Abstract
Cryptopatches (CPs) and isolated lymphoid follicles (ILFs) are organized intestinal lymphoid tissues that develop postnatally in mice and include stromal cells expressing the receptor activator of nuclear factor kappa-B ligand (RANKL). We investigated how stromal RANKL influences the development and differentiation of CPs and ILFs by analyzing the development of these lymphoid structures in knockout mice lacking RANKL. We found that RANKL−/− mice had a fourfold reduction in the overall density of CPs in the small intestine compared to control mice, with the largest decrease in the proximal small intestine. No B cells were present in CPs from the small intestine of RANKL−/− mice and ILF formation was completely blocked. In sharp contrast, colonic ILFs containing B cells were present in RANKL−/− mice. Stromal cells within CPs in the small intestine of RANKL−/− mice did not express CXCL13 (originally called B lymphocyte chemoattractant) and often lacked other normally expressed stromal cell antigens, whereas colonic lymphoid aggregates in RANKL−/− mice retained stromal CXCL13 expression. The CXCL13-dependent maturation of precursor CPs into ILFs is differentially regulated in the small intestine and colon, with an absolute requirement for RANKL only in the small intestine.
Lymphoid organogenesis is dependent on a series of complex interactions involving multiple cell adhesion molecules, chemokines, cytokines, and their receptors.1 The development of each type of organized lymphoid tissue is characterized by a differential degree of reliance on individual mediators. For example, receptor activator of NF-κB ligand (RANKL) is a tumor necrosis factor (TNF) superfamily cytokine that is essential for the development of some types of lymphoid tissue (eg, lymph nodes), but dispensable for other lymphoid structures such as Peyer's patches (PPs) and thymus.2,3 In lymph node development, RANKL has an obligate role in the establishment of the positive feedback loops involving lymphotoxin (LT) α1β2-producing lymphoid tissue inducer (LTi) cells, and LTβR-expressing lymphoid tissue organizer cells.4,5
Besides PPs, the gut-associated lymphoid tissue of mice also includes two additional types of smaller organized lymphoid tissues, cryptopatches (CPs) and isolated lymphoid follicles (ILFs).6 CPs, the smaller of these lymphoid tissues, are found in the deep lamina propria adjacent to the crypts where they develop postnatally starting on day 14. Under normal homeostatic conditions, there are approximately 1500 CPs in the small intestine and 150 in the colon.6–8 An ILF is a single B-cell follicle that develops a germinal center on maturation and can serve as an inductive site for IgA responses.9,10 A single ILF fills up an entire villus and is covered by a follicle-associated epithelium (FAE) containing M cells, that is similar to the FAE of PPs. A normal adult mouse has approximately 100 to 200 ILFs in the small intestine and 50 in the colon.9 Recently, it has been suggested that CPs and ILFs are not distinct types of intestinal lymphoid tissue, but rather two extremes on a continuum of solitary intestinal lymphoid tissue (SILT).11 Current evidence suggests that the number of SILT structures in the intestine is normally stable after postnatal development is completed.8 Instead, changes to the size and composition of the SILT occur in response to changes in the status of the commensal flora. Although CPs are present in germ-free mice, the development of ILFs in the small intestine from these precursor CPs requires signals originating with the commensal enteric flora.7,12–14
We have previously shown that RANKL is expressed on stromal cells found throughout CPs, but is preferentially expressed by stromal cells located in the subepithelial dome of ILFs and PPs.15 We also showed RANKL appears on stromal cells later in ontogeny than other stromal cell antigens including the follicular dendritic cell marker FDC-M1, vascular cell adhesion molecule 1 (VCAM-1), and CD157, and can still be induced when LTα1β2 signaling through the LTβR is blocked. In this study, we used RANKL−/− mice to further investigate the role of RANKL in the development of CPs and ILFs, and found that in the absence of RANKL small intestinal, but not colonic, CPs fail to express CXCL13, recruit B cells, or develop into ILFs.
Materials and Methods
Mice
RANKL−/− mice on a C57BL/6 background were obtained from a breeding colony maintained in a conventional, specific pathogen–free mouse facility at Emory University. This colony was established with mice provided by Dr. Yongwon Choi at the University of Pennsylvania (Philadelphia, PA). Mice heterozygous for the RANKL null mutation were also backcrossed to BALB/cByJ mice (The Jackson Laboratory, Bar Harbor, ME) for at least four generations to allow intercrossing of male C57BL/6 RANKL+/− mice and female BALB/c RANKL+/− mice, and production of RANKL−/− mice and littermate controls on a background roughly equivalent to (C57BL/6 × BALB/c)F1 mice. A higher fraction of RANKL−/− mice offspring on the F1 background survived into adulthood. All experiments using RANKL−/− mice were done with mice on a C57BL/6 background and/or mice on a (C57BL/6 × BALB/c)F1 background, as indicated in the figure legends. Since equivalent results were obtained with RANKL−/− mice on both backgrounds, the quantitative data were pooled for the figures. All control mice used were littermates to the RANKL−/− mice, and these controls included both wild-type mice (RANKL+/+) and heterozygous mice (RANKL+/−). Genotyping of mice for the RANKL null mutation was done using a three PCR primer system as previously described.16 The mice used were at least 8 weeks old. All animal studies were reviewed and approved by the Emory University Institutional Animal Care and Use Committee.
Antibodies
The monoclonal antibodies used for immunofluorescence detection of mouse cells on frozen sections included PE- and FITC-anti-Thy-1 (53-2.1; eBioscience, San Diego, CA), PE-anti-CD157 (BP-3; eBioscience), biotin-anti-VCAM-1 (429; BD Biosciences, San Diego, CA), PE-anti-CD11c (HL3; eBioscience), allophycocyanin- and FITC-anti-B220 (RA3-6B2; eBioscience), unconjugated FDC-M1 (BD Biosciences), and unconjugated anti-glycoprotein 2 (GP2) (2F11-C3; MBL International, Woburn, MA). Biotinylated antibodies were detected using streptavidin conjugated to Alexa488 or Alexa647 (Invitrogen, Carlsbad, CA). Unconjugated anti-GP2 was detected with Alexa546-conjugated goat anti-rat IgG (Invitrogen). Unconjugated FDC-M1 was detected with biotinylated goat anti-rat IgG (Invitrogen) followed by horseradish peroxidase-conjugated streptavidin and tyramide signal amplification (PerkinElmer, Waltham, MA). CXCL13 was detected with biotinylated polyclonal goat anti-mouse CXCL13 (R&D Systems, Minneapolis, MN) followed by horseradish peroxidase-conjugated streptavidin and tyramide signal amplification.
