Abstract
Antiplatelet agents are the mainstay treatment in the prevention and management of atherothrombotic complications. However, a substantial interpatient variability in response to clopidogrel has been reported. Furthermore, patients with coronary artery disease and lesser platelet inhibition in response to clopidogrel are at increased risk for cardiovascular events. Clopidogrel after absorption requires two-step oxidation by the hepatic cytochrome P450 to generate its active metabolite. Polymorphisms of genes encoding the cytochrome enzymes and P-glycoprotein involved in clopidogrel absorption are regarded as major determinants of the interindividual variability in the clopidogrel-induced platelet inhibition. In our review we discuss the prevalence and clinical significance of various alleles of the genes: CYP2C19 and ABCB1 in the setting of coronary artery disease. Allele CYP2C19*2 is associated with excess of ischaemic events including myocardial infarction and stent thrombosis. On the other hand, CYP2C19*17 allele poses a serious threat of bleeding. Data concerning the prognostic value of genetic variant 3435C→T of ABCB1 remain inconclusive.
Keywords: Clopidogrel, Platelets, Polymorphism, Interindividual variability, Cytochrome P450
Introduction
Several large clinical trials have shown that clopidogrel effectively prevents thrombotic events in patients with atherosclerotic vascular disease [1–4]. Clopidogrel administration combined with aspirin is a standard therapy in acute coronary syndromes and after stent implantation.
However, pharmacodynamic response to clopidogrel has substantial interpatient variability, and patients with coronary disease and lesser platelet inhibition in response to clopidogrel appear to be at increased risk for cardiovascular events [5–11].
There are several possible mechanisms of clopidogrel response variability or “resistance” [12, 13]. If a patient is not taking clopidogrel or taking it only intermediately, the patient will appear by platelet function testing to be hyporesponsive or resistant to clopidogrel. Other drugs may interfere with clopidogrel metabolism through hepatic cytochrome P450. Single nucleotide polymorphism may modulate platelet response to clopidogrel therapy [14]. Other factors modifying response to clopidogrel should be also taken in account, e.g. accelerated platelet turnover, with introduction into bloodstream of newly formed, drug-unaffected platelets that can strongly influence antiplatelet effect of clopidogrel [15]. Numerous clinical conditions such as elderly age, diabetes mellitus, decreased left ventricular function, renal failure, and acute coronary syndrome are associated with an impaired antiplatelet action of clopidogrel. Treatment failure unrelated to a lack of clopidogrel effectiveness cannot be excluded because arterial thrombosis is a multifactorial phenomenon, not solely dependent on P2Y12 activation [16–19].
In this review we discuss genetic background of platelet response to clopidogrel and its clinical significance in patients with coronary artery disease.
Genetic background of clopidogrel metabolism
Clopidogrel, like all thienopyridines, is an inactive prodrug. It requires two-step oxidation by the hepatic cytochrome P450 to generate its active thiol metabolite. The metabolite targets and irreversibly inhibits P2Y12 receptor. Esterases shunt the remaining prodrug to an inactive pathway. The genes encoding the cytochrome enzymes are polymorphic, and there are some genetic variants that diminish transformation into the active metabolite (Fig. 1). Several studies have established that CYP2C19 genotyping identifies more than 90% of poor clopidogrel metabolizers, indicating good genotype–phenotype agreement [10, 20].
Fig. 1.
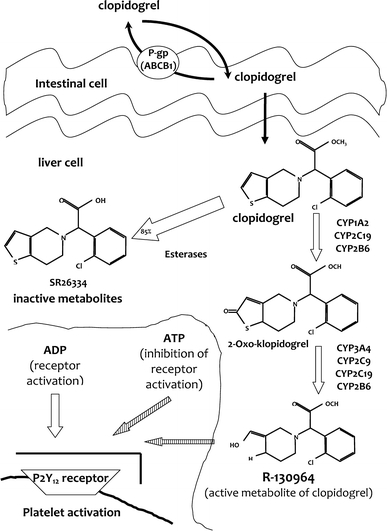
Genetic determinants of clopidogrel metabolism
There are 25 known polymorphic variants of the CYP2C19 gene, with the frequency varying among different ethnic groups [21]. Some of these genetic variants strongly influence the enzyme function (Table 1). According to the allele present, the enzymatic activity of CYP2C19 may be normal, reduced, or increased, resulting in different plasma concentration of clopidogrel’s active metabolite (Table 2) [22–24]. The CYP2C19*1 allele is the normal (wild-type) copy that has full enzymatic activity. The CYP2C19*2 and CYP2C19*3 alleles are the most common variants resulting in complete loss of enzymatic activity. Carriers of the *2 and *3 alleles have reduced metabolism of clopidogrel and demonstrate diminished clopidogrel-induced platelet inhibition. Other genetic variants of CYP2C19-*4 and *5, also result in no enzymatic activity, but their effect on platelet inhibition by clopidogrel has not been fully documented [25–27]. Finally, the CYP2C19*17 allele results in increased CYP2C19 activity, higher production of active metabolite, and improved clopidogrel-induced platelet inhibition [23, 24, 28].
Table 1.
