Abstract
AIM: To investigate the efficacy of rebamipide in a rat model of colitis and restitution of intestinal epithelial cells in vitro.
METHODS: Acute colitis was induced with trinitrobenzene sulfonic acid (TNBS) in male Wistar rats. Rats received intrarectal rebamipide treatment daily starting on day 7 and were sacrificed on day 14 after TNBS administration. The distal colon was removed to evaluate the various parameters of inflammation. Moreover, wound healing assays were used to determine the enhanced restitution of rat intestinal epithelial (RIE) cells treated with rebamipide.
RESULTS: Intracolonic administration of rebamipide accelerated TNBS-induced ulcer healing. Increases in the wet weight of the colon after TNBS administration were significantly inhibited by rebamipide. The wound assay revealed that rebamipide enhanced the migration of RIE cells through phosphorylation of extracellular signal-regulated kinase (ERK) and activation of Rho kinase.
CONCLUSION: Rebamipide enema healed intestinal injury by enhancing restitution of RIE cells, via ERK activation. Rebamipide might be a novel therapeutic approach for inflammatory bowel disease.
Keywords: Rebamipide, Experimental colitis, Intestinal epithelial cells, Extracellular signal-regulated kinase, Rho kinase
INTRODUCTION
Inflammatory bowel disease (IBD), including ulcerative colitis (UC) and Crohn’ s disease (CD), is a chronic and recurrent intestinal inflammatory disorder whose precise pathogenesis remains unknown[1]. In patients with IBD, a variety of cells and soluble factors mediate extensive mucosal damage, and epithelial cell damage is frequently observed[2]. Generally, after sustaining mucosal injury, the intestinal epithelium rapidly reestablishes its integrity via restitution, proliferation, and differentiation of epithelial cells[3]. Among these three steps, restitution is thought to be most critical for intestinal mucosal healing[4]. Therefore, the promotion of restitution remains an important therapeutic target. Along these lines, various molecules involved in regenerating the intestinal epithelium are currently under consideration for clinical use[5].
Rebamipide is an amino acid derivative of 2(1H)-quinolinone, and is a gastric mucosal protective and ulcer-healing agent that has been widely used for treatment of acute and chronic gastritis and gastric ulcer. It is already known that rebamipide has anti-inflammatory properties including scavenging of free radicals, suppression of pro-inflammatory cytokine production, inhibition of inflammatory cell migration and adherence, and promotion of prostaglandin and mucus production[6]. Recently, rebamipide has been used as a gastric protective agent and for treatment of UC[7]. First, Makiyama et al[8] have reported that rebamipide enema had an anti-inflammatory effect in a patient with proctitis-type UC. They also have reported the efficacy of rebamipide enemas in active distal UC and proctitis in a prospective study[9]. Furthermore, it has been demonstrated that rebamipide enema is safe and useful in corticosteroid-refractory or -dependent patients with the active distal type of UC[10]. In addition, Matsumoto et al[11] have reported that rebamipide enema ameliorates disease activity in patients with left-side ischemic colitis. Thus, these clinical data suggest that rebamipide shows promise in terms of its potential for repairing intestinal injury. However, the detailed molecular mechanism of action of rebamipide against intestinal inflammation remains unclear.
Therefore, in the present study, we aimed to assess the effect of rebamipide in intestinal inflammation by using the trinitrobenzene sulfonic acid (TNBS)-induced colitis model, a well-accepted IBD model. Furthermore, we analyzed the possible mechanisms involved in rebamipide-mediated mucosal restitution by using a well-established model of intestinal epithelial wound healing in vitro[12,13].
MATERIALS AND METHODS
Reagents
All chemicals were prepared immediately before use. Rebamipide {2-(4-chlorobenzoylamino)-3[2-(1H)-quinolinon-4-yl] propionic acid} was a kind gift from Otsuka Pharmaceutical Co. Ltd. (Tokyo, Japan). TNBS and 3, 3’, 5, 5’-tetramethylbenzidine were obtained from Wako Pure Chemicals (Osaka, Japan). We used MEK1/2 inhibitor as an extracellular signal-regulated kinase (ERK) inhibitor (U0126; BIOMOL International LP, Plymouth Meeting, PA, United States) and Y27632 [(1)-(R)-trans-4-(1-aminoethyl)-N-(4-pyridyl)cyclohexanecarboxamide dihydrochloride] as the Rho kinase inhibitor (Biaffin GmbH and Co KG, Kassel, Germany). All other chemicals were of the highest quality commercially available.
In vivo study animals
Male Wistar rats weighing 180-200 g were obtained from Shimizu Laboratory Supplies Co. Ltd. (Kyoto, Japan). The animals were housed at 22 °C in a controlled environment with 12 h of artificial light per day, and were allowed access to rat chow and water ad libitum. The animals were maintained and all experimental procedures were carried out in accordance with the National Institutes of Health (NIH) guidelines for the use of experimental animals. All experimental protocols were approved by the Animal Care Committee of the Kyoto Prefectural University of Medicine (Kyoto, Japan).