Immunofluorescence Staining of Frozen Sections
The small intestine and colon were excised, placed in cold PBS, and then opened longitudinally. For horizontal sections of the small intestine or colon, three small sheets of tissue (approximately 15 × 20 mm) were stacked and covered with TissueTek optical cutting temperature freezing medium (VWR Scientific, Radnor, PA). Swiss rolls of small intestine or colon were prepared for vertical sections. PPs were excised from surrounding tissue and blocked separately. For anti-GP2 staining, colon tissue was fixed in buffered formalin for 10 minutes before blocking in optical cutting temperature compound. The tissue blocks were quickly frozen in cold 2-methylbutane on dry ice. Frozen sections of 6-μm thickness were cut with a cryostat, air dried overnight, and fixed for 10 minutes in acetone at −20°C. Endogenous peroxidase activity was quenched with 0.3% H2O2 in PBS for 30 minutes at 37°C. Sections were rinsed with PBS and blocked with TNB buffer (PerkinElmer). To obtain optimal staining results when staining intestinal tissues for the soluble chemokine CXCL13, the tissue was fixed in situ by perfusion of mice with 3 mL of PBS, followed by 5 mL of 4% paraformaldehyde and then 3 mL of 5% sucrose as previously described.17
Quantitative Analysis of CP and ILF Development
CPs and ILFs in mice were quantitated by counting the number of intestinal lymphoid aggregates on H&E- or anti-Thy-1–stained sections and using ImageJ software (http://rsb.info.nih.gov/ij) to calculate their density (expressed as aggregates per square centimeter of crypt lamina propria area) and the area of individual aggregates. In some experiments, the observed lymphoid aggregates were also assigned to one of six classes based on size (Class I: <5000 μm2; Class II: 5000 to 10,000 μm2; Class III: 10,000 to 15,000 μm2; Class IV: 15,000 to 20,000 μm2; Class V: 20,000 to 50,000 μm2; Class VI: >50,000 μm2), as previously described.8,14
In Utero Treatment of Mice with LTβR-Ig
To generate mice without PPs that develop increased numbers of intestinal ILFs postnatally, timed pregnant C57BL/6 RANKL+/− mice, which had been mated to C57BL/6 RANKL−/− mice, were injected i.v. with purified human LTβR-Ig fusion protein as described previously.15 Injections of 100 μg of LTβR-Ig were given on embryonic days 14 and 16. The RANKL+/− and RANKL−/− offspring were sacrificed at 8 weeks of age, and tissue from the small intestine was analyzed. In all of the in utero–treated mice, the absence of PPs was verified by gross examination.
Recombinant Mouse RANKL
A fusion protein of glutathione S-transferase (GST) and mouse RANKL (137–316) was generated using a bacterial expression construct as previously described.18 The GST-RANKL fusion protein was administered to RANKL null mice by initial i.p. and s.c. injections of 100 μg of GST-RANKL followed by s.c. injections of 100 μg per day for the next 4 days. Recombinant GST prepared from empty pGEX-5X-1 vector was used as a control for GST-RANKL.
Statistical Analysis
Differences between the mean values for groups were analyzed by either two-tailed analysis of variance (for multiple groups) or two-tailed Student's t-test as calculated using Prism (GraphPad Software, San Diego, CA). A P value of < 0.01 was considered significant.
Results
The Small Intestine of RANKL−/− Mice Has a Reduced Density of CPs with Decreased Size
CPs can be easily identified in sections from the small intestine by staining for Thy-1–expressing LTi cells. The mean density of small CP-sized aggregates in the pericryptal lamina propria of control mice was 19.5 aggregates per cm2, with no difference in density between the jejunum and ileum. In RANKL−/− mice, the overall density of CPs was significantly reduced (Figure 1, A and B) to 4.8 aggregates per cm2. In addition, the density of CPs in RANKL−/− mice was higher in the distal small intestine compared to the proximal small intestine (Figure 1B). CPs from RANKL−/− mice were also smaller compared to CPs in control mice (Figure 1C). Control small intestinal CPs had a mean area of 21,626 μm2, whereas CPs in RANKL−/− mice averaged just 5410 μm2, or approximately a fourfold decrease.
Figure 1.
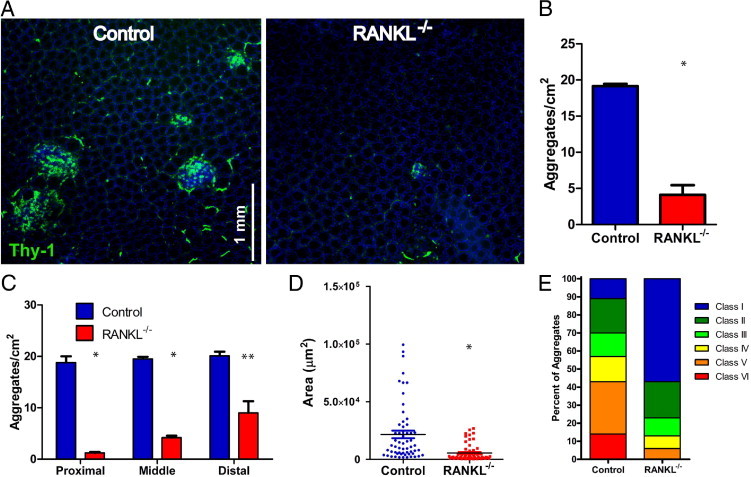
Cryptopatches are less frequent and smaller in the small intestine of RANKL−/− mice. A: Horizontal sections of the pericryptal lamina propria in the distal ileum from RANKL−/− mice and littermate controls were stained with anti–Thy-1 and DAPI. Scale bar = 1 mm. B:RANKL−/− mice had a reduced density of small intestinal lymphoid aggregates. C: The reduction in the mean density of small intestinal lymphoid aggregates in RANKL−/− mice was greatest in the proximal small intestine (proximal = 0 to 10 cm; middle = 10 to 20 cm; distal = beyond 20 cm). D: The average size of small intestinal lymphoid aggregates was reduced in RANKL−/− mice. E: Divided bar graph depicting the size distribution of small intestinal lymphoid aggregates in RANKL−/− and control mice by assigning them to one of six previously described classes based on their size.11 This analysis used three RANKL−/− mice on a C57BL/6 background and two RANKL−/− mice on an F1 equivalent background with a matched number of littermate controls. *P ≤ 0.001, **P ≤ 0.01 (compared to control mice by t-test).