The most frequent CYP2C19 alleles and their impact on the enzyme function
| CYP2C19 allele | Nucleotide change | Impact on the enzyme function | Prevalence in different populations (%) |
|---|---|---|---|
| *1 | None (wildtype) | Normal | |
| *2 | 681G > A | Inactive | 25–55 |
| *3 | 636G > A | Inactive | 1–7 |
| *4 | 1A > G | Inactive | <1 |
| *5 | 1297C > T | Inactive | <1 |
| *17 | 991A > G | Increased | 4–41 |
Table 2.
Expected effects of CYP2C19 polymorphism on clopidogrel metabolism
| Genotype | Expected effect on clopidogrel metabolism |
|---|---|
| CYP2C19*1/*1 | Normal metabolizer—the subject is expected to demonstrate effective antiplatelet response to a standard dose of clopidogrel |
| CYP2C19*1/*2 | Intermediate metabolizer—the subject exhibits approximately half-normal enzyme activity, thus an increased dosage of clopidogrel or an alternative treatment should be considered |
| CYP2C19*1/*3 | |
| CYP2C19*1/*4 | |
| CYP2C19*1/*5 | |
| CYP2C19*2/*17 | Normal/intermediate metabolizer—the subject is likely to have metabolic activity between normal and intermediate metabolizers. In general, the response to a standard dose of clopidogrel should be sufficient |
| CYP2C19*3/*17 | |
| CYP2C19*4/*17 | |
| CYP2C19*5/*17 | |
| CYP2C19*2/*2 | Poor metabolizer—the subject is characterized by a greatly decreased enzyme activity and may require an alternative treatment or higher than standard dosage of clopidogrel to achieve required antiplatelet effect |
| CYP2C19*2/*3 | |
| CYP2C19*2/*4 | |
| CYP2C19*2/*5 | |
| Or other configuration of these alleles | |
| CYP2C19*1/*17 | Rapid metabolizer—the subject have elevated enzyme activity and may be exposed to a higher concentration of active drug metabolites resulting in increased bleeding risk |
| CYP2C19*17/*17 | Ultra rapid metabolizer—the subject have markedly elevated enzyme activity. For prodrugs, like clopidogrel, ultra rapid metabolizers are exposed to a higher concentration of active drug metabolites and may require lower than standard dosage because of increased risk of bleeding |
The prevalence of different CYP2C19 alleles varies by ethnicity (Figs. 2, 3, Table 1). In the study published by Sibbing et al. [29] 73% of patients in the German population were CYP2C19 wild-type homozygotes (CYP2C19*1/*1), 25% were heterozygotes with one CYP2C19*2 allele and only 2% were homozygotes (CYP2C19*2/*2). Almost the same frequencies were found by Collet et al. [22] in the French population. Due to the rare occurrence of the CYP2C19*2/*2 genotype, majority of clinical analyses are based on the comparison between wild-type homozygotes (CYP2C19*1/*1) and carriers of at least one allele CYP2C19*2 (*1/*2 or *2/*2). CYP2C19*2 mutant allele are more common in other populations—present in approximately 40% of Africans, and more than 55% of East Asians [9]. The proportion of patients who carry at least one copy of CYP2C19*3 allele in Caucasians, Africans, and Asians, is <1, <1, and 7%, respectively. Other loss-of-function genetic variants of CYP2C19-*4 and *5 are rare in all ethnicities (<1%) [22]. Relatively high frequencies of CYP2C19*17 allele were found: in Germans 41% (36% heterozygotes and 5% homozygotes), in French 20%, both in Swedes and Ethiopians 18%, and only in 4% of Chinese [23, 24, 28].
Fig. 2.
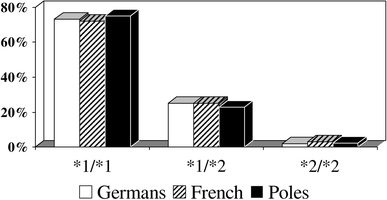
CYP2C19*2 genotype frequency in the German (n = 2485), French (n = 259), and Polish (n = 347) populations [22, 29, and unpublished data of the authors]. *1-allele CYP2C19*1; *2-allele CYP2C19*2
Fig. 3.
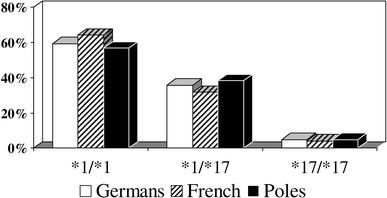
CYP2C19*17 genotype frequency in the German (n = 1524), French (n = 598), and Polish (n = 347) populations [24, 28, and unpublished data of the authors]. *1-CYP2C19*1, *17-allele CYP2C19*17
The most frequent genetic functional variants of cytochrome P450 2C19 enzyme: CYP2C19*2 and CYP2C19*17 are important contributors to the wide interindividual variability of the antiplatelet effect of clopidogrel (Table 2). The first studies in this field concerned CYP2C19 681 G > A*2 allelic variant encoding a cryptic splice site resulting in loss of CYP2C19 enzyme activity.
Polymorphism of ABCB1, a gene encoding the P-glycoprotein involved in clopidogrel absorption, is another emerging issue in the field of cardiovascular genetics. The genetic variant 3435C→T is associated with impaired function of the intestinal drug-efflux transporter. The prevalence of homozygotes CC, heterozygotes CT, and homozygotes TT in the French population (n = 2,188) was reported to be 25.8, 48.0, and 26.2%, respectively [30].
