Abstract
Nearly 12% of US children are exposed to intense adverse experiences. Research has demonstrated that these experiences can negatively impact adult health, often resulting in psychopathology. Less attention, however, is given to the impact of childhood adverse experiences on childhood health and wellbeing. Using a rodent model of chronic juvenile stress (restraint 6h daily from postnatal day 20–41), we report that chronic stress has significant immediate morbidities in both males and females during this developmental window. Specifically, we demonstrate that chronic juvenile stress produces depressive-like behavior and significant neuronal remodeling of brain regions likely involved in these behavioral alterations: the hippocampus, prefrontal cortex and amygdala. Chronically stressed males and females exhibit anhedonia, increased locomotion when exposed to novelty, and altered coping strategies when exposed to acute stress. Coincident with these behavioral changes, we report simplification of dendrites in the hippocampus and prefrontal cortex and concurrent hypertrophy of dendrites in the amygdala. Taken together, these results demonstrate that chronically stressed juveniles exhibit aberrant behavioral responses to acute challenges that occur in conjunction with stress-induced remodeling of brain regions intimately involved in regulating emotionality and stress reactivity. Further, the absence of sex differences in our reported stress responses, likely speaks to the decreased sensitivity of immature HPA regulating brain regions to sex hormones.
Background
Toxic stressors are repeated, intense adverse experiences. Nearly, nine million children in the United States are exposed to such adverse experiences (Finkelhor et al., 2005). The vast majority of research investigating the sequelae of chronic childhood stress focuses on its lasting effects on adult mental health, and far less attention is given to its more immediate effects, those morbidities occurring during childhood and adolescence. Research involving rodent models has documented increased anxiety (Pohl et al., 2007), anhedonia (Pohl et al., 2007), impaired spatial memory (Isgor et al., 2004), exaggerated corticosterone secretion (Barha et al. 2010; Isgor et al., 2004; Pohl et al., 2007), and altered volume of hippocampal subfields (Isgor et al., 2004) in juveniles exposed to chronic stress. Still unknown is whether chronic juvenile stress produces a full spectrum of depressive behavior and whether brain regions outside the hippocampus are affected.
Our goal, therefore, was to further delineate the immediate sequelae of chronic juvenile stress by focusing on the juvenile and adolescent period. First, we evaluated the effects of chronic juvenile stress on a broader spectrum of depressive-like and anxiety-like behaviors. Next, we assessed for the physiologic effects related to chronic juvenile stress. Third, we investigated dendritic architecture in three interconnected stress-sensitive limbic system brain regions that have been associated with the pathogenesis of depression: the hippocampus, amygdala and prefrontal cortex. Finally, as sex differences in stress reactivity have been well documented in adults, we compared the sequelae of juvenile chronic stress in male and female adolescents.
Methods
Animals
Time-pregnant Sprague-Dawley rats obtained from Charles River Laboratories (Kingston, NY) arrived at our animal facility on gestational day 13. The dams were singly housed. Two days after delivery (postnatal day 2-PND2), litters were culled to 12 pups with equal number of males and females where possible. Litters were left undisturbed except to provide bi-weekly routine husbandry. On PND20 litters were weaned and housed in same-sex groups of 2–3 rats per cage. Animals were maintained on a 12h light-dark schedule (lights on from 0600h to 1800h) and the ambient temperature was maintained at 21 ± 2°C. Food and water were available ad libitum.
Chronic Restraint Stress
Cages were randomly assigned to a control or chronic restraint stress (CRS) group. Beginning on PND20, CRS rats were placed in snuggly-fitted wire mesh restrainers for six hours daily (initiated between the hours of 800h and 1100h) for a period of 21 days. Control rats were left undisturbed in their home cages during this three-week period. One week prior to behavioral testing, control rat were handled for three minutes daily to minimize stress reaction to manipulation during behavioral testing.
Sucrose Preference Testing
Testing took place on the last two days of chronic restraint stress, during the dark phase of the light cycle. During the first 24 hours of testing two water bottles were placed directly adjacent to each other in the wiretops of the animal cages. One bottle contained tap water and the other bottle contained a 2% sucrose solution. During the second 24h, the position of the two bottles was switched to prevent place preference contributing to results. The bottles were weighed prior to placement in the cages, at the end of 24 hours and at the end of 48 hours. The percent sucrose consumption was calculated as:
Elevated Plus Maze
Twenty-four hours after the last restraint session control and CRS rats were observed in an elevated plus maze. This maze consisted of a plus-shaped platform with two open arms and two closed arms. The closed arms were surrounded by 37 cm high opaque walls on three sides. The open and closed arms were located directly opposite each other. Each rat was placed on the central platform facing a closed arm and allowed to explore the maze for a period of five minutes. The maze was cleaned with a 50% ethanol solution at the conclusion of each trial. Trials were recorded and analyzed using the Noldus Ethovision XT software (Noldus Information Technology, Leesburg, VA, USA). Arm entry was defined as the central portion of the rat’s body entering the arm. The number of entries and duration of time spent in the center, open and closed-arms was recorded. Ratio of open arm duration to open arm and closed arm duration was calculated.
Forced Swim Test
Within 15 minutes of completing the EPM, animals were subjected to forced swim. Rats were individually placed in a water-filled cylindrical acrylic container measuring 45cm high × 38cm wide. The depth of the water was 30cm and the temperature was 25 ± 1ºC. The animals were forced to swim for 12 minutes on day one (pre-test) and then 24 hours later the animals were forced to swim for five minutes (test). The swim sessions were videotaped and scored at a later point for latency to immobility, total immobility duration and struggling duration. Immobility was defined as lack of movement of three paws and minimal movement of the 4th. Struggling was defined as vigorous movement of all four paws. The scorer was blinded to the experimental group and sex of the animal.