RANKL−/− Mice Do Not Develop B Cell–Containing ILF
Surprisingly, all of the small intestinal lymphoid aggregates observed in the RANKL−/− mice lacked B cells (Figure 2A). Because no small intestinal lymphoid aggregates aside from PPs contained B cells in RANKL−/− mice, all non-PP lymphoid aggregates in RANKL−/− mice can be classified as CPs. To further assess the apparent size difference of CPs between control and RANKL−/− mice, a classification system for SILT based on their cross-sectional area originally developed by Pabst et al14 was applied to the analysis of small intestinal lymphoid aggregates from RANKL−/− and control mice. Both Classes I and II SILTs are typically CPs with few, if any, B220+ cells. Classes III, IV, and V have progressively larger B220+ clusters and can be deemed immature ILFs. Mature ILFs with full-sized B-cell follicles typically fall into the Class VI category. Although the SILT structures in control mice showed the expected degree of diversity and were distributed among all six classes, 80% of the RANKL−/− aggregates fell into Class I and Class II, consistent with most of these structures being small CPs (Figure 1C). The RANKL−/− aggregates falling into Classes III, IV, and V, which are typically the immature ILF classes, lacked B cells and therefore do not qualify as ILFs. Even the largest lymphoid aggregates in the RANKL−/− mice that filled up an entire villus contained no B cells (Figure 2B). The classification of 20% of the lymphoid aggregates from RANKL−/− mice as Class III to V based solely on their area points out the ability of these CPs to expand without attracting B cells.
Figure 2.
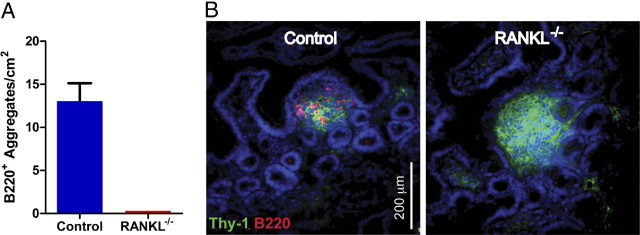
Cryptopatches in the small intestine of RANKL−/− mice do not contain any B cells. A: Density of lymphoid aggregates containing B220+ cells in the small intestine of RANKL−/− and littermate control mice. B: Horizontal sections of the pericryptal lamina propria in the distal ileum were stained with anti-Thy-1, anti-B220, and DAPI. Representative images show that B220+ cells were absent in all of the RANKL−/− lymphoid aggregates. Scale bar = 200 μm. This analysis used three RANKL−/− mice on a C57BL/6 background and two RANKL−/− mice on an F1 equivalent background with a matched number of littermate controls.
In Utero LTβR-Ig Treatment Fails to Restore ILF Development in RANKL−/− Mice
Because ILF development is dependent on the status of the commensal gut flora, most conventionally housed mice on a C57BL/6 background fail to develop large numbers of ILFs. ILF development in wild-type mice can be enhanced by in utero treatment with LTβR-Ig, which blocks formation of PPs leading to a compensatory increase in the number of ILFs.15,19 Pregnant RANKL+/− female mice that had been bred with RANKL−/− male mice were treated with LTβR-Ig. The resultant RANKL−/− and control RANKL+/− offspring lacked PPs, indicating the treatment successfully blocked PP development. Similar to previous studies,19 in utero LTβR-Ig treatment of RANKL+/− mice resulted in a threefold increase in the number of aggregates (Figure 3A). These aggregates included a high percentage of ILFs that had a greater average size than aggregates in untreated mice (Figure 3B). This increase skewed the distribution of lymphoid aggregates, with an increased number of Class IV to VI structures (Figure 3C). However, the RANKL−/− mice showed no increase in the number of small intestinal lymphoid aggregates after in utero LTβR-Ig treatment (Figure 3A). The aggregates present in the LTβR-Ig–treated RANKL−/− mice were not increased in size compared to untreated RANKL−/− mice (Figure 3, B and C) and still lacked any B cells (data not shown).
Figure 3.
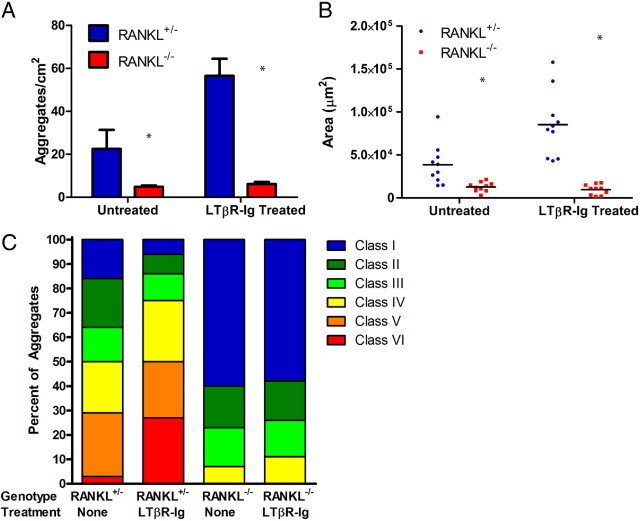
In utero LTβR-Ig treatment does not restore isolated lymphoid follicle development in the small intestine of RANKL−/− mice. In utero LTβR-Ig treatment increased both the density (A) and average size of lymphoid aggregates (B) in RANKL+/− mice, but the density and size of the aggregates were not increased in RANKL−/− mice. C: Divided bar graph depicting the size distribution of small intestinal lymphoid aggregates shows the shift to larger classes after LTβR-Ig treatment in RANKL+/− mice, but not RANKL−/− mice. This analysis used three mice of each genotype on a C57BL/6 background. *P ≤ 0.001 (compared to RANKL+/− mice by analysis of variance).
RANKL−/− Mice Develop Colonic Aggregates That Include B-Cell Follicles
Previous studies on CP and ILF development have largely focused on either the small intestine or the colon, rather than comparing formation of these lymphoid aggregates in both tissues. We investigated whether the defects in CP and ILF development found in the small intestine of RANKL−/− mice were also present in the colon. The colonic lamina propria in RANKL−/− and littermate control mice had a similar density of Thy-1+ clusters (Figure 4, A and B). On average RANKL−/− colonic aggregates were roughly one third of the size of control colonic aggregates, with an average area of 27,954 μm2 compared to 84,160 μm2, respectively. Assigning these aggregates to Classes I to VI using the SILT classification revealed that the smaller classes of aggregates (Class II to IV) were increased in frequency at the expense of the Class VI aggregates. Despite the smaller average size of colonic lymphoid aggregates in RANKL−/− mice, these aggregates had a frequency of B220+ cells similar to colonic lymphoid aggregates in control mice (Figure 4C). The colonic ILFs in RANKL−/− mice included some mature ILFs covered by a FAE, demonstrating that the full spectrum of ILF development can be completed in the colon in the absence of RANKL. However, M cells, as identified by their expression of the M cell–specific marker GP2,20 were present in the FAE overlaying colonic ILFs in RANKL+/− mice, but not in RANKL−/− mice (Figure 4D). We previously showed that differentiation of M cells in the PP FAE requires RANKL.18
Figure 4.
