Abstract
In multiple sclerosis (MS), myelin-specific T cells are normally associated with destruction of myelin and axonal damage. However, in acute MS plaque, remyelination occurs concurrent with T-cell infiltration, which raises the question of whether T cells might stimulate myelin repair. We investigated the effect of myelin-specific T cells on oligodendrocyte formation at sites of axonal damage in the mouse hippocampal dentate gyrus. Infiltrating T cells specific for myelin proteolipid protein stimulated proliferation of chondroitin sulfate NG2–expressing oligodendrocyte precursor cells early after induction via axonal transection, resulting in a 25% increase in the numbers of oligodendrocytes. In contrast, T cells specific for ovalbumin did not stimulate the formation of new oligodendrocytes. In addition, infiltration of myelin-specific T cells enhanced the sprouting response of calretinergic associational/commissural fibers within the dentate gyrus. These results have implications for the perception of MS pathogenesis because they show that infiltrating myelin-specific T cells can stimulate oligodendrogenesis in the adult central nervous system.
T cell infiltration, demyelination, and axonal damage are central pathologic features of multiple sclerosis (MS). Whereas the primary immune attack on oligodendrocytes and myelin is effected by T cells,1,2 remyelination occurs in acute plaques, also in the presence of T cells.3,4 Remyelination depends on chondroitin sulfate NG2–expressing adult oligodendrocyte precursor cells (OPCs).5,6 OPCs retain the capacity to proliferate and differentiate into myelinating oligodendrocytes in response to toxic or inflammatory demyelination7–9 and other forms of central nervous system (CNS) injury such as ischemia,10 spinal cord injury,11,12 axonal lesions,13,14 and inflammation.15 During differentiation, OPCs down-regulate NG2 as cells acquire markers of mature oligodendrocytes such as 2′,3′-cyclic nucleotide 3′-phosphodiesterase (CNP).16
The axonal damage that occurs within and distal to the acute MS lesion can be modeled in the hippocampal dentate gyrus by transection of the perforant pathway (PP), resulting in degeneration of the PP axons and their myelin sheaths in the outer part of the molecular layer.17–19 PP lesions also induce proliferation of OPCs, which results in formation of new oligodendrocytes.14 These newly formed oligodendrocytes are presumed to myelinate the axonal sprouts that extend from other afferent fiber systems in the dentate gyrus20,21 such as the associational/commissural afferents from the calretinergic hilar mossy cells.20,22,23 Indeed, in stratum radiatum of the hippocampal CA3 region, lesion-induced axonal sprouting is associated with formation of more oligodendrocytes and more myelin.24
Because remyelination ultimately fails in MS,25 it is assumed that autoimmune demyelination reduces the capacity for myelin repair.26,27 We investigated the effect of myelin-specific T cells on the formation of oligodendrocytes in the dentate gyrus of mice subjected to PP transection. Via adoptive transfer of T cells specific for myelin proteolipid protein (PLP) before axonal lesioning, infiltration of T cells into the dentate gyrus was significantly enhanced, compared with limited T-cell infiltration in PP-lesioned mice with adoptive transfer of ovalbumin (OVA)–specific T cells or lesioned naïve mice. A significantly higher increase in the number of postproliferative oligodendrocytes was observed in the PP-lesioned TPLP-recipient mice than in PP-lesioned TOVA-recipient and naïve mice. Furthermore, the increased oligodendrogenesis was preceded by increased proliferation of NG2+ OPCs in the dentate gyrus. These changes correlated with an increased clearance of myelin debris and increased sprouting of calretinergic associational/commissural fibers. Our results demonstrate that myelin-specific T cells can stimulate oligodendrogenesis in vivo.
Materials and Methods
Animals
Female SJL mice aged 8 to 10 weeks were obtained from Taconic Europe A/S (Ejby, Denmark) and maintained in a pathogen-free temperature- and humidity-controlled environment with a 12-hour light-dark cycle, and were provided with food and water ad libitum. Experiments were approved by the National Danish Animal Care Committee (J.nr. 192000/561-272 and J.nr. 192000/51-272).
Transfer of T Cells
TPLP and TOVA were generated as previously described.28 Donor mice were immunized via two s.c. injections, at the base of the tail and in the flank, with 50 μL emulsion of Mycobacterium tuberculosis H37 RA (2 mg/mL) (Difco Laboratories, Inc., Detroit, MI) in incomplete Freund's adjuvant solution (Difco Laboratories, Inc.) and PLP139–151 (1 mg/mL) (KJ Ross-Petersen ApS, Klampenborg, Denmark) or ovalbumin (30 mg/mL) (Sigma-Aldrich Corp., St. Louis, MO). Lymph nodes were collected on day 11, and cells were cultured for 4 days in RPMI-1640 medium (Invitrogen Corp., Carlsbad, CA) containing 10% fetal bovine serum (Invitrogen Corp.), 2 mmol/L l-glutamine (Sigma-Aldrich Corp.), 50 μmol/L 2-mercaptoethanol (Bie & Berntsen A/D, Herlev, Denmark), and 5 μg/mL PLP. Proliferation was measured using the Vybrant MTT Cell Proliferation Assay Kit (Invitrogen Corp.). TPLP and TOVA cultures showed equal proliferation rates before cells were collected on a Ficoll-Hypaque gradient (Amersham Pharmacia Biotech, Inc., Piscataway, NJ), counted, and injected i.v. into recipient mice (6 × 106 blasts per mouse or 28% to 30% of the cells injected). TPLP- and TOVA-recipient mice (TPLP and TOVA mice, respectively) were weighed and clinically evaluated daily. TOVA mice demonstrated no symptoms. TPLP mice reached experimental allergic encephalomyelitis (EAE) grade 0 to 2 before termination at 7 days post lesion (11 days post transfer). For studies of cytokine expression of T cells in vitro, TPLP and TOVA cells were generated, cultivated, and harvested as described above, transferred into TRIzol (Invitrogen Corp.), and stored at −80°C. Lymph node cells from naïve mice served as controls.
PP Transection
Anterograde axonal degeneration was induced via stereotactic transection of the PP using a wire knife.14 Mice were PP lesioned at 4 days post transfer, when myelin-specific T cells usually enter the CNS after i.v. injection.29 Mice were euthanized at 2 days post lesion (11 TPLP, 8 TOVA, and 10 naïve) and 7 days post lesion (7 TPLP, 8 TOVA, and 6 naïve). Unlesioned TOVA and TPLP mice (n = 6 and 8, respectively) were euthanized at 11 days post transfer, when TPLP mice demonstrated symptoms of EAE grade 0 to 2. Unmanipulated mice (n = 6) and unlesioned the contralateral dentate gyrus served as controls. To study the cytokine profile using quantitative PCR (qPCR), a similar set of animals was generated (n = 8 to 12 per group). For investigations of cellular proliferation and differentiation, animals received a i.p. bolus injection of BrdU (5′-bromo-2′-deoxyuridine) dissolved in isotonic saline solution (50 mg/kg) at 2 days post lesion. Animals euthanized at day 2 received the injection 1 hour before sacrifice.
Fixation and Tissue Processing
Mice were deeply anesthetized using 0.05 mL pentobarbital (200 mg/mL) and perfused through the left ventricle using 5 mL 0.15 mmol/L Sørensen phosphate buffer (pH 7.4) followed by 20 mL 4% paraformaldehyde in 0.15 mmol/L Sørensen phosphate buffer (pH 7.4). The brains were postfixed in 4% paraformaldehyde for 1½ hours, immersed in 20% sucrose overnight, frozen, and serially cut into 16-μm parallel cryostat sections. For qPCR, animals were perfused using 20 mL 0.15 mmol/L Sørensen phosphate buffer, and the ipsilateral hippocampus was dissected and stored in TRIzol at −80°C. For in situ hybridization, PP-lesioned TPLP, TOVA, and naïve mice (n = 3 or 4 per group) were decapitated, and the brains were frozen, cut as above, and stored at −80°C.
Validation of PP Lesion
The quality of the lesion was controlled using Fluoro-Jade staining.30 Only mice that demonstrated a dense band of green fluorescence in the outer molecular layer, reflecting complete transection of both the medial and lateral PP, were included in the study.30,31
Diaminobenzidine Immunohistochemistry
Neurofilament (NF) was detected by using primary monoclonal rat anti-mouse phosphorylated NF antibody (MAB5448; dilution 1:100; Chemicon International, Inc., Temecula, CA), secondary biotinylated species-specific monoclonal goat anti-rat antibody (RPN 1005; dilution 1:200; Amersham Pharmacia Biotech, Inc.), and streptavidin-horseradish peroxidase (P397; dilution 1:200; Dako A/S, Glostrup, Denmark), as described by Nielsen et al.14 T cells were visualized as described for NF using monoclonal rat anti-human CD3 antibody (MCA1477; dilution 1:200; AbD Serotec, Ltd., Kidlington, Oxfordshire, England). Cross-reactivity for murine CD3 was confirmed via location of CD3+ cells to the periarteriolar sheath in murine spleen sections. OPCs were visualized using polyclonal rabbit raised against chondroitin sulfate proteoglycan NG2 (AB5320; dilution 1:500; Chemicon International, Inc.). Oligodendrocytes and myelin were visualized using monoclonal mouse anti-rat Rip antibody (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA) recognizing 2′, 3′-cyclic nucleotide 3′-phosphodiesterase.32,33 The antibody was biotinylated by Karsten Skjødt, University of Southern Denmark (Odense) and used at a concentration of 0.05 mg/mL. NG2 and CNP stainings were counterstained using toluidine blue to facilitate cell counting. Calretinergic fibers were visualized using polyclonal rabbit anti-calretinin antibody (7699/3H; dilution 1:10.000; Swant, Inc., Marly, Switzerland) diluted in 10% fetal bovine serum containing 1% triton. Rinses were extended to 3 × 2 hours. Primary and secondary antibodies were incubated overnight at 4°C.
Diaminobenzidine/Alkaline Phosphatase Double Immunohistochemistry
After staining for NG2 or CNP, sections were rinsed in 2× standard saline citrate solution [300 mmol/L NaCl and 30 mmol/L and sodium citrate (pH 8.0)] for 2 × 15 minutes at room temperature, incubated in a 49% solution of formamide in 2× standard saline citrate solution for 2 hours at 60°C, rinsed in 2× standard saline citrate solution for 2 × 5 minutes at 60°C, and incubated in 2N HCl in 0.05 mmol/L Tris-buffered saline solution for 30 minutes at 37°C. Sections were rinsed and incubated using monoclonal rat anti-BrdU antibody (AB6326; dilution 1:100; Abcam Ltd., Cambridge, England), which was detected using biotinylated goat anti-rat Ig antibody (RPN 1005; dilution 1:200; Amersham Pharmacia Biotech, Inc.), and alkaline phosphatase–conjugated streptavidin (P396; Dako A/S), and incubated in an alkaline phosphatase developer as previously described.34
Double Immunofluorescence Histochemistry
Calretinergic fibers were detected using Alexa 488–conjugated sodium arsenite (S-32354; dilution 1:500; Invitrogen Corp.) along with fluorescence detection of myelin basic protein (MBP) using Alexa 594–conjugated goat anti-rabbit Ig (A-11012; dilution 1:500; Invitrogen Corp.) as described by Nielsen et al.35 The cellular nuclei were visualized using the nucleic acid stain DAPI (D3571; Invitrogen Corp.), which was added in a concentration of 300 nmol/L to the Tris-buffered saline solution during the last rinse.
Non-specific staining was controlled for by incubation without the primary antibody, with an isotype-specific control (rat IgG1 or rat IgG2b; Nordic Biosite AB, Täby, Sweden) or with rabbit (X902) or goat (X907) serum (both from Dako A/S), and exhibited no staining.
