 1.9) and COPD patients (5.0 ± 1.0). One-half maximal dyspnea occurred after 9.8 ± 1.1 (normal) and 8.1 ± 1.6 (COPD) min [P < 0.05]. The respective threshold times for NE and Epi rises were also comparable in normal subjects (11 ± 1.9 and 12 ± 1.1 min) and COPD patients (9 ± 2.4 and 12 ± 1.4 min). NE and Epi thresholds occurred significantly later than Borg dyspnea-score thresholds in both groups (P < 0.01).
VO2 thresholds (percentage of VO2 max) for Borg dyspnea score, NE and Epi elevations were 23 ± 5, 56 ± 10 and 60 ± 10 in normal men, and 34 ± 6, 60 ± 9 and 60 ± 9 in COPD patients. Only the Borg-dyspnea VO2 thresholds differed in the two study groups by being higher in COPD (P < 0.001).
Maximal-exercise performance
Normal subjects achieved significantly higher maximal values of VE, HR and VO2 than COPD patients (Table 2), thus verifying fitness differences. Percentage maximal VO2, expressed as a percentage of the predicted VO2 maximum, did not differ, viz. 85 ± 13% in normal and 70 ± 19% in COPD (P = NS). To illustrate functional differences, at an absolute VO2 of 9.8 ± 3.3 mL/kg/min, the Borg score was 4.0 ± 2.9 in COPD vs 1.3 ± 1.1 in normal subjects (P < 0.01).
TABLE 2.
Performance at Maximal Exercise
| Variable | Normal (N = 8) | COPD (N = 9) | P value |
|---|---|---|---|
| VE at end exercise (mL/kg/min) | 72 ± 14 | 39 ± 13 | < 0.001 |
| VE (% predicted maximum) | 49 ± 11 | 77 ± 20 | < 0.001 |
| HRmax (beats/min) | 161 ± 14 | 133 ± 13 | < 0.001 |
| HR (% predicted maximum) | 96 ± 6.0 | 86 ± 7.0 | < 0.010 |
| VO2 max (mL/kg/min) | 27 ± 4.3 | 17 ± 4.0 | < 0.001 |
| VO2 (% predicted maximum) | 85 ± 13 | 70 ± 19 | NS |
|
|
|||
Data are the mean ± SEM. NS denotes P ≥ 0.01.
VE, minute ventilation; HR, heart rate, VO2 max, maximal oxygen consumption.
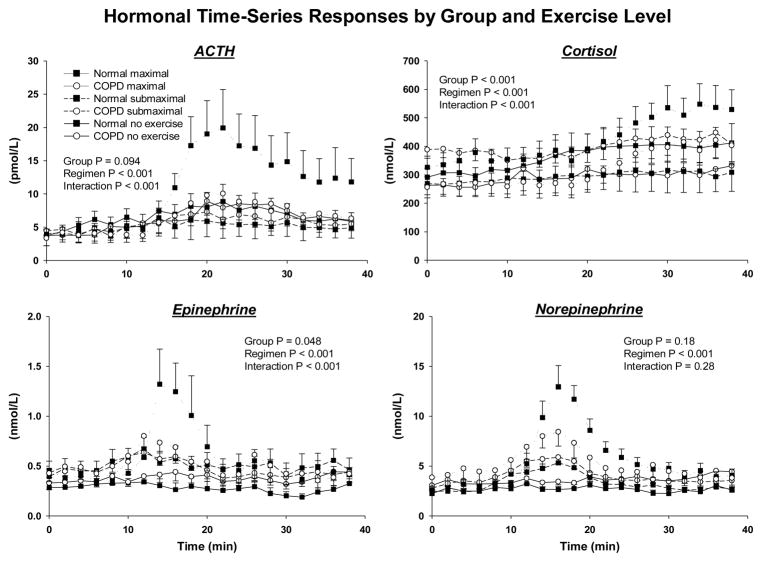
Figure 1 depicts (mean ± SEM) hormone concentration profiles over time in the two groups (normal, COPD) for each of 3 exercise levels (none, submaximal, maximal) and for each of four hormones (ACTH, cortisol, Epi, NE). Normal subjects, but not COPD patients, manifested distinct hormone peaks during maximal exercise at 14 min (Epi), 16 min (NE), 22 min (ACTH) and 34 min (cortisol). Results of ANCOVA of the 6 time series in each panel are given showing group contrasts for cortisol (P < 0.001), Epi (P = 0.048) and a similar trend for ACTH (P = 0.094). Exercise regimen was a significant factor for all 4 of ACTH, cortisol, Epi and NE responses (each P < 0.001). Group-by-regimen interactions were highly significant for all 3 of ACTH, cortisol and Epi (each P < 0.001). In all cases, COPD was associated with lower responses to exercise over time. The covariate (no exercise effect) was a significant predictor for the same 3 hormones (each P < 0.001).