Blood Collection
Thirty minutes after termination of the second session of the FST, rats were decapitated. Trunk blood was collected into BD Vacutainer ® K3 EDTA collection Tubes (Becton Dickinson and Company, Franklin Lakes, NJ). Blood was stored on ice until centrifuged for 15 minutes at 1350 x g at 4°C. Plasma was removed and stored at −80°C until assayed for corticosterone.
Golgi Procedure and Histology
Following decapitation brains were rapidly removed and processed according to FD Rapid Golgistain Kit instructions (FD NeuroTechnologies, Baltimore, MD). These staining procedures and methods have previously been established as successfully staining hippocampal pyramidal cells (Kleen et al., 2006; McLaughlin et al., 2005). Slides containing the Golgi-impregnated sections were coded before analysis and the code was not broken until the analysis was complete. To be selected for analysis, Golgi impregnated pyramidal neurons had to possess the following characteristics: 1. Hippocampus: the neuron had to be in the segment of the cornu ammonis 3 (CA3) subregion proximal to the dentate gyrus of the dorsal hippocampus. The CA3 segment examined excluded the CA3 curvature and ended at the imaginary line connecting the ends of the dorsal and ventral blades of the dentate gyrus. Prefrontal cortex: the neuron had to be within layer II/III and within the boundaries of the anterior cingulated cortex and pre-limbic area. Basolateral Amygdala: the neuron had to be within the basolateral nucleus of the amygdala and have a spiny pyramidal-like phenotype (McDonald, 1982; Vyas et al., 2006; Vyas et al., 2002) 2. Cell bodies had to be located in the middle third of the tissue section to avoid analysis of impregnated neurons that extended largely into other sections. 3. Dark and consistent impregnation throughout the extent of all of the dendrites and 4. Relative isolation from neighboring impregnated cells.
Neuronal cell tracing and branch point analysis was performed using NeuroExplorer and NeuroLeucida software (MicroBrightfield, Inc. Williston, VT). For each rat, an average length and branch point quantification was calculated. A total of twenty-four rats were included in this analysis: six rats from each of the four experimental groups.
Corticosterone radioimmunoassay
The corticosterone radioimmunoassay (RIA) was performed using a commercially available kit (ImmunoChem™ Double Antibody, MP Biomedicals, Orangeburg, NY). The assay has a sensitivity of 1 ng/ml and intra- and inter-coefficients of variation of less than 10%.
Statistical Analysis
All parametric data was analyzed using two-way ANOVA with sex and stress as factors. Significant results were further analyzed using Bonferroni posttest and where appropriate, post hoc pair wise comparisons were made using unpaired Student’s t-test. Data are presented as mean ± S.E.M. Statistical significance was set at P<0.05.
Results
Physiologic effects of chronic restraint stress during the adolescent period
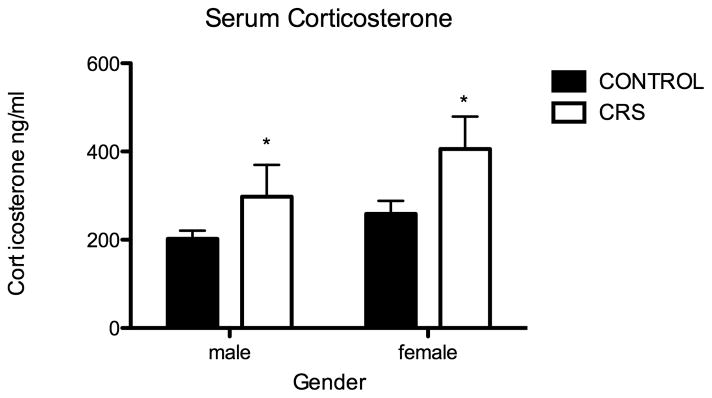
As has been described in adult rats, CRS during the adolescent period results in decreased weight gain, thymic atrophy and adrenal hypertrophy (Table 1). Of note, during the adolescent period chronic stress seems to inhibit weight gain to a greater degree than occurs during adulthood. After three weeks of chronic restraint stress, juveniles exposed to chronic stress weigh 30% less than non-stressed rats, whereas the weight difference in chronically stressed adults is only 8% less than controls (Eiland and McEwen, 2010). In regard to thymic and adrenal weights, in adolescents, we found a significant effect of sex. Specifically, as has been documented in adults (Pilipovic et al., 2008; Pitychoutis and Papadopoulou-Daifoti, 2010), adrenal weight as a percentage of body weight was greater for females than males (two-way ANOVA F1,27=22.69, P<0.0001). A different effect of sex was noted in thymic weights as a percentage of body weight. There was no baseline difference between male and females, however females did not exhibit stress-induced atrophy. Finally, CRS during the adolescent period effects hypothalamo-pituitary-adrenal axis (HPA) reactivity; CRS rats exhibited significantly higher serum corticosterone levels 30 minutes following forced swim (Two-way ANOVA F1,23=5.68, P=0.03: Figure 1). Sex did not significantly effect stress-induced corticosterone secretion.
Table 1.
Physiologic Stress Responses
| Control Male | CRS Male | Control Female | CRS Female | |
|---|---|---|---|---|
| Body Weight | 221.9 ± 8.5 | 155.1 ± 4.5* | 174.1 ± 6.7 | 126.8 ± 5.2* |
| Relative Adrenal Weight | 0.020 ± 0.001 | 0.025 ± 0.001* | 0.026 ± 0.002‡ | 0.032 ± 0.001*‡ |
| Relative Thymus Weight | 0.30 ± 0.02 | 0.24 ± 0.01* | 0.31 ± 0.01 | 0.31 ± 0.02 |
stress effect, P<0.0001
sex effect, P<0.0001
Figure 1.
Juvenile chronic restraint stress (CRS) produces exaggerated stress induced corticosterone secretion. Serum corticosterone (ng/ml) 30 minutes following forced swim, CONT males (n=8) 202.4, CONT females (n=8) 258.9, CRS males (n=7) 297.7, CRS females (n=5) 406.0; stress effect two-way ANOVA F1,23=5.68, *P=0.03.