Induction of colitis
TNBS-induced colitis was established using the method of Morris et al[14]. The rats were lightly anesthetized with pentobarbital following a 48-h fast, and then a rubber catheter (outer diameter, 2 mm) was inserted via the anus, such that the tip was 8 cm from the anus. TNBS dissolved in 50% ethanol (120 mg/mL) was instilled into the lumen of the colon via the catheter (volume, 0.25 mL). Following the instillation of TNBS at 30 mg per rat, the anus was occluded with a clip for 1 h.
Treatment protocol
All animals were randomized into groups that received rebamipide or physiological saline vehicle. We focused on the effects of rebamipide during healing after colonic mucosal injury. One percent rebamipide was intrarectally administered (2 mL/kg) twice daily starting on day 7 after induction of colitis, until day 14.
Evaluation of colonic damage
The rats were sacrificed on day 14 and the distal colon was removed and opened by longitudinal incision. The wet colon weight was measured immediately thereafter. As indices of inflammation, damage was estimated macroscopically as the sum of the mucosal score. The mucosal score was rated on a six-point scale (0-5) according to the criteria established by Morris et al[14] (Table 1). The degree of colitis was evaluated by an independent observer who did not have previous knowledge of the treatment. For the histological examination, formalin-fixed tissue was stained with hematoxylin and eosin. The colon histological score was evaluated using the histopathological grading system of Ameho et al[15] by an observer blinded to the treatment. This grading, which takes into account the degree of infiltration, the presence of erosion, ulceration, or necrosis, and the depth and surface of the lesion, is scaled from 0 to 6.
Table 1.
Criteria for scoring gross morphological damage of the colon[12]
| Mucosal score | Gross morphology |
| 0 | No damage |
| 1 | Localized hyperemia, but no ulcers |
| 2 | Liner ulcers with no significant inflammation |
| 3 | Liner ulcers with inflammation at one site |
| 4 | Two or more sites of ulceration and/or inflammation |
| 5 | Two or more major sites of inflammation and ulceration extending > 1 cm along the length of the colon |
In vitro study of intestinal epithelial cell line
Non-transformed rat intestinal epithelial (RIE) cells were grown in a 1:1 mixture of Dulbecco’ s Modified Eagle’ s Medium and Ham’ s F12 medium supplemented with 5% heat-inactivated fetal bovine serum, 2 mmol/L glutamine, 100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin. The cells were incubated at 37 °C in a humidified atmosphere supplemented with 5% CO2. RIE cells were trypsinized and seeded into 60-mm culture dishes. Experiments were performed when the cells reached confluency.
Wound assay
Wound assays were performed using a previously described method with minor modifications[16]. Confluent monolayers of RIE cells in 60-mm culture dishes were washed with phosphate-buffered saline, and cells were cultured for an additional 24 h in serum-free medium. Subsequently, cell monolayers were disrupted using an extra long 10-μL pipette tip (Pelican Life Sciences, San Diego, CA, United States), followed by a cycle of washing in serum-free medium to yield a cell-free zone in the culture dishes. The process of migration was monitored using an inverted phase-contrast microscope at 0, 6 and 12 h after induction of the artificial wound. Changes in the cell-free zone were analyzed with the ImageJ software (Wayne Rashband; NIH, Bethesda, MD, United States), and this analysis was performed by the same individual under blind conditions to prevent observer bias. To investigate the effects of rebamipide on RIE cell migration, the cells were co-incubated with rebamipide (2 mmol/L) after wound induction. Furthermore, to investigate involvement of the ERK signaling pathway and the Rho kinase pathway, cells were co-treated with U0123 (10 µmol/L) or Y27632 (1 μmol/L) after wounding.
Western blotting analysis
To determine whether rebamipide was involved in the ERK signaling pathway, proteins were obtained from RIE cells at 0, 5, 15 and 20 min after stimulation with 2 mmol/L rebamipide. The total proteins were mixed with SDS sample buffer. The samples were then subjected to 10% SDS-PAGE and blotted onto a polyvinylidene fluoride membrane (Atto Corporation, Tokyo, Japan). The membrane was blocked with 2% bovine serum albumin in Tris-buffered saline that contained 0.1% Tween (TBS-T) at room temperature for 30 min. Western blotting analysis was carried out using rabbit polyclonal anti-p44/42, phospho-p44/42 (1:1000), and actin antibody (1:1000) as an internal control at room temperature for 1 h. After three washes with TBS-T, the membrane was incubated with anti-rabbit IgG–horseradish peroxidase (1:3000; GE Healthcare UK Ltd., Little Chalfont, Bucks, United Kingdom) at room temperature for 45 min. The signals were visualized using an ECL kit (GE Healthcare) according to the manufacturer’ s instructions.
Statistical analysis
Results are presented as the mean ± SE. Overall differences between groups were determined by one-way ANOVA. Whenever one-way ANOVA was significant, differences between individual groups were analyzed by Bonferroni’ s multiple comparisons test. Differences of P < 0.05 were considered significant. All analyses were performed using the GraphPad Prism 4 program (San Diego, CA, United States) for a Macintosh computer.