Relation of CYP2C19*2 allele to the platelet inhibition by clopidogrel
Hulot et al. [25] evaluated platelet aggregation during and after a 7-day course of clopidogrel according to the CYP2C19 genotype in healthy volunteers. There were huge differences in ex vivo ADP-induced platelet aggregation between carriers and noncarriers of the CYP2C19*2 mutant allele encoding a deficient drug metabolizing enzyme—a very good response after a 7-day course of clopidogrel in wild-type homozygotes (CYP2C19*1/*1) and impaired responsiveness in carriers of CYP2C19*2 mutant allele [25].
Mega et al. [9] showed that carriers of at least one CYP2C19 reduced-function allele had relative reductions of 32.4 and 9% in plasma concentration of the active metabolite of clopidogrel and maximal platelet aggregation, respectively, as compared with non-carriers. The pharmacokinetic and pharmacodynamic effects of CYP2C19 reduced-function allele on the response to clopidogrel were observed after a loading dose (either 300 or 600 mg) and during the administration of a maintenance dose (75 mg). Carriers of a reduced-function CYP2B6 allele also tended to have a lower pharmacokinetic and pharmacodynamic response to clopidogrel [9].
These observations were confirmed in a group of 797 consecutive patients undergoing percutaneous coronary intervention, who were followed for 1 year [21]. Patients carrying at least one CYP2C19*2 allele are more prone to high platelet ADP-induced residual platelet aggregation [21].
Impact of CYP2C19*2 polymorphism on the clinical outcome
Because of the profound impact of genetic variation in CYP2C19 activity on clopidogrel-induced inhibition of platelet aggregation, there has been considerable investigation in extending these observations to clinical outcomes.
The great impact of CYP2C19 polymorphism on clinical outcome was shown by Collet et al. [22] in a population of young patients after myocardial infarction treated with clopidogrel. The finding of a strong relation between presence of CYP2C19 reduced-function mutant allele (CYP2C19*2) and recurrent coronary events was consistent in a subset analysis, with both stent-related and stent-independent coronary events. The primary endpoint (composite of death, myocardial infarction, and urgent coronary revascularization) occurred more frequently in carriers of mutant allele than in non-carriers (HR 3.69; 95% CI 1.69–8.05; P = 0.0005), as did stent thrombosis (HR 6.02; 95% CI 1.81–20.04; P = 0.0009). Multivariable analysis revealed that the presence of CYP2C19*2 allele was the only independent predictor of cardiovascular events (HR 4.04; 95% CI 1.81–9.02; P = 0.0006) in young patients receiving clopidogrel after myocardial infarction [22].
Another study of 2485 consecutive patients after coronary stent placement revealed more than threefold higher 30-day incidence of stent thrombosis in CYP2C19*2 mutant allele carriers (*1/*2 or *2/*2) as compared with CYP2C19*1 wild-type homozygous patients (P = 0.007) [29]. The study also demonstrated that CYP2C19*2 carrier status is an independent predictor for the occurrence of stent thrombosis. Besides stent thrombosis, the cumulative incidence of ST-segment elevation myocardial infarction was significantly higher in mutant allele carriers versus wild-type homozygotes, so was the incidence of ischaemic stroke. These results are corroborated by a gene-dose effect of the CYP2C19*2 allele with homozygous allele carriers (*2/*2) exhibiting the highest risk of stent thrombosis [29].
Similarly, Małek et al. [31] observed suboptimal reperfusion and increased all-cause mortality in carriers of the CYP2C19*2 allele treated with primary percutaneous coronary intervention. The findings are in line with pharmacodynamic [21, 32] and pharmacokinetic [33, 34] investigations showing the strongest attenuation of platelet response to clopidogrel for the mutant CYP2C19 allele homozygotes (*2/*2).
The next proof of clinical significance of CYP2C19 polymorphism in patients treated with clopidogrel comes from the genetic substudy of TRITON-TIMI 38 (Trial to assess improvement in therapeutic outcomes by optimizing platelet inhibition with prasugrel TIMI 38) trial [9]. The study provides genetic data for almost 1,500 patients treated with clopidogrel. 395 (27.1%) of them were carriers of at least one CYP2C19 reduced-function allele. In concordance with pharmacokinetic and pharmacodynamic findings, the risk of primary efficacy outcome of death from cardiovascular causes, myocardial infarction, or stroke was significantly higher in carriers as compared to noncarriers—Fig. 4 (hazard ratio for carriers, 1.53; 95% confidence interval, 1.07–2.19; P = 0.01). No significant associations between any other CYP genotypes and clinical outcome were observed. CYP2C19*2 allele carriers had a higher rate of stent thrombosis than noncarriers (2.6 vs. 0.8%; hazard ratio for carriers 3.09; 95% confidence interval, 1.19–8.00; P = 0.02) [9].
Fig. 4.