Chronic stress produces a distinct behavioral phenotype in adolescents
In adult rats, chronic stress produces a depressive-like phenotype characterized by anhedonia, increased anxiety-like behavior, and behavioral despair as measured in the forced swim test (McEwen, 2008; McEwen and Magarinos, 2001). We investigated whether CRS during the juvenile period would produce a similar phenotype.
Anhedonia
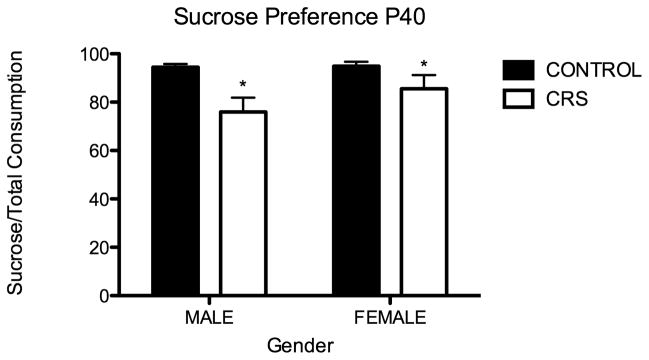
During the dark cycle of the last two days of CRS, adolescent rats were presented with two bottles: one contained tap water and the other contained a 2% sucrose solution. The percentage of sucrose consumed in each cage was calculated for the 48-hour period. As measured by percent sucrose consumption, adolescent CRS rats in comparison to controls, exhibited anhedonia; they drank significantly less of a 2% sucrose solution (two-way ANOVA F1,11=9.31, P=0.01: Figure 2). There was not a significant effect of sex. It should be noted that CRS rats drank significantly more tap water than controls (Student t test P=0.01).
Figure 2.
Juvenile chronic restraint stress (CRS) produces anhedonia in males and females. Percent consumption of 2% sucrose solution: CONT males 94.5% (n=8, 3 cages), CONT females 94.8% (n=8, 4 cages), CRS males 76% (n=8, 4 cages), Female CRS 85.6% (n=8, 4 cages). Stress effect two-way ANOVA F1,11=9.31, *P=0.01.
Anxiety-like behavior
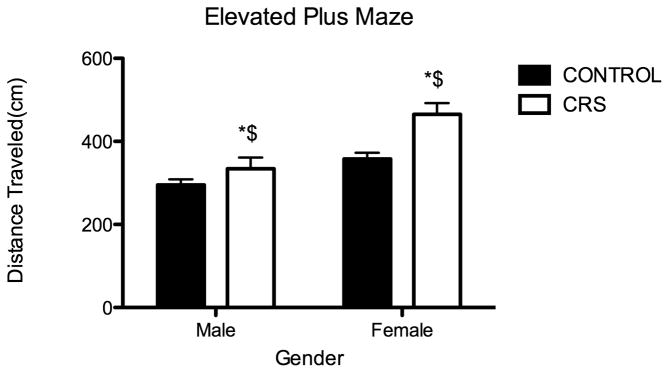
Twenty-four hours after the last CRS session, rats were observed in the elevated plus maze; quantitative analysis is shown in Figure 3. As measured by the amount of time spent in the open arms of the elevated plus maze, there was not a significant difference between control and CRS groups in anxiety-like behavior. However, in comparison to controls, CRS animals exhibited a significant increase in locomotion while in the EPM (Two-way ANOVA F1,25=11.94, P=0.002, N=7 for all groups except CONT males N=8). As has been previously documented in juvenile rats, there was a significant effect of sex on locomotion; females travelled significantly greater distances than males (Two-way ANOVA F1,25=20.86, P=0.0001).
Figure 3.
Juvenile chronic restraint stress (CRS) significantly increases locomotion. Groups were observed in EPM 24 hours after the last CRS session. CRS significantly increased the distance traveled in the EPM: CONT males 295.4 cm, CONT females 357.7 cm, CRS males 334.2 cm, CRS females 465.3 cm. Two-way ANOVA F1,25=11.94, *P=0.002, n=7 for all groups except CONT males n=8. In addition to the observed effect of stress on locomotion, females exhibited greater locomotion than males. Two-way ANOVA F1,25=20.86, $P=0.0001.
Behavioral Despair-like Behavior
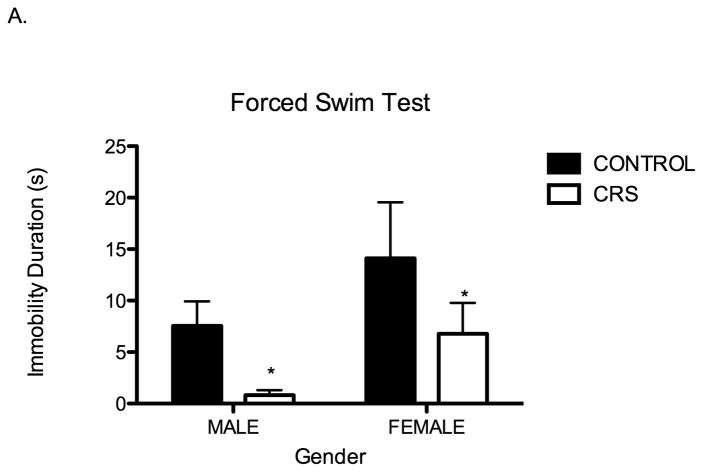
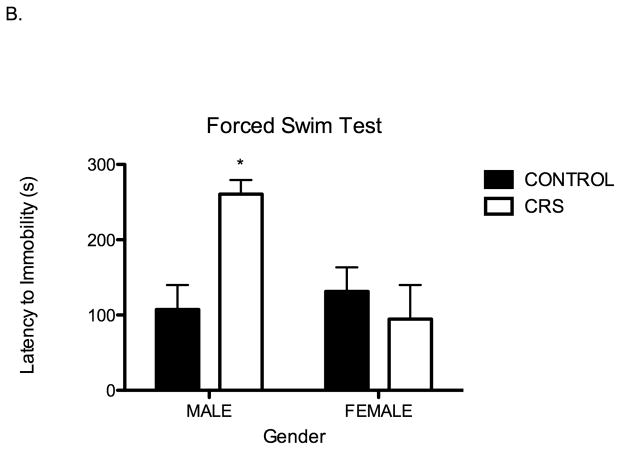
Following observation in the EPM, rats were subjected to forced swim. We found that forced swim behavior of juvenile rats is also influenced by chronic stress. First, in comparison to controls, chronically stressed juvenile rats were immobile for significantly shorter periods of time (Two-way ANOVA F1,28=4.41, P=0.04; Figure 4A). Second, in regard to latency to immobility, there was a significant interaction between stress and sex whereby chronically stressed juvenile males exhibited a significantly longer latency to immobility: two-way ANOVA F1,26=8.06, P=0.01 Figure 4B. The latency to immobility of chronically stressed females was indistinguishable from controls.