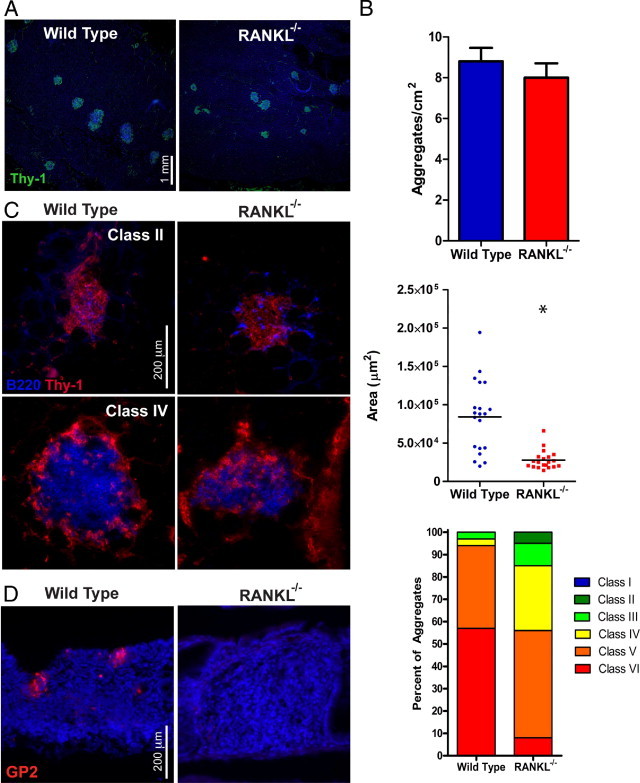
Colonic lymphoid aggregates in RANKL−/− mice include B-cell follicles. A: Horizontal sections of colon were stained with anti–Thy-1 and DAPI, revealing a similar density of lymphoid aggregates in RANKL−/− and littermate control mice. Scale bar = 1 mm. B: The density of colonic lymphoid aggregates in RANKL−/− mice was not significantly different from control mice, but the average size of the lymphoid aggregates was significantly reduced. C: Representative images of Class II and Class IV lymphoid aggregates show that follicles of B220+ cells were present in lymphoid aggregates of both RANKL−/− and control mice. Scale bar = 200 μm. This analysis used three RANKL−/− mice on a C57BL/6 background and two RANKL−/− mice on an F1 equivalent background with a matched number of littermate controls. *P ≤ 0.001 (compared to control mice by t-test). D: Representative images of colonic ILF stained with anti-GP2 and DAPI show M cells are present in RANKL+/−, but not RANKL−/− FAE. Scale bar = 200 μm.
The Density and Distribution of CD11c+ Cells Are Not Perturbed in RANKL−/− CP
CD11c+ dendritic cells account for 20% to 30% of the cells in the small intestinal CP of adult mice.7 These CD11c+ cells are concentrated at the border of CPs, with the Thy-1+ LTi cells predominating centrally.7,21 During the postnatal development of CP, clusters of CD11c+ cells associate with CP before the influx of B cells to generate ILF begins.22 We examined the distribution of CD11c+ cells in RANKL−/− CPs to ascertain whether a failure to recruit normal numbers of CD11c+ cells or a perturbation in the distribution of CD11c+ cells might be a contributor to the failure of RANKL−/− CPs to progress to ILF development. CPs in adult RANKL−/− mice had a density and distribution of CD11c+ cells that was unchanged from CPs in control mice (Figure 5).
Figure 5.
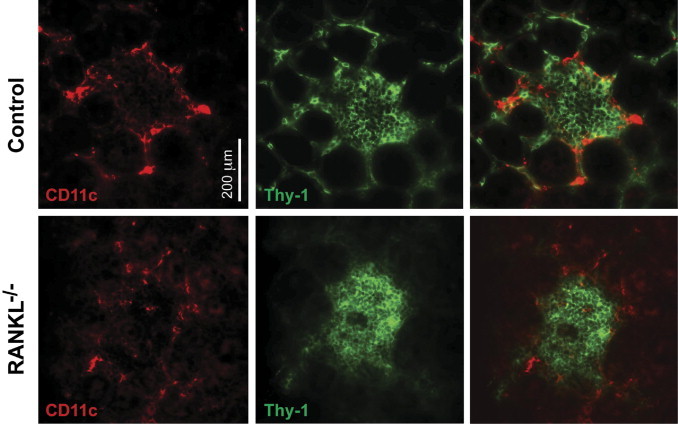
RANKL−/− cryptopatches have a normal density and distribution of CD11c+ cells. Cryptopatches in horizontal sections of small intestine from RANKL−/− and control small intestine were stained for Thy-1 and CD11c. The density and peripheral distribution of CD11c+ cells were similar in RANKL−/− cryptopatches and control cryptopatches. Scale bar = 200 μm. The representative images shown are from a RANKL−/− mouse on an F1 equivalent background and a littermate control, but similar results were obtained with two RANKL−/− mice and controls on a C57BL/6 background.
CXCL13 Is Not Expressed within CPs in the Small Intestine of RANKL−/− Mice
CXCL13 is a chemokine expressed by stromal cells in organized lymphoid structures, including CPs and ILFs, that plays a critical role in the recruitment of B cells.23 Intestinal tissue from RANKL−/− mice and controls was stained with antibodies to CXCL13. In control mice, CXCL13 was found on stromal cells in all B-cell follicles in the gut, including both small intestinal and colonic aggregates (Figure 6). In RANKL−/− mice, CXCL13 was not detected in RANKL−/− small intestinal aggregates (Figure 6A), but remained present in the colonic lymphoid aggregates that contained B-cell–containing follicles (Figure 6B).
Figure 6.
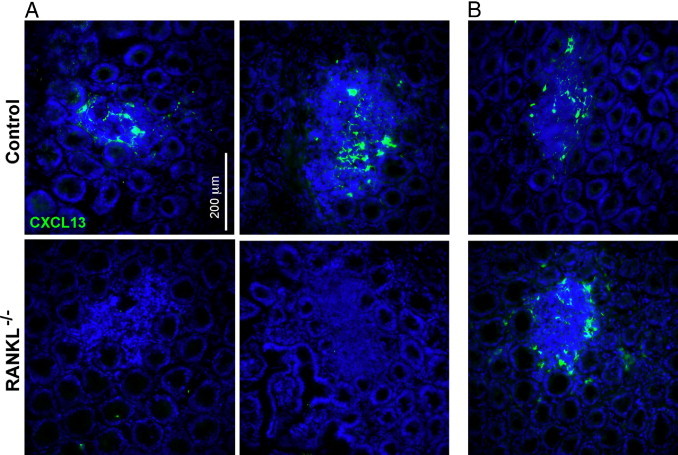
CXCL13-expressing cells are absent from small intestinal lymphoid aggregates in RANKL−/− mice. Horizontal sections of small intestine (A) and colon (B) were stained for CXCL13 expression. Representative images demonstrate that CXCL13-expressing cells were only detected in the colon of RANKL−/− mice. Scale bar = 200 μm. This analysis used three RANKL−/− mice on an F1 equivalent background and three littermate controls.