In Situ Hybridization
A mixture of two alkaline phosphatase–labeled DNA probes (DNA Technology A/S, Risskov, Denmark) complementary to bases 169 to 196 (5′-GGCTTTCAATGACTGTGCCGTGGCAGTA-3′) and 530 to 557 (5′-CGCTTCCTGAGGCTGGATTCCGGCAACA-3′) was used for detection of interferon-γ (IFN-γ) cDNA. Probe specificity was confirmed by hybridizing with each probe alone or with a probe mixture, showing identical regional and cellular localization of the in situ signal but with a stronger signal in sections hybridized with the probe mixture. In addition, sections pretreated with RNase A (27-0323-01; Amersham Pharmacia Biotech, Inc.) before hybridization or hybridized using a 100-fold excess of unlabeled IFN-γ probe mixture or buffer alone were devoid of signal (see Supplemental Figure S1B at http://ajp.amjpathol.org).
qPCR
RNA extraction, cDNA synthesis, and qPCR were performed on dissected hippocampi36 using a sequence detection system (PRISM 7300; Applied Biosystems, Inc., Foster City, CA) and using either a Taqman probe (TAG Copenhagen A/S, Copenhagen, Denmark) or SYBR Green (Cambrex Bio Science Walkersville, Inc., Walkersville, MD) as fluorescent reporter molecules. A standard curve was prepared from a fivefold dilution series of cDNA from spinal cord obtained from animals with symptoms of EAE. Primers and probes for tumor necrosis factor (TNF)–α and IFN-γ and the ε-chain of the CD3 complex were used as previously described.34,37 For IL-4, we used forward primer 5′-AAACATGGGAAAACTCCA-3′ and reverse primer 5′-CAGCTTATCGATGAATCCA-3′); for IL-10, forward primer 5′-AGGACTTTAAGGGTTACT-3′ and reverse primer 5′-AATGCTCCTTGATTTCTG-3′; for IL-17, forward primer 5′-GCTTCATCTGTGTCTCTG-3′ and reverse primer 5′-GAACGGTTGAGGTAGTCT-3′). For NG2, we used forward primer 5′-TCCCGGAGAGAGGTGGAAGAG-3′ and reverse primer 5′-GGTCCATCTCTGAGGCATTAGC, and probe 5′-AAGGCGTCTGTCTGTGTCTCACTTCCATCA-3′. For insulin-like growth factor-1 (IGF-1), we used forward primer 5′-CCGAGGGGC TTTTACTTCAACAA-3′ and reverse primer 5′-CGGAAGCAACACTCATCCACAA-3′. For brain-derived neurotrophic factor (BDNF), we used forward primer 5′-GGCCCAACGAAGAAAACCAT-3′ and reverse primer 5′-AGCATCACCCGGGAAGTGT-3′. Specificity of the PCR primer/probe set was validated as described in Meldgaard et al.36 Test gene data were normalized to the reference gene hypoxanthine phosphoribosyltransferase 1 and calibrated to a pool of control samples from animals not operated on. mRNA expression was normalized to the expression of unmanipulated controls, which was set to 1.0. IFN-γ, IL-4, IL-17, and BDNF mRNA expressions were normalized to the expression in unlesioned TOVA mice because we were unable to detect IFN-γ, IL-4, IL-17, or BDNF mRNA from these cytokines in the hippocampus of unlesioned naïve mice. In cases in which mRNA was undetectable (more than 40 cycles or undetermined), the fold increase was set at zero. Changes in mRNA level less than twofold were not subjected to statistical analysis.36
Cell Counting
Cell counting in the molecular layer of the temporal dentate gyrus of PP-lesioned mice was performed using the computer-assisted cast-grid microscope system (CAST2; Visiopharm A/S, Hoersholm, Denmark). The CD3+ cells were defined by their brown plasmalemma. NG2+ cells were defined by a toluidine blue–stained nucleus surrounded by a brown plasmalemma from which more than one process extended. CNP+ cells were defined by a toluidine blue–stained nucleus surrounded by a brown plasmalemma. BrdU+NG2+ cells and BrdU+CNP+ cells were defined as NG2+ and CNP+ cells with a bluish black nucleus. To count the cells using the method of Nielsen et al.,14 cells were counted in the molecular layer of the dentate gyrus in 10 parallel sections 160 μm apart using a counting frame of 4900 μm2 (fraction of total area, 61%) along the rostrocaudal axis of the hippocampus, starting in the section in which the dentate gyrus first demonstrated a typical U shape (−1900 μm from the bregma), as previously reported.14 At least 150 to 200 single-labeled NG2+ or CNP+ cells were counted per animal. To account for potential differences in the quality of the stainings, cell numbers are reported as the ratio between cell numbers obtained from the deafferented ipsilateral molecular layer compared with cell numbers obtained from the contralateral molecular layer (NG2i/c and CNPi/c). Because of the virtual absence of T cells in the contralateral molecular layer, the total number of counted CD3+ T cells is given.
Area Estimations
To estimate the area of the inner and outer molecular layers, calretinin-stained sections were analyzed using the CAST2 system. The number of points hitting the inner and outer molecular layers (P) was counted on sections with a mean distance (t) of 160 μm. The sum of the areas of various brain sections was calculated by multiplying the total number of counted points (P) with the computer-given factor of area per point (Apoint = 6.8582 mm2). The total area (Atotal) was calculated using the formula Atotal = ΣP × Apoint × t. Results are given as the ratio between the area of the inner molecular layer and the entire molecular layer.
Statistical Analysis
Results are given as mean ± SD. Comparison of medians in two groups was performed using the Mann-Whitney rank sum test. For multiple comparisons, Kruskal-Wallis one-way analysis of variance was performed, followed by Dunn's multiple comparisons test. Statistical analyses were performed using Prism 4.0b software for Macintosh (GraphPad Software, Inc., San Diego, CA). P values are indicated as follows: *P < 0.05, **P < 0.01, and ***P < 0.001.
Results
Afferent Fibers with Potential for Sprouting Persist after PP Lesioning
Staining for NF directly visualized the PP fibers terminating as a broad band of NF+ fibers in the outer molecular layer (Figure 1, A and C). As expected, this band of NF+ fibers was completely obliterated by transection of the PP, which focused attention on the NF+ fiber systems that persisted in the inner and outer molecular layers of the dentate gyrus after PP lesioning (Figure 1, B and D). Furthermore, sections from all PP-lesioned TPLP, TOVA, and naïve mice were stained with Fluoro-Jade for visualization of the area of degenerating axons and terminals. As expected, Fluoro-Jade staining demonstrated a sharply demarcated band of green fluorescence in the outer part of the molecular layer of the ipsilateral dentate gyrus, at both 2 and 7 days post lesion, in contrast to the non-deafferented contralateral molecular layer, which demonstrated no staining (Figure 1, E and F).
Figure 1.
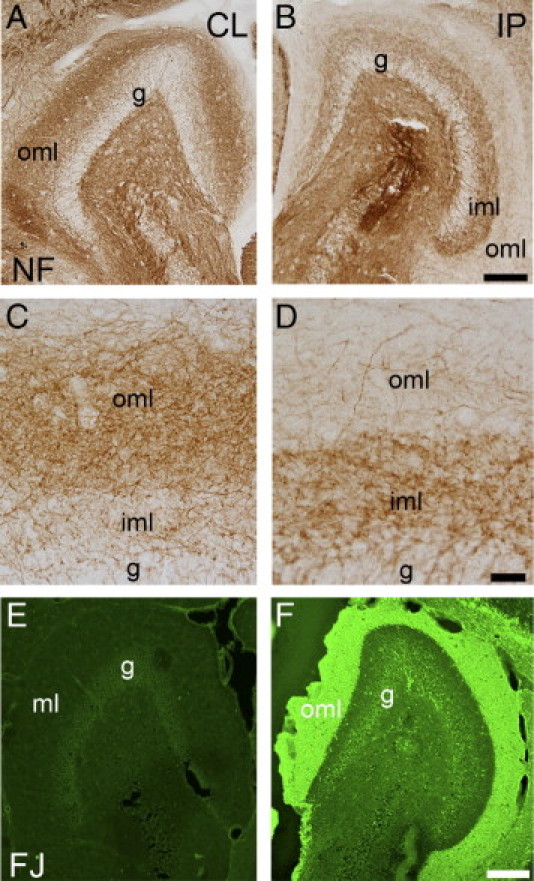
Afferent fibers with potential for sprouting persist after PP lesioning. A–D: Staining for NF at 7 days post lesion demonstrates the NF+ fibers in intact contralateral dentate gyrus (A and C) and the reduced amount of NF+ fibers in the outer molecular layer (oml) of the deafferented ipsilateral (IP) dentate gyrus (B and D). The NF+ fibers that persist in the outer molecular layer may represent calretinergic and cholinergic afferent fibers and other afferent fibers not part of the PP. E and F: Fluoro-Jade staining of the dentate gyrus at 7 days post lesion. The anterograde axonal and terminal degeneration is visualized as an intense band of green fluorescence in the deafferented outer molecular layer in the ipsilateral dentate gyrus (E). No degeneration is observed in the contralateral dentate gyrus (F). CL, contralateral layer; g, granule cell layer; iml, inner molecular layer; ml, molecular layer. Scale bars: 20 μm (C and D); 150 μm (A, B, E, and F).
Myelin-Specific T Cells Infiltrate Zones of Axonal Degeneration
To verify that myelin-specific T cells infiltrated the outer molecular layer at the time of maximal OPC proliferation and remained during the period of OPC differentiation,14 we studied T-cell infiltration in TPLP, TOVA, and naïve mice at 2 and 7 days post lesion (Figure 2A). Compared with few infiltrating CD3+ T cells in PP-lesioned TOVA and naïve mice, there was a significantly higher infiltration of CD3+ T cells in the dentate gyrus and surrounding meninges in TPLP mice at both 2 and 7 days post lesion (Figure 2, A and B; see also Supplemental Table S1 at http://ajp.amjpathol.org). In line with these observations, hippocampi from TPLP mice also demonstrated a significantly higher increase in CD3ε mRNA expression than did hippocampi from TOVA and naïve mice at both 2 and 7 days post lesion (Figure 2B; see also Supplemental Table S1 at http://ajp.amjpathol.org). The number of T cells and CD3ε mRNA expression in PP-lesioned naïve mice and unlesioned TPLP and TOVA mice were close to the baseline in unlesioned naïve mice (see Supplemental Table S1 at http://ajp.amjpathol.org).
Figure 2.
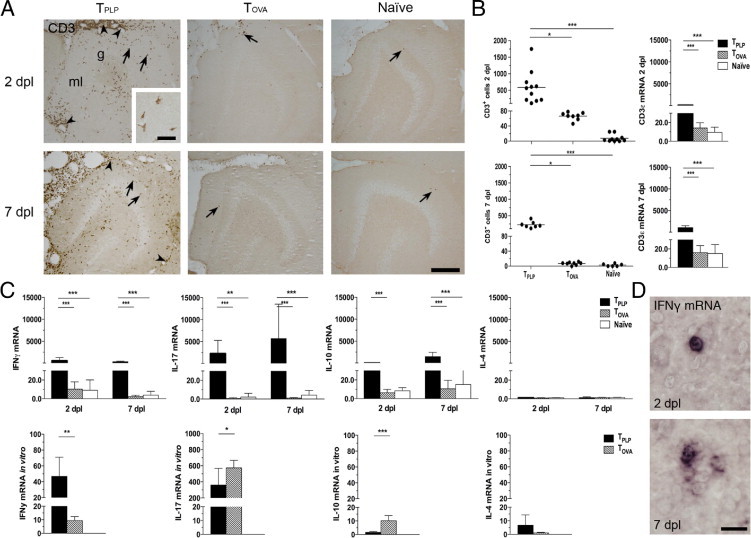
T cells infiltrate zones of axonal degeneration in lesioned TPLP mice. A: Increased infiltration of CD3+ T cells (arrows) in the molecular layer (ml) of PP-lesioned TPLP animals compared with scarce T cells in TOVA and naïve mice at 2 and 7 days post lesion. Note the meningeal infiltration, which is most pronounced in lesioned TPLP animals (arrowheads). g, granule cell layer. Scale bars: 10 μm; 250 μm (inset). B: Cell counting data demonstrate an enhanced number of CD3+ T cells in the molecular layer, and qPCR demonstrated enhanced expression of CD3ε mRNA in hippocampi of TPLP mice compared with TOVA and naïve mice at 2 and 7 days post lesion. Lines represent the mean. *P < 0.05; ***P < 0.001. C: qPCR detection demonstrates enhanced mRNA expression of IFN-γ, IL-17, and IL-10 but not IL-4 in hippocampi of TPLP mice compared with TOVA and naïve mice at 2 and 7 days post lesion (top row) and in TPLP mice compared with TOVA mice in vitro (bottom row). TOVA mice exhibited only a modest increase in IFN-γ in vivo and in vitro. Results are given as mean ± SD. *P < 0.05; **P < 0.01; ***P < 0.001. D:In situ hybridization demonstrates scarce IFN-γ mRNA–expressing cells in the hippocampus proper at 2 and 7 days post lesion. Scale bar = 20 μm.