RESULTS
Therapeutic effect of rebamipide on TNBS colitis
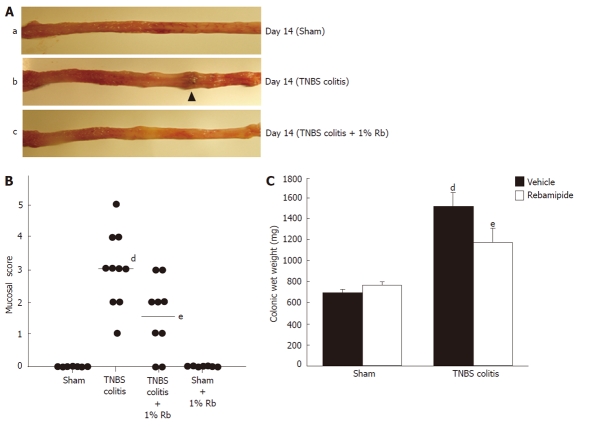
In rats exposed to TNBS, macroscopic findings in the colon demonstrated severe colitis with hyperemia, edema, thickening, ulceration, and necrosis. It has already been demonstrated that the lesion area reaches its maximum on day 2 or 3 after TNBS treatment, after which it decreases in a time-dependent manner[13,17]. In order to focus on the effects of mucosal healing by rebamipide enema, all rats were administered either placebo or rebamipide solution starting on day 7 after induction of TNBS, until day 14. On day 14, severe colitis with thickening of the mucosa and ulceration were still observed in the placebo group (Figure 1A). In contrast, rats treated with 1% rebamipide showed smaller erosions and mild edema in the colon (Figure 1A). Thus, the colonic mucosal damage score on day 14 had significantly increased due to TNBS administration in the sham-treated group. Increases in the mucosal damage score were significantly inhibited by treatment with 1% rebamipide (Figure 1B). Furthermore, the colonic wet weight was significantly increased in the TNBS colitis group. This increase was significantly decreased by treatment with 1% rebamipide (Figure 1C).
Figure 1.
Effects of 1% rebamipide on macroscopic findings, mucosal damage score, and wet colon weight on day 14 after trinitrobenzene sulfonic acid-induced injury. A: Severe colitis was induced with hyperemia, edema, thickening, ulceration, and necrosis in trinitrobenzene sulfonic acid (TNBS)-colitis rats (b) compared to sham-operated rats (a). These changes were reduced in rats treated with 1% rebamipide (TNBS-colitis rats treated with 1% rebamipide) (c). B: A 1% rebamipide enema was administrated twice daily starting on day 7 after induction of colitis, until day 14. Rats were sacrificed on day 14, and the mucosal damage score was evaluated. Data are expressed as a scatter plot. dP < 0.01 vs sham-treated rats. eP < 0.05 vs TNBS-induced colitis rats receiving the vehicle. C: Rats were sacrificed on day 14 and the distal colon was removed, after which, the wet colon weight was immediately measured. Data represent the mean ± SE of seven rats. dP < 0.01 vs sham-treated rats receiving the vehicle. eP < 0.01 vs TNBS-induced colitis rats receiving the vehicle.
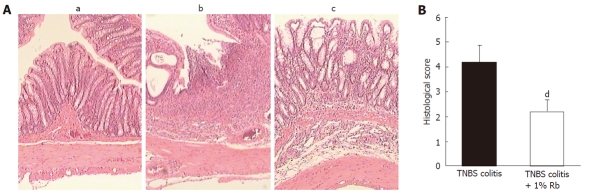
The therapeutic effects of 1% rebamipide enema were also confirmed by histological examination. Figure 2A shows the representative histological features of a normal colon (day 0) and those of the control group (day 14) and the rebamipide-treated group (day 14). TNBS administration induced marked thickening of the colonic wall, with transmural infiltration and aggregation of numerous inflammatory cells (Figure 2A), which is in contrast to the features of the normal colon, which does not show transmural infiltration or aggregation of inflammatory cells (Figure 2A). On the contrary, in rats treated with 1% rebamipide, inhibition of both mural wall thickening and inflammatory cell infiltration was observed (Figure 2A). More importantly, rebamipide enema promoted restitution of the colonic epithelium in the ulcerative area. The histological score was increased in the TNBS colitis group, and this increase was significantly inhibited by treatment with 1% rebamipide (Figure 2B).
Figure 2.
Effects of 1% rebamipide on histological findings in the colon on day 14 after trinitrobenzene sulfonic acid-induced injury. A: Histological appearance of colonic tissue in sham-operated rats (a), trinitrobenzene sulfonic acid (TNBS)-colitis rats (b), and TNBS-colitis rats treated with 1% rebamipide (c). Histological examination revealed that TNBS administration induced marked thickening of the colonic wall, which was associated with transmural infiltration of inflammatory cells. In contrast, both mural wall thickening and infiltration of inflammatory cells were inhibited in rats treated with 1% rebamipide. Hematoxylin and eosin staining (× 40). B: Histological score was evaluated. Data represent the mean ± SE of six rats. dP < 0.01 vs TNBS-colitis rats receiving the vehicle.