Figure 1.
Mean (± SEM) time-concentration profiles in normal untrained men (N = 8) and patients with COPD (N = 9) studied at rest (baseline) and during submaximal (35 ± 5% of VO2 max) or maximal exercise. Blood was sampled at 2-min intervals for 40 minutes for measurements of ACTH (top left), cortisol (top right), Epi (bottom left) and NE (bottom right).
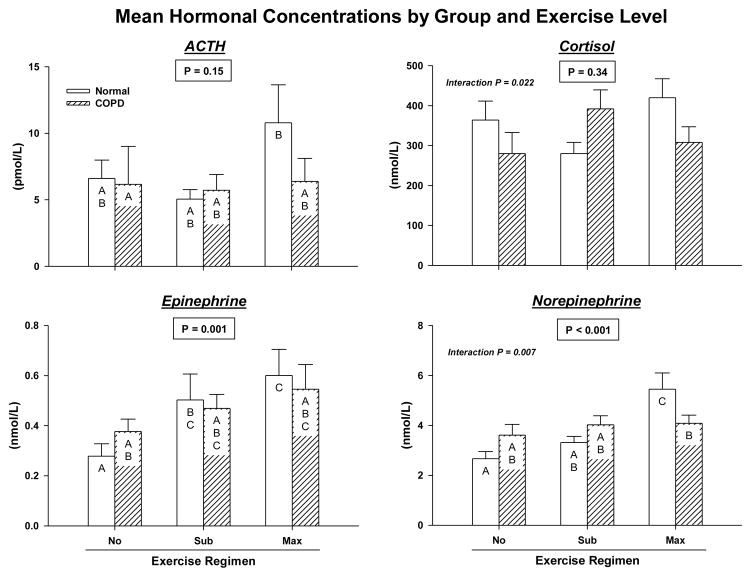
Time-averaged (40 min) mean hormone concentrations were examined first by two-way ANCOVA. Group (normal vs COPD) significantly determined mean ACTH concentrations (lower in COPD) during exercise (P = 0.044): Figure 2A. Exercise intensity (none, submaximal, maximal) determined mean Epi and NE levels (both P ≤ 0.001). There were significant interactions between group and exercise level for mean cortisol (P = 0.022) and NE (P = 0.007) concentrations. Post hoc multiple-comparison testing of time-averaged cortisol responses disclosed no further contrasts. Interactions between cohort and exercise level were due to: (i) failure of COPD (but not normal) subjects to elevate mean NE concentrations during maximal compared with no exercise (P = 0.11 COPD vs P < 0.001 normal); and (ii) lower mean NE values in COPD than healthy participants at maximal (P = 0.010) but not submaximal (P = 0.998) exercise.
Figure 2.
Mean (Panel A) and peak (Panel B) plasma concentrations of ACTH, cortisol, Epi and NE assessed by two-way ANCOVA. Means with unshared (unique) alphabetic superscripts (such as A vs C, but not A vs AB) are significantly different by Tukey’s honestly significantly different multiple-comparison test (P < 0.05). Open and hatched bars denote control and COPD, respectively.