Cytokine Expression of Infiltrating T Cells
To determine the activation state of the infiltrating T cells, we investigated the cytokine expression in hippocampi of PP-lesioned TPLP, TOVA, and naïve mice at both 2 and 7 days post lesion. The TPLP mice demonstrated a several hundred-fold to several thousand-fold increase in IFN-γ, IL-17, and IL-10 mRNA expression at both observation times, compared with a few-fold increase in PP-lesioned TOVA and naïve mice (Figure 2C; see also Supplemental Table S1 at http://ajp.amjpathol.org). In contrast, IL-4 mRNA demonstrated no significant changes in mRNA expression in any of the groups (Figure 2C; see also Supplemental Table S1 at http://ajp.amjpathol.org).
Although the fold increase in IFN-γ mRNA in the hippocampi of TPLP mice was huge, in situ detection clearly demonstrated that IFN-γ mRNA was confined to single cells located in the hippocampus (Figure 2D; see also Supplemental Figure S1A at http://ajp.amjpathol.org), while being more abundant in the meninges and in the scattered perivascular infiltrates present in the brain stem and cerebellum (see Figure S1B at http://ajp.amjpathol.org). IFN-γ mRNA expressing cells were not detected in PP-lesioned TOVA and naïve mice (see Supplemental Figure S1A at http://ajp.amjpathol.org), which was in line with the variable detection of IFN-γ mRNA in these mice (see Supplemental Table S1 at http://ajp.amjpathol.org).
To investigate whether the increase in IFN-γ, IL-17, and IL-10 mRNA expression was the result of CNS–T cell interaction, cytokine mRNA expression was also quantified in TPLP and TOVA mice and in lymph node cells from naïve mice, the latter being included for control (see Supplemental Table S1 at http://ajp.amjpathol.org). TPLP mice were characterized by a particularly high expression of IFNγ, but also expressed high concentrations of IL-17 mRNA and some IL-4 mRNA (Figure 2C). TOVA mice also expressed high concentrations of IL-17 mRNA and some IFN-γ and IL-10 mRNA, consistent with the findings of others.28
Considered together, the cytokine profile and pattern of infiltration with CD3+ T cells in PP-lesioned TPLP mice suggested the presence of subpopulations of T cells consistent with a Th1 response, but also Th17 and, possibly regulatory T cells response, similar to that of EAE lesions,38 although here associated with enhanced oligodendrogenesis rather than inflammatory demyelination.
Myelin-Reactive T Cells Affect the OPC Response to Axonal Lesions
To study the effect of activated myelin-specific T cells on the axonal lesion–induced response of the NG2+ OPC population, we compared the morphologic and numeric OPC response in PP-lesioned TPLP, TOVA, and naïve mice, initially focusing on mice surviving 2 days post lesion. Characteristic of the OPC response in the PP-lesioned TPLP mice, the NG2+ cells located at the transition between the inner and outer molecular layers had redirected their processes so that they radiated deep into the T cell–infiltrated outer molecular layer at 2 days post lesion (Figure 3A). This response was distinctly different from the OPC response in the PP-lesioned TOVA and naïve mice, in which the OPCs had transformed into hypertrophic and hyper-ramified cells, with their processes radiating in all directions (Figure 3A).
Figure 3.
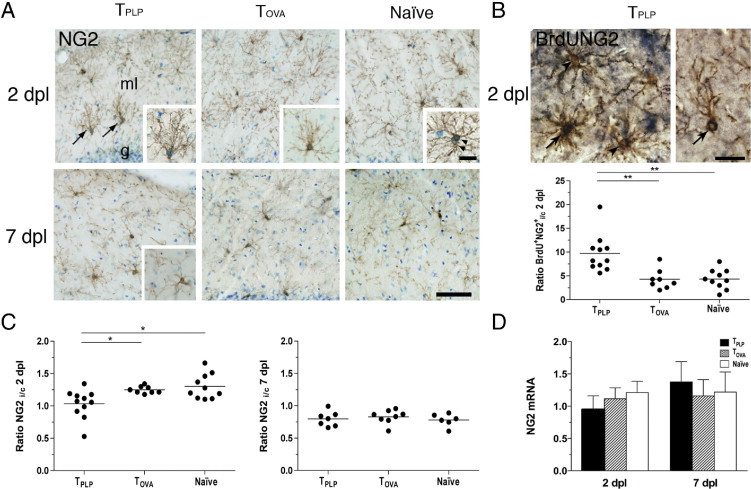
Myelin-reactive T cells stimulate OPC proliferation and differentiation in response to lesioning. A: NG2 staining demonstrates activated NG2+ OPC at 2 days post lesion. Hypertrophic cells (inset in TOVA) and cellular double profiles (inset in Naïve) are observed in all groups of PP-lesioned mice. In TPLP mice, NG2+ cells additionally extended their processes into the area of T cell infiltration (arrows and inset). At 7 days post lesion, the NG2+ cells exhibited a similar structure in all groups, but were difficult to distinguish. Sections were counterstained with toluidine blue for visualization of cellular nuclei. g, granule cell layer; ml, molecular layer. Scale bars: 25 μm; 10 μm (inset). B: BrdU incorporation into mitotic NG2+ cell (left arrow) together with nonmitotic NG2+ cells (left arrowheads) at 2 days post lesion. BrdU incorporation was also observed in NG2+ cells extending their processes into the outer molecular layer (right arrow). Scale bar = 10 μm. Ipsilateral and contralateral ratios of BrdU+NG2+ cells at 2 days post lesion demonstrate a significantly elevated proliferation in TPLP mice compared with TOVA and naïve mice. Lines mark the mean. **P < 0.01. C: NG2+ cells given as ipsilateral and contralateral ratios in the molecular layer in TPLP mice at 2 days post lesion failed to show lesion-induced proliferation. At 7 days post lesion, there was no longer any difference between the groups. D: qPCR analysis of the hippocampus demonstrated a trend toward decreased mRNA expression of NG2 in TPLP mice at 2 days post lesion, whereas no difference was observed at 7 days post lesion. Results are given as mean ± SD.
Because we had shown previously that NG2+ cells in the contralateral hippocampus are unaffected by the lesion,14 we calculated the lesion-induced increase in the number of OPCs as a ratio, NG2i/c, of the cell numbers in the ipsilateral and contralateral molecular layers. Quantification demonstrated a comparable increase in the NG2i/c ratio of 1.25 and 1.30 in TOVA and naïve mice, respectively, at 2 days post lesion (Figure 3C; see also Supplemental Table S1 at http://ajp.amjpathol.org). This represented 25% and 30% increases in OPC numbers in the PP-lesioned TOVA and naïve mice, identical to previous findings in PP-lesioned naïve mice.14 In contrast, the TPLP mice, demonstrating an NG2i/c ratio of 1.04 at 2 days post lesion, completely failed to demonstrate a lesion-induced increase in OPC numbers (Figure 3C; see also Supplemental Table S1 at http://ajp.amjpathol.org). This was additionally supported by comparison of the lesion-induced increase in NG2 mRNA levels in the various groups of mice, which showed a trend toward unchanged NG2 mRNA expression at 2 days post lesion in TPLP mice compared with the 1.2-fold increase in PP-lesioned naïve mice (Figure 3D; see also Supplemental Table S1 at http://ajp.amjpathol.org).
At 7 days post lesion, both the NG2i/c ratio and NG2 mRNA expression in PP-lesioned TPLP mice were comparable to the values obtained in PP-lesioned TOVA and naïve mice (Figure 3, C and D; see also Supplemental Table S1 at http://ajp.amjpathol.org). Furthermore, as previously reported in PP-lesioned naïve mice,14 the OPCs were less distinctly stained in all groups of mice (Figure 3A), possibly because of protease-mediated cleavage of the ectodomain of NG2 chondroitin sulfate.39
Lesion-Induced OPC Proliferation Is Stimulated by Myelin-Reactive T Cells
The abrogation of the axonal lesion–induced increase in the NG2+ OPC population in the TPLP mice at 2 days post lesion might be explained by either reduced proliferation or increased proliferation and accelerated differentiation of OPCs. Therefore, we investigated OPC proliferation by giving mice a bolus injection of the thymidine analog BrdU at 1 hour before termination at 2 days post lesion. The TPLP mice demonstrated a significantly higher (10-fold) increase in BrdU+NG2+ OPCs, compared with an approximately fourfold increase in TOVA and naïve mice (Figure 3B; see also Supplemental Table S1 at http://ajp.amjpathol.org). Furthermore, many of the activated NG2+ OPCs extending their processes into the T cell–infiltrated outer molecular layer had incorporated BrdU (Figure 3B), which demonstrated that they had recently proliferated. The OPC responses in PP-lesioned TOVA and naïve mice were comparable, with scattered hypertrophic and highly branched cells co-labeled for BrdU and NG2 (data not shown). Numerous single-labeled BrdU+ cells were also observed, mainly corresponding to the nuclei of mitotic microglia.30 A few BrdU+NG2+ OPCs were also observed in the contralateral hippocampus of PP-lesioned TPLP, TOVA, and naïve mice, reflecting baseline proliferation.5,14,40 These observations showed that myelin-specific T cells significantly stimulated the proliferation of NG2+ OPCs. This suggested that the abrogation of the lesion-induced increase in the NG2+ cell population in TPLP mice at 2 days post lesion was due to accelerated differentiation of recently proliferated OPCs.
Increased OPC Proliferation Translates into Increased Numbers of Oligodendrocytes
To clarify whether the increased proliferation of OPCs at 2 days post lesion translated into an increased number of newly generated oligodendrocytes at 7 days post lesion, we quantified the number of CNP+ cells and the number of BrdU+CNP+ cells in TPLP, TOVA, and naïve mice at 7 days post lesion. Results are given as a ratio of CNP+ or BrdU+CNP+ cells in the ipsilateral and contralateral molecular layers. Although examination of CNP-stained sections obtained from TPLP, TOVA, and naïve mice revealed no differences in cellular structure (Figure 4A), PP lesions in TPLP mice resulted in a CNPi/c ratio of 1.25, which was significantly increased compared with an unchanged CNPi/c ratio in PP-lesioned TOVA and naïve mice (Figure 4B; see also Supplemental Table S1 at http://ajp.amjpathol.org). This corresponded to a 25% increase in CNP+ oligodendrocytes in the TPLP mice, compared with unchanged numbers in TOVA and naïve mice. Furthermore, compared with TOVA and naïve mice, the TPLP mice demonstrated a significantly higher BrdU+CNP+i/c ratio (Figure 4, C and D). Thus, 28% of the CNP+ cells had incorporated BrdU in TPLP mice, compared with 12% in TOVA mice and 14% in naïve mice. Observations of BrdU+CNP+ cells extending their processes toward myelinated fibers in TPLP mice (Figure 4C) but not in TOVA and naïve mice (data not shown) suggested that the recently proliferated oligodendrocytes were engaged in myelination and that this phenomenon was most frequent in PP-lesioned mice that had received myelin-specific T cells.
Figure 4.