Stromal Cells in Small Intestinal CPs from RANKL−/− Mice Fail to Express Several LTβR-Dependent Stromal Cell Antigens
We previously reported that all CPs in wild-type mice include stromal cells that express FDC-M1, VCAM-1, and CD157.15 Although all CPs in the RANKL−/− small intestine included FDC-M1+ stromal cells, most of these CPs did not include any stromal cells expressing VCAM-1 or CD157 (Figure 7A). None of the small intestinal CPs from RANKL−/− mice examined had both VCAM-1+ and CD157+ stromal cells, the pattern found in all control CPs. In 84% of the CPs from RANKL−/− mice, no stromal cell expression of VCAM-1 or CD157 was detected. In the other 16% of CPs, only CD157+ stromal cells were found. In contrast with the stromal cell abnormalities observed in small intestinal CPs from RANKL−/− mice, colonic lymphoid aggregates from RANKL−/− mice and control mice contained stromal cells expressing VCAM-1 and CD157 to the same extent as in small intestinal CPs and ILFs from control mice (Figure 7B). To determine whether the altered pattern of stromal cell antigen expression in the small intestinal CPs of RANKL−/− mice could be corrected by providing an exogenous source of RANKL, RANKL−/− mice were treated with 100 μg of GST-RANKL for 5 days. Although this treatment was previously shown to be sufficient to restore M cell differentiation in the PPs of RANKL−/− mice,18 it failed to normalize stromal cell antigen expression in the small intestinal CPs or bring about recruitment of B cells into the CPs (data not shown).
Figure 7.
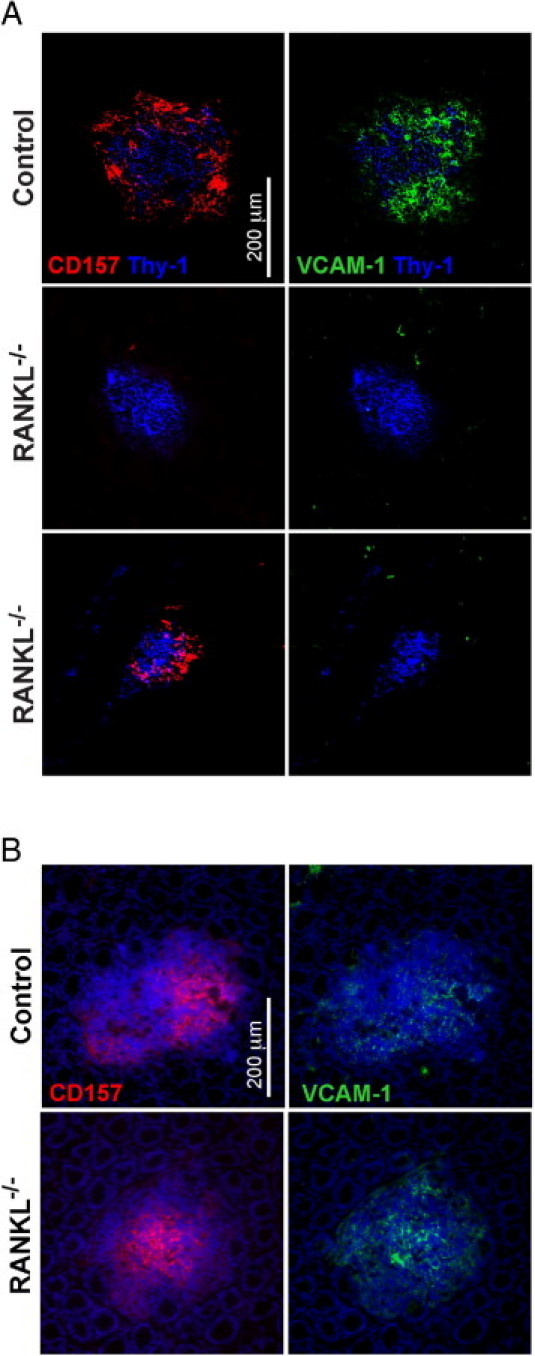
Stromal cells in small intestinal but not colonic cryptopatches from RANKL−/− mice do not express several LTβR-dependent antigens. A: Horizontal sections of small intestine stained for VCAM-1 and CD157 revealed that most RANKL−/− cryptopatches lack expression of both of these antigens found on stromal cells in all cryptopatches in littermate control mice. Scale bar = 200 μm. B: Horizontal sections of colon stained with anti-VCAM-1, anti-CD157, and DAPI showed all aggregates in RANKL−/− mice and control mice included both VCAM-1+ and CD157+ stromal cells. Scale bar = 200 μm. The representative images shown are from two RANKL−/− mice on an F1 equivalent background and two littermate controls.
Discussion
The early stages of lymphoid tissue development involve exchange of a series of cytokine-mediated signals between LTi cells and lymphoid tissue organizer cells. RANKL has a critical role in these interactions as evidenced by the complete absence of lymph nodes in RANKL-deficient mice.2,3 Not all secondary lymphoid tissues fail to develop in the absence of RANKL. Intestinal PPs are present in RANKL−/− mice, although they are smaller and exhibit a profound deficiency in the formation of M cells.18 The contribution of RANKL to the development of the organized lymphoid tissues of the intestine that develop postnatally (ie, CPs and ILFs) has not been previously examined.
The absence of RANKL results in multiple abnormalities in CP and ILF development in the small intestine, but does not compromise development of these structures in the colon. Small intestinal CPs from RANKL−/− mice are both fewer in number and smaller compared to CPs from wild-type mice. Furthermore, the CPs that remain in the small intestine of RANKL−/− mice do not include any B cells, and no ILFs form in the small intestine. Even when in utero LTβR-Ig treatment was used as a stimulus to enhance the extent of postnatal ILF development, no small intestinal ILF development was observed in RANKL−/− mice. In contrast, colonic CPs from RANKL−/− mice were present at a normal density and are capable of maturing into ILFs. The development of colonic ILFs, but not small intestinal ILFs, in RANKL−/− mice demonstrates that ILF development is differentially regulated in the small intestine and colon.