Effects of rebamipide treatment as determined by wound assay using RIE cells
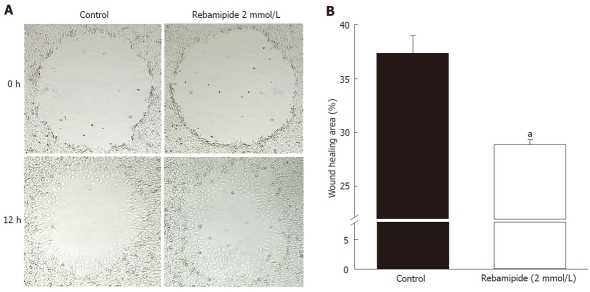
To investigate the effect of rebamipide on restitution of the intestinal epithelium, we performed a wound assay using RIE cells. The cell-free area at the wound site gradually decreased in a time-dependent manner, and complete recovery was observed 15 h after wound induction (data not shown). Restitution was significantly enhanced by rebamipide treatment (Figure 3A). Treatment with 2 mmol/L rebamipide accelerated wound healing compared to the control group (Figure 3B).
Figure 3.
Restitution of rat intestinal epithelial cells around an artificially created wound in control and rebamipide-treated groups. A: Restitution of rat intestinal epithelial (RIE) cells was evaluated using a wound assay. The denuded area of RIE cells recovered in a time-dependent manner after wound induction. Restitution of the denuded area was promoted by rebamipide at 12 h after wound induction. B: To investigate the effects of rebamipide on RIE cell migration, cells were co-incubated with 2 mmol/L rebamipide after wound induction. Wound repair in 2 mmol/L rebamipide-treated cells occurred significantly earlier than it did in the controls. Datas represent the mean ± SE of four experiments. aP < 0.05 vs controls.
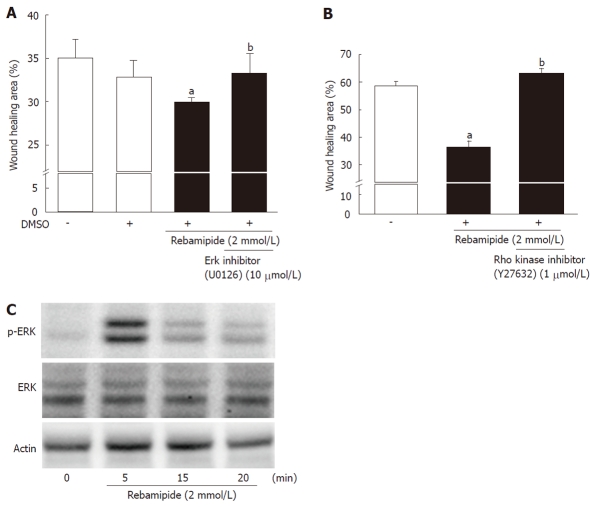
To investigate whether the promotion of restitution by rebamipide was involved in ERK signaling, RIE cells were stimulated with 2 mmol/L rebamipide by using a specific ERK inhibitor (U01263). As shown in Figure 4A, the ERK inhibitor blocked the promotion of wound healing by rebamipide. Moreover, to confirm the involvement of the ERK signaling pathway in the enhanced restitution associated with rebamipide treatment, we performed western blot analysis using phosphorylation-status-dependent and -independent antibodies against ERK1/2 (44 and 42 kDa) at 0, 5, 15 and 20 min after treatment with 2 mmol/L rebamipide. The western blots revealed ERK phosphorylation in RIE cells 5 min after rebamipide treatment (Figure 4B). Furthermore, to examine the role of Rho kinase in rebamipide-enhanced restitution, RIE cells were stimulated with rebamipide by using Y27632, a Rho kinase inhibitor (1 μmol/L). The inhibition of Rho kinase canceled the promotion of wound healing by rebamipide treatment.
Figure 4.
Involvement of extracellular signal-regulated kinase and Rho in rebamipide-treated rat intestinal epithelial cells. A: Rat intestinal epithelial (RIE) cells were treated with rebamipide (2 mmol/L) with or without an extracellular signal-regulated kinase inhibitor (U01263, 10 μmol/L) after wound induction. The wound healing area (12 h later) was then monitored. Data represent the mean ± SE of four experiments. aP < 0.05 vs controls. bP < 0.05 vs the 2 mmol/Lrebamipide-treated group. B: Expression of p44/42 and phospho-p44/42 in RIE cells incubated with rebamipide (2 mmol/L) was measured using western blot analysis. Actin antibody was used as an internal control. Representative data from three observations is shown. C: RIE cells were treated with rebamipide (2 mmol/L) with or without Rho kinase inhibitor (Y27632, 1 μmol/L) after wound induction. The wound healing area (6 h later) was then monitored. Data represent the mean ± SE of four experiments. aP < 0.05 vs controls. bP < 0.05 vs the 2 mmol/L rebamipide-treated group. DMSO: Dimethyl sulfoxide.