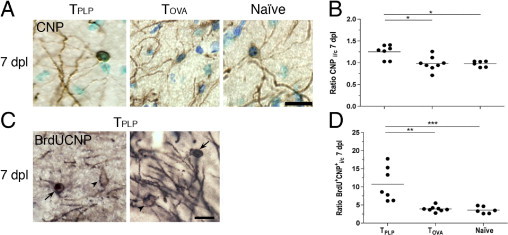
Enhanced oligodendrogenesis in TPLP mice at 7 days post lesion. A: CNP-stained sections demonstrated no differences in oligodendrocyte structure (inset) in TPLP, TOVA, and naïve mice at 7 days post lesion, but increased clearance of myelin debris in TPLP mice. Sections were counterstained using toluidine blue. Scale bar = 10 μm. B: Ipsilateral and contralateral ratios of CNP+ cells in the outer molecular layer at 7 days post lesion demonstrate an increased ratio in TPLP mice. Lines mark the mean. *P < 0.05. C: Postproliferative BrdU+CNP+ oligodendrocyte (left arrow) in TPLP mouse at 7 days post lesion, together with a non-dividing CNP+ oligodendrocyte (left arrowhead). BrdU+CNP+ cells with processes extending toward myelinated fibers (right arrow) together with single-labeled cells (right arrowhead). Scale bar = 10 μm. D: Ipsilateral and contralateral ratios of BrdU+CNP+ cells at 7 days post lesion demonstrate increased ratios in TPLP mice compared with TOVA and naïve mice. Lines mark the mean. **P < 0.01; ***P < 0.001.
Myelin-Reactive T Cells Enhance Clearance of Myelin Debris
Because we had previously demonstrated that PP lesions result in accumulation of myelin debris in the outer molecular layer at 7 days post lesion41 and that microglial clearance of myelin debris is stimulated by infiltrating TMBP,35 we also investigated the clearance of CNP+ myelin debris in PP-lesioned TPLP, TOVA, and naïve mice (see Supplemental Figure S2A at http://ajp.amjpathol.org). Using the scoring system of Nielsen et al,19 we observed that the amount of myelin debris was significantly lower in TPLP mice than in TOVA and naïve mice at 7 days post lesion (see Supplemental Figure S2B at http://ajp.amjpathol.org). Inasmuch as myelin debris is eventually cleared from the outer molecular layer after PP lesioning in naïve mice,42 we concluded that myelin-specific TPLP-cells accelerated the clearance of myelin debris, as previously reported for TMBP-cells.19
Sprouting of Calretinergic Fibers Is Enhanced in TPLP-Infiltrated Dentate Gyrus
It has been well documented that the associational/commissural afferents from the calretinergic hilar mossy cells sprout from the inner molecular layer into the deafferented outer molecular layer after PP lesioning, which is detectable a few weeks after lesioning.20,23 Therefore, we wondered whether the sprouting of these fibers might be accelerated by myelin-specific T cells, because this could contribute to explaining the increased oligodendrogenesis in the PP-lesioned TPLP recipient mice at 7 days post lesion. Comparison of the extent of the lesion-induced sprouting in TPLP mice, and TOVA and naïve mice demonstrated that the calretinergic band in TPLP mice occupied 32% of the molecular layer at 7 days post lesion, which was significantly more than 28% in TOVA and 27% in naïve mice at 7 days post lesion (P < 0.05 for both groups) (Figure 5, A and B). Furthermore, in TPLP mice, the calretinergic band was no longer sharply demarcated, as observed in TOVA and naïve mice, but was fuzzy (Figure 5A), which reflects translaminar sprouting of calretinergic fibers into the deep part of the outer molecular layer.23
Figure 5.
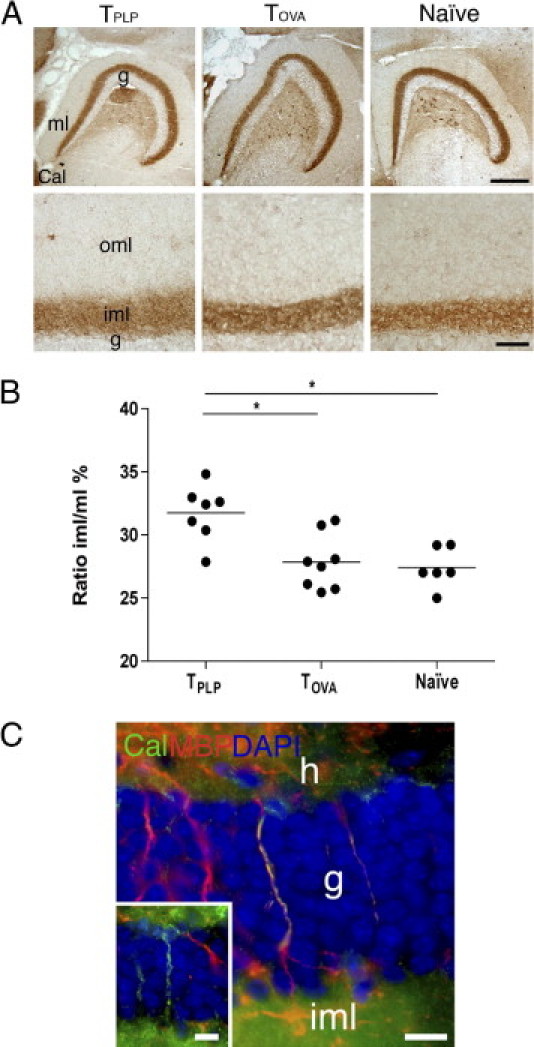
Enhanced lesion-induced sprouting of calretinergic fibers in TPLP-infiltrated dentate gyrus. A: Calretinin staining of hilar mossy cells and their terminals at 7 days post lesion. Note in the TOVA and naïve mice the well-defined border between the inner (iml) and outer (oml) molecular layers. In TPLP mice, this border is fuzzy, reflecting calretinergic fibers sprouting from the inner molecular layer and translaminarly into the outer molecular layer. g, granule cell layer; ml, molecular layer. Scale bars: 200 μm; 20 μm (inset). B: Area estimation demonstrates an increased inner molecular layer–molecular layer ratio in TPLP mice compared with TOVA and naïve mice at 7 days post lesion. Lines mark the mean. *P < 0.05. C: Double immunofluorescence labeling for calretinin (green) and MBP (red) demonstrates co-localization of MBP to a calretinin+ fiber (yellow) transversing the granule cell layer (g; blue). Unmyelinated calretinin+ fibers are also observed (inset). h, Hilus. Scale bars = 10 μm.
Demonstrations by others that sprouting of calretinergic fibers is normally a late phenomenon20,23 would predict similarly sized calretinergic bands at the early 7 days post lesion time. Indeed, we observed that calretinergic band sizes in PP-lesioned and unlesioned naïve mice at 7 days post lesion were comparable (94,700 μm2 versus 89,300 μm2; difference not significant). The possibility of lesion-induced shrinkage of the molecular layer obscuring changes in band size in TPLP mice was eliminated in that lesion-induced shrinkage of the molecular layer in TPLP, TOVA, and naïve mice was comparable (382,100 μm2 versus 326,200 μm2 versus 328,100 μm2; difference not significant for all comparisons). Considered together, the results suggested that the myelin-specific T cells significantly accelerated lesion-induced sprouting of calretinergic fibers into the outer molecular layer.
Because the commissural calretinergic fibers are myelinated, we investigated the myelination of the associational projections in our mice. It was observed that calretinergic fibers traversing the granule cell layer co-localized with MBP (Figure 5C), although single-labeled MBP+ and calretinin+ fibers were also observed. This demonstrated that at least parts of the associational fibers were myelinated.
Enhanced and Prolonged TNF but Not IGF-1 or BDNF mRNA Expression in TPLP Mice
Because TNF stimulates myelination,43 we investigated the production of TNF mRNA in PP-lesioned TPLP, TOVA, and naïve mice. At 2 days post lesion, TPLP mice demonstrated an 80-fold increase in TNF mRNA expression, which was significantly higher than the approximately 30-fold increase observed in TOVA and naïve mice (Figure 6A; see also Supplemental Table S1 at http://ajp.amjpathol.org). It was remarkable that at 7 days post lesion, TPLP mice demonstrated a close to 100-fold increase, which was significantly higher than that in both TOVA and naïve mice, which at that time had returned to close to baseline levels (Figure 6A; see also Supplemental Table S1 at http://ajp.amjpathol.org). Because both T cells and microglia and macrophages can produce TNF,44 we also investigated TNF mRNA expression in T cells in vitro, and observed that, compared with TOVA mice, TPLP mice demonstrated higher TNF mRNA expression (Figure 6A).
Figure 6.
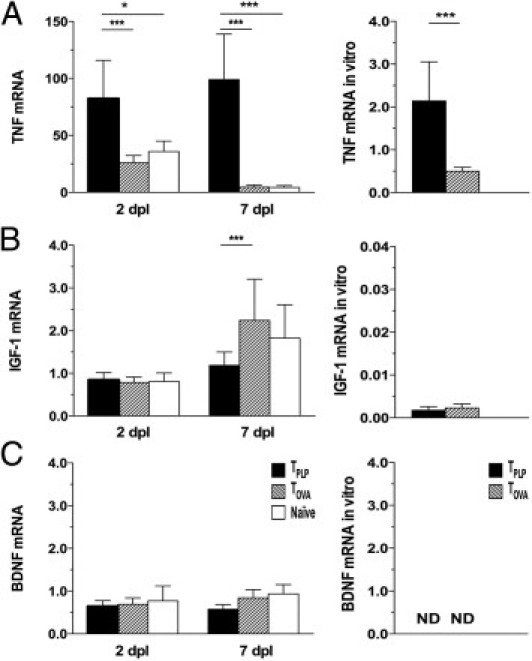
Enhanced expression of TNF but not IGF-1 or BDNF in TPLP mice. A: TNF mRNA expression was continuously elevated in TPLP mice from 2 days post lesion through 7 days post lesion, compared with TOVA and naïve mice, and in vitro TPLP cultures compared with TOVA cultures. B: IGF-1 mRNA expression demonstrated no changes at 2 days post lesion in TPLP, TOVA, and naïve mice. In contrast, IGF-1 mRNA expression was elevated in TOVA and naïve animals at 7 days post lesion but not in PP-lesioned TPLP mice. IGF-1 mRNA expression was low in TPLP and TOVA mice in vitro. C: BDNF mRNA expression showed no changes at 2 and 7 days post lesion in TPLP, TOVA, and naïve mice. BDNF mRNA expression was not detected in vitro. *P < 0.05; **P < 0.01; ***P < 0.001. ND, not detected.
We next investigated the expression of IGF-1 mRNA, which exerts trophic effects on neurons and glial cells, in particular in the OPC population.45,46 At 2 days post lesion, IGF-1 mRNA expression remained at baseline levels in TPLP, TOVA, and naïve mice (Figure 6B; see also Supplemental Table S1 at http://ajp.amjpathol.org). In contrast, at 7 days post lesion, when TOVA and naïve mice demonstrated a close to twofold increase in IGF-1 mRNA expression (P < 0.01 and P < 0.01, respectively; see also Supplemental Table S1 at http://ajp.amjpathol.org), TPLP mice failed to demonstrate a lesion-induced increase in IGF-1 mRNA expression (Figure 6B; see also Supplemental Table S1 at http://ajp.amjpathol.org). IGF-1 mRNA was detected in TPLP and TOVA cells in vitro; however, this expression was lower than in lymph node cells from naïve mice (Figure 6B).
Inasmuch as T cells produce BDNF in MS lesions47 and BDNF is involved in oligodendrogenesis48 and in neuronal plasticity,49 we investigated the mRNA expression of BDNF. Although BDNF mRNA was not detected in lymph node cells from TPLP, TOVA, and naïve mice in vitro (Figure 6C), BDNF mRNA expression was readily detected in the hippocampus of TPLP, TOVA, and naïve mice at 2 and 7 days post lesion. However, the expression remained at the same high baseline level as in unmanipulated controls (Figure 6C; see also Supplemental Table S1 at http://ajp.amjpathol.org).