The most striking feature of the RANKL−/− small intestinal CPs was the complete absence of any B-cell clusters within these CPs. Our results indicate that loss of CXCL13 expression in the stroma of the CPs may be a pivotal factor in the inability of these structures to recruit and incorporate B cells, allowing maturation into ILFs. CXCL13 has been identified as an important factor in the initiation of lymphoid tissue development by virtue of its ability to attract LTi cells to sites of lymphoid organogenesis.24–26 In embryonic lymph node development, stimulation of neurons elicits retinoic acid production leading to CXCL13 expression by lymph node organizer cells, thus triggering the initial formation of the lymph node anlagen without a requirement for LT-α.27 CXCL13 also contributes to the formation of B-cell follicles in nasopharynx-associated lymphoid tissue and omental milky spots.28,29 Intestinal CPs and ILFs also depend on CXCL13 for B-cell recruitment and maturation into ILFs. CXCL13−/− mice have normal numbers of CPs, but lack B220+ ILFs, thus showing the dependence on CXCL13 for the accumulation and organization of B cells, but not the initial development of CPs.22 Knocking out the CXCR5 receptor for CXCL13 results in a similar phenotype: CXCR5−/− mice lack mature ILFs, although some aggregates still have a limited number of B cells.8 Increasing the local concentration of CXCL13 through transgenic expression by gut epithelial cells results in an increase in B-cell accumulation and ILF development in the jejunum and ileum.30 Although the overexpression of CXCL13 in these mice also increased the number of RORγt+ LTi cells, there was no increase in the absolute number of intestinal aggregates. The conspicuous absence of CXCL13 in small intestinal aggregates in RANKL−/− mice reveals that expression of CXCL13 by stromal cells in small intestinal CPs is dependent on RANKL. This dependency does not extend to colonic lymphoid aggregates from the RANKL−/− mice, since CXCL13 expression is maintained and ILFs still develop.
The requirement for RANKL to achieve CXCL13 expression in small intestinal CPs may be a consequence of RANKL's demonstrated ability to promote LTα1β2 expression. During the fetal development of lymph nodes, RANKL is not required for the earliest steps in lymph node development. Instead, RANKL participates in a positive feedback loop involving LTα1β2 that is required for lymph node development to continue.31 CP development is thought to closely resemble fetal lymph node development in many respects, and CPs also contain RORγt+ LTi cells.32,33 RANKL is also not required for the initial step in CP development, as RANKL−/− mice still have some CPs, although the reduced number of CPs in RANKL−/− mice reveals RANKL is an important factor in achieving full CP development. Growth of developing CPs relies on the continued cross talk between LTi cells, which express RANK, RANKL and CXCR5, and VCAM-1+ organizer cells that express RANKL and CXCL13 upon LTβR signaling.34,35 Decreased LTα1β2 expression caused by absence of RANKL may lead to loss of CXCL13 expression, which in turn inhibits further CP development and prevents their maturation into ILFs.
Our results also show the small intestinal CPs that develop in RANKL−/− mice fail to express stromal antigens such as VCAM-1 and CD157 that are normally present on the stroma of CPs in wild-type mice.15 In previous studies, we showed that aly/aly mice lacking functional NF-κB-inducing kinase (NIK) or wild-type mice in which LTα1β2 signaling was blocked with LTβR-Ig also had defective stromal antigen expression in CPs, failing to express VCAM-1, CD157, and FDC-M1, but retaining RANKL expression. The CPs from the aly/aly mice also failed to develop into ILFs. In aggregate, these findings point to a requirement in the small intestine for multiple factors, including RANKL and LTα1β2, for normal stromal cell differentiation in CPs and the development of ILFs from these CPs.
A major difference between the small intestine and the colon is the increase in the density of the commensal flora in the colon. Although signals derived from the enteric microflora are not required for CP development, commensal bacteria influence the size of CPs, because germ-free wild-type mice were found to have smaller CPs.14 Sensing of the enteric microflora by nucleotide-binding oligomerization domain protein 1 (NOD1) was identified as a critical signal driving the differentiation of CPs into immature ILFs.36 Although RANKL−/− mice are normally colonized by gut commensals, we previously showed they have less than 2% of the normal number of antigen-sampling M cells compared to wild-type mice.18 This profound M cell deficit in RANKL−/− mice likely results in decreased antigen-sampling of the commensal flora, thereby impairing the NOD1-initiated differentiation of CPs into immature ILFs. Follicular dendritic cells isolated from PP have the capacity to respond directly to environmental stimuli, including retinoic acid and intestinal bacteria, by increasing production of both CXCL13 and BAFF,37 indicating that stromal cells are able to directly sense the presence of the commensal enteric flora. In wild-type mice, the density of CPs is relatively uniform in different portions of the small intestine despite a gradient in the density of the enteric microflora between the proximal and distal end. The increase in the density of CPs in the distal small intestine of RANKL−/− mice compared to the proximal small intestine parallels the increase in the density of the commensal flora. The proximal-to-distal gradient of CP density in the small intestine of RANKL−/− mice and the lack of stromal CXCL13 expression suggest that the density of the commensal flora has the potential to influence the extent of CP development in the small intestine, but this effect is normally overridden by the contribution of other signals. In the colon, the density of the commensal flora may be high enough that direct induction of CXCL13 expression on stromal cells in lymphoid aggregates is sufficient to override the requirement for RANKL evident in the small intestine. The contribution of RANKL to colonic lymphoid aggregate development is limited to influencing the extent of B-cell recruitment and the final size attained by ILFs.
In summary, we have shown that the development of ILFs from precursor CPs in the small intestine is dependent on RANKL for the expression of CXCL13. Surprisingly, this requirement for RANKL for induction of CXCL13 and ILF development did not extend to colonic lymphoid aggregates. The differential dependence on RANKL for development of lymphoid structures in the small intestine and colon suggests that other molecules involved in CP and ILF development may also be differentially used between these tissues. Previous studies have revealed that the status of the commensal enteric flora exerts a far greater influence on the number of ILFs that develop in the small intestine compared to the colon.38 Our findings reveal a new role for RANKL in CP and ILF formation in the small intestine and also provide new evidence that the molecular pathways that contribute to the initiation and maturation of these lymphoid aggregates are not identical in the small intestine and colon.
Footnotes
Supported by grants from the National Institutes of Health (DK64730 to I.R.W., and DK64399 supporting the Imaging Core Facility of the Emory Digestive Diseases Research Development Center) and the Crohn's & Colitis Foundation of America (Senior Research Award to I.R.W.).
References
- 1.Mebius R.E. Organogenesis of lymphoid tissues. Nat Rev Immunol. 2003;3:292–303. doi: 10.1038/nri1054. [DOI] [PubMed] [Google Scholar]
- 2.Kong Y.Y., Yoshida H., Sarosi I., Tan H.L., Timms E., Capparelli C., Morony S., Oliveira-dos-Santos A.J., Van G., Itie A., Khoo W., Wakeham A., Dunstan C.R., Lacey D.L., Mak T.W., Boyle W.J., Penninger J.M. OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature. 1999;397:315–323. doi: 10.1038/16852. [DOI] [PubMed] [Google Scholar]
- 3.Yoshida H., Naito A., Inoue J., Satoh M., Santee-Cooper S.M., Ware C.F., Togawa A., Nishikawa S. Different cytokines induce surface lymphotoxin-αβ on IL-7 receptor-α cells that differentially engender lymph nodes and Peyer's patches. Immunity. 2002;17:823–833. doi: 10.1016/s1074-7613(02)00479-x. [DOI] [PubMed] [Google Scholar]
- 4.Ruddle N.H., Akirav E.M. Secondary lymphoid organs: responding to genetic and environmental cues in ontogeny and the immune response. J Immunol. 2009;183:2205–2212. doi: 10.4049/jimmunol.0804324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roozendaal R., Mebius R.E. Stromal cell-immune cell interactions. Annu Rev Immunol. 2011;29:23–43. doi: 10.1146/annurev-immunol-031210-101357. [DOI] [PubMed] [Google Scholar]
- 6.Taylor R.T., Williams I.R. Lymphoid organogenesis in the intestine. Immunol Res. 2005;33:167–182. doi: 10.1385/IR:33:2:167. [DOI] [PubMed] [Google Scholar]
- 7.Kanamori Y., Ishimaru K., Nanno M., Maki K., Ikuta K., Nariuchi H., Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where clusters of c-kit+ IL-7R+ Thy1+ lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–1459. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velaga S., Herbrand H., Friedrichsen M., Jiong T., Dorsch M., Hoffmann M.W., Forster R., Pabst O. Chemokine receptor CXCR5 supports solitary intestinal lymphoid tissue formation. B cell homing, and induction of intestinal IgA responses, J Immunol. 2009;182:2610–2619. doi: 10.4049/jimmunol.0801141. [DOI] [PubMed] [Google Scholar]
- 9.Hamada H., Hiroi T., Nishiyama Y., Takahashi H., Masunaga Y., Hachimura S., Kaminogawa S., Takahashi-Iwanaga H., Iwanaga T., Kiyono H., Yamamoto H., Ishikawa H. Identification of multiple isolated lymphoid follicles on the antimesenteric wall of the mouse small intestine. J Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- 10.Lorenz R.G., Newberry R.D. Isolated lymphoid follicles can function as sites for induction of mucosal immune responses. Ann N Y Acad Sci. 2004;1029:44–57. doi: 10.1196/annals.1309.006. [DOI] [PubMed] [Google Scholar]
- 11.Pabst O., Herbrand H., Worbs T., Friedrichsen M., Yan S., Hoffmann M.W., Korner H., Bernhardt G., Pabst R., Forster R. Cryptopatches and isolated lymphoid follicles: dynamic lymphoid tissues dispensable for the generation of intraepithelial lymphocytes. Eur J Immunol. 2005;35:98–107. doi: 10.1002/eji.200425432. [DOI] [PubMed] [Google Scholar]
- 12.Fagarasan S., Muramatsu M., Suzuki K., Nagaoka H., Hiai H., Honjo T. Critical roles of activation-induced cytidine deaminase in the homeostasis of gut flora. Science. 2002;298:1424–1427. doi: 10.1126/science.1077336. [DOI] [PubMed] [Google Scholar]
- 13.Lorenz R.G., Chaplin D.D., McDonald K.G., McDonough J.S., Newberry R.D. Isolated lymphoid follicle formation is inducible and dependent upon lymphotoxin-sufficient B lymphocytes, lymphotoxin β receptor, and TNF receptor I function. J Immunol. 2003;170:5475–5482. doi: 10.4049/jimmunol.170.11.5475. [DOI] [PubMed] [Google Scholar]
- 14.Pabst O., Herbrand H., Friedrichsen M., Velaga S., Dorsch M., Berhardt G., Worbs T., Macpherson A.J., Forster R. Adaptation of solitary intestinal lymphoid tissue in response to microbiota and chemokine receptor CCR7 signaling. J Immunol. 2006;177:6824–6832. doi: 10.4049/jimmunol.177.10.6824. [DOI] [PubMed] [Google Scholar]
- 15.Taylor R.T., Patel S.R., Lin E., Butler B.R., Lake J.G., Newberry R.D., Williams I.R. Lymphotoxin-independent expression of TNF-related activation-induced cytokine by stromal cells in cryptopatches, isolated lymphoid follicles, and Peyer's patches. J Immunol. 2007;178:5659–5667. doi: 10.4049/jimmunol.178.9.5659. [DOI] [PubMed] [Google Scholar]
- 16.Kim D., Mebius R.E., MacMicking J.D., Jung S., Cupedo T., Castellanos Y., Rho J., Wong B.R., Josien R., Kim N., Rennert P.D., Choi Y. Regulation of peripheral lymph node genesis by the tumor necrosis factor family member TRANCE. J Exp Med. 2000;192:1467–1478. doi: 10.1084/jem.192.10.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dauner J.G., Chappell C.P., Williams I.R., Jacob J. Perfusion fixation preserves enhanced yellow fluorescent protein and other cellular markers in lymphoid tissues. J Immunol Methods. 2009;340:116–122. doi: 10.1016/j.jim.2008.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Knoop K.A., Kumar N., Butler B.R., Sakthivel S.K., Taylor R.T., Nochi T., Akiba H., Yagita H., Kiyono H., Williams I.R. RANKL is necessary and sufficient to initiate development of antigen-sampling M cells in the intestinal epithelium. J Immunol. 2009;183:5738–5747. doi: 10.4049/jimmunol.0901563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Newberry R.D., McDonough J.S., McDonald K.G., Lorenz R.G. Postgestational lymphotoxin/lymphotoxin β receptor interactions are essential for the presence of intestinal B lymphocytes. J Immunol. 2002;168:4988–4997. doi: 10.4049/jimmunol.168.10.4988. [DOI] [PubMed] [Google Scholar]
- 20.Hase K., Kawano K., Nochi T., Pontes G.S., Fukuda S., Ebisawa M., Kadokura K., Tobe T., Fujimura Y., Kawano S., Yabashi A., Waguri S., Nakato G., Kimura S., Murakami T., Iimura M., Hamura K., Fukuoka S., Lowe A.W., Itoh K., Kiyono H., Ohno H. Uptake through glycoprotein 2 of FimH+ bacteria by M cells initiates mucosal immune response. Nature. 2009;462:226–230. doi: 10.1038/nature08529. [DOI] [PubMed] [Google Scholar]
- 21.Lügering A., Kucharzik T., Soler D., Picarella D., Hudson J.T., 3rd, Williams I.R. Lymphoid precursors in intestinal cryptopatches express CCR6 and undergo dysregulated development in the absence of CCR6. J Immunol. 2003;171:2208–2215. doi: 10.4049/jimmunol.171.5.2208. [DOI] [PubMed] [Google Scholar]
- 22.McDonald K.G., McDonough J.S., Dieckgraefe B.K., Newberry R.D. Dendritic cells produce CXCL13 and participate in the development of murine small intestine lymphoid tissues. Am J Pathol. 2010;176:2367–2377. doi: 10.2353/ajpath.2010.090723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohl L., Henning G., Krautwald S., Lipp M., Hardtke S., Bernhardt G., Pabst O., Forster R. Cooperating mechanisms of CXCR5 and CCR7 in development and organization of secondary lymphoid organs. J Exp Med. 2003;197:1199–1204. doi: 10.1084/jem.20030169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Honda K., Nakano H., Yoshida H., Nishikawa S., Rennert P., Ikuta K., Tamechika M., Yamaguchi K., Fukumoto T., Chiba T., Nishikawa S.I. Molecular basis for hematopoietic/mesenchymal interaction during initiation of Peyer's patch organogenesis. J Exp Med. 2001;193:621–630. doi: 10.1084/jem.193.5.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Finke D., Acha-Orbea H., Mattis A., Lipp M., Kraehenbuhl J. CD4+CD3− cells induce Peyer's patch development: role of α4β1 integrin activation by CXCR5. Immunity. 2002;17:363–373. doi: 10.1016/s1074-7613(02)00395-3. [DOI] [PubMed] [Google Scholar]
- 26.Luther S.A., Ansel K.M., Cyster J.G. Overlapping roles of CXCL13, interleukin 7 receptor α, and CCR7 ligands in lymph node development. J Exp Med. 2003;197:1191–1198. doi: 10.1084/jem.20021294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.van de Pavert S.A., Olivier B.J., Goverse G., Vondenhoff M.F., Greuter M., Beke P., Kusser K., Hopken U.E., Lipp M., Niederreither K., Blomhoff R., Sitnik K., Agace W.W., Randall T.D., de Jonge W.J., Mebius R.E. Chemokine CXCL13 is essential for lymph node initiation and is induced by retinoic acid and neuronal stimulation. Nat Immunol. 2009;10:1193–1199. doi: 10.1038/ni.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rangel-Moreno J., Moyron-Quiroz J., Kusser K., Hartson L., Nakano H., Randall T.D. Role of CXC chemokine ligand 13, CC chemokine ligand (CCL) 19, and CCL21 in the organization and function of nasal-associated lymphoid tissue. J Immunol. 2005;175:4904–4913. doi: 10.4049/jimmunol.175.8.4904. [DOI] [PubMed] [Google Scholar]
- 29.Rangel-Moreno J., Moyron-Quiroz J.E., Carragher D.M., Kusser K., Hartson L., Moquin A., Randall T.D. Omental milky spots develop in the absence of lymphoid tissue-inducer cells and support B and T cell responses to peritoneal antigens. Immunity. 2009;30:731–743. doi: 10.1016/j.immuni.2009.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marchesi F., Martin A.P., Thirunarayanan N., Devany E., Mayer L., Grisotto M.G., Furtado G.C., Lira S.A. CXCL13 expression in the gut promotes accumulation of IL-22-producing lymphoid tissue-inducer cells, and formation of isolated lymphoid follicles. Mucosal Immunol. 2009;2:486–494. doi: 10.1038/mi.2009.113. [DOI] [PubMed] [Google Scholar]
- 31.Vondenhoff M.F., Greuter M., Goverse G., Elewaut D., Dewint P., Ware C.F., Hoorweg K., Kraal G., Mebius R.E. LTβR signaling induces cytokine expression and up-regulates lymphangiogenic factors in lymph node anlagen. J Immunol. 2009;182:5439–5445. doi: 10.4049/jimmunol.0801165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eberl G., Marmon S., Sunshine M.J., Rennert P.D., Choi Y., Littman D.R. An essential function for the nuclear receptor RORγt in the generation of fetal lymphoid tissue inducer cells. Nat Immunol. 2004;5:64–73. doi: 10.1038/ni1022. [DOI] [PubMed] [Google Scholar]
- 33.Eberl G. Inducible lymphoid tissues in the adult gut: recapitulation of a fetal developmental pathway? Nat Rev Immunol. 2005;5:413–420. doi: 10.1038/nri1600. [DOI] [PubMed] [Google Scholar]
- 34.Katakai T., Suto H., Sugai M., Gonda H., Togawa A., Suematsu S., Ebisuno Y., Katagiri K., Kinashi T., Shimizu A. Organizer-like reticular stromal cell layer common to adult secondary lymphoid organs. J Immunol. 2008;181:6189–6200. doi: 10.4049/jimmunol.181.9.6189. [DOI] [PubMed] [Google Scholar]
- 35.Suto H., Katakai T., Sugai M., Kinashi T., Shimizu A. CXCL13 production by an established lymph node stromal cell line via lymphotoxin-β receptor engagement involves the cooperation of multiple signaling pathways. Int Immunol. 2009;21:467–476. doi: 10.1093/intimm/dxp014. [DOI] [PubMed] [Google Scholar]
- 36.Bouskra D., Brezillon C., Berard M., Werts C., Varona R., Boneca I.G., Eberl G. Lymphoid tissue genesis induced by commensals through NOD1 regulates intestinal homeostasis. Nature. 2008;456:507–510. doi: 10.1038/nature07450. [DOI] [PubMed] [Google Scholar]
- 37.Suzuki K., Kawamoto S., Maruya M., Fagarasan S. GALT: organization and dynamics leading to IgA synthesis. Adv Immunol. 2010;107:153–185. doi: 10.1016/B978-0-12-381300-8.00006-X. [DOI] [PubMed] [Google Scholar]
- 38.Kweon M.N., Yamamoto M., Rennert P.D., Park E.J., Lee A.Y., Chang S.Y., Hiroi T., Nanno M., Kiyono H. Prenatal blockage of lymphotoxin β receptor and TNF receptor p55 signaling cascade resulted in the acceleration of tissue genesis for isolated lymphoid follicles in the large intestine. J Immunol. 2005;174:4365–4372. doi: 10.4049/jimmunol.174.7.4365. [DOI] [PubMed] [Google Scholar]