Figure 4.
Juvenile chronic restraint stress (CRS) alters swim behavior during forced swim; CRS rats have significantly shorter immobility duration and prolonged latency to immobility. A. Immobility duration for CONT males 7.6 s, CONT females 14.1 s, CRS males 0.8 s, CRS females 6.8 s. Stress effect two-way ANOVA F1,28=4.41, *P=0.04, n= 8 for all groups. B. There is a significant interaction between sex and stress for latency to immobility; there is only a significant effect of chronic stress on latency to immobility in males. CONT males 107.3 s, CONT females 131.4 s, CRS males 260.0 s, CRS females 94.63 s. Two-way ANOVA F1,28=8.06, *P=0.01, n=8 for all groups.
Stress-induced dendritic remodeling is not sex specific in adolescent rats
Aside from assessing the physiologic and behavioral sequelae of chronic stress during the adolescent period, we examined the effect of CRS on dendritic architecture of pyramidal neurons in three limbic brain regions: the hippocampus, prefrontal cortex and amygdala. In these brain regions, stress-induced plasticity has been extensively studied and well characterized in adult rats, but not previously examined during adolescence.
Hippocampus
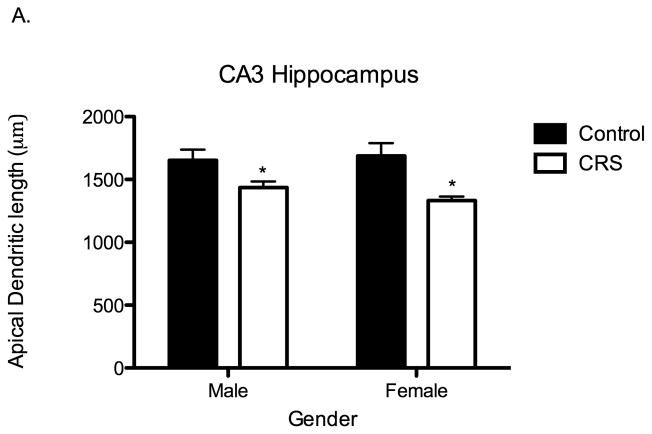
We examined apical dendritic length and branch points of pyramidal neurons in the CA3 subregion of the hippocampus. In comparison to controls, CRS rats had significantly decreased apical dendritic length (Two-way ANOVA F1,19=16.11, P=0.0007; Figure 5A) and significantly fewer branch points (Two-way ANOVA F1,16=5.1, P=0.04). Contrary to what has been found in adult rats, there was not a significant effect of sex on stress-induced dendritic remodeling. During adolescence, both male and female rats subjected to CRS exhibit significant apical dendritic structural simplification.
Figure 5.
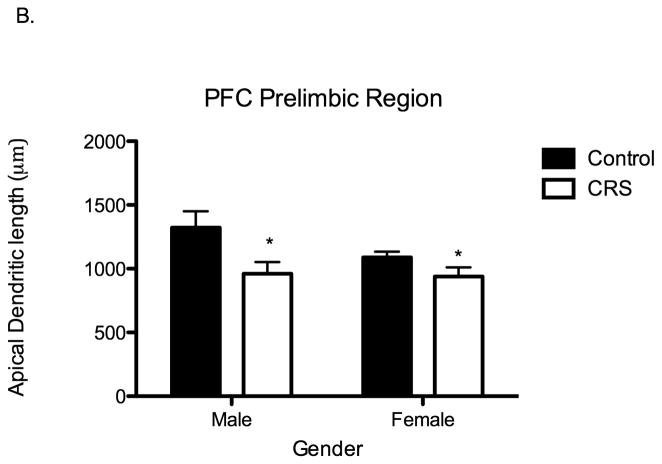
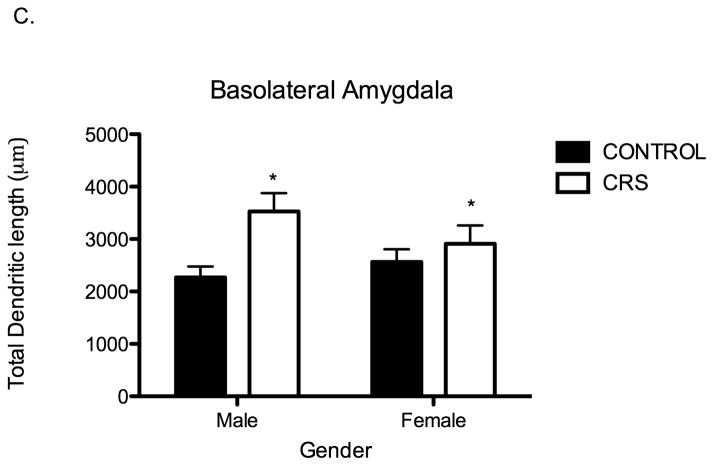
Juvenile chronic restraint stress (CRS) produces apical dendritic remodeling in the CA3 region of the hippocampus (CA3), the prelimbic prefrontal cortex (PFC) and in the basolateral amygdala (BLA) of both males and females. A. CRS significantly shortens apical dendritic length of CA3 pyramidal neurons: CONT males 1653 μms, CONT females 1688 μms, CRS males 1435 μms, CRS females 1332 μms. Two-way ANOVA F1,19=16.11, P=0.0007, n= 6 for all groups except control males n=5. B. CRS significantly shortens apical dendrites in the PFC: CONT males 1322 μms, CONT females 1089 μms, CRS males 961 μms, CRS females 939 μms. Two-way ANOVA F1,18=8.25, P=0.01, CONT groups n= 5, CRS groups n=6. C. CRS significantly increases total dendritic material of pyramidal-like neurons in the BLA. CONT males 2266 μms, CONT females 2558 μms, CRS males 3526 μms, CRS females 2911 μms. Two-way ANOVA F1,16=7.48, P=0.01, n= 5 for all groups.