Considered together, the results indicate that TNF might have a role in the increased OPC proliferation in PP-lesioned TPLP-infiltrated hippocampus.
Discussion
The major finding of this study is that CNS-infiltrating myelin-specific T cells, via increased OPC proliferation, stimulate adult oligodendrogenesis. The results also indicate that several different mechanisms contribute to the oligodendrotrophic effect of myelin-specific T cells.
The high mRNA expression of IFN-γ, IL-17, and IL-10 in the hippocampus of the PP-lesioned TPLP mice suggested the presence of activated Th1 and Th-17 cells, and possibly T-regs, in the mice that demonstrated the oligodendrotrophic effect. Traditionally, induction of inflammatory lesions in EAE is associated with CD4+ T cells producing IFN-γ and IL-17, whereas IL-10 may have a role in the recovery of EAE.38 Unlike IFN-γ and IL-17, both of which were abundantly expressed in TPLP in vitro, the in vitro expression of IL-10 mRNA in TPLP mice was low. The observed in vivo expression of IL-10 mRNA in the PP-lesioned TPLP mice may reflect that IL-10 can be produced by both T cells and microglia and macrophages and that production is enhanced by both cell types via T-cell interaction.50 IL-10 has been associated with protection of oligodendrocytes in vitro51 and promotion of remyelination in vivo, via either enhanced oligodendrogenesis52 or enhanced clearance of myelin debris by microglia phagocytosis,53 which was observed in the present study and in previous studies.35 That we detected only low levels of IL-17 and IL-10 mRNA in the hippocampus of PP-lesioned TOVA mice despite the high levels detected in vitro likely reflects the low number of T cells detected in these animals or the lack of secondary activation by specific antigen recognition. In combination, the demonstration of increased levels of mRNA levels of IFNγ, IL-17, and of IL-10 in the PP-lesioned TPLP mice and the low expression of these cytokines in the PP-lesioned TOVA mice indicates that IFN-γ, IL-17, and IL-10 might contribute to the oligodendrotrophic effect of the myelin-reactive T cells.
Inflammation in MS and EAE is associated with expression of IFN-γ by CD4+ and CD8+ T cells,54–56 both of which were abundant in the PP-lesioned mice receiving myelin-specific T cells.19 Whereas IFN-γ is undetectable in the normal perfused CNS, it increases many fold during T cell–driven CNS inflammation.57,58 This is in line with our observation of absence of IFN-γ mRNA in hippocampi from perfused unlesioned naïve mice and of strongly elevated mRNA levels of IFN-γ in TPLP mice. Unlike in a previous study using reverse transcription-PCR,59 we detected a transient increase in IFN-γ mRNA expression in TOVA and naïve mice at 2 days post lesion, which declined at 7 days post lesion, corresponding to CD3 mRNA expression. However, IFN-γ mRNA was not detectable in all mice, and the fold increase was small. Other studies have demonstrated that IFN-γ can both inhibit differentiation and induce apoptosis of OPCs,60,61 as well as protect mature oligodendrocytes against cuprizone-induced destruction62 and inflammatory destruction,63 which suggests that the effect of IFN-γ on the oligodendrocyte population is context-dependent. However, although our finding of a several hundred–fold increase in IFN-γ mRNA expression in the PP-lesioned TPLP mice raises the possibility of a role for IFN-γ in the oligodendrotrophic effect of the myelin-reactive T cells, it is noteworthy that IFN-γ mRNA detectable via in situ hybridization was expressed by rare cells, presumed to be T cells, located outside of the molecular layer of the dentate gyrus. This observation allows that T cell–derived factors other than IFN-γ might stimulate oligodendrogenesis in this model.
At present, knowledge of the function of IL-17 is limited. It may have a role in, yet not be required for, development of EAE64,65 and MS, inasmuch as it has been shown to be produced in T cells and astrocytes in active MS lesions.66 In the present study, we did not investigate the origin of IL-17 mRNA in the hippocampus; however, it is striking that both TPLP and TOVA mice produced high amounts of IL-17 mRNA in vitro. Furthermore, IL-17 induces TNF production in macrophages67 and enhances oligodendrocyte apoptosis in vitro, which contradicts the enhanced oligodendrogenesis observed here in vivo. However, inasmuch as a recent in vitro study demonstrated that IL-17 enhances phagocytosis by macrophages,68 it is possible that the enhanced oligodendrogenesis observed in the present study might be attributed to IL-17 stimulating microglial phagocytosis of myelin debris. It has been suggested that IL-17 is a marker for the Th17 subset of T cells, which may exert effector function via several other mediators.69
The present study also demonstrated that myelin-specific T cells stimulate sprouting of intact calretinergic fibers. In the mouse, sprouting is particularly well documented for the calretinergic axons of hilar mossy cells.20 These fibers sprout translaminarly from the inner molecular layer into the deafferented outer molecular layer after creation of a PP lesion, although normally this is not manifested until a few weeks after PP lesioning.23 Unlike the unmyelinated cholinergic septo-hippocampal afferents that also sprout after creation of a PP lesion,70 the present study demonstrates that at least part of the calretinergic fibers, presumably associational fibers, that traverse the granule cell layer are myelinated. This adds to observations by others that demonstrated that the commissural calretinergic fibers are myelinated.22 Our observation of an increased sprouting response of calretinergic fibers from the inner molecular layer into the deafferented outer molecular layer at 7 days post lesion in the TPLP mice, but not in the TOVA and naïve mice, correlates well with the translaminar sprouting usually observed several weeks after PP lesioning.23 Inasmuch as ultrastructural studies have suggested that the process of axonal elongation is usually first initiated at 4 to 6 days post lesion and completed after 12 days post lesion,21,71 it seems likely that the myelin-specific T cells accelerated the sprouting of the calretinergic fibers. Given our recent demonstration of adult oligodendrogenesis in areas of axonal sprouting in non-deafferented zones of the hippocampus of PP-lesioned mice,24 the accelerated sprouting of the calretinergic fibers might itself stimulate oligodendrogenesis. Furthermore, we observed that myelin-specific TPLP cells enhanced the clearance of myelin debris, probably due to stimulation of microglial macrophage myelin phagocytosis, as we have previously reported for myelin-specific TMBP cells.35 Because myelin debris is a potent inhibitor of axonal outgrowth,72–74 axonal sprouting may be additionally stimulated by the accelerated removal of myelin debris, making the neural tissue more permissive to the axonal sprouts.72–74 Furthermore, myelin debris itself inhibits remyelination via inhibition of OPC differentiation.74,75
The results show that the increase in oligodendrogenesis is linked to increased proliferation of the NG2+ OPC population and to morphologic changes specific to the TPLP mice. Although proliferation is a characteristic feature of OPC activation,14,16,76 these cells also respond by changing their structure and level of expression of NG2. Morphologic changes have been reported to be characteristic of the OPC response to inflammation,77 and may have been exaggerated by the abundance of activated myelin-specific T cells in the dentate gyrus in TPLP mice. Recently, it has become clear that NG2+ cells form classic synapses with cortical neurons78 and that neurons, via axon collaterals, co-activate NG2 cells along with their postsynaptic neurons.79–81 In principle, integration of NG2+ cells in the axonal circuitry places the NG2+ cells in a unique position to sense the disappearance of presynaptic input, because it occurs in the outer molecular layer after PP lesion, and to sense stimuli from even single sprouting axons. Furthermore, there is evidence that synaptic mechanisms modulate both division and migration of NG2 cells.82,83 These findings correlate well with the observation of division of NG2+ cells in areas of axonal sprouting in the non-deafferented hippocampus, which results in an increased number of oligodendrocytes and length of myelinated fibers several weeks after PP lesioning.24 Although we observed a few BrdU+CNP+ cells extending processes toward CNP+ myelinated fibers, suggestive of myelination, a thorough study of myelination of sprouting axons would require an extended survival time.21,71
In line with studies that have demonstrated that autoimmune infiltration can exert beneficial effects on injured neurons,47,84 we report here that infiltration with myelin-specific T cells can stimulate proliferation and differentiation of OPCs. Our observations also raise the possibility that several different mechanisms contribute to the oligodendrotrophic effect of myelin-specific T cells. In addition to IFN-γ, subpopulations of T cells in inflammatory lesions produce BDNF,84–86 which in addition to its multiple beneficial effects on neurons87 is a potent stimulus of remyelination88 and neuronal plasticity.49 That we did not detect BDNF mRNA expression in vitro suggests that BDNF in vivo is produced by CNS neurons and glial cells, which is in line with in situ hybridization studies that demonstrated high BDNF mRNA expression, in particular in hippocampal neurons.89 After PP lesioning, transient up-regulation in mRNA expression has been observed in the granular neurons.90,91 It is, therefore, possible that the unchanged mRNA levels observed in TPLP mice may be the result of a dilution bias because qPCR performed on the entire hippocampus, as in the present study, might underestimate the lesion-induced local changes in cellular mRNA expression. It is noteworthy that hippocampal sprouting after injury does not seem to depend on BDNF.92,93
TNF induces injury to both oligodendrocytes and myelin43,94,95 and promotes OPC differentiation and remyelination.43 In the CNS, TNF is expressed primarily by microglia or infiltrating macrophages in response to injury,96 and is induced by inflammatory cytokines such as IFN-γ97 or by cell-cell contact with activated myelin-reactive T cells.98,99 Using the PP lesion model, our group has previously demonstrated a transient increase in TNF mRNA expression levels at 2 days post lesion,34 which was increased by the presence of IFN-γ.100 This was confirmed in the present study, in which a marked increase in TNF mRNA expression was observed in TPLP mice at 2 days post lesion and persisted at 7 days post lesion. Microglial production of TNF has been previously associated with increased phagocytosis of myelin debris in microglia in vitro.101,102 As reported for IL-10 mRNA, the elevated TNF mRNA expression was concurrent with enhanced clearance of myelin debris, which has previously been attributed to microglial phagocytosis in PP-lesioned TMBP mice.35
Although we were not able to detect any changes in IGF-1 mRNA expression at the time of maximal OPC proliferation at 2 days post lesion, as much as twofold up-regulation in TOVA and naïve mice was observed at 7 days post lesion. This corresponds well to in situ hybridization results that demonstrated elevated IGF-1 mRNA expression in the deafferented outer molecular layer up to 12 days post lesion in rats.46,103 IGF-1 is expressed in glial cells and neurons in normal CNS.104,105 As mentioned for BDNF, the inability to detect a T cell–mediated effect on IGF-1 mRNA expression at 2 and 7 days post lesion in TPLP mice could also be due to dilution bias. An effect of the myelin-specific T cells on IGF-1 signaling in OPCs could also be accomplished by up-regulation of bindings sites on target cells, as has been demonstrated for IGF-1 binding sites after PP lesioning.106 Thus, T cells might stimulate oligodendrogenesis through lesion-reactive microglia by different mechanisms, and a role for BDNF and IGF-1 cannot be excluded by our findings. This was recently exemplified by IFN-γ–stimulated microglia that demonstrated a phenotype alternately beneficial or detrimental to formation of oligodendrocytes, depending on co-expression of other cytokines.107 Thus, cytokines produced by subpopulations of T cells may have far more wide-ranging effects within the neural tissue than has been predicted.
In conclusion, findings of the present study demonstrate that myelin-specific T cells can stimulate OPC proliferation and oligodendrogenesis in vivo. Inasmuch as most of the current therapeutic strategies for MS are immunomodulatory, these findings are of importance not only for understanding T cell–mediated effects in MS but also for future refinement of therapeutic strategies.
Acknowledgments
We thank Lene Jørgensen and Sussanne Petersen for technical assistance, Karsten Skjødt for assistance in biotinylating the CNP antibody, and the European Cooperation in Science and Technology (COST) Action BM0603 Inflammation in Brain Disease (NEURINFNET) for discussions and for networking support.
Footnotes
Supported by the Danish MS Society, the Michaelsen Foundation, the Graduate School of Immunology, the Danish Medical Association Research Foundation, the Lundbeckfoundation, the Leo Nielsen Foundation, Bent Bøgh and Wife's Foundation, the Foundation for Promotion of Medical Research, the Frimodt-Heineke Foundation, the King Christian X Foundation, the Merchant Brogaard Foundation, the Senior Hospital Physician's Foundation, the Karen A Tholstrup Foundation, the Foundation for Neurological Research, the Frimodt Foundation, the Hede Nielsen Foundation, the Kurt Bønnelycke's Foundation, the Torben og Alice Nielsen Foundation, the Fru Lily Benthine Lund Foundation, the Jacob Madsen and Wife Olga Madsen Foundation, the Kathrine and Vigo Skovgaards Foundation, the Beckett-Foundation, the Warwara Larsen Foundation, and a grant from Alfred Helsted, MD.
Supplemental material for this article may be found on http://ajp.amjpathol.org or at doi: 10.1016/j.ajpath.2011.06.006.
Supplementary data
IFNγ mRNA expression visualized by in situ hybridization. A: A few cells expressing IFN-γ mRNA were detected in the hilus of the dentate gyrus and in the meninges of the hippocampal and choroid fissures (arrowheads) at 2 and 7 days post lesion in TPLP mice but not in TOVA or naïve mice. g, Granule cell layer; ml, molecular layer. B–G:In situ hybridization controls. Photomicrographs demonstrate a perivascular cuff in the cerebellum in PP-lesioned TPLP mouse after in situ hybridization with probe 1 (B), probe 2 (C), and a combination of both probes (D). Note the infiltrating cells (arrowheads) in the perivascular space exhibiting a stronger signal in sections hybridized using the mixed probes. Sections hybridized using a 100-fold excess of unlabeled IFN probe mixture (E), pretreated with RNase A (F) or buffer alone (G) were devoid of signal. Scale bars: 250 μm (A); 40 μm (B–G).
Enhanced clearance of myelin debris in TPLP mice. A: Photomicrographs of CNP-stained sections obtained from TPLP, TOVA, and naïve mice at 7 days post lesion. Although myelin debris was present in high amounts in TOVA and naïve mice (J, K, M, N), this was less pronounced in TPLP mice. g, granular cell layer; ml, molecular layer. Scale bars 25 μm; 200 μm. B: Scoring demonstrated that PP-lesioned TPLP mice contained less myelin debris than did PP-lesioned TOVA and naïve mice at 7 days after lesion. Lines indicate medians. **p< 0.01; ***p<0.001.
References
- 1.Weiner H.L. Multiple sclerosis is an inflammatory T-cell–mediated autoimmune disease. Arch Neurol. 2004;61:1613–1615. doi: 10.1001/archneur.61.10.1613. [DOI] [PubMed] [Google Scholar]
- 2.Lucchinetti C., Brück W., Parisi J., Scheithauer B., Rodriguez M., Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Ann Neurol. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 3.Wolswijk G. Oligodendrocyte precursor cells in the demyelinated multiple sclerosis spinal cord. Brain. 2002;125:338–349. doi: 10.1093/brain/awf031. [DOI] [PubMed] [Google Scholar]
- 4.Raine C.S., Wu E. Multiple sclerosis: remyelination in acute lesions. J Neuropathol Exp Neurol. 1993;52:199–204. [PubMed] [Google Scholar]
- 5.Dawson M.R., Polito A., Levine J.M., Reynolds R. NG2-expressing glial progenitor cells: an abundant and widespread population of cycling cells in the adult rat CNS. Mol Cell Neurosci. 2003;24:476–488. doi: 10.1016/s1044-7431(03)00210-0. [DOI] [PubMed] [Google Scholar]
- 6.French-Constant C., Raff M.C. Proliferating bipotential glial progenitor cells in adult rat optic nerve. Nature. 1986;319:499–502. doi: 10.1038/319499a0. [DOI] [PubMed] [Google Scholar]
- 7.Blakemore W.F., Franklin R.J. Remyelination in experimental models of toxin-induced demyelination. Curr Topic Microbiol Immunol. 2008;318:193–212. doi: 10.1007/978-3-540-73677-6_8. [DOI] [PubMed] [Google Scholar]
- 8.Levine J.M., Reynolds R. Activation and proliferation of endogenous oligodendrocyte precursor cells during ethidium bromide–induced demyelination. Exp Neurol. 1999;160:333–347. doi: 10.1006/exnr.1999.7224. [DOI] [PubMed] [Google Scholar]
- 9.Reynolds R., Dawson M., Papadopoulos D., Polito A., Di Bello I.C., Pham-Dinh D., Levine J. The response of NG2-expressing oligodendrocyte progenitors to demyelination in MOG-EAE and MS. J Neurocytol. 2002;31:523–536. doi: 10.1023/a:1025747832215. [DOI] [PubMed] [Google Scholar]
- 10.Matsumoto H., Kumon Y., Watanabe H., Ohnishi T., Shudou M., Chuai M., Imai Y., Takahashi H., Tanaka J. Accumulation of macrophage-like cells expressing NG2 proteoglycan and Iba1 in ischemic core of rat brain after transient middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2008;28:149–163. doi: 10.1038/sj.jcbfm.9600519. [DOI] [PubMed] [Google Scholar]
- 11.Jones L.L., Sajed D., Tuszynski M.H. Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J Neurosci. 2003;23:9276–9288. doi: 10.1523/JNEUROSCI.23-28-09276.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lytle J.M., Vicini S., Wrathall J.R. Phenotypic changes in NG2+ cells after spinal cord injury. J Neurotrauma. 2006;23:1726–1738. doi: 10.1089/neu.2006.23.1726. [DOI] [PubMed] [Google Scholar]
- 13.Dehn D., Burbach G.J., Schafer R., Deller T. NG2 upregulation in the denervated rat fascia dentata following unilateral entorhinal cortex lesion. Glia. 2006;53:491–500. doi: 10.1002/glia.20307. [DOI] [PubMed] [Google Scholar]
- 14.Nielsen H.H., Ladeby R., Drojdahl N., Peterson A.C., Finsen B. Axonal degeneration stimulates the formation of NG2+ cells and oligodendrocytes in the mouse. Glia. 2006;54:105–115. doi: 10.1002/glia.20357. [DOI] [PubMed] [Google Scholar]
- 15.Schonberg D.L., Popovich P.G., McTigue D.M. Oligodendrocyte generation is differentially influenced by toll-like receptor (TLR) 2 and TLR4-mediated intraspinal macrophage activation. J Neuropathol Exp Neurol. 2007;66:1124–1135. doi: 10.1097/nen.0b013e31815c2530. [DOI] [PubMed] [Google Scholar]
- 16.Dawson M.R., Levine J.M., Reynolds R. NG2-expressing cells in the central nervous system: are they oligodendroglial progenitors? J Neurosci Res. 2000;61:471–479. doi: 10.1002/1097-4547(20000901)61:5<471::AID-JNR1>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 17.Jensen M.B., Gonzalez B., Castellano B., Zimmer J. Microglial and astroglial reactions to anterograde axonal degeneration: a histochemical and immunocytochemical study of the adult rat fascia dentata after entorhinal perforant path lesions. Exp Brain Res. 1994;98:245–260. doi: 10.1007/BF00228413. [DOI] [PubMed] [Google Scholar]
- 18.Kwidzinski E., Mutlu L.K., Kovac A.D., Bunse J., Goldmann J., Mahlo J., Aktas O., Zipp F., Kamradt T., Nitsch R., Bechmann I. Self-tolerance in the immune privileged CNS: lessons from the entorhinal cortex lesion model. J Neural Transm Suppl. 2003 doi: 10.1007/978-3-7091-0643-3_2. –4929. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen H.H., Ladeby R., Fenger C., Toft-Hansen H., Babcock A.A., Owens T., Finsen B. Enhanced microglia clearance of myelin debris in T-cell infiltrated central nervous system. J Neuropathol Exp Neurol. 2009;68:845–856. doi: 10.1097/NEN.0b013e3181ae0236. [DOI] [PubMed] [Google Scholar]
- 20.Phinney A.L., Calhoun M.E., Woods A.G., Deller T., Jucker M. Stereological analysis of the reorganization of the dentate gyrus following entorhinal cortex lesion in mice. Eur J Neurosci. 2004;19:1731–1740. doi: 10.1111/j.1460-9568.2004.03280.x. [DOI] [PubMed] [Google Scholar]
- 21.Steward O., Vinsant S.L. The process of reinnervation in the dentate gyrus of adult rats: a quantitative electron microscopic analysis of terminal proliferation and reactive synaptogenesis. J Comp Neurol. 1983;214:370–386. [Google Scholar]
- 22.Blasco-Ibanez J.M., Freund T.F. Distribution, ultrastructure, and connectivity of calretinin-immunoreactive mossy cells of the mouse dentate gyrus. Hippocampus. 1997;7:307–320. doi: 10.1002/(SICI)1098-1063(1997)7:3<307::AID-HIPO6>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 23.Del Turco D., Woods A.G., Gebhardt C., Phinney A.L., Jucker M., Frotscher M., Deller T. Comparison of commissural sprouting in the mouse and rat fascia dentata after entorhinal cortex lesion. Hippocampus. 2003;13:685–699. doi: 10.1002/hipo.10118. [DOI] [PubMed] [Google Scholar]
- 24.Drojdahl N., Nielsen H.H., Gardi J.E., Wree A., Peterson A.C., Nyengaard J.R., Eyer J., Finsen B. Axonal plasticity elicits long-term changes in oligodendroglia and myelinated fibers. Glia. 2010;58:29–42. doi: 10.1002/glia.20897. [DOI] [PubMed] [Google Scholar]
- 25.Patrikios P., Stadelmann C., Kutzelnigg A., Rauschka H., Schmidbauer M., Laursen H., Sorensen P.S., Bruck W., Lucchinetti C., Lassmann H. Remyelination is extensive in a subset of multiple sclerosis patients. Brain. 2006;129:3165–3172. doi: 10.1093/brain/awl217. [DOI] [PubMed] [Google Scholar]
- 26.Ludwin S.K. Chronic demyelination inhibits remyelination in the central nervous system: an analysis of contributing factors. Lab Invest. 1980;43:382–387. [PubMed] [Google Scholar]
- 27.Niehaus A., Shi J., Grzenkowski M., Diers-Fenger M., Archelos J., Hartung H.P., Toyka K., Bruck W., Trotter J. Patients with active relapsing-remitting multiple sclerosis synthesize antibodies recognizing oligodendrocyte progenitor cell surface protein: implications for remyelination. Ann Neurol. 2000;48:362–371. [PubMed] [Google Scholar]
- 28.Krakowski M.L., Owens T. Naïve T lymphocytes traffic to inflamed central nervous system, but require antigen recognition for activation. Eur J Immunol. 2000;30:1002–1009. doi: 10.1002/(SICI)1521-4141(200004)30:4<1002::AID-IMMU1002>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 29.Flugel A., Berkowicz T., Ritter T., Labeur M., Jenne D.E., Li Z., Ellwart J.W., Willem M., Lassmann H., Wekerle H. Migratory activity and functional changes of green fluorescent effector cells before and during experimental autoimmune encephalomyelitis. Immunity. 2001;14:547–560. doi: 10.1016/s1074-7613(01)00143-1. [DOI] [PubMed] [Google Scholar]
- 30.Dissing-Olesen L., Ladeby R., Nielsen H.H., Toft-Hansen H., Dalmau I., Finsen B. Axonal lesion-induced microglial proliferation and microglial cluster formation in the mouse. Neuroscience. 2007;149:112–122. doi: 10.1016/j.neuroscience.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 31.Matthews D.A., Cotman C., Lynch G. An electron microscopic study of lesion-induced synaptogenesis in the dentate gyrus of the adult rat. I: Magnitude and time course of degeneration. Brain Res. 1976;115:1–21. doi: 10.1016/0006-8993(76)90819-2. [DOI] [PubMed] [Google Scholar]