DISCUSSION
In the present study, we demonstrated that rebamipide enema promoted wound healing in rats with TNBS-induced colonic ulceration. In this model, the area of colonic ulceration peaked on day 2 or 3 after TNBS treatment, and subsequent amelioration was observed in a time-dependent manner. In this study, rats were treated with 1% rebamipide enema starting on day 7 after TNBS injury induction, until day 14. Rebamipide clearly accelerated colonic wound healing under these conditions. These findings are consistent with previous results showing the beneficial effects of rebamipide enema in patients with active UC[7-10], as well as with the results of a study using another experimental colitis model treated with rebamipide enema[18-20].
Rebamipide is a gastric protective and ulcer healing agent that was developed in Japan. It is used clinically in Japan in combination with acid suppressive agents for gastric mucosal protection, acute and chronic gastritis treatment, and gastroduodenal ulcer healing. More interestingly, accumulating evidence suggests that rebamipide exerts protective and healing effects on other tissues. In fact, rebamipide has been shown to be effective in the treatment of patients with UC. In addition, the therapeutic effect of rebamipide may not be limited to the colon alone: indeed, this agent has been demonstrated by both clinical and basic research as effective for the treatment of stomatitis[21] and pulmonary[22], renal[23] and liver damage[24-26], and it also provides corneal protection[27-29].
The mechanisms responsible for amelioration of colitis by rebamipide have not been fully elucidated but may involve inhibition of reactive oxygen species production[19,20,30], suppression of neutrophil accumulation[31,32], increases in trans-epithelial electrical resistance[33], and induction of hepatocyte growth factor expression[34]. However, it remains unclear whether rebamipide enhances restitution of RIE cells. It is already known that rebamipide enhances gastric epithelial restitution, but the same has yet to be proven in intestinal epithelial restitution. In cases of intestinal mucosal injury, restitution is an important step in re-establishing mucosal integrity, and restitution is the most rapid post-injury response; in effect, restitution restores the continuity of the intestinal epithelial layer, primarily by redistribution of epithelial cells. This process is completed by the migration of local epithelial cells along the underlying matrix, which does not require epithelial cell proliferation[35,36]. In this study, to assess the effects of rebamipide on intestinal epithelial restitution, the round wound assay was evaluated using RIE cells. Our results showed that 2 mmol/L rebamipide treatment promoted the restitution of RIE cells.
With regard to the role of the ERK signaling pathway in epithelial restitution, several studies have been reported in which activation of the ERK signaling pathway played an important role in epithelial wound closure. In the present study, rebamipide promoted ERK phosphorylation. This result is in agreement with the results of Gazel et al[37] and Wang et al[38]. Moreover, rebamipide-promoted restitution was suppressed by U01263, a specific ERK inhibitor. These data indicate that accelerated restitution by rebamipide is at least partly mediated by the ERK pathway. Tanigawa et al[39] have demonstrated that rebamipide induces ERK phosphorylation in gastric cancer cells and inhibits cell growth through Smad signaling. Although they used gastric cancer cells, they also found that rebamipide induced phosphorylation of ERK.
Furthermore, we investigated whether rebamipide-promoted restitution was related to Rho kinase activation. Rho kinase has been identified as one of the effectors of the small GTP-binding protein Rho. Accumulating evidence has demonstrated that the Rho/Rho kinase pathway plays an important role in various cellular functions, including cell contraction, cell proliferation, gene expression, and especially cell migration[40,41]. With regard to restitution of IE cells, Santos et al[42] have found that Rho protein is one of the essential elements of a mechanism by which growth factors induce cell migration to restore mucosal integrity, and Rao et al[43] have demonstrated that activation of Rho kinase results in increased phosphorylation of the myosin light chain, which leads to cell migration. In this investigation, we used Y27632, which has been widely used as a specific inhibitor of the Rho-associated coiled-coil forming protein serine/threonine kinase family of protein kinases[44]. Co-treatment with Y27632 cancelled the effect of rebamipide on the restitution of RIE cells. These data indicate that rebamipide enhanced the restitution of RIE cells via ERK phosphorylation and Rho kinase activation. However, the detailed mechanism of rebamipide-induced ERK and Rho kinase activation remains unknown. Further investigations are needed to elucidate this mechanism.
In summary, the present study indicates that treatment with rebamipide can promote the healing of TNBS-induced intestinal injury, which is associated with acceleration of intestinal epithelial restitution. The present results suggest that rebamipide has great potential as a new therapeutic agent for the treatment of inflammation-associated intestinal injury.
COMMENTS
Background
Ulcerative colitis (UC) is a chronic and recurrent disorder of the colon and rectum. While the precise pathogenesis of UC remains unknown, medical management of patients with acute exacerbation of UC symptoms focuses on achieving remission by inhibiting intestinal inflammation and repairing mucosal injury. However, some patients with inflammatory bowel disease do not respond, or respond incompletely, to the existing treatments. Therefore, it is important to investigate new anti-inflammatory strategies.
Research frontiers
Rebamipide is a gastric mucosal protective and ulcer-healing agent, and has been used for treatment of UC. However, the detailed mechanism of action of rebamipide against intestinal inflammation such as UC remains unclear. In this study, the authors investigated the therapeutic efficacy of rebamipide in an experimental rat model of colitis and evaluated the restitution of intestinal epithelial cells treated with rebamipide in vitro.