Prefrontal Cortex
Just as hippocampal pyramidal neurons exhibit stress-induced remodeling, pyramidal neurons in the prefrontal cortex (PFC) of adult rats exhibit stress-induced structural remodeling (Liston et al., 2006; Radley et al., 2006). Accordingly, we examined pyramidal neurons in the prelimbic and infralimbic regions of the PFC of chronically stressed adolescent rats. Within the prelimbic region of the PFC, in comparison to controls, adolescent CRS rats had significantly shorter apical dendrites (Two-way ANOVA F1,18=8.25, P=0.01; Figure 5B) and fewer apical dendritic branch points (Two-way ANOVA F1,18=6.55, P=0.02). Further, there was a significant interaction between stress and sex in regard to the number of apical dendritic branch points (two-way ANOVA F1,18=4.48, P=0.05). Specifically, at baseline, females had significantly fewer branch points than males and did not exhibit a further reduction in branch points following chronic stress exposure. Within the infralimbic region of the PFC, there were no significant differences between control and CRS rats in apical dendritic length or branch points. Finally, within both the prelimbic and infralimbic regions of the PFC, there were no significant differences between control and CRS rats in basal dendritic length or branch points.
Amygdala
Pyramidal-like neurons in the basolateral nucleus of the amygdala (BLA) exhibit stress-induced structural remodeling that is distinct from the remodeling that occurs in the hippocampus and PFC. Pyramidal-like neurons in the BLA of adult rats subjected to chronic stress exhibit stress-induced hypertrophy; there is an increase in total dendritic material following chronic stress exposure (Vyas et al., 2002; Vyas et al., 2004). We, therefore, examined whether pyramidal-like neurons of adolescent rats subjected to chronic stress undergo similar stress-induced hypertrophy. We found that in comparison to controls, adolescents subjected to CRS exhibit increased total dendritic material in the BLA (Two-way ANOVA F1,16=7.48, P=0.01; Figure 5C). There was not a significant effect of stress or stress on branch points (stress: Two-way ANOVA F1,18=1.71, P=0.2, sex: Two-way ANOVA F1,18=1.76, P=0.2). As we found in the hippocampus and PFC, sex did not have a significant effect on stress-induced structural remodeling.
Finally, we investigated whether there was a correlation of structural remodeling between these stress-sensitive brain regions. We found a significant positive correlation between apical dendritic length of pyramidal neurons in the hippocampus and PFC (R2=0.29, P=0.03, N=16) and a significant negative correlation between hippocampal pyramidal neurons and pyramidal like neurons in the BLA (R2=0.34, P=0.02). The dendritic length of pyramidal cells in the PFC did not correlate with pyramidal-like cells in the BLA.
Discussion
In comparison to our knowledge of the effects of chronic stress in adults, there is a paucity of information regarding the sequelae of chronic stress during the juvenile and adolescent period. Our goal, therefore, was to investigate the immediate physiologic, behavioral and neuroanatomical effects of chronic stress during this period. We report that chronic stress in adolescent rats impairs growth, alters HPA reactivity, produces depressive-like behavior and leads to remodeling of pyramidal neurons in the hippocampus, prefrontal cortex and amygdala in both males and females. Our results suggest significant immediate physiologic effects and morbidities related to juvenile chronic stress, thereby warranting continued investigation of the effects of stress during this developmental period.
Juvenile stress alters behavior in a distinct fashion than occurs during adulthood
We found that juveniles exposed to chronic restraint stress (CRS) exhibit anhedonia and increased locomotion in response to novelty and acute stress exposure. Anhedonia is one of the most important criteria for diagnosis of major depressive disorder in both adult and pediatric populations (American Psychiatric Association., 2000). Anhedonia has been documented in chronically stressed adult rats, while reports on chronically stressed juveniles have been less clear. Specifically, anhedonia has not previously been reported in chronically stressed juveniles males (Pohl et al., 2007; Toth et al., 2008), it has only been reported in chronically stressed juvenile females using a 10% sucrose solution (Pohl et al., 2007). We report anhedonia in both males and females using a 2.0% sucrose solution. Our data may differ from prior reports based on the concentration of sucrose used, we used 2.0% compared to 0.2% used by Toth et al (2008), and 7% and 10% solutions used by Pohl et al (2007). In addition, our results may differ based on differences in stressors employed. We used chronic restraint stress: 21 days of restraint for 6 hours a day. This compares to Toth et al, using chronic mild stress for 28 days and Pohl et al, using severe sporadic stress over 28 days.