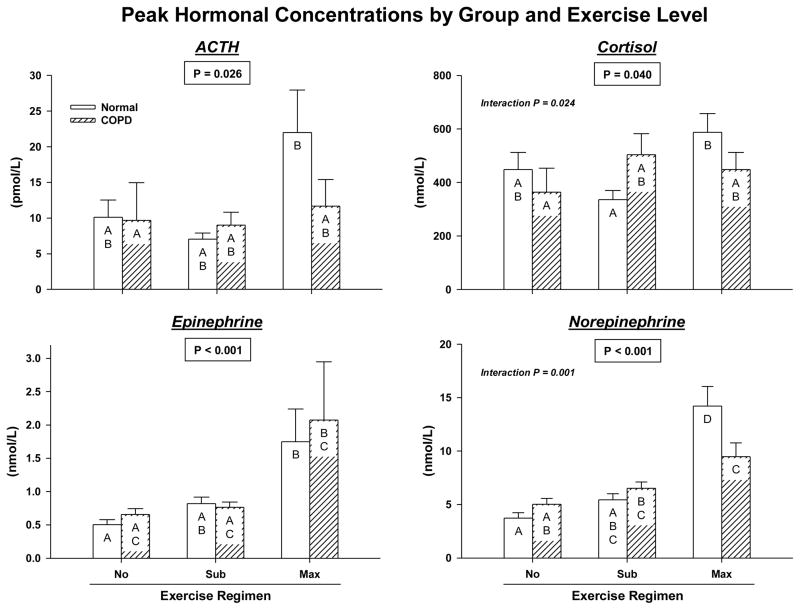
Peak ACTH concentrations were influenced by both group (P = 0.036) and exercise gradation (P = 0.026): Figure 2B. In particular, peak ACTH values in COPD were lower. Group did not determine peak levels of the other 3 hormones (0.10 < P < 0.90). However, there were significant main effects of exercise level on cortisol (P = 0.040), Epi (P < 0.001) and NE (P < 0.001). By post hoc analysis, maximal (compared with no) exercise increased peak concentrations of each hormone; viz., ACTH (P= 0.034), cortisol (P= 0.037), Epi (P < 0.001) and NE (P < 0.001) in the combined groups. Submaximal exercise stimulated peak NE release (P = 0.003 vs rest) with a similar trend for peak Epi (P = 0.070). Maximal exceeded submaximal exercise effects for peak NE (P < 0.001) and Epi (P = 0.005) with an analogous trend-level effect for ACTH (P = 0.075). All covariate effects were P ≤ 0.001. There were significant group-by-exercise interactions for peak cortisol (P= 0.024) and peak NE (P = 0.001) concentrations. In particular, only healthy participants had greater peak cortisol (P = 0.048) and NE (P < 0.001) concentrations after maximal than submaximal exercise. By Tukey’s post hoc multiple-comparison test, normal subjects attained higher peak NE concentrations during maximal exercise than COPD patients (P = 0.001).
The mechanisms underlying exercise’s stimulation of peak hormone concentrations were assessed next by deconvolution analysis. Exercise intensity strongly and positively influenced pulsatile ACTH secretion (P = 0.007), pulsatile NE secretion (P < 0.001), and the mass of NE released per pulse (P < 0.001). Estimated half-lives of NE and Epi did not differ by group or exercise level (medians 2.6 and 2.8 min, respectively). An unexpected mechanistic insight was abbreviation of cortisol secretory bursts (decreased mode) by maximal exercise in both groups (P = 0.025). In particular, cortisol secretory-burst mode averaged 3.2 ± 0.51 min during maximal and 6.4 ± 0.94 min during submaximal exercise
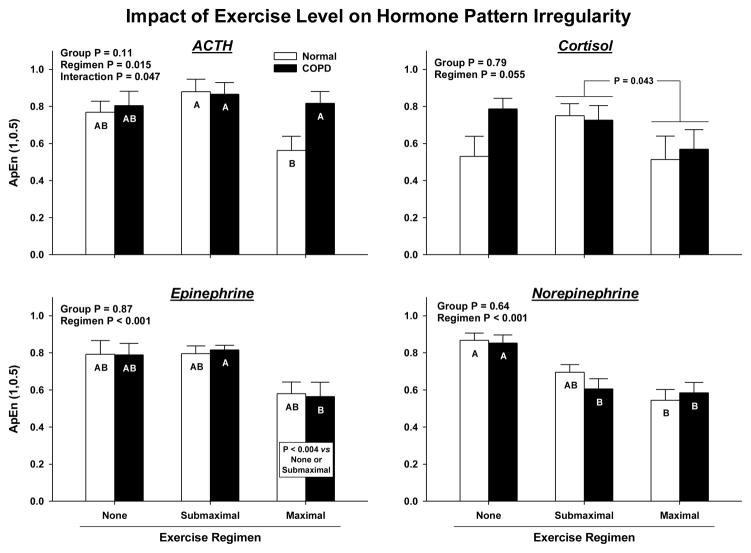
Approximate entropy (ApEn) was used as a measure of physiological irregularity. Figure 3 shows that there were no group effects on ApEn. Exercise intensity was associated with reduced ApEn for each of ACTH (P = 0.015), cortisol (P = 0.055), Epi (P < 0.001) and NE (P < 0.001). ApEn values of ACTH (P = 0.011), cortisol (P = 0.043) and Epi (P = 0.001) were lower during maximal than submaximal exercise. Decreased ApEn would denote decreased pattern irregularity (increased relative orderliness). In addition, exercise interacted with group to determine ACTH irregularity (P = 0.047 for interaction). Specifically, ACTH irregularity decreased less in COPD than normal subjects with maximal exercise (Tukey’s test P = 0.042).
Figure 3.
ApEn (approximate entropy) estimates of ACTH, cortisol, Epi and NE secretory irregularity, a quantifiable measure of altered feedback/feedforward strength. Higher ApEn defines greater irregularity (greater process randomness). Data are presented otherwise as defined in Figure 2.
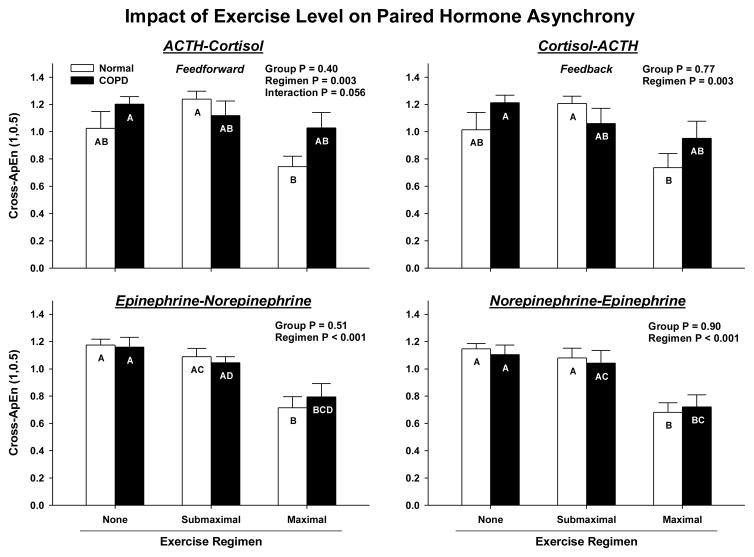
Cross-ApEn provides an objective measure of joint asynchrony, wherein higher values denote more asynchrony (less pairwise synchrony). ACTH-cortisol feedforward cross-ApEn fell (synchrony increased) during maximal compared with submaximal exercise in normal subjects only (P = 0.003 exercise effect, P = 0.056 interaction): Figure 4. Cortisol-ACTH feedback cross-ApEn declined with exercise intensity (P = 0.003), but without any interaction between intensity and group (P = 0.108). Epi-NE and NE-Epi cross-ApEn also decreased at P < 0.001 with maximal exercise. Other hormone pairs (ACTH-Epi, ACTH-NE, Epi-ACTH, NE-ACTH) exhibited similar lowering of cross-ApEn during maximal exercise (P < 0.005, except for Epi-ACTH cross-ApEn where P= 0.021).
Figure 4.
Cross-ApEn estimates of joint (pairwise) asynchrony between ACTH and cortisol (feedforward), cortisol and ACTH (feedback) and analogously for NE-ACTH and ACTH-NE. Higher cross-ApEn quantifies greater synchrony (less pattern coordination). See data presentation format in Figure 2.
DISCUSSION
Baseline (resting) ACTH, cortisol and catecholamine concentrations sampled over 40 min were similar in normal subjects and patients with COPD. However, exercise evoked significant group distinctions. Peak ACTH, cortisol and Epi concentrations failed to rise in COPD, and peak NE concentrations increased less than in healthy subjects. Impaired ACTH and NE responses were due to selective attenuation of pulsatile hormone secretion and failure to regularize (as quantified by ApEn) ACTH secretion patterns. The abnormal responses in COPD could not be attributed to lower fitness per se, since percentage VO2 max values were comparable in the two study cohorts and similar to those in other studies [9]. Diminished endocrine responses were also not due to reduced dyspneic stress, since COPD patients at any given gradation of exercise reported more severe dyspnea (perceived exertion) as expected [23].