Innovations and breakthroughs
The present study indicated that treatment with rebamipide could promote the healing of trinitrobenzene sulfonic acid-induced intestinal injury, which has been associated with acceleration of intestinal epithelial restitution through extracellular signal-regulated kinase and Rho kinase activation. The present results suggest that rebamipide has great potential as a new therapeutic agent for the treatment of inflammation-associated intestinal injury.
Applications
By understanding how rebamipide inhibits intestinal inflammation and promotes healing of the intestinal injury, rebamipide may represent a future therapeutic agent for treatment of patients with UC.
Terminology
Rho kinase has been identified as one of the effectors of the small GTP-binding protein Rho. Accumulating evidence has demonstrated that the Rho/Rho kinase pathway plays an important role in various cellular functions, including cell contraction, cell proliferation, gene expression, and especially, cell migration.
Peer review
This is an interesting, well-designed study with good documentation of results.
Footnotes
Supported by A Grant-in-Aid for Scientific Research (B) to Toshikazu Yoshikawa (Grant No. 21390184) and Challenging Exploratory Research to Yuji Naito (No. 08101559) from the Japan Society for the Promotion of Science; A City Area Program to Toshikazu Yoshikawa and Yuji Naito from Ministry of Education, Culture, Sports, Science and Technology, Japan; An Adaptable and Seamless Technology Transfer Program through target-driven R&D to Yuji Naito from Japan Science and Technology Agency
Peer reviewer: Ibrahim A Al Mofleh, Professor, Deaprtment of Medicine, College of Medicine, King Saud University, PO Box 2925, 11461 Riyadh, Saudi Arabia
S- Editor Tian L L- Editor Kerr C E- Editor Li JY
References
- 1.Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 2.Fiocchi C. Inflammatory bowel disease: etiology and pathogenesis. Gastroenterology. 1998;115:182–205. doi: 10.1016/s0016-5085(98)70381-6. [DOI] [PubMed] [Google Scholar]
- 3.Podolsky DK. Mucosal immunity and inflammation. V. Innate mechanisms of mucosal defense and repair: the best offense is a good defense. Am J Physiol. 1999;277:G495–G499. doi: 10.1152/ajpgi.1999.277.3.G495. [DOI] [PubMed] [Google Scholar]
- 4.Okamoto R, Watanabe M. Cellular and molecular mechanisms of the epithelial repair in IBD. Dig Dis Sci. 2005;50 Suppl 1:S34–S38. doi: 10.1007/s10620-005-2804-5. [DOI] [PubMed] [Google Scholar]
- 5.van Deventer SJ. Small therapeutic molecules for the treatment of inflammatory bowel disease. Gut. 2002;50 Suppl 3:III47–III53. doi: 10.1136/gut.50.suppl_3.iii47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Naito Y, Yoshikawa T. Rebamipide: a gastrointestinal protective drug with pleiotropic activities. Expert Rev Gastroenterol Hepatol. 2010;4:261–270. doi: 10.1586/egh.10.25. [DOI] [PubMed] [Google Scholar]
- 7.Skrypnyk IN. Diagnostic and treatment algorithms of ulcerative colitis in Ukraine. Dig Dis. 2009;27:550–554. doi: 10.1159/000233296. [DOI] [PubMed] [Google Scholar]
- 8.Makiyama K, Takeshima F, Kawasaki H, Zea-Iriarte WL. Anti-inflammatory effect of rebamipide enema on proctitis type ulcerative colitis: a novel therapeutic alternative. Am J Gastroenterol. 2000;95:1838–1839. doi: 10.1111/j.1572-0241.2000.02154.x. [DOI] [PubMed] [Google Scholar]
- 9.Makiyama K, Takeshima F, Hamamoto T. Efficacy of rebamipide enemas in active distal ulcerative colitis and proctitis: a prospective study report. Dig Dis Sci. 2005;50:2323–2329. doi: 10.1007/s10620-005-3055-1. [DOI] [PubMed] [Google Scholar]
- 10.Miyata M, Kasugai K, Ishikawa T, Kakumu S, Onishi M, Mori T. Rebamipide enemas-new effective treatment for patients with corticosteroid dependent or resistant ulcerative colitis. Dig Dis Sci. 2005;50 Suppl 1:S119–S123. doi: 10.1007/s10620-005-2816-1. [DOI] [PubMed] [Google Scholar]