In addition to anhedonia, we report that chronic stress during the juvenile period alters responses to novelty and acute stress. In response to exposure to a novel environment (the elevated plus maze), we report significantly increased locomotion in both male and female juveniles exposed to chronic stress. There was a significant effect of sex, with females exhibiting greater locomotion. The same finding has been reported following chronic social stress; stressed juvenile females exhibit significantly greater locomotion than stressed males (McCormick et al., 2008).
Aside from increasing locomotion, CRS did not affect anxiety-like behavior as measured by open arm entry in the EPM. The ability of stress to elicit anxiety-like behavior in the juvenile rat seems to be influenced by the type of stress and sex of the subject. Specifically, similar to our finding of no difference in open arm entry following CRS, others have reported no difference in open arm entry in juvenile males exposed to social isolation(Leussis and Andersen, 2008), social instability (McCormick et al., 2008), chronic mild stress (Pohl et al., 2007) and unpredictable stress (Maslova et al., 2002). In contrast, males exposed to severe sporadic stress show greater open arm entry (Pohl et al., 2007). Overall, in juvenile males, chronic stress seems to have little influence on EPM open arm entry. The effect of stress on open arm entry in juvenile females is inconsistent; stressed females exhibit open arm entry that is increased, decreased or unchanged. Specifically, severe sporadic stress (Pohl et al., 2007) and social isolation combined with social instability (McCormick et al., 2008) increase open arm entry, while social isolation alone decreases open arm entry (Leussis and Andersen, 2008) and chronic mild stress has no effect on open arm entry (Pohl et al., 2007). What accounts for the differences in behavior across these studies is unclear. Rats were all stressed and tested in the early to mid-adolescent period so that maturity is likely not the factor. Further, these inconsistencies were also present within the same strain of rats. Perhaps the duration and severity of the stressors contributed to differences in EPM behavior of female rats.
In regard to acute stress exposure, forced swim, we report that chronic juvenile stress differentially alters behavioral responses in males and females. Both male and female juveniles exposed to chronic restraint stress, exhibit decreased mobility time. In contrast, the effect of chronic restraint stress on latency to immobility is sex specific during the juvenile period; it is prolonged only in stressed males. The latency to immobility in stressed females is indistinguishable from that of controls. As we reported that juvenile chronic stress increased locomotor activity in the EPM, so it seems that decreased immobility during forced swim also represents stress-enhanced locomotion.
Most studies that have looked at behavior of chronically stressed juveniles during forced swim have found no differences from controls (Maslova et al.; Mathews et al., 2008; Toth et al., 2008); however, in these studies only a single exposure to forced swim was performed. In contrast, we performed the classical Porsolt two-day exposure paradigm. We identified one study that used the same two day forced swim paradigm to assess juveniles exposed to social isolation from P30-P35. In this study, stressed males exhibited greater immobility duration and decreased latency to immobility(Leussis and Andersen, 2008). Despite using identical forced swim paradigms our results were in direct contrast with this groups. The differences in duration of stress may account for this disparity; 5 days of social isolation versus 21 days of chronic restraint. Consider that adult studies examining the effect of chronic restraint stress on forced swim behavior demonstrate alterations in immobility time that are dependent on the chronicity of restraint. Specifically, seven sessions of 2h immobilization increases immobility duration (Cancela et al., 1991), whereas longer periods of restraint (2.5h for 11 days (Platt and Stone, 1982) or 4.5 h for 14 days (Swiergiel et al., 2007)) decreases immobility duration. Interestingly, in these studies, the effect of shorter periods of restraint seem to be influenced by opiates, whereas the effects of longer periods of restraint seem influenced by corticotrophin releasing factor and β-adrenergic receptors.
Juvenile stress leads to non-sex dependent pyramidal cell remodeling
Finally, we examined pyramidal cell anatomy in the hippocampus, prefrontal cortex and amygdala of chronically stressed juveniles. We examined these brain regions because they are all stress sensitive (Eiland and McEwen, 2010; Liston et al., 2006; McLaughlin et al., 2007; Radley et al., 2006; Vyas et al., 2002), play a role in modulating the stress response (Conrad, 2006; Joels and de Kloet, 1994; McEwen, 2008), have been implicated in the pathophysiology of depression (Hercher et al., 2009; Nestler et al., 2002; Price and Drevets, 2009) and are interconnected. In regard to chronic juvenile stress, we report that both males and females exhibit pyramidal cell atrophy in the CA3 region of the hippocampus and the prelimbic region of the PFC. Similarly, we found that both male and female chronically stressed juveniles exhibit increased dendritic material in the basolateral amygdala. The mechanism by which stress exerts these effects is not fully understood; however, in adults, corticosterone, glutamate, serotonin, and neurotrophins contribute to remodeling of dendrites in these brain regions (Joels et al., 2003; McEwen, 1999; McEwen and Magarinos, 2001).
Of particular interest, there was no difference between juvenile males and females in the effects of stress on dendritic remodeling. In adults, substantial sex-based differences in this process have been documented. Specifically, adult females do not exhibit retraction of apical dendrites in CA3 neurons in response to chronic stress (McEwen and Milner, 2007), and within the prefrontal cortex, females have actually been shown to exhibit dendritic expansion in amygdala-projecting neurons in response to chronic stress (Shansky et al., 2010). These sex differences are likely attributable to sex hormones as the presence or absence of estrogen can modulate the effects of stress on dendritic remodeling (McLaughlin et al., 2009; Shansky et al., 2010). During the prepubescent period, sex differences in remodeling may not exist secondary to an immature hypothalamic-pituitary-gonadal axis that produces relatively low estrogen levels (Apter, 1997). Perhaps the lack of significant sex-based differences we report in stressed male and female juvenile rats parallels clinical data whereby there are not significant sex-based differences in prevalence of depression in children until after the onset of puberty, when estrogen levels rise and depression becomes more prevalent in females (Angold et al., 1999; Bhatia and Bhatia, 2007; Emslie et al., 2005; Rushton et al., 2002).