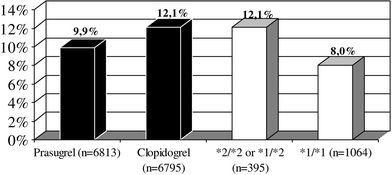
Primary end point (cardiovascular death, myocardial infarction, stroke) in the TRITON-TIMI 38 trial (clopidogrel and prasugrel arms—black bars) and in the TRITON-TIMI 38 genetic substudy in clopidogrel arm in relation to CYP2C19 polymorphism (white bars) [9, 36]. *1-allele CYP2C19*1, *2-allele CYP2C19*2
Results of the pharmacokinetic and pharmacodynamic studies mentioned above, combined by Mega et al. [9] with clinical observations, provided strong evidence linking polymorphism in the CYP2C19 gene with a reduced exposure to the active clopidogrel metabolite, less platelet inhibition, and as the consequence less protection from ischemic events.
Well known TRITON-TIMI 38 study showed significantly better results in the prasugrel group as compared to the whole group of patients treated with clopidogrel in terms of primary efficacy outcome of cardiovascular death, myocardial infarction, and stroke [35]. Interestingly, the primary efficacy outcome in patients with favourable genotype (noncarriers of CYP2C19*2 allele) treated with prasugrel was comparable to this observed in patients assigned to prasugrel therapy irrespectively of the genotype (Fig. 4). In contrast to this finding, carriers of this mutant allele treated with clopidogrel demonstrated a significantly higher incidence of primary efficacy outcome as compared to wild-type homozygotes (P = 0.01) [9, 36].
There are several other studies concerning the clinical significance of CYP2C19 polymorphism. Pooled data from 10 studies (11,959 patients) displayed a 30% increase in the risk of major adverse cardiovascular events in carriers of CYP2C19*2 [27]. This single gene variant was also associated with an 80% excess in mortality (6,225 patients) and 3.5-fold higher risk of stent thrombosis (4,905 patients). This increased risk was apparent in both heterozygotes and homozygotes and was independent of the baseline cardiovascular risk [27]. Similarly, a recent meta-analysis of 9,685 patients on clopidogrel therapy treated predominantly with coronary stenting clearly indicates unfavorable clinical outcome in carriers of CYP2C19*2 when compared to noncarriers (Fig. 5) [36]. On the other hand, a combined analysis of the genetic substudies of CURE (clopidogrel in unstable angina to prevent recurrent events) and ACTIVE (atrial fibrillation clopidogrel trial with irbesartan for prevention of vascular events) trials suggests that in patients on clopidogrel therapy treated mostly conservatively, CYP2C19 polymorphism may not play any major clinical role [37].
Fig. 5.
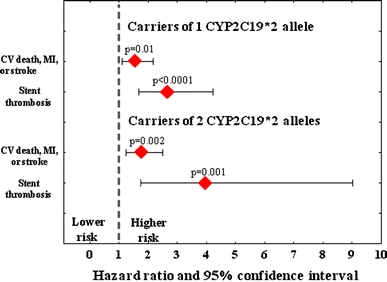
Occurrence of ischaemic events in patients treated with clopidogrel in relation to the CYP2C19*2 polymorphism in the metaanalysis (n = 9685) conducted by Mega et al. [37]. CV cardiovascular death, MI myocardial infarction
Theoretical considerations imply that a concomitant use of proton pump inhibitors may competitively inhibit activation of clopidogrel by CYP2C19, thereby attenuating its antiplatelet effect. However, a subanalysis of TRITON-TIMI 38 trial failed to establish any clinical impact of CYP2C19 polymorphism in patients treated with clopidogrel and proton pump inhibitors [38]. Furthermore, in two recent ex vivo studies coadministration of clopidogrel and proton pump inhibitors led to a decreased antiplatelet effect in all genetic subsets [39, 40]. However, the attenuation of clopidogrel antiplatelet activity was paradoxically least pronounced in carriers of a loss-of-function allele.
Potential strategies dedicated to overcome genetic clopidogrel resistance
Numerous strategies to facilitate platelet inhibition in carriers of a reduced-function alleles were proposed. The simplest ones include an increase of clopidogrel loading and/or maintenance doses, and switching to ticlopidine. Although such changes decrease platelet aggregation, their favourable clinical impact has not been convincingly proven so far [41, 42].
Other therapeutic options include switching to a more potent P2Y12 inhibitor, use of hepatic cytochrome P450—independent agents, and additional blockade of alternative platelet activation pathways.
As mentioned previously, according to the genetic substudy of TRITON-TIMI 38 trial, prasugrel confers clinical benefits only in clopidogrel poor metabolizers [9].
On the other hand, ticagrelor, a direct and reversible P2Y12 inhibitor, decreases prevalence of ischaemic events irrespectively of the CYP2C19 genotype, as shown in the genetic substudy of PLATO (the platelet inhibition and patient outcomes) trial [43]. Cangrelor and elinogrel are other potent reversible P2Y12 inhibitors which do not require activation and may be particularly effective in clopidogrel poor metabolizers. However, in two large-scale international phase III trials in patients undergoing percutaneous coronary interventions intravenous therapy with cangrelor was comparable in terms of the clinical efficiency to 600 mg of oral clopidogrel [44] and intravenous pretreatment with cangrelor before administration of a loading dose of clopidogrel was not superior to placebo in reducing ischaemic events [45]. Interestingly, elinogrel is suitable for both acute intravenous and long-term oral therapy. In an unpublished phase IIb clinical study—INNOVATE-PCI, elinogrel when compared with clopidogrel achieved a more rapid and potent platelet inhibition with similar rates of bleedings and clinical and biological efficacy end-points in patients treated with elective coronary stenting.
Cilostazol, an inhibitor of phosphodiesterase 3, when added to a standard clopidogrel maintenance dose, suppresses ADP-dependent platelet aggregation more effectively than a high clopidogrel maintenance dose in patients with high post-treatment platelet reactivity undergoing coronary stenting [46]. However, no genetic analysis was performed in this study.
Similarly, periprocedural glycoprotein IIb/IIIa receptor blockade with tirofiban in patients undergoing elective coronary stenting successfully reduced ischemic events among those resistant to clopidogrel and/or aspirin [47]. In this study patients were assigned to tirofiban or placebo on the basis of platelet function evaluation with a point-of-care assay, without CYP2C19 genotyping.
Significance of CYP2C19*17 polymorphism
In 2006 Sim et al. [23] identified a novel allele (CYP2C19*17) carrying mutations: –806C > T and –3402C > T. In an experimental study they showed increased transcriptional activity of the CYP2C19*17 allele in vivo in mice. The presence of CYP2C19*17 allele results in ultrarapid metabolism of all CYP2C19 substrates.
Frere et al. [24] evaluated the impact of clopidogrel extensive metabolism in the presence of CYP2C19*17 allelic variant on platelet response to the drug in patients suffering from non-ST elevation acute coronary syndrome. The observed frequency of the CYP2C19*17 allele in the group of 449 patients was 20%. The CYP2C19*17 allele carriers exhibited the lowest vasodilator stimulated phosphoprotein phosphorylation index PRI VASP (mean ± SD 49.7 ± 23.7 vs. 55.9 ± 22.8), testifying a better platelet response to a 600 mg loading dose of clopidogrel [24]. These ex vivo results were confirmed in vivo by Sibbing et al. [28].
They assessed the impact of CYP2C19*17 on ADP-induced platelet aggregation, the risk of bleeding, and stent thrombosis occurrence in clopidogrel-treated patients undergoing percutaneous coronary intervention. The study population consisted of 1524 patients. Both heterozygous (*1/*17; n = 546) and homozygous (*17/*17; n = 76) allele carriers had significantly lower ADP-induced platelet aggregation values as compared with wild-type homozygotes. CYP2C19*17 allele carriage was also identified as an independent risk factor of bleeding (odds ratio for carriers of at least 1 allele vs. noncarriers 1.85; 95% confidence interval 1.19–2.86; P = 0.006). However, no significant influence of CYP2C19*17 on the occurrence of stent thrombosis was found [28].
Clinical consequences of ABCB1 polymorphism
Although the genetic variant 3435C→T of ABCB1 is associated with impaired function of the intestinal drug-efflux transporter, the data concerning its clinical significance remain inconclusive. Simon et al. [30] analyzing patients presenting with myocardial infarction and receiving clopidogrel from a nationwide French registry found that carriers of two variant alleles 3435C → T of had a higher rate of cardiovascular events (death from any cause, nonfatal stroke, or myocardial infarction) at 1 year than those with the ABCB1 wild-type genotype (CC at nucleotide 3435) (15.5 vs. 10.7%; adjusted hazard ratio 1.72; 95% confidence interval 1.20–2.47). However, in an subanalysis of patient undergoing coronary angioplasty the ABCB1 polymorphism was not an independent predictor of outcome. Furthermore, recently published genetic substudies of TRITON-TIMI 38 and PLATO trials demonstrated contrasting results [43, 48]. Mega at al. [48] noted an increased risk of recurrent ischaemic events in carriers of two variant alleles 3435C→T treated with clopidogrel. According to their observations, in healthy individuals such genotype was linked with less platelet inhibition [48]. In contrast, Wallentin at al. [43] observed even a numerically higher rate of primary efficacy events for the high-expression group (patients with the ABCB1 3435 CC genotype) who were on clopidogrel.
Mutations in the P2Y12 receptor
Besides CYP2C19 and ABCB1 mutations, polymorphism of the P2Y12 receptor can at least theoretically cause clopidogrel non-responsiveness. Cattaneo described several infrequent genetically determined defects of the platelet P2Y12 receptor leading to a mild-moderate bleeding diathesis [49]. However, in a genetic analysis of the FAST-MI registry including patients with an acute myocardial infarction receiving clopidogrel therapy, none of the selected single-nucleotide polymorphisms in the P2Y12 receptor was associated with a risk of an adverse outcome [30].
Is it time for routine genotyping in patients on clopidogrel therapy?
Most of commercially available genotyping assays are precluded from clinical use for establishing the optimal drug choice in the acute setting since they require sophisticated methodology, highly-skilled staff and several day period before the final results are obtained. However, first point-of-care assays for the CYP2C19 genotyping, have been recently introduced. They include the AmpliChip Cyp450 test (Roche Diagnostics GmbH, Mannheim, Germany), Infinity (AutoGenomics, Carlsbad, CA, US), and Verigene System (Nanosphere, Northbrook, IL, US). These tests can be performed on small amounts of whole blood with a turnaround time of about 3–8 h.
It should be also taken into account that platelet function is dynamic in individual patients because of the influence of variable external factors, while the influence of genetic variants is intrinsically constant. Thus, it may be reasonable to consider both genotyping and platelet function measurement to assess ischaemic risk and to facilitate antiplatelet therapy [50].
Finally, it has not been proven in prospective, adequately-powered, randomized trials so far that a decision-making process based on the genotyping of variants involved in the clopidogrel metabolism improves clinical outcome. Several such projects (ELEVATE-TIMI 56, GIANT, RAPID GENE, TARGET-PCI) are currently ongoing.
Conclusions
Different combinations of genetic variants may generate high interpatient variability in clopidogrel metabolism. The genetically determined variability in clopidogrel metabolism results in differences of clinical outcome in patients on antiplatelet therapy with this drug. However, due to limited data supporting this strategy, a routine adoption of genotyping into clinical practice to guide antiplatelet therapy cannot be currently recommended.
Conflict of interest
None.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
References
- 1.Bertrand ME, Rupprecht HJ, Urban P, Gershlick AH, CLASSIC Investigators Double-blind study of the safety of clopidogrel with and without a loading dose in combination with aspirin compared with ticlopidine in combination with aspirin after coronary stenting: the clopidogrel aspirin stent international cooperative study (CLASSICS) Circulation. 2000;102:624–629. doi: 10.1161/01.cir.102.6.624. [DOI] [PubMed] [Google Scholar]
- 2.Mehta SR, Yusuf S, Peters RJ, et al. Effect of pretreatment with clopidogrel and aspirin followed by long-therm therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527–533. doi: 10.1016/S0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- 3.Steinhubl SR, Berger PB, Mann JT, 3rd, et al. Early and sustained dual oral antiplatelet therapy following percutaneous coronary intervention: a randomized controlled trial. JAMA. 2002;288:2411–2420. doi: 10.1001/jama.288.19.2411. [DOI] [PubMed] [Google Scholar]
- 4.Sabatine MS, Cannon CP, Gibson CM, et al. Effect of clopidogrel pretreatment before percutaneous coronary intervention in patients with ST-elevation myocardial infarction treated with fibrinolytics: the PCI-CLARITY study. JAMA. 2005;294:1224–1232. doi: 10.1001/jama.294.10.1224. [DOI] [PubMed] [Google Scholar]
- 5.Barsky AA, Arora RR. Clopidogrel resistance: myth or reality? J Cardiovasc Pharmacol Ther. 2006;11:47–53. doi: 10.1177/107424840601100104. [DOI] [PubMed] [Google Scholar]
- 6.Husted S. New developments in oral antiplatelet therapy. Eur Heart J Supp. 2007;9:D20–D27. doi: 10.1093/eurheartj/sum012. [DOI] [Google Scholar]
- 7.Müller I, Besta F, Schulz C, Massberg S, Schönig A, Gawaz M. Prevalence of clopidogrel non-responders among patients with stable angina pectoris scheduled for elective coronary stent placement. Thromb Haemost. 2003;89:783–787. [PubMed] [Google Scholar]
- 8.Serebruany VL, Steinhubl SR, Berger PB, Malinin AI, Bhatt DL, Topol EJ. Variability in platelet responsiveness to clopidogrel among 544 individuals. J Am Coll Cardiol. 2005;45:246–251. doi: 10.1016/j.jacc.2004.09.067. [DOI] [PubMed] [Google Scholar]
- 9.Mega JL, Close SL, Wiviott SD, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N Engl J Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 10.Kubica J, Koziński M, Grześk G. Mechanizmy działania leków przeciwpłytkowych. Folia Cardiol Excerpta. 2009;4:10–17. [Google Scholar]
- 11.Matetzky S, Shenkman B, Guetta V, et al. Clopidogrel resistance is associated with increased risk of recurrent atherothrombotic events in patients with acute myocardial infarction. Circulation. 2004;109:3171–3175. doi: 10.1161/01.CIR.0000130846.46168.03. [DOI] [PubMed] [Google Scholar]
- 12.Cattaneo M. Aspirin and clopidogrel: efficacy, safety, and the issue of drug resistance. Arterioscler Thromb Vasc Biol. 2004;24:1980–1987. doi: 10.1161/01.ATV.0000145980.39477.a9. [DOI] [PubMed] [Google Scholar]
- 13.Michelson AD. P2Y12 antagonism. Promises and challenges. Arterioscler Thromb Vasc Biol. 2008;28:s33–s38. doi: 10.1161/ATVBAHA.107.160689. [DOI] [PubMed] [Google Scholar]
- 14.Bryniarski L, Pelc-Nowicka A, Zabojszcz M, Mirek-Bryniarska E. Dual antiplatelet therapy and antithrombotic treatment: recommendations and controversies. Cardiol J. 2009;16:179–189. [PubMed] [Google Scholar]
- 15.Koziński M, Bielis L, Wiśniewska-Szmyt J, et al. Increased morning ADP-dependent platelet aggregation persists despite dual antiplatelet therapy in patients with first ST-segment elevation myocardial infarction: preliminary report. Cardiol J. 2008;15:530–536. [PubMed] [Google Scholar]
- 16.Kubica A. Problemy długotrwałej terapii przeciwpłytkowej po implantacji stentu do tętnicy wieńcowej. Post Kardiol Interw. 2009;5:158–161. [Google Scholar]
- 17.Trzos E, Uznańska B, Rechciński T, Krzemińska-Pakuła M, Bugała M, Kurpesa M. Myocardial infarction in young people. Cardiol J. 2009;16:307–311. [PubMed] [Google Scholar]
- 18.Kasprzak M, Koziński M, Bielis L, et al. Pantoprazole may enhance antiplatelet effect of enteric-coated aspirin in patients with acute coronary syndrome. Cardiol J. 2009;16:535–544. [PubMed] [Google Scholar]
- 19.Kralev S, Krause B, Papavassiliu T, et al. Clinical outcome of patients with diabetes presenting with ST-elevation myocardial infarction and treated with concomitant use of glycoprotein IIb/IIIa inhibitors. Cardiol J. 2009;16:234–240. [PubMed] [Google Scholar]
- 20.von Beckerath N, Taubert D, Pogatsa-Murray G, Schömig E, Kastrati A, Schömig A. Absorption, metabolization, and antiplatelet effects of 300-, 600-, and 900-mg loading doses of clopidogrel: results of the ISAR-CHOICE (intracoronary stenting and antithrombotic regimen: choose between 3 high oral doses for immediate clopidogrel effect) trial. Circulation. 2005;112:2946–2950. doi: 10.1161/CIRCULATIONAHA.105.559088. [DOI] [PubMed] [Google Scholar]
- 21.Trenk D, Hochholzer W, Fromm MF, et al. Cytochrome P450 2C19 681G >A polymorphism and high on-clopidogrel platelet reactivity associated with adverse 1-year clinical outcome of elective percutaneous coronary intervention with drug-eluting or bare-metal stents. J Am Coll Cardiol. 2008;51:1925–1934. doi: 10.1016/j.jacc.2007.12.056. [DOI] [PubMed] [Google Scholar]
- 22.Collet JP, Hulot JS, Pena A, et al. Cytochrome P450 2C19 polymorphism in young patients treated with clopidogrel after myocardial infarction: a cohort study. Lancet. 2009;373:309–317. doi: 10.1016/S0140-6736(08)61845-0. [DOI] [PubMed] [Google Scholar]
- 23.Sim SC, Risinger C, Dahl ML, et al. A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther. 2006;79:103–113. doi: 10.1016/j.clpt.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 24.Frére C, Cuisset T, Gaborit B, Alessi MC, Hulot JS. The CYP2C19*17 allele is associated with better platelet response to clopidogrel in patients admitted for non-ST acute coronary syndrome. J Thromb Haemost. 2009;7:1409–1411. doi: 10.1111/j.1538-7836.2009.03500.x. [DOI] [PubMed] [Google Scholar]
- 25.Hulot JS, Bura A, Villard E, et al. Cytochrome P450 2C19 loss-of-function polymorphism is a major determinant of clopidogrel responsiveness in healthy subjects. Blood. 2006;108:2244–2247. doi: 10.1182/blood-2006-04-013052. [DOI] [PubMed] [Google Scholar]
- 26.Storey RF. Clopidogrel in acute coronary syndrome: to genotype or not? Lancet. 2009;373:276–278. doi: 10.1016/S0140-6736(08)61846-2. [DOI] [PubMed] [Google Scholar]
- 27.Hulot JS, Collet JP, Silvain J, et al. Cardiovascular risk in clopidogrel-treated patients according to cytochrome P450 2C19*2 loss-of-function allele or proton pump inhibitor co-administration. a systematic meta-analysis. J Am Coll Cardiol. 2010;56:134–143. doi: 10.1016/j.jacc.2009.12.071. [DOI] [PubMed] [Google Scholar]
- 28.Sibbing D, Koch W, Gebhard D, et al. Cytochrome 2C19*17 allelic variant, platelet aggregation, bleeding events, and stent thrombosis in clopidogrel-treated patients with coronary stent placement. Circulation. 2010;121:512–518. doi: 10.1161/CIRCULATIONAHA.109.885194. [DOI] [PubMed] [Google Scholar]
- 29.Sibbing D, Stegherr J, Latz W, et al. Cytochrome P450 2C19 loss-of-function polymorphism and stent thrombosis following percutaneous coronary intervention. Eur Heart J. 2009;30:916–922. doi: 10.1093/eurheartj/ehp041. [DOI] [PubMed] [Google Scholar]
- 30.Simon T, Verstuyft C, Mary-Krause M, et al. Genetic determinants of response to clopidogrel and cardiovascular events. N Engl J Med. 2009;360:363–375. doi: 10.1056/NEJMoa0808227. [DOI] [PubMed] [Google Scholar]
- 31.Małek LA, Przyłuski J, Spiewak M, et al. Cytochrome P450 2C19 polymorphism, suboptimal reperfusion and all-cause mortality in patients with acute myocardial infarction. Cardiology. 2010;117:81–87. doi: 10.1159/000320093. [DOI] [PubMed] [Google Scholar]