- 11.Matsumoto S, Tsuji K, Shirahama S. Rebamipide enema therapy for left-sided ischemic colitis patients accompanied by ulcers: open label study. World J Gastroenterol. 2008;14:4059–4064. doi: 10.3748/wjg.14.4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lotz MM, Rabinovitz I, Mercurio AM. Intestinal restitution: progression of actin cytoskeleton rearrangements and integrin function in a model of epithelial wound healing. Am J Pathol. 2000;156:985–996. doi: 10.1016/S0002-9440(10)64966-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Takagi T, Naito Y, Okuda T, Uchiyama K, Adachi S, Mizushima K, Handa O, Kokura S, Ichikawa H, Yoshikawa T. Ecabet sodium promotes the healing of trinitrobenzene-sulfonic-acid-induced ulceration by enhanced restitution of intestinal epithelial cells. J Gastroenterol Hepatol. 2010;25:1259–1265. doi: 10.1111/j.1440-1746.2010.06263.x. [DOI] [PubMed] [Google Scholar]
- 14.Morris GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803. [PubMed] [Google Scholar]
- 15.Ameho CK, Adjei AA, Harrison EK, Takeshita K, Morioka T, Arakaki Y, Ito E, Suzuki I, Kulkarni AD, Kawajiri A, et al. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut. 1997;41:487–493. doi: 10.1136/gut.41.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Petit V, Boyer B, Lentz D, Turner CE, Thiery JP, Vallés AM. Phosphorylation of tyrosine residues 31 and 118 on paxillin regulates cell migration through an association with CRK in NBT-II cells. J Cell Biol. 2000;148:957–970. doi: 10.1083/jcb.148.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bell CJ, Gall DG, Wallace JL. Disruption of colonic electrolyte transport in experimental colitis. Am J Physiol. 1995;268:G622–G630. doi: 10.1152/ajpgi.1995.268.4.G622. [DOI] [PubMed] [Google Scholar]
- 18.Murai R, Kanbe T, Mukoyama T, Shimomura T, Hashiguchi K, Yoshida Y, Tsuchiya H, Hoshikawa Y, Kurimasa A, Shiota G. Effect of rectal administration of rebamipide on dextran sulfate sodium-induced colitis: role of hepatocyte growth factor. Inflamm Res. 2007;56:240–245. doi: 10.1007/s00011-007-6100-z. [DOI] [PubMed] [Google Scholar]
- 19.Nakamura M, Takahashi T, Matsumoto T, Atsuda K, Hibi N, Matsui H, Yamada H, Tsuchimoto K. Direct autoradiographic evidence that rebamipide interacts with neutrophils in dextran sulfate sodium induced colitis in rats. Dig Dis Sci. 2005;50 Suppl 1:S113–S118. doi: 10.1007/s10620-005-2815-2. [DOI] [PubMed] [Google Scholar]
- 20.Okayama M, Tsubouchi R, Nishio H, Kato S, Takeuchi K. Protective effect of intra-rectal administration of rebamipide on dextran sulfate sodium-induced rat colitis. Digestion. 2004;70:240–249. doi: 10.1159/000083716. [DOI] [PubMed] [Google Scholar]
- 21.Matsuda T, Ohno S, Hirohata S, Miyanaga Y, Ujihara H, Inaba G, Nakamura S, Tanaka S, Kogure M, Mizushima Y. Efficacy of rebamipide as adjunctive therapy in the treatment of recurrent oral aphthous ulcers in patients with Behçet’ s disease: a randomised, double-blind, placebo-controlled study. Drugs R D. 2003;4:19–28. doi: 10.2165/00126839-200304010-00002. [DOI] [PubMed] [Google Scholar]
- 22.Ro JY, Kim JY, Kim KH. The inhibitory mechanism of rebamipide on the mediator release in the guinea pig lung mast cells activated with specific antigen-antibody reactions. Pharmacology. 2001;63:175–184. doi: 10.1159/000056130. [DOI] [PubMed] [Google Scholar]
- 23.Saad SY, Najjar TA, Al-Sohaibani MO. The effect of rebamipide on cisplatin-induced nephrotoxicity in rats. Pharmacol Res. 2000;42:81–86. doi: 10.1006/phrs.2000.0662. [DOI] [PubMed] [Google Scholar]
- 24.Hong KW, Kim KE, Rhim BY, Lee WS, Kim CD. Effect of rebamipide on liver damage and increased tumor necrosis factor in a rat model of endotoxin shock. Dig Dis Sci. 1998;43:154S–159S. [PubMed] [Google Scholar]
- 25.Lee SM, Kim KH. Rebamipide ameliorates hepatic dysfunction induced by ischemia/reperfusion in rats. Eur J Pharmacol. 1995;294:41–46. doi: 10.1016/0014-2999(95)00507-2. [DOI] [PubMed] [Google Scholar]
- 26.Tokuhara K, Hamada Y, Tanaka H, Yamada M, Ozaki T, Matsui K, Kamiyama Y, Nishizawa M, Ito S, Okumura T. Rebamipide, anti-gastric ulcer drug, up-regulates the induction of iNOS in proinflammatory cytokine-stimulated hepatocytes. Nitric Oxide. 2008;18:28–36. doi: 10.1016/j.niox.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 27.Ríos JD, Shatos M, Urashima H, Tran H, Dartt DA. OPC-12759 increases proliferation of cultured rat conjunctival goblet cells. Cornea. 2006;25:573–581. doi: 10.1097/01.ico.0000208819.24990.0d. [DOI] [PubMed] [Google Scholar]