Conclusions
Overall our research has demonstrated significant morbidity in behavioral, physiologic and neuronal domains related to chronic stress exposure during the juvenile period. The diminished body weight coupled with significant remodeling of neurons in the hippocampus, prefrontal cortex and amygdala suggest significant alterations in growth and stress hormones, excitatory amino acids and neurotrophins during a critical period of development. There is significant evidence demonstrating that early life stress sets the stage for maladaptive changes later in life, but we have demonstrated that chronic stress during the juvenile period produces contemporaneous morbidities that are as significant as those documented in chronically stressed adults. Together these findings support the need for further investigation of the immediate effects of chronic juvenile stress, as elucidating how stress differentially impacts the developing brain, may lead to more efficacious therapies in this population and substantially decrease lifetime morbidity.
Acknowledgments
We thank Dr. Susan Vannucci for her input regarding study design and implementation.
Role of Funding Sources
Funding for this study was provided by a Weill Cornell Medical College Friedman Clinical Scholarship in Newborn Medicine and by a National Institute of Mental Health (NIMH) Diversity Supplement 3R01MH041256-22S1. Neither Weill Cornell Medical College nor the NIMH had a further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Footnotes
Contributions
Author Lisa Eiland designed the study, participated in all experimental procedures, including behavioral studies, tissue collection and processing, performed dendritic cell tracing, performed statistical analysis and wrote this manuscript. Author Johnny Ramroop assisted in all behavioral experiments and tissue processing. Author Matthew Hill traced amygdala neurons and edited manuscript. Author Jasmine Manley, assisted in behavioral studies and maternal separation procedure. Author Bruce McEwen provided input for study design, analysis of data and edited manuscript. All authors have approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- American Psychiatric Association. Diagnostic criteria from DSM-IV-TR. xii. Washington, D.C: American Psychiatric Association; 2000. p. 370. [Google Scholar]
- Angold A, Costello EJ, Erkanli A, Worthman CM. Pubertal changes in hormone levels and depression in girls. Psychol Med. 1999;29(5):1043–53. doi: 10.1017/s0033291799008946. [DOI] [PubMed] [Google Scholar]
- Apter D. Development of the hypothalamic-pituitary-ovarian axis. Ann N Y Acad Sci. 1997;816:9–21. doi: 10.1111/j.1749-6632.1997.tb52125.x. [DOI] [PubMed] [Google Scholar]
- Barha CK, Brummelte S, Lieblich SE, Galea LA. Chronic restraint stress in adolescence differentially influences hypothalamic-pituitary-adrenal axis function and adult hippocampal neurogenesis in male and female rats. Hippocampus. 2010 doi: 10.1002/hipo.20829. [DOI] [PubMed] [Google Scholar]
- Bhatia SK, Bhatia SC. Childhood and adolescent depression. Am Fam Physician. 2007;75(1):73–80. [PubMed] [Google Scholar]
- Cancela LM, Rossi S, Molina VA. Effect of different restraint schedules on the immobility in the forced swim test: modulation by an opiate mechanism. Brain Res Bull. 1991;26(5):671–5. doi: 10.1016/0361-9230(91)90159-h. [DOI] [PubMed] [Google Scholar]
- Conrad CD. What is the functional significance of chronic stress-induced CA3 dendritic retraction within the hippocampus? Behav Cogn Neurosci Rev. 2006;5(1):41–60. doi: 10.1177/1534582306289043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, McEwen BS. Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus. 2010 doi: 10.1002/hipo.20862. [DOI] [PubMed] [Google Scholar]
- Emslie GJ, Ryan ND, Wagner KD. Major depressive disorder in children and adolescents: clinical trial design and antidepressant efficacy. J Clin Psychiatry. 2005;66(Suppl 7):14–20. [PubMed] [Google Scholar]
- Finkelhor D, Ormrod R, Turner H, Hamby SL. The victimization of children and youth: a comprehensive, national survey. Child Maltreat. 2005;10(1):5–25. doi: 10.1177/1077559504271287. [DOI] [PubMed] [Google Scholar]
- Hercher C, Turecki G, Mechawar N. Through the looking glass: examining neuroanatomical evidence for cellular alterations in major depression. J Psychiatr Res. 2009;43(11):947–61. doi: 10.1016/j.jpsychires.2009.01.006. [DOI] [PubMed] [Google Scholar]
- Isgor C, Kabbaj M, Akil H, Watson SJ. Delayed effects of chronic variable stress during peripubertal-juvenile period on hippocampal morphology and on cognitive and stress axis functions in rats. Hippocampus. 2004;14(5):636–48. doi: 10.1002/hipo.10207. [DOI] [PubMed] [Google Scholar]
- Joels M, de Kloet ER. Mineralocorticoid and glucocorticoid receptors in the brain. Implications for ion permeability and transmitter systems. Prog Neurobiol. 1994;43(1):1–36. doi: 10.1016/0301-0082(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Joels M, Verkuyl JM, Van Riel E. Hippocampal and hypothalamic function after chronic stress. Ann N Y Acad Sci. 2003;1007:367–78. doi: 10.1196/annals.1286.036. [DOI] [PubMed] [Google Scholar]
- Kleen JK, Sitomer MT, Killeen PR, Conrad CD. Chronic stress impairs spatial memory and motivation for reward without disrupting motor ability and motivation to explore. Behav Neurosci. 2006;120(4):842–51. doi: 10.1037/0735-7044.120.4.842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leussis MP, Andersen SL. Is adolescence a sensitive period for depression? Behavioral and neuroanatomical findings from a social stress model. Synapse. 2008;62(1):22–30. doi: 10.1002/syn.20462. [DOI] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26(30):7870–4. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maslova LN, Bulygina VV, Amstislavskaya TG. Prolonged social isolation and social instability in adolescence in rats: immediate and long-term physiological and behavioral effects. Neurosci Behav Physiol. 40(9):955–63. [Google Scholar]