Borg-dyspnea scores increased rapidly after about 6 min of exercise at 36 ± 6% (COPD) and 23 ± 5% (controls) VO2 max, akin to other studies [24]. It would seem that COPD patients report less subjective dyspnea acutely in their chronic dyspneic state. Based upon 2-min sampling, the timing of peak hormone responses was later: viz., Epi (14 min), NE (16 min), ACTH (22 min) and cortisol (34 min). This sequence is consistent with evidence that central adrenergic outflow triggers corticotropic-axis activation [25]. Reduced responsiveness could suggest that this pathway is impaired in patients with COPD. Catecholamine drive of ACTH is central, because peripheral infusions of Epi or NE do not elicit ACTH or cortisol secretion [26]. Greater hypoxia in COPD would not explain impaired sympathoadrenal responses, since severe hypoxia stimulates rather than inhibits NE, Epi, ACTH and cortisol secretion [5, 10]. A possibility is that COPD patients release less CRH, which would drive less ACTH secretion [27, 28], and/or produce more somatostatin and natriuretic peptide [29, 30], which would inhibit CRH-driven ACTH secretion more significantly [11, 31, 32]. Because exercise can induce ACTH secretion even during constant CRH infusion [33], other agonists like arginine vasopressin may be important as well [27].
Another finding was that maximal exercise enforces more regular patterns (lower ApEn) of ACTH, cortisol, NE and Epi release. This was true in both study groups, except for ACTH regularization, which failed to occur in COPD patients. Based upon empirical and theoretic considerations, regularization of ACTH secretion requires muting of glucocorticoid feedback [19, 34, 35]. Thus, COPD patients might be unable to disinhibit cortisol’s feedback onto ACTH during exercise. Withdrawal not only of glucocorticoid but also of GABAergic inhibition may be required for ACTH release during exercise [36]. Whatever the proximate mechanisms, corticotropic and adrenergic responses are proportional to relative workload (power output), expressible as percentage VO2 max [10, 37–39]. The latter was similar in normal volunteers and COPD patients, indicating that other mechanisms are responsible for decreased corticotropic responses in COPD. A possibility is that the mechanisms are similar to those in chronic fatigue syndrome [40].
Caveats include the possibility of carryover or training-like effects of the three exercise sessions. However, all studies were scheduled at least 72 hr apart, and the same order of study was applied to both groups. Moreover, an additional rest session separated the active-exercise bouts. Although BMI strongly inhibits the effect of exercise on GH secretion, its effects on exercise-stimulated ACTH and cortisol secretion are less well delineated. Whereas many factors might influence exercise effects, precautionary exclusions were concomitant use of mineralocorticoid-receptor antagonists (such as spironolactone) or calcium-channel antagonists (such as diltiazem) [41, 42], and weight loss or the use of glucocorticoids within 3 mo. ACE inhibitors were also disallowed [38]. Although all COPD subjects denied recent smoking, acute nicotine exposure would be expected to potentiate rather than attenuate ACTH and cortisol release [43]. The statistical similarity of ages would not necessarily exclude a less evident influence of a small age difference on the hormonal data. The study was not powered to answer this question.
In conclusion, men with stable COPD fail to achieve normal exercise-induced corticotropic axis and adrenomedullary outflow. The basis for joint defects in the amplitude and the regularity of stress-hormone release will require further study.
Acknowledgments
We thank Samuel Rudolph and Jill Smith for support of manuscript preparation; Ashley Bryant for data analysis and graphics; and Salem Veterans Affairs Hormone Laboratory for assay assistance. Supported in part via the Center for Translational Science Activities (CTSA) Grant Number 1 UL 1 RR024150 from the National Center for Research Resources (Rockville, MD), and DK073148 and DK050456 (Metabolic Studies Core of the Minnesota Obesity Center) from the National Institutes of Health (Bethesda, MD).
Abbreviations
- NE
Norepinephrine
- Epi
Epinephrine
- ACTH
Adrenocorticotropic hormone
- COPD
Chronic Obstructive Pulmonary Disease
- ANCOVA
Analysis of Covariance
- ApEn
Approximate Entropy
- FEV
Forced expiratory volume
- Hg
Hemoglobin
- HR
Heart rate
- VO2
Oxygen consumption
- VE
minute ventilation
- BP
Blood pressure
- EDTA
Ethylenediaminetetraacetic acid
- RIA
Radioimmunoassay
- CV
Cardiovascular
- HSD
Honestly significant difference
- BMI
Body mass index
- CRH
Corticotropin-releasing hormone
- ACE inhibitors
Angiotensin-converting enzyme inhibitors
Footnotes
Disclosures: None. Authors have nothing to declare.