- 32.Frere C, Cuisset T, Morange PE, et al. Effect of cytochrome p450 polymorphisms on platelet reactivity after treatment with clopidogrel in acute coronary syndrome. Am J Cardiol. 2008;101:1088–1093. doi: 10.1016/j.amjcard.2007.11.065. [DOI] [PubMed] [Google Scholar]
- 33.Brandt JT, Close SL, Iturria SJ, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007;5:2429–2436. doi: 10.1111/j.1538-7836.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 34.Umemura K, Furuta T, Kondo K. The common gene variants of CYP2C19 affect pharmacokinetics and pharmacodynamics to an active metabolite of clopidogrel in healthy subjects. J Thromb Haemost. 2008;6:1439–1441. doi: 10.1111/j.1538-7836.2008.03050.x. [DOI] [PubMed] [Google Scholar]
- 35.Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357:2001–2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 36.Mega JL, Simon T, Collet JP, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–1830. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paré G, Mehta SR, Yusuf S, et al. Effects of CYP2C19 genotype on outcomes of clopidogrel treatment. N Engl J Med. 2010;363:1704–1714. doi: 10.1056/NEJMoa1008410. [DOI] [PubMed] [Google Scholar]
- 38.O’Donoghue ML, Braunwald E, Antman EM, et al. Pharmacodynamic effect and clinical efficacy of clopidogrel and prasugrel with or without a proton-pump inhibitor: an analysis of two randomised trials. Lancet. 2009;374:989–997. doi: 10.1016/S0140-6736(09)61525-7. [DOI] [PubMed] [Google Scholar]
- 39.Furuta T, Iwaki T, Umemura K. Influences of different proton pump inhibitors on the anti-platelet function of clopidogrel in relation to CYP2C19 genotypes. Br J Clin Pharmacol. 2010;70:383–392. doi: 10.1111/j.1365-2125.2010.03717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee JK (2010) Clopidogrel–PPI interaction more relevant in noncarriers of CYP2C19 loss-of-function gene. http://www.theheart.org/article/1120273.do
- 41.Maeda A, Ando H, Asai T, et al. Differential impacts of CYP2C19 gene polymorphisms on the antiplatelet effects of clopidogrel and ticlopidine. Clin Pharmacol Ther. 2011;89:229–233. doi: 10.1038/clpt.2010.268. [DOI] [PubMed] [Google Scholar]
- 42.Neubauer H, Kaiser AF, Endres HG, et al. Tailored antiplatelet therapy can overcome clopidogrel and aspirin resistance—the bochum clopidogrel and aspirin plan (BOCLA-Plan) to improve antiplatelet therapy. BMC Med. 2011;9:3. doi: 10.1186/1741-7015-9-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wallentin L, James S, Storey RF, et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–1328. doi: 10.1016/S0140-6736(10)61274-3. [DOI] [PubMed] [Google Scholar]
- 44.Harrington RA, Stone GW, Mc Nulty S, et al. Platelet inhibition with cangrelor in patients undergoing PCI. N Engl J Med. 2009;361:2318–2329. doi: 10.1056/NEJMoa0908628. [DOI] [PubMed] [Google Scholar]
- 45.Bhatt DL, Lincoff AM, Gibson CM, et al. Intravenous platelet blockade with cangrelor during PCI. N Engl J Med. 2009;361:2330–2341. doi: 10.1056/NEJMoa0908629. [DOI] [PubMed] [Google Scholar]
- 46.Jeong YH, Lee SW, Choi BR, et al. Randomized comparison of adjunctive cilostazol versus high maintenance dose clopidogrel in patients with high post-treatment platelet reactivity: results of the ACCEL-RESISTANCE (adjunctive cilostazol versus high maintenance dose clopidogrel in patients with clopidogrel resistance) randomized study. J Am Coll Cardiol. 2009;53:1101–1109. doi: 10.1016/j.jacc.2008.12.025. [DOI] [PubMed] [Google Scholar]
- 47.Valgimigli M, Campo G, de Cesare N, et al. Intensifying platelet inhibition with tirofiban in poor responders to aspirin, clopidogrel, or both agents undergoing elective coronary intervention: results from the double-blind, prospective, randomized tailoring treatment with tirofiban in patients showing resistance to aspirin and/or resistance to clopidogrel study. Circulation. 2009;119:3215–3222. doi: 10.1161/CIRCULATIONAHA.108.833236. [DOI] [PubMed] [Google Scholar]
- 48.Mega JL, Close SL, Wiviott SD, et al. Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet. 2010;376:1312–1319. doi: 10.1016/S0140-6736(10)61273-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cattaneo M. The platelet P2Y12 receptor for adenosine diphosphate: congenital and drug-induced defects. Blood. 2011;117:2102–2112. doi: 10.1182/blood-2010-08-263111. [DOI] [PubMed] [Google Scholar]
- 50.Gurbel PA, Tantry US, Shuldiner AR, Kereiakes DJ. Genotyping: one piece of the puzzle to personalize antiplatelet therapy. J Am Coll Cardiol. 2010;56:112–116. doi: 10.1016/j.jacc.2010.04.008. [DOI] [PubMed] [Google Scholar]