- Maslova LN, Bulygina VV, Markel AL. Chronic stress during prepubertal development: immediate and long-lasting effects on arterial blood pressure and anxiety-related behavior. Psychoneuroendocrinology. 2002;27(5):549–61. doi: 10.1016/s0306-4530(01)00092-0. [DOI] [PubMed] [Google Scholar]
- Mathews IZ, Wilton A, Styles A, McCormick CM. Increased depressive behaviour in females and heightened corticosterone release in males to swim stress after adolescent social stress in rats. Behav Brain Res. 2008;190(1):33–40. doi: 10.1016/j.bbr.2008.02.004. [DOI] [PubMed] [Google Scholar]
- McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187(2):228–38. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- McDonald AJ. Neurons of the lateral and basolateral amygdaloid nuclei: a Golgi study in the rat. J Comp Neurol. 1982;212(3):293–312. doi: 10.1002/cne.902120307. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Stress and hippocampal plasticity. Annu Rev Neurosci. 1999;22:105–22. doi: 10.1146/annurev.neuro.22.1.105. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Central effects of stress hormones in health and disease: Understanding the protective and damaging effects of stress and stress mediators. Eur J Pharmacol. 2008;583(2–3):174–85. doi: 10.1016/j.ejphar.2007.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Magarinos AM. Stress and hippocampal plasticity: implications for the pathophysiology of affective disorders. Hum Psychopharmacol. 2001;16(S1):S7–S19. doi: 10.1002/hup.266. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Milner TA. Hippocampal formation: shedding light on the influence of sex and stress on the brain. Brain Res Rev. 2007;55(2):343–55. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Conrad CD. Chronic stress- and sex-specific neuromorphological and functional changes in limbic structures. Mol Neurobiol. 2009;40(2):166–82. doi: 10.1007/s12035-009-8079-7. [DOI] [PubMed] [Google Scholar]
- McLaughlin KJ, Baran SE, Wright RL, Conrad CD. Chronic stress enhances spatial memory in ovariectomized female rats despite CA3 dendritic retraction: possible involvement of CA1 neurons. Neuroscience. 2005;135(4):1045–54. doi: 10.1016/j.neuroscience.2005.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin KJ, Gomez JL, Baran SE, Conrad CD. The effects of chronic stress on hippocampal morphology and function: an evaluation of chronic restraint paradigms. Brain Res. 2007;1161:56–64. doi: 10.1016/j.brainres.2007.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler EJ, Barrot M, DiLeone RJ, Eisch AJ, Gold SJ, Monteggia LM. Neurobiology of depression. Neuron. 2002;34(1):13–25. doi: 10.1016/s0896-6273(02)00653-0. [DOI] [PubMed] [Google Scholar]
- Pilipovic I, Vidic-Dankovic B, Perisic M, Radojevic K, Colic M, Todorovic V, Leposavic G. Sexual dimorphism in the catecholamine-containing thymus microenvironment: a role for gonadal hormones. J Neuroimmunol. 2008;195(1–2):7–20. doi: 10.1016/j.jneuroim.2007.12.006. [DOI] [PubMed] [Google Scholar]
- Pitychoutis PM, Papadopoulou-Daifoti Z. Of depression and immunity: does sex matter? Int J Neuropsychopharmacol. 2010;13(5):675–89. doi: 10.1017/S1461145710000465. [DOI] [PubMed] [Google Scholar]
- Platt JE, Stone EA. Chronic restraint stress elicits a positive antidepressant response on the forced swim test. Eur J Pharmacol. 1982;82(3–4):179–81. doi: 10.1016/0014-2999(82)90508-8. [DOI] [PubMed] [Google Scholar]
- Pohl J, Olmstead MC, Wynne-Edwards KE, Harkness K, Menard JL. Repeated exposure to stress across the childhood-adolescent period alters rats’ anxiety- and depression-like behaviors in adulthood: The importance of stressor type and gender. Behav Neurosci. 2007;121(3):462–74. doi: 10.1037/0735-7044.121.3.462. [DOI] [PubMed] [Google Scholar]
- Price JL, Drevets WC. Neurocircuitry of mood disorders. Neuropsychopharmacology. 2009;35(1):192–216. doi: 10.1038/npp.2009.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16(3):313–20. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Rushton JL, Forcier M, Schectman RM. Epidemiology of depressive symptoms in the National Longitudinal Study of Adolescent Health. J Am Acad Child Adolesc Psychiatry. 2002;41(2):199–205. doi: 10.1097/00004583-200202000-00014. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex. 2010;20(11):2560–7. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swiergiel AH, Zhou Y, Dunn AJ. Effects of chronic footshock, restraint and corticotropin-releasing factor on freezing, ultrasonic vocalization and forced swim behavior in rats. Behav Brain Res. 2007;183(2):178–87. doi: 10.1016/j.bbr.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Toth E, Gersner R, Wilf-Yarkoni A, Raizel H, Dar DE, Richter-Levin G, Levit O, Zangen A. Age-dependent effects of chronic stress on brain plasticity and depressive behavior. J Neurochem. 2008;107(2):522–32. doi: 10.1111/j.1471-4159.2008.05642.x. [DOI] [PubMed] [Google Scholar]
- Vyas A, Jadhav S, Chattarji S. Prolonged behavioral stress enhances synaptic connectivity in the basolateral amygdala. Neuroscience. 2006;143(2):387–93. doi: 10.1016/j.neuroscience.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22(15):6810–8. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vyas A, Pillai AG, Chattarji S. Recovery after chronic stress fails to reverse amygdaloid neuronal hypertrophy and enhanced anxiety-like behavior. Neuroscience. 2004;128(4):667–73. doi: 10.1016/j.neuroscience.2004.07.013. [DOI] [PubMed] [Google Scholar]