Author contributions
Ali Iranmanesh: wrote and reviewed paper
Dudley F. Rochester: conducted experiments
Jing Liu: conducted experiments
Johannes D. Veldhuis: Corresponding author. Wrote the paper. Performed mathematical analyses.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Kraemer WJ, Patton JF, Knuttgen HG, et al. Hypothalamic-pituitary-adrenal responses to short-duration high-intensity cycle exercise. J Appl Physiol. 1989;66:161–166. doi: 10.1152/jappl.1989.66.1.161. [DOI] [PubMed] [Google Scholar]
- 2.Raastad T, Bjoro T, Hallen J. Hormonal responses to high- and moderate-intensity strength exercise. Eur J Appl Physiol. 2000;82:121–128. doi: 10.1007/s004210050661. [DOI] [PubMed] [Google Scholar]
- 3.Henriksen JH, Christensen NJ, Kok-Jensen A, et al. Increased plasma noradrenaline concentration in patients with chronic obstructive lung disease: relation to haemodynamics and blood gases. Scand J Clin Lab Invest. 1980;40:419–427. doi: 10.3109/00365518009101864. [DOI] [PubMed] [Google Scholar]
- 4.Raguso CA, Luthy C. Nutritional status in chronic obstructive pulmonary disease: Role of hypoxia. Nutrition. 2011;27:138–143. doi: 10.1016/j.nut.2010.07.009. [DOI] [PubMed] [Google Scholar]
- 5.Raff H, Levy SA. Renin-angiotensin II-aldosterone and ACTH-cortisol control during acute hypoxemia and exercise in patients with chronic obstructive pulmonary disease. Am Rev Respir Dis. 1986;133:396–399. doi: 10.1164/arrd.1986.133.3.396. [DOI] [PubMed] [Google Scholar]
- 6.Wouters EF, Creutzberg EC, Schols AM. Systemic effects in COPD. Chest. 2002;121:127S–130S. doi: 10.1378/chest.121.5_suppl.127s. [DOI] [PubMed] [Google Scholar]
- 7.Zarkovic M, Ignjatovic S, Dajak M, et al. Cortisol response to ACTH stimulation correlates with blood interleukin 6 concentration in healthy humans. Eur J Endocrinol. 2008;159:649–652. doi: 10.1530/EJE-08-0544. [DOI] [PubMed] [Google Scholar]
- 8.de Diego Acosta AM, Garcia JC, Fernandez-Pastor VJ, et al. Influence of fitness on the integrated neuroendocrine response to aerobic exercise until exhaustion. J Physiol Biochem. 2001;57:313–320. doi: 10.1007/BF03179825. [DOI] [PubMed] [Google Scholar]
- 9.Killian KJ, LeBlanc P, Martin DH, et al. Exercise capacity and ventilatory, circulatory, and symptom limitation in patients with chronic airflow limitation. Am Rev Respir Dis. 1992;146:935–940. doi: 10.1164/ajrccm/146.4.935. [DOI] [PubMed] [Google Scholar]
- 10.Kjaer M, Bangsbo J, Lortie G, et al. Hormonal response to exercise in humans: influence of hypoxia and physical training. Am J Physiol. 1988;254:R197–R203. doi: 10.1152/ajpregu.1988.254.2.R197. [DOI] [PubMed] [Google Scholar]
- 11.Adnot S, Andrivet P, Chabrier PE, et al. Plasma levels of atrial natriuretic factor, renin activity, and aldosterone in patients with chronic obstructive pulmonary disease. Response to O2 removal and to hyperoxia. Am Rev Respir Dis. 1990;141:1178–1184. doi: 10.1164/ajrccm/141.5_Pt_1.1178. [DOI] [PubMed] [Google Scholar]
- 12.Raasch W, Wittmershaus C, Dendorfer A, et al. Angiotensin II inhibition reduces stress sensitivity of hypothalamo-pituitary-adrenal axis in spontaneously hypertensive rats. Endocrinol. 2006;147:3539–3546. doi: 10.1210/en.2006-0198. [DOI] [PubMed] [Google Scholar]
- 13.Mador MJ, Rodis A, Magalang UJ. Reproducibility of Borg scale measurements of dyspnea during exercise in patients with COPD. Chest. 1995;107:1590–1597. doi: 10.1378/chest.107.6.1590. [DOI] [PubMed] [Google Scholar]
- 14.Veldhuis JD, Iranmanesh A, Lizarralde G, et al. Amplitude modulation of a burst-like mode of cortisol secretion subserves the circadian glucocorticoid rhythm in man. Am J Physiol. 1989;257:E6–E14. doi: 10.1152/ajpendo.1989.257.1.E6. [DOI] [PubMed] [Google Scholar]
- 15.Veldhuis JD, Iranmanesh A, Johnson ML, et al. Amplitude, but not frequency, modulation of ACTH secretory bursts gives rise to the nyctohemeral rhythm of the corticotropic axis in man. J Clin Endocrinol Metab. 1990;71:452–463. doi: 10.1210/jcem-71-2-452. [DOI] [PubMed] [Google Scholar]
- 16.Keenan DM, Roelfsema F, Biermasz N, et al. Physiological control of pituitary hormone secretory-burst mass, frequency and waveform: a statistical formulation and analysis. Am J Physiol. 2003;285:R664–R673. doi: 10.1152/ajpregu.00195.2003. [DOI] [PubMed] [Google Scholar]
- 17.Veldhuis JD, Keenan DM, Pincus SM. Motivations and methods for analyzing pulsatile hormone secretion. Endocr Rev. 2008;29:823–864. doi: 10.1210/er.2008-0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu PY, Keenan DM, Kok P, et al. Sensitivity and specificity of pulse detection using a new deconvolution method. Am J Physiol Endo Metab. 2009;297:E538–E544. doi: 10.1152/ajpendo.00071.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Veldhuis JD, Straume M, Iranmanesh A, et al. Secretory process regularity monitors neuroendocrine feedback and feedforward signaling strength in humans. Am J Physiol. 2001;280:R721–R729. doi: 10.1152/ajpregu.2001.280.3.R721. [DOI] [PubMed] [Google Scholar]
- 20.Liu PY, Pincus SM, Keenan DM, et al. Analysis of bidirectional pattern synchrony of concentration-secretion pairs: implementation in the human testicular and adrenal axes. Am J Physiol. 2005;288:R440–R446. doi: 10.1152/ajpregu.00414.2004. [DOI] [PubMed] [Google Scholar]
- 21.Zar JH. Biostatistical analysis. 3. Upper Saddle River, NJ: Prentice Hall; 1996. [Google Scholar]
- 22.O’Brien PC. The appropriateness of analysis of variance and multiple-comparison procedures. Biometrics. 1983;39:787–794. [PubMed] [Google Scholar]
- 23.O’Donnell DE, Bertley JC, Chau LK, et al. Qualitative aspects of exertional breathlessness in chronic airflow limitation: pathophysiologic mechanisms. Am J Respir Crit Care Med. 1997;155:109–115. doi: 10.1164/ajrccm.155.1.9001298. [DOI] [PubMed] [Google Scholar]
- 24.Kearon MC, Summers E, Jones NL, et al. Effort and dyspnoea during work of varying intensity and duration. Eur Respir J. 1991;4:917–925. [PubMed] [Google Scholar]
- 25.Buckingham JC, Hodges JR. Production of corticotrophin releasing hormone by the isolated hypothalamus of the rat. J Physiol. 1977;272:469–479. doi: 10.1113/jphysiol.1977.sp012054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oberbeck R, Schurmeyer T, Hosch W, et al. Epinephrine or norepinephrine fail to influence pituitary-adrenal secretion in man. Horm Metab Res. 1996;28:142–146. doi: 10.1055/s-2007-979147. [DOI] [PubMed] [Google Scholar]
- 27.Alexander SL, Irvine CH, Ellis MJ, et al. The effect of acute exercise on the secretion of corticotropin-releasing factor, arginine vasopressin, and adrenocorticotropin as measured in pituitary venous blood from the horse. Endocrinol. 1991;128:65–72. doi: 10.1210/endo-128-1-65. [DOI] [PubMed] [Google Scholar]
- 28.Nussey SS, Page SR, Peterson DB, et al. Corticotrophin-releasing hormone (CRH1-41) stimulates the secretion of adrenocorticotrophin, vasopressin and oxytocin but not adrenocotricotrophin precursors: evidence from petrosal sinus sampling in man. Clin Endocrinol. 1991;34:51–56. doi: 10.1111/j.1365-2265.1991.tb01735.x. [DOI] [PubMed] [Google Scholar]