- 28.Urashima H, Okamoto T, Takeji Y, Shinohara H, Fujisawa S. Rebamipide increases the amount of mucin-like substances on the conjunctiva and cornea in the N-acetylcysteine-treated in vivo model. Cornea. 2004;23:613–619. doi: 10.1097/01.ico.0000126436.25751.fb. [DOI] [PubMed] [Google Scholar]
- 29.Tanito M, Takanashi T, Kaidzu S, Yoshida Y, Ohira A. Cytoprotective effects of rebamipide and carteolol hydrochloride against ultraviolet B-induced corneal damage in mice. Invest Ophthalmol Vis Sci. 2003;44:2980–2985. doi: 10.1167/iovs.02-1043. [DOI] [PubMed] [Google Scholar]
- 30.Sakurai K, Osaka T, Yamasaki K. Protection by rebamipide against acetic acid-induced colitis in rats: relationship with its antioxidative activity. Dig Dis Sci. 1998;43:125S–133S. [PubMed] [Google Scholar]
- 31.Iwai A, Iwashita E. Changes in colonic inflammation induced by dextran sulfate sodium (DSS) during short- and long-term administration of rebamipide. Dig Dis Sci. 1998;43:143S–147S. [PubMed] [Google Scholar]
- 32.Kishimoto S, Haruma K, Tari A, Sakurai K, Nakano M, Nakagawa Y. Rebamipide, an antiulcer drug, prevents DSS-induced colitis formation in rats. Dig Dis Sci. 2000;45:1608–1616. doi: 10.1023/a:1005525313856. [DOI] [PubMed] [Google Scholar]
- 33.Nakashima T, Maeda T, Nagamoto H, Kumakura T, Takai M, Mori T. Rebamipide enema is effective for treatment of experimental dextran sulfate sodium induced colitis in rats. Dig Dis Sci. 2005;50 Suppl 1:S124–S131. doi: 10.1007/s10620-005-2817-0. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi M, Takada H, Takagi K, Kataoka S, Soma R, Kuwayama H. Gastric restitution is inhibited by dexamethasone, which is reversed by hepatocyte growth factor and rebamipide. Aliment Pharmacol Ther. 2003;18 Suppl 1:126–132. doi: 10.1046/j.1365-2036.18.s1.19.x. [DOI] [PubMed] [Google Scholar]
- 35.Taupin D, Podolsky DK. Trefoil factors: initiators of mucosal healing. Nat Rev Mol Cell Biol. 2003;4:721–732. doi: 10.1038/nrm1203. [DOI] [PubMed] [Google Scholar]
- 36.Wong WM, Playford RJ, Wright NA. Peptide gene expression in gastrointestinal mucosal ulceration: ordered sequence or redundancy? Gut. 2000;46:286–292. doi: 10.1136/gut.46.2.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gazel A, Nijhawan RI, Walsh R, Blumenberg M. Transcriptional profiling defines the roles of ERK and p38 kinases in epidermal keratinocytes. J Cell Physiol. 2008;215:292–308. doi: 10.1002/jcp.21394. [DOI] [PubMed] [Google Scholar]
- 38.Wang Z, Yang H, Tachado SD, Capó-Aponte JE, Bildin VN, Koziel H, Reinach PS. Phosphatase-mediated crosstalk control of ERK and p38 MAPK signaling in corneal epithelial cells. Invest Ophthalmol Vis Sci. 2006;47:5267–5275. doi: 10.1167/iovs.06-0642. [DOI] [PubMed] [Google Scholar]
- 39.Tanigawa T, Pai R, Arakawa T, Tarnawski AS. Rebamipide inhibits gastric cancer cell growth. Dig Dis Sci. 2007;52:240–247. doi: 10.1007/s10620-006-9226-x. [DOI] [PubMed] [Google Scholar]
- 40.Cetin S, Ford HR, Sysko LR, Agarwal C, Wang J, Neal MD, Baty C, Apodaca G, Hackam DJ. Endotoxin inhibits intestinal epithelial restitution through activation of Rho-GTPase and increased focal adhesions. J Biol Chem. 2004;279:24592–24600. doi: 10.1074/jbc.M313620200. [DOI] [PubMed] [Google Scholar]
- 41.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 42.Santos MF, McCormack SA, Guo Z, Okolicany J, Zheng Y, Johnson LR, Tigyi G. Rho proteins play a critical role in cell migration during the early phase of mucosal restitution. J Clin Invest. 1997;100:216–225. doi: 10.1172/JCI119515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rao JN, Guo X, Liu L, Zou T, Murthy KS, Yuan JX, Wang JY. Polyamines regulate Rho-kinase and myosin phosphorylation during intestinal epithelial restitution. Am J Physiol Cell Physiol. 2003;284:C848–C859. doi: 10.1152/ajpcell.00371.2002. [DOI] [PubMed] [Google Scholar]
- 44.Ishizaki T, Uehata M, Tamechika I, Keel J, Nonomura K, Maekawa M, Narumiya S. Pharmacological properties of Y-27632, a specific inhibitor of rho-associated kinases. Mol Pharmacol. 2000;57:976–983. [PubMed] [Google Scholar]