- 32.Friedman B., Hockfield S., Black J.A., Woodruff K.A., Waxman S.G. In situ demonstration of mature oligodendrocytes and their processes: an immunocytochemical study with a new monoclonal antibody, Rip. Glia. 1989;2:380–390. doi: 10.1002/glia.440020510. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe M., Sakurai Y., Ichinose T., Aikawa Y., Kotani M., Itoh K. Monoclonal antibody Rip specifically recognizes 2′,3′-cyclic nucleotide 3′-phosphodiesterase in oligodendrocytes. J Neurosci Res. 2006;84:525–533. doi: 10.1002/jnr.20950. [DOI] [PubMed] [Google Scholar]
- 34.Fenger C., Drojdahl N., Wirenfeldt M., Sylvest L., Jorgensen O.S., Meldgaard M., Lambertsen K.L., Finsen B. Tumor necrosis factor and its p55 and p75 receptors are not required for axonal lesion-induced microgliosis in mouse fascia dentata. Glia. 2006;54:591–605. doi: 10.1002/glia.20405. [DOI] [PubMed] [Google Scholar]
- 35.Nielsen H.H., Ladeby R., Fenger C., Toft-Hansen H., Babcock A.A., Owens T., Finsen B. Enhanced microglial clearance of myelin debris in T cell–infiltrated central nervous system. J Neuropathol Exp Neurol. 2009;68:845–856. doi: 10.1097/NEN.0b013e3181ae0236. [DOI] [PubMed] [Google Scholar]
- 36.Meldgaard M., Fenger C., Lambertsen K.L., Pedersen M.D., Ladeby R., Finsen B. Validation of two reference genes for mRNA level studies of murine disease models in neurobiology. J Neurosci Methods. 2006;156:101–110. doi: 10.1016/j.jneumeth.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 37.Lambertsen K.L., Gregersen R., Meldgaard M., Clausen B.H., Heibol E.K., Ladeby R., Knudsen J., Frandsen A., Owens T., Finsen B. A role for interferon-gamma in focal cerebral ischemia in mice. J Neuropathol Exp Neurol. 2004;63:942–955. doi: 10.1093/jnen/63.9.942. [DOI] [PubMed] [Google Scholar]
- 38.Kennedy M.K., Torrance D.S., Picha K.S., Mohler K.M. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992;149:2496–2505. [PubMed] [Google Scholar]
- 39.Trotter J., Karram K., Nishiyama A. NG2 cells: properties, progeny and origin. Brain Res Rev. 2010;63:72–82. doi: 10.1016/j.brainresrev.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Levison S.W., Young G.M., Goldman J.E. Cycling cells in the adult rat neocortex preferentially generate oligodendroglia. J Neurosci Res. 1999;57:435–446. [PubMed] [Google Scholar]
- 41.Jensen M.B., Hegelund I.V., Poulsen F.R., Owens T., Zimmer J., Finsen B. Microglial reactivity correlates to the density and the myelination of the anterogradely degenerating axons and terminals following perforant path denervation of the mouse fascia dentata. Neuroscience. 1999;93:507–518. doi: 10.1016/s0306-4522(99)00139-6. [DOI] [PubMed] [Google Scholar]
- 42.Buss A., Schwab M.E. Sequential loss of myelin proteins during Wallerian degeneration in the rat spinal cord. Glia. 2003;42:424–432. doi: 10.1002/glia.10220. [DOI] [PubMed] [Google Scholar]
- 43.Arnett H.A., Mason J., Marino M., Suzuki K., Matsushima G.K., Ting J.P. TNF alpha promotes proliferation of oligodendrocyte progenitors and remyelination. Nat Neurosci. 2001;4:1116–1122. doi: 10.1038/nn738. [DOI] [PubMed] [Google Scholar]
- 44.Lambertsen K.L., Meldgaard M., Ladeby R., Finsen B. A quantitative study of microglial-macrophage synthesis of tumor necrosis factor during acute and late focal cerebral ischemia in mice. J Cereb Blood Flow Metab. 2005;25:119–135. doi: 10.1038/sj.jcbfm.9600014. [DOI] [PubMed] [Google Scholar]
- 45.Mason J.L., Goldman J.E. A2B5+ and O4+ cycling progenitors in the adult forebrain white matter respond differentially to PDGF-AA, FGF-2, and IGF-1. Mol Cell Neurosci. 2002;20:30–42. doi: 10.1006/mcne.2002.1114. [DOI] [PubMed] [Google Scholar]
- 46.Guthrie K.M., Nguyen T., Gall C.M. Insulin-like growth factor-1 mRNA is increased in deafferented hippocampus: spatiotemporal correspondence of a trophic event with axon sprouting. J Comp Neurol. 1995;352:147–160. doi: 10.1002/cne.903520111. [DOI] [PubMed] [Google Scholar]
- 47.Stadelmann C., Kerschensteiner M., Misgeld T., Brück W., Hohlfeld R., Lassmann H. BDNF and gp145trkB in multiple sclerosis brain lesions: neuroprotective interactions between immune and neuronal cells? Brain. 2002;125:75–85. doi: 10.1093/brain/awf015. [DOI] [PubMed] [Google Scholar]
- 48.McTigue D.M., Horner P.J., Stokes B.T., Gage F.H. Neurotrophin-3 and brain-derived neurotrophic factor induce oligodendrocyte proliferation and myelination of regenerating axons in the contused adult rat spinal cord. J Neurosci. 1998;18:5354–5365. doi: 10.1523/JNEUROSCI.18-14-05354.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramer M.S. Endogenous neurotrophins and plasticity following spinal deafferentiation. Exp Neurol. 2010 doi: 10.1016/j.expneurol.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 50.Chabot S., Williams G., Hamilton M., Sutherland G., Yong V.W. Mechanisms of IL-10 production in human microglia–T cell interaction. J Immunol. 1999;162:6819–6828. [PubMed] [Google Scholar]
- 51.Broderick C., Duncan L., Taylor N., Dick A.D. IFN-gamma and LPS-mediated IL-10–dependent suppression of retinal microglial activation. Invest Ophthalmol Vis Sci. 2000;41:2613–2622. [PubMed] [Google Scholar]
- 52.Yang J., Jiang Z., Fitzgerald D.C., Ma C., Yu S., Li H., Zhao Z., Li Y., Ciric B., Curtis M., Rostami A., Zhang G.X. Adult neural stem cells expressing IL-10 confer potent immunomodulation and remyelination in experimental autoimmune encephalitis. J Clin Invest. 2009;119:3678–3691. doi: 10.1172/JCI37914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith M.E., van der Maesen K., Somera F.P. Macrophage and microglial responses to cytokines in vitro: phagocytic activity, proteolytic enzyme release, and free radical production. J Neurosci Res. 1998;54:68–78. doi: 10.1002/(SICI)1097-4547(19981001)54:1<68::AID-JNR8>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 54.Liblau R.S., Singer S.M., McDevitt H.O. Th1 and Th2 CD4+ T cells in the pathogenesis of organ-specific autoimmune diseases. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 55.Zamvil S., Nelson P., Trotter J., Mitchell D., Knobler R., Fritz R., Steinman L. T-cell clones specific for myelin basic protein induce chronic relapsing paralysis and demyelination. Nature. 1985;317:355–358. doi: 10.1038/317355a0. [DOI] [PubMed] [Google Scholar]
- 56.Goverman J. Autoimmune T cell responses in the central nervous system. Nat Rev Immunol. 2009;9:393–407. doi: 10.1038/nri2550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Popko B., Corbin J.G., Baerwald K.D., Dupree J., Garcia A.M. The effects of interferon-gamma on the central nervous system. Mol Neurobiol. 1997;14:19–35. doi: 10.1007/BF02740619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Imitola J., Chitnis T., Khoury S.J. Cytokines in multiple sclerosis: from bench to bedside. Pharmacol Ther. 2005;106:163–177. doi: 10.1016/j.pharmthera.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 59.Jensen M.B., Poulsen F.R., Finsen B. Axonal sprouting regulates myelin basic protein gene expression in denervated mouse hippocampus. Int J Dev Neurosci. 2000;18:221–235. doi: 10.1016/s0736-5748(99)00091-x. [DOI] [PubMed] [Google Scholar]
- 60.Chew L.J., King W.C., Kennedy A., Gallo V. Interferon-gamma inhibits cell cycle exit in differentiating oligodendrocyte progenitor cells. Glia. 2005;52:127–143. doi: 10.1002/glia.20232. [DOI] [PubMed] [Google Scholar]
- 61.Lin W., Kemper A., Dupree J.L., Harding H.P., Ron D., Popko B. Interferon-gamma inhibits central nervous system remyelination through a process modulated by endoplasmic reticulum stress. Brain. 2006;129:1306–1318. doi: 10.1093/brain/awl044. [DOI] [PubMed] [Google Scholar]
- 62.Gao X., Gillig T.A., Ye P., D'Ercole A.J., Matsushima G.K., Popko B. Interferon-gamma protects against cuprizone-induced demyelination. Mol Cell Neurosci. 2000;16:338–349. doi: 10.1006/mcne.2000.0883. [DOI] [PubMed] [Google Scholar]
- 63.Lin W., Bailey S.L., Ho H., Harding H.P., Ron D., Miller S.D., Popko B. The integrated stress response prevents demyelination by protecting oligodendrocytes against immune-mediated damage. J Clin Invest. 2007;117:448–456. doi: 10.1172/JCI29571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haak S., Croxford A.L., Kreymborg K., Heppner F.L., Pouly S., Becher B., Waisman A. IL-17A and IL-17F do not contribute vitally to autoimmune neuro-inflammation in mice. J Clin Invest. 2009;119:61–69. doi: 10.1172/JCI35997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Park H., Li Z., Yang X.O., Chang S.H., Nurieva R., Wang Y.H., Wang Y., Hood L., Zhu Z., Tian Q., Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Tzartos J.S., Friese M.A., Craner M.J., Palace J., Newcombe J., Esiri M.M., Fugger L. Interleukin-17 production in central nervous system-infiltrating T cells and glial cells is associated with active disease in multiple sclerosis. Am J Pathol. 2008;172:146–155. doi: 10.2353/ajpath.2008.070690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jovanovic D.V., Di Battista J.A., Martel-Pelletier J., Jolicoeur F.C., He Y., Zhang M., Mineau F., Pelletier J.P. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- 68.Silverpil E, Glader P, Hansson M, Linden A: Impact of interleukin-17 on macrophage phagocytosis of apoptotic neutrophils and particles. Inflammation 34:1–9 [DOI] [PubMed]
- 69.Codarri L., Gyulveszi G., Tosevski V., Hesske L., Fontana A., Magnenat L., Suter T., Becher B. RORγt drives production of the cytokine GM-CSF in helper T cells, which is essential for the effector phase of autoimmune neuroinflammation. Nat Immunol. 2011;12:560–567. doi: 10.1038/ni.2027. [DOI] [PubMed] [Google Scholar]
- 70.Stanfield B.B., Cowan W.M. The sprouting of septal afferents to the dentate gyrus after lesions of the entorhinal cortex in adult rats. Brain Res. 1982;232:162–170. doi: 10.1016/0006-8993(82)90619-9. [DOI] [PubMed] [Google Scholar]
- 71.Meier S., Brauer A.U., Heimrich B., Nitsch R., Savaskan N.E. Myelination in the hippocampus during development and following lesion. Cell Mol Life Sci. 2004;61:1082–1094. doi: 10.1007/s00018-004-3469-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schwab M.E. Nogo and axon regeneration. Curr Opin Neurobiol. 2004;14:118–124. doi: 10.1016/j.conb.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 73.McPhail L.T., Borisoff J.F., Tsang B., Hwi L.P., Kwiecien J.M., Ramer M.S. Protracted myelin clearance hinders central primary afferent regeneration following dorsal rhizotomy and delayed neurotrophin-3 treatment. Neurosci Lett. 2007;411:206–211. doi: 10.1016/j.neulet.2006.09.076. [DOI] [PubMed] [Google Scholar]
- 74.Schwab M.E. Myelin-associated inhibitors of neurite growth and regeneration in the CNS. Trends Neurosci. 1990;13:452–456. doi: 10.1016/0166-2236(90)90098-u. [DOI] [PubMed] [Google Scholar]