- 29.Iranmanesh A, Carpenter PC, Mielke K, et al. Putative somatostatin suppression potentiates adrenocorticotropin secretion driven by ghrelin and human corticotropin-releasing hormone. J Clin Endocrinol Metab. 2007;92:3653–3659. doi: 10.1210/jc.2007-0523. [DOI] [PubMed] [Google Scholar]
- 30.Huang WS, Lee MS, Perng HW, et al. Circulating brain natriuretic peptide values in healthy men before and after exercise. Metabolism. 2002;51:1423–1426. doi: 10.1053/meta.2002.35194. [DOI] [PubMed] [Google Scholar]
- 31.Kellner M, Wiedemann K, Holsboer F. Atrial natriuretic factor inhibits the CRH-stimulated secretion of ACTH and cortisol in man. Life Sci. 1992;50:1835–1842. doi: 10.1016/0024-3205(92)90543-x. [DOI] [PubMed] [Google Scholar]
- 32.Lawrence DL, Skatrud JB, Shenker Y. Effect of hypoxia on atrial natriuretic factor and aldosterone regulation in humans. Am J Physiol. 1990;258:E243–E248. doi: 10.1152/ajpendo.1990.258.2.E243. [DOI] [PubMed] [Google Scholar]
- 33.Smoak B, Deuster P, Rabin D, et al. Corticotropin-releasing hormone is not the sole factor mediating exercise-induced adrenocorticotropin release in humans. J Clin Endocrinol Metab. 1991;73:302–306. doi: 10.1210/jcem-73-2-302. [DOI] [PubMed] [Google Scholar]
- 34.Veldhuis JD, Iranmanesh A, Naftolowitz D, et al. Corticotropin secretory dynamics in humans under low glucocorticoid feedback. J Clin Endocrinol Metab. 2001;86:5554–5563. doi: 10.1210/jcem.86.11.8046. [DOI] [PubMed] [Google Scholar]
- 35.Pincus SM, Huang WM. Approximate entropy: Statistical properties and applications. Commun Statist-Theory Meth. 1992;21:3061–3077. [Google Scholar]
- 36.Major JM, Laughlin GA, Kritz-Silverstein D, et al. Insulin-like growth factor-I and cancer mortality in older men. J Clin Endocrinol Metab. 2010;95:1054–1059. doi: 10.1210/jc.2009-1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oleshansky MA, Zoltick JM, Herman RH, et al. The influence of fitness on neuroendocrine responses to exhaustive treadmill exercise. Eur J Appl Physiol Occup Physiol. 1990;59:405–410. doi: 10.1007/BF02388620. [DOI] [PubMed] [Google Scholar]
- 38.Luger A, Deutster PA, Kyle SB, et al. Acute hypothalamic-pituitary-adrenal responses to the stress of treadmill exercise. Physiologic adaptations to physical training. N Engl J Med. 1987;316:1309–1315. doi: 10.1056/NEJM198705213162105. [DOI] [PubMed] [Google Scholar]
- 39.Hill EE, Zack E, Battaglini C, et al. Exercise and circulating cortisol levels: the intensity threshold effect. J Endocrinol Invest. 2008;31:587–591. doi: 10.1007/BF03345606. [DOI] [PubMed] [Google Scholar]
- 40.Demitrack MA, Dale JK, Straus SE, et al. Evidence for impaired activation of the hypothalamic-pituitary-adrenal axis in patients with chronic fatigue syndrome. J Clin Endocrinol Metab. 1991;73:1224–1234. doi: 10.1210/jcem-73-6-1224. [DOI] [PubMed] [Google Scholar]
- 41.Wellhoener P, Born J, Fehm HL, et al. Elevated resting and exercise-induced cortisol levels after mineralocorticoid receptor blockade with canrenoate in healthy humans. J Clin Endocrinol Metab. 2004;89:5048–5052. doi: 10.1210/jc.2004-0086. [DOI] [PubMed] [Google Scholar]
- 42.Kono K, Okutani R, Tsuda S, et al. Effects of diltiazem on renin-aldosterone and ACTH-adrenocortical function during upper abdominal surgery. J Clin Anesth. 1990;2:407–414. doi: 10.1016/0952-8180(90)90027-z. [DOI] [PubMed] [Google Scholar]
- 43.Kirschbaum C, Scherer G, Strasburger CJ. Pituitary and adrenal hormone responses to pharmacological, physical, and psychological stimulation in habitual smokers and nonsmokers. Clin Investig. 1994;72:804–810. doi: 10.1007/BF00180552. [DOI] [PubMed] [Google Scholar]