- 75.Kotter M.R., Li W.W., Zhao C., Franklin R.J. Myelin impairs CNS remyelination by inhibiting oligodendrocyte precursor cell differentiation. J Neurosci. 2006;26:328–332. doi: 10.1523/JNEUROSCI.2615-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Blakemore W.F. Regeneration and repair in multiple sclerosis: the view of experimental pathology. J Neurol Sci. 2008;265:1–4. doi: 10.1016/j.jns.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 77.Di Bello I.C., Dawson M.R., Levine J.M., Reynolds R. Generation of oligodendroglial progenitors in acute inflammatory demyelinating lesions of the rat brain stem is associated with demyelination rather than inflammation. J Neurocytol. 1999;28:365–381. doi: 10.1023/a:1007069815302. [DOI] [PubMed] [Google Scholar]
- 78.Gallo V., Mangin J.M., Kukley M., Dietrich D. Synapses on NG2-expressing progenitors in the brain: multiple functions? J Physiol. 2008;586:3767–3781. doi: 10.1113/jphysiol.2008.158436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bergles D.E., Roberts J.D., Somogyi P., Jahr C.E. Glutamatergic synapses on oligodendrocyte precursor cells in the hippocampus. Nature. 2000;405:187–191. doi: 10.1038/35012083. [DOI] [PubMed] [Google Scholar]
- 80.Mangin J.M., Kunze A., Chittajallu R., Gallo V. Satellite NG2 progenitor cells share common glutamatergic inputs with associated interneurons in the mouse dentate gyrus. J Neurosci. 2008;28:7610–7623. doi: 10.1523/JNEUROSCI.1355-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Muller J., Reyes-Haro D., Pivneva T., Nolte C., Schaette R., Lubke J., Kettenmann H. The principal neurons of the medial nucleus of the trapezoid body and NG2(+) glial cells receive coordinated excitatory synaptic input. J Gen Physiol. 2009;134:115–127. doi: 10.1085/jgp.200910194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuan X., Eisen A.M., McBain C.J., Gallo V. A role for glutamate and its receptors in the regulation of oligodendrocyte development in cerebellar tissue slices. Development. 1998;125:2901–2914. doi: 10.1242/dev.125.15.2901. [DOI] [PubMed] [Google Scholar]
- 83.Tong X.P., Li X.Y., Zhou B., Shen W., Zhang Z.J., Xu T.L., Duan S. Ca(2+) signaling evoked by activation of Na(+) channels and Na(+)/Ca(2+) exchangers is required for GABA-induced NG2 cell migration. J Cell Biol. 2009;186:113–128. doi: 10.1083/jcb.200811071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hammarberg H., Lidman O., Lundberg C., Eltayeb S.Y., Gielen A.W., Muhallab S., Svenningsson A., Linda H., van Der Meide P.H., Cullheim S., Olsson T., Piehl F. Neuroprotection by encephalomyelitis: rescue of mechanically injured neurons and neurotrophin production by CNS-infiltrating T and natural killer cells. J Neurosci. 2000;20:5283–5291. doi: 10.1523/JNEUROSCI.20-14-05283.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Besser M., Wank R. Cutting edge: clonally restricted production of the neurotrophins brain-derived neurotrophic factor and neurotrophin-3 mRNA by human immune cells and Th1/Th2-polarized expression of their receptors. J Immunol. 1999;162:6303–6306. [PubMed] [Google Scholar]
- 86.Kerschensteiner M., Gallmeier E., Behrens L., Leal V.V., Misgeld T., Klinkert W.E., Kolbeck R., Hoppe E., Oropeza-Wekerle R.L., Bartke I., Stadelmann C., Lassmann H., Wekerle H., Hohlfeld R. Activated human T cells, B cells, and monocytes produce brain-derived neurotrophic factor in vitro and in inflammatory brain lesions: a neuroprotective role of inflammation? J Exp Med. 1999;189:865–870. doi: 10.1084/jem.189.5.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chao M.V. Neurotrophins and their receptors: a convergence point for many signalling pathways. Nat Rev Neurosci. 2003;4:299–309. doi: 10.1038/nrn1078. [DOI] [PubMed] [Google Scholar]
- 88.Linker R.A., Lee D.H., Demir S., Wiese S., Kruse N., Siglienti I., Gerhardt E., Neumann H., Sendtner M., Luhder F., Gold R. Functional role of brain-derived neurotrophic factor in neuroprotective autoimmunity: therapeutic implications in a model of multiple sclerosis. Brain. 2010;133:2248–2263. doi: 10.1093/brain/awq179. [DOI] [PubMed] [Google Scholar]
- 89.Hofer M., Pagliusi S.R., Hohn A., Leibrock J., Barde Y.A. Regional distribution of brain-derived neurotrophic factor mRNA in the adult mouse brain. EMBO J. 1990;9:2459–2464. doi: 10.1002/j.1460-2075.1990.tb07423.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Burbach G.J., Dehn D., Del Turco D., Deller T. Quantification of layer-specific gene expression in the hippocampus: effective use of laser microdissection in combination with quantitative RT-PCR. J Neurosci Methods. 2003;131:83–91. doi: 10.1016/s0165-0270(03)00232-2. [DOI] [PubMed] [Google Scholar]
- 91.Lapchak P.A., Araujo D.M., Hefti F. BDNF and trkB mRNA expression in the rat hippocampus following entorhinal cortex lesions. Neuroreport. 1993;4:191–194. doi: 10.1097/00001756-199302000-00019. [DOI] [PubMed] [Google Scholar]
- 92.Forster E., Naumann T., Deller T., Straube A., Nitsch R., Frotscher M. Cholinergic sprouting in the rat fascia dentata after entorhinal lesion is not linked to early changes in neurotrophin messenger RNA expression. Neuroscience. 1997;80:731–739. doi: 10.1016/s0306-4522(97)00054-7. [DOI] [PubMed] [Google Scholar]
- 93.Bender R., Heimrich B., Meyer M., Frotscher M. Hippocampal mossy fiber sprouting is not impaired in brain-derived neurotrophic factor-deficient mice. Exp Brain Res. 1998;120:399–402. doi: 10.1007/s002210050413. [DOI] [PubMed] [Google Scholar]
- 94.Cammer W. Effects of TNFalpha on immature and mature oligodendrocytes and their progenitors in vitro. Brain Res. 2000;864:213–219. doi: 10.1016/s0006-8993(00)02178-8. [DOI] [PubMed] [Google Scholar]
- 95.Taupin V., Renno T., Bourbonniere L., Peterson A.C., Rodriguez M., Owens T. Increased severity of experimental autoimmune encephalomyelitis, chronic macrophage/microglial reactivity, and demyelination in transgenic mice producing tumor necrosis factor-alpha in the central nervous system. Eur J Immunol. 1997;27:905–913. doi: 10.1002/eji.1830270416. [DOI] [PubMed] [Google Scholar]
- 96.Gregersen R., Lambertsen K., Finsen B. Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 2000;20:53–65. doi: 10.1097/00004647-200001000-00009. [DOI] [PubMed] [Google Scholar]
- 97.Renno T., Krakowski M., Piccirillo C., Lin J.Y., Owens T. TNF-alpha expression by resident microglia and infiltrating leukocytes in the central nervous system of mice with experimental allergic encephalomyelitis: regulation by Th1 cytokines. J Immunol. 1995;154:944–953. [PubMed] [Google Scholar]
- 98.Chabot S., Williams G., Yong V.W. Microglial production of TNF-alpha is induced by activated T lymphocytes: involvement of VLA-4 and inhibition by interferonbeta-1b. J Clin Invest. 1997;100:604–612. doi: 10.1172/JCI119571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dasgupta S., Jana M., Liu X., Pahan K. Role of very-late antigen-4 (VLA-4) in myelin basic protein-primed T cell contact-induced expression of proinflammatory cytokines in microglial cells. J Biol Chem. 2003;278:22424–22431. doi: 10.1074/jbc.M301789200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Jensen M.B., Hegelund I.V., Lomholt N.D., Finsen B., Owens T. IFNgamma enhances microglial reactions to hippocampal axonal degeneration. J Neurosci. 2000;20:3612–3621. doi: 10.1523/JNEUROSCI.20-10-03612.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bruck W., Bruck Y., Friede R.L. TNF-alpha suppresses CR3-mediated myelin removal by macrophages. J Neuroimmunol. 1992;38:9–17. doi: 10.1016/0165-5728(92)90085-y. [DOI] [PubMed] [Google Scholar]
- 102.Smith M.E. Phagocytic properties of microglia in vitro: implications for a role in multiple sclerosis and EAE. Microsc Res Tech. 2001;54:81–94. doi: 10.1002/jemt.1123. [DOI] [PubMed] [Google Scholar]
- 103.Woods A.G., Guthrie K.M., Kurlawalla M.A., Gall C.M. Deafferentiation-induced increases in hippocampal insulin-like growth factor-1 messenger RNA expression are severely attenuated in middle aged and aged rats. Neuroscience. 1998;83:663–668. doi: 10.1016/s0306-4522(97)00539-3. [DOI] [PubMed] [Google Scholar]
- 104.Valenzuela C.F., Kazlauskas A., Weiner J.L. Roles of platelet-derived growth factor in the developing and mature nervous systems. Brain Res Brain Res Rev. 1997;24:77–89. doi: 10.1016/s0165-0173(97)00012-x. [DOI] [PubMed] [Google Scholar]
- 105.Chesik D., De Keyser J., Wilczak N. Insulin-like growth factor system regulates oligodendroglial cell behavior: therapeutic potential in CNS. J Mol Neurosci. 2008;35:81–90. doi: 10.1007/s12031-008-9041-2. [DOI] [PubMed] [Google Scholar]
- 106.Kar S., Baccichet A., Quirion R., Poirier J. Entorhinal cortex lesion induces differential responses in [125I]insulin-like growth factor I, [125I]insulin-like growth factor II and [125I]insulin receptor binding sites in the rat hippocampal formation. Neuroscience. 1993;55:69–80. doi: 10.1016/0306-4522(93)90455-o. [DOI] [PubMed] [Google Scholar]
- 107.Butovsky O., Ziv Y., Schwartz A., Landa G., Talpalar A.E., Pluchino S., Martino G., Schwartz M. Microglia activated by IL-4 or IFN-gamma differentially induce neurogenesis and oligodendrogenesis from adult stem/progenitor cells. Mol Cell Neurosci. 2006;31:149–160. doi: 10.1016/j.mcn.2005.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IFNγ mRNA expression visualized by in situ hybridization. A: A few cells expressing IFN-γ mRNA were detected in the hilus of the dentate gyrus and in the meninges of the hippocampal and choroid fissures (arrowheads) at 2 and 7 days post lesion in TPLP mice but not in TOVA or naïve mice. g, Granule cell layer; ml, molecular layer. B–G:In situ hybridization controls. Photomicrographs demonstrate a perivascular cuff in the cerebellum in PP-lesioned TPLP mouse after in situ hybridization with probe 1 (B), probe 2 (C), and a combination of both probes (D). Note the infiltrating cells (arrowheads) in the perivascular space exhibiting a stronger signal in sections hybridized using the mixed probes. Sections hybridized using a 100-fold excess of unlabeled IFN probe mixture (E), pretreated with RNase A (F) or buffer alone (G) were devoid of signal. Scale bars: 250 μm (A); 40 μm (B–G).
Enhanced clearance of myelin debris in TPLP mice. A: Photomicrographs of CNP-stained sections obtained from TPLP, TOVA, and naïve mice at 7 days post lesion. Although myelin debris was present in high amounts in TOVA and naïve mice (J, K, M, N), this was less pronounced in TPLP mice. g, granular cell layer; ml, molecular layer. Scale bars 25 μm; 200 μm. B: Scoring demonstrated that PP-lesioned TPLP mice contained less myelin debris than did PP-lesioned TOVA and naïve mice at 7 days after lesion. Lines indicate medians. **p< 0.01; ***p<0.